Abstract
African Americans (AAs) and obese individuals have increased thrombotic risk. This study evaluated the effectiveness and safety of rivaroxaban versus warfarin in obese, AAs with nonvalvular atrial fibrillation (NVAF) or venous thromboembolism (VTE). Optum® De-Identified Electronic Health Record (EHR) data was used to perform separate propensity-score matched analyses of adult, oral anticoagulant (OAC)-naïve AAs with NVAF or acute VTE, respectively; who had a body mass index≥30kg/m2 and ≥12-months EHR activity with ≥1-encounter before OAC initiation. Cox regression was performed and reported as hazard ratios (HRs) with 95% confidence intervals (CIs). For the NVAF analysis, 1,969 rivaroxaban- and 1,969 warfarin-users were matched. Rivaroxaban was not associated with a difference in stroke/systemic embolism versus warfarin (HR = 0.88, 95%CI = 0.60-1.28), but less major bleeding (HR = 0.68, 95%CI = 0.50-0.94) was observed. Among 683 rivaroxaban-users with VTE, 1:1 matched to warfarin-users, rivaroxaban did not alter recurrent VTE (HR = 1.36, 95%CI = 0.79-2.34) or major bleeding (HR = 0.80, 95%CI = 0.37-1.71) risk versus warfarin at 6-months (similar findings observed at 3- and 12-months). Rivaroxaban appeared to be associated with similar thrombotic, and similar or lower major bleeding risk versus warfarin in these obese, AA cohorts.
Keywords: rivaroxaban, warfarin, African American, obesity, atrial fibrillation, venous thromboembolism
Introduction
Black patients appear genetically predisposed to an increased risk of thrombosis compared to other races including Asians, Hispanics and Caucasians.1,2 This increased risk of thrombotic events extends to stroke secondary to atrial fibrillation3 and acute venous thromboembolism (VTE).4–6 In addition to increased genetic risk, ∼50% of black patients are obese (body mass index (BMI) ≥30 kg/m2),7 placing them at further risk of thrombotic events.8,9
Not only are black patients at a higher risk of thrombotic events, but they may also experience a poorer pharmacologic response to certain oral anticoagulants.10,11 Black patients treated with warfarin often take longer to achieve a therapeutic international normalized ratio (INR) compared to other races10 and spend less time (<50%) in the therapeutic target range.11 Similar results have been observed in obese patients, regardless of race, taking warfarin; with longer times to achieve therapeutic INRs and requirements for higher warfarin doses compared to patients of normal weight.12 Conversely, body weight and BMI appear to have a limited impact on the pharmacokinetics/ pharmacodynamics, effectiveness, safety or tolerability of rivaroxaban.13–15
Prior studies have compared rivaroxaban and warfarin in either an African American or an obese population with non-valvular atrial fribilation (NVAF) or experiencing an acute VTE.16–22 These studies have suggested rivaroxaban is associated with similar or better outcomes compared to warfarin. A paucity of studies has evaluated the effectiveness and safety of rivaroxaban and warfarin in obese, African American patients with NVAF or acute VTE.
The goal of this study was to evaluate the effectiveness and safety of rivaroxaban versus warfarin in obese, African American patients with NVAF or suffering an acute VTE.
Methods
US Optum® De-Identified electronic health record (EHR) data from November 1, 2010 through September 30, 2018 was used to perform two separate (one in NVAF patients, a second in patients experiencing an acute VTE) analyses.23 The Optum EHR database includes longitudinal patient-level medical record data for 97 million patients seen at ∼700 hospitals and ∼7,000 clinics across the United States. The database includes records of prescriptions as prescribed and administered, laboratory results, vital signs, body measurements, diagnoses and procedures.
To be included in either analysis, patients had to be a self-reported African American adult, have ≥12-months of EHR activity prior to the index date (initiation of oral anticoagulant), received care documented in the EHR database from ≥1 provider in the 12-months prior and be obese (have a baseline BMI ≥30 kg/m2 per the National Heart, Lung and Blood Institute using anthropometric measurements closest to the index date) and within the 12-month baseline period).24 The NVAF analysis included patients with ≥1 claim for atrial fibrillation (without evidence of valvular heart disease) during the baseline period, newly prescribed rivaroxaban or warfarin after November 2011 (rivaroxaban’s US approval date for NVAF) and absent any alternative indication for oral anticoagulation use (e.g., venous thromboembolism, hip or knee replacement surgery) or any prior oral anticoagulation utilization per written prescription or patient self-report at baseline. The VTE analysis included patients admitted to the hospital, emergency department or observation unit for acute VTE,25 who were prescribed rivaroxaban or warfarin as their first oral anticoagulant within 7-days after the thrombotic event, initiated on rivaroxaban or warfarin after November 2012 (rivaroxaban’s US approval date for acute VTE treatment) and absent any alternative indication for oral anticoagulation use (e.g., NVAF, hip or knee replacement surgery), any prior oral anticoagulation utilization per written prescription or patient self-report at baseline or evidence of active cancer. The last exclusion criterion was imposed as cancer-associated thrombosis is typically treated as a distinct clinical entity and has its own unique treatment pathways (which de-emphasizes this study’s control, warfarin).
For each analysis (NVAF and VTE, separately) patients prescribed rivaroxaban were 1:1 matched to patients prescribed warfarin based on propensity scores calculated via multivariable logistic regression.26 Propensity scores were estimated separately based upon commonly used variables and accepted risk factors for differential oral anticoagulation exposure specific to the disease state being evaluated (65 covariates for NVAF and 40 for VTE) including demographics, comorbidities and concurrent outpatient co-medications identified during the baseline period (Tables 1 and 2, eTables 1-3). In addition, both analyses forced exact matching on oral anticoagulant initiation year. The presence of residual differences in measured covariates following cohort matching (using a caliper = 0.20 standard deviations of the logit of the propensity score) were assessed by calculating absolute standardized differences (<0.1 considered well-balanced for each covariate).26 Propensity score matching was performed using the “MatchIT” package and R statistical software (version 3.4.3, The R Project for Statistical Computing).
Table 1.
Baseline Characteristics of 1:1 Propensity Score Matched Rivaroxaban and Warfarin Non-Valvular Atrial Fibrillation Cohorts.
| Rivaroxaban, % (N = 1,969) | Warfarin, % (N = 1,969) | Standardized difference | |
|---|---|---|---|
| Demographics | |||
| Female | 49.26 | 50.33 | −0.0213 |
| Age ≤ 64 years | 55.31 | 52.51 | 0.0567 |
| Age 65-74 years | 26.87 | 28.49 | −0.0369 |
| Age ≥ 75 years | 17.83 | 18.99 | −0.0323 |
| EGFR < 15 mL/minute | 0.51 | 0.61 | −0.0158 |
| EGFR 15-29 mL/minute | 3.61 | 4.93 | −0.0758 |
| EGFR 30-59 mL/minute | 18.18 | 20.67 | −0.0662 |
| EGFR 60-89 mL/minute | 41.75 | 40.83 | 0.0186 |
| EGFR ≥ 90 mL/minute | 32.76 | 29.30 | 0.0722 |
| EGFR unknown | 3.10 | 3.56 | −0.0262 |
| Creatinine clearance < 50 mL/minute† | 5.84 | 8.02 | † |
| BMI 30-34.9 kg/m2 | 39.26 | 38.55 | 0.0147 |
| BMI 35-39.9 kg/m2 | 26.36 | 26.76 | −0.0092 |
| BMI ≥ 40 kg/m2 | 34.38 | 34.69 | −0.0064 |
| Past Medical History* | |||
| Asthma | 14.32 | 14.17 | 0.0042 |
| CABG | 6.09 | 6.70 | −0.0266 |
| COPD | 15.59 | 17.27 | −0.0469 |
| Diabetes mellitus | 45.10 | 46.42 | −0.0265 |
| Heart failure | 22.55 | 25.95 | −0.0813 |
| Hypertension | 86.19 | 85.27 | 0.0271 |
| Myocardial infarction | 9.70 | 11.48 | −0.0615 |
| Peripheral vascular disease | 8.68 | 9.90 | −0.0439 |
| Percutaneous coronary intervention | 6.75 | 6.91 | −0.0061 |
| History of GI bleed | 0.51 | 0.81 | −0.0475 |
| Inflammatory bowel diseases | 0.61 | 0.71 | −0.0145 |
| Liver disease | 1.98 | 1.83 | 0.0115 |
| Coagulopathy | 2.84 | 2.69 | 0.0098 |
| GERD | 20.47 | 20.47 | 0.0000 |
| Anemia | 18.44 | 21.23 | −0.0739 |
| History of ischemic stroke | 8.08 | 9.70 | −0.0614 |
| Sleep apnea | 26.66 | 27.22 | −0.0125 |
| Smoker | 15.08 | 16.10 | −0.0271 |
| Hemorrhoids | 3.15 | 2.84 | 0.0182 |
| Alcohol abuse | 0.36 | 0.30 | 0.0089 |
| Anxiety | 10.11 | 9.80 | 0.0099 |
| Depression | 0.96 | 1.02 | −0.0049 |
| Psychosis | 0.81 | 0.86 | −0.0058 |
| Rheumatoid arthritis | 7.57 | 7.01 | 0.0215 |
| Osteoarthritis | 25.39 | 25.95 | −0.0128 |
| Headache | 6.65 | 6.25 | 0.0169 |
| Diverticulitis | 5.03 | 4.62 | 0.0190 |
| H. pylori | 0.61 | 0.36 | 0.0348 |
| Hypothyroidism | 0.81 | 1.02 | −0.0210 |
| Proteinuria | 0.91 | 1.22 | −0.0337 |
| Dementia | 1.68 | 1.98 | −0.0259 |
| Carotid stenosis | 2.49 | 2.64 | −0.0098 |
| Tumor | 5.64 | 5.99 | −0.0156 |
| Lymph | 0.76 | 0.76 | 0.0000 |
| Metastasis | 0.96 | 1.02 | −0.0051 |
| Medications | |||
| Aspirin | 42.81 | 44.18 | −0.0277 |
| P2Y12 inhibitors | 7.52 | 7.87 | −0.0135 |
| NSAID | 20.98 | 20.72 | 0.0060 |
| Celecoxib | 1.73 | 1.17 | 0.0433 |
| ACEI/ARB | 64.50 | 65.16 | −0.0138 |
| Beta blocker | 72.57 | 72.73 | −0.0034 |
| Diltiazem | 20.62 | 20.47 | 0.0037 |
| Verapamil | 1.98 | 2.13 | −0.0103 |
| DHP Calcium channel blocker | 36.21 | 37.13 | −0.0189 |
| Loop diuretic | 39.06 | 42.61 | −0.0737 |
| Thiazide diuretic | 35.14 | 35.25 | −0.0021 |
| Digoxin | 9.65 | 8.63 | 0.0370 |
| Amiodarone | 10.87 | 12.19 | −0.0430 |
| Dronedarone | 1.93 | 1.42 | 0.0341 |
| Other antiarrhythmic | 8.23 | 7.21 | 0.0368 |
| Statin | 52.41 | 55.92 | −0.0701 |
| Other antihyperlipidemic | 5.08 | 4.88 | 0.0093 |
| Noninsulin antidiabetic | 31.69 | 32.40 | −0.0152 |
| GLP1 agonists | 1.93 | 1.63 | 0.0208 |
| Insulin | 18.94 | 20.16 | −0.0314 |
| SSRI or SNRI | 10.77 | 10.67 | 0.0033 |
| Other antidepressant | 8.58 | 8.79 | −0.0071 |
| Proton pump inhibitor | 29.56 | 30.27 | −0.0157 |
| Histamin-2 receptor antagonist | 8.18 | 9.09 | −0.0335 |
| Systemic corticoids | 18.23 | 19.25 | −0.0257 |
| Hypnotic | 4.77 | 3.91 | 0.0410 |
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; DHP, dihydropyridine; DVT, deep vein thrombosis; EGFR, estimated glomerular filtration rate; GERD, gastroesophageal reflux disease; GI, gastrointestinal; GLP-1, glucagonlike peptide; NSAID, non-steroidal anti-inflammatory drugs; PE, pulmonary embolism; SSRI, selective serotonin reuptake inhibitor; SNRI, selective norepinephrine reuptake inhibitor; VTE, venous thromboembolism.
* History of bariatric surgery and history intracranial hemorrhage were considered for inclusion in the propensity score model, but the incidences of these covariates were 0% in both groups.
† EGFR and not creatinine clearance was used in the propensity score model.
Table 2.
Baseline Characteristics of 1:1 Propensity Score Matched Rivaroxaban and Warfarin Venous Thromboembolism Cohorts.
| Rivaroxaban, % (N = 683) | Warfarin, % (N = 683) | Standardized difference | |
|---|---|---|---|
| Demographics | |||
| Female | 62.96 | 64.13 | −0.0244 |
| PE ± DVT | 21.96 | 23.57 | −0.0402 |
| EGFR < 60 mL/minute | 11.86 | 12.15 | −0.0096 |
| Creatinine clearance < 15 mL/minute† | 0.00 | 0.29 | † |
| Age ≥ 60 years | 23.72 | 25.18 | −0.0350 |
| BMI ≥ 35 kg/m2 | 56.80 | 60.61 | −0.0867 |
| Past Medical History* | |||
| Chronic lung disease | 23.43 | 22.84 | 0.0139 |
| Heart failure | 4.98 | 6.73 | −0.0771 |
| Hypertension | 56.37 | 56.52 | −0.0029 |
| Diabetes mellitus | 25.77 | 27.53 | −0.0408 |
| PVD or carotid stenosis | 3.95 | 4.54 | −0.0280 |
| MI, PCI, or CABG | 7.47 | 8.78 | −0.0512 |
| Thrombophilia | 0.88 | 0.73 | 0.0128 |
| GERD | 18.89 | 18.74 | 0.0037 |
| Anemia | 21.08 | 23.87 | −0.0674 |
| Sleep apnea | 16.98 | 16.98 | 0.0000 |
| Smoker | 23.87 | 24.89 | −0.0239 |
| Anxiety | 14.20 | 11.57 | 0.0743 |
| Depression | 1.61 | 1.32 | 0.0239 |
| Psychosis | 0.59 | 0.59 | 0.0000 |
| Arthritis | 26.50 | 28.11 | −0.0286 |
| Helicobacter pylori | 0.15 | 0.15 | 0.0000 |
| Varicose veins | 0.88 | 1.61 | −0.0730 |
| Paralysis | 1.76 | 2.78 | −0.0836 |
| Medications | |||
| Aspirin | 22.55 | 23.72 | −0.0280 |
| P2Y12 inhibitors | 2.49 | 3.22 | −0.0465 |
| NSAID | 37.04 | 36.60 | 0.0087 |
| ACEI/ARB | 34.26 | 34.85 | −0.0125 |
| Beta blocker | 26.50 | 25.33 | 0.0274 |
| Calcium channel blocker | 23.13 | 22.99 | 0.0035 |
| Diuretic | 33.38 | 34.41 | −0.0220 |
| Statin | 22.40 | 22.11 | 0.0071 |
| Other antihyperlipidemic | 2.20 | 1.76 | 0.0328 |
| Noninsulin antidiabetic | 17.13 | 19.47 | −0.0618 |
| Insulin | 9.52 | 10.10 | −0.0208 |
| SSRI or SNRI | 11.86 | 12.01 | −0.0046 |
| Other antidepressants | 9.81 | 9.22 | 0.0205 |
| Acid suppressive therapy | 26.06 | 26.65 | −0.0134 |
| Systemic corticoids | 16.84 | 17.72 | −0.0234 |
| Estrogen | 13.18 | 14.49 | −0.0393 |
| Hypnotic | 4.10 | 2.93 | 0.0566 |
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass graft; DVT, deep vein thrombosis; EGFR, estimated glomerular filtration rate; GERD, gastroesophageal reflux disease; GI, gastrointestinal; MI, myocardial infarction; NSAID, non-steroidal anti-inflammatory drugs; PE, pulmonary embolism; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SSRI, selective serotonin reuptake inhibitor; SNRI, selective norepinephrine reuptake inhibitor; VTE, venous thromboembolism.
* History of bariatric surgery, gastrointestinal bleed and intracranial hemorrhage were considered for inclusion in the propensity score model, but the incidences of these covariates were 0% in both groups.
† EGFR and not creatinine clearance was used in the propensity score model.
For the NVAF analysis, the primary effectiveness endpoint was the incidence of stroke or systemic embolism (SSE), defined by a corresponding inpatient discharge diagnosis code in the primary position. For the acute VTE analysis, the primary endpoint was the incidence of recurrent VTE (defined by an appropriate inpatient discharge diagnosis code in the primary position). In both analyses, the primary safety endpoint was major bleeding as defined by the Cunningham algorithm.27 The Cunningham algorithm utilizes International Classification of Diseases (ICD) billing codes to identify cerebral, gastrointestinal, genitourinary and other types of bleeding resulting in hospitalization.27 Secondary endpoints included extracranial and intracranial bleeding separately and ischemic stroke (only for the NVAF analysis). An intent-to-treat analysis approach was used in all cases. NVAF patients were followed until endpoint occurrence, end-of-EHR activity or end of available follow-up. Acute VTE patients were followed for up to 3-, 6- or 12-months, or until endpoint occurrence or end of available follow-up.
Descriptive statistics were used to analyze baseline characteristics with categorical data reported as percentages and continuous data as medians with 25%, 75% ranges. Cox proportional hazards regression models were fit to compare event rates over time for the matched rivaroxaban and warfarin cohorts. Results of Cox regression were reported as hazard ratios (HRs) with 95% confidence intervals (CIs). Cox proportional hazards regression analysis were performed using IBM SPSS version 26.0 (IBM Corp, Armonk, NY). The proportional hazard assumption was tested based on Schoenfeld residuals and was found valid for all outcomes. A p-value <0.05 was considered statistically significant unless otherwise noted.
Results
NVAF Analysis
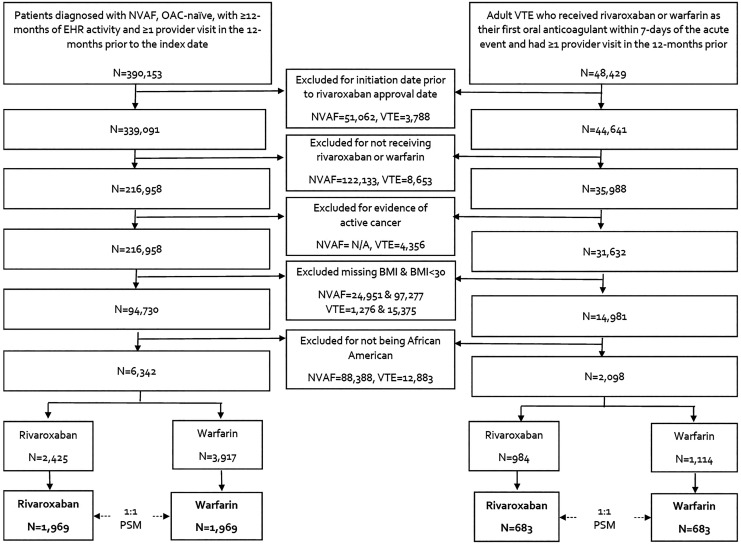
In total, 6,342 African American patients with NVAF and a BMI≥30 kg/m2 met inclusion criteria of which 2,425 were prescribed rivaroxaban and 3,917 were prescribed warfarin (Figure 1). After 1:1 propensity score matching, 1,969 rivaroxaban and 1,969 warfarin users were included in the analysis. The median time of available follow-up was 2.3 years (25%, 75% range of 1.1, 3.7 years). The median age of this population was 63 (55, 72) years, the median CHA2DS2-VASc score was 3 (2, 4), median CHADS2 score was 2 (1, 3) and median modified HASBLED score was 2 (1, 3) (Table 1). In the rivaroxaban cohort, there were 115 patients with an estimated creatinine clearance <50 mL/minute, of these, 54 were prescribed low dose (15 mg) rivaroxaban on the index date with the rest prescribed 20 mg once daily. In the remaining 1,854 patients with an estimated creatinine clearance ≥50 mL/minute, 266 were prescribed rivaroxaban 15 mg once daily on the index with the rest prescribed 20 mg once daily. Over half of patients (51.2%) received antiplatelet therapy at baseline including aspirin and/or P2Y12 inhibitors. Most patients also had a history of atherosclerotic disease or risk factors for atherosclerosis (e.g., diabetes, hyperlipidemia, hypertension, smoking, obesity). Thirty-nine percent of patients had a BMI 30.0-34.9 kg/m2, 27% a BMI 35.0-39.9 kg/m2, and 35% a BMI ≥40 kg/m2. Baseline characteristics all had a standardized difference <0.1 between the rivaroxaban and warfarin cohorts.
Figure 1.
Flow diagram of nonvalvular atrial fibrillation and venous thromboembolism patient inclusion and exclusion. BMI indicates body mass index; EHR, electronic health record; N/A, not applicable; NVAF, nonvalvular atrial fibrillation; OAC, oral anticoagulation; PSM, propensity score matching; VTE, venous thromboembolism.
The rate of SSE or ischemic stroke alone was not found to be significantly different between rivaroxaban and warfarin users (HR = 0.88, 95% = 0.68-1.22 and HR = 0.85, 95%CI = 0.58-1.26, respectively); however, rivaroxaban prescription was associated with a significant 32% relative reduction in the risk of major bleeding (95%CI = 6%-50%) (Figure 2). Rivaroxaban was associated with a reduced risk of extracranial hemorrhage compared to warfarin (HR = 0.66, 95%CI = 0.48-0.92), however there was no difference in intracranial hemorrhage (HR = 0.90, 95%CI = 0.35-2.33).
Figure 2.
Incidence and hazard ratio of nonvalvular atrial fibrillation outcomes. CI indicates confidence interval; HR, hazard ratio.
Acute VTE Analysis
There were 2,098 African American patients with acute VTE and a BMI≥30 kg/m2 that met inclusion criteria of which 984 were prescribed rivaroxaban and 1,114 were prescribed warfarin (Figure 1). Following 1:1 propensity score matching, 683 rivaroxaban and 683 warfarin users were included in the analysis. All rivaroxaban patients started therapy at 15 mg twice a day. No rivaroxaban patient had a creatinine clearance <15 mL/minute. The index event was a pulmonary embolism (PE) ± deep vein thrombosis (DVT) in 22.8% of patients, median age was 49 (39, 59) years, 63.6% of patients were female, 26.0% were on an antiplatelet agent (either aspirin or P2Y12 inhibitor), 24.6% were receiving antihyperlipidemic agents, 13.8% were taking estrogen therapy and 24.4% were smokers at baseline. Forty-one percent of patients had a BMI 30.0-34.9 kg/m2, 27% a BMI 35.0-39.9 kg/m2, and 32% a BMI ≥40 kg/m2. Median RIETE score was 1 (0, 1), All baseline characteristics had a standardized difference <0.1 between cohorts.
No differences were seen between rivaroxaban and warfarin cohorts for the recurrent VTE or major bleeding endpoints at any time point (Figure 3). This was also true when comparing rivaroxaban to warfarin cohorts for extracranial hemorrhage alone. The incidence of intracranial hemorrhages was low in both cohorts with no events in the rivaroxaban cohort through 12-months and one event in the warfarin cohort that occurred 15 days after anticoagulation initiation.
Figure 3.
Incidence and hazard ratio of venous thromboembolism outcomes. CI indicates confidence interval; HR, hazard ratio. *Hazard ratio and confidence intervals cannot be calculated due to zero events in the rivaroxaban cohort.
Discussion
This study utilized real-world EHR data on over 5,300 obese African American patients with either NVAF or acute VTE. In the NVAF population, rivaroxaban was found to be associated with a 32% relative risk reduction in major bleeding and a similar risk of SSE compared to warfarin. Ischemic stroke and intracranial hemorrhage were not significantly different between the cohorts, however there was 34% reduction in extracranial hemorrhage associated with rivaroxaban. In the VTE population, a similar risk for both recurrent VTE and major bleeding was found between rivaroxaban and warfarin users. There was no significant difference when comparing extracranial hemorrhage between groups, and only one intracranial hemorrhage which occurred in the warfarin cohort.
These results build upon the results of previous studies of African American and of obese patients16–22 in order to investigate a population likely at higher risk for poor outcomes than either population alone. A sub-analysis of the ROCKET-AF randomized trial16 and an observational study17 of black and/or African American patients with NVAF (regardless of BMI) found rivaroxaban to be associated with a similar16 or up to a 23% (95%CI = 1%-40%)17 reduced risk of SSE compared to warfarin. These studies also found a similar or reduced risk (up to 16%17) of major bleeding with rivaroxaban versus warfarin. Observational NVAF studies focused on obese patients (all morbidly obese, BMI≥40 kg/m2)19–20 have shown rivaroxaban to be associated with at least similar relative risks of SSE and major bleeding compared to warfarin.
In a single, retrospective observational VTE study of African American patients,18 rivaroxaban was not associated with significant difference in the relative risks of recurrent VTE or major bleeding versus warfarin. The EINSTEIN-DVT/PE randomized clinical trials bodyweight sub-analysis21 and two observational studies19,22 investigated VTE in obese21 or morbidly obese19,22 patients and found similar relative risks of recurrent VTE and major bleeding between rivaroxaban and warfarin cohorts.
This study has limitations that need to be discussed. Given the nature of non-randomized studies, biases including confounding, misclassification and sampling may reduce internal validity.28 Furthermore, residual confounding is possible due to unobserved or unmeasured covariates. Additionally, as we were reliant on billing codes for identifying the nature of index VTE and due to inconsistencies in testing and/or reporting of DVT in patients with PE, it is was not possible for us to further stratify cases of PE (as PE alone or with concurrent DVT) at the time of hospitalization. As the Optum® EHR database utilized includes only US patients, the results of this study are less generalizable to non-US populations (thus African American and black patients were distinguished in this paper). Both analyses had relatively small populations (<4,000 patients) due to their narrow target population of study (i.e., obese African Americans) which imposed some constraints on the propensity score modeling. A substantial number of patients in the analyses (nearly 50 and 25% for NVAF and VTE, respectively) were receiving antiplatelet therapy. These proportions of patients are higher than those observed in the ROCKET AF and EINSTEIN clinical trials16,29,30; however, other clinical trials and real-world registry studies30–32 have reported similar or even higher rates of antiplatelet use. It is possible, that both the use of this EHR database which captures patient self-reported over-the-counter medication use (such as aspirin) and the higher risk nature of this study population (obese and African American) may account for the increased use of antiplatelet agents observed. Importantly, the proportion of patients receiving antiplatelet therapies at baseline in the present study were similar between the rivaroxaban and warfarin cohorts after matching (standardized differences ≤0.0465), making it an unlikely confounder in the analysis. Moreover, previous study data comparing rivaroxaban to warfarin for NVAF29 and acute VTE30 suggest concomitant antiplatelet therapy does not impact rivaroxaban’s relative effectiveness or safety. Time in therapeutic international normalized ratio (INR) was not calculated for this study; however, the preponderance of evidence suggests both NVAF and acute VTE patients managed outside of randomized trials spend <60% of time in target range.33,34 Given that this study was based on real-world EHR data, a similar time in therapeutic INR would be anticipated. Furthermore, in the rivaroxaban cohort for NVAF, approximately 14% of patients were underdosed and 3% overdosed which may have affected the outcome of major bleeding. Optum® EHR covers insured and uninsured patients, however the database does not cover all institutions and therefore it is possible that certain follow-up events could have been missed. Finally, an EHR entry to initiate an oral anticoagulant does not necessarily mean a patient filled their prescription and/or took it. Moreover, as the Optum® EHR does not provide data on adjudicated prescription claims from pharmacies, traditional methods for assessing adherence and persistence in large observational studies could not be implemented. As a result, results were only analyzed using an intent-to-treat approach.
In summary, this study found that African Americans diagnosed with either NVAF or acute VTE in conjunction with obesity, and prescribed rivaroxaban, had similar effectiveness compared to patients prescribed warfarin. NVAF patients prescribed rivaroxaban displayed a reduced rate in the risk of major bleeding, while patients with an acute VTE displayed a similar rate in the risk of major bleeding, compared to patients prescribed warfarin. This is one of the first studies to evaluate the effectiveness and safety of rivaroxaban compared to warfarin in African American patients with either NVAF or acute VTE and obesity. These results are consistent with data supporting rivaroxaban’s US prescribing labeling.35
Supplemental Material
Supplemental Material, Costa_STROBE_RvW_in_AA_with_Obesity_and_NVAF for Rivaroxaban Versus Warfarin for Management of Obese African Americans With Non-Valvular Atrial Fibrillation or Venous Thromboembolism: A Retrospective Cohort Analysis by Olivia S. Costa, Jan Beyer-Westendorf, Veronica Ashton, Dejan Milentijevic, Kenneth Todd Moore, Thomas J. Bunz and Craig I. Coleman in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, Costa_Supplement_RvW_in_AA_with_Obesity_and_NVAF for Rivaroxaban Versus Warfarin for Management of Obese African Americans With Non-Valvular Atrial Fibrillation or Venous Thromboembolism: A Retrospective Cohort Analysis by Olivia S. Costa, Jan Beyer-Westendorf, Veronica Ashton, Dejan Milentijevic, Kenneth Todd Moore, Thomas J. Bunz and Craig I. Coleman in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Authors’ Note: OSC, CIC, JBW, VA, DM and KTM conceptualized and designed the study. OSC, CIC and TJB analyzed the data. Data interpretation was done by all authors. The manuscript was written primarily by OSC and CIC; all authors contributed to revisions. All authors substantially contributed to this project, read and approved the manuscript and assume responsibility for the contents of the manuscript. Data used in this study were obtained from Optum® under a license to Janssen Pharmaceuticals (and provided to Dr. Coleman under a third-party agreement) and are not publicly available. The use of Optum clinical EHR database has been reviewed by the New England Institutional Review Board (IRB) and has been determined to be exempt from broad IRB approval, as this research project did not involve human subject research. This study has not been presented at a conference.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. C. I. Coleman has received grant funding and consultancy fees from Janssen Scientific Affairs LLC, Titusville, NJ, Bayer AG, Berlin, Germany; Portola Pharmaceuticals, South San Francisco, CA; and speaker fees from Medscape Inc. J. Beyer-Westendorf has grant funding and consultancy fees from Bayer AG, Berlin, Germany; Boehringer Ingelheim Pharmaceuticals, Ridgefield, CT; Pfizer New York, NY; Daiichi Sankyo, Basking Ridge, NJ; and Portola Pharmaceuticals, South San Francisco, CA. V. Ashton and D. Milentijevic are employees of Janssen Scientific Affairs LLC, Titusville, NJ. K.T. Moore is an employee of Janssen Pharmaceuticals Inc., Titusville, NJ. O. S. Costa and T. J. Bunz have no declarations-of-interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Janssen Scientific Affairs LLC, Titusville, NJ.
ORCID iD: Olivia S. Costa  https://orcid.org/0000-0001-6775-1499
https://orcid.org/0000-0001-6775-1499
Craig I. Coleman  https://orcid.org/0000-0003-4868-7158
https://orcid.org/0000-0003-4868-7158
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Hernandez W, Gamazon ER, Smithberger E, et al. Novel genetic predictors of venous thromboembolism risk in African Americans. Blood. 2016;127(15):1923–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edelstein LC, Simon LM, Montoya RT, et al. Racial differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376. Nat Med. 2013;19(12):1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White H, Boden-Albala B, Wang C, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: The Northern Manhattan Study. Circulation. 2005;111(10):1327–1331. [DOI] [PubMed] [Google Scholar]
- 4. Dowling NF, Austin H, Dilley A, Whitsett C, Evatt BL, Hooper WC. The epidemiology of venous thromboembolism in Caucasians and African Americans: The GATE Study. J Thromb Haemost. 2003;1(1):80–87. [DOI] [PubMed] [Google Scholar]
- 5. Schneider D, Lilienfeld DE, Im W. The epidemiology of pulmonary embolism: racial contrasts in incidence and in-hospital case fatality. J Natl Med Assoc. 2006;98(12):1967–1972. [PMC free article] [PubMed] [Google Scholar]
- 6. White RH, Kennan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123(4):S11–17. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. Published 2020 Accessed May 27, 2020 https://www.cdc.gov/obesity/data/adult.html
- 8. Wang TJ, Praise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–2477. [DOI] [PubMed] [Google Scholar]
- 9. Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med. 2005;118(9):978–980. [DOI] [PubMed] [Google Scholar]
- 10. Yong C, Xu X, Than C, et al. Racial disparities in warfarin time in INR therapeutic range in patients with atrial fibrillation: findings from the TREAT-AF study. Circulation. 2013;128(22):A14134. [Google Scholar]
- 11. Shen AY, Yao JF, Brar SS, Jorgensen MB, Wang X, Chen W. Racial/ethnic differences in ischemic stroke rates and the efficacy of warfarin among patients with atrial fibrillation. Stroke. 2008;39(10):2736–2743. [DOI] [PubMed] [Google Scholar]
- 12. Wallace JL, Reaves AB, Tolley EA, et al. Comparison of initial warfarin response in obese patients versus non obese patients. J Thromb Thrombolysis. 2013:36(1);96–101. [DOI] [PubMed] [Google Scholar]
- 13. Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. J Clin Pharmacol. 2007;47(2):218–226. [DOI] [PubMed] [Google Scholar]
- 14. Balla SR, Cyr DD, Lokhnygina Y, et al. Relation of risk of stroke in patients with atrial fibrillation to body mass index (from patients treated with rivaroxaban and warfarin in the rivaroxaban once daily oral direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation trial). Am J Cardiol. 2017;119(12):1989–1996. [DOI] [PubMed] [Google Scholar]
- 15. Tittl L, Endig S, Marten S, Reitter A, Beyer-Westendorf I, Beyer-Westendorf J. Impact of BMI on clinical outcomes of NOAC therapy in daily care—results of the prospective Dresden NOAC registry (NCT01588119). Int J Cardiol. 2018;262:85–91. [DOI] [PubMed] [Google Scholar]
- 16. Patel MR, Mahaffey KW, Garg J, et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. [DOI] [PubMed] [Google Scholar]
- 17. Coleman CI, Thompson S, Ashton V, Palladino M, Bunz TJ. Rivaroxaban versus warfarin in African American patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2020;75(11):abstract 338. [DOI] [PubMed] [Google Scholar]
- 18. Costa OS, Thompson S, Ashton V, Palladino M, Bunz TJ, Coleman CI. Rivaroxaban versus warfarin for treatment and prevention of recurrence of venous thromboembolism in African American patients: a retrospective cohort analysis. Thromb J. 2020;18:6 doi:10.1186/s12959-020-00219-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kushnir M, Choi Y, Eisenberg R, et al. Efficacy and safety of direct oral factor Xa inhibitors compared with warfarin in patients with morbid obesity: a single-centre retrospective analysis of chart data. Lancet Haematol. 2019;6(7):e359–e365. [DOI] [PubMed] [Google Scholar]
- 20. Peterson ED, Ashton V, Chen YW, Wu B, Spyropoulos AC. Comparative effectiveness, safety, and costs of rivaroxaban and warfarin among morbidly obese patients with atrial fibrillation. Am Heart J. 2019;212:113–119. [DOI] [PubMed] [Google Scholar]
- 21. Di Nisio M, Vedovati MC, Riera-Mestre A, et al. Treatment of venous thromboembolism with rivaroxaban in relation to body weight. A sub-analysis of the EINSTEIN DVT/PE studies. Thromb Haemost. 2016;116(4):739–746. [DOI] [PubMed] [Google Scholar]
- 22. Spyropoulos AC, Ashton V, Chen YW, Wu B, Peterson ED. Rivaroxaban versus warfarin treatment among morbidly obese patients with venous thromboembolism: comparative effectiveness, safety, and costs. Thromb Res. 2019;182:159–166. [DOI] [PubMed] [Google Scholar]
- 23. Optum. Optum EHR Offering. Optum Inc; 2018. Accessed July 28, 2019 https://www.optum.com/campaign/ls/data-new-era-of-visibility/download.html [Google Scholar]
- 24. National Heart, Lung and Blood Institute. Classification of overweight and obesity by BMI, waist circumference, and associated disease risks. Accessed May 27, 2020 https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi_dis.htm
- 25. White RH, Garcia M, Sadeghi B, et al. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb Res. 2010;126(1):61–67. [DOI] [PubMed] [Google Scholar]
- 26. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gandhi SK, Salmon W, Kong SX, Zhao SZ. Administrative databases and outcomes assessment: an overview of issues and potential utility. J Manag Care Spec Pharm. 1999;5(3):215–222. [Google Scholar]
- 29. Shah R, Helkamp A, Lokhnygina Y, et al. Use of concomitant aspirin in patients with atrial fibrillation: findings from the ROCKET AF trial. Am Heart J. 2016;179:77–86. [DOI] [PubMed] [Google Scholar]
- 30. Valeriani E, Porreca E, Weitz JI, Schulman S, Candeloro M, Di Nisio M. Impact of concomitant antiplatelet therapy on the efficacy and safety of direct oral anticoagulants for acute venous thromboembolism: systemic review and meta-analysis. J Thromb Haemost. 2020;18(7):1661–1671. [DOI] [PubMed] [Google Scholar]
- 31. Larsen TB, Skjeth F, Kjældgaard JN, Lip GYH, Nielsen PB, Søgaard M. Effectiveness and safety of rivaroxaban and warfarin in patients with unprovoked venous thromboembolism: a propensity-matched nationwide cohort study. Lancet Haematol. 2017;4(5):e237–244. [DOI] [PubMed] [Google Scholar]
- 32. Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GY. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mearns ES, White CM, Kohn CG, et al. Quality of vitamin K antagonist control and outcomes in atrial fibrillation patients: a meta-analysis and meta-regression. Thromb J. 2014;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mearns ES, Kohn CG, Song JS, et al. Meta-analysis to assess the quality of international normalized ratio control and associated outcomes in venous thromboembolism patients. Thromb Res. 2014;134(2):310–319. [DOI] [PubMed] [Google Scholar]
- 35. XARELTO® (rivaroxaban) Tablets, for Oral Use. Janssen Pharmaceuticals, Inc; 2020. Accessed May 27, 2020 http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Costa_STROBE_RvW_in_AA_with_Obesity_and_NVAF for Rivaroxaban Versus Warfarin for Management of Obese African Americans With Non-Valvular Atrial Fibrillation or Venous Thromboembolism: A Retrospective Cohort Analysis by Olivia S. Costa, Jan Beyer-Westendorf, Veronica Ashton, Dejan Milentijevic, Kenneth Todd Moore, Thomas J. Bunz and Craig I. Coleman in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, Costa_Supplement_RvW_in_AA_with_Obesity_and_NVAF for Rivaroxaban Versus Warfarin for Management of Obese African Americans With Non-Valvular Atrial Fibrillation or Venous Thromboembolism: A Retrospective Cohort Analysis by Olivia S. Costa, Jan Beyer-Westendorf, Veronica Ashton, Dejan Milentijevic, Kenneth Todd Moore, Thomas J. Bunz and Craig I. Coleman in Clinical and Applied Thrombosis/Hemostasis