Abstract
Objective
Population screening can facilitate early diagnosis of dementia and improve disease management. This study examined the effects of a screening campaign for neurodegenerative disorders on the early diagnosis of dementia using 2-year follow-up data.
Methods
A 5-day screening campaign was conducted that comprised neurological, neuropsychological and other specialist examinations. Identification of alterations during the neurological examination was followed-up by further diagnostic examinations to confirm the neurological impairment.
Results
Neurological alterations were observed in 39% of the screened subjects, who were mostly diagnosed with mild cognitive impairment and referred to a dementia and cognitive disorders centre. Suspicion of neurological impairment was a risk factor for inclusion in a specific neurological ambulatory follow-up and a condition for exemption from payment for medical examinations.
Conclusions
Neurodegenerative screening initiatives should include subjects selected by general practitioners. It would be useful to create a network including primary care physicians and cognitive disorder centres. Telemedicine tools (e.g., teleconsulting) could also be used to facilitate early diagnosis.
Keywords: Screening campaign, public health, prevention, neuroepidemiology, dementia, neurodegenerative disorders, mild cognitive impairment, general practitioner
Introduction
The most recent global estimates indicate that the burden of neurodegenerative disorders is the first cause of disability and the second cause of death, and that such disorders have greatly increased over the past 25 years owing to population aging.1 The Italian National Institute of Statistics estimated that in 2015, the mortality rate from neurological causes was 4.63 per 10,000 individuals and 19.37 per 10,000 individuals aged over 65 years.2
Early diagnosis of neurological impairment is crucial to ensure optimal disease management. Recently, there has been great progress in the identification of potential biomarkers using neuroimaging and electrophysiology techniques,3–6 as well as genetic and proteomic analysis,7,8 to help neurologists provide early diagnoses. However, the biggest stumbling block to early diagnosis is identifying symptoms that can alert the patient or their carer to visit a general practitioner (GP) or neurological centre.9 Although some neurological diseases present clear symptoms and are easily recognizable (e.g., headache and epilepsy), others, such as multiple sclerosis, Parkinson’s disease, brain tumours and dementia are more difficult to assess owing to their complexity. However, as such diseases are very disabling, regular accurate neurological checkups could facilitate their early identification.
Dementia is characterized by several symptoms, such as behavioural changes, deficits of space–time orientation and language, and memory loss, which is the most obvious symptom.10 Dementia starts with an initial symptomatic stage on the cognitive decline continuum called mild cognitive impairment (MCI), which is characterized by objective cognitive impairment that is not severe enough that the person requires help to perform their usual activities of daily living.11 This neurodegenerative condition is often not considered an illness but an inevitable part of aging.12 For this reason, dementia diagnosis usually occurs after the disease has progressed and the patient is at least partially dependent on a caregiver,13 causing a range of unmet needs for patients with dementia and their families.14 However, timely diagnosis could support patients and caregivers in optimal management of the disease.15 Moreover, dementia also has a substantial economic effect on healthcare systems. It has been estimated that the global economic costs of dementia were approximately $818 billion in 2015.16 Thus, it is essential to develop local and national policy strategies for early diagnosis of dementia to improve care and reduce costs.
The first challenge is probably to train the population to promptly recognize the initial symptoms of dementia. This would facilitate early diagnosis and improve dementia care. Moreover, education can help to break down the stigma associated with dementia, which manifests as discrimination, stereotypes and prejudice.12 In the absence of appropriate information about the identification of MCI and dementia symptoms, a screening campaign could be useful in helping to overcome this social stigma. Indeed, dementia can be diagnosed at an early stage through screening.17 However, screening population campaigns have some disadvantages, especially in terms of healthcare system costs, as they require dedicated facilities and staff.13
The study aim was to describe a neurodegenerative screening campaign, focusing on a 2-year follow-up of participants in one neurologic ambulatory service.
Methods
Overview
A 5-day free neurological screening was performed in Messina in March 2017 by the IRCCS Centro Neurolesi Bonino Pulejo of Messina, Sicily. The screening took place during ‘Brain Awareness Week’, a global campaign aimed to increase public awareness of brain research.18 A week before this initiative, a marketing campaign was conducted to encourage participation. The campaign was advertised using high visibility posters placed around the hospital, and information in local newspapers and on a local television channel. Moreover, a dedicated information point placed behind the hospital offered information about neurodegenerative diseases and the screening initiative, including the screening process.
Inclusion and exclusion criteria
For this study, we selected subjects who had completed the whole screening programme. Exclusion criteria were i) a diagnosis of dementia or other neurological or psychiatric illness in the 24 months prior to the screening visit; ii) aged younger than 18 years; iii) inability to understand and communicate needs; iv) mental disability.
Screening procedure and clinical assessment
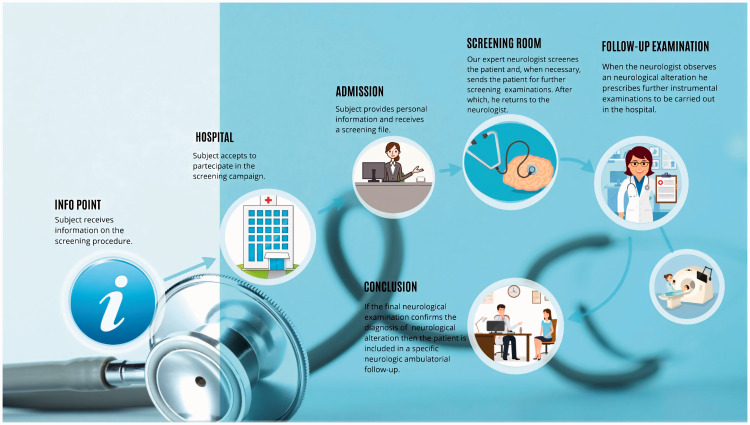
Figure 1 shows the screening process. A reception desk was placed at the hospital entrance for personnel to collect participant information and to schedule examinations as part of an individual screening course. After having provided written informed consent for the treatment of personal data, each participant received an assessment form on which medical staff noted all clinical information obtained during the screening. The default screening programme included the collection of anamnestic data and a neurological examination performed by an expert neurologist. On the basis of the clinical picture, the neurologist could schedule other specialist evaluations as part of the screening process (e.g., cardiological and ear, nose and throat evaluations) and order instrumental examinations such as electroencephalogram (EEG), electrocardiogram (ECG), evoked potential (EP), carotid Doppler ultrasonography (CDU) and neuropsychological tests. If the subject reported possible memory alterations, then he/she was also evaluated using standard clinical and psychometric tests of dementia (the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA)), which were administered by skilled neuropsychologists to assess global cognitive functioning.19,20 These screening tests provide measures of orientation, registration (immediate memory), short-term memory (but not long-term memory) and language functioning.
Figure 1.
Step-by-step description of the population screening procedure.
After the screening process was complete, the participant returned to the neurologist. The neurologist examined the subject’s assessment form and either made a diagnosis of alteration or prescribed further diagnostic examinations. These were scheduled by the hospital personnel as follow-up examinations and available for a fee.
All the follow-up exams were performed from June 2017 to June 2018, according to hospital availability and patient clinical need. The exams included an in-depth neurological evaluation in a specific clinical area (e.g., epilepsy, movement disorders, headache, multiple sclerosis, psychiatry, dementia), instrumental examinations for in-depth neuropsychological testing (e.g., the Trail Making Test, the Attentive Matrices test and the Rey Auditory Verbal Learning Test), magnetic resonance imaging (MRI), computed tomography, polysomnography and echocardiograms. EEG, EP, CDU, cardiac evaluation and ECG were also performed if these had not been administered during the screening.
At the end of these follow-up examinations, a final neurological examination was performed. Subjects with a confirmed diagnosis of neurological impairment were admitted to a specific neurologic ambulatory care facility of the hospital.
Ethics committee approval was not needed, because this was an observational study.
Outcomes
The primary outcome was the percentage of subjects who completed the screening process, as a measure of adherence to the campaign. The secondary outcome was the effect of this public health service on early detection of neurological disorders, measured as the percentage of diagnosis of neurological alteration and the percentage of subjects referred to a neurologic ambulatory care facility. We also assessed which examinations were most useful for early diagnosis of neurological impairment, as well as possible risk factors.
Statistical analysis
Statistical analysis was performed using Stata, Version 14.1 (Stata Corp. LP, College Station, TX, USA). The level of significance was 0.05. Results for continuous variables were expressed as means ± standard deviations and results for categorical variables expressed as frequencies and percentages. The X2 test with continuity correction was used to assess statistical differences in proportions, and the unpaired Student t-test was used to compare continuous variables. Logistic regression was used to estimate the odds ratio (OR) and associated 95% confidence intervals (CI) of being included in a specific neurologic ambulatory follow-up in the screened population with suspected neurological alteration, after adjustment for age, sex and payment exemption for medical examinations.
Results
Attendance at the screening campaign
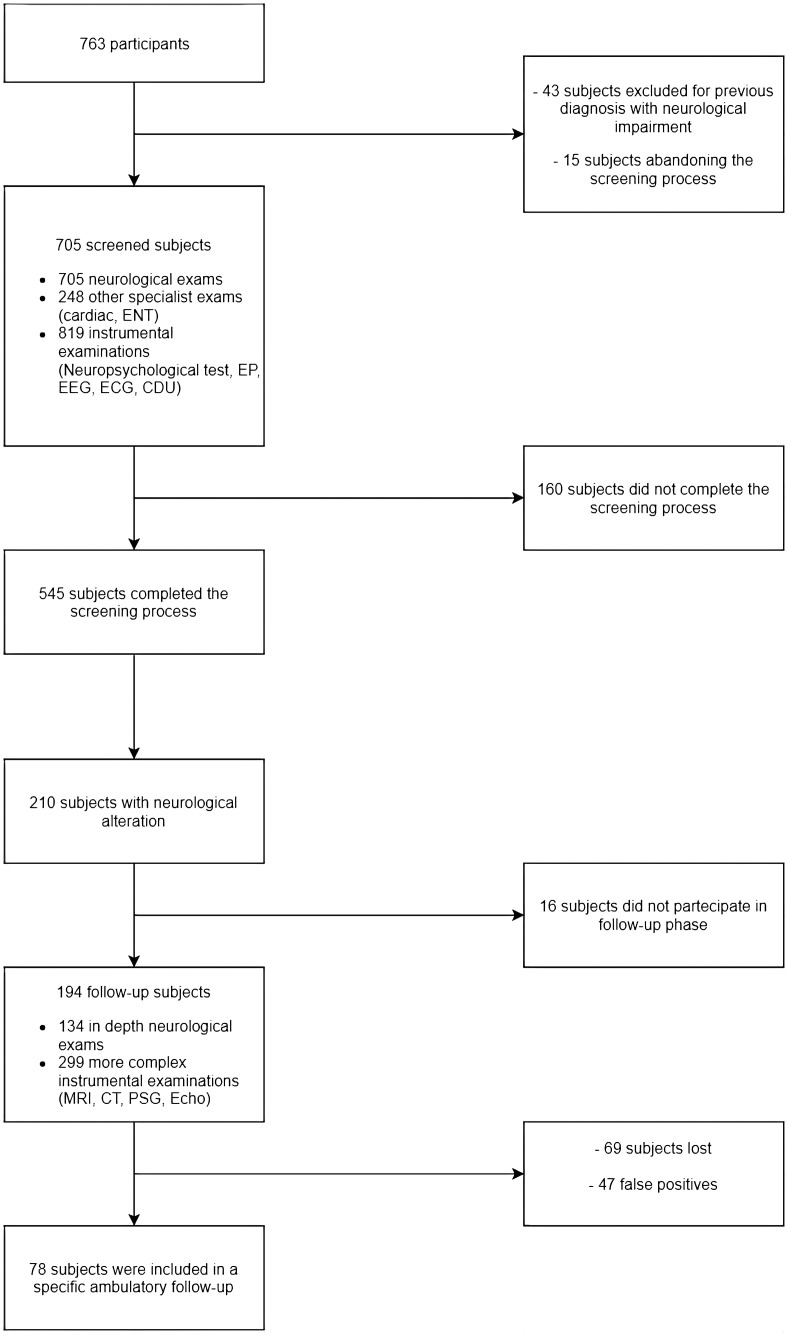
Figure 2 shows the participant selection procedure. A total of 763 subjects agreed to participate in this free neurological screening campaign. Forty-three subjects were excluded because of a previous diagnosis of neurological impairment, and 15 subjects dropped out of the screening process before it begun. Thus, only 705 participants were screened (293 men and 412 women, mean age 61.9 ± 13.9 years). Approximately 77.3% of these (545 subjects) completed the screening, and 160 subjects dropped out before obtaining a diagnosis. A neurological alteration was observed in 210 of these 545 subjects (38.53%).
Figure 2.
Participant selection procedure for the whole screening process.
ENT: ear, nose and throat evaluation; EEG: electroencephalogram; ECG: electrocardiogram; EP: evoked potential; CDU: carotid Doppler ultrasonography; MRI: magnetic resonance imaging; CT: computed tomography; PSG: polysomnography; Echo: echocardiogram.
Participant characteristics
As shown in Table 1, subjects with an alteration were significantly older than those without any alteration (P < 0.01). However, these two groups did not differ on exemption from medical service payment.
Table 1.
Characteristics of subjects who completed the screening campaign.
| Characteristics | Participants |
P-value (presence vs. absence of alteration) | ||
|---|---|---|---|---|
| All screened | Presence of alteration | Absence of alteration | ||
| 545 | 210 (38.53) | 335 (61.47) | ||
| Male, N (%) | 240 (44.04) | 95 (45.24) | 145 (43.28) | X2 (1) = 0.13; P = 0.72 |
| Age, mean ± SD years | 64.22 ± 13.04 | 66.16 ± 11.17 | 63.01 ± 13.97 | t (512) = 2.91; P < 0.01 |
| Exempt from payment, N (%) | 410 (75.23) | 167 (79.52) | 243 (72.54) | X2 (1) = 3.02; P = 0.08 |
SD: standard deviation.
Most of the subjects screened (410/545) were exempt from payment.
Screening examinations
All subjects screened received a neurological examination and, on the neurologist’s request, 248 specialist and 819 instrumental examinations were carried out. A neuropsychological test was the most common additional examination (58.58%), followed by ECG (35.89%), cardiac evaluation (19.72%), EEG (16.70%) and EP (16.17%). Approximately 80% of subjects with a diagnosis of neurological alteration underwent a neuropsychological test (Figure 3). We found a significant association between the presence of an alteration and having undergone a neuropsychological test (X2 (1) = 44.81; P < 0.001). In contrast, there was no association between the presence of an alteration and having undergone a different instrumental examination (i.e. EEG, EP, ECG, CDU).
Figure 3.
Examinations performed during the screening week. Red bars represent the proportion of subjects without neurological alteration; the blue bars represent the proportion of subjects with neurological alteration.
Attendance at the follow-up examinations
Although the follow-up examinations were not free, a large proportion of subjects with suspected neurological impairment participated in this further investigation phase (92.4%), a proportion significantly higher than the proportion of subjects who completed the initial screening process: X2 (1) = 22.7; P < 0.001.
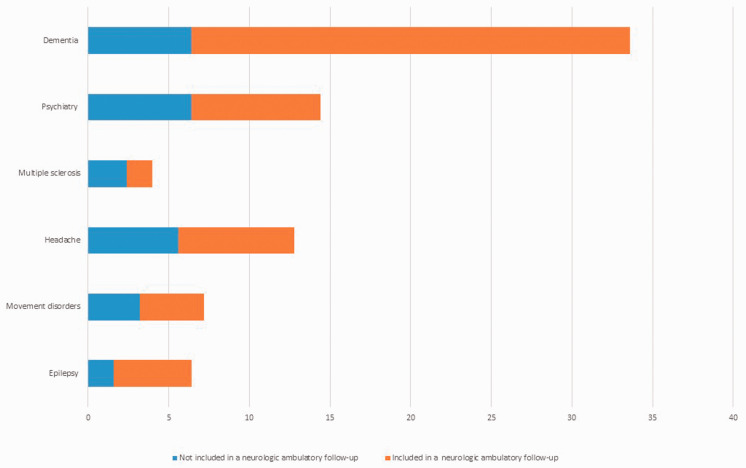
During the follow-up, subjects underwent 134 in-depth neurological evaluations, 59 other specialist evaluations and 299 instrumental examinations. Approximately 50% of subjects underwent an MRI, which was the most common instrumental examination performed, followed by neuropsychological tests (approximately 44%). Of the specialist ambulatory care facilities, the most frequent referrals occurred for the dementia and cognitive disorders centre (approximately 34%), followed by psychiatry (14.4%) and headache (12.8%) services, as shown in Figure 4.
Figure 4.
Examinations performed at the specialist ambulatory care facilities during the follow-up phase. Red bars represent the proportion of subjects with subsequent inclusion in the ambulatory follow-up (true positive); the blue bars represent the proportion of subjects not included in the follow-up (false positive). Data for lost subjects were not included.
Inclusion in a neurologic ambulatory follow-up
At 2 years after the screening campaign, 122 of 545 screened subjects (22.4%) attended the specific neurologic ambulatory care facilities of our hospital. Of these, 78 subjects (63.9%) were included at the end of the follow-up examination phase (true positives), whereas 44 subjects showed no symptoms of alteration during the screening (false negatives). We found that the probability of early attendance at a neurologic ambulatory care facility differed significantly according to the outcome of the screening process: X2 (1) = 50.74; P < 0.001. As shown in Table 2, suspected neurological impairment was a risk factor for inclusion in a specific neurologic ambulatory follow-up. Approximately 41% of the subjects included in a specific neurologic ambulatory follow-up attended the dementia and cognitive disorders centre with a diagnosis of MCI (and 68% were included at the end of the follow-up examination phase).
Table 2.
Logistic regression analysis of inclusion vs. not inclusion in a neurologic ambulatory follow-up after adjustment for sex, age and payment exemption
| Coefficient | OR | [95% CI] | P-value |
|---|---|---|---|
| Neurological alteration | 4.31 | 2.80–6.64 | <0.001 |
| Sex | 1.03 | 0.67–1.60 | 0.880 |
| Age | 1.01 | 0.98–1.03 | 0.617 |
| Exemption | 1.92 | 0.02–0.23 | 0.057 |
OR: odds ratio; CI: confidence interval.
Discussion
To the best of our knowledge, this is the first southern Italian neurodegenerative screening initiative aimed at providing public healthcare services for citizens.
The use of population screening methods for early diagnosis of neurodegenerative disorders is controversial. The benefits of screening initiatives differ according to the disease. For example, early diagnosis of brain tumour could improve prognosis more than early diagnosis of neurodegenerative diseases like dementia, for which there is no effective cure.12 However, the progression and management of the disease depend on the timely initiation of treatment. Thus, early detection of dementia or MCI, facilitated by screening programmes, may help patients and caregivers to make decisions about all aspects of patient care in management of the disease.21 However, prior to screening, there should be a consideration of whether the advantages of the screening quantitatively outweigh its disadvantages, to evaluate its effectiveness and utility.13
Screening initiatives can entail several negative aspects (e.g., false positives and misdiagnoses), which in turn generate secondary effects such as anxiety and/or depression in people without dementia. Similarly, a correct diagnosis can lead to depression, acquisition of stigmatizing labels and reduced quality of life.22 Furthermore, the patient could receive a therapy that they do not need, and be directed to expensive services and treatments.23 Additionally, population screening campaigns can be very costly for both individuals and healthcare systems.24 Given all these factors, the benefits of neurodegenerative population screening remain uncertain.
Our results showed a neurological alteration in approximately 39% of screened subjects, and a significant association between neurological alteration detected during screening and early diagnosis of impairment. Furthermore, we found that this early diagnosis of neurological alteration was a risk factor for inclusion in a specific neurologic ambulatory follow-up, particularly referral to the dementia and cognitive disorders centre with a diagnosis of MCI.
Although the most common instrumental examination was MRI, a comparison of examinations performed for subjects with and those without suspected neurological impairment showed that the neuropsychological examination was essential in diagnosing neurological impairment in approximately 80% of cases. Approximately 57% of subjects who underwent neuropsychological screening were admitted to the dementia and cognitive disorders centre, indicating that cognitive screening methods may have a future in primary care. Indeed, GPs play an essential role in disease diagnosis and management and are the first step toward early diagnosis of dementia. GPs can train patients to recognize the first symptoms, request neurological examinations if there is suspected impairment and support patients with dementia and their families to manage the disease after diagnosis. Borson et al.21 found increased rates of dementia diagnoses after screening. However, not many people with positive screening diagnoses undergo a further diagnostic assessment by their GPs, and often they choose not to consult a specialist.25
In Australia, GPs use routine memory tests such as the MMSE to assess patients with complaints of memory loss.26 However, the accuracy of these tests depends on the patient’s age and educational level; it is possible to obtain false positives in people with lower educational levels and false negatives in individuals with higher educational levels.27
The cost of medical examinations is one reason for people avoiding regular health checks. However, our findings showed that 75% of participants were exempt from payment for medical examinations. Hence, these subjects could have prevented their clinical condition free of charge. Indeed, we observed high participation in the follow-up examination phase although it was not free. This result could inform new socioeconomic hypotheses of health behaviour, which could be explored in future studies.
Screening campaigns are usually designed to prevent life-threatening illnesses or manage them at early, non-lethal stages.28 Their overall efficacy depends both on substantial adherence of the screenable population and on statistically equivalent participation of all relevant social groups. At the development phase, costs have to be balanced with benefits. However, the effectiveness of the screening should be greater than mere cost-saving. The costs to the health system of case identification, diagnosis and intervention should be economically balanced with the medical cost savings that may result from the screening programme, both as anticipated benefits and as other opportunities for public health programmes.29 Bovet et al.30 recommend the investigation of the potential cost-effectiveness of community-based screening programmes, in addition to related awareness campaigns. In line with their idea, our findings suggest that a neurological screening programme could be cost-effective if it focuses on participants who have one or several risk factors.
This study has some limitations, such as the lack of a follow-up for those subjects who completed the screening with a diagnosis of alteration but were lost from our hospital. Such subjects may have opted to visit a different hospital or not to have treatment at all. However, the main study limitation is the lack of screening campaign costs. The screening campaign described in this study involved more than 20 personnel units that included neurologists, cardiologists, neuropsychologists, neurophysiopathologists, technicians, nurses and administrative staff, who participated for a whole working week using three screening rooms and a range of medical instrumentation. There were also advertising costs, and costs for the staff who scheduled the follow-up examinations. However, this screening was an initiative planned for ‘Brain Awareness Week’, the principal aim of which is to increase public awareness of the progress and benefits of brain research, and progress in the diagnosis, treatment and prevention of brain disorders such as dementia. In future work, ‘cause finding’ (i.e. appropriate research based on clinical suspicion) may be a reasonable compromise to reduce costs of screening campaigns. This involves actively searching for at-risk people, rather than waiting for them to present with symptoms or signs of active disease. In this screening method, neurological investigations and neuropsychological tests are conducted only for those subjects who present important clinical risk factors and are more likely to develop a disease, rather than for the whole population.31 A screening intervention for dementia must take into account many possible negative aspects. For example, not all cases of MCI develop Alzheimer’s disease, which complicates the possibility of estimating disease progression.32
Conclusions
It is important to provide early diagnosis of neurodegenerative impairments such as dementia so that treatments can be started promptly.
Neurological screening population programmes can facilitate early diagnosis, although they are not always cost-effective. However, screening costs and potential participant harm could be reduced by focusing on suspected disease, as identified by GPs, using memory tests such as the MMSE and MoCA. Thus, it would be useful to create a network of GPs and cognitive disorder centres that could facilitate initial neurological consultations. Telemedicine tools (e.g., teleconsulting) could also be used to confirm the suspicion of dementia and obtain an early diagnosis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Francesco Corallo https://orcid.org/0000-0003-4862-3832
Marcella Di Cara https://orcid.org/0000-0002-4275-4340
References
- 1.GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017; 16: 877–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ISTAT. Health for all-Italia. https: //www.istat.it/it/archivio/14562 (2019, accessed 11 July 2019).
- 3.Lowe VJ, Lundt ES, Albertson SM, et al. Neuroimaging correlates with neuropathologic schemes in neurodegenerative disease. Alzheimers Dement 2019; 19: 1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat S, Acharya UR, Hagiwara Y, et al. Parkinson's disease: cause factors, measurable indicators, and early diagnosis. Comput Biol Med 2018; 102: 234–241. [DOI] [PubMed] [Google Scholar]
- 5.Fiscon G, Weitschek E, Cialini A, et al. Combining EEG signal processing with supervised methods for Alzheimer's patients classification. BMC Med Inform Decis Mak 2018; 18: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horvath A, Szucs A, Csukly G, et al. EEG and ERP biomarkers of Alzheimer's disease: a critical review. Front Biosci 2018; 23: 183–220. [DOI] [PubMed] [Google Scholar]
- 7.Xu L, Chen Y, Shen T, et al. Genetic analysis of PICK1 gene in Alzheimer's disease: a study for finding a new gene target. Front Neurol 2019; 9: 1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong X, Wang J, Carlsson C, et al. A strategy for discovery and verification of candidate biomarkers in cerebrospinal fluid of preclinical Alzheimer's disease. Front Mol Neurosci 2019; 11: 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Barlas CL, Thompson Y, et al. Caregivers’ experience of decision-making regarding diagnostic assessment following cognitive screening of older adults. J Aging Res 2018; 835: 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pittam G, Allaby M. Screening for dementia: can screening bring benefits to those with unrecognised dementia, their carers and society? An appraisal against UKNSC criteria. Oxford: Solutions for Public Health, 2014, pp.4–29. [Google Scholar]
- 11.Benbow SM, Jolley D. Dementia: stigma and its effects. Neurodegener Dis Manag 2012; 2: 165–172. [Google Scholar]
- 12.Brayne C, Kelly S. Against the stream: early diagnosis of dementia, is it so desirable? BJPsych Bull 2019; 43: 123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brayne C, Fox C, Boustani M. Dementia screening in primary care: is it time? JAMA. Erratum in JAMA 2008; 299: 634. [DOI] [PubMed] [Google Scholar]
- 14.De Cola MC, Lo Buono V, Mento A, et al. Unmet needs for family caregivers of elderly people with dementia living in Italy: what do we know so far and what should we do next? Inquiry 2017; 54: 46958017713708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment-beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256: 240–246. [DOI] [PubMed] [Google Scholar]
- 16.Prince M, Wimo A, Guerchet M, et al. World Alzheimer Report 2015: the global impact of dementia. An analysis of prevalence, incidence, costs and trends. London, UK: Alzheimer’s Disease International, 2015, pp.6–80. [Google Scholar]
- 17.Alzheimer’s Society: Dementia out of the shadows. https: //www.alzheimers.org.uk/sites/default/files/2018-08/out_of_the_shadows.pdf?fileID=454 (2008, accessed 18 May 2019).
- 18.Dana Foundation. http: //dana.org/BAW/ (2019, accessed 5 June 2019).
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 20.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 21.Borson S, Scanlan J, Hummel J, et al. Implementing routine cognitive screening of older adults in primary care: process and impact on physician behavior. J Gen Intern Med 2007; 22: 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milne A. Dementia screening and early diagnosis: the case for and against. Health Risk Soc 2010; 1: 65–76. [Google Scholar]
- 23.McCartney M. “Case finding” in dementia is simply screening with no evidence of benefit. BMJ 2014; 349: 4791. [DOI] [PubMed] [Google Scholar]
- 24.Martin S, Kelly S, Khan A, et al. Attitudes and preferences towards screening for dementia: a systematic review of the literature. BMC Geriatr 2015; 15: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borson S, Frank L, Bayley PJ, et al. Improving dementia care: the role of screening and detection of cognitive impairment. Alzheimers Dement 2013; 9: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panegyres PK, Berry R, Burchell J. Early dementia screening. Diagnostics (Basel) 2016; 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvin JE, Fagan AM, Holtzman DM, et al. Relationship of dementia screening tests with biomarkers of Alzheimer’s disease. Brain 2010; 133: 3290–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burioni R, Contucci P, Fedele M, et al. Enhancing participation to health screening campaigns by group interactions. Sci Rep 2015; 5: 9904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Research Council. Reducing the odds: preventing perinatal transmission of HIV in the United States. Washington, DC: National Academies Press, 1999, pp.15–237. [PubMed] [Google Scholar]
- 30.Bovet P, Hirsiger P, Emery F, et al. Impact and cost of a 2-week community-based screening and awareness program for diabetes and cardiovascular risk factors in a Swiss canton. Diabetes Metab Syndr Obes 2011; 4: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson JMG, Jungner G. Principles and practice of screening for disease . Geneva: World Health Organization; https: //apps.who.int/iris/bitstream/handle/10665/37650/WHO_PHP_34.pdf?sequence=17 (1968, accessed 4 August 2019). [Google Scholar]
- 32.Lin JS, O'Connor E, Rossom RC, et al. Screening for cognitive impairment in older adults: an evidence update for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US), 2013. [PubMed] [Google Scholar]