Dear editor
Walsh et al. recently published a review in this journal focusing on the duration of infectiousness in SARS-CoV-2 infected individuals.1 Based on available data from SARS-CoV-2 culture studies and contract tracing studies included in this review, the authors reported that patients with mild-to-moderate Coronavirus Disease 2019 (COVID-19) are considered to be without infectious potential beyond day 10 after the onset of symptoms. In contrast, immunocompromised and severe-to-critical patients may have prolonged viral shedding and, thus, may also provide prolonged infectiousness. Data addressing prolonged viral shedding and potential spread of SARS-CoV-2 are matter of public concern. Whereas isolation precautions as recommended by the United States Centers of Disease Control and Prevention2 and the European health care authorities3 are considered to fit for most SARS-CoV-2 infected patients, uncertainty remains in those patients with underlying immunodeficiency. Walsh et al. included a total of 15 relevant studies, but only two of them identified immunosuppressed patients from whom SARS-CoV-2 was isolated for up to 20 days beyond onset of disease.4 , 5 Here, we report SARS-CoV-2 positive viral culture 7 weeks after onset of COVID-19 in a patient with an underlying immunosuppressive disorder, so-called X chromosome-linked agammaglobulinemia (XLA), demonstrating the potential of prolonged SARS-CoV-2 spreading beyond widely accepted isolation precautions.
Our patient had been tested positive for SARS-CoV-2 ribonucleic acid (RNA) by reverse transcription real-time polymerase chain reaction (RT-PCR) from upper respiratory specimen first on March 11 2020,. At this point of time, symptoms comprised fever and fatigue. In the patient's medical history, XLA, obstructive respiratory disorder, impaired alveolar diffusion capacity and non-cystic fibrosis bronchiectasis were recorded. Due to worsening of fever, cough and dyspnea, the patient required for hospitalization 16 days after the initial diagnosis of COVID-19. The patient received antibiotic treatment with amoxicillin/clavulanic acid and azithromycin, was switched to piperacillin/tazobactam, followed by moxifloxacin and meropenem. Based on local recommendations valid at this point of time, the patient was treated with hydroxychloroquine and then by an antiretroviral combination of lopinavir/ritonavir. Furthermore, posaconazole was administered for Aspergillus positive sputum culture and intravenous immunoglobulin substitution (IVIG) was performed regarding the absence of endogenous antibody production in underlying XLA. Due to persistent fever up to 40.4 °C, progressive respiratory insufficiency and deterioration of laboratory parameters expressing an increasing inflammatory activity, the patient was transmitted to the Intensive Care Unit (ICU) on April 9. Interleukin-6 (IL-6) receptor blockade by tocilizumab and convalescent plasma were administered on April 10. No adverse effects related to this treatment regimen were recorded. The rationale behind this approach was to restrain the inflammatory response by IL-6 blockade and to provide neutralizing COVID-19 immunoglobulins by convalescent plasma. Afterwards, we observed a rapid recovery regarding clinical and laboratory parameters. Ferritin and C-reactive protein (CRP) significantly declined as compared to pre-transfusion whereas the lymphocyte count returned to normal. We observed an increase in IL-6 after treatment followed by a fast and almost complete decline within the next days. Body temperature did not exceed a limit of 38 °C as compared to measurements of up to 40.4 °C pre-transfusion. Oxygen demand decreased resulting in an increase of PaO2/FiO2 ratio (143 before versus 223 after treatment). A chest radiograph showed a significant decline in infiltrative opacities. On April 15, five days after tocilizumab and convalescent plasma administration and five weeks after the initial diagnosis of COVID-19, SARS-CoV-2 RNA was not detectable for the first time. The patient showed progressive clinical recovery, but an alternating course of three negative followed by three positive SARS-CoV-2 RT-PCR results was subsequently observed. Convalescent plasma transfusion was repeated on April 21. Despite full clinical recovery, SARS-CoV-2 RT-PCR showed six positive and also six negative SARS-CoV-2 RT-PCR results in an alternating order in the time span between April 23 and May 4. SARS-CoV-2 PCR from oropharyngeal swabs and sputum obtained on April 24 showed 103 (sputum) to 105 (oropharyngeal swabs) SARS-CoV-2 copies/mL. A SARS-CoV-2 PCR-positive oropharyngeal swab with a low cycle threshold (Ct) value of 25 was inoculated onto Vero E6 cells for viral culture. A cytopathic effect was observed four days after inoculation, and the presence of SARS-CoV-2 in the cell culture supernatant was confirmed by RT-PCR (Ct value of 16 at a 10-fold higher dilution than the original swab). In contrast to this finding, previously published reports revealed that the viral burden measured in respiratory specimens obtained from mild coronavirus disease 2019 (COVID-19) cases declined after onset of symptoms and was considered without infectious potential beyond day 9 or 10 of symptoms with less than 105 viral ribonucleic acid (RNA) copies/mL of sputum.6 , 7 Based on the clinical improvement and three negative follow up SARS-CoV-2 PCR results the patient was discharged on May 5 and isolated at home. Almost 2 weeks later a SARS-CoV-2 PCR showed 500 copies/mL in transport medium (containing the oropharyngeal swab) and the viral culture was negative. Fig. 1 presents an overview by timeline from the initial diagnosis of COVID-19 up until negative viral culture.
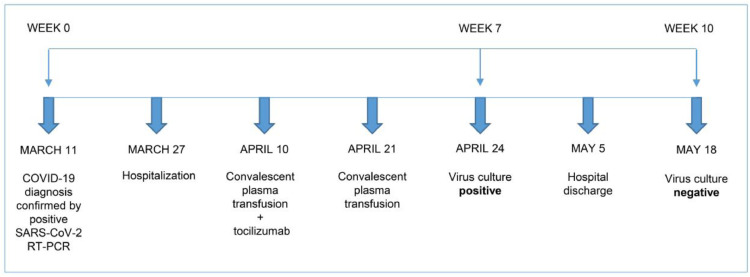
Fig. 1.
Timeline presenting milestones of disease from onset of COVID 19 up until negative virus culture. Our patient was diagnosed with COVID-19 based on a positive result from SARS-CoV-2 RT-PCR first on March 11. Admission to the hospital was required on March 27 due to progressive clinical deterioration. Administration of an experimental treatment approach comprising convalescent plasma transfusion and interleukin-6 receptor blockade by tocilizumab was performed on April 10 and convalescent plasma was administered for a second time on April 21. A virus culture within week 7 of disease still showed a positive result and, thus, confirmed prolonged viral shedding in this patient. Viral culture control in week 10 presented a negative result. COVID-19 = coronavirus disease 2019; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; RT-PCR = real-time polymerase chain reaction.
In summary, we have to assume that in our patient shedding of infectious SARS-CoV-2 stopped between week 7 and 10 of disease. Our patient suffers from XLA, also known as Bruton's agammaglobulinemia, which is caused by a mutation in Bruton's tyrosine kinase resulting in an inability of endogenous antibody production du to a developmental arrest of pre B cells.8 In contrast with a previously described 3-day mild course of COVID-19 in a patient with underlying XLA,9 our patient experienced a prolonged and severe course COVID-19 requiring ICU, oxygen support, treatment of cytokine storm with tocilizumab and administration of convalescent plasma to overcome a lacking antibody response. To conclude, our case demonstrates that in immunocompromised patients caution is warranted in applying generally accepted clinical management and public health strategies to prevent the spread of COVID-19. Additionally, our observations in a patient with severe COVID-19 in underlying immunodeficiency encourages the conclusion drawn by Walsh et al. requiring for further reserch in certain subgroups, particularly.
Declaration of Competing Interest
None to declare.
Acknowledgments
Acknowledgments
None to declare.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Walsh K.A., Spillane S., Comber L., Cardwell K., Harrington P., Connell J. The duration of infectiousness of individuals infected with SARS-COV-2. J Infect. 2020 doi: 10.1016/j.jinf.2020.10.009. . In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Symptom-based strategy to discontinue isolation for persons with COVID-19 [cited 2020. Available from: https://www.cdc.gov/coronavirus/2019-ncov/community/strategy-discontinue-isolation.html.
- 3.European Centre for Disease Prevention and Control (ECDC). Guidance for discharge and ending isolation in the context of widespread community transmission of COVID-19 – first update [cited 2020. Available from: https://www.ecdc.europa.eu/en/publications-data/covid-19-guidance-discharge-and-ending-isolation.
- 4.van Kampen JJ vdVD, Fraaij P.L., Haagmans B.L., Lamers M.M., Okba N., van den Akker J.P., Endeman H., Gommers D.A., Cornelissen J.J., Hoek R.A., van der Eerden M.M., Hesselink D.A., Metselaar H.J., Verbon A., de Steenwinkel J.E., Aron G.I., van Gorp E.C., van Boheemen S., Voermans J.C., Boucher C.A., Molenkamp R., Koopmans M.P., Geurtsvankessel C., van der Eijk A.A. Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants. medRxiv. 2020 doi: 10.1101/2020.06.08.20125310. . Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decker A., Welzel M., Laubner K., Grundmann S., Kochs G., Panning M. Prolonged SARS-CoV-2 shedding and mild course of COVID-19 in a patient after recent heart transplantation. Am J Transplant. 2020 doi: 10.1111/AJT.16133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 7.Arons M.M., Hatfield K.M., Reddy S.C., Kimball A., James A., Jacobs J.R. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shillitoe B., Gennery A. X-linked agammaglobulinaemia: outcomes in the modern era. Clin Immunol. 2017;183:54–62. doi: 10.1016/j.clim.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Quinti I., Lougaris V., Milito C., Cinetto F., Pecoraro A., Mezzaroma I. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146(1):P211–P213. doi: 10.1016/j.jaci.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]