Abstract
Patients who have undergone revascularization with a cryopreserved cadaveric arterial allograft (CCAA) require lifelong surveillance because of the risk of allograft failure. The reported long-term complications of these grafts include thrombosis, anastomotic pseudoaneurysm, and graft disruption. We have described a case in which a CCAA developed a nonanastomotic pseudoaneurysm at the site of a previously ligated branch vessel and was repaired using a covered stent graft. This case demonstrates that spontaneous rupture of CCAA branches is a late complication that can occur when using these grafts and that endovascular methods are an option for repair.
Keywords: Cadaveric allograft, Graft infection, Pseudoaneurysm
Treatment of a prosthetic graft infection is challenging. The treatment goal is to remove all prosthetic material and to re-establish blood flow.1 One option used for revascularization has been cryopreserved cadaveric arterial allografts (CCAAs). These CCAAs provide readily available conduits that are resistant to infection.1 The long-term outcomes of these grafts are unknown; however, late complications, such as aneurysmal dilation, anastomotic pseudoaneurysm formation, and graft thrombosis, have been reported.2 We have presented the case of a patient who developed a spontaneous pseudoaneurysm in the midportion of the left iliac limb of a cadaveric aortoiliac graft. This pseudoaneurysm was treated using a covered stent graft and had remained patent at the latest follow-up examination at 18 months. The patient provided written informed consent for the report of the images and clinical details of her case.
Case report
The patient was a 67-year-old woman with a medical history of hypertension, peripheral arterial disease, and an abdominal aortic aneurysm that had been previously treated with an aortic endograft. The aortic endograft had developed a graft infection 3 months after the initial procedure. This had prompted endograft explanation and extra-anatomic reconstruction with a polytetrafluoroethylene (PTFE) axillary bifemoral artery bypass. However, at 6 months after this procedure, the patient had presented to our institution with concern for bilateral groin wound infections. A computed tomography scan demonstrated fluid collections at the site of the bilateral groin incisions and gas near the left femoral anastomosis. These findings prompted replacement of the distal portion of the axillary bifemoral artery bypass with a cadaveric aortoiliac graft. The axillary portion of the PTFE graft was left in place because it was not infected, and we did not want to expose the patient to reoperative axillary exploration. Aortic reconstruction was not performed because of concerns for dense intra-abdominal adhesions. The cadaveric aortoiliac graft was prepared for implantation by oversewing of the aortic and iliac side branches with 5-0 Prolene suture (Ethicon, Inc, Somerville, NJ). The axillary graft to cadaveric aortic anastomosis was completed using Gore-Tex CV6 suture (WL Gore and Associates, Newark, Del), and the anastomosis between the cadaveric iliac limbs and the common femoral arteries was completed using 5-0 Prolene suture (Ethicon, Inc). The remainder of the distal PTFE graft was explanted. The wound and tissue cultures were negative for infection. However, a clinical suspicion was present for a graft infection, and the patient was discharged with a prescription for trimethoprim/sulfamethoxazole (Bactrim; Roche, Basel, Switzerland). The patient stopped the antibiotics 5 months later. At 4 years after this surgery, she developed spontaneous pain in her abdomen and denied any symptoms of an infection. An arterial duplex ultrasound scan was performed, which demonstrated a pseudoaneurysm (Fig 1) of the midportion of the iliac limb of the cadaveric aortoiliac graft. The patient had a normal white blood cell count and negative blood cultures. A computed tomography angiogram (Fig 2) revealed that a pseudoaneurysm measuring 15 × 18 mm was present at the site of the internal iliac artery origin on the cadaveric graft. She had no clinical or radiologic signs to suggest graft infection. Therefore, we decided to treat this pseudoaneurysm using a stent graft to cover its origin. An incision was made over the axillary portion of the graft, and a 7F sheath was placed in the antegrade direction. Therapeutic heparin was administered, and the origin of the cadaveric left internal iliac artery was visualized (Fig 3). A size discrepancy was present, with the diameter of the cadaveric common iliac artery measuring 9 mm and the diameter of the cadaveric external iliac artery measuring 7 mm. We decided to place a 10 mm × 50 mm Viabahn stent (WL Gore and Associates). We decided to use a self-expanding stent because we were concerned about the failure of a balloon expandable stent secondary to external compression in this superficial location. The stent was postdilated in the proximal aspect using a 10-mm × 20-mm Armada angioplasty balloon (Abbott, Lake Bluff, Ill) and in the distal aspect using a 8-mm × 20-mm Armada angioplasty balloon (Abbott). After postdilation of the stent, filling of the pseudoaneurysm no longer occurred (Fig 4). Immediately postoperatively, the patient's symptoms had improved, and she was discharged on hospital day 4 with instructions to take aspirin and clopidogrel (Plavix; Bristol-Myers Squibb–Sanofi Pharmaceuticals, New York, NY). This patient has been followed up with arterial duplex ultrasound scans every 6 months and has not had any recurrence of her symptoms. The stent has remained patent for 18 months postoperatively.
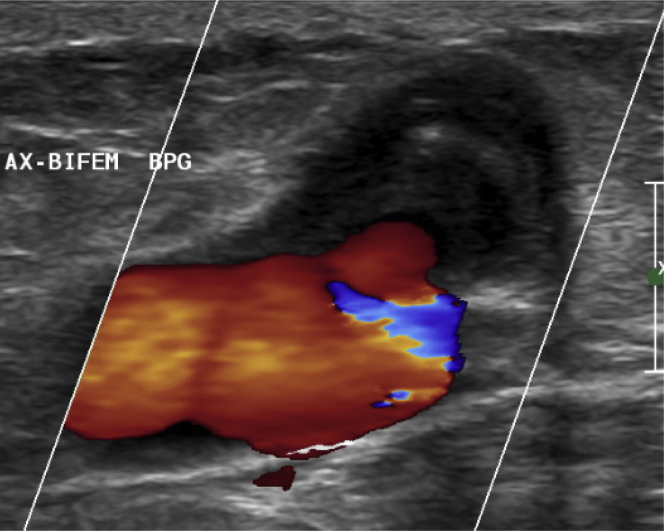
Fig 1.
Arterial duplex ultrasound scan of the pseudoaneurysm arising from the cadaveric cryopreserved left iliac artery.
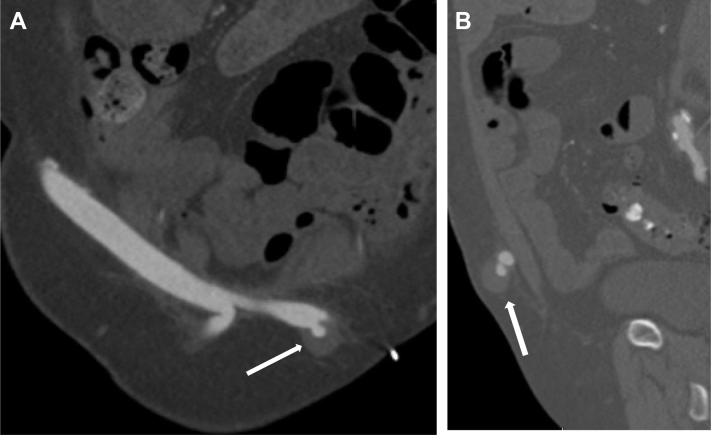
Fig 2.
Computed tomography angiograms, coronal view (A) and sagittal view (B), of pseudoaneurysm (arrow) arising from the cadaveric cryopreserved left internal iliac artery.
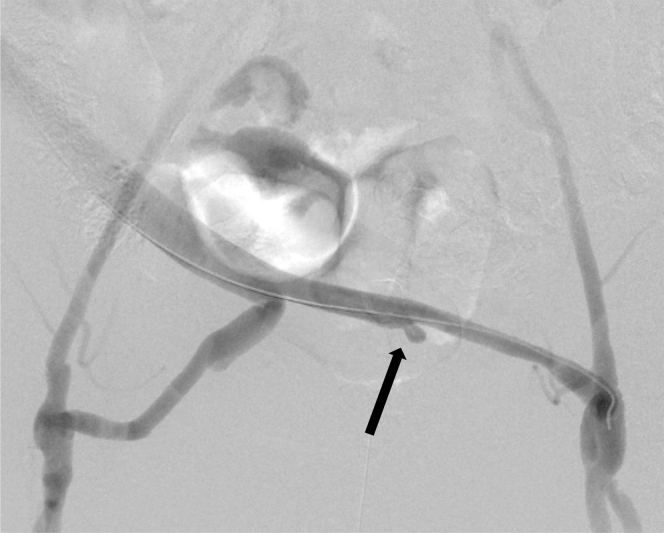
Fig 3.
Angiogram of the cadaveric cryopreserved aortoiliac graft showing the pseudoaneurysm arising at the site of the left internal iliac artery (arrow).
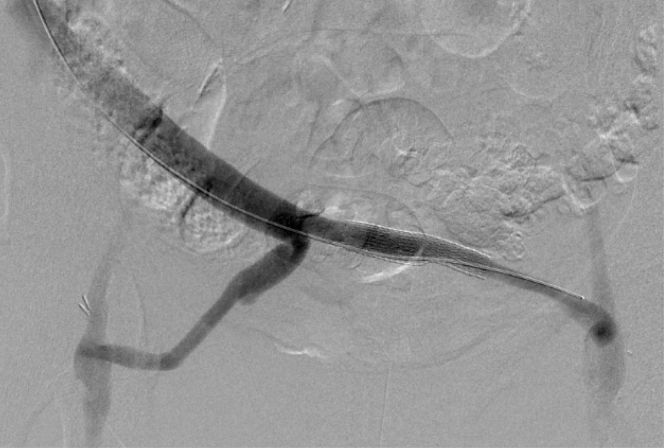
Fig 4.
Angiogram of the cadaveric cryopreserved aortoiliac graft after placement of the stent graft and resolution of blood flow into the pseudoaneurysm.
Discussion
The treatment of a prosthetic bypass graft infection is complicated. The standard practice of extra-anatomic bypass, followed by explanation of the infected conduit and wide debridement has resulted in poor outcomes. This technique offers a 50% to 60% 5-year patency rate, a 6% to 15% reinfection rate, and a 10% to 20% 3-year amputation rates.2 These results prompted the use of cadaveric allografts because of their presumed lower infection risk and superior patency.2 However, fresh aortoiliac cadaveric grafts have had a 26.4% rate of late aneurysmal degeneration.3 The use of cryopreservation techniques has improved the mechanical strength of cadaveric grafts compared with fresh grafts and has decreased the risk of aneurysmal formation.4 However, 29% of CCAAs will result in late complications.2 These have included graft thrombosis, graft stenosis, anastomotic pseudoaneurysm formation, aneurysmal degeneration, and allograft disruption.2 Nonanastomotic pseudoaneurysm formation can result from allograft disruption. The potential causes of allograft disruption include mechanical weakening of the graft wall secondary to gradual fragmentation and the loss of elastic fibers,2 “slip off” of a ligated allograft branch, and pressure injury at the site of branch ligation.5
Treatment of the late complications of CCAAs using open surgical techniques has presented a difficult challenge owing to the presence of dense adhesions.6 Thus, endovascular methods have been proposed to treat the late complications of CCAA. Stenosis of CCAAs has been treated endoluminally with angioplasty and stents.7 Petrunic et al8 reported a false aneurysm formation at the distal anastomosis of a previous aortic reconstruction with a CCAA. The false aneurysm formation was treated by placement of a bifurcated endovascular aneurysm repair device, which excluded the false aneurysm. Bustamante et al6 had used an iliac limb stent graft from an endovascular aneurysm repair device to repair a pseudoaneurysm from the midportion of an iliac limb and aortoiliac CCAA that had been used to repair an infected aortobifemoral artery bypass.
The case we have presented further supports the use of endovascular techniques to treat the late complications of CCAA. The present findings highlight that the CCAA branch vessels have the potential to rupture over time and can form pseudoaneurysms. The initial concern in the present patient was that the pseudoaneurysm was secondary to an infectious process. However, the patient had had a normal white blood cell count and negative blood cultures. Also, the CCAA on the computed tomography scan did not have a surrounding fluid collection, and it appeared that the pseudoaneurysm had originated from a previous branch vessel. Thus, we believed that an infectious etiology was less likely and that the pseudoaneurysm had most likely resulted from gradual weakening of the branch vessel and its eventual rupture. The use of a stent graft to exclude this pseudoaneurysm offered a minimally invasive solution. Also, placement of the stent graft did not compromise the patency of the CCAA.
Conclusions
Pseudoaneurysm formation secondary CCAA branch rupture is a potential complication that requires long-term graft surveillance. Stent grafts offer an endovascular treatment strategy for these pseudoaneurysms. Follow-up with an arterial duplex ultrasonography at 6-month intervals is a reasonable assessment strategy.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Lejay A., Delay C., Girsowicz E., Chenesseau B., Bonnin E., Ghariani M. Cryopreserved cadaveric arterial allograft for arterial reconstruction in patients with prosthetic graft infection. Eur J Vasc Endovasc Surg. 2017;54:636–644. doi: 10.1016/j.ejvs.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Minga Lowampa E., Holemans C., Stiennon L., Van Damme H., Defraigne J.O. Late fate of cryopreserved arterial allografts. Eur J Vasc Endovasc Surg. 2016;52:696–702. doi: 10.1016/j.ejvs.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Vogt P.R. Arterial allografts in treating aortic graft infections: something old, something new. Semin Vasc Surg. 2011;24:227–233. doi: 10.1053/j.semvascsurg.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Szilagyi D.E., Rodriguez F.J., Smith R.F., Elliott J.P. Late fate of arterial allografts: observations 6 to 15 years after implantation. Arch Surg. 1970;101:721–733. doi: 10.1001/archsurg.1970.01340300077014. [DOI] [PubMed] [Google Scholar]
- 5.Vogt P.R., Brunner-LaRocca H.P., Lachat M., Ruef C., Turina M.I. Technical details with the use of cryopreserved arterial allografts for aortic infection: influence on early and midterm mortality. J Vasc Surg. 2002;35:847–852. doi: 10.1067/mva.2002.118818. [DOI] [PubMed] [Google Scholar]
- 6.Bustamante M., Gomez-Dermit V., Garcia I., Ponton A., Revuelta J.M., Gonzalez-Tutor A. Endoluminal repair of a pseudoaneurysm in a patient with cryopreserved arterial allograft of the iliac vessel. Ann Vasc Surg. 2009;23:410.e17–410.e20. doi: 10.1016/j.avsg.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Brown K., Heyer K., Rodriguez H., Eskandari M., Pearce W., Morasch M. Arterial reconstruction with cyropreserved human allografts in the setting of infection: a single-center experience with midterm follow-up. J Vasc Surg. 2008;49:660–666. doi: 10.1016/j.jvs.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 8.Petrunic M., Drazen P., Mestrovic T., Haluzan D., Mahecic T. Endovascular treatment of a late false aneurysm complication open suprarenal mycotic aneurysm repair with a cryopreserved aortic homograft. Ann Vasc Surg. 2019;56:350.e5–350.e8. doi: 10.1016/j.avsg.2018.07.055. [DOI] [PubMed] [Google Scholar]