Abstract
Background and Purpose
Epidemiological and experimental studies suggest that microbial exposure in early childhood is linked with reduced risk to suffer asthma. Thus microbial components with immunoregulatory capabilities might serve as a preventive strategy for allergic asthma. Recently, it was identified that Streptococcus pneumoniae aminopeptidase N (PepN) could suppress T cell effector function. We sought to investigate the effect of PepN on murine allergic asthma and elucidate the underlying mechanism.
Experimental Approach
The effects of intranasal administration of PepN during or before sensitization were examined in ovalbumin (OVA)‐induced murine allergic asthma. The roles of CD11b+ dendritic cells in PepN treated OVA‐induced allergic asthma were evaluated by flow cytometry, cytokines detection and adoptive transfer. Moreover, the numbers of lung type 2 innate lymphoid cells (ILC2s) were also detected.
Key Results
Administration of PepN during or before sensitization attenuated type‐2 airway inflammation (eosinophilia, mucus hypersecretion, Th2 cytokines production and IgE production) in allergic asthma mice. PepN reduced lung accumulation of CD11b+ dendritic cells, which was accompanied by diminished dendritic cell‐attracting chemokine CCL20 production as well as CCL17 and CCL22, which are Th2‐cell chemokines predominantly produced by CD11b+ dendritic cells. Adoptive transfer of BM‐derived CD11b+ dendritic cells abolished the inhibitory effect of PepN on OVA‐induced type‐2 airway inflammation. The numbers of lung ILC2s were decreased in asthmatic mice receiving PepN.
Conclusion and Implications
PepN alleviated type‐2 inflammation in OVA‐induced allergic asthma mice, which was mediated by regulation of lung CD11b+ dendritic cells. Our study provides a novel strategy for the prevention of allergic asthma.
Keywords: allergic asthma, dendritic cells, Streptococcus pneumoniae aminopeptidase N, type 2 innate lymphoid cells
Abbreviations
- BALF
bronchoalveolar lavage fluid
- BMDC
bone marrow‐derived dendritic cell
- cDC
conventional dendritic cell
- DC
dendritic cell
- H&E
haematoxylin–eosin
- ILC2
type 2 innate lymphoid cell
- LN
lymph node
- OVA
ovalbumin
- PAS
periodic acid‐Schiff
- PepN
aminopeptidase N
- Spn
Streptococcus pneumoniae
What is already known
Microbial exposure in early childhood is linked with reduced risk to suffer asthma.
Spn aminopeptidase N has been identified as an inhibitory component that suppressed T cell function.
What does this study add
Spn aminopeptidase N attenuates type‐2 airway inflammation in OVA‐induced murine allergic asthma.
Spn aminopeptidase N reduces the numbers of lung CD11b+ dendritic cells and ILC2s.
What is the clinical significance
Spn aminopeptidase N may have potential for the prevention of allergic asthma.
1. INTRODUCTION
The prevalence of allergic asthma has been increasing dramatically in affluent countries over the last 50 years, with approximately 100 million persons affected globally (Baldacci et al., 2015). Currently, allergic asthma has been controlled with pharmaceuticals and allergen‐specific immunotherapy. However, anti‐asthma medications remain palliative and specific immunotherapy requires a long duration before a beneficial action is observed. Accordingly, it is urgent to develop a new strategy for allergic asthma prevention or treatment.
Epidemiological studies have suggested that early life contact to complex microbial communities such as traditional dairy farms reduces the risk of allergies and asthma (Milligan, Matsui, & Sharma, 2016). The increasing experimental studies have reported that bacterial infection (e.g. H. pylori, Lactobacillus rhamnosus and Bifidobacterium) can inhibit allergic asthma (Raftis et al., 2018; Spacova et al., 2019; van Wijck et al., 2018). However, initial clinical studies with living bacteria as strategies for allergic asthma prevention revealed poor effects (Schmidt et al., 2019). In contrast, the use of bacterial components with definable physicochemical properties make it easier to assess the dose–response relationships, probably due to the higher bioavailability and lower variability of clinical responses (Jatzlauk, Bartel, Heine, Schloter, & Krauss‐Etschmann, 2017). Therefore, using immunomodulatory bacterial components might provide a new therapeutic approached for the treatment of allergic asthma. Thus far, only very few bacterial components such as flagellin B and D‐tryptophan have been shown to attenuate asthma in mice (Kepert et al., 2017; Shim et al., 2016). However, they may still have poor effects when applied clinically. Therefore, exploring new bacterial components to provide more such candidates for subsequent clinical studies is needed.
Streptococcus pneumoniae (Spn) vaccine has been linked with decreased asthma‐related hospitalizations (Ansaldi et al., 2005; Thorburn et al., 2010) and,treatment with S. pneumoniae inhibited allergic airways disease in mice (Preston et al., 2011; Thorburn, Foster, Gibson, & Hansbro, 2012). Thus we hypothesised that S. pneumoniae may have a particular components that can suppress the development of allergic asthma. Given that allergic asthma is a disease of T cell immune dysfunction, Spn aminopeptidase N (PepN) focused our attention since the discovery of its role in negatively regulating T cell receptor signalling in a mouse influenza virus infection model (Blevins et al., 2017). However, whether PepN can suppress excessive T cell immune responses in allergic asthma and thus protect against allergic asthma remains unknown.
Upon allergen stimulation, increased airway epithelial cell secretions of CCL2, CCL8 and CCL20 lead to the recruitment of dendritic cells to lung (Gill, 2012; O'Boyle, Brain, Kirby, & Ali, 2007; Tiberio et al., 2018). Lung CD11b+ dendritic cells that acquire allergens and migrate to the mediastinal lymph nodes induce naïve CD4+ T cells to Th2 polarization (Plantinga et al., 2013). Meanwhile, Lung CD11b+ dendritic cells produce chemokines CCL17 and CCL22, attracting Th2 cells to the lung (Gill, 2012; Medoff et al., 2009). Activated Th2 cells release abundant Th2 cytokines IL‐4, IL‐5 and IL‐13, inducing enhanced IgE production, airway eosinophilia, mucus hypersecretion and airway hyperresponsiveness, which are typical features of allergic asthma (Islam & Luster, 2012; Lambrecht & Hammad, 2015; Locksley, 2010). Recently, lung type 2 innate lymphoid cells are known to drive allergic asthma by releasing IL‐5 and IL‐13 (Anderson et al., 2016; Lund, Walford, & Doherty, 2013). Besides, type 2 innate lymphoid cells can also license CD11b+ dendritic cells to enhance Th2 cell responses during allergic airway inflammation (Halim et al., 2016). Here, we aimed to address the effect of PepN on ovalbumin (OVA)‐induced murine allergic asthma and investigate the underlying mechanism.
2. METHODS
2.1. Animals
Female, 6‐ to 8‐week‐old BALB/c mice (RRID:IMSR_ORNL:BALB/cRl) were purchased from the Animal Experimental Center of Chongqing Medical University. All mice were housed under specific pathogen‐free conditions in Chongqing Medical University; the mice were housed at 22 ± 1°C with 12 h light–dark cycles and fed with a regular chow diet and water under standard conditions (specific‐pathogen‐free) with air filtration. All experiments were performed in consistent with the Institutional Animal Care and Use Committee's guidelines at the Chongqing Medical University. Animal studies are reported in compliance with the ARRIVE guidelines (Percie du Sert et al., 2020) and with the recommendations made by the British Journal of Pharmacology (Lilley, Stanford et al., 2020).
2.2. Experimental protocols for allergic asthma and PepN intervention
The animal studies are reported in compliance with the ARRIVE guidelines as per the recommendations of the British Journal of Pharmacology. A block randomization technique was used to randomize the animals into groups of equal sample sizes. However, in some cases, mice reached euthanasia criteria during the acute inflammation or treatments so were not able to be included in analyses. The investigators were blinded to the treatments. The group size was determined by our previous work on other questions with comparable analysis methods and power analysis. The numbers of mice used for each experimental group are shown in the figure legends. The experimental groups were designed as follows:‐ sham induction by the sterile PBS control group, asthma model group with OVA (Sigma‐Aldrich, St. Louis, MO, USA) induction and asthma model with PepN treatment group. For OVA‐induced allergic asthma, mice were intraperitoneally sensitized with 100 μg OVA suspended in 1 mg of aluminium hydroxide (Thermo Scientific, Waltham, MA, USA) in 200 μl of sterile PBS on days 0 and 7, followed by eight aerosol challenges with 5% (w/v) OVA in PBS for 30 min per day from day 14 to 21 (Yan et al., 2019). The control group mice were treated in the same way with PBS at the same time points. Recombinant Spn PepN was produced in E. coli with <0.10 EU per 1 μg of the protein. For treatment of PepN during sensitization, mice were anaesthetized by intraperioneally injected 1.5%(w/v) pentobarbital sodium and intranasally (i.n.) instilled with 30 μl of different doses of PepN on days 2, 6 and 10. For treatment of PepN before sensitization, mice were anaesthetized and intranasally instilled with 30 μl of 50 μg of PepN on days 10, 6 and 2. Mice were euthanized by intraperioneally injected 1.5% pentobarbital sodium for analysis 24 h after the last challenge.
2.3. Analysis of bronchoalveolar lavage fluid (BALF)
Tracheal intubation was performed using an indwelling needle after mice were anaesthetized. bronchoalveolar lavage fluid was acquired by lavaging five times with 1 ml of PBS. The recovered bronchoalveolar lavage fluid was collected and centrifuged at 800× g for 5 min at 4°C. The cell free supernatant was used for detection of cytokines or chemokines by ELISA kits. The cell pellets were resuspended in 1 ml of PBS and the total numbers of inflammatory cells were counted by modified Neubauer Counter under microscope. After cytospin and Wright's staining, neutrophils, eosinophils, lymphocytes and macrophages were differentially counted. Two hundred cells were counted for each sample and the absolute numbers of each cell type were calculated.
2.4. Histology
The thoracic cavity was dissected after mice were anaesthetized. The lungs were removed and fixed in a 4% paraformaldehyde solution. They were subjected to dehydration, transparency, waxing, embedding and sectioning. Finally, the samples were stained with haematoxylin–eosin (H&E) to observe lung inflammatory cell infiltrations or with periodic acid‐Schiff (PAS) to examine mucus secretion of goblet cells under a Nikon E200 microscope. The production of mucus was scored as described previously (Xu et al., 2018) and PAS‐stained goblet cells in airway epithelium were measured double blind using a numerical scoring system (0 = <5% goblet cells; 1 = 5–25%; 2 = 25–50%; 3 = 50–75%; 4 = >75%). The sum of the airway scores from each lung was divided by the number of airways examined (20–30 per mouse) and expressed as mucus score in arbitrary units.
2.5. ELISA
IFN‐γ, IL‐4, IL‐5, IL‐13, IL‐17A and IgE levels were detected by commercial ELISA kits (Biolegend, San Diego, CA, USA). IL‐13, CCL20 and CCL24 were measured by ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
2.6. qPCR
RNA from lungs was extracted using RNAiso Plus reagent (Takara, Shiga, Japan), then reversely transcribed using a PrimeScript™ RT reagent Kit (Takara, Shiga, Japan) according to the manufacturer's protocol. cDNA was analysed by qPCR using a SYBR Premix Ex Taq kit (Takara, Shiga, Japan). GAPDH was used as an internal reference for analysis. The primers used for qPCR in this study are listed in Table 1.
TABLE 1.
Primers used for qPCR
| Gene symbols | Primer Sequence (5′–3′) |
|---|---|
| GADPH |
F: TGGAAAGCTGTGGCGTGATG R: TACTTGGCAGGTTTCTCCAGG |
| CCL2 |
F: GATGCAGTTAACGCCCCACT R: ACCCATTCCTTCTTGGGGTC |
| CCL8 |
F: GCCAGATAAGGCTCCAGTCA R: TGCCTGGAGAAGATTAGGGGA |
| CCL20 |
F: TTTGTGTCCCAGTGGACTTGT R: AATCCTTCCACTAAGCGCCC |
| CCL17 |
F: CAATGTAGGCCGAGAGTGCT R: CCTGGAACACTCCACTGAGG |
| CCL22 |
F: TTCTCTGCCCCACACCTTTG R: GCAGCTCCCACAACCATCTA |
2.7. Flow cytometry
Single‐cell suspensions of the lungs were prepared first as described below. After the lungs were perfused with 20 ml sterile PBS, removed the lungs from mice, cut into approximately 1 mm3 pieces and incubated with 2 ml digestion buffer (RPMI 1640 containing 1 mg·ml−1 collagenase IV [Sigma‐Aldrich, St. Louis, MO, USA] and 5 U·ml−1 DNase I [Sigma‐Aldrich, St. Louis, MO, USA]) at 37°C for 1 h. Then a 70‐μm cell strainer was used to filter the digested tissue and red blood cells were lysed using red blood cell lysis buffer and collected cells by centrifugation. Next, after washed in PBS twice, cells in lung tissue were incubated with the purified anti‐mouse CD16/32 antibody (eBioscience, San Diego, CA, USA, Cat#14‐0161‐85, RRID: AB_467134) for 20 min at 4°C. For dendritic cells and type 2 innate lymphoid cells surface staining, single‐cell suspensions of the lungs were incubated with corresponding flow cytometry antibodies for 30 min. For type 2 innate lymphoid cells intracellular staining, cells were fixed with a fixation buffer (Biolegend, San Diego, CA, USA) and treated with permeabilization buffer (Biolegend, San Diego, CA, USA), further stained intracellularly with anti‐mouse IL‐5 antibody (Biolegend, San Diego, CA, USA, Cat#504311, RRID:AB_2563161). After washing with PBS, cells were detected and analysed on FACSCanto II (BD Biosciences). For Th2 cell staining, single cells were stained with CD3 (Biolegend, San Diego, CA, USA, Cat#100236, RRID:AB_2561456) and CD4 (Biolegend, San Diego, CA, USA, Cat#116004, RRID:AB_313689) antibodies for 30 min, then fixed with a fixation buffer (Biolegend, San Diego, CA, USA), followed by treatment with intracellular staining permeabilization buffer (Biolegend, San Diego, CA, USA). Subsequently, the cell was stained with anti‐mouse IL‐4 (Biolegend, San Diego, CA, USA, Cat#504104, RRID:AB_315318) antibody for 30 min. After washing with PBS, cells were detected on FACSCanto II (BD Biosciences, Franklin, NJ, USA) and analysed using FlowJo software (Tree Star Inc., OR, USA, RRID:SCR_008520).
2.8. Bone marrow‐derived dendritic cells generation and culture
Bone marrow‐derived dendritic cells were generated from female BABL/c mice. Bone marrow cells were isolated from the femurs and tibias with sterile PBS and cultured in RPMI 1640 medium supplemented with 100 U·ml−1 penicillin, 50 μg·ml−1 streptomycin, 10% FBS (Sciencell, San Diego, Los Angeles, USA), 20 ng·ml−1 GM‐CSF (R&D Systems, Minneapolis, MN, USA) and 10 ng·ml−1 IL‐4 (PeproTech, London, U.K.). On day 3, an equal volume of medium was replaced and 80% of the medium was replaced on day 5. On day 7, floating nonadherent bone marrow‐derived dendritic cells were collected. For adoptive transfer experiments, CD11c+ CD11b+ bone marrow‐derived dendritic cells were sorted by a CD11c MicroBeads Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and a CD11b MicroBeads Kit (Miltenyi Biotec, Bergisch Gladbach, Germany).
2.9. Adoptive transfer experiment with CD11c+CD11b+ bone marrow‐derived dendritic cells
During the allergic asthma model, viable 3 × 105 CD11c+ CD11b+ bone marrow‐derived dendritic cells were adoptively transferred to PepN‐treated allergic asthma mice through intranasal administration 2 h before the first challenge on the allergic asthma model of day 14. Then all mice were killed for analysis 24 h after the last challenge.
2.10. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology. The statistical analysis was undertaken only for studies where each group size was at least n = 5. Analyses were blinded to group identity. The declared group sizes are the number of individual mice. The declared group size (n) is the number of independent values and that statistical analysis was done using these independent values. Units of y‐axes are direct linear measures of physical values except for qPCR. Data normalization was only conducted for qPCR analysis. The data were shown as means ± SEM. Data were subjected to Grubbs' outlier test; outliers were excluded in data analysis and presentation and the number of outliers excluded in analysis was indicated within the figure legend. Differences between two independent groups were analysed by Student's t test or Mann–Whitney U test. A P value <0.05 was considered significantly different. While comparing multiple independent groups, post hoc tests were used only if F in ANOVA achieved a P value of <0.05 and there was no significant variance inhomogeneity. The data and statistical analyses were performed in GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA, RRID:SCR_002798).
2.11. Materials
The following anti‐mouse antibodies were used for analysis of dendritic cells from mouse lungs:‐ CD11c‐FITC (eBioscience, San Diego, CA, USA, Cat#11‐0114‐81, RRID:AB_464939), I‐A/I‐E‐PerCp/Cy5.5 (BD Pharmingen, San Jose, CA, USA, Cat#562363, RRID:AB_11153297), CD11b‐APC (BD Pharmingen, San Jose, CA, USA, Cat#561690, RRID:AB_10897015), CD103‐BV421 (Biolegend, San Diego, CA, USA, Cat#121422, RRID:AB_2562901), CD86‐PE (eBioscience, San Diego, CA, USA, Cat#12‐0862‐81, RRID:AB_465767) and CD40‐PE/Cy7 (Biolegend, San Diego, CA, USA, Cat#124622, RRID:AB_10897812). For staining type 2 innate lymphoid cells, Lineage Cocktail‐FITC (Biolegend, San Diego, CA, USA, Cat#133301), CD90.2‐APC (Biolegend, San Diego, CA, USA, Cat#140312, RRID:AB_10640728), ST2‐PE (Biolegend, San Diego, CA, USA, Cat#145304, RRID:AB_2561915), CD127‐PerCP/Cy5.5 (Biolegend, San Diego, CA, USA, Cat#121114, RRID: AB_1134205) and IL‐5‐BV421 (Biolegend, San Diego, CA, USA, Cat#504311, RRID: AB_2563161) were used. For staining Th2 cells, CD3‐APC (Biolegend, San Diego, CA, USA, Cat#100236, RRID:AB_2561456), CD4‐FITC (Biolegend, San Diego, CA, USA, Cat#116004, RRID:AB_313689) and IL‐4‐PE (Biolegend, San Diego, CA, USA, Cat#504104, RRID:AB_315318) were used.
2.12. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3. RESULTS
3.1. Administration of PepN during sensitization attenuated airway inflammation in OVA‐induced allergic asthma
To find out the effect that PepN may have on allergic asthma, intranasal administrations of different doses of PepN were executed during OVA sensitization (Figure 1a). We firstly assessed the numbers of total cells and eosinophils and Th2 cytokines levels in bronchoalveolar lavage fluid. The numbers of total cells and eosinophils did not differ between treating with or without 0.1 μg of PepN. This was the same for IL‐5 and IL‐13 levels in bronchoalveolar lavage fluid (Figure 1b,c). Treatment with 10 μg of PepN obviously reduced the eosinophils numbers and IL‐5 and IL‐13 levels in bronchoalveolar lavage fluid, but no effect on the numbers of total cells in bronchoalveolar lavage fluid (Figure 1b,c). However, 50 μg of PepN significantly suppressed all above indicators (Figure 1b,c). Consequently, 50 μg of PepN was chosen to be used in the subsequent studies.
FIGURE 1.
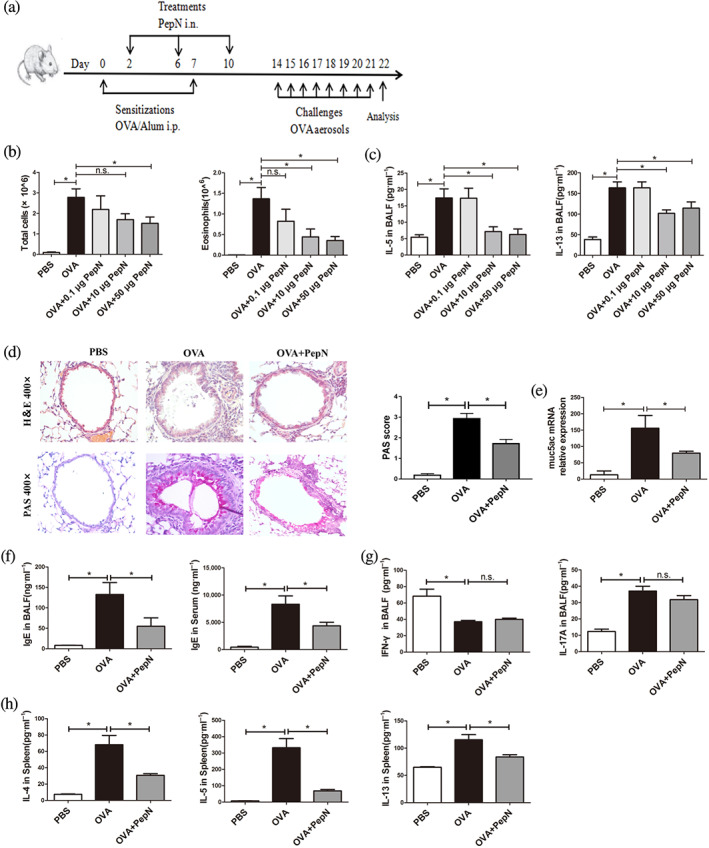
Effect of administration of different doses (0.1, 10 and 50 μg) of PepN during sensitization on airway inflammation of OVA‐induced allergic asthma. (a) Experimental protocol. (b) Total inflammatory cells and eosinophils in bronchoalveolar lavage fluid. (c) Th2 cytokine (IL‐5 and IL‐13) levels in bronchoalveolar lavage fluid (BALF). (d) Photomicrographs of H&E‐ and PAS‐stained lung tissue sections of mice (400× magnification) and semi‐quantified PAS score. (e) Muc5ac mRNA expression levels in mouse lung tissues. (f) IgE levels in BALF and serum. (g) IFN‐γ and IL‐17A levels in BALF. (h) Th2 cytokines (IL‐4, IL‐5 and IL‐13) levels in spleen cells re‐stimulated with 200 μg·ml−1 OVA for 72 h. The results were representative of three independent experiments. Data were presented as means ± SEM (n = 6–8). * P < 0.05, n.s., not significant
We then determined the capacity of PepN to suppress the additional features of allergic asthma. H&E‐stained lung sections showed that OVA exposure induced thickened bronchial mucosa and increased peri‐bronchial inflammatory cells infiltration compared to PBS‐treated mice, while this inflammation was decreased after treatment with PepN (Figure 1d). PAS staining also showed reduced goblet cell hyperplasia and mucus secretion in the lungs of PepN‐treated mice compared to allergic asthma mice (Figure 1d). This phenomenon was associated with reduced mRNA expression of muc5ac in lung tissues of PepN‐treated mice (Figure 1e). Moreover, increased IgE levels in bronchoalveolar lavage fluid and serum of allergic asthma mice were significantly diminished by PepN treatment (Figure 1f). In addition, we measured Th1‐ and Th17‐associated cytokines IFN‐γ and IL‐17A in bronchoalveolar lavage fluid and found that they were not significantly different in allergic asthma mice with or without PepN treatment (Figure 1g). To further test the effect of PepN on OVA‐specific Th2 responses, we re‐stimulated cells from the spleen with OVA allergen in vitro and found that spleen cells from PepN‐treated mice had significantly less Th2 cytokine (IL‐4, IL‐5 and IL‐13) production than did spleen cells from allergic asthma mice (Figure 1h). Collectively, these results corroborated the observation that administration of PepN during sensitization effectively suppressed airway eosinophilic inflammation and aberrant Th2 immune responses in allergic asthma.
3.2. Administration of PepN during sensitization reduced the lung accumulation of CD11b+ dendritic cells in allergic asthma mice
In response to allergen stimulation, lung conventional dendritic cells capture allergens and then migrate to the mediastinal lymph nodes to initiate Th2 inflammatory responses (Plantinga et al., 2013). As PepN reduced OVA‐induced Th2 responses in allergic asthma mice, we therefore investigated whether PepN affected lung conventional dendritic cells accumulation. Lung conventional dendritic cells are identified as CD11c+ MHCII+ cells and they are further segregated as cDC1 (CD103+ dendritic cells) and cDC2 (CD11b+ dendritic cells) (Eisenbarth, 2019). CD11b+ dendritic cells specialize in the presentation of antigens to CD4+ T cells, while CD103+ dendritic cells professionally present antigens to CD8+ T cells (Deckers, De Bosscher, Lambrecht, & Hammad, 2017; Yi et al., 2018). Allergic asthma mice had significantly increased the numbers of conventional dendritic cells and CD11b+ dendritic cells than did PBS control mice in the lung, while CD103+ dendritic cells numbers did not increase (Figure 2a,b) and the number of CD11b+ dendritic cells that plays a vital role in allergic asthma was decreased in mice treated with PepN (Figure 2a,b). When detecting dendritic cells activation markers, we found that neither CD11b+ dendritic cells nor CD103+ dendritic cells in PepN treated mice had different mean fluorescence intensity (MFI) of CD40 and CD86 compared with their counterparts from allergic asthma mice (Figure 2c). Moreover, the results showed that PepN did not affect the percentages of CD11b+ dendritic cells activation markers CD40 and CD86 in the lungs of allergic asthma mice (Figure 2d). These findings suggested that PepN reduced the lung accumulation of CD11b+ dendritic cells in allergic asthma mice without directly acting on their activation.
FIGURE 2.
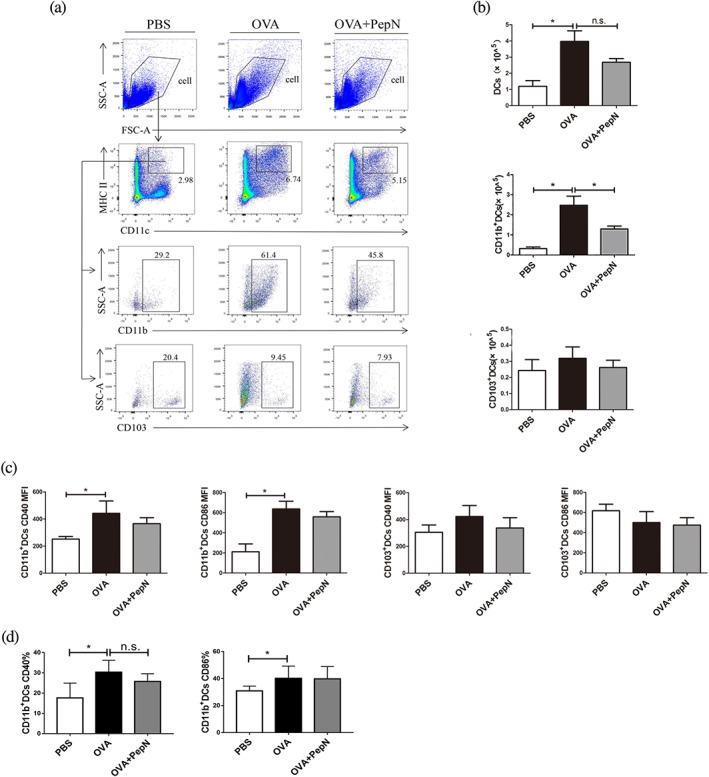
Administration of 50 μg of PepN during sensitization reduced the number of CD11b+ dendritic cells (DCs) in lungs of allergic asthma mice. (a) Representative flow diagram showing the lung DCs (CD11c+ MHCII+) and DCs subsets population. (b) Total numbers of lung DCs and DCs subsets. (c) The mean fluorescence intensity (MFI) of CD40 and CD86 expression on DCs. (d) The percentages of CD40 and CD86 on CD11b+ DCs. The results were representative of two independent experiments. Data were presented as means ± SEM (n = 6–8). * P < 0.05, , n.s., not significant
3.3. PepN impaired CD11b+ dendritic cells‐related chemokines production in lung of allergic asthma mice
To further explore the effect of PepN on lung CD11b+ dendritic cells, we firstly measured the expression of chemokines which has been linked to the recruitment of dendritic cells to the lung upon allergen exposure. CCL2 and CCL8 are the ligands for CCR2, a chemokine receptor expressed on inflammatory dendritic cells (O'Boyle et al., 2007; Tiberio et al., 2018). OVA sensitization and challenge induced increased lung mRNA expressions of CCL2 and CCL8, however both of which remained unchanged after mice receiving PepN (Figure 3a). Notably, the mRNA expression of another chemokine CCL20, which is the ligand for CCR6 to recruit immature dendritic cells was significantly decreased in PepN‐treated lung tissue (Figure 3a) (Gill, 2012). In keeping with the mRNA expression, PepN also reduced the protein levels of CCL20 in both bronchoalveolar lavage fluid and lung homogenate (Figure 3b). Besides, lung CD11b+ dendritic cells serve as the predominant source of the chemokines CCL17 and CCL22 which have been reported to recruit effector Th2 cells to the lungs upon allergen challenge (Gill, 2012; Medoff et al., 2009). Thus, we detected the lung mRNA expression of CCL17 and CCL22 and found they were both decreased in allergic asthma mice receiving PepN treatment (Figure 3c). These results offered further proof of reduced accumulation of lung CD11b+ dendritic cells in PepN‐treated mice.
FIGURE 3.
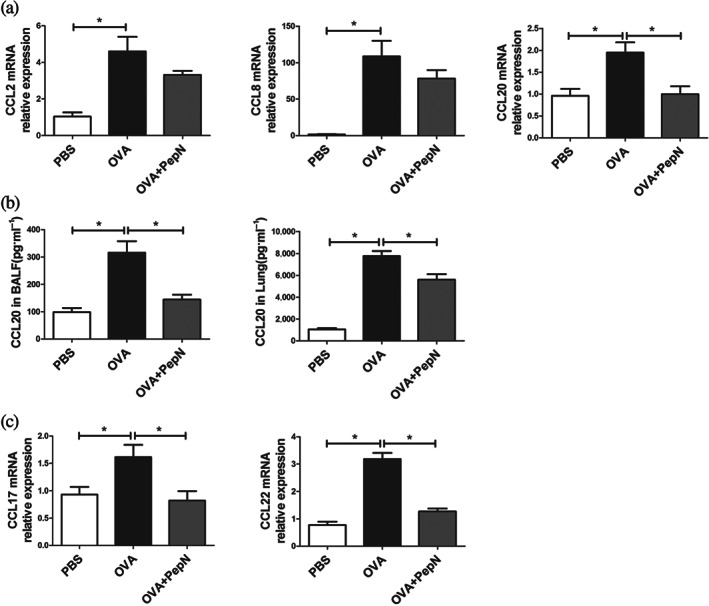
Administration of 50 μg of PepN reduced CD11b+ dendritic cell (DCs)‐related chemokines production in lungs of allergic asthma mice. (a) mRNA expression levels of CCL2, CCL8 and CCL20 in lung tissues. (b) The concentrations of CCL20 in bronchoalveolar lavage fluid (BALF) and lung tissues. (c) mRNA expression levels of CCL17 and CCL22 in lung tissues. The results were representative of three independent experiments. Data were presented as means ± SEM (n = 6–8). * P < 0.05
3.4. Adoptive transfer of bone marrow‐derived CD11b+ dendritic cells restored airway inflammation and Th2 responses in PepN‐treated mice
As we have shown, administration of PepN during sensitization reduced the accumulation of lung CD11b+ dendritic cells without affecting their activation, we therefore conducted adoptive transfer experiments to further verified whether the diminished allergic responses in PepN‐treated mice were directly due to impaired lung recruitment of CD11b+ dendritic cells (Figure 4a). The transfer of viable 3 × 105 bone marrow‐derived CD11b+ dendritic cells restored asthma features in PepN‐treated mice, as manifested by increased numbers of bronchoalveolar lavage fluid total inflammatory cells (Figure 4b), aggravated peri‐bronchial inflammation in lung tissues and mucus production (Figure 4c), elevated secretion of IL‐5 in bronchoalveolar lavage fluid (Figure 4d), as well as boosted level of total IgE in bronchoalveolar lavage fluid (Figure 4e), to comparable extent observed in allergic asthma mice. In addition, there were also increased trends of eosinophils numbers and IL‐13 production in bronchoalveolar lavage fluid and IgE levels in serum (Figure 4b,d,e). Moreover, the lung reconstitution CD11b+ dendritic cells caused a remarkable increase of the mRNA expression of CCL17 in lungs compared to PepN‐treatment group (Figure 4f), the mRNA expression of CCL22 in lungs also had an increased trend (Figure 4f). Furthermore, the reduced number of Th2 cells in PepN‐treated allergic asthma mice was also increased after CD11b+ dendritic cells transfer (Figure 4g). Altogether, these results demonstrated that the protective effect of PepN on OVA‐induced allergic asthma was mediated by reduced lung recruitment of CD11b+ dendritic cells.
FIGURE 4.
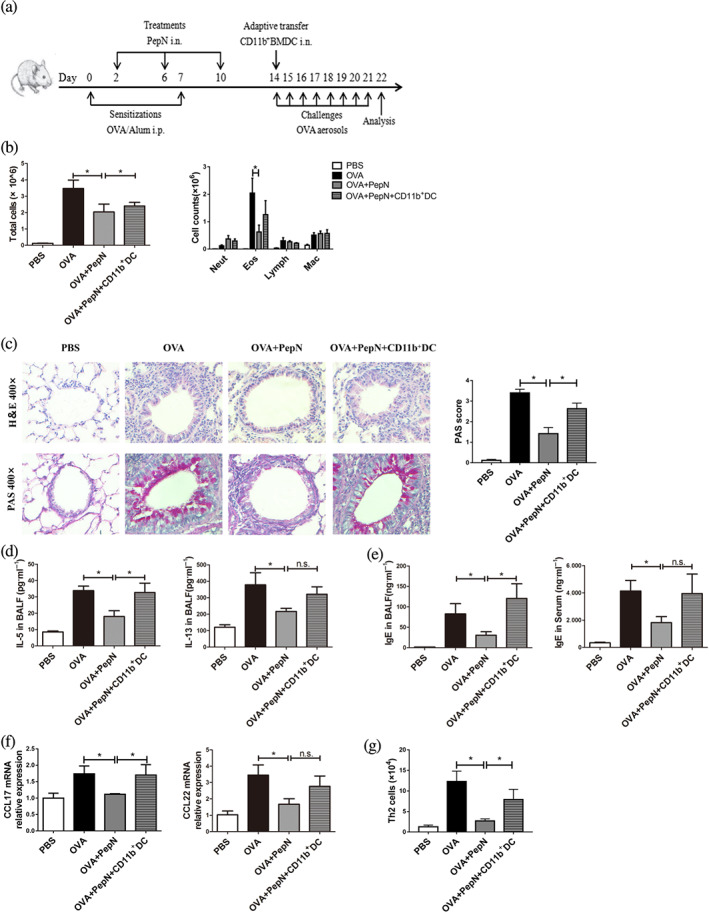
Adoptive transfer of bone marrow (B)‐derived CD11b+ dendritic cell (DCs) restored airway inflammation and Th2 responses in 50 μg of PepN‐treated mice. (a) Experimental protocol. (b) Total inflammatory cells and the differential cell counts in bronchoalveolar lavage fluid (BALF). (c) Photomicrographs of H&E‐ and PAS‐stained lung tissue sections of mice (400× magnification) and semi‐quantified PAS score. (d) Th2 cytokines (IL‐5 and IL‐13) levels in BALF. (e) IgE levels in BALF and serum. (f) CCL17 and CCL22 mRNA expression levels of in lung tissues. (g) The number of Th2 cells in lung tissue. An outlier of the lymphocyte data was excluded from the OVA group and CD11b+ DCs transfer group, respectively. Similar results were obtained in two independent experiments. Data were presented as means ± SEM (n = 6–8). * P < 0.05, n.s., not significant
3.5. PepN reduced accumulation of type 2 innate lymphoid cells in lung tissue of allergic asthma mice
Research showed that lung type 2 innate lymphoid cells could drive eosinophil airway inflammation by releasing high levels of the Th2 cytokines IL‐5 and IL‐13 (Lund et al., 2013) and type 2 innate lymphoid cells could license CD11b+ dendritic cells to potentiate Th2 immune responses (Halim et al., 2016). Therefore, we explored whether PepN affects the number of lung type 2 innate lymphoid cells in allergic asthma. The type 2 innate lymphoid cells were defined as viable cells that were lineage− CD90.2+ ST2+ CD127+ and the numbers of which were tested by flow cytometry (Figure 5a). The results showed that OVA sensitization and challenge significantly increased the number of type 2 innate lymphoid cells in lung tissues of asthma mice, yet this was obviously blunted after PepN treatment (Figure 5b). To further determine whether PepN affect cytokine production in type 2 innate lymphoid cells, we performed intracellular staining of IL‐5 in type 2 innate lymphoid cells. As shown in Figure 5c, the increased number of IL5+ type 2 innate lymphoid cells in lung of allergic asthma mice was significantly reduced after treatment with PepN. As IL‐33 is essential for the activation and proliferation of type 2 innate lymphoid cells (Anderson et al., 2016), we detected the expression of IL‐33 and found that PepN dramatically inhibited the mRNA and protein levels of lung IL‐33 in allergic asthma mice (Figure 5d). These data indicated that reduced allergic response in PepN‐treated allergic asthma mice was related to impaired type 2 innate lymphoid cells.
FIGURE 5.
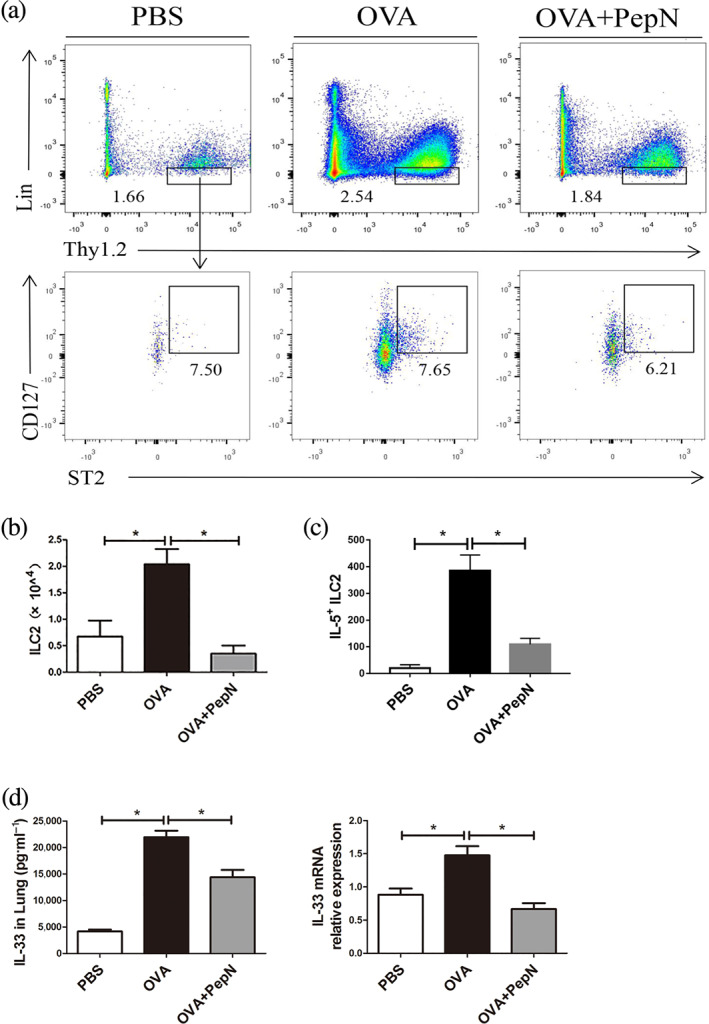
Administration of 50 μg of PepN reduced accumulation of type 2 innate lymphoid cells (ILC2s) in lung tissues of allergic asthma mice. (a) Representative flow diagram showing the lung ILC2 population (Lin−Thy1.2+ST2+CD127+). (b) Total number of lung ILC2s. (c) The number of IL5+ ILC2s in lung tissue. (d) mRNA and protein expression levels of IL‐33 in mouse lung tissues. Data were presented as means ± SEM (n = 6–8). * P < 0.05
3.6. PepN suppressed OVA‐induced allergic asthma when administered before sensitization
Epidemiological studies have suggested that early life contact to complex microbial communities reduces the risk of allergies and asthma (Milligan et al., 2016). To explore the prophylactic effect of PepN on allergic asthma, the mice were subjected to OVA sensitization and challenge after intranasal administrations of PepN (Figure 6a). The results showed that the numbers of total cells and eosinophils in bronchoalveolar lavage fluid, peri‐bronchial inflammation, mucus secretion, production of OVA‐induced IL‐5, IL‐13, CCL24 in bronchoalveolar lavage fluid and levels of IgE in bronchoalveolar lavage fluid and serum were dramatically decreased in the presence of PepN (Figure 6b–e), suggesting that PepN could also ameliorate OVA‐induced allergic asthma when administered before sensitization.
FIGURE 6.
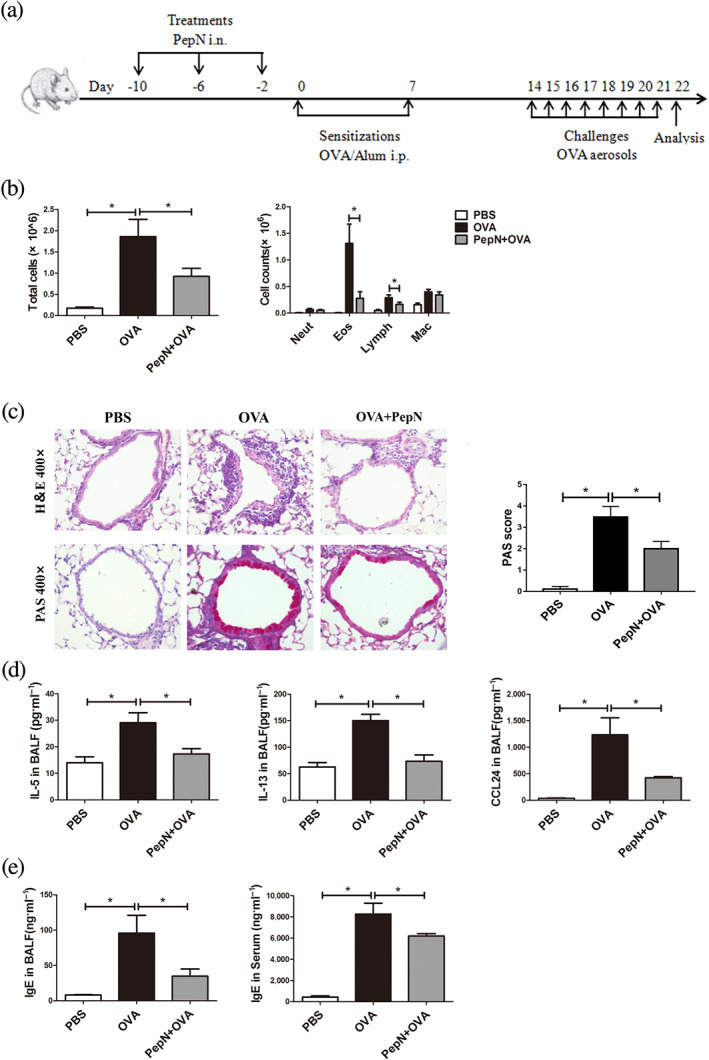
Administration of 50 μg of PepN suppressed OVA‐induced allergic asthma when administered before sensitization. (a) Experimental protocol. (b) Total inflammatory cells and the differential cell counts in bronchoalveolar lavage fluid (BALF). (c) Photomicrographs of H&E‐ and PAS‐stained lung tissue sections of mice (400× magnification) and semi‐quantified PAS score. (d) Th2 cytokines (IL‐5 and IL‐13) and eotaxin‐2 (CCL24) levels in bronchoalveolar lavage fluid (BALF). (e) IgE levels in BALF and serum. Data were presented as means ± SEM (n = 6–8). * P < 0.05
4. DISCUSSION
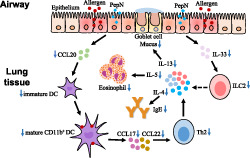
In the present work, for the first time, we confirmed that Spn aminopeptidase N (PepN) could clearly attenuate OVA‐induced allergic asthma in mice when administrated during or before sensitization and was accompanied by reduced eosinophilia in the airways, diminished Th2 inflammatory responses, less mucus production and decreased IgE production. We further demonstrated that the protective effect of PepN on allergic asthma was partially mediated by inhibiting the lung recruitment of CD11b+ dendritic cells. Besides, we also found that PepN reduced accumulation of type 2 innate lymphoid cells in lung tissue of allergic asthma mice, which provided another underlying mechanism for protective effect of PepN on allergic asthma (Figure 7).
FIGURE 7.
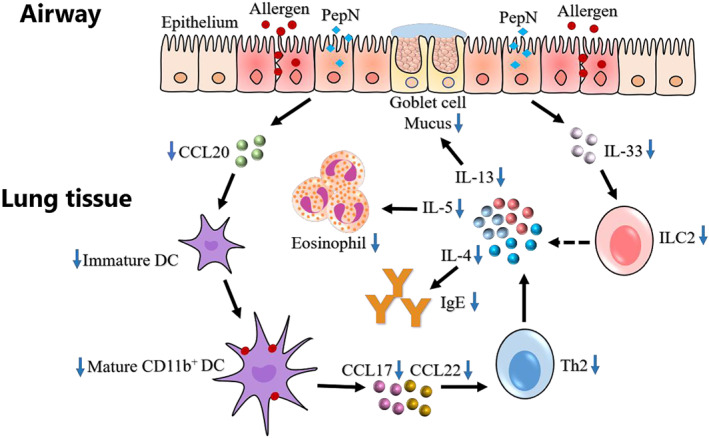
Graphical summary of the protective effect of PepN on allergic asthma. In allergic asthma, PepN inhibited the production of epithelium‐derived immature dendritic cell (DCs) chemokine CCL20, which reduced the recruitment of lung CD11b+ DCs. The decrease in lung CD11b+ DCs recruitment led to the decrease in the uptake of lung allergen OVA; therefore, the Th2 immune response (increased Th2 cytokines IL‐4, IL‐5, IL‐13, enhanced IgE level, eosinophilia and mucus hypersecretion) initiated by CD11b+ DCs was reduced. Besides, PepN also inhibited the production of epithelium‐derived IL‐33, which reduced the number of lung type 2 innate lymphoid cell (ILC2s). The reduced ILC2s may directly reduce the Th2 immune response by reducing the production of Th2 cytokines IL‐4, IL‐5 and IL‐13 (dashed line)
Spn aminopeptidase N, a class of exoproteases that selectively excise amino acid residues from the N‐terminus of proteins and polypeptides to produce free amino acids. A recent research indicated the ability of PepN to inhibit T cell effector function via regulation of T cell receptor signalling (Blevins et al., 2017), whereas its role in Th2‐mediated allergic asthma is unknown. Here, we first assessed the effect of PepN on allergic asthma. We showed that intranasal administration of PepN during sensitization prominently suppressed cardinal characteristics of allergic asthma, including eosinophilia, mucus hypersecretion, enhanced levels of IgE in bronchoalveolar lavage fluid and serum and increased OVA‐specific Th2 responses (Figure 7). Additionally, we did not observe any difference of representative Th1 or Th17 cytokines IFN‐γ and IL‐17A in bronchoalveolar lavage fluid between allergic asthma and PepN treatment mice, indicating that PepN might directly suppress Th2‐dominant type‐2 airway inflammation without affecting Th1 or Th17 type immune responses.
It has long been established that lung dendritic cells play a central role in the development of allergic asthma (Deckers et al., 2017). Lung conventional dendritic cells are segregated as CD11b+ dendritic cells and CD103+ dendritic cells (Eisenbarth, 2019), CD11b+ dendritic cells migrate to the mediastinal lymph node to induce Th2 polarization upon OVA exposure, while CD103+ dendritic cells are responsible to induce Th1 deviation (Furuhashi et al., 2012) and lung tolerance to allergens (Khare et al., 2013). Coinciding with these studies, we observed a smaller number of lung CD11b+ dendritic cells in PepN‐treated allergic asthma mice, while the number of CD103+ dendritic cells kept unchanged. Meanwhile, CCL17 and CCL22 are crucial Th2‐cell chemokines predominantly secreted by CD11b+ dendritic cells in mice challenged with allergen, we found that their expressions were significantly decreased in PepN‐treated lung tissue and these supported the results that PepN treatment reduced the numbers of lung CD11b+ dendritic cells in allergic asthma mice (Figure 7).
It has been reported that chemokines produced by epithelial cells affect lung dendritic cells recruitment. Upon allergen stimulation, airway epithelial cells secret CCL20 to initiate recruitment of immature dendritic cells to the lung (Gill, 2012). Lung immature CD11b+ dendritic cells are activated by uptaking allergen, then migrating to the mediastinal lymph nodes as mature CD11b+ dendritic cells to initiate Th2 inflammatory responses (Plantinga et al., 2013). Our results showed that PepN reduced production of lung CCL20 at both protein and mRNA level. Therefore, we hypothesized that PepN may inhibit the response of epithelial cells to OVA and reduce the production of CCL20, thus indirectly inhibiting lung immature CD11b+ dendritic cells recruitment (Figure 7). Besides, we did not observe any changes of CD11b+ dendritic cells activation markers CD40 or CD86 in the presence of PepN treatment, suggesting that PepN did not directly affect the activation of CD11b+ dendritic cells. Consequently, we speculate that the decrease in CD11b+ dendritic cells number led to the decrease in the uptake of lung allergen OVA, hence the Th2 immune response initiated by CD11b+ dendritic cells was reduced along with allergic asthma in mice (Figure 7).
Adoptive transfer of bone marrow‐derived CD11b+ dendritic cells rescued peribronchial inflammation, mucus secretion, IgE level, productions of type‐2 cytokines, Th2 cell‐recruiting chemokines and Th2 cell number and partially rescued numbers of eosinophil and total inflammatory cell, indicating that the protective effect of PepN on allergic asthma is partially mediated by CD11b+ dendritic cells. Besides, we observed that the number of lymphocytes was not obviously changed, so we speculate that CD11b+ dendritic cells transfer mainly enhanced the polarization of Th2 cells in PepN‐treated asthmatic mice and inhibited other lymphocyte subpopulations, probably the polarization of regulatory T cells, because our preliminary data showed that PepN treatment could increase the number of regulatory T cells in lung tissue of asthmatic mice (data were not showed).
Type 2 innate lymphoid cells are situated mainly at mucosal surfaces and they are vital in bridging the innate and adaptive immunity (Halim et al., 2016; Skevaki & Renz, 2018). Once activated by the epithelia‐derived cytokines IL‐33, IL‐25 and TSLP these cells release large amounts of IL‐5 and IL‐13 inducing a strongly eosinophil airway inflammation (Lund et al., 2013). In our study, we found that PepN reduced the numbers of type 2 innate lymphoid cells and production of IL‐33 in lung tissue of allergic asthma mice. Consequently, reduced numbers of type 2 innate lymphoid cells in PepN‐treated mice might directly provide a potential mechanism for protective effect of PepN on OVA‐induced allergic asthma. Additionally, a recent study found that type 2 innate lymphoid cells act upstream of dendritic cells; type 2 innate lymphoid cell‐deficient and type 2 innate lymphoid cell‐depleted mice both had remarkably fewer lung CD11b+ dendritic cells during allergic airway inflammation (Halim et al., 2016). This led us to hypothesize that reduced lung CD11b+ dendritic cells numbers in PepN‐treated mice were in part influenced by decreased type 2 innate lymphoid cells. However, whether reduced lung CD11b+ dendritic cells was virtually influenced by decreased type 2 innate lymphoid cells in PepN treated allergic asthma mice requires to be further explored (Figure 7).
Epidemiological studies have suggested that early life contact to complex microbial communities reduces the risk of allergies and asthma. Study by Schulke et al. showed that the fusion protein of flagellin A and OVA suppressed the Th2 response and prevented murine intestinal allergy when administrated during or before sensitization (Schulke et al., 2011). Park et al. also investigated the effect of a sphingosine‐1‐phosphate 2 antagonist on allergic asthma in mice when treated during or before sensitization (Park & Im, 2019). In this context, we mainly studied the effect of S. pneumoniae aminopeptidase N (PepN) on allergic asthma when administrated during OVA sensitization and its mechanism and we also performed a model of prophylactic administration of PepN to further confirmed the protective effect of PepN on allergic asthma.
In summary, our results identified Spn aminopeptidase N effectively suppressed OVA‐induced allergic asthma when administrated during or before sensitization. Moreover, we demonstrated that the inhibition of PepN on allergic asthma was partially mediated by reduced lung CD11b+ dendritic cells. Also, the reduced lung type 2 innate lymphoid cells may play an important role in attenuated allergic responses of PepN‐treated mice. Therefore, our study supplies a new theoretical basis for the hygienic hypothesis that exposure to microbes in early life reduces the risk of allergic diseases and also provides a novel strategy for prevention of allergic asthma.
AUTHOR CONTRIBUTIONS
W.X. supervised and funded the work. W.X. and G.W. designed the studies and wrote the paper. G.W. and X.Z. performed animal experiments. X.C. and J.W. performed data analysis. J.Y. and L.W. performed cell experiments. S.S. and Y.Q. carried out data statistical analysis. H.W. and Y.Y. provided advice in experiments. All authors read and approved of the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design and Analysis and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
ACKNOWLEDGEMENTS
This work was supported by the Natural Science Foundation of China (81671639). We thank the School of Laboratory Medicine, Chongqing Medical University, Chongqing, China and all the authors involved in this study.
Wu G, Zhang X, Chen X, et al. Streptococcus pneumoniae aminopeptidase N regulates dendritic cells that attenuates type‐2 airway inflammation in murine allergic asthma. Br J Pharmacol. 2020;177:5063–5077. 10.1111/bph.15216
Guangying Wu and Xuemei Zhang should be considered joint first author.
Contributor Information
Guangying Wu, Email: 18716251466@163.com.
Wenchun Xu, Email: xuwen@cqmu.edu.cn.
REFERENCES
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Watts, V. (2019). The concise guide to pharmacology 2019/20: Enzymes. British Journal of Pharmacology, 176(S1), S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, E. L. , Kobayashi, T. , Iijima, K. , Bartemes, K. R. , Chen, C. C. , & Kita, H. (2016). IL‐33 mediates reactive eosinophilopoiesis in response to airborne allergen exposure. Allergy, 71(7), 977–988. 10.1111/all.12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansaldi, F. , Turello, V. , Lai, P. , Bastone, G. , De Luca, S. , Rosselli, R. , … Icardi, G. (2005). Effectiveness of a 23‐valent polysaccharide vaccine in preventing pneumonia and non‐invasive pneumococcal infection in elderly people: A large‐scale retrospective cohort study. The Journal of International Medical Research, 33(5), 490–500. 10.1177/147323000503300503 [DOI] [PubMed] [Google Scholar]
- Baldacci, S. , Maio, S. , Cerrai, S. , Sarno, G. , Baiz, N. , Simoni, M. , … Study, H. (2015). Allergy and asthma: Effects of the exposure to particulate matter and biological allergens. Respiratory Medicine, 109(9), 1089–1104. 10.1016/j.rmed.2015.05.017 [DOI] [PubMed] [Google Scholar]
- Blevins, L. K. , Parsonage, D. , Oliver, M. B. , Domzalski, E. , Swords, W. E. , & Alexander‐Miller, M. A. (2017). A novel function for the Streptococcus pneumoniae aminopeptidase N: Inhibition of T cell effector function through regulation of TCR signaling. Frontiers in Immunology, 8, 1610 10.3389/fimmu.2017.01610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers, J. , De Bosscher, K. , Lambrecht, B. N. , & Hammad, H. (2017). Interplay between barrier epithelial cells and dendritic cells in allergic sensitization through the lung and the skin. Immunological Reviews, 278(1), 131–144. 10.1111/imr.12542 [DOI] [PubMed] [Google Scholar]
- Eisenbarth, S. C. (2019). Dendritic cell subsets in T cell programming: Location dictates function. Nature Reviews. Immunology, 19(2), 89–103. 10.1038/s41577-018-0088-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi, K. , Suda, T. , Hasegawa, H. , Suzuki, Y. , Hashimoto, D. , Enomoto, N. , … Chida, K. (2012). Mouse lung CD103+ and CD11βhigh dendritic cells preferentially induce distinct CD4+ T‐cell responses. American Journal of Respiratory Cell and Molecular Biology, 46(2), 165–172. 10.1165/rcmb.2011-0070OC [DOI] [PubMed] [Google Scholar]
- Gill, M. A. (2012). The role of dendritic cells in asthma. The Journal of Allergy and Clinical Immunology, 129(4), 889–901. 10.1016/j.jaci.2012.02.028 [DOI] [PubMed] [Google Scholar]
- Halim, T. Y. , Hwang, Y. Y. , Scanlon, S. T. , Zaghouani, H. , Garbi, N. , Fallon, P. G. , & McKenzie, A. N. (2016). Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nature Immunology, 17(1), 57–64. 10.1038/ni.3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, S. A. , & Luster, A. D. (2012). T cell homing to epithelial barriers in allergic disease. Nature Medicine, 18(5), 705–715. 10.1038/nm.2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatzlauk, G. , Bartel, S. , Heine, H. , Schloter, M. , & Krauss‐Etschmann, S. (2017). Influences of environmental bacteria and their metabolites on allergies, asthma, and host microbiota. Allergy, 72(12), 1859–1867. 10.1111/all.13220 [DOI] [PubMed] [Google Scholar]
- Kepert, I. , Fonseca, J. , Muller, C. , Milger, K. , Hochwind, K. , Kostric, M. , … Krauss‐Etschmann, S. (2017). D‐tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. The Journal of Allergy and Clinical Immunology, 139(5), 1525–1535. 10.1016/j.jaci.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Khare, A. , Krishnamoorthy, N. , Oriss, T. B. , Fei, M. , Ray, P. , & Ray, A. (2013). Cutting edge: Inhaled antigen upregulates retinaldehyde dehydrogenase in lung CD103+ but not plasmacytoid dendritic cells to induce Foxp3 de novo in CD4+ T cells and promote airway tolerance. Journal of Immunology, 191(1), 25–29. 10.4049/jimmunol.1300193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht, B. N. , & Hammad, H. (2015). The immunology of asthma. Nature Immunology, 16(1), 45–56. 10.1038/ni.3049 [DOI] [PubMed] [Google Scholar]
- Lilley, E. , Stanford, S. C. , Kendall, D. E. , Alexander, S. P. , Cirino, G. , Docherty, J. R. , … Ahluwalia, A. (2020). ARRIVE 2.0 and the British Journal of Pharmacology: Updated guidance for 2020. British Journal of Pharmacology, 177(16), 3611–3616. 10.1111/bph.15178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley, R. M. (2010). Asthma and allergic inflammation. Cell, 140(6), 777–783. 10.1016/j.cell.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, S. , Walford, H. H. , & Doherty, T. A. (2013). Type 2 innate lymphoid cells in allergic disease. Current Immunology Reviews, 9(4), 214–221. 10.2174/1573395510666140304235916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff, B. D. , Seung, E. , Hong, S. , Thomas, S. Y. , Sandall, B. P. , Duffield, J. S. , … Luster, A. D. (2009). CD11b+ myeloid cells are the key mediators of Th2 cell homing into the airway in allergic inflammation. Journal of Immunology, 182(1), 623–635. 10.4049/jimmunol.182.1.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan, K. L. , Matsui, E. , & Sharma, H. (2016). Asthma in urban children: Epidemiology, environmental risk factors, and the public health domain. Current Allergy and Asthma Reports, 16(4), 33 10.1007/s11882-016-0609-6 [DOI] [PubMed] [Google Scholar]
- O'Boyle, G. , Brain, J. G. , Kirby, J. A. , & Ali, S. (2007). Chemokine‐mediated inflammation: Identification of a possible regulatory role for CCR2. Molecular Immunology, 44(8), 1944–1953. 10.1016/j.molimm.2006.09.033 [DOI] [PubMed] [Google Scholar]
- Park, S. J. , & Im, D. S. (2019). Blockage of sphingosine‐1‐phosphate receptor 2 attenuates allergic asthma in mice. British Journal of Pharmacology, 176(7), 938–949. 10.1111/bph.14597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percie du Sert, N. , Hurst, V. , Ahluwalia, A. , Alam, S. , Avey, M. T. , Baker, M. , … Würbel, H. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biology, 18(7), e3000410 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga, M. , Guilliams, M. , Vanheerswynghels, M. , Deswarte, K. , Branco‐Madeira, F. , Toussaint, W. , … Lambrecht, B. N. (2013). Conventional and monocyte‐derived CD11b+ dendritic cells initiate and maintain T helper 2 cell‐mediated immunity to house dust mite allergen. Immunity, 38(2), 322–335. 10.1016/j.immuni.2012.10.016 [DOI] [PubMed] [Google Scholar]
- Preston, J. A. , Thorburn, A. N. , Starkey, M. R. , Beckett, E. L. , Horvat, J. C. , Wade, M. A. , … Hansbro, P. M. (2011). Streptococcus pneumoniae infection suppresses allergic airways disease by inducing regulatory T‐cells. The European Respiratory Journal, 37(1), 53–64. 10.1183/09031936.00049510 [DOI] [PubMed] [Google Scholar]
- Raftis, E. J. , Delday, M. I. , Cowie, P. , McCluskey, S. M. , Singh, M. D. , Ettorre, A. , & Mulder, I. E. (2018). Bifidobacterium breve MRx0004 protects against airway inflammation in a severe asthma model by suppressing both neutrophil and eosinophil lung infiltration. Scientific Reports, 8(1), 12024 10.1038/s41598-018-30448-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, R. M. , Pilmann Laursen, R. , Bruun, S. , Larnkjaer, A. , Molgaard, C. , Michaelsen, K. F. , & Host, A. (2019). Probiotics in late infancy reduce the incidence of eczema: A randomized controlled trial. Pediatric Allergy and Immunology, 30(3), 335–340. 10.1111/pai.13018 [DOI] [PubMed] [Google Scholar]
- Schulke, S. , Burggraf, M. , Waibler, Z. , Wangorsch, A. , Wolfheimer, S. , Kalinke, U. , … Scheurer, S. (2011). A fusion protein of flagellin and ovalbumin suppresses the TH2 response and prevents murine intestinal allergy. The Journal of Allergy and Clinical Immunology, 128(6), 1340–1348e1312. 10.1016/j.jaci.2011.07.036 [DOI] [PubMed] [Google Scholar]
- Shim, J. U. , Lee, S. E. , Hwang, W. , Lee, C. , Park, J. W. , Sohn, J. H. , … Koh, Y. I. (2016). Flagellin suppresses experimental asthma by generating regulatory dendritic cells and T cells. The Journal of Allergy and Clinical Immunology, 137(2), 426–435. 10.1016/j.jaci.2015.07.010 [DOI] [PubMed] [Google Scholar]
- Skevaki, C. , & Renz, H. (2018). Advances in mechanisms of allergic disease in 2017. The Journal of Allergy and Clinical Immunology, 142(6), 1730–1739. 10.1016/j.jaci.2018.09.027 [DOI] [PubMed] [Google Scholar]
- Spacova, I. , Petrova, M. I. , Fremau, A. , Pollaris, L. , Vanoirbeek, J. , Ceuppens, J. L. , … Lebeer, S. (2019). Intranasal administration of probiotic Lactobacillus rhamnosus GG prevents birch pollen‐induced allergic asthma in a murine model. Allergy, 74(1), 100–110. 10.1111/all.13502 [DOI] [PubMed] [Google Scholar]
- Thorburn, A. N. , Foster, P. S. , Gibson, P. G. , & Hansbro, P. M. (2012). Components of Streptococcus pneumoniae suppress allergic airways disease and NKT cells by inducing regulatory T cells. Journal of Immunology, 188(9), 4611–4620. 10.4049/jimmunol.1101299 [DOI] [PubMed] [Google Scholar]
- Thorburn, A. N. , O'Sullivan, B. J. , Thomas, R. , Kumar, R. K. , Foster, P. S. , Gibson, P. G. , & Hansbro, P. M. (2010). Pneumococcal conjugate vaccine‐induced regulatory T cells suppress the development of allergic airways disease. Thorax, 65(12), 1053–1060. 10.1136/thx.2009.131508 [DOI] [PubMed] [Google Scholar]
- Tiberio, L. , Del Prete, A. , Schioppa, T. , Sozio, F. , Bosisio, D. , & Sozzani, S. (2018). Chemokine and chemotactic signals in dendritic cell migration. Cellular & Molecular Immunology, 15(4), 346–352. 10.1038/s41423-018-0005-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijck, Y. , de Kleijn, S. , John‐Schuster, G. , Mertens, T. C. J. , Hiemstra, P. S. , Muller, A. , … Taube, C. (2018). Therapeutic application of an extract of Helicobacter pylori ameliorates the development of allergic airway disease. Journal of Immunology, 200(5), 1570–1579. 10.4049/jimmunol.1700987 [DOI] [PubMed] [Google Scholar]
- Xu, F. , Luo, M. , He, L. , Cao, Y. , Li, W. , Ying, S. , … Shen, H. (2018). Necroptosis contributes to urban particulate matter‐induced airway epithelial injury. Cellular Physiology and Biochemistry, 46(2), 699–712. 10.1159/000488726 [DOI] [PubMed] [Google Scholar]
- Yan, J. , Zhang, X. , Sun, S. , Yang, T. , Yang, J. , Wu, G. , … Xu, W. (2019). miR‐29b reverses T helper 1 cells/T helper 2 cells imbalance and alleviates airway eosinophils recruitment in OVA‐induced murine asthma by targeting inducible co‐stimulator. International Archives of Allergy and Immunology, 180(3), 182–194. 10.1159/000501686 [DOI] [PubMed] [Google Scholar]
- Yi, S. , Zhai, J. , Niu, R. , Zhu, G. , Wang, M. , Liu, J. , … Tang, H. (2018). Eosinophil recruitment is dynamically regulated by interplay among lung dendritic cell subsets after allergen challenge. Nature Communications, 9(1), 3879 10.1038/s41467-018-06316-9 [DOI] [PMC free article] [PubMed] [Google Scholar]


