Abstract
Several mechanisms allow for cargo internalization into cells within membrane-bound endocytic carriers. How these internalization processes couple to specific pathways of intracellular distribution remains poorly explored. Here, we review uptake reactions that are independent of the conventional clathrin machinery. We discuss how these link to retrograde trafficking from endosomes to the Golgi apparatus and exemplify biological situations in which the polarized secretion capacity of the Golgi apparatus allows for retrograde cargoes to be delivered to specialized areas of the plasma membrane, such as the leading edge of migratory cells or the immunological synapse of immune cells. We also address the evidence that allows to position apicobasal polarity of epithelial cells in this context. The underlying theme is thereby the functional coupling between specific types of endocytosis to intracellular retrograde trafficking for protein cargoes that need to be localized in a highly polarized and dynamic manner to plasmalemmal subdomains.
Keywords: Endocytosis, Intracellular trafficking, Retrograde transport, Polarity, Glycobiology, Galectin, Shiga toxin
Introduction
The plasma membrane delimitates eukaryotic cells from their environment. Owing to its capacity to internalize cargoes from the extracellular space or the cell surface, endocytosis controls a multitude of cellular functions, ranging from nutrient uptake and signaling to cell migration and neurotransmission [1].
The plasma membrane also protects cells from extracellular insults. Yet, pathogens (e.g. viruses) and pathogenic agents (e.g. protein toxins) have found ways to breach this barrier to gain access to the intracellular space to exert their harmful action and/or to replicate.
In this review, we will first summarize endocytic mechanisms with an emphasis on those—often still poorly characterized—that do not depend on the conventional clathrin machinery. We will then discuss how these relate to the polarized distribution of molecules within cells. We will notably focus on retrograde trafficking from endosomes to the Golgi apparatus, which allows cargo proteins that follow this pathway to have access to the polarized secretion capacity of the Golgi for targeted delivery to specialized areas of the plasma membrane, such as the leading edge in migratory cells, or the immunological synapse in lymphocytes or specialized antigen presenting cells.
Endocytic mechanisms—focus on nonclathrin uptake processes
Clathrin-dependent endocytosis remains the best characterized endocytosis pathway [1]. The clathrin triskelion is recruited to the plasma membrane via adaptor proteins, which directly bind consensus signals found in the cytosolic tails of cargo proteins. On the basis of its self-assembly properties and the recruitment of curvature inducers such as epsins and BAR-domain proteins, the clathrin coat drives the formation of clathrin-coated pits from which clathrin-coated vesicles detach in a process that depends of the pinchase dynamin [2].
Several endocytic processes continue to operate efficiently even when the clathrin pathway is blocked [3, 4, 5]. Since their discovery in the early 1980s, it has been a conundrum to know how cargo proteins are recruited and membranes bent in the absence of the clathrin coat. In the following, we discuss particularly well explored examples for which elements of response to these key questions have been proposed. Owing to length limitations of the current review, we unfortunately cannot be fully exhaustive.
Fast endophilin-mediated endocytosis
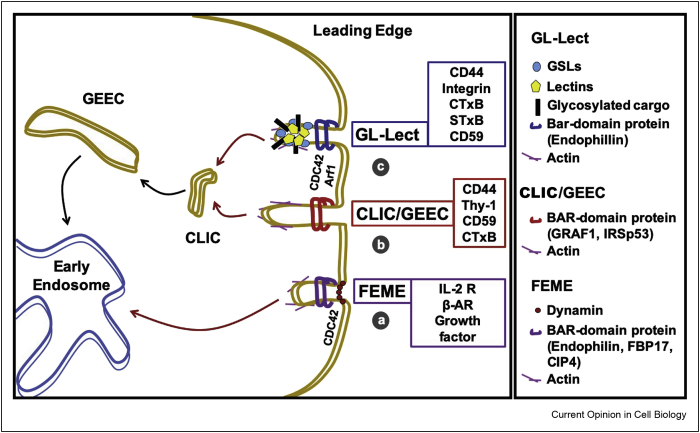
A first model for clathrin-independent endocytosis, termed fast endophilin-mediated endocytosis (FEME), relies on the membrane curvature-active BAR (Bin/amphiphysin/Rvs)-domain protein family member endophilin [6] (Figure 1a). FEME occurs preferentially in the leading edge of migrating cells is triggered by ligands and used by a number of plasma membrane proteins, including heterotrimeric G-proteins, growth factor receptors, and IL-2 receptor. Recently, it was described that the small GTPase Cdc42 brings two BAR-domain proteins to the plasma membrane, FBP17 and CIP4, which then recruit the phosphatase SHIP2 and lamellipodin to drive the local production of PIP2 and thereby, the enrichment of endophilin [7].
Figure 1.
Clathrin-independent endocytic processes. Schematic representation of the leading edge of a migratory cell where processes of clathrin-independent endocytosis mainly operate, while the clathrin pathway remains unpolarized. (a) FEME, fast endophilin-mediated endocytosis. FEME relies on the BAR-domain protein family member endophilin and other BAR domain proteins. This endocytic process is used by cargo proteins such as IL-2R, β-adrenergic receptor (β-AR), and growth factors. (b) CLIC/GEEC endocytosis. Clathrin-independent carriers are short tubular often crescent-shaped endocytic carriers that mature into glycophosphatidylinositol-anchored protein-enriched early endocytic compartments. CLIC/GEEC endocytosis is regulated by the GTPase activating factor GRAF1 and the small GTPase CDC42, among others, for the cellular uptake of cargoes like the hyaluronic acid receptor CD44, the GPI-anchored proteins CD59 and Thy-1, and the bacterial cholera toxin. (c) GL-Lect hypothesis. Molecular hypothesis according to which sugar-binding proteins of pathogenic (e.g. the GSL-binding B-subunits of Shiga and cholera toxin, STxB and CTxB, respectively, or the GSL-binding VP1 capsid protein of SV40) or cellular origin (e.g. galectins) reorganize glycolipids to which they bind in a way such as to drive the biogenesis of tubular endocytic pits from which CLICs are generated for the cellular uptake of pathogens (e.g. SV40 virus), pathogenic products (e.g. Shiga and cholera toxins), or cellular proteins (e.g. CD44, integrins, CD59) that are recruited by the galectins.
Clathrin-independent carrier/glycosylphosphatidylinositol-anchored protein-enriched early endocytic compartment
Another model for clathrin-independent endocytosis involves short sometimes crescent-shaped tubular clathrin-independent carriers (CLICs) [8] that then mature into glycosylphosphatidylinositol (GPI)-anchored protein-enriched early endocytic compartments (GEECs) [5] (Figure 1b). This CLIC/GEEC process has initially been described for the ganglioside-binding B-subunit of cholera toxin, GPI-anchored proteins (such as CD59 and Thy-1), the transmembrane protein CD44 and a major fraction of internalized fluid phase. CLIC/GEEC endocytosis is regulated by the small GTPases Arf1 and CDC42 [9,10], the GTPase activating factor GRAF1 [11], the actin nucleation factor ARP2/3 [12], and the BAR domain protein IRSp53 [13]. CLIC/GEEC endocytosis is dynamin-independent for the endogenous cargoes that have been analyzed [5] and not strictly dynamin-dependent for exogenous cargoes such as cholera [8] and Shiga toxins (Ref. [14], see below).
Glycolipid-lectin
How membrane bending might be operated in at least some processes of CLIC/GEEC endocytosis has been addressed at the examples of Shiga and cholera toxins and the cellular CD44 and α5β1 integrin, which all are found in CLICs (Refs. [8,15] and unpublished). The glycosphingolipid (GSL)-binding homopentameric B-subunits of Shiga and cholera toxin (termed STxB and CTxB, respectively) induce tubular endocytic pits on cells and model membranes as a first step of their internalization [16]. This relies on curvature active properties of the B-subunit GSL complexes [17] and the capacity to undergo membrane-mediated clustering [18]. The scission process is not strictly dynamin-dependent [8,14] and involves other scission modalities [14,19].
The capacity to drive narrow membrane bending in interaction with GSLs leading to the formation of tubular endocytic pits and CLICs has also been observed for cellular lectins of the galectin family [20] (Figure 1c). In the specific case of galectin-3 (Gal3), the lectin binds as a monomer to carbohydrates on cargo proteins such as CD44 or α5β1 integrin. Gal3 then oligomerizes and thereby gains to capacity to also interact with GSLs to drive membrane bending and the biogenesis of tubular endocytic pits from which CLICs detach for the cellular uptake of the cargoes. A similar sequence of events has recently also been described for galectin-8 and GSL-dependent cellular endocytosis of CD166 [21]. This mechanism has been termed glycolipid-lectin (GL-Lect) hypothesis [4].
All these clathrin-independent processes—that is, FEME, CLIC/GEEC, and GL-Lect—have in common that they are particularly sensitive to interference with the activity of the actin cytoskeleton and the organization of the membrane into raft-type nanodomains. It also appears noteworthy that some cargoes and trafficking factors are overlapping between these endocytic modalities. We tentatively favor a view according to which clathrin-independent endocytic processes are driven by elements of molecular machinery that are recruited in an interchangeable way according to physiological needs [22].
Another emerging theme from these studies is that different forms of endocytic uptake couple to different intracellular distribution schemes, sometimes for the same receptor in the same cells. The molecular mechanisms (ligand concentrations, post-translational modifications, conformational changes, etc…) underlying this complexity often still remain to be elucidated. In the following sections, we will address specifically one aspect of this riddle: a possible link between clathrin-independent endocytosis, retrograde trafficking, and cell polarity.
Clathrin-independent endocytosis and cell polarity
Several lines of evidence indicate that clathrin-independent endocytosis is linked to cell polarity. Well-established cargoes of nonclathrin uptake processes are localized and internalized in a polarized manner: The leading edge of migratory cells for (i) CD44 leading to extracellular matrix interaction and persistent cell migration, (ii) the GPI-anchored protein Thy-1 for cell–cell interaction, and (iii) β1 integrin for cell adhesion [15]; the apical membrane of epithelial Madin-Darby canine kidney (MDCK) cells for Thy-1 [23]; the basolateral membrane in hepatic epithelial cells for the GPI-anchored protein CD59 for its transcytosis to the apical side [24] (Figure 2g).
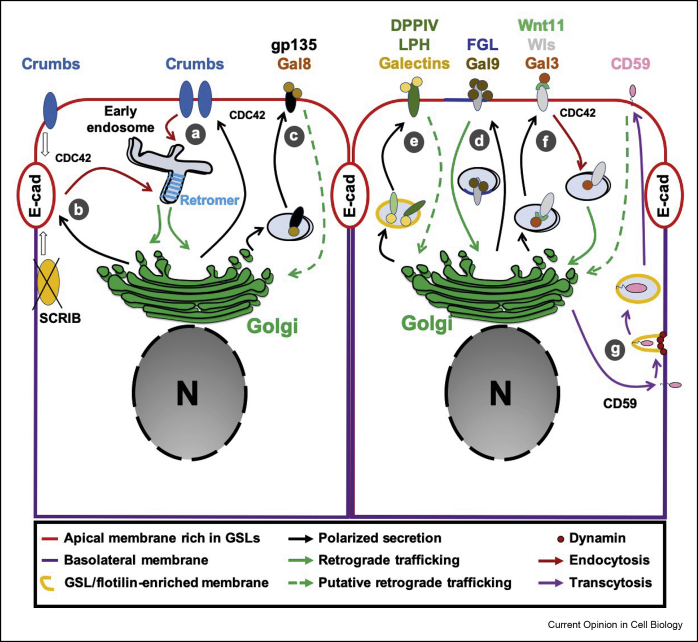
Figure 2.
Retrograde trafficking in the polarized epithelium. Apicobasal polarity determinants: Note the polarized orientation of the Golgi facing the apical membrane. (a) The transmembrane protein Crumbs (Crbs) is an apicobasolateral polarity determinant that together with the Cdc42-Par6-aPKC protein complex controls the establishment and maintenance of apical domain identity. In Drosophila, Crumbs uses the retromer-dependent retrograde route for its apical transport/recycling, which is crucial for apical integrity. (b) Both the apical Crumbs and the lateral Scribble (SCRIB) scaffold protein are needed to stabilize the junction protein E-cadherin (E-cad) at the sub-apical membrane. When silenced, SCRIB induces an accumulation of E-cad within retromer positive structures before reaching the Golgi compartment, suggesting that in certain circumstances the retrograde machinery is involved in the polarized secretion of E-cad. (c) Apically located glycocalyx/gp135 is another key protein in the epithelium. In kidney epithelial cells, galectin-8 (Gal8) binds gp135 in a carbohydrate-dependent manner for post-Golgi delivery to the apical surface to regulate lumen formation. Whether Gal8 traffics from the apical membrane back to the Golgi for a new round of apical secretion of gp135 is an intriguing possibility. Polarized secretion: (d) Extracellular galectin-9 (Gal9) contributes to apicobasolateral polarity. Gal9 binds to the GSL Forssman antigen (FGL) and undergoes cycles of retrograde transport and polarized apical secretion. (e) Similarly to Gal9, other galectins including Gal3 and Gal4 are required for the maintenance of epithelial polarity by controlling the flotillin-dependent polarized secretion of apical proteins such as DPPIV and LPH. In contrast to Gal9, it has not yet been analyzed whether these galectins also undergo retrograde trafficking. (f) Wnt11 is specifically secreted to the apical surface in a process that involves Gal3. As part of its functional cycle, the Wnt receptor Wntless (Wls) undergoes retrograde transport to bind newly synthetized Wnt in the Golgi for subsequent polarized secretion. Whether Gal3 also contributes to the retrograde transport of Wls still needs to be investigated. (g) The GPI-anchored protein CD59 is first transported to the basolateral membrane before being transcytosed to the apical side in a clathrin-independent but dynamin and flotillin-dependent manner. A link between this transcytotic route and the retrograde pathway presents an interesting possibility.
The apicobasal polarization of the colorectal cancer cell line Caco-2 is an important feature for the proper binding of the plant toxin ricin, a nonclathrin cargo, to the basolateral membrane [25]. In MDCK cells, clathrin-independent endocytosis of ricin occurs equally efficiently at both the apical and basolateral surfaces [26]. Upon cAMP stimulation, however, binding and uptake now preferentially operate at the apical membrane, indicating that both processes are subjected to regulation.
Key regulators of the nonclathrin uptake machinery are also localized in a polarized manner. Once activated, FEME asymmetrically operates at the leading edge of migrating cells, whereas clathrin-coated pits exhibit a nonpolarized distribution [6]. FEME component Endo-A2 mediates endothelial cell migration to ensure sprout angiogenesis by regulating the clathrin-independent endocytosis of ligand-activated VEGFR2 at the leading edge [27]. Endo-A2 silencing strongly alters the front-rear asymmetry, notably by repositioning the Golgi behind the nucleus, away from the leading-edge, suggesting a major function for FEME during persistent cell migration [27].
Similarly, CLIC structures were shown to be preferentially distributed at the leading edge of migrating mouse embryonic fibroblast, where the CLIC/GEEC regulator CDC42 is located [15]. Interestingly, CDC42 is an essential contributor in the initiation and maintenance of cell polarity, by acting as an epithelial polarity determinant that notably regulates the proper apical localization of podocalyxin and E-cadherin [28,29], and polarized cell migration in the context of wound-healing [30]. The CLIC/GEEC regulator GRAF1 is also implicated in cell polarity, regulating the orientation of cell spreading in migrating cells and apicobasal polarity during lumen formation [31].
Lipid-rafts play critical roles in cell polarity, especially in migrating cells where they preferentially localize at the leading edge [32], such as during chemoattractant-induced neutrophil polarization to recruit calcium-dependent calpain 2 [33]. GSLs also exhibit a polarized distribution. In migrating mouse embryonic fibroblasts, the polarized localization of the GM1 ganglioside at the leading edge [34] is maintained through Rho/mDia-mediated microtubule stabilization and further regulated by integrin-activated focal adhesion kinase (FAK) [35]. In contrast, GM1 is enriched at the uropod at the rear of migrating T lymphocytes, together with CD44 and β1 integrin, whereas the leading-edge is enriched in GM3 [36]. This differential ganglioside distribution is key for T-cell polarization and is further regulated by cholesterol and the actin cytoskeleton [35,36]. The GD3 ganglioside shares a similar leading-edge localization in melanoma cells where it controls the polarized recruitment of the lipid raft-associated neogenin, which is essential for cell migration and invasion [37].
Interestingly, GSLs are also crucial in the establishment and the maintenance of apicobasal polarization. In Caenorhabditis elegans, GSLs act as determinants to initiate apical domain identity [38]. In mice, their loss alters the identity of apical brush border membrane, and GSL-depleted animals fail to take nutrients up from the intestinal lumen, which is likely because of deficiency in clathrin-independent endocytosis [39].
As suggested by the GL-Lect hypothesis, GSLs function together with galectins for the clathrin-independent construction of endocytic pits [4]. The latter also appear to be important for cell polarization. In kidney, Gal8 binds in a carbohydrate-dependent manner to podocalyxin (gp135) for its post-Golgi delivery to the apical surface such as to specify lumen identity [40] (Figure 2c). In kidney tubules, Gal9 preferentially binds sialylated glycoproteins for apical delivery [41]. Similarly, Gal3 enables the polarized transport of key apical markers, that is the dipeptidyl peptidase IV (DPPIV) and lactase-phlorizin hydrolase (LPH) enzymes [42], and Gal4 was found in post-Golgi flotillin-containing membrane fractions that are responsible of apical delivery [43] (Figure 2e). Of note, Gal3 [20], Gal4 [43], and Gal9 [44], all interact with GSLs in the establishment/maintenance of cell polarity, which highlights the possibility that the GL-Lect process plays a specific role in this context (Figure 2d,e).
In the following section, we discuss evidence that links clathrin-independent endocytosis to retrograde transport in the context of cell polarity.
Retrograde trafficking and cell polarity
The Golgi apparatus faces the leading edge in migrating cells [34] and the apical membrane in epithelial cells [45]. The Golgi is thereby strategically positioned in a way such that it facilitates polarized secretion to these specialized areas of the plasma membrane. Retrograde transport from the plasma membrane to the Golgi apparatus allows in some cases for cell surface proteins to be subjected again to this polarized secretion program such as to keep them dynamically localized to corresponding plasmalemmal subdomains [46,47]. In the following, we will discuss links between clathrin-independent endocytosis, retrograde transport, and cell polarity.
A possible link between clathrin-independent endocytosis and retrograde trafficking has been noticed for protein toxins, that is bacterial Shiga and cholera toxins, the plant toxin ricin [48], and viruses or viral products, that is adeno-associated virus (AAV) [49], human papillomavirus [50], and HIV [51]. The transferrin receptor was among the first cellular proteins for which it was shown that they undergo retrograde transport [52]. Only a small fraction of transferrin receptor is transported to the Golgi, however, which is mirrored by a small fraction that binds to Gal3 [53] and that enters cells by clathrin-independent endocytosis [21]. Whether these fractions correspond to each other remains to be established.
Apicobasal polarity
In association with MIG-14/Wntless, Wnt morphogen undergoes retrograde transport to the Golgi apparatus [54], which is regulated by the polarity determinants Cdc42, the Cdc42-associated PAR-3/PAR-6/aPKC complex, as well as the Cdc42-dependent actin assembly F-BAR domain proteins TOCA-1 and TOCA-2 [55]. Different endocytic processes seem to be operating here: Clathrin for uptake at the basolateral membrane of MDCK cells [56]; clathrin-independent for the canonical Wnt3a; clathrin-dependent for the noncanonical basolateral Wnt5 [57]; and nonclathrin and Gal3-dependent for the apical secretion/localization of Wnt11 [4,56], putatively via the GL-Lect mechanism [4] (Figure 2f).
In Drosophila, the retromer complex strongly contributes to apical polarity by regulating the retrograde transport/recycling of the apical membrane determinant Crumbs [58] (Figure 2a). In MDCK cells, Crumbs together with Scribbles stabilizes the epithelial junction protein E-cadherin. Upon Scribble (SCRIB) silencing, internalized E-cadherin accumulates in a retromer-dependent manner in the Golgi [59] (Figure 2b). This interaction is likely conserved in C. elegans, contributing to Wnt signaling during polarized neuronal migration [60].
Front-rear polarity
The retrograde pathway plays an essential role in the polarized localization of β1 integrin to the leading edge of migrating cells [47]. Interfering with retrograde transport leads to the redistribution of β1 integrin and the inhibition of persistent cell migration, whereas imposing high front-rear polarization stimulates retrograde transport of β1 integrin [47] (Figure 3a). Both clathrin-dependent and clathrin-independent uptake processes have previously been documented for β1 integrin [61, 62, 63], the latter involving the GL-Lect mechanism based on Gal3 and GSLs [20,63]. The relationship between these entry modes and retrograde transport has not yet been assessed.
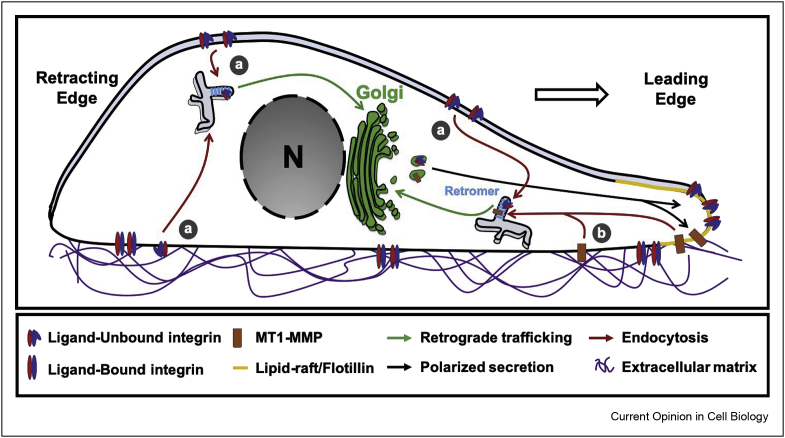
Figure 3.
Retrograde trafficking in migratory cells, During migration (directionality indicated by thick arrow), the cell exhibits two distinct domains: the retracting rear and the migration front, also known as leading edge. Here again, the Golgi apparatus is localized in a polarized manner such that it directly faces the leading edge. (a) The nonligand-bound integrin α5β1 uses the retrograde route to the Golgi before being re-secreted in a polarized manner to the leading edge. Of note, α5β1 integrin uses both clathrin-dependent and clathrin-independent endocytosis processes. The possible link between retrograde transport and the modality of endocytosis remains unexplored. (b) The matrix metalloproteinase MT1-MMP also follows a similar retrograde transport and polarized secretion cycle. Of note, MT1-MMP expression is regulated by the clathrin-independent cargo CD147, and both are recruited to the same lipid raft nanodomains. Other metalloproteinases such as MMP9 are also found at the leading edge and are positively regulated by Gal3. Altogether, this may suggest a general scheme where adhesion molecules and metalloproteinases cooperate and share similar endocytic and intracellular delivery pathways, further regulated by galectins to facilitate efficient cell migration.
The matrix metalloproteinase MT1-MMP undergoes retrograde transport before being secreted back to the plasma membrane [64] (Figure 3b). The clathrin-independent cargo protein CD147 [65] stimulates MT1-MMP expression within lipid-raft enriched invadopodia [66], suggesting that this may occur as the result of polarized secretion from the Golgi to this specialized area of the plasma membrane, following prior retrograde transport.
Immunological and neuronal synapse
Upon T-cell receptor (TCR) activation, T-lymphocytes build a highly polarized structure: the immunological synapse (Figure 4). The linker for activation of T cells (LAT), a key organizer of signalosome formation, undergoes retrograde transport to the Golgi to be secreted in a polarized manner to the immune synapse [46] (Figure 4a). LAT and T-cell signaling components such as the TCR are associated with lipid rafts [67], and it was recently reported that the TCR is internalized in a clathrin-independent manner [68] (Figure 4b). These findings again reinforce the hypothesis of a tight relationship between clathrin-independent endocytosis and retrograde transport-dependent polarized secretion as a common theme to several biological processes involving specialized areas of the plasma membrane.
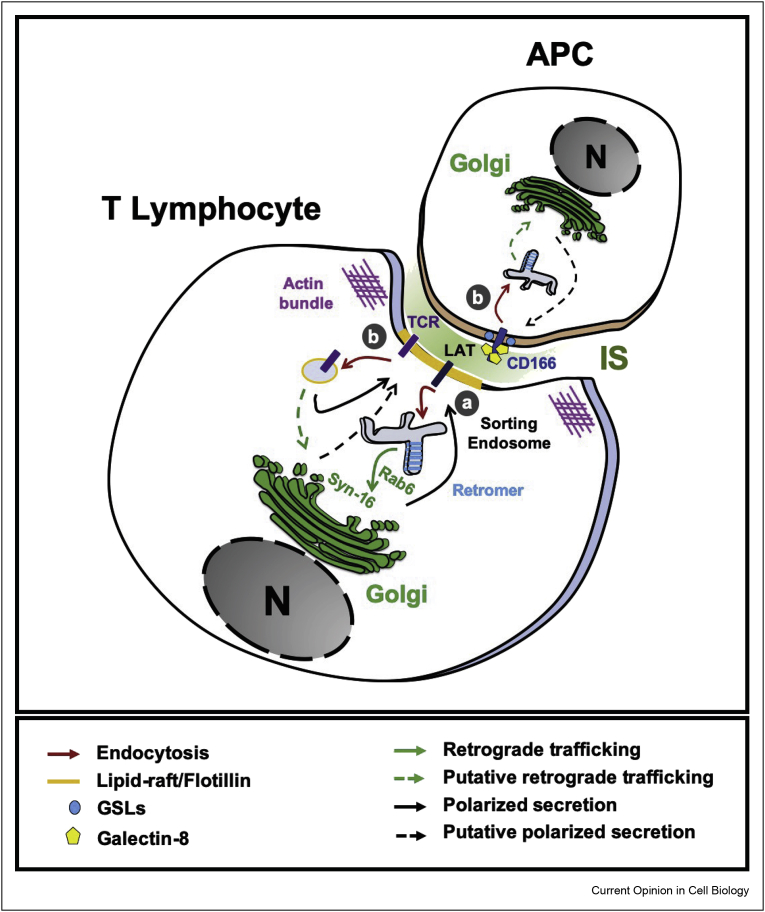
Figure 4.
Retrograde trafficking in immune cells. T-cell receptor (TCR) activation occurs in the presence of antigen presenting cells (APC). During this event, T lymphocytes build specialized membrane domains, termed immunological synapses (IS) to which the linker for activation of T-cell (LAT) localizes in a polarized and dynamic manner to organize the signalosome. Here again, the Golgi apparatus is localized in a polarized manner such that it directly faces the IS. (a) LAT efficiently undergoes Rab6, retromer, and Synt16-dependent retrograde trafficking to the Golgi compartment for subsequent polarized secretion to the IS, a trafficking loop that is required for efficient T cell activation. (b) LAT and other T-cell components such as TCR as well as APC cargoes such as CD166 have been reported to be internalized in a clathrin-independent manner. Whether similar to LAT the other cargoes also undergo retrograde transport for their dynamic localization to the IS remains to be studied directly.
In C. elegans, glutamate receptor is localized in a polarized manner toward the dendrites, and retrograde trafficking is required to ensure efficient postsynaptic activity [69]. Both clathrin-dependent and clathrin-independent endocytosis have been implicated in glutamate receptor turnover [70]. Which of these internalization modes couples to the retrograde route has yet to be addressed.
Concepts and perspectives
In this review, we have pointed to a possible link between the clathrin-independent construction of endocytic sites at the plasma membrane and specific types of intracellular distribution, notably via the retrograde route. Further work is required to further establish this concept and to identify molecular mechanisms that can explain how such coupling might be operated.
We have also addressed the role of retrograde trafficking in the establishment and maintenance of cell polarity, by enabling the polarized secretion of reinternalized cargoes to specialized areas of the plasma membrane within different cellular contexts, such as the leading edge of migratory cells and the immunological synapse of activated T cells. We expect that this concept will apply more widely in the realm of cell polarity, including apicobasal polarity of epithelial cells.
Further investigation of all these aspects is likely to benefit domains of molecular cell biology research that are still relatively poorly explored, such as the role of carbohydrates and raft lipids in the dynamic compartmentalization of biological functions.
Conflict of interest statement
Nothing declared.
Acknowledgements
The authors acknowledge support by grants from European Research Council (advanced grant 340485), Human Frontier Science Program (RGP0029-2014), Swedish Research Council (K2015-99X-22877-01-6), Mizutani Foundation for Glycosciences (reference n° 200014), Agence Nationale de la Recherche (ANR-16-CE23-0005-02, ANR-19-CE13-0001-01), Institut National Du Cancer (n° 2018-1-PLBIO-01-ICR-1, n° 2019-1-PL BIO-05-CEA-1), Plan Cancer program LipoCanPredict. The Johannes team is member of Labex CelTisPhyBio (ANR-11-LBX-0038) and Idex Paris Sciences et Lettres (ANR-10-IDEX-0001-02 PSL).
This review comes from a themed issue on Membrane Trafficking
Edited by Frances M. Brodsky and Jennifer L. Stow
Contributor Information
Massiullah Shafaq-Zadah, Email: massiullah.shafaq-zadah@curie.fr.
Ludger Johannes, Email: ludger.johannes@curie.fr.
References
- 1.Doherty G.J., McMahon H.T. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 2.Antonny B., Burd C., De Camilli P., Chen E., Daumke O., Faelber K., Ford M., Frolov V.A., Frost A., Hinshaw J.E. Membrane fission by dynamin: what we know and what we need to know. EMBO J. 2016;35:2270–2284. doi: 10.15252/embj.201694613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira A.P.A., Boucrot E. Mechanisms of carrier formation during clathrin-independent endocytosis. Trends Cell Biol. 2018;28:188–200. doi: 10.1016/j.tcb.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Johannes L., Wunder C., Shafaq-Zadah M. Glycolipids and lectins in endocytic uptake processes. J Mol Biol. 2016;428:4792–4818. doi: 10.1016/j.jmb.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 5.Mayor S., Parton R.G., Donaldson J.G. Clathrin-independent pathways of endocytosis. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a016758. a016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucrot E., Ferreira A.P., Almeida-Souza L., Debard S., Vallis Y., Howard G., Bertot L., Sauvonnet N., McMahon H.T. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature. 2015;517:460–465. doi: 10.1038/nature14067. [DOI] [PubMed] [Google Scholar]
- Chan Wah Hak L., Khan S., Di Meglio I., Law A.L., Lucken-Ardjomande Hasler S., Quintaneiro L.M., Ferreira A.P.A., Krause M., McMahon H.T., Boucrot E. FBP17 and CIP4 recruit SHIP2 and lamellipodin to prime the plasma membrane for fast endophilin-mediated endocytosis. Nat Cell Biol. 2018;20:1023–1031. doi: 10.1038/s41556-018-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration of the sequential recruitment of BAR-domain proteins in the process of FEME. CDC42 initiates the recruitment of the two BAR-domain proteins FBP17 and CIP4 to facilitate the clustering of SHIP1/2 phosphatases and lamellipodin, which in turn control the recruitment of endophillin to drive FEME-mediated carrier formation upon receptor activation. This study provides impressive insights into the staging of molecular events at the plasma membrane leading to the preparation of domains from which carrier formation is then initiated according to the FEME mechanism.
- 8.Kirkham M., Fujita A., Chadda R., Nixon S.J., Kurzchalia T.V., Sharma D.K., Pagano R.E., Hancock J.F., Mayor S., Parton R.G. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168:465–476. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumari S., Mayor S. ARF1 is directly involved in dynamin-independent endocytosis. Nat Cell Biol. 2008;10:30–41. doi: 10.1038/ncb1666. [DOI] [PubMed] [Google Scholar]
- 10.Sabharanjak S., Sharma P., Parton R.G., Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 11.Lundmark R., Doherty G.J., Howes M.T., Cortese K., Vallis Y., Parton R.G., McMahon H.T. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr Biol. 2008;18:1802–1808. doi: 10.1016/j.cub.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathe M., Muthukrishnan G., Rae J., Disanza A., Thattai M., Scita G., Parton R.G., Mayor S. Small GTPases and BAR domain proteins regulate branched actin polymerisation for clathrin and dynamin-independent endocytosis. Nat Commun. 2018;9:1835. doi: 10.1038/s41467-018-03955-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study on endocytic carrier formation in clathrin- and dynamin-independent CLIC/GEEC endocytosis. Concomitant CDC42-mediated recruitment of IRSp53 and ARF1-dependent inhibition of PICK1 are both required to induce branched actin nucleation, leading to the formation of clathrin-independent carriers. Exciting findings on how BAR domain proteins and small GTPases function together to regulate actin polymerization during CLIC/GEEC endocytosis.
- 13.Kast D.J., Dominguez R. IRSp53 coordinates AMPK and 14-3-3 signaling to regulate filopodia dynamics and directed cell migration. Mol Biol Cell. 2019;30:1285–1297. doi: 10.1091/mbc.E18-09-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renard H.F., Simunovic M., Lemiere J., Boucrot E., Garcia-Castillo M.D., Arumugam S., Chambon V., Lamaze C., Wunder C., Kenworthy A.K. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature. 2015;517:493–496. doi: 10.1038/nature14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howes M.T., Kirkham M., Riches J., Cortese K., Walser P.J., Simpson F., Hill M.M., Jones A., Lundmark R., Lindsay M.R. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J Cell Biol. 2010;190:675–691. doi: 10.1083/jcb.201002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romer W., Berland L., Chambon V., Gaus K., Windschiegl B., Tenza D., Aly M.R., Fraisier V., Florent J.C., Perrais D. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–675. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- 17.Watkins E.B., Majewski J., Chi E.Y., Gao H., Florent J.C., Johannes L. Shiga toxin induces lipid compression: a mechanism for generating membrane curvature. Nano Lett. 2019;19:7365–7369. doi: 10.1021/acs.nanolett.9b03001. [DOI] [PubMed] [Google Scholar]
- 18.Pezeshkian W., Gao H., Arumugam S., Becken U., Bassereau P., Florent J.C., Ipsen J.H., Johannes L., Shillcock J.C. Mechanism of Shiga toxin clustering on membranes. ACS Nano. 2017;11:314–324. doi: 10.1021/acsnano.6b05706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simunovic M., Manneville J.B., Renard H.F., Evergren E., Raghunathan K., Bhatia D., Kenworthy A.K., Voth G.A., Prost J., McMahon H.T. Friction mediates scission of tubular membranes scaffolded by BAR proteins. Cell. 2017;170:172–184 e111. doi: 10.1016/j.cell.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using combined theoretical, model membrane, and cell-based approaches, a consistent model is proposed to explain earlier observations on a role of the BAR domain protein endophilin and the molecular motor dynein in membrane scission (DOI:10.1038/nature14064). This newly discovered mechanism, termed friction-driven scisson, is based on the friction between the BAR domain protein scaffold and the tense tubular membrane on which motor proteins such as dynein actively pull. This elegant study highlights a new concept in membrane scission, which is likely to be particularly relevant for processes of clathrin-independent endocytosis which often are little reliant on the conventional pinchase dynamin.
- 20.Lakshminarayan R., Wunder C., Becken U., Howes M.T., Benzing C., Arumugam S., Sales S., Ariotti N., Chambon V., Lamaze C. Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers. Nat Cell Biol. 2014;16:595–606. doi: 10.1038/ncb2970. [DOI] [PubMed] [Google Scholar]
- Renard H.F., Tyckaert F., Lo Guidice C., Hirsch T., Velades Cruz C.A., Lemaigre C., Shafaq-Zadah M., Wunder C., Wattiez R., Johannes L., van der Bruggen P., Alsteens D., Morsomme P. Endophilin-A3 and galectin-8 control the clathrin-independent endocytosis of CD166. Nat Commun. 2020;11:1457. doi: 10.1038/s41467-020-15303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper highlights the discovery of the clathrin-independent endocytosis of the glycoprotein CD166, which is expressed on various antigen presenting cells. Surprisingly, CD166 uptake is dependent on the BAR domain protein endophilin-A3, and not the other endophilin isoforms. It also depends on galectin-8 and glycosphingolipids to regulate cancer cell migration according to the glycolipid-lectin (GL-Lect) hypothesis. It is quite unexpected that endocytosis according to the GL-Lect hypothesis is tuned with such precision according to the isoform of a given BAR domain protein family member.
- 22.Johannes L., Wunder C., Bassereau P. Bending “on the rocks” – a cocktail of biophysical modules to build endocytic pathways. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a016741. a016741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson J.M., Fasel N., Kraehenbuhl J.P. Polarity of endogenous and exogenous glycosyl-phosphatidylinositol-anchored membrane proteins in Madin-Darby canine kidney cells. J Cell Sci. 1990;96(Pt 1):143–149. doi: 10.1242/jcs.96.1.143. [DOI] [PubMed] [Google Scholar]
- 24.Ait-Slimane T., Galmes R., Trugnan G., Maurice M. Basolateral internalization of GPI-anchored proteins occurs via a clathrin-independent flotillin-dependent pathway in polarized hepatic cells. Mol Biol Cell. 2009;20:3792–3800. doi: 10.1091/mbc.E09-04-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackman M.R., Ellis J.A., Gray S.R., Shurety W., Luzio J.P. Cell polarization is required for ricin sensitivity in a Caco-2 cell line selected for ricin resistance. Biochem J. 1999;341(Pt 2):323–327. [PMC free article] [PubMed] [Google Scholar]
- 26.Eker P., Holm P.K., van Deurs B., Sandvig K. Selective regulation of apical endocytosis in polarized Madin-Darby canine kidney cells by mastoparan and cAMP. J Biol Chem. 1994;269:18607–18615. [PubMed] [Google Scholar]
- Genet G., Boye K., Mathivet T., Ola R., Zhang F., Dubrac A., Li J., Genet N., Henrique Geraldo L., Benedetti L. Endophilin-A2 dependent VEGFR2 endocytosis promotes sprouting angiogenesis. Nat Commun. 2019;10:2350. doi: 10.1038/s41467-019-10359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors have identified the BAR-domain protein endophilin-A2 as part of a new clathrin-independent mechanism in VEGFR endocytosis. Upon VEGF stimulation, this BAR-domain protein plays a crucial role in the establishment of front-rear polarity, a prerequisite for endothelial cell migration and sprouting angiogenesis. This study remarkably depicts the link between clathrin-independent but endophilin-dependent endocytosis and polarized cell migration in the context of sprouting angiogenesis.
- 28.Bryant D.M., Datta A., Rodriguez-Fraticelli A.E., Peranen J., Martin-Belmonte F., Mostov K.E. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shafaq-Zadah M., Brocard L., Solari F., Michaux G. AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine. Development. 2012;139:2061–2070. doi: 10.1242/dev.076711. [DOI] [PubMed] [Google Scholar]
- 30.Etienne-Manneville S., Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 31.Vidal-Quadras M., Holst M.R., Francis M.K., Larsson E., Hachimi M., Yau W.L., Peranen J., Martin-Belmonte F., Lundmark R. Endocytic turnover of Rab8 controls cell polarization. J Cell Sci. 2017;130:1147–1157. doi: 10.1242/jcs.195420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guirland C., Suzuki S., Kojima M., Lu B., Zheng J.Q. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron. 2004;42:51–62. doi: 10.1016/s0896-6273(04)00157-6. [DOI] [PubMed] [Google Scholar]
- 33.Nuzzi P.A., Senetar M.A., Huttenlocher A. Asymmetric localization of calpain 2 during neutrophil chemotaxis. Mol Biol Cell. 2007;18:795–805. doi: 10.1091/mbc.E06-09-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bisel B., Calamai M., Vanzi F., Pavone F.S. Decoupling polarization of the Golgi apparatus and GM1 in the plasma membrane. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080446. e80446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palazzo A.F., Eng C.H., Schlaepfer D.D., Marcantonio E.E., Gundersen G.G. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 2004;303:836–839. doi: 10.1126/science.1091325. [DOI] [PubMed] [Google Scholar]
- 36.Gomez-Mouton C., Abad J.L., Mira E., Lacalle R.A., Gallardo E., Jimenez-Baranda S., Illa I., Bernad A., Manes S., Martinez A.C. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci U S A. 2001;98:9642–9647. doi: 10.1073/pnas.171160298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneko K., Ohkawa Y., Hashimoto N., Ohmi Y., Kotani N., Honke K., Ogawa M., Okajima T., Furukawa K., Furukawa K. Neogenin, defined as a GD3-associated molecule by enzyme-mediated activation of radical sources, confers malignant properties via intracytoplasmic domain in melanoma cells. J Biol Chem. 2016;291:16630–16643. doi: 10.1074/jbc.M115.708834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H., Abraham N., Khan L.A., Hall D.H., Fleming J.T., Gobel V. Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat Cell Biol. 2011;13:1189–1201. doi: 10.1038/ncb2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jennemann R., Kaden S., Sandhoff R., Nordstrom V., Wang S., Volz M., Robine S., Amen N., Rothermel U., Wiegandt H. Glycosphingolipids are essential for intestinal endocytic function. J Biol Chem. 2012;287:32598–32616. doi: 10.1074/jbc.M112.371005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim H., Yu C.Y., Jou T.S. Galectin-8 regulates targeting of Gp135/podocalyxin and lumen formation at the apical surface of renal epithelial cells. FASEB J. 2017;31:4917–4927. doi: 10.1096/fj.201601386R. [DOI] [PubMed] [Google Scholar]
- 41.Mo D., Costa S.A., Ihrke G., Youker R.T., Pastor-Soler N., Hughey R.P., Weisz O.A. Sialylation of N-linked glycans mediates apical delivery of endolyn in MDCK cells via a galectin-9-dependent mechanism. Mol Biol Cell. 2012;23:3636–3646. doi: 10.1091/mbc.E12-04-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delacour D., Koch A., Ackermann W., Eude-Le Parco I., Elsasser H.P., Poirier F., Jacob R. Loss of galectin-3 impairs membrane polarisation of mouse enterocytes in vivo. J Cell Sci. 2008;121:458–465. doi: 10.1242/jcs.020800. [DOI] [PubMed] [Google Scholar]
- 43.Delacour D., Gouyer V., Zanetta J.P., Drobecq H., Leteurtre E., Grard G., Moreau-Hannedouche O., Maes E., Pons A., Andre S. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol. 2005;169:491–501. doi: 10.1083/jcb.200407073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mishra R., Grzybek M., Niki T., Hirashima M., Simons K. Galectin-9 trafficking regulates apical-basal polarity in Madin-Darby canine kidney epithelial cells. Proc Natl Acad Sci U S A. 2010;107:17633–17638. doi: 10.1073/pnas.1012424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bacallao R., Antony C., Dotti C., Karsenti E., Stelzer E.H., Simons K. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J Cell Biol. 1989;109:2817–2832. doi: 10.1083/jcb.109.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpier J.M., Zucchetti A.E., Bataille L., Dogniaux S., Shafaq-Zadah M., Bardin S., Lucchino M., Maurin M., Joannas L.D., Magalhaes J.G. Rab6-dependent retrograde traffic of LAT controls immune synapse formation and T cell activation. J Exp Med. 2018;215:1245–1265. doi: 10.1084/jem.20162042. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors have provided pioneering findings on the involvement of the retrograde transport pathway during T lymphocyte activation. In that context, the linker for activation of T cells (LAT) undergoes retrograde transport to the Golgi from where it is secreted in a polarized manner to the immunological synapse. In combination with findings on migrating cells [47], this study highlights the exciting possibility of a link between retrograde trafficking and polarized secretion to specialized areas of the plasma membrane.
- 47.Shafaq-Zadah M., Gomes-Santos C.S., Bardin S., Maiuri P., Maurin M., Iranzo J., Gautreau A., Lamaze C., Caswell P., Goud B. Persistent cell migration and adhesion rely on retrograde transport of beta(1) integrin. Nat Cell Biol. 2016;18:54–64. doi: 10.1038/ncb3287. [DOI] [PubMed] [Google Scholar]
- 48.Johannes L., Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135:1175–1187. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Nonnenmacher M.E., Cintrat J.C., Gillet D., Weber T. Syntaxin 5-dependent retrograde transport to the trans-Golgi network is required for adeno-associated virus transduction. J Virol. 2015;89:1673–1687. doi: 10.1128/JVI.02520-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schelhaas M., Shah B., Holzer M., Blattmann P., Kuhling L., Day P.M., Schiller J.T., Helenius A. Entry of human papillomavirus type 16 by actin-dependent, clathrin- and lipid raft-independent endocytosis. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002657. e1002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blot G., Janvier K., Le Panse S., Benarous R., Berlioz-Torrent C. Targeting of the human immunodeficiency virus type 1 envelope to the trans-Golgi network through binding to TIP47 is required for env incorporation into virions and infectivity. J Virol. 2003;77:6931–6945. doi: 10.1128/JVI.77.12.6931-6945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snider M.D., Rogers O.C. Intracellular movement of cell surface receptors after endocytosis: resialylation of asialo-transferrin receptor in human erythroleukemia cells. J Cell Biol. 1985;100:826–834. doi: 10.1083/jcb.100.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carlsson M.C., Bengtson P., Cucak H., Leffler H. Galectin-3 guides intracellular trafficking of some human serotransferrin glycoforms. J Biol Chem. 2013;288:28398–28408. doi: 10.1074/jbc.M113.487793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J., Liu J., Norris A., Grant B.D., Wang X. A novel requirement for ubiquitin-conjugating enzyme UBC-13 in retrograde recycling of MIG-14/Wntless and Wnt signaling. Mol Biol Cell. 2018;29:2098–2112. doi: 10.1091/mbc.E17-11-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai Z., Grant B.D. A TOCA/CDC-42/PAR/WAVE functional module required for retrograde endocytic recycling. Proc Natl Acad Sci U S A. 2015;112:E1443–E1452. doi: 10.1073/pnas.1418651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamamoto H., Awada C., Hanaki H., Sakane H., Tsujimoto I., Takahashi Y., Takao T., Kikuchi A. The apical and basolateral secretion of Wnt11 and Wnt3a in polarized epithelial cells is regulated by different mechanisms. J Cell Sci. 2013;126:2931–2943. doi: 10.1242/jcs.126052. [DOI] [PubMed] [Google Scholar]
- 57.Bandmann V., Mirsanaye A.S., Schafer J., Thiel G., Holstein T., Mikosch-Wersching M. Membrane capacitance recordings resolve dynamics and complexity of receptor-mediated endocytosis in Wnt signalling. Sci Rep. 2019;9:12999. doi: 10.1038/s41598-019-49082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pocha S.M., Wassmer T., Niehage C., Hoflack B., Knust E. Retromer controls epithelial cell polarity by trafficking the apical determinant Crumbs. Curr Biol. 2011;21:1111–1117. doi: 10.1016/j.cub.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 59.Lohia M., Qin Y., Macara I.G. The Scribble polarity protein stabilizes E-cadherin/p120-catenin binding and blocks retrieval of E-cadherin to the Golgi. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051130. e51130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wassmer T., Attar N., Harterink M., van Weering J.R., Traer C.J., Oakley J., Goud B., Stephens D.J., Verkade P., Korswagen H.C. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell. 2009;17:110–122. doi: 10.1016/j.devcel.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palamidessi A., Frittoli E., Garre M., Faretta M., Mione M., Testa I., Diaspro A., Lanzetti L., Scita G., Di Fiore P.P. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 62.Vassilieva E.V., Gerner-Smidt K., Ivanov A.I., Nusrat A. Lipid rafts mediate internalization of beta1-integrin in migrating intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G965–G976. doi: 10.1152/ajpgi.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furtak V., Hatcher F., Ochieng J. Galectin-3 mediates the endocytosis of beta-1 integrins by breast carcinoma cells. Biochem Biophys Res Commun. 2001;289:845–850. doi: 10.1006/bbrc.2001.6064. [DOI] [PubMed] [Google Scholar]
- 64.Wang X., Ma D., Keski-Oja J., Pei D. Co-recycling of MT1-MMP and MT3-MMP through the trans-Golgi network. Identification of DKV582 as a recycling signal. J Biol Chem. 2004;279:9331–9336. doi: 10.1074/jbc.M312369200. [DOI] [PubMed] [Google Scholar]
- 65.Eyster C.A., Higginson J.D., Huebner R., Porat-Shliom N., Weigert R., Wu W.W., Shen R.F., Donaldson J.G. Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic. 2009;10:590–599. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grass G.D., Bratoeva M., Toole B.P. Regulation of invadopodia formation and activity by CD147. J Cell Sci. 2012;125:777–788. doi: 10.1242/jcs.097956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Varshney P., Yadav V., Saini N. Lipid rafts in immune signalling: current progress and future perspective. Immunology. 2016;149:13–24. doi: 10.1111/imm.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compeer E.B., Kraus F., Ecker M., Redpath G., Amiezer M., Rother N., Nicovich P.R., Kapoor-Kaushik N., Deng Q., Samson G.P.B. A mobile endocytic network connects clathrin-independent receptor endocytosis to recycling and promotes T cell activation. Nat Commun. 2018;9:1597. doi: 10.1038/s41467-018-04088-w. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors have highlighted the existence of a tight relation at the immunological synapse of T cells between clathrin-independent endocytosis and polarized recycling. Remarkably, the T cell receptor (TCR) is internalized via clathrin-independent processes, and incorporated into mobile long-lived endocytic tubules containing the lipid-membrane organizer flotillin, which is necessary for T cell activation. This thorough study provides further evidence on a tight relationship between clathrin-independent endocytosis and polarized recycling within activated T lymphocytes, which might mirror the finding on LAT [46].
- 69.Zhang D., Dubey J., Koushika S.P., Rongo C. RAB-6.1 and RAB-6.2 promote retrograde transport in C. elegans. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149314. e0149314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glodowski D.R., Chen C.C., Schaefer H., Grant B.D., Rongo C. RAB-10 regulates glutamate receptor recycling in a cholesterol-dependent endocytosis pathway. Mol Biol Cell. 2007;18:4387–4396. doi: 10.1091/mbc.E07-05-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]