Abstract
Autophagy is characterized by the formation of double-membrane vesicles called autophagosomes, which deliver bulk cytoplasmic material to the lytic compartment of the cell for degradation. Autophagosome formation is initiated by assembly and recruitment of the core autophagy machinery to distinct cellular sites, referred to as phagophore assembly sites (PAS) in yeast or autophagosome formation sites in other organisms. A large number of autophagy proteins involved in the formation of autophagosomes has been identified; however, how the individual components of the PAS are assembled and how they function to generate autophagosomes remains a fundamental question. Here, we highlight recent studies that provide molecular insights into PAS organization and the role of the endoplasmic reticulum and the vacuole in autophagosome formation.
Keywords: ATG1, ATG2, ATG5, ATG12, ATG13, ATG16, ATG17, ATG18, ATG29, ATG31, VAC8, Autophagosome, Phagophore, Isolation membrane, Autophagosome formation site, Phagophore assembly site, Pre-autophagosomal structure, PAS, ER, Vacuole, Autophagy, Lipid transfer, Organelle contact site, Tether, Phase separation, Liquid droplet
Introduction
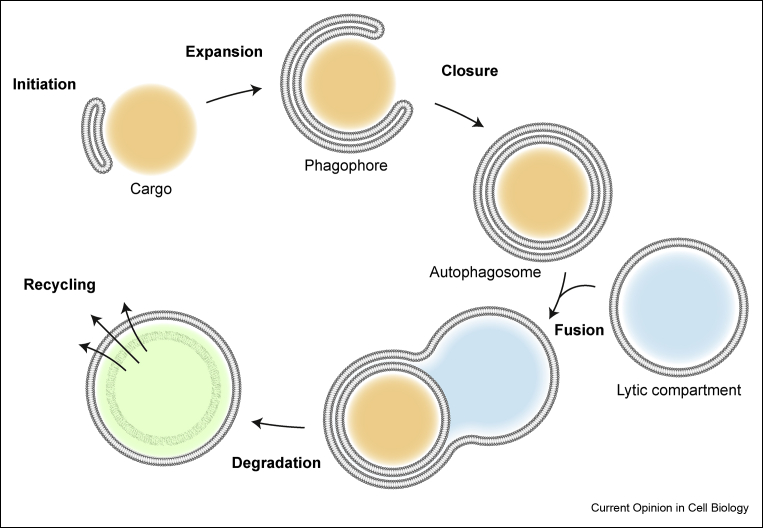
Autophagy is a highly conserved eukaryotic pathway for degradation and recycling of intracellular components. During macroautophagy (hereafter referred to as autophagy), bulk cytoplasmic material is sequestered by a double-membrane structure, the so-called phagophore (also known as the isolation membrane), which expands around the cargo and finally forms a sealed, double-membrane vesicle known as the autophagosome. Fusion of the autophagosome with the lytic compartment (the vacuole in yeast and plants, the lysosome in mammals) leads to the degradation and recycling of the cargo [1] (Fig. 1).
Figure 1.
Overview of the autophagy pathway. Autophagosome biogenesis is initiated with the nucleation of the phagophore, a double-membrane structure. Expansion of the phagophore leads to the engulfment of the autophagic cargo. The edges of the phagophore are eventually closed to form a sealed autophagosome. The outer membrane of the completed autophagosome fuses with a lytic compartment, resulting in the degradation of the inner autophagosomal membrane and the autophagic cargo by resident hydrolyses. Finally, the resulting macromolecules are released back into the cytosol.
Nutrient starvation and other stress conditions induce the random, nonselective degradation of cytoplasmic material to recycle building blocks required for cellular survival, a process that is known as bulk autophagy. Conversely, during selective autophagy, specific cargo is targeted by dedicated autophagy receptors, which promote the in situ formation of an autophagosome and the selective sequestration of the cargo.
The molecular mechanism underlying the biogenesis of autophagosomes has been a major focus in the field of autophagy for the last two decades. Combined efforts have led to the identification and characterization of a large number of players involved in autophagosome formation, yet for many of these proteins, the molecular function remains largely unknown.
In this review, we focus on the cellular structure that functions in autophagosome formation. We briefly discuss how the concept of an autophagosome formation site emerged and then summarize recent advances regarding composition, organization and functions of this structure. We focus on recent work done in Saccharomyces cerevisiae; however, many of the concepts have also been confirmed in mammalian cells.
Autophagosomes are formed at the phagophore assembly site
Autophagy is initiated by the assembly of the core autophagy machinery proteins, both in yeast and mammals. This assembly has been most extensively studied in yeast and is termed as the pre-autophagosomal structure or phagophore assembly site (both terms are abbreviated as PAS).
The term pre-autophagosomal structure was first used in yeast to describe a punctate, perivacuolar structure visible in fluorescence microscopy where various proteins of the autophagy machinery co-localized. Importantly, this structure was demonstrated to be required and directly involved in the formation of autophagosomes [2,3]. To date, more than 40 autophagy-related (Atg) proteins have been identified, mainly by genetic screens in S. cerevisiae, and named Atg1 to Atg42 (reviewed in Ref. [4]).
A systematic analysis of the interdependencies between the core Atg proteins revealed the organization principles of PAS formation and allowed the development of a hierarchy diagram of Atg protein recruitment. However, analysis of the spatial organization of the PAS and the phagophore was beyond the resolving power of light microscopy [5].
The next milestone in understanding the PAS was reached when two independent studies demonstrated that different PAS factors display distinct localization patterns in regard to the phagophore [6,7]. Importantly, both studies found that the PAS not only associates with the vacuole but also with endoplasmic reticulum (ER) exit sites, a specialized subdomain of the ER, which plays a functional role during autophagosome formation.
Initially, it was unclear if the PAS represents a membranous precursor structure that matures into an autophagosome or if the PAS and the phagophore are two different yet associated structures. Accumulating evidence favors the latter model, suggesting that the PAS constitutes a distinct cellular structure that functions in the production of autophagosomes but does not convert into an autophagosome. This is supported by the observation that Atg proteins forming the PAS associate with the growing phagophore to promote autophagosome formation but dissociate after completion of the autophagosome [8,9]. In 2007, it was suggested to use the term ‘phagophore assembly site’ as an alternative interpretation for the acronym PAS to highlight that the PAS is not equivalent to the phagophore but rather a hybrid structure between the core autophagy machinery and the phagophore [10,11]. Although the term pre-autophagosomal structure was introduced first [3], the aforementioned reasoning justifies the more descriptive term phagophore assembly site.
In the next section, we will highlight and discuss recent work that has substantially improved our understanding of the PAS as an autophagosome formation site.
Initiation and maturation of the PAS
Assembly of the PAS initiates autophagy and defines the site of autophagosome formation. One of the initial events in PAS formation is the generation of an oligomeric protein assembly by action of one of the two scaffold proteins Atg11 and Atg17, and other members of the Atg1 kinase complex [12]. During selective autophagy, cargo is recognized by dedicated cargo receptor proteins, which then recruit Atg11 to induce PAS formation. In bulk autophagy, when no defined cargo is present, multiple interactions between members of the Atg1 kinase complex lead to the formation of higher-order oligomeric assemblies [13]. In both cases, local clustering of Atg1 results in trans-autophosphorylation and induction of kinase activity, which is considered a hallmark of PAS initiation [13, 14, 15]. A recent study proposes that the early PAS in bulk autophagy undergoes phase separation to form liquid droplets [16]. The authors suggest that the higher-order assembly of the Atg1 complex induces phase separation and that this process is mainly controlled by the phosphorylation status of Atg13. It will be interesting to study if and how phase separation influences maturation of the PAS and if the PAS remains phase separated throughout autophagosome formation.
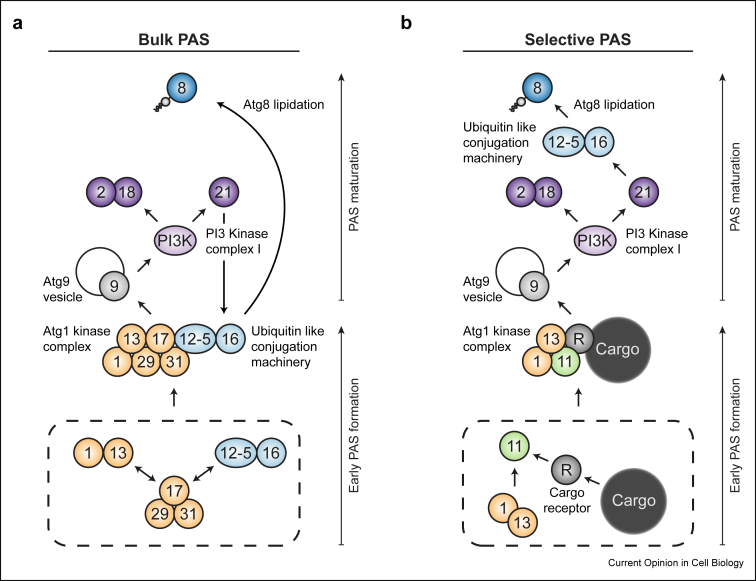
After formation of an early PAS structure, the PAS matures by hierarchical recruitment of downstream Atg proteins. First, the transmembrane protein Atg9, embedded in small vesicles, is recruited, serving as an additional recruitment platform for other PAS factors [17]. Next, the phosphatidylinositol (PI) 3-kinase (PI3K) complex I, which contains the autophagy-specific subunit Atg14, accumulates at the PAS and catalyzes PI phosphorylation to generate PI3-phosphate (PI3P). The Atg2–Atg18 module engages with the PAS in a PI3P-dependent manner. Independently of this module, the Atg12—Atg5–Atg16 ubiquitin-like conjugation machinery is recruited [18]. This requires, at least during selective autophagy, the PI3P binding protein Atg21, which directly interacts with Atg16. Finally, the Atg12—Atg5–Atg16 complex catalyzes conjugation of the ubiquitin-like protein Atg8 to phosphatidylethanolamine lipids present in the phagophore, which is a prerequisite for autophagosome formation [19,20] (Figure 2a,b).
Figure 2.
Hierarchical model of bulk PAS (a) and selective PAS (b) assembly in yeast. Formation of the PAS is initiated by an oligomeric assembly of the Atg1 kinase complex, which constitutes the early PAS and marks the site of autophagosome formation. Recruitment of the downstream PAS factors matures the early PAS into a structure that is competent for phagophore formation. Recently, Harada et al. [21] discovered an alternative pathway for bulk PAS recruitment of the Atg12—Atg5–Atg16 complex via Atg17, which, in addition, appears to contribute to early PAS formation. Arrows indicate that the PAS recruitment of a protein or a protein complex is affected by the presence or action of others. This model depicts the core autophagy machinery discussed in this review; however, it does not represent a complete account of all factors present at the PAS that are involved in autophagosome formation. Circles containing numbers correspond to the respective Atg proteins. The PI3 kinase complex I comprises Vps15, Vps30/Atg6, Vps34, Atg14 and Atg38.
Recently an alternative, PI3P-independent pathway for the recruitment of the Atg12—Atg5–Atg16 complex to the bulk autophagy PAS has been discovered, mediated by the interaction of Atg17 with Atg12 [21]. Atg17 is involved in the formation of the early PAS upstream of PI3K complex recruitment, indicating that this newly described pathway acts before and independent of Atg21. However, both pathways seem to act cooperatively since only the combination of an Atg21 deletion with mutations that abolish the interaction between Atg17 and Atg12 resulted in a complete block of bulk autophagy. Surprisingly, interaction between Atg17 and Atg12 appears to also stimulate PAS formation, which attributes a novel noncanonical role to the Atg12—Atg5–Atg16 complex at a very early step of PAS formation (Fig. 2a). Moreover, this observation raises an intriguing question: how does the Atg12—Atg5–Atg16 complex influence the liquid-like properties of the early PAS?
Organization of the spatial environment for autophagosome formation
Although the PAS had initially been described as a perivacuolar structure, the functional relevance of the PAS–vacuole connection had remained elusive for a long time. Recent findings revealed that the vacuolar protein Vac8 is required for establishing a stable connection between the bulk PAS and the vacuole that persists throughout phagophore expansion until fusion of the autophagosome with the vacuole [22]. In bulk autophagy, deletion of Vac8 causes a reduced PAS initiation rate, formation of smaller autophagosomes and an autophagosome–vacuole fusion defect [22]. Similarly, selective autophagy is severely impaired by loss of Vac8 [23, 24, 25], demonstrating that the PAS–vacuole connection is required also for selective autophagy. Vac8 directly interacts with Atg13, a member of the Atg1 kinase complex, thereby establishing the PAS–vacuole connection [22,26]. This model has recently been confirmed in vitro by using a reconstituted Atg1 kinase complex and Vac8 tethered to giant unilamellar vesicles [16]. How exactly the PAS–vacuole connection is involved in phagophore formation remains to be understood.
In addition to the perivacuolar localization, autophagosomes form at the ER. Atg2 was found to localize to contact points between the phagophore and the ER [6,7,27]. Recent studies identified two membrane-binding domains located in the N-terminal and C-terminal regions of yeast Atg2 and its mammalian homolog ATG2A, which both are required for autophagy [28,29]. The C-terminal domain was found to mediate recruitment of the Atg2–Atg18 complex to the PAS, whereas the N-terminal domain has been suggested to establish the PAS–ER connection [29].
Atg9 confines Atg2 to the phagophore extremity, where the phagophore contacts the ER. Loss of the Atg2–Atg9 interaction results in Atg2 being present on the entire phagophore and expansion of the phagophore–ER contact area. This situation does not completely block phagophore expansion, but it prevents formation of sealed autophagosomes [30]. Although an Atg2 knockout does not completely abolish the PAS–ER connection in vivo [30], it is clear that Atg2 plays a key role in connecting the phagophore with the ER membrane. These observations raise the question whether Atg2 is sufficient to establish phagophore–ER contact sites, which other factors contribute to this connection, and why it is important to confine the contact sites to the extremity of the phagophore.
Atg2 recruitment to the PAS depends on Atg9 and PI3P production, which are hierarchically downstream of Atg13, the known interaction partner of Vac8. Thus, it might appear that the PAS first forms at the vacuole and subsequently establishes a connection with the ER. However, neither loss of the vacuole connection by deletion of Vac8 nor loss of close association with the ER by inhibition of ER exit sites prevents early PAS formation, suggesting that PAS initiation is not strictly dependent on these connections [6,22]. In contrast, the ER connection is essential for phagophore expansion and the vacuolar connection is required for the efficient formation of normal-sized autophagosomes. These findings support the idea that the PAS organizes a subcellular niche between the ER and the vacuole for autophagosome formation.
Phagophore nucleation and expansion
Phagophore nucleation requires a precursor membrane. It is plausible that either vesicles recruited to the PAS serve as a membrane seed or that a membrane expanding from an existing organelle is pinched off. Lipid transfer and fusion with additional vesicles could then drive phagophore expansion.
Because Atg9 vesicles belong to the core PAS components, it is reasonable to speculate that these structures constitute a phagophore seed [31]. Indeed, Atg9 has been described to be part of the phagophore in yeast [32]. However, in mammals, ATG9 appears to only transiently interact with the phagophore [33], functioning in the recruitment of auxiliary factors or acting as a membrane transporter [34].
Even less is known about how phagophore expansion is achieved and many cellular membranes have been suggested to act as lipid donors for this process [35]. Recently also COPII vesicles have been implicated as a potential membrane source for autophagosomes but their contribution to autophagosome formation remains to be investigated [36, 37, 38, 39]. Recent studies demonstrating the ability of yeast Atg2 and mammalian ATG2A to tether high curvature vesicles and mediate lipid transfer between such vesicles shed light on a potential function of phagophore–ER contact sites [27,40,41]. To drive phagophore expansion, lipid flow would need to be directional from the ER to the phagophore. However, lipid transfer by Atg2 in vitro does not appear to consume energy and in consequence it was suggested to be bidirectional, indicating that additional mechanisms act in concert with Atg2 to facilitate phagophore expansion by lipid flow from the ER.
In yeast, autophagosomes have been reported to form in about 10 min [42]. Therefore, autophagy requires rapid and extensive membrane delivery to the phagophore, raising the question whether this demand could actually be satisfied by lipid transfer alone. An Atg2 mutant allele defective in lipid transfer but proficient in PAS and membrane binding could help to study the impact of Atg2-mediated lipid transfer on phagophore expansion in vivo.
Is the PAS a distinct and conserved cellular structure that generates autophagosomes?
In yeast, formation of an autophagosome is always preceded by the formation of the PAS. Assembly of the PAS not only marks the site of autophagosome formation but also defines the cargo destined for degradation. Furthermore, the PAS establishes organelle contact sites with the vacuole and the ER, thereby creating a subcellular environment that is required for autophagosome biogenesis. During autophagosome formation, the PAS closely associates with the phagophore, forming a hybrid structure. After initiation of phagophore formation, subcomplexes of the PAS associate with different regions of the phagophore to mediate membrane expansion and ultimately formation of a sealed autophagosome. However, likely these complexes remain distinct entities that only interact with the phagophore, because most Atg proteins have been reported to be absent from mature autophagosomes. Indeed, the majority of Atg proteins bound to the outer autophagosomal membrane have been reported to dissociate after phagophore closure before autophagosome–vacuole fusion. These findings suggest that the PAS disassembles and that PAS factors are recycled to participate in a new round of autophagosome formation (Fig. 3).
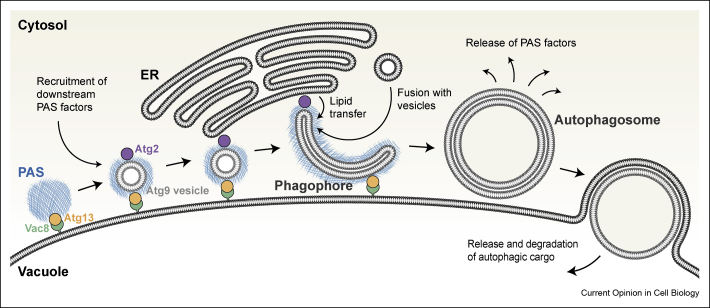
Figure 3.
Model of autophagosome formation in yeast. Autophagy is initiated by formation of the early PAS in the vicinity of the vacuole. Additional PAS factors are recruited to the early PAS in a hierarchical manner and a connection to the ER is established. Recently, it has been shown that the interaction of the early PAS factor Atg13 with the vacuolar membrane protein Vac8 stably anchors the PAS at the vacuole throughout autophagosome formation [22]. Furthermore, it has been demonstrated that the late PAS factor Atg2 establishes the PAS–ER connection [29,30]. This spatial arrangement creates a subcellular niche between the ER and the vacuole for autophagosome formation. Phagophore formation is nucleated by Atg9 vesicles or another membrane seed and fusion of vesicles has been suggested to drive phagophore growth. In a recent study from Osawa et al. [41], Atg2 has been found to possess lipid transfer activity, which describes a novel mechanism of phagophore expansion and provides molecular insights into the role of the phagophore–ER connection. PAS factors associate with the phagophore to create a PAS–phagophore hybrid structure that persists throughout autophagosome formation until the edge of the phagophore closes and a sealed autophagosome is formed. PAS factors are released from the autophagosome before the outer autophagosomal membrane fuses with the vacuole.
The PAS itself is a complex oligomeric protein assembly generated by the concerted action of many Atg proteins and additional factors (Fig. 2). Interestingly, mutations that block phagophore expansion, like deletion of Atg2, do not necessarily prevent assembly of the core autophagy machinery to form a PAS, indicating that a nonfunctional PAS can form even in the absence of autophagosome formation. Based on the available data, the PAS can therefore be considered as a distinct and highly dynamic cellular structure with the purpose to generate autophagosomes.
This model raises the question whether the autophagosome generating structure, the PAS, is conserved. In fact, accumulating evidence suggests a high degree of conservation from yeast to mammals, although the term PAS is still almost exclusively used in yeast. Mammalian homologues have been identified for most of the core ATG genes in yeast and for many a homologous function has already been confirmed. Moreover, in mammals, many of the ATG proteins also co-localize at punctate structures and the hierarchy of the ATG protein assembly has been reported to be similar to yeast [43]. Specialized subdomains of the ER are now considered to be the major site of autophagosome formation in yeast, mammals and also other eukaryotes [6,44]. Close association of the lytic compartment with forming autophagosomes, which is a hallmark of the PAS in S. cerevisiae, has so far only been described for yeast. In contrast to yeast, where typically one or a few PAS per cell are present, many such punctate structures can be observed in other organisms. These two major differences likely represent species-specific adaptations of autophagy in yeast that are not conserved, nonetheless, they do not contradict the existence of an otherwise conserved PAS. Rather, this implies that certain characteristics of the PAS, such as its cellular localization, might be variable to meet the specific requirements on autophagy arising in different species, cell types or types of autophagy. In yeast, the PAS simultaneously establishes two distinct organelle contact sites, with the ER and the vacuole, creating a confined space for autophagosome formation. Whether such a spatial arrangement is functionally conserved in other eukaryotes and whether a confined space also needs to be established, will be interesting to address in the future. Based on the presented evidence, we favor the model that the PAS indeed represents a conserved structure, although further work in higher eukaryotes is required to support this notion.
Conclusion
During recent years, we have gained a basic understanding of the composition and function of the PAS; however, the details of how exactly the individual components function to propagate the sequestration of cargo by the de novo formation of an autophagosome remains less well understood. We anticipate that further work on the PAS–phagophore hybrid structure will not only provide novel insight on the molecular mechanisms of autophagosome formation but will also unveil variations and different requirements of this process owed to different situations where autophagy takes place.
Conflicts of interest
Nothing declared.
Acknowledgements
The authors would like to thank Raffaela Torggler and Dorotea Fracchiolla for critical reading of the manuscript. The Kraft laboratory has received funding from the Vienna Science and Technology Fund (WWTF, VRG10-001) from the FWF Austrian Science Fund (grant numbers P25522-B20, P28113-B28 and W1261), from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement number 769065), from the European Union's Horizon 2020 research and innovation program (grant agreement number 765912), from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—(Project-ID 403222702—SFB 1381 and Project-ID 259130777—SFB 1177) and from the EMBO Young Investigator Program. This work reflects only the authors' view and the European Union's Horizon 2020 research and innovation programme is not responsible for any use that may be made of the information it contains.
This review comes from a themed issue on Membrane Trafficking
Edited by Frances M. Brodsky and Jennifer L. Stow
References
- 1.Yin Z., Pascual C., Klionsky D.J. Autophagy: machinery and regulation. Microb Cell. 2016;3:588–596. doi: 10.15698/mic2016.12.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J., Huang W.-P., Stromhaug P.E., Klionsky D.J. Convergence of multiple autophagy and cytoplasm to vacuole targeting components to a perivacuolar membrane compartment prior to de novo vesicle formation. J Biol Chem. 2002;277:763–773. doi: 10.1074/jbc.M109134200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki K., Kirisako T., Kamada Y., Mizushima N., Noda T., Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20:5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki K., Kubota Y., Sekito T., Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Gene Cell. 2007;12:209–218. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- 6.Graef M., Friedman J.R., Graham C., Babu M., Nunnari J. ER exit sites are physical and functional core autophagosome biogenesis components. MBoC. 2013;24:2918–2931. doi: 10.1091/mbc.E13-07-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki K., Akioka M., Kondo-Kakuta C., Yamamoto H., Ohsumi Y. Fine mapping of autophagy-related proteins during autophagosome formation in Saccharomyces cerevisiae. J Cell Sci. 2013;126:2534–2544. doi: 10.1242/jcs.122960. [DOI] [PubMed] [Google Scholar]
- 8.Cebollero E., van der Vaart A., Zhao M., Rieter E., Klionsky D.J., Helms J.B., Reggiori F. Phosphatidylinositol-3-phosphate clearance plays a key role in autophagosome completion. Curr Biol. 2012;22:1545–1553. doi: 10.1016/j.cub.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheong H., Nair U., Geng J., Klionsky D.J. The Atg1 kinase complex is involved in the regulation of protein recruitment to initiate sequestering vesicle formation for nonspecific autophagy in Saccharomyces cerevisiae. MBoC. 2007;19:668–681. doi: 10.1091/mbc.E07-08-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskelinen E.-L. The mystery of the membranes. Autophagy. 2008;4:3–4. doi: 10.4161/auto.5180. [DOI] [PubMed] [Google Scholar]
- 11.Xie Z., Klionsky D.J. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 12.Papinski D., Kraft C. Regulation of autophagy by signaling through the Atg1/ULK1 complex. J Mol Biol. 2016;428:1725–1741. doi: 10.1016/j.jmb.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto H., Fujioka Y., Suzuki S.W., Noshiro D., Suzuki H., Kondo-Kakuta C., Kimura Y., Hirano H., Ando T., Noda N.N. The intrinsically disordered protein Atg13 mediates supramolecular assembly of autophagy initiation complexes. Dev Cell. 2016;38:86–99. doi: 10.1016/j.devcel.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Kamber R.A., Shoemaker C.J., Denic V. Receptor-bound targets of selective autophagy use a scaffold protein to activate the Atg1 kinase. Mol Cell. 2015;59:372–381. doi: 10.1016/j.molcel.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torggler R., Papinski D., Brach T., Bas L., Schuschnig M., Pfaffenwimmer T., Rohringer S., Matzhold T., Schweida D., Brezovich A. Two independent pathways within selective autophagy converge to activate Atg1 kinase at the vacuole. Mol Cell. 2016;64:221–235. doi: 10.1016/j.molcel.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 16••.Fujioka Y., Alam J.M., Noshiro D., Mouri K., Ando T., Okada Y., May A.I., Knorr R.L., Suzuki K., Ohsumi Y. Phase separation organizes the site of autophagosome formation. Nature. 2020;578:301–305. doi: 10.1038/s41586-020-1977-6. [DOI] [PubMed] [Google Scholar]; The early PAS in yeast bulk autophagy is formed by higher-order assemblies of the Atg1 kinase complex. Here Fujioka et al. discover liquid-liquid phase separation of the early bulk PAS in vivo. Furthermore, in vitro reconstitution experiments using purified proteins of the Atg1 kinase complex demonstrate that phase separation can be controlled by the phosphorylation status of Atg13, as phosphorylated Atg13 prevents formation of liquid-like condensates.
- 17.Noda T., Kim J., Huang W.-P., Baba M., Tokunaga C., Ohsumi Y., Klionsky D.J. Apg9p/Cvt7p is an integral membrane protein required for transport vesicle formation in the cvt and autophagy pathways. J Cell Biol. 2000;148:465–480. doi: 10.1083/jcb.148.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizushima N., Noda T., Ohsumi Y. Apg16p is required for the function of the Apg12p–Apg5p conjugate in the yeast autophagy pathway. EMBO J. 1999;18:3888–3896. doi: 10.1093/emboj/18.14.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farré J.-C., Subramani S. Mechanistic insights into selective autophagy pathways: lessons from yeast. Nat Rev Mol Cell Biol. 2016;17:537–552. doi: 10.1038/nrm.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lystad A.H., Simonsen A. Phosphoinositide-binding proteins in autophagy. FEBS (Fed Eur Biochem Soc) Lett. 2016;590:2454–2468. doi: 10.1002/1873-3468.12286. [DOI] [PubMed] [Google Scholar]
- 21••.Harada K., Kotani T., Kirisako H., Sakoh-Nakatogawa M., Oikawa Y., Kimura Y., Hirano H., Yamamoto H., Ohsumi Y., Nakatogawa H. Two distinct mechanisms target the autophagy-related E3 complex to the pre-autophagosomal structure. eLife. 2019;8 doi: 10.7554/eLife.43088. e43088. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors describe an alternative mechanism for the recruitment of the autophagy-related E3 complex to the bulk PAS in yeast via direct interaction with Atg17. In addition, they discover a novel function of the E3 complex during the initiation step of bulk PAS assembly. These findings provide mechanistic insight into PAS assembly in yeast.
- 22••.Hollenstein D.M., Gómez-Sánchez R., Ciftci A., Kriegenburg F., Mari M., Torggler R., Licheva M., Reggiori F., Kraft C. Vac8 spatially confines autophagosome formation at the vacuole in S. cerevisiae. J Cell Sci. 2019;132 doi: 10.1242/jcs.235002. [DOI] [PMC free article] [PubMed] [Google Scholar]; In yeast, autophagosomes are generated in the vicinity of the vacuole. This study reports that Vac8 constitutes a vacuolar tether that stably anchors the PAS and growing autophagosomes to the vacuole. Loss of the PAS–vacuole connection results in the formation of fewer and smaller autophagosomes and inefficient autophagosome–vacuole fusion, demonstrating the importance of this organelle contact site for autophagosome formation.
- 23.Kiššová I.B., Salin B., Schaeffer J., Bhatia S., Manon S., Camougrand N. Selective and non-selective autophagic degradation of mitochondria in yeast. Autophagy. 2007;3:329–336. doi: 10.4161/auto.4034. [DOI] [PubMed] [Google Scholar]
- 24.van Zutphen T., Todde V., de Boer R., Kreim M., Hofbauer H.F., Wolinski H., Veenhuis M., van der Klei I.J., Kohlwein S.D. Lipid droplet autophagy in the yeast Saccharomyces cerevisiae. MBoC. 2013;25:290–301. doi: 10.1091/mbc.E13-08-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y.-X., Catlett N.L., Weisman L.S. Vac8p, a vacuolar protein with armadillo repeats, functions in both vacuole inheritance and protein targeting from the cytoplasm to vacuole. J Cell Biol. 1998;140:1063–1074. doi: 10.1083/jcb.140.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott S.V., Nice D.C., Nau J.J., Weisman L.S., Kamada Y., Keizer-Gunnink I., Funakoshi T., Veenhuis M., Ohsumi Y., Klionsky D.J. Apg13p and Vac8p are part of a complex of phosphoproteins that are required for cytoplasm to vacuole targeting. J Biol Chem. 2000;275:25840–25849. doi: 10.1074/jbc.M002813200. [DOI] [PubMed] [Google Scholar]
- 27.Valverde D.P., Yu S., Boggavarapu V., Kumar N., Lees J.A., Walz T., Reinisch K.M., Melia T.J. ATG2 transports lipids to promote autophagosome biogenesis. J Cell Biol. 2019;218:1787–1798. doi: 10.1083/jcb.201811139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chowdhury S., Otomo C., Leitner A., Ohashi K., Aebersold R., Lander G.C., Otomo T. Insights into autophagosome biogenesis from structural and biochemical analyses of the ATG2A-WIPI4 complex. Proc Natl Acad Sci U S A. 2018;115:E9792–E9801. doi: 10.1073/pnas.1811874115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Kotani T., Kirisako H., Koizumi M., Ohsumi Y., Nakatogawa H. The Atg2-Atg18 complex tethers pre-autophagosomal membranes to the endoplasmic reticulum for autophagosome formation. Proc Natl Acad Sci Unit States Am. 2018;115:10363–10368. doi: 10.1073/pnas.1806727115. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work presents a biochemical analysis of various Atg2 truncation mutants and demonstrates that two different lipid binding regions of Atg2 mediate the interaction of Atg2 with the phagophore and the ER membrane, thereby providing insight into how phagophore–ER contact sites are formed.
- 30••.Gómez-Sánchez R., Rose J., Guimarães R., Mari M., Papinski D., Rieter E., Geerts W.J., Hardenberg R., Kraft C., Ungermann C. Atg9 establishes Atg2-dependent contact sites between the endoplasmic reticulum and phagophores. J Cell Biol. 2018;217:2743–2763. doi: 10.1083/jcb.201710116. [DOI] [PMC free article] [PubMed] [Google Scholar]; Phagophore–ER contact sites have been demonstrated to be essential for the formation of autophagosomes, but it remained unclear how this connection is established. This work demonstrates that Atg2 plays a key role in connecting the phagophore with the ER membrane. Furthermore, interaction of Atg2 with Atg9 restricts the localization of Atg2 on the phagophore, which is required for the formation of closed autophagosomes.
- 31.Mari M., Griffith J., Rieter E., Krishnappa L., Klionsky D.J., Reggiori F. An Atg9-containing compartment that functions in the early steps of autophagosome biogenesis. J Cell Biol. 2010;190:1005–1022. doi: 10.1083/jcb.200912089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto H., Kakuta S., Watanabe T.M., Kitamura A., Sekito T., Kondo-Kakuta C., Ichikawa R., Kinjo M., Ohsumi Y. Atg9 vesicles are an important membrane source during early steps of autophagosome formation. J Cell Biol. 2012;198:219–233. doi: 10.1083/jcb.201202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orsi A., Razi M., Dooley H.C., Robinson D., Weston A.E., Collinson L.M., Tooze S.A. Dynamic and transient interactions of Atg9 with autophagosomes, but not membrane integration, are required for autophagy. MBoC. 2012;23:1860–1873. doi: 10.1091/mbc.E11-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Judith D., Jefferies H.B.J., Boeing S., Frith D., Snijders A.P., Tooze S.A. ATG9A shapes the forming autophagosome through Arfaptin 2 and phosphatidylinositol 4-kinase IIIβ. J Cell Biol. 2019;218:1634–1652. doi: 10.1083/jcb.201901115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlsson S.R., Simonsen A. Membrane dynamics in autophagosome biogenesis. J Cell Sci. 2015;128:193–205. doi: 10.1242/jcs.141036. [DOI] [PubMed] [Google Scholar]
- 36.Ge L., Zhang M., Kenny S.J., Liu D., Maeda M., Saito K., Mathur A., Xu K., Schekman R. Remodeling of ER-exit sites initiates a membrane supply pathway for autophagosome biogenesis. EMBO Rep. 2017;18:1586–1603. doi: 10.15252/embr.201744559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeong Y.-T., Simoneschi D., Keegan S., Melville D., Adler N.S., Saraf A., Florens L., Washburn M.P., Cavasotto C.N., Fenyö D. The ULK1-FBXW5-SEC23B nexus controls autophagy. eLife. 2018;7 doi: 10.7554/eLife.42253. e42253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shima T., Kirisako H., Nakatogawa H. COPII vesicles contribute to autophagosomal membranes. J Cell Biol. 2019;218:1503–1510. doi: 10.1083/jcb.201809032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan D., Cai Y., Wang J., Zhang J., Menon S., Chou H.-T., Ferro-Novick S., Reinisch K.M., Walz T. The EM structure of the TRAPPIII complex leads to the identification of a requirement for COPII vesicles on the macroautophagy pathway. Proc Natl Acad Sci Unit States Am. 2013;110:19432–19437. doi: 10.1073/pnas.1316356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maeda S., Otomo C., Otomo T. The autophagic membrane tether ATG2A transfers lipids between membranes. eLife. 2019;8 doi: 10.7554/eLife.45777. e45777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Osawa T., Kotani T., Kawaoka T., Hirata E., Suzuki K., Nakatogawa H., Ohsumi Y., Noda N.N. Atg2 mediates direct lipid transfer between membranes for autophagosome formation. Nat Struct Mol Biol. 2019;26:281. doi: 10.1038/s41594-019-0203-4. [DOI] [PubMed] [Google Scholar]; In this study, Osawa et al. report the crystal structure of the N-terminal region of Schizosaccharomyces pombe Atg2 and identify an open hydrophobic cavity that is able to accommodate phospholipids. They further demonstrate that S. cerevisiae Atg2 possesses lipid transfer activity in vitro, which depends on its ability to tether liposomes. These findings provide insights into the molecular function of Atg2 at the phagophore–ER contact sites. Lipid transfer activity was also demonstrated for mammalian ATG2A [27,40].
- 42.Xie Z., Nair U., Klionsky D.J. Atg8 controls phagophore expansion during autophagosome formation. MBoC. 2008;19:3290–3298. doi: 10.1091/mbc.E07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Itakura E., Mizushima N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian Atg proteins. Autophagy. 2010;6:764–776. doi: 10.4161/auto.6.6.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King J.S. Autophagy across the eukaryotes. Autophagy. 2012;8:1159–1162. doi: 10.4161/auto.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]