Abstract
Three-dimensional (3D) bioprinting has demonstrated great potential for the fabrication of biomimetic human tissues and complex graft materials. This technology utilizes bioinks composed of cellular elements placed within a biomaterial. Mesenchymal stromal cells (MSCs) are an attractive option for cell selection in 3D bioprinting. MSCs can be isolated from a variety of tissues, can pose vast proliferative capacity and can differentiate to multiple committed cell types. Despite their promising properties, the use of MSCs has been associated with several drawbacks. These concerns are related to the ex vivo manipulation throughout the process of 3D bioprinting. The herein manuscript aims to present the current evidence surrounding these events and propose ways to minimize the risks to the patients following widespread expansion of 3D bioprinting in the medical field.
Keywords: mesenchymal stromal cells, ex vivo expansion, bioprinting, additive manufacturing, 3D bioprinting
Introduction
With an increasing aging population the need to regenerate diseased tissues or replace tissues and organs lost due to trauma or surgery is increasing (Colwill et al., 2008; International Population Reports, 2016). There is already a lack of supply of sufficient organ donations and tissue grafts which is likely to worsen in the future (Yanagi et al., 2017; American Transplant Foundation, 2018). Tissue engineering that was introduced in the last few decades generally employs the seeding of scaffolds with cells (Langer and Vacanti, 1993). This process is associated with inhomogeneous distribution of cells within the scaffold, which can also affect subsequent engineered construct survival, integration and function (Gao et al., 2014). It was previously hypothesized that inhomogeneous seeding could prevent some cells from nutrients and oxygen resulting in poor function (Melchels et al., 2010).
The recent advent of three-dimensional (3D) bioprinting has brought about new possibilities to advance tissue engineering and regenerative medicine. Three-dimensional bioprinting involves the use of cells that are mixed with a carrier material while in liquid form with subsequent solidification of such material by using one of a number of cross-linking techniques. This mixture, known as bioink may also include growth factors (Ashammakhi et al., 2019a, b) or other additives such as osteoconductive materials (Byambaa et al., 2017; Ashammakhi et al., 2019c). Three-dimensional bioprinting techniques and bioinks have evolved tremendously over the last two decades, to address the need to create complex biomimetic tissue constructs (Mandrycky et al., 2016; Figure 1).
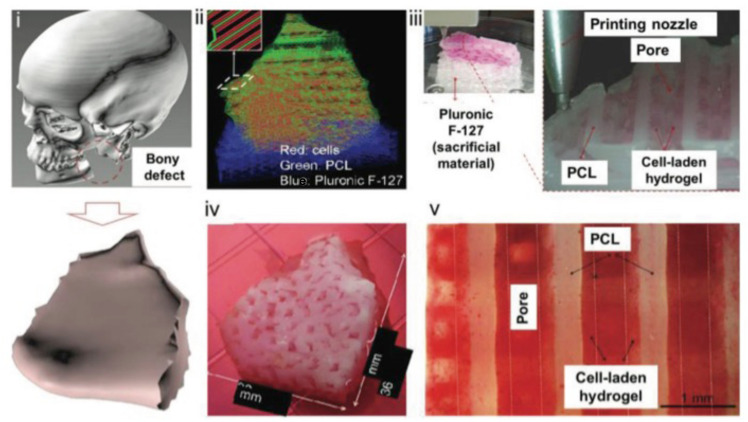
FIGURE 1.
The pathway of creating complex 3D printed structures. (i) Modeling of a mandibular defect with the use of patients CT scans. (ii) Construction of 3D architecture. (iii) 3D printing process. (iv) Culture of the graft. (v) Differentiation of the cells to osteoblasts. Reproduced with permission from Kang et al. (2016).
Cells used in bioinks have represented one of the major challenges faced by tissue engineers because of their limited availability (Freimark et al., 2010), proliferation (Willerth and Sakiyama-Elbert, 2008), and differentiation potential (Tuszynski et al., 2014). While already differentiated cells could be ideal, their harvest can cause donor site morbidity while often perform poorly with ex vivo manipulation. Alternative cell sources of cells include embryonic or reprogrammed cells. These cell types are associated with many challenges (Bongso et al., 2008; Trounson and McDonald, 2015) and concerns. The biggest concern shared by physicians and other care providers, regulatory bodies and industry as a whole is the safety of stem cell therapeutics for use in patients (Goldring et al., 2011). Mesenchymal stem cells on the other hand, have gained popularity and represent a cell type of choice for many experimental and clinical studies in tissue engineering.
MSCs in 3D Bioprinting
Mesenchymal stromal cells (MSCs) represent one of the most popular types of cells used in tissue engineering today. In fact, their clinical use is so strong today that are used in more than 700 clinical trials listed on US clinical trials. This is because MSCs have potential to differentiate into a wide variety of cell types (Sasaki et al., 2008) but also due of their wide availability from different sources such as the bone marrow (Gnecchi and Melo, 2009), adipose tissue (Katz et al., 2005), blood vessels (Kuznetsov et al., 2001), muscle (Young et al., 1995) as well as rather “embryonic” tissues such as amniotic fluid (Tsai et al., 2004) and cord blood (Bieback et al., 2004). MSCs actively participate in the regeneration of tissues and provide substitute cells for those that expire (Pintus et al., 2018). Following injury MSCs mobilize to distant sites and either provide reparative cells and/or secrete trophic factors to promote healing. In addition, MSCs pose anti-inflammatory and immunomodulatory capacity as can improve inflammation and restore or inhibit the functions of immune cells (Pintus et al., 2018). MSCs can be easily expanded ex vivo to provide clinically relevant numbers prior to use. Although their exact function is not fully elucidated, MSCs have been used widely in tissue engineering instead of pluripotent stem cells (embryonic or induced pluripotent stem cells) which possess their own concerns and more complex processing techniques (Porada et al., 2006).
In 3D bioprinting, MSCs remains a popular cell type for the use in bioink. Their use is not limited to bone (Ong et al., 2018), cartilage (Bae et al., 2018), and adipose tissue (Qi et al., 2018) but MSCs are considered and used in many other 3D bioprinting applications. In fact, in addition to bone and cartilage, MSCs were used in 3D bioprinting of muscle (Phillippi et al., 2008), aortic valve (Kang et al., 2017), cardiovascular tissue (Ryu et al., 2015), neural tissues (Jakab et al., 2010), tendons and ligaments (Rak Kwon et al., 2020), and others (Tasnim et al., 2018). Thus, the objective of this review is to examine the literature on 3D bioprinting that utilized MSCs and examine accumulated data pertaining to the safety of MSCs in 3D bioprinting in various pre-, intra-, and post-printing stages. Discussion of findings is included, challenges highlighted, and future directions are outlined.
Pre-Printing
The generation of reliable MSC-based 3D bioprinting products requires first an in-depth understanding of the MSC physiology. MSC physiology is complex and it is influenced by the local microenvironment. For example, some researchers have shown that MSCs have tumor-suppressing properties (Khakoo et al., 2006; Cousin et al., 2009; Ho et al., 2013). On the contrary, MSCs can also favor tumor progression by promoting tumor angiogenesis, maturation of tumor vasculature and expansion through the secretion of a wide range of bioactive biomolecules (Kucerova et al., 2010; Suzuki et al., 2011; Huang et al., 2013). The reason for such dual roles is largely obscure. Together with MSC physiology, the target tissue micro-architectural topography, physiology, mechanical properties have to be elucidated. This will dictate the porosity, stiffness, orientation of the scaffold components and depict the exact location of the cellular components (Daly et al., 2017).
In addition to robust understanding of MSC physiology, further work on developing methodologies that safeguard high viability and ensure safety of grafts is needed. Literature suggests that the success of potential application of MSCs is closely related to the number of MSCs (Hernigou et al., 2005a, b). The expansion of the cells raises several concerns involving the extent of the expansion (expansion induces deprivation of MSCs properties), the effect of culture conditions, culture media and tissue culture plastics on the cells as well as the effect of cryopreservation on MSCs (Sotiropoulou et al., 2006; Pountos et al., 2007). The need for supplementation of the culture media with cytokines and chemokines in high non-physiologic concentrations is unknown whether it can affect their long-term properties. Worrying reports are available suggesting, that ex vivo expansion of MSCs can induce spontaneous malignant transformation into cells with tumorigenic potential (Rubio et al., 2005). Even more disturbing are the reports of occasional sarcoma formation in patients receiving bone marrow treatment and those undergone autologous fat graft (Perrot et al., 2010).
Printing Process
Characteristics of 3D Bioprinting Methods in Brief
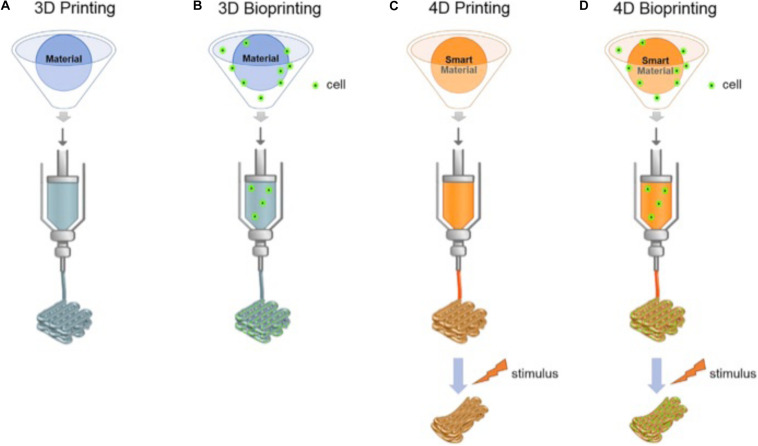
There are several 3D printing techniques among which the most commonly used for 3D bioprinting are extrusion, laser-based (Catros et al., 2011), inkjet (Cui et al., 2010), stereolithography (Wang et al., 2015) and electrospinning-based printing (Khalil and Sun, 2009; Wüst et al., 2011; Dababneh and Ozbolat, 2014; Figure 2). The same technologies can be used to create smart 3D-bioprinted structures able to respond to the environment; commonly referred to as four-dimensional (4D) bioprinting (Figure 3). Extrusion 3D bioprinting or pressure-assisted bioprinting uses hydrogel bioinks extruded from a syringe in a continuous trace through a fine nozzle (Maher et al., 2009; Bhuthalingam et al., 2015; Irvine et al., 2015). In most extrusion bioprinters, the nozzle can move on y-z axes with the substrate collector plate moving in the x-axis to produce the final structure (Maher et al., 2009; Bhuthalingam et al., 2015; Irvine et al., 2015). Extrusion bioprinting delivers good homogeneity of bioinks, can deliver very high cell densities and does not require any specific environmental conditions (can be carried out at room temperature) (Atala and Yoo, 2015; Bishop et al., 2017). The overall resolution is rather poor compared to other techniques (minimum feature size is generally over 100 μm) (Leberfinger et al., 2017). Despite this, the technique has been used to create complex structures but MSCs survival was as low as 40% due to apoptosis and cell deformation.
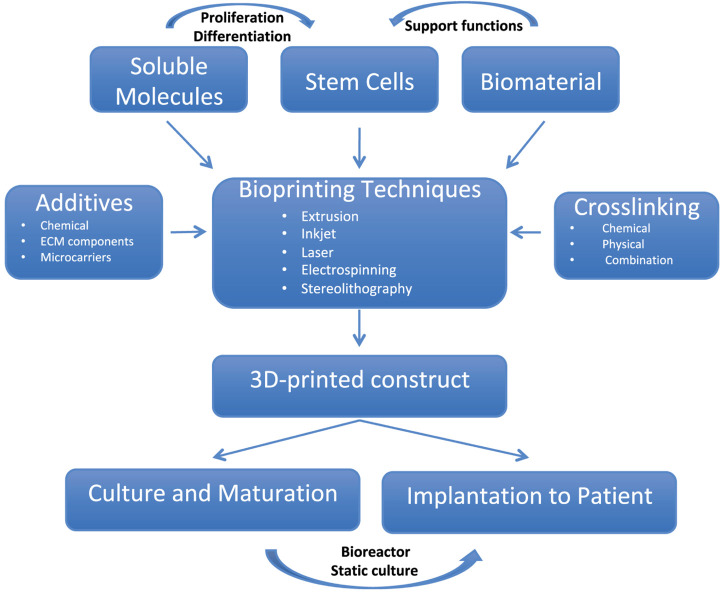
FIGURE 2.
The pathways and components of 3D bioprinting process.
FIGURE 3.
The different printing technologies (3D, 3D bioprinting, 4D, and 4D bioprinting). (A,B) shows conventional 3D printing and bioprinting techniques. (C,D) For-dimensional bioprinting is defined as 3D printing of cell-laden materials in which the printed structures would be able to respond to external stimulus due to stimuli-responsive bioinks or internal cell forces. Reproduced with permission from Ashammakhi et al. (2018).
Laser bioprinting uses a pulsed nanosecond or ultraviolet (UV) like wavelength laser as a source of energy to stimulate the upper surface of an energy absorbing metal film, which is usually made of a layer of titanium or gold (Catros et al., 2011). This metal film is coated with bioink on its lower surface and acts as a donor film. Stimulation of the upper surface of the metal film causes vaporization, creating a pressure bubble that drives the bioink from the donor film onto a substrate plate containing a biopolymer (Stolberg and McCloskey, 2009; Jana and Lerman, 2015; Irvine and Venkatraman, 2016; Li et al., 2016). The biopolymer functions to aid in sustaining growth and cellular adhesion of the cells after transfer from the donor film (Catros et al., 2011; Trombetta et al., 2017). The precise resolution is influenced by a number of factors including the energy emitted by the laser, printing speed, viscosity and thickness of the bioink layer on the donor film and its rheological properties, shape and organization of the structure and substrate wettability (Guillemot et al., 2010a, b; Li et al., 2016). Despite that, this is a scaffold-free technique reaching resolutions between 10 and 50 μm. Some studies managed to achieve a resolution of a single cell per droplet. This method negates the shearing stress experienced by cells during deposition down a narrow print head or nozzle (Murphy and Atala, 2014; Mandrycky et al., 2016; Keriquel et al., 2017). The potential of laser bioprinting has been demonstrated in a number of studies (Barron et al., 2004; Guillemot et al., 2010a, b).
Inkjet bioprinting arose from the adaptation of conventional desktop inkjet printers. It is a noncontact printing process where a droplet of bioink is deposited through the print head on demand, under the control of a thermal or piezoelectric actuator. This type of multi-cell printing is known as drop on demand (Irvine and Venkatraman, 2016). The resolution is in the region of up to 50 μm (Mandrycky et al., 2016). Thermal actuation is the more commonly used method for inkjet bioprinting where droplets of bioink are generated by an electric current. The thermal actuator element reaches temperatures in excess of up to 300°C, allowing a vapor bubble to generate sufficient pulse pressure to expel the bioink from the print head (Cui et al., 2010). This could potentially impart both shear and thermal stress on the cells (Irvine and Venkatraman, 2016; Li et al., 2016). The requirement to use low viscosity inks to prevent blockage of the print-head prevents the use of a number of efficacious bioinks. In contrast to the thermal, the piezoelectric actuation produces a transient pressure to eject the droplets on to the substrate. It produces more homogenous droplets than thermal actuation, but some authors reported greater levels of cell damage (Seetharam, 1991; Nakamura et al., 2005; Saunders et al., 2007).
Stereolithography is another 3D bioprinting technique that can be used to generate 3D constructs. This technique involves the solidification of a cell-laden photo-crosslinkable polymer solution in a layer-by-layer fashion, and it is controlled by a moveable stage along the z-axis (Murphy and Atala, 2014). In stereolithography, 3D complex structures can be produced without the need for a printhead that moves in x–y direction. In this process, a digital micromirror device (DMD) which allows highly precise patterns to be created, is used to control selectively crosslinking of bioink in z direction (Heinrich et al., 2019). This selective crosslinking method by light does not lead to any cell shear stress, making it possible to achieve higher cell viability in produced constructs. However, the use of transparent bioinks is required in stereolithography in order to achieve uniform crosslinking. This restricts the cell density that can be used in the bioink (Minteer et al., 2013). Despite this limitation, the technique has a great potential because of high speed, high resolution (∼1 μm) and controllability of the internal and external architecture of the resulting construct (Gruene et al., 2011a, b; Kang et al., 2017).
Electrospinning is a high-resolution fabrication method that can be used to produce thin fibers (Heinrich et al., 2019). During the process of electrospinning, a high voltage is applied to the ejected polymeric solution from the syringe. When the electrostatic repulsion starts to overcome the surface tension of the solution, the solution begins to evaporate and it is subsequently solidified during transit to form fibers (Ashammakhi et al., 2008; Bhardwaj and Kundu, 2010). Thin fiber-based constructs can be produced by this technique. Recently, this process technique has been modified for bioprinting by adding cells and controlling the process of fiber arrangement in the resulting structure. One of the primary features of electrospinning-based bioprinting (EBB) is the shorter collecting range of fibers (around 0.5–3 mm) in comparison to traditional electrospinning. This allows for more controllable deposition of electrospun materials with less applied voltage than usually used in conventional electrospinning (Heinrich et al., 2019). Visser et al., 2015; have recently used electrospinning-based bioprinting technology to enhance GelMA hydrogel mechanical strength by reinforcing high-porosity poly(ε-caprolactone) (Visser et al., 2015). The rigidity of GelMA hydrogels increased 30 times by 7–214 kPa while its elastic properties were preserved (Visser et al., 2015). However, the main restrictions of EBB are the fast spinning of fibers, resulting in a spatially unstable 3D structures and the high processing temperature and voltage, which is challenging to cells contained in the electrospun material.
Cell Death During 3D Bioprinting
The viability of the cells can be influenced by a number of factors. These include the storage of the cells in the printer, the thermal damage during the printing process and the mechanical forces exerted during bioprinting. Table 1 shows documented survival rates following 3D bioprinting.
TABLE 1.
Studies presenting the survival rates of cells used as bioink for 3D-bioprinting applications.
| Author, year | 3D Printer | Cell types | Survival rates | Comments/Other findings |
| Inkjet bioprinting | ||||
| Christensen et al. (2015) | Thermal inkjet printing | Chinese hamster ovary cells and primary embryonic motor neurons from ventral cords of 14-day embryos from pregnant Sprague-Dawley rats | Greater than 90% cellular viability after printing. | |
| Saunders et al. (2007) | Piezoelectric drop-on-demand inkjet printing | HT 1080 human fibroblasts | Cellular survival of 94–98%. | Survival rates decrease with increased printing pulse amplitude. Sampled printed at 40v demonstrated survival rates that could not be distinguished from unprinted control samples. |
| Cui et al. (2010) | Thermal inkjet printing | Green fluorescent protein expressing Chinese hamster ovary cells | Average cellular viability was 89%. | No significant difference in viability was observed in different cellular concentrations of ink. Printed cell number correlated with increasing cellular ink concentrations. |
| Christensen et al. (2015) | Inkjet based free form fabrication | NIH 3T3 mouse fibroblasts | Post printed cellular viability was 92.4% immediately after printing and 90.8% after 24 h of incubation. | |
| Levato et al. (2014) | Bioscaffolder system (Levato et al., 2014) | Mesenchymal stem cells from 2 to 4 weeks old Lewis rats | Post dispensing viability was 80% after 1 day and more than 90% after 3 days. | Pre-seeded particles suspended in the gels had the lowest number of viable cells (60%) after 1 day of culture, which increased to 90% after 3 days. |
| Du et al. (2015) | Inkjet with four independent z-axis-controlled ink reservoirs | Bone mesenchymal stem cells from 4-weeks-old male adult Sprague-Dawley rats | Cellular viability of > 90% was seen during printing | CBD-BMP2-collagen microfibers induced BMSC differentiation into osteocytes within 14 days more efficiently than the osteogenic medium. |
| Extrusion bioprinting | ||||
| Zhao et al. (2014) | Microextrusion printing | HeLa cells | Post printed viability of the HeLa cells in constructs was 94.9% ± 2.2% with parameters of 10 mm3 min–1 extrusion speed, 250 μm nozzle inner diameter, 10°C chamber temperature and 25°C nozzle temperature. | Comparisons of 3D and 2D tumor models of HeLa cells show a higher cellular proliferation rate and more simulated tumor characteristics with 3D printing |
| Zhao et al., 2015 | Four nozzle microextrusion printing | A549 cells | Cell survival rate was > 90% for all rheological conditions at a holding temperature of 20° | For all concentrations of bioink used in microextrusion printing, a holding temperature of 20° should be used. Optimum holding times were variable, dependent upon bioink concentration |
| Laser assisted bioprinting | ||||
| Barron et al. (2005) | BioLPTM Biological Laser Printing | Human osteosarcoma cells | After six days of incubation, cells demonstrated a 100% viability | |
| Koch et al. (2010) | Laser based printing based on laser assisted forward transfer (LIFT) | Skin cell lines (fibroblasts, keratinocytes); Human mesenchymal stem cells | 98% ± 1% standard error of the mean (skin cells) and 90% ± 10% (hMSC). | No increase in apoptosis or DNA fragmentation was seen with the use of LIFT. hMSC phenotype was maintained as proven by fluorescence activated cell sorting analysis. |
| Hopp et al. (2012) | Femtosecond KrF laser in laser assisted forward transfer (LIFT) | Human neuroblastoma, chronic myeloid leukemia and osteogenic sarcoma cell lines and primary astroglial rat cells | Short-term and long-term survival for neuroblastoma and astroglial cells was 65–70%. Long term survival of osteosarcoma cells was low, while myeloid leukemia cells did not tolerate the procedure under the conditions. | |
| Stereolithography bioprinting | ||||
| Arcaute et al. (2006) | Stereolithography bioprinting | Human dermal fibroblasts | Cell viability was at least 87% at 2 and 24 h following fabrication. | |
| Raman et al. (2016) | High-resolution projection stereolithography bioprinting | fibroblasts (3T3), myoblasts (C2C12), endothelial (C166), and bone marrow stromal (D1) cells | Cells encapsulated in the lower molecular weight polymer demonstrate a viability of 70% ± 10%, whereas cells encapsulated in the higher molecular weight polymer demonstrate a viability of 93% ± 3% on day 1 after printing for 3T3 cells. In the long term (2 weeks) cell viability in low molecular weight does not significantly change, but cell viability in high molecular weight significantly increases. | |
| Electrospinning-based bioprinting | ||||
| Visser et al. (2015) | Electrospinning-based bioprinting | Chondrocytes | Chondrocytes maintained high cell viability (∼80%) on days 1 and 7. | |
Cell storage and conditions during the printing process can potentially affect cell viability. During this process the cells are required to be stable and in media that could allow them to recover from the effects of cells-detaching solution (i.e., Trypsin, TrypLE, collagenase or others) and the stress exerted on them during the detachment process (i.e., centrifugation, washing, etc.). It is known that these methods can affect cell survival, phenotype and differentiation potential (Parvin et al., 2012; Tsuji et al., 2017). In addition, the effect of prolonged bioprinting protocols would require stable media and stable cell conditions. At present, there are limited studies in this field.
Thermal injury to cells is another area of concern. For example, during inkjet printing, where temperatures exceed 200 °, studies have shown that the bioink temperatures are raised by just 4–10° (Cui et al., 2010) and this does not significantly adversely affect the viability of mammalian cells (Suzuki et al., 2011). This heating effect is thought to be temporary (∼5 μs), with less than 8% of the cells being lysed during bioprinting (Cui et al., 2010). Similar results were reported for the heat shock of the laser pulse where the cell survival, proliferation and differentiation were comparable to those of controls at 5 days in cell culture (Gruene et al., 2011b).
In addition to the potential thermal damage, the mechanical stress should be also taken into account. Cells are known to respond to mechanical stress by changing their gene expression and cell function. Among many cells’ adaptation mechanisms activated, MSCs activates several intracellular signaling cascades, including kinases (PKB, MAPK, FAK), β-catenin, GTPases (Thompson et al., 2012). Chang et al. (2008), found that cellular viability is inversely related to extrusion pressure, with as little as 40% viability found at the extremes of high pressure. Mechanical pressure observed in inkjet printing has been demonstrated to promote the differentiation of MSCs toward bone and cartilage lineages (Shav and Einav, 2010). In contrast, the shear stress produced in extrusion techniques promotes differentiation toward both endothelial and bone tissues (Stolberg and McCloskey, 2009). The choice of the 3D technology is mostly done on the basis of required resolution and the target tissue as well as other factors. Lee et al. (2015) suggested that laser assisted and inkjet bioprinting may be preferable to extrusion bioprinting in most circumstances, but where circumstances necessitate the use of bioink with a high viscosity, extrusion bioprinting may be necessary. In these circumstances, the effects of sheer stress may be countered by modification of the bioink composition, e.g., by the inclusion of thinning polymers and the control of back pressure during the printing process (Mackay et al., 1998).
Bioink Characteristics and Cellular Adhesion
The primary aim in preparing a bioink is the biomimicry of the extracellular matrix, which creates a microenvironment that is optimal for cellular adhesion, proliferation and differentiation. An ideal bioink will maintain its printed structure integrity, be crosslinkable and can undergo degradation. It must accommodate cells, and sustain their integrity and viability throughout the printing process (Irvine and Venkatraman, 2016; Grungor-Ozkerim et al., 2018). It should also have the specific mechanical, physicochemical, rheological and biological properties needed for printability and for the preservation of cellular phenotype (Byambaa et al., 2017). Skardal and Atala (2015) highlighted that most biocompatible bioinks which were able to bear the vertical weight of emerging structures either produced toxic macromolecules during the setting process or required a toxic solvent for setting itself.
Porosity and interconnectivity are also two essential factors. Pore size, shape and volume are all influential in the behavior of cells following adhesion to the scaffold structure. Matsiko et al. (2015), found that pore size correlates with cellular organization, mineralization and the development and assembly of collagen I. Greater porosity and more interconnectivity allow for better matrix deposition and transportation of oxygen and other essential substrates into the center of the scaffold, promoting better ingrowth of tissue. Domingos et al. (2013), concluded that the morphology of printed cells did not appear to be influenced by the topology of pores, but that cell viability and proliferation were strongly affected by the size and shape of the pores, with large quadrangular pores resulting in the best viability and proliferation of human MSCs.
Scaffold stiffness has also been noted to play an integral role in the terminal differentiation of cells. MSCs have been observed to differentiate into cell types that best fit the microenvironment supported by the mechanical properties of the attachment surface or matrix. Differentiation toward an osteogenic lineage is observed in cells adhering to a rigid surface (34 kPa), compared with a more elastic surface (0.1–1 kPa), where MSCs display a tendency to differentiate toward a neuronal lineage (Engler et al., 2006; Lane et al., 2014). In relatively soft hydrogels (2.5–5 kPa), a differentiation toward adipogenesis is observed (Arany et al., 2010). This offers the possibility for the modification of bioink matrices and scaffolds to induce a specific lineage differentiation. Gao et al. (2015), produced a bioink that was optimized for bone and cartilage regeneration. The ink, made from a hybrid of polyethylene glycol and gel dimethylacrylate, had a compressive modulus of 1–2 MPa when printed, significantly stiffer than previously used hydrogels. MSCs printed in this hydrogel demonstrated a greater propensity toward osteocyte and chondrocyte lineage (Gao et al., 2015), but only in the context of specific extracellular matrix (Rowlands et al., 2008) and cross-linking conditions (Das et al., 2015).
It has been previously suggested that a scaffold can guide MSCs toward a specific lineage. In cases where the aim is to maintain stemness, bioinert hydrogels should be used. This avoids creating an environment that may be favorable to one particular lineage of cells. One such example of a bioinert hydrogel is alginate (Irvine and Venkatraman, 2016) which retains the stemness of printed stem cells (Blaeser et al., 2016). However, caution must be exercised when using bioinert hydrogels, as proliferative capabilities and movement are reduced, which may promote anoikis (Carrow et al., 2015), however, this may be overcome by the addition of the integrin binding peptide arginyl-glycyl-aspartic acid (RGD) moieties to bioinert alginates which increases cellular interaction whilst maintaining stemness (Carrow et al., 2015). Hyaluronic acid is an alternative to alginate, with proven clinical efficacy (Ozbolat and Hospodiuk, 2015). In contrast to alginate, hyaluronic acid promotes MSC attachment and maintains multipotency and proliferation through CD44 receptors (Cao et al., 2016), with the added benefit of adaptation to promote a specific lineage differentiation. One such example is the use of hyaluronic acid in cardiogenesis (Mairim et al., 2012). Where bioinert inks have been used, MSCs can be differentiated by incubation with soluble factors that direct maturation to a specific lineage in a similar fashion to culture additives (Irvine and Venkatraman, 2016). To remove reliance on extrinsic factors, additives can be included in bioink. For example, alginate bioinks have been modified with the addition of hydroxyapatite in the context of bone regeneration (Wüst et al., 2014). In vivo murine models of alginate scaffolds containing biphasic calcium phosphate particles (consisting of hydroxyapatite and β-tricalcium phosphate) displayed greater osteogenic differentiation than scaffolds having no biphasic calcium triphosphate (Wang et al., 2007).
The Effect of Cross-Linking
Three-dimensionally bioprinted extracellular matrix may lack the required stability and integrity to support contained cells. Crosslinking is often an essential step and a number of physical, biological and chemical crosslinking techniques have been proposed over the years. The aim of these techniques is to enhance the mechanical and biological properties of the grafts preventing the cell-mediated contraction. Crosslinking induces chemical or physical links between the polymer chains of the scaffold and can be achieved by using UV light, dehydrothermal treatment, or treatment with sodium citrate, sodium tripolyphosphate, sulfosuccinic acid, oxalic acid, glutaraldehyde, genipin, or carbodiimide (Lew et al., 2007; Pfeiffer et al., 2008; Jóźwiak et al., 2017; Vining et al., 2019).
Crosslinking can affect several of the cellular functions, including proliferation, differentiation and cellular ability to attach to a scaffold (Davidenko et al., 2015). Kim et al., investigated the effect of different crosslinking techniques on immortalized human corneal epithelial cells, human skin fibroblasts, primary bovine corneal endothelial cells and immortalized human retinal pigment epithelial cells (Kim et al., 2014). The authors reported different toxicity levels with the least toxic being with mononitroalcohols and glyceraldehide, intermediate toxicity being with nitrodiol and nitrotriol, and highest toxicity being with glutaraldehyde, paraformaldehyde, genipin, and bronopol. Several studies have also defined the critical concentration over which the agent induces cytotoxic effect (Wang and Stegemann, 2011; Muzzarelli et al., 2015). On the contrary, some studies suggest that crosslinking can have a positive effect on cellular function. Raucci et al. (2015), studies the effect of citric acid crosslinked cellulose containing hydrogel on the osteogenic differentiation of MSCs. The authors revealed enhanced hydrophilicity and roughness of the hydrogel together with a stimulation of osteogenic differentiation as demonstrated by enhanced expression of bone markers such as osteopontin and osteocalcin. In addition to the direct effect of the crosslinking on MSCs, the physical properties of the extracellular matrix can regulate the response and phenotypes of the cells (Kyle et al., 2019).
Despite many promising studies, to date, there is no gold standard method for cross-linking 3D printed biomimetic materials. In cases where multiple bioinks are used, tuning the scaffold microstructure through crosslinking of multiple biomaterials without affecting its properties will require significant improvement in our 3D printing technology. In tissues where biodegradation or regeneration is required, like for example in 3D bioprinting of bone, the mechanical properties of scaffolds are negatively correlated with their biodegradation profile (Oryan et al., 2018). Finally, one major concern is the potential inflammatory reaction following implantation. It is shown that the cross-linking methods can induce an immune reaction, initiate M1 macrophage response and inhibition of M2 macrophage polarization, reduced cell infiltration, increased proinflammatory cytokine expression and peri-implantation fibrosis (Delgado et al., 2015), which should be carefully considered and solutions devised.
Post-Printing
Following 3D printing, cell-laden scaffolds will require incubation prior to implantation. This raises the question of how the nutrients and wastes will be exchanged to support the cells until implantation. For a thin construct, this can be done through a static culture through diffusion; however, functioning vasculature will be required for larger constructs. Dynamic culturing can provide continuous infiltrating flow of medium and/or compressive/tensile loading, which is most beneficial for cartilage and bone tissue engineering (Butler et al., 2009). In case the technology reaches the stage of creating vasculature (Shahabipour et al., 2020), research would be needed to determine if blood would be an adequate medium to facilitate nutrients and waste exchange.
In addition to the nutrient supply, cells will require time to attach onto the scaffold. It has been previously shown that post-fabrication incubation for long periods can increase the mechanical strength of the construct due the function of the cells and further tissue development (Butler et al., 2009). If photopolymerization is used to harden the bioink, it is unknown whether it can cause cytotoxicity due to the photoinitiators and ultraviolet light. Visible light-sensitive photoinitiators are reported to cause less cytotoxicity but this area is poorly explored (Lim et al., 2016; Mondschein et al., 2017).
Future Directions and Conclusion
Three-dimensional bioprinting technology has achieved growing popularity for its favorable potential. There is impressive progress with the pertinent techniques supporting the view that in the near future organ manufacturing will be a reality. Three-dimensional bioprinting can find application in organ and graft transplantation by overcoming the issues of immune rejection and reducing the cost of grafts and could be used to establish platforms for research and drug screening.
MSCs are one of the most popular cell type in tissue engineering and are involved in more than half of the clinical trials since 2000 (Yuan et al., 2019). These cells are most likely to be the main component of 3D bioprinting. In order to preserve and deliver MSCs advantages, it is essential to mimic there in vivo microenvironment throughout the 3D biofabrication process (Baker and Chen, 2012). In addition, the availability of nutrients and oxygen remains high and similar to that in the body (Melchels et al., 2010; Ashammakhi et al., 2020). This seems to be the only way for the cells retain their phenotype, adhesion, metabolism, and response signaling (Baker and Chen, 2012).
Despite the great progress we have seen in understanding the biology of target tissues in humans, our knowledge is still based on animal biology. Understanding MSC biology is also crucial and it is in fact the most difficult challenge. This will allow us to direct the efforts creating more physiologically relevant structures. MSCs for example could be used in high densities when creating biomimetic cartilage and bone tissues or in lower densities as supporting cells in other applications. Before, however, we are in a position to discuss such matters we would have to decode our biology in health and disease in humans raises significant ethical issues. Once 3D bioprinting reaches a position of manufacturing complex biomimetic tissues, such as organs and large grafts, an appropriate regulatory framework will be required. Hints that this is imminent are shown in many studies which produced complex grafts. Ethical issues include the ownership of prototypes, the harvesting and type of cells and biomaterials, research as well as commercialization of produced constructs. Regulation in terms of safety is also needed including the biocompatibility of bioinks, long-term safety of grafts and the ex vivo manipulation of cells.
The optimal ex vivo conditions prior to printing should be established. In our view, minimizing the ex vivo journey of the cells is crucial. Harvesting and printing the cells in the same sitting could only be done with knowledge of specific markers for MSC, which we lack at present. This is feasible for other cell types with, such as for example the hematopoietic stem cells, which are currently used without manipulation in cancer patients following whole body irradiation (Bazinet and Popradi, 2019). For MSCs however, at present there is a lack of robust techniques for cell isolation and purification that do not affect MSCs biology and then cell preservation strategies. To this end, one of the major drawbacks is the unavailability of reliable culture media, as current research is merely based on animal derived sera. Serum free media or the use of autologous serum can be an alternative but further research is needed in this matter. In addition, the identification of biomimetic matrices mimicking the native tissue composition and allowing cellular growth and differentiation is required. Finally, conditions under which the 3D constructs will survive following printing potentiate dangers and can jeopardize the whole process. A solution would include developing new bioinks and bioprinters that allow high-resolution fabrication process would diminish the need for post-fabrication culture. Only addressing the aforementioned challenges will safeguard the feasibility and safety of 3D bioprinting for regenerative medicine applications.
Author Contributions
LB, NT, HM, AE, NA, and IP contributed to the research, writing, editing and formatting of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. AE acknowledges funding from TUBITAK-2219 (1059B19170 0093).
References
- American Transplant Foundation (2018). American Transplant Foundation. Available at: www.americantransplantfoundation.org (accessed November 7, 2019). [Google Scholar]
- Arany P. R., Shvartsman D., Huebsch N., Mao A. S., Ali O. A., Bencherif S. A., et al. (2010). Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 9 518–526. 10.1038/nmat2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaute K., Mann B., Wicker R. (2006). Stereolithography of three-dimensional bioactive poly(ethylene glycol) constructs with encapsulated cells. Ann. Biomed. Eng. 34 1429–1441. 10.1007/s10439-006-9156-y [DOI] [PubMed] [Google Scholar]
- Ashammakhi N., Ahadian S., Darabi M. A., El Tahchi M., Lee J., Suthiwanich K., et al. (2019a). Minimally invasive and regenerative therapeutics. Adv. Mater. 31:e1804041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashammakhi N., Hasan A., Kaarela O., Byambaa B., Sheikhi A., Gaharwar A. K., et al. (2019b). Advancing frontiers in bone bioprinting. Adv. Healthc. Mater. 8:e1801048. [DOI] [PubMed] [Google Scholar]
- Ashammakhi N., Ahadian S., Xu C., Montazerian H., Ko H., Nasiri R., et al. (2019c). Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio 1:100008. 10.1016/j.mtbio.2019.100008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashammakhi N., Ahadian S., Zengjie F., Suthiwanich K., Lorestani F., Orive G., et al. (2018). Advances and future perspectives in 4D bioprinting. Biotechnol. J. 13:e1800148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashammakhi N., Darabi M. A., Kehr N. S., Erdem A., Hu S. K., Dokmeci M. R., et al. (2020). Advances in controlled oxygen generating biomaterials for tissue engineering and regenerative therapy. Biomacromolecules 21 56–72. [DOI] [PubMed] [Google Scholar]
- Ashammakhi N., Ndreu N., Wimpenny Y. (2008). Advancing tissue engineering by using electrospun nanofibers. Regen. Med. 3 547–574. 10.2217/17460751.3.4.547 [DOI] [PubMed] [Google Scholar]
- Atala A., Yoo J. J. (2015). Essentials of 3D Biofabrication and Translation [Internet]. San Diego, CA: Elsevier Scienc. [Google Scholar]
- Bae S. W., Lee K. W., Park J. H., Lee J., Jung C. R., Yu J., et al. (2018). 3D bioprinted artificial trachea with epithelial cells and chondrogenic-differentiated bone marrow-derived mesenchymal stem cells. Int. J. Mol. Sci. 19:1624. 10.3390/ijms19061624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. M., Chen C. S. (2012). Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues. J. Cell. Sci. 125(Pt 13), 3015–3024. 10.1242/jcs.079509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron J., Krizman D., Ringeisen B. (2005). Laser printing of single cells: statistical analysis. Cell Viabil. Stress 33 121–130. 10.1007/s10439-005-8971-x [DOI] [PubMed] [Google Scholar]
- Barron J., Wu P., Ladouceur H., Ringeisen B. (2004). Biological laser printing: a novel technique for creating heterogeneous 3-dimensional cell patterns. Biomed. Microdev. 6 139–147. 10.1023/b:bmmd.0000031751.67267.9f [DOI] [PubMed] [Google Scholar]
- Bazinet A., Popradi G. (2019). A general practitioner’s guide to hematopoietic stem-cell transplantation. Curr. Oncol. 26 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj N., Kundu S. C. (2010). Electrospinning: a fascinating fiber fabrication technique. Biotechnol. Adv. 28 325–347. 10.1016/j.biotechadv.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Bhuthalingam R., Lim P. Q., Irvine S., Agrawal A., Mhaisalkar P., An J., et al. (2015). A novel 3D printing method for cell alignment and differentiation. Int. J. Bioprint. 1 57–65. [Google Scholar]
- Bieback K., Kern S., Klüter H., Eichler H. (2004). Critical parameters for the isolation of mesenchymal stem cells from umbilical cord Blood. Stem Cells 22 625–634. 10.1634/stemcells.22-4-625 [DOI] [PubMed] [Google Scholar]
- Bishop E. S., Mostafa S., Pakvasa M., Luu H. H., Lee M. J., Wolf J. M., et al. (2017). 3-D bioprinting technologies in tissue engineering and regenerative medicine: current and future trends. Genes Dis. 4 185–195. 10.1016/j.gendis.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaeser A., Duarte Campos D. F., Puster U., Richtering W., Stevens M. M., Fischer H. (2016). Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv. Healthc. Mater. 5 326–333. 10.1002/adhm.201500677 [DOI] [PubMed] [Google Scholar]
- Bongso A., Fong C.-Y., Gauthaman K. (2008). Taking stem cells to the clinic: major challenges. J. Cell. Biochem. 105 1352–1360. 10.1002/jcb.21957 [DOI] [PubMed] [Google Scholar]
- Butler D. L., Goldstein S. A., Guldberg R. E., Guo X. E., Kamm R., Laurencin C. T., et al. (2009). The Impact of Biomechanics in Tissue Engineering and Regenerative Medicine. Larchmont, NY: Mary Ann Liebert, Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byambaa B., Annabi N., Yue K., Trujillo-de Santiago G., Alvarez M. M., Jia W., et al. (2017). Bioprinted osteogenic and vasculogenic patterns for engineering 3D bone tissue. Adv. Healthc. Mater. 6:1700015. 10.1002/adhm.201700015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Heazlewood S. Y., Williams B., Cardozo D., Nigro J., Oteiza A., et al. (2016). The role of CD44 in fetal and adult hematopoietic stem cell regulation. Haematologica 101 26–37. 10.3324/haematol.2015.135921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow J. K., Kerativitayanan P., Jaiswal M. K., Lokhande G., Gaharwar A. K. (2015). “Chapter 13-polymers for bioprinting,” in Essentials of 3D Biofabrication and Translation, ed. Haley M. (Amsterdam: Elsevier Inc; ). [Google Scholar]
- Catros S., Fricain J.-C., Guillotin B., Pippenger B., Bareille R., Remy M., et al. (2011). Laser-assisted bioprinting for creating on-demand patterns of human osteoprogenitor cells and nano-hydroxyapatite. Biofabrication 3:025001 10.1088/1758-5082/3/2/025001 [DOI] [PubMed] [Google Scholar]
- Chang R., Nam J., Sun W. (2008). Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication–based direct cell writing. Tissue Eng. Part A 14 41–48. 10.1089/ten.2007.0004 [DOI] [PubMed] [Google Scholar]
- Christensen K., Xu C., Chai W., Zhang Z., Fu J., Huang Y. (2015). Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 112 1047–1055. 10.1002/bit.25501 [DOI] [PubMed] [Google Scholar]
- Colwill J. M., Cultice J. M., Kruse R. L. (2008). Will generalist physician supply meet demands of an increasing and aging population? Health Aff. 27 w232–w241. [DOI] [PubMed] [Google Scholar]
- Cousin B., Ravet E., Poglio S., De Toni F., Bertuzzi M., Lulka H., et al. (2009). Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One 4:e6278. 10.1371/journal.pone.0006278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Dean D., Ruggeri Z. M., Boland T. (2010). Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol. Bioeng. 106 963–969. 10.1002/bit.22762 [DOI] [PubMed] [Google Scholar]
- Dababneh A. B., Ozbolat I. T. (2014). Bioprinting technology: a current state-of-the-art review. J. Manufact. Sci. Eng. 136:61016. [Google Scholar]
- Daly A. C., Freeman F. E., Gonzalez-Fernandez T., Critchley S. E., Nulty J., Kelly D. J. (2017). 3D bioprinting for cartilage and osteochondral tissue engineering. Adv. Healthc. Mater. 6:1700298. 10.1002/adhm.201700298 [DOI] [PubMed] [Google Scholar]
- Das S., Pati F., Choi Y.-J., Rijal G., Shim J.-H., Kim S. W., et al. (2015). Bioprintable, cell-laden silk fibroin–gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 11 233–246. 10.1016/j.actbio.2014.09.023 [DOI] [PubMed] [Google Scholar]
- Davidenko N., Schuster C. F., Bax D. V., Raynal N., Farndale R. W., Best S. M., et al. (2015). Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 25 131–142. 10.1016/j.actbio.2015.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado L. M., Bayon Y., Pandit A., Zeugolis D. I. (2015). To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B Rev. 21 298–313. 10.1089/ten.teb.2014.0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos M., Intranuovo F., Russo T., De Santis R., Gloria A., Ambrosio L., et al. (2013). The first systematic analysis of 3D rapid prototyped poly(ε-caprolactone) scaffolds manufactured through BioCell printing: the effect of pore size and geometry on compressive mechanical behaviour and in vitro hMSC viability. Biofabrication 5:045004 10.1088/1758-5082/5/4/045004 [DOI] [PubMed] [Google Scholar]
- Du M., Chen B., Meng Q., Liu S., Zheng X., Zhang C., et al. (2015). 3D bioprinting of BMSC-laden methacrylamide gelatin scaffolds with CBD-BMP2-collagen microfibers. Biofabrication 7:044104 10.1088/1758-5090/7/4/044104 [DOI] [PubMed] [Google Scholar]
- Engler A. J., Sen S., Sweeney H. L., Discher D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell 126 677–689. 10.1016/j.cell.2006.06.044 [DOI] [PubMed] [Google Scholar]
- Freimark D., Pino-Grace P., Pohl S., Weber C., Wallrapp C., Geigle P., et al. (2010). Use of encapsulated stem cells to overcome the bottleneck of cell availability for cell therapy approaches. Trans. Med. Hemother. 37 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G., Schilling A., Hubbell K., Yonezawa T., Truong D., Hong Y., et al. (2015). Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 37 2349–2355. 10.1007/s10529-015-1921-2 [DOI] [PubMed] [Google Scholar]
- Gao G., Schilling A. F., Yonezawa T., Wang J., Dai G., Cui X. (2014). Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol. J. 9 1304–1311. 10.1002/biot.201400305 [DOI] [PubMed] [Google Scholar]
- Gnecchi M., Melo L. G. (2009). Bone marrow-derived mesenchymal stem cells: Isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol. Biol. 482 281–294. 10.1007/978-1-59745-060-7_18 [DOI] [PubMed] [Google Scholar]
- Goldring C. E., Duffy P. A., Benvenisty N., Andrews P. W., Ben-David U., Eakins R., et al. (2011). Assessing the safety of stem cell therapeutics. Cell Stem Cell 8 618–628. 10.1016/j.stem.2011.05.012 [DOI] [PubMed] [Google Scholar]
- Gruene M., Deiwick A., Koch L., Schlie S., Unger C., Hofmann N., et al. (2011a). Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng. Part C Methods 17 79–87. 10.1089/ten.tec.2010.0359 [DOI] [PubMed] [Google Scholar]
- Gruene M., Pflaum M., Deiwick A., Koch L., Schlie S., Unger C., et al. (2011b). Adipogenic differentiation of laser-printed 3D tissue grafts consisting of human adipose-derived stem cells. Biofabrication 3:015005 10.1088/1758-5082/3/1/015005 [DOI] [PubMed] [Google Scholar]
- Guillemot F., Souquet A., Catros S., Guillotin B., Lopez J., Faucon M., et al. (2010a). High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 6 2494–2500. 10.1016/j.actbio.2009.09.029 [DOI] [PubMed] [Google Scholar]
- Guillemot F., Souquet A., Catros S., Guillotin B. (2010b). Laser-assisted cell printing: principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine 5 507–515. 10.2217/nnm.10.14 [DOI] [PubMed] [Google Scholar]
- Grungor-Ozkerim P. S., Inci I., Zhang Y. S., Khademhosseini A., Dokmeci M. R. (2018). Bioinks for 3D bioprinting: an overview. Biomater. Sci. 6 915–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich M. A., Liu W., Jimenez A., Yang J., Akpek A., Liu X., et al. (2019). 3D bioprinting: from benches to translational applications. Small 15:e1805510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernigou P., Poignard A., Beaujean F., Rouard H. (2005a). Percutaneous autologous bone-marrow grafting for nonunions: influence of the number and concentration of progenitor cells. J. Bone Joint Surg. Am. 87 1430–1437. 10.2106/00004623-200507000-00003 [DOI] [PubMed] [Google Scholar]
- Hernigou P., Poignard A., Manicom O., Mathieu G., Rouard H. (2005b). The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J. Bone Joint Surg. Br. 87 896–902. 10.1302/0301-620x.87b7.16289 [DOI] [PubMed] [Google Scholar]
- Ho I. A., Toh H. C., Ng W. H., Teo Y. L., Guo C. M., Hui K. M., et al. (2013). Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells 31 146–155. 10.1002/stem.1247 [DOI] [PubMed] [Google Scholar]
- Hopp B., Smausz T., Szabó G., Kolozsvári L., Kafetzopoulos D., Fotakis C., et al. (2012). Femtosecond laser printing of living cells using absorbing film-assisted laser-induced forward transfer. Opt. Eng. 51:014302 10.1117/1.oe.51.1.014302 [DOI] [Google Scholar]
- Huang W.-H., Chang M.-C., Tsai K.-S., Hung M.-C., Chen H.-L., Hung S.-C. (2013). Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene 32 4343–4354. 10.1038/onc.2012.458 [DOI] [PubMed] [Google Scholar]
- Hyeong J. L., Yong B. K., Seung H. A., Ji-Seon L., Jang c., Yoon H., et al. (2015). A New Approach for Fabricating Collagen/ECM-Based Bioinks Using Preosteoblasts and Human Adipose Stem Cells. Weinheim: Wiley Subscription Services, Inc. [Google Scholar]
- International Population Reports (2016). Aging World : 2015: International Population Reports. Series WP;2016 ASI 2546-17.21;Census P95/16-1. Available online at: https://statistical.proquest.com/statisticalinsight/result/pqpresultpage.previewtitle?docType=PQSI&titleUri=/content/2016/2546-17.21.xml (accessed November 7, 2019). [Google Scholar]
- Irvine S., Agrawal A., Lee B., Chua H., Low K., Lau B., et al. (2015). Printing cell-laden gelatin constructs by free-form fabrication and enzymatic protein crosslinking. Biomed. Microdev. 17 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine S. A., Venkatraman S. S. (2016). Bioprinting and differentiation of stem cells. Molecules 21:1188. 10.3390/molecules21091188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab K., Norotte C., Marga F., Murphy K., Vunjak-Novakovic G., Forgacs G., et al. (2010). Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2:022001 10.1088/1758-5082/2/2/022001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana S., Lerman A. (2015). Bioprinting a cardiac valve. Biotechnol. Adv. 33 1503–1521. 10.1016/j.biotechadv.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Jóźwiak T., Filipkowska U., Szymczyk P., Rodziewicz J., Mielcarek A. (2017). Effect of ionic and covalent crosslinking agents on properties of chitosan beads and sorption effectiveness of Reactive Black 5 dyeReactive and Functional Polymers. Reacti. Funct. Polym. 114 58–74. 10.1016/j.reactfunctpolym.2017.03.007 [DOI] [Google Scholar]
- Kang H.-W., Lee S. J., Ko I. K., Kengla C., Yoo J. J., Atala A. (2016). A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 34 312–319. 10.1038/nbt.3413 [DOI] [PubMed] [Google Scholar]
- Kang L., Armstrong P., Lee L., Duan B., Kang K., Butcher J. (2017). Optimizing photo-encapsulation viability of heart valve cell types in 3D printable composite hydrogels. Ann. Biomed. Eng. 45 360–377. 10.1007/s10439-016-1619-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. J., Tholpady A., Tholpady S. S., Shang H., Ogle R. C. (2005). Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells 23 412–423. 10.1634/stemcells.2004-0021 [DOI] [PubMed] [Google Scholar]
- Keriquel V., Oliveira H., Rémy M., Ziane S., Delmond S., Rousseau B., et al. (2017). In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 7:1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakoo A. Y., Pati S., Anderson S. A., Reid W., Elshal M. F., Rovira I. I., et al. (2006). Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J. Exp. Med. 203 1235–1247. 10.1084/jem.20051921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil S., Sun W. (2009). Bioprinting endothelial cells with alginate for 3D tissue constructs. J. Biomech. Eng. 131:111002. [DOI] [PubMed] [Google Scholar]
- Kim M., Takaoka A., Hoang Q. V., Trokel S. L., Paik D. C. (2014). Pharmacologic alternatives to riboflavin photochemical corneal cross-linking: a comparison studyof cell toxicity thresholds. Invest. Ophthalmol. Vis. Sci. 55 3247–3257. 10.1167/iovs.13-13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch L., Kuhn S., Sorg H., Gruene M., Schlie S., Gaebel R., et al. (2010). Laser printing of skin cells and human stem cells. Tissue Eng. Part C Methods 16 847–854. 10.1089/ten.tec.2009.0397 [DOI] [PubMed] [Google Scholar]
- Kucerova L., Matuskova M., Hlubinova K., Altanerova V., Altaner C. (2010). Tumor cell behaviour modulation by mesenchymal stromal cells. Mol. Cancer 9:129. 10.1186/1476-4598-9-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov S. A., Mankani M. H., Gronthos S., Satomura K., Bianco P., Robey P. G. (2001). Circulating skeletal stem cells. J. Cell Biol. 153 1133–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle H. V., Stafford A., Mooney D. J. (2019). Sequential modes of crosslinking tune viscoelasticity of cell-instructive hydrogels. Biomaterials 188 187–197. 10.1016/j.biomaterials.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane S. W., Williams D. A., Watt F. M. (2014). Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 32 795–803. 10.1038/nbt.2978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R., Vacanti J. P. (1993). Tissue engineering. Science 260 920–926. [DOI] [PubMed] [Google Scholar]
- Leberfinger A. N., Ravnic D. J., Dhawan A., Ozbolat I. T. (2017). Concise review: bioprinting of stem cells for transplantable tissue fabrication. pISSN 6 1940–1948. 10.1002/sctm.17-0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Kim Y. B., Ahn S. H., Lee J., Jang C. H., Yoon H., et al. (2015). A new approach for fabricating collagen/ECM-based bioinks using preosteoblasts and human adipose stem cells. Adv. Healthc. Mater. 4 1359–1368. 10.1002/adhm.201500193 [DOI] [PubMed] [Google Scholar]
- Levato R., Visser J., Planell J. A., Engel E., Malda J., Mateos-Timoneda M. A. (2014). Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 6:035020 10.1088/1758-5082/6/3/035020 [DOI] [PubMed] [Google Scholar]
- Lew D. H., Liu P. H., Orgill D. P. (2007). Optimization of UV cross-linking density for durable and nontoxic collagen gag dermal substitute. J. Biomed. Mater. Res. B Appl. Biomater. 82 51–56. 10.1002/jbm.b.30704 [DOI] [PubMed] [Google Scholar]
- Li J., Chen M., Fan X., Zhou H. (2016). Recent advances in bioprinting techniques: approaches, applications and future prospects. J. Transl. Med. 14:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K. S., Schon B. S., Mekhileri N. V., Brown G. C. J., Chia C. M., Prabakar S., et al. (2016). New visible-light photoinitiating system for improved print fidelity in gelatin-based bioinks. ACS Biomater. Sci. Eng. 2 1752–1762. 10.1021/acsbiomaterials.6b00149 [DOI] [PubMed] [Google Scholar]
- Mackay A. M., Beck S. C., Murphy J. M., Barry F. P., Chichester C. O., Pittenger M. F., et al. (1998). Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 4 415–428. [DOI] [PubMed] [Google Scholar]
- Maher P., Keatch R., Donnelly K., Mackay R., Paxton J. (2009). Construction of 3D biological matrices using rapid prototyping technology. Rapid Prototyp. J. 15 204–210. 10.1108/13552540910960307 [DOI] [Google Scholar]
- Mairim A. S., Chen Y. H., Wong T. Y., Bittencourt V., Lin Y. C., Huang L. (2012). Hyaluronan regulates cell behavior: A potential niche matrix for stem cells. Biochem. Res. Int. 2012:346972-11. 10.1155/2012/346972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrycky C., Wang Z., Kim K., Kim D. -H. (2016). 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 34 422–434. 10.1016/j.biotechadv.2015.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsiko A., Gleeson J. P., O’Brien F. J. (2015). Scaffold mean pore size influences mesenchymal stem cell chondrogenic differentiation and matrix deposition. Tissue Eng. Part A 21 486–497. 10.1089/ten.tea.2013.0545 [DOI] [PubMed] [Google Scholar]
- Melchels F. P., Barradas A. M., van Blitterswijk C. A., de Boer J., Feijen J., Grijpma D. W., et al. (2010). Effects of the architecture of tissue engineering scaffolds on cell seeding and culturing. Acta Biomater. 6 4208–4217. 10.1016/j.actbio.2010.06.012 [DOI] [PubMed] [Google Scholar]
- Minteer D., Marra K. G., Rubin J. P. (2013). Adipose-derived mesenchymal stem cells: biology, and potential applications. Adv. Biochem. Eng. Biotechnol. 129 59–71. 10.1007/10_2012_146 [DOI] [PubMed] [Google Scholar]
- Mondschein R. J., Kanitkar A., Williams C. B., Verbridge S. S., Long T. E., et al. (2017). Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 140 170–188. 10.1016/j.biomaterials.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Murphy S. V., Atala A. (2014). 3D bioprinting of tissues and organs. Nat. Biotechnol. 32 773–785. 10.1038/nbt.2958 [DOI] [PubMed] [Google Scholar]
- Muzzarelli R. A., El Mehtedi M., Bottegoni C., Aquili A., Gigante A. (2015). Genipin-crosslinked chitosan gels and scaffolds for tissue engineering and regeneration of cartilage and bone. Mar. Drugs 13 7314–7338. 10.3390/md13127068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Kobayashi A., Takagi F., Watanabe A., Hiruma Y., Ohuchi K., et al. (2005). Biocompatible inkjet printing technique for designed seeding of individual living Cells. Tissue Eng. 11 1658–1666. 10.1089/ten.2005.11.1658 [DOI] [PubMed] [Google Scholar]
- Ong C. S., Yesantharao P., Huang C. Y., Mattson G., Boktor J., Fukunishi T., et al. (2018). 3D bioprinting using stem cells. Pediatr. Res. 83 223–231. 10.1038/pr.2017.252 [DOI] [PubMed] [Google Scholar]
- Oryan A., Kamali A., Moshiri A., Baharvand H., Daemi H. (2018). Chemical crosslinking of biopolymeric scaffolds: current knowledge and future directions of crosslinked engineered bone scaffolds. Int. J. Biol. Macromol. 107(Pt A):678–688. 10.1016/j.ijbiomac.2017.08.184 [DOI] [PubMed] [Google Scholar]
- Ozbolat I. T., Hospodiuk M. (2015). Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 76 321–343. 10.1016/j.biomaterials.2015.10.076 [DOI] [PubMed] [Google Scholar]
- Parvin S., Noorjahan B. A., Abdul M. A., Abdul R. O., Maryam M., Ehsan J., et al. (2012). Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton’s jelly. In Vitro Cell Dev. Biol. Anim. 48 75–83. 10.1007/s11626-011-9480-x [DOI] [PubMed] [Google Scholar]
- Perrot P., Rousseau J., Bouffaut A. L., Rédini F., Cassagnau E., Deschaseaux F., et al. (2010). Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS One 5:e10999. 10.1371/journal.pone.0010999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer E., Vickers S. M., Frank E., Grodzinsky A. J., Spector M. (2008). The effects of glycosaminoglycan content on the compressive modulus of cartilage engineered in type II collagen scaffolds. Osteoarthr. Cartil. 16 1237–1244. 10.1016/j.joca.2008.02.014 [DOI] [PubMed] [Google Scholar]
- Phillippi J. A., Miller E., Weiss L., Huard J., Waggoner A., Campbell P., et al. (2008). Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cells 26 127–134. 10.1634/stemcells.2007-0520 [DOI] [PubMed] [Google Scholar]
- Pintus E., Baldassarri M., Perazzo L., Natali S., Ghinelli D., Buda R., et al. (2018). Stem cells in osteochondral tissue engineering. Adv. Exp. Med. Biol. 1058 359–372. 10.1007/978-3-319-76711-6_16 [DOI] [PubMed] [Google Scholar]
- Porada C., Zanjani E. D., Almeida-Porad G. (2006). Adult mesenchymal stem cells: a pluripotent population with multiple applications. Stem Cell Res. Ther. 1 365–369. 10.2174/157488806778226821 [DOI] [PubMed] [Google Scholar]
- Pountos I., Corscadden D., Emery P., Giannoudis P. V. (2007). Mesenchymal stem cell tissue engineering: Techniques for isolation, expansion and application. Injury 38:S33 10.1016/S0020-1383(08)70006-8 [DOI] [PubMed] [Google Scholar]
- Qi D., Wu S., Kuss M. A., Shi W., Chung S., Deegan P. T., et al. (2018). Mechanically robust cryogels with injectability and bioprinting supportability for adipose tissue engineering. Acta Biomater. 74 131–142. 10.1016/j.actbio.2018.05.044 [DOI] [PubMed] [Google Scholar]
- Rak Kwon D., Jung S., Jang J., Park G. Y., Suk Moon Y., Lee S. C., et al. (2020). A 3-dimensional bioprinted scaffold with human umbilical cord blood-mesenchymal stem cells improves regeneration of chronic full-thickness rotator cuff tear in a rabbit model. Am. J. Sports Med. 48 947–958. 10.1177/0363546520904022 [DOI] [PubMed] [Google Scholar]
- Raman R., Bhaduri B., Mir M., Shkumatov A., Lee M. K., Popescu G., et al. (2016). High-resolution projection microstereolithography for patterning of neovasculature. Adv. Healthcare Mater. 5 610–619. 10.1002/adhm.201500721 [DOI] [PubMed] [Google Scholar]
- Raucci M. G., Alvarez-Perez M. A., Demitri C., Giugliano D., De Benedictis V., Sannino A., et al. (2015). Effect of citric acid crosslinking cellulose-based hydrogels on osteogenic differentiation. J. Biomed. Mater. Res. A. 103 2045–2056. 10.1002/jbm.a.35343 [DOI] [PubMed] [Google Scholar]
- Rowlands A. S., George P. A., Cooper-White J. J. (2008). Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. Am. J. Physiol. Cell Physiol. 295 1037–1044. 10.1152/ajpcell.67.2008 [DOI] [PubMed] [Google Scholar]
- Rubio D., Garcia-Castro J., Martin M. C., de la Fuente R., Cigudosa J. C., Lloyd A. C., et al. (2005). Spontaneous human adult stem cell transformation. Cancer Res. 65 3035–3039. 10.1158/0008-5472.CAN-04-4194 [DOI] [PubMed] [Google Scholar]
- Ryu S., Yoo J., Jang Y., Jin H., Jung SY., Park J., et al. (2015). Nanothin coculture membranes with tunable pore architecture and thermoresponsive functionality for transfer-printable stem cell-derived cardiac sheets. ACS Nano 9 10186–10202. 10.1021/acsnano.5b03823 [DOI] [PubMed] [Google Scholar]
- Sasaki M., Abe R., Fujita Y., Ando S., Inokuma D., Shimizu H. (2008). Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by trans differentiation into multiple skin cell type. J. Immunol. 180 2581–2587. 10.4049/jimmunol.180.4.2581 [DOI] [PubMed] [Google Scholar]
- Saunders R. E., Gough J. E., Derby B. (2007). Delivery of human fibroblast cells by piezoelectric drop-on-demand inkjet printing. Biomaterials 29 193–203. 10.1016/j.biomaterials.2007.09.032 [DOI] [PubMed] [Google Scholar]
- Seetharam R. (1991). “Purification and analysis of recombinant proteins,” in Bioprocess Technology (New York, NY: Dekker; ). [PubMed] [Google Scholar]
- Shav D., Einav S. (2010). The effect of mechanical loads in the differentiation of precursor cells into mature cells. Ann. N. Y. Acad. Sci. 1188 25–31. 10.1111/j.1749-6632.2009.05079.x [DOI] [PubMed] [Google Scholar]
- Shahabipour F., Ashammakhi N., Oskuee R. K., Bonakdar S., Hoffman T., Shokrgozar M. A., et al. (2020). Key components of engineering vascularized 3-dimensional bioprinted bone constructs. Transl Res. 216 57–76 10.1016/j.trsl.2019.08.010 [DOI] [PubMed] [Google Scholar]
- Skardal A., Atala A. (2015). Biomaterials for Integration with 3-D Bioprinting. Biomed. Eng. 43 730–746. 10.1007/s10439-014-1207-1 [DOI] [PubMed] [Google Scholar]
- Sotiropoulou P. A., Perez S. A., Salagianni M., Baxevanis C. N., Papamichail M. (2006). Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 24 462–471. 10.1634/stemcells.2004-0331 [DOI] [PubMed] [Google Scholar]
- Stolberg S., McCloskey K. E. (2009). Can shear stress direct stem cell fate? Cell Culture Tissue Eng. 25 10–19. 10.1002/btpr.124 [DOI] [PubMed] [Google Scholar]
- Suzuki K., Sun R., Origuchi M., Kanehira M., Takahata T., Itoh J., et al. (2011). “Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization,” in Molecular Medicine, Vol. 17 (Cambridge, MA: ), 579–587. 10.2119/molmed.2010.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasnim N., De la Vega L., Kumar S. A., Abelseth L., Matthew Alonzo M., et al. (2018). 3D bioprinting stem cell derived tissues. Cell Mol. Bioeng. 11 219–240. 10.1007/s12195-018-0530-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. R., Rubin J., Rubin C. T. (2012). Mechanical regulation of signaling pathways in bone. Gene 503 179–193. 10.1016/j.gene.2012.04.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta R., Inzana J., Schwarz E., Kates S., Awad H. (2017). 3D printing of calcium phosphate ceramics for bone tissue engineering and drug delivery. Ann. Biomed. Eng. 45 23–44. 10.1007/s10439-016-1678-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trounson A., McDonald C. (2015). Stem cell therapies in clinical trials: Progress and challenges. Cell Stem Cell 17 11–22. 10.1016/j.stem.2015.06.007 [DOI] [PubMed] [Google Scholar]
- Tsai M. S., Lee J. L., Chang Y. J., Hwang S. M. (2004). Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum. Reprod. 19 1450–1456. 10.1093/humrep/deh279 [DOI] [PubMed] [Google Scholar]
- Tsuji K., Ojima M., Otabe K., Horie M., Koga H., Sekiya I., et al. (2017). Effects of different cell-detaching methods on the viability and cell surface antigen expression of synovial mesenchymal stem cells. Cell Transplant. 26 1089–1102. 10.3727/096368917X694831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski M. H., Wang Y., Graham L., Gao M., Wu D., Brock J., et al. (2014). Neural stem cell dissemination after grafting to CNS injury sites. Cell 156 388–389. 10.1016/j.cell.2014.01.016 [DOI] [PubMed] [Google Scholar]
- Vining K. H., Stafford A., Mooney D. J. (2019). Sequential modes of crosslinking tune viscoelasticity of cell-instructive hydrogels. Biomaterials 188 187–197. 10.1016/j.biomaterials.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser J., Melchels F., Jeon J. (2015). Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 6:6933. 10.1038/ncomms7933 [DOI] [PubMed] [Google Scholar]
- Wang H. L., Zuo Y., Li Y., Ma J., Cheng S. L. (2007). Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials 28 3338–3348. 10.1016/j.biomaterials.2007.04.014 [DOI] [PubMed] [Google Scholar]
- Wang L., Stegemann J. P. (2011). Glyoxal crosslinking of cell-seeded chitosan/collagen hydrogels for bone regeneration. Acta Biomater. 7 2410–2417. 10.1016/j.actbio.2011.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Abdulla R., Parker B., Samanipour R., Ghosh S., Kim K. (2015). A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 7:045009 10.1088/1758-5090/7/4/045009 [DOI] [PubMed] [Google Scholar]
- Willerth S. M., Sakiyama-Elbert S. (2008). Cell therapy for spinal cord regeneration. Drug. Deliv. Rev. 60 263–276. 10.1016/j.addr.2007.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst S., Godla M. E., Müller R., Hofmann S. (2014). Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 10 630–640. 10.1016/j.actbio.2013.10.016 [DOI] [PubMed] [Google Scholar]
- Wüst S., Müller R., Hofmann Boss S. S. (2011). Controlled positioning of cells in biomaterials – approaches towards 3D tissue printing. J. Funct. Biomater. 2:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Jin J., Gregory C., Hickman J. J., Boland T. (2005). Inkjet printing of viable mammalian cells. Biomaterials 26 93–99. 10.1016/j.biomaterials.2004.04.011 [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Nakayama K., Taguchi T., Enosawa S., Tamura T., Yoshimaru K., et al. (2017). In vivo and ex vivo methods of growing a liver bud through tissue connection. Sci. Rep. 7:14085. 10.1038/s41598-017-14542-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. E., Mancini M. L., Wright R. P., Smith J. C., Black A. C., Jr, Reagan C. R., et al. (1995). Mesenchymal stem cells reside within the connective tissues of many organs. Dev. Dyn. 202 137–144. 10.1002/aja.1002020205 [DOI] [PubMed] [Google Scholar]
- Yuan X., Logan T. M., Ma T. (2019). Metabolism in human mesenchymal stromal cells: A missing link between hMSC Biomanufacturing and therapy? Front. immunol. 10:977. 10.3389/fimmu.2019.00977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Li Y., Mao S., Sun W., Yao R. (2015). The influence of printing parameters on cell survival rate and printability in microextrusion-based 3D cell printing technology. Biofabrication 7:045002 10.1088/1758-5090/7/4/045002 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Yao R., Ouyang L., Ding H., Zhang T., Zhang K., et al. (2014). Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 6:035001 10.1088/1758-5082/6/3/035001 [DOI] [PubMed] [Google Scholar]