Abstract
Successful vaccines against specific pathogens often require multiple immunizations and adjuvant usage. Yet, assessing the protective efficacy of different immunization regimens with adjuvanted Toxoplasma gondii vaccines remains elusive. In this study, we investigated the vaccine efficacy induced by CpG-ODN-adjuvanted T. gondii virus-like particles (VLPs) after challenge infection with T. gondii (ME49) in mice (BALB/c) upon one, two, and three immunizations. Immunization with adjuvanted T. gondii VLPs induced higher levels of T. gondii-specific IgG and/or IgA antibody responses, germinal center (GC) B cells, total B cells, and CD4+ and CD8+ T cells compared with unadjuvanted VLPs. Increasing the number of immunizations was strongly correlated with enhanced protective immunity against T. gondii in mice, with the highest protection being demonstrated in mice thrice-immunized with either adjuvanted T. gondii VLPs or VLPs alone. Notably, lesser bodyweight reductions and cerebral cyst counts were observed in mice receiving multiple immunizations with the adjuvanted VLPs, thereby confirming the effectiveness of adjuvanted boost immunizations. These results demonstrated that multiple immunizations with T. gondii VLPs is an effective approach, and the CpG-ODN can be developed as an effective adjuvant for T. gondii VLP vaccines.
Keywords: Toxoplasma gondii, virus-like particle, vaccine, CpG-ODN
1. Introduction
Toxoplasma gondii is an obligate intracellular parasite widely distributed across the globe and infects a vast array of mammals including humans [1,2,3]. T. gondii infection in pregnant women and acquired immune deficiency syndrome (AIDS) patients can have severe consequences such as spontaneous abortion and encephalitis [4,5,6]. To date, clinical T. gondii vaccines remain commercially unavailable. While therapeutic interventions are possible, their applications are hindered by toxicity and other side effects [7,8]. To address these limitations, multitudes of T. gondii vaccine studies are currently being conducted using DNA, protein subunit, inactivated, and attenuated vaccines to develop an effective toxoplasmosis vaccine [9].
Conflicting protective efficacy results have been reported through numerous vaccine studies. All of the mice immunized with the DNA vaccine encoding the T. gondii surface antigen 1 (SAG1) survived upon T. gondii (ME49) challenge infection [10], whereas none of the mice immunized with the DNA vaccine expressing T. gondii superoxide dismutase (SOD) survived [11]. Survival discrepancies were also observed from mice immunized with various subunit vaccines before challenge infection with T. gondii ME49 [12,13,14]. In contrast to the DNA or recombinant subunit vaccines, immunizing the mice with attenuated T. gondii ensured that all of the immunized mice survived following challenge infection with a lethal dose of ME49 [15,16]. Though the protective efficacies of the live attenuated vaccines appear promising, the safety aspects of these vaccines are of concern since attenuated T. gondii can revert to the highly pathogenic wild type [14]. As a safer alternative, we generated several virus-like particle (VLP) T. gondii vaccines conferring 100% protection against a lethal dose of T. gondii ME49 strain in mice [17,18,19,20]. Although all of the immunized mice survived in our previous studies, incomplete removal of cerebral cysts and bodyweight loss upon challenge infection from these mice indicated that further improvements to the VLP vaccines are needed to minimize disease manifestation.
Synthetic oligodeoxynucleotides containing unmethylated CpG motifs (CpG-ODNs) have been shown to act as immunologic adjuvants in mice, which enhances humoral and cellular responses induced by co-administered vaccines [21,22]. C-Class CpG-ODNs induce strong interferon-alpha (IFN-α) production from the plasmacytoid dendritic cell (pDC) as well as B-cell stimulation [22]. To this extent, combining the highly immunogenic T. gondii VLP vaccines with CpG-ODN adjuvants could confer enhanced protection with close to no symptoms. Multiple immunizations are of utmost importance for adequate adaptive immunity induction. An assessment of the protective efficacy of different immunization regimens with adjuvanted T. gondii VLPs vaccines is urgently needed.
In the current study, mice were intranasally immunized with the CpG-ODN-adjuvanted VLPs once, twice, or thrice and the resulting immune responses were assessed. We found that the highest protection was found from mice thrice-immunized with adjuvanted T. gondii VLPs. Our findings highlight the importance of this multi-immunization approach and adjuvant CpG usage in eliciting potent antibody responses and protection.
2. Materials and Methods
2.1. Mice and Parasite
Female, 6–8-week-old, BALB/c mice were purchased from NARA Biotech (Seoul, Korea) and maintained in the animal facility at Kyung Hee University. All animal experiments were performed following the institutional animal care and use institutional animal care and use committee (IACUC) guidelines (permit number: KHUASP (SE)-18-050). T. gondii ME49 and RH strains were maintained and used for experimental infections as previously described [23,24].
2.2. VLP Vaccine and Reagents
TG146 VLP vaccine expressing T. gondii IMC, ROP18, and MIC8 was produced in insect cells as described previously [18,20]. The multi-antigenic TG146 VLPs were aliquoted and stored at −80 °C until use. Lyophilized CpG-ODN (ODN 2395) was purchased from InvivoGen (San Diego, CA, USA) and reconstituted using ultra-pure water following the manufacturer’s protocol. CpG-ODN adjuvants were aliquoted and stored at −20 °C until use.
2.3. Immunization and T. gondii ME49 Infection
To determine the efficacy of the CpG-ODN adjuvant against T. gondii ME49, BALB/c mice (n = 6 per group) were intranasally immunized with T. gondii VLPs (100 μg) alone or T. gondii VLPs + CpG ODN (100 μg + 5 μg). After prime immunization, mice were immunized with the same doses of either T. gondii VLPs alone or T. gondii VLPs + CpG ODN at 4-week intervals for second and third immunizations. Immune sera were collected at 1 and 4 weeks after each immunization. At 4 weeks after the final immunization, mice immunized once, twice, or thrice were orally infected with a lethal dose of T. gondii ME49 (One + Cha, Two + Cha, Three + Cha). After challenge infection, mice were monitored for 35 days to record body weight changes and survival rates. To compare the protective efficacy and immunological responses upon T. gondii ME49 infection, mice were euthanized 35 days post-infection (dpi) and mucosal antibodies, spleen, mesenteric lymph nodes (MLNs), and brain tissues were collected.
2.4. Sample Preparation
To determine mucosal immune responses, mouse mucosal samples (feces, intestine, urine, and vaginal samples) and brains of the immunized mice were collected at day 35 after T. gondii ME49 infection. Ten pieces of feces were collected from each mouse and 100 μL of phosphate-buffered saline (PBS) was added per 0.1 g of feces. For intestinal sample preparation, a 5 cm long duodenum tissue starting from the pyloric sphincter was acquired from each mouse. Duodenum was longitudinally incised and resuspended in 800 μL PBS. Urine samples from mice undergoing euthanasia were carefully collected for use. Vaginal secretions were collected by washing the vagina twice with 200 μL of PBS using a pipette. All mucosal samples were incubated for 1 h at 37 °C, then centrifuged for 10 min at 5000 rpm. Following the centrifugation process, supernatants were collected for the antibody assay. Brain tissues were homogenized using a syringe in 400 μL of PBS and centrifuged at 8000 rpm. Supernatants were used to measure cerebral antibody and cytokine responses. Remaining brain tissues were used to quantify brain cysts and their sizes as previously described [25,26].
2.5. ELISA for Antibody Responses and Cytokine Levels
T. gondii-specific antibody and cytokine levels were determined by ELISA as reported previously [18,26]. Diluted immune sera (1:100), urine (1:3), vaginal samples (1:3), fecal supernatants (1:3), intestine samples (1:100), and brain supernatants (undiluted) were used as primary antibodies. Briefly, 96-well plates were coated with T. gondii RH (2 μg/mL) and blocked prior to incubation with the primary antibodies listed above. Anti-mouse IgG and IgA secondary antibodies conjugated with horseradish peroxidase (HRP) were used to detect the antigen-specific responses. The production of pro-inflammatory cytokines interferon-gamma (IFN-γ) and interleukin-6 (IL-6) were measured from the brain supernatants using the cytokine ELISA kits (BD Biosciences, San Jose, CA, USA).
2.6. Flow Cytometry
Single cell suspensions of splenocytes and MLN cells acquired from mice were used for fluorescence-activated cell sorting (FACS) analysis as previously described [26]. Cells were unstimulated or stimulated with T. gondii RH antigens (1 μg/mL) for 2 h, treated with CD16/32 antibody for blocking Fc receptors, and subsequently incubated with fluorophore-labeled antibodies specific for anti-mouse CD3 (PE-Cy7), CD4 (FITC), CD8 (PE), GL7 (PE), B220 (FITC), IgD (PE), and CD19 (PE-Cy7). All antibodies were purchased from Invitrogen (Carlsbad, CA, USA) and BD Biosciences (San Diego, CA, USA). Cell acquisition and data analysis were performed using BD Accuri C6.
2.7. Statistical Analysis
GraphPad Prism version 5 software was used for statistical analysis. Data sets were presented as mean ± SD. One-way ANOVA with Tukey’s post hoc test or two-way ANOVA with Bonferroni’s post hoc test was used to test for statistical significance between each group. p values of less than 0.05 were considered statistically significant (* p < 0.05, ** p < 0.01, *** p < 0.001).
3. Results
3.1. T. gondii-Specific IgG and IgA Antibody Responses in Sera
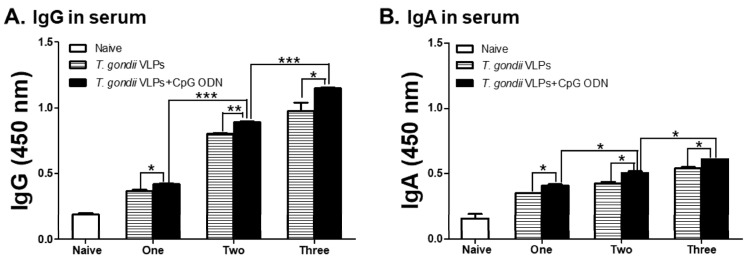
To determine whether the CpG-ODN adjuvant can enhance the efficacy of the T. gondii VLP vaccine after multiple immunizations, mice were immunized with either T. gondii VLPs alone or CpG-ODN-adjuvanted T. gondii VLPs for one, two, or three times. As expected, CpG-ODN-adjuvanted T. gondii VLP vaccination induced higher levels of T. gondii-specific IgG (Figure 1A) and IgA (Figure 1B) antibodies compared with unadjuvanted T. gondii VLPs upon one, two, and three immunizations. A positive correlation between antibody inductions and the number of immunizations was observed as anticipated. The highest IgG and IgA responses were observed from the sera of mice immunized thrice, while the lowest antibody inductions were found in mice immunized once. Notably, regardless of the vaccination schedule, the presence of CpG-ODN adjuvants effectively bolstered the serum antibody responses.
Figure 1.
Immunization with CpG-ODN-adjuvanted Toxoplasma gondii virus-like particles (VLPs) enhances T. gondii-specific IgG (A) and IgA (B) antibody production in the sera upon one, two, and three immunizations. Groups of mice (n = 6 per group) were intranasally immunized one, two, or three times with T. gondii VLPs alone or CpG-ODN-adjuvanted T. gondii VLP vaccine. Booster vaccinations were provided at 4-week intervals. Mouse sera were collected at 4 weeks after each immunization to detect the levels of IgG (A) and IgA (B) antibodies by ELISA. Results are presented as mean ± SD and statistical significances were denoted using asterisks (* p < 0.05, ** p < 0.01, and *** p < 0.001).
3.2. T. gondii—Specific IgG and IgA Antibody Responses in Mucosal Samples
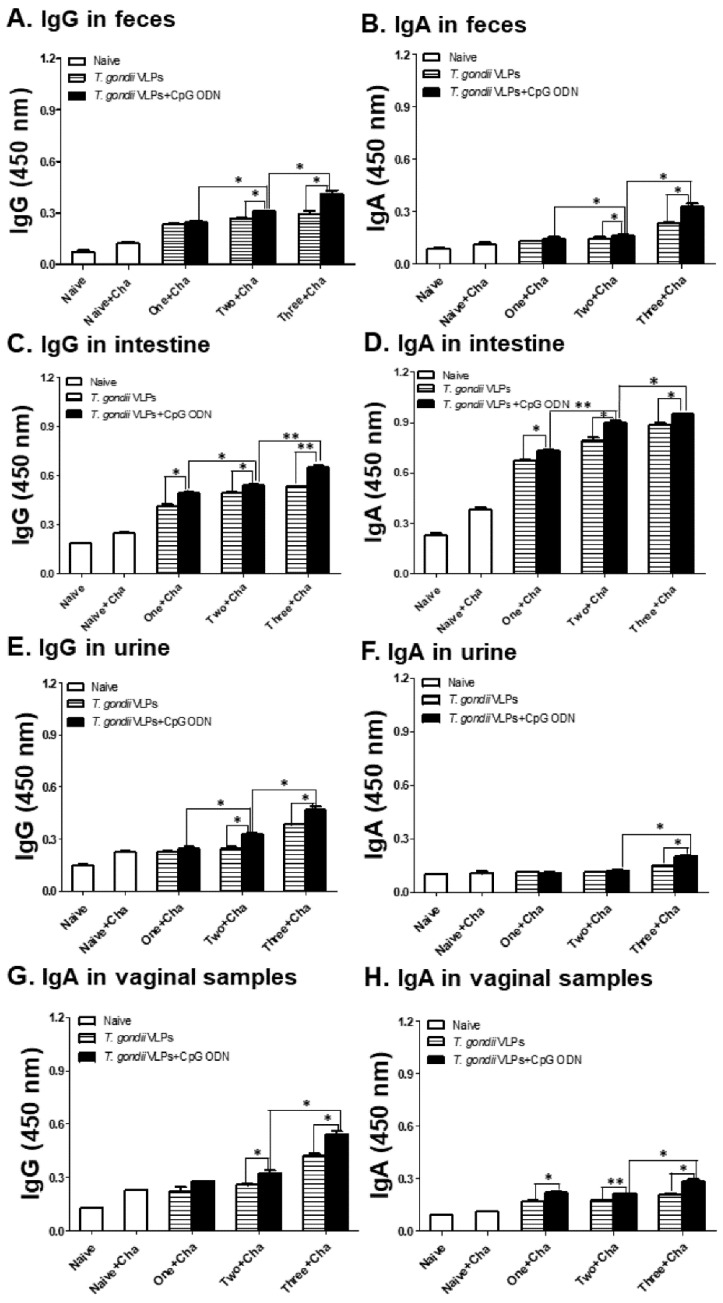
Mucosal antibody responses from naive, naive-challenged (Naïve + Cha), and immunized mice were determined using feces (Figure 2A,B), intestines (Figure 2C,D), urine (Figure 2E,F), and vaginal secretions (Figure 2G,H). Immunization with the adjuvanted T. gondii VLP vaccine elicited higher levels of T. gondii-specific IgG and IgA antibody responses compared with unadjuvanted T. gondii VLPs, indicating adjuvant effects in inducing IgG and IgA antibody responses in all mucosal samples. The highest IgG and IgA antibody responses were elicited upon three immunizations in intestine samples after challenge infection with T. gondii.
Figure 2.
IgG and IgA antibody responses in mucosal samples after immunization with T. gondii VLPs alone or adjuvanted VLPs. Mice (n = 6 per group) were intranasally immunized with T. gondii VLPs alone or adjuvanted T. gondii VLP vaccine one, two, or three times. IgG and IgA mucosal antibody responses from feces (A,B), intestines (C,D), urine (E,F), and vaginal samples (G,H) were determined post-challenge with T. gondii ME49. Results are presented as mean ± SD and statistical significances were denoted using asterisks (* p < 0.05 and ** p < 0.01).
3.3. Immunization with T. gondii VLPs Alone or Adjuvanted VLPs Induces CD4+ and CD8+ T-Cell Expansion
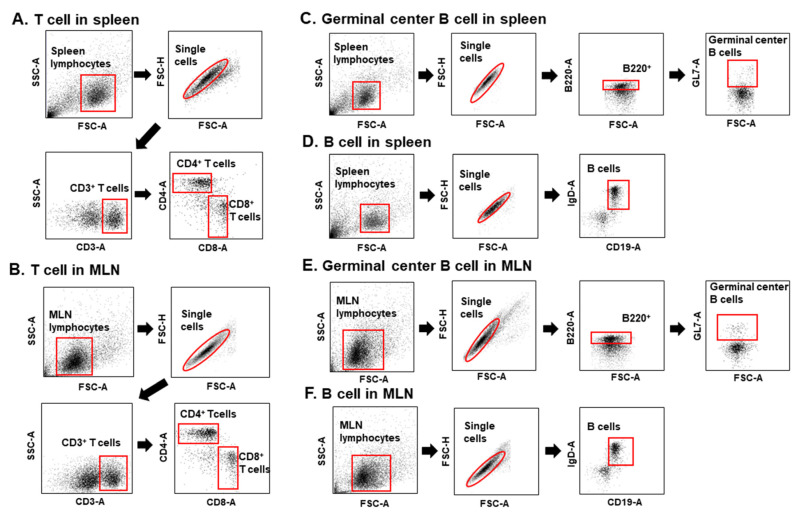
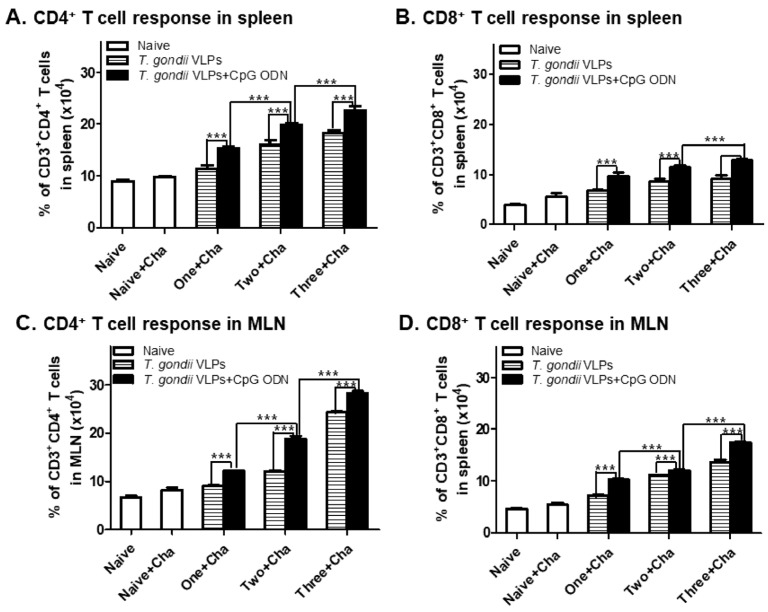
At 35 dpi, spleens and MLNs were collected from mice immunized once, twice, or thrice with either unadjuvanted T. gondii VLPs or CpG-ODN-adjuvanted T. gondii VLPs to determine the effect of adjuvants on CD4+ and CD8+ T-cell frequency. To identify the cells and quantify the percentage, CD4+ T cells, CD8+ T cells, and germinal center B cells in spleen and MLNs were gated as illustrated below (Figure 3). CpG-ODN-adjuvanted T. gondii VLP vaccination showed high levels of CD4+ (Figure 4A,C) and CD8+ (Figure 4B,D) T-cell frequencies in spleen and MLNs compared with T. gondii VLPs alone, indicating that the adjuvant CpG-ODN played a crucial role in inducing CD4+ and CD8+ T-cell responses. Enhancing T-cell expansion in both spleen and MLNs was dependent on adjuvant usage and the number of immunizations.
Figure 3.
The gating strategy of CD4+ T cells, CD8+ T cells, and B cells in spleen and mesenteric lymph nodes (MLNs). Spleen and MLN cells from mice (n = 6) were collected at 35 dpi upon challenge infection with T. gondii ME49 and stained with phenotype-specific antibodies (CD3, CD4, CD8, B220, and GL7). The gating strategies depicted above were applied to identify CD4+ and CD8+ T cells (A,B), germinal center B cells, and B cells from both spleen and MLNs (C–F).
Figure 4.
Immunization with CpG-ODN-adjuvanted T. gondii VLPs enhances CD4+ (A,C) and CD8+ (B,D) T-cell frequencies. BALB/c mice (n = 6 per group) immunized one, two, or three times were challenge-infected with T. gondii ME49, 4 weeks after the last immunization. Spleen and MLN tissues were harvested at 35 dpi and then cell phenotypes were determined by flow cytometry with or without T. gondii antigen stimulation. Results are presented as mean ± SD and statistical significances were denoted using asterisks (*** p < 0.001).
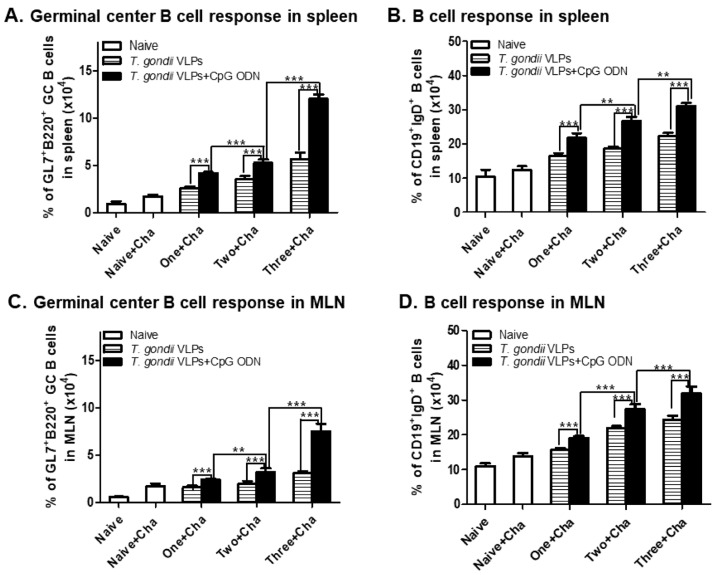
3.4. CpG-ODN-Adjuvanted VLP Vaccination Induced Germinal Center (GC) B-Cell and B-Cell Responses
CpG-ODN adjuvants are known to be potent immune stimulators that activate GC B cells and B cells. To evaluate the CpG-ODN adjuvant effects on GC B-cell and B-cell response inductions, murine splenocytes and MLN cells were harvested at 35 dpi with T. gondii ME49. As seen in Figure 5, adjuvanted VLP vaccination induced higher levels of GC B cells (Figure 5A,C) and B cells (Figure 5B,D) in spleen and MLNs. Induced GC B-cell and B-cell responses were proportional to the number of immunizations, with mice receiving three immunizations showing better responses than mice immunized once or twice. GC B-cell induction was the highest in the spleen (12%) and B cells were induced to the highest extent in MLNs (33.8%), both of which were observed in mice immunized thrice with the adjuvanted VLPs.
Figure 5.
Immunization with the adjuvanted T. gondii VLPs enhances antigen-specific B-cell responses. Immunized mice (n = 6 per group) were infected with T. gondii ME49, 4 weeks after the last immunization. Spleen and MLN tissues were harvested at 35 dpi and then cell phenotypes were determined by flow cytometry. The percentage of GC B (A,C) cells or B cells (B,D) was quantified by subtracting unstimulated cells from stimulated cells. Results are presented as mean ± SD and statistical significances were denoted using asterisks (** p < 0.01 and *** p < 0.001).
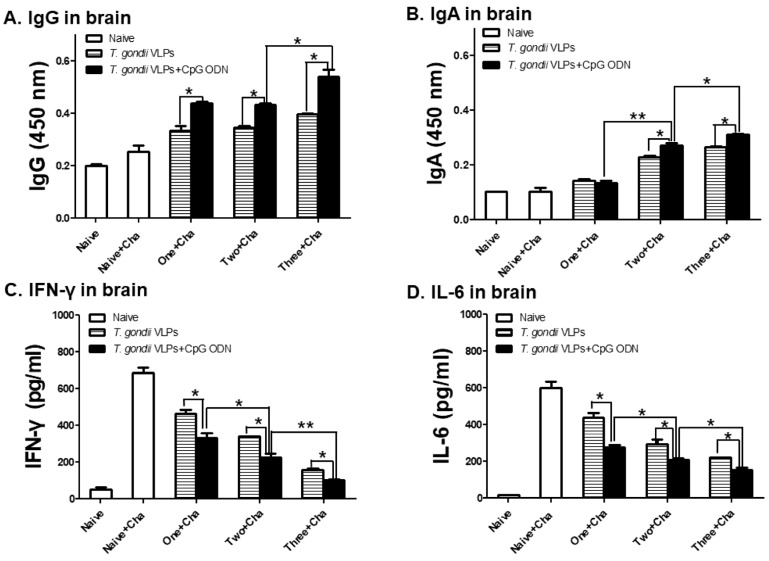
3.5. Antibody and Pro-Inflammatory Cytokine Responses in the Brain
Following T. gondii VLP immunization with or without adjuvants, brains of mice were collected at 35 dpi to evaluate antibody responses and pro-inflammatory cytokine production. Adjuvanted T. gondii VLPs elicited greater T. gondii-specific IgG (Figure 6A) and IgA (Figure 6B) responses in the brain in comparison with unadjuvanted T. gondii VLPs, with the highest antibody inductions occurring in mice immunized thrice. Immunization with the adjuvanted T. gondii VLPs lessened the production of pro-inflammatory cytokines IFN-γ (Figure 6C) and IL-6 (Figure 6D) compared with unadjuvanted T. gondii VLPs alone. T. gondii infection in unimmunized mice resulted in the highest production of these pro-inflammatory cytokines, while a marked decline in their levels became evident with the increasing number of immunizations.
Figure 6.
Antibody responses and pro-inflammatory cytokine responses in the brain upon one, two, and three immunizations. Mice (n = 6 per group) were immunized once, twice, or thrice with either adjuvanted or unadjuvanted T. gondii VLPs, and their brains were collected at 35 dpi with T. gondii. Brain supernatants were prepared and pro-inflammatory cytokines were measured by ELISA. Results (A–D) are presented as mean ± SD and statistical significances were denoted using asterisks (* p < 0.05 and ** p < 0.01).
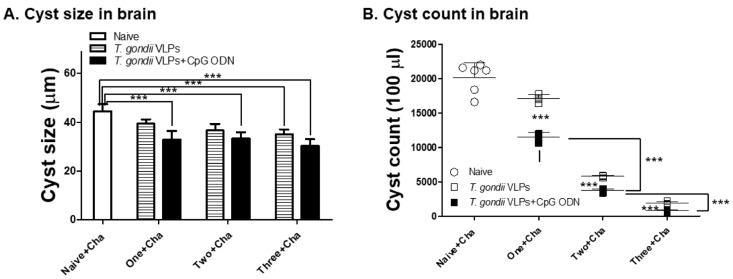
3.6. Adjuvanted T. gondii VLP Vaccination Significantly Reduced Cyst Counts upon Challenge Infection with T. gondii ME49
To further assess the effects of T. gondii VLPs formulated with CpG-ODN adjuvants, key parameters indicating protection such as cyst size and their counts in the brain were measured. Different groups of mice received a different number of immunizations with or without CpG-ODN adjuvants. Upon challenge infection, mice were sacrificed at 35 dpi to evaluate reductions in brain cyst size and their counts under the microscope. Cyst sizes observed from the brains of mice immunized once, twice, or thrice were 35.1 μm, 35.6 μm, and 30.3 μm, respectively (Figure 7A). Compared with the cysts observed from unimmunized control (44.8 μm), significant reductions in cyst sizes were detected from immunized mice which were unaffected by adjuvants. Cyst formation was suppressed to a greater extent in mice immunized with T. gondii VLPs formulated with CpG-ODN adjuvants than those receiving T. gondii VLPs alone (Figure 7B). Cyst counts from immunization with T. gondii VLPs alone once, twice, or thrice were 17,100, 5870, and 2010, respectively. Immunization with adjuvant-formulated VLPs once, twice, or thrice further lessened these counts to 13,400, 3750, and 807, respectively. These results indicated that adjuvant usage and multiple immunizations have a profound effect on reducing total cyst counts.
Figure 7.
CpG-ODN-adjuvanted T. gondii VLP immunization reduced T. gondii cyst sizes and counts. Immunized mice (n = 6 per group) were challenge-infected 4 weeks after the last immunization and sacrificed 35 dpi. The cyst sizes (A) and counts (B) of T. gondii in the brains were measured. Results are presented as mean ± SD and statistical significances were denoted using asterisks (*** p < 0.001).
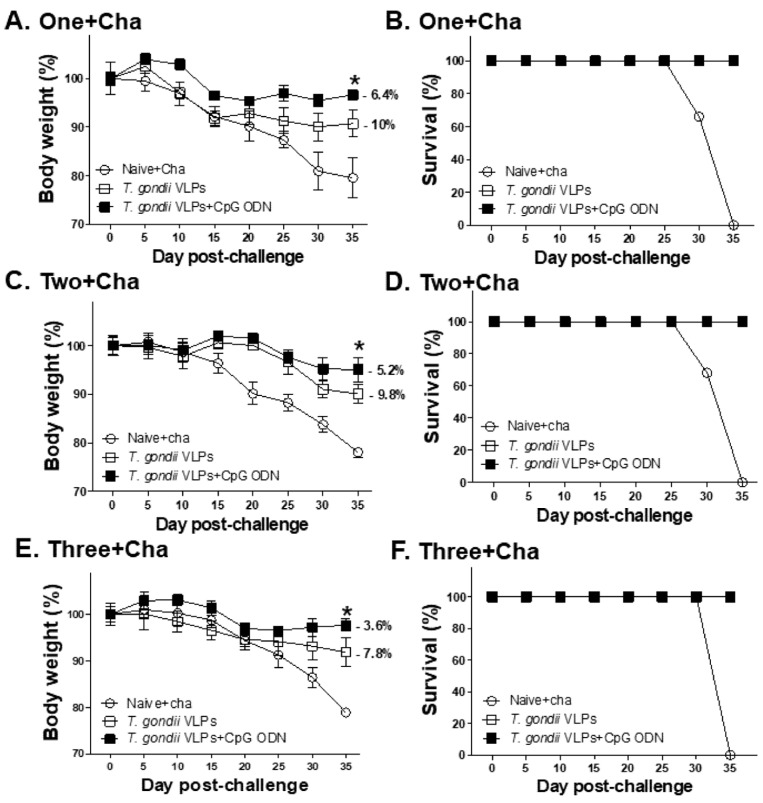
3.7. Protective Efficacy Induced by T. gondii VLPs with or without Adjuvant CpG-ODN
To determine the effects of adjuvants and multiple immunizations on improving protective efficacy, immunized mice were challenged with a lethal dose of T. gondii ME49, 4 weeks after the last immunization. Bodyweight changes (Figure 8A,C,E) and survival rates (Figure 8B,D,F) were monitored for 35 days after challenge infection. Naive mice underwent gradual bodyweight loss, eventually reaching the humane intervention point by 35 dpi. On the contrary, all of the immunized mice survived and were subjected to lesser weight loss than the unimmunized control. Notably, increasing the number of immunizations lessened the bodyweight loss and this was further reduced through adjuvant incorporation. Mice immunized thrice with VLPs alone or adjuvanted VLPs experienced 7.8% and 3.6% bodyweight loss, respectively. Nearly two-fold differences in the percentage of bodyweight loss were observed between mice immunized twice and thrice, indicating the importance of adjuvant and multiple immunizations in inducing protection.
Figure 8.
Protective efficacy induced by T. gondii VLPs alone and adjuvanted T. gondii VLPs following multiple immunizations. Immunized mice (n = 6 per group) were challenge-infected after the final immunization and monitored for 35 days. Bodyweight reductions (A,C,E) and survival rates (B,D,F) from mice immunized once, twice, or thrice were measured during this period. Results are presented as mean ± SD and statistical significances were denoted using asterisks (* p < 0.05).
4. Discussion and Conclusions
Successful vaccine development requires adjuvants that can induce enhanced humoral and cellular immunity [27,28]. In our previous study, immunization with the T. gondii VLPs alone following the prime–boost regimen successfully induced protection by eliciting both humoral and cellular immunity [17,18,26,29]. We hypothesized that introducing the CpG-ODN adjuvant into T. gondii VLP vaccine formulation could induce enhanced protective immune responses, thereby improving the overall vaccine efficacy. Thus, in this study, the effect of the CpG-ODN adjuvant was evaluated using three different immunization regimens in mice, and the resulting protection against T. gondii infection was compared.
The toll-like receptor 9 (TLR9) agonist C-class CpG-ODN embodies the features of both classes A and B, enabling potent antibody induction and cytokine secretion by activating dendritic cells (DCs) and B cells [30,31]. The absence of adverse side effects even in higher eukaryotic organisms such as primates further validates its use as a vaccine adjuvant. Evidently, CpG-ODN-adjuvanted recombinant hepatitis B virus vaccine has recently been approved for clinical use [32]. Thus far, several studies have reported the protective efficacies of protein and DNA vaccines formulated with CpG-ODN adjuvants [33,34,35,36]. In our current study, we found that the efficacy of the CpG-ODN-adjuvanted T. gondii VLP vaccine was significantly enhanced compared with unadjuvanted VLPs after one, two, and three immunizations.
Immunization regimen is another important aspect contributing to vaccine efficacy. The prime–boost strategy has been used in our previous studies where significantly higher levels of IgG and/or IgA antibody responses were elicited after boost compared to the prime, resulting in a significant reduction of cerebral cyst counts and body weight loss upon challenge infection with T. gondii ME49 [17,18,26,29]. Recently, a clinical study assessing the efficacy of a pertussis vaccine reported that enhanced vaccine effectiveness was observed from subjects receiving two vaccine boosters compared with those receiving a single booster vaccination [37]. In our current study, increased number of immunizations using CpG-ODN-adjuvanted T. gondii VLPs significantly increased the protective humoral and cellular immune responses.
Intranasal (IN) immunization is known to elicit mucosa immunity as well as systemic immunity [38]. Mucosal immunity induction is particularly important since T. gondii transmission mainly occurs through oral ingestion of the pathogen [39,40]. The CpG-ODN adjuvant has been proved to be a promising mucosal adjuvant [41,42] and induces better protective mucosal immunity after IN immunization [43,44]. In the current study, upon intranasal immunization, mucosal IgG and IgA antibodies were found to be detected in various mucosal sites. Among them, the highest parasite-specific IgG and IgA antibody responses were detected from intestinal samples of mice immunized three times with the adjuvanted VLPs. Secretions of these antibodies are crucial for protection, as they often serve as the first line of defense against pathogens [45,46]. In line with this notion, the least number of brain cysts were found in mice receiving three immunizations with the adjuvanted VLPs, which possessed the highest level of mucosal antibodies.
Spleen and MLNs are secondary lymphoid organs that activate lymphocytes and initiate an adaptive immune response [47]. The adaptive immunity induced by vaccination depends on the presence of T cells and B cells. In our current study, T. gondii-specific CD4+ T cells, CD8+ T cells, GC B cells, and B-cell responses in both spleen and MLNs were found to be higher in adjuvanted VLP vaccination compared with unadjuvanted VLPs, which were significantly enhanced after two or three immunizations.
Pro-inflammatory cytokines induced by T. gondii infection tend to exacerbate the disease itself or symptoms associated with the disease [25,48,49]. After infection with T. gondii ME49, pro-inflammatory cytokines appear most frequently in the brain. In our current study, adjuvanted VLP vaccination showed significant reductions of pro-inflammatory cytokines IFN-γ and IL-6 in the brain compared with unadjuvanted VLPs. The induction of inflammatory cytokines correlated with bodyweight reduction and cyst counts. The lowest cytokine levels were detected from mice immunized thrice with the adjuvanted VLPs, which correspondingly had the least bodyweight loss and cyst counts. Lesser number of immunizations resulted in greater pro-inflammatory cytokine production, cyst counts, and bodyweight loss as indicated by mice immunized once or twice.
In summary, our results demonstrated that the adjuvants and multiple immunizations are necessary for developing an effective T. gondii VLP vaccine. Multiple immunizations with the CpG-ODN-adjuvanted VLPs conferred protection against a lethal dose of T. gondii ME49 by eliciting strongly enhanced cellular and humoral immunity.
Author Contributions
Conceptualization, F.-S.Q.; methodology, H.-J.K., S.-H.L. and M.-J.K.; validation, F.-S.Q.; formal analysis, H.-J.K. and F.-S.Q.; investigation, H.-J.K. and F.-S.Q.; resources, H.P., H.J. and E.-K.M.; data curation, H.J.K.; writing—original draft preparation, H.-J.K. and F.-S.Q.; writing—review and editing, K.-B.C. and F.-S.Q.; visualization, H.-J.K.; supervision, F.-S.Q.; project administration, F.-S.Q.; funding acquisition, F.-S.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation of Korea (NRF) (2018R1A6A1A03025124, 2018R1A2B6003535), and the Ministry of Health & Welfare, Republic of Korea (HI15C2928, HV20C0085).
Conflicts of Interest
The authors declare no conflict of interest. Hyunwoo Park and Hui Jin are from Health Park Co., Ltd., the company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Black M.W., Boothroyd J.C. Lytic Cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 2000;64:607–623. doi: 10.1128/MMBR.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tenter A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: From Animals to Humans. Int. J. Parasitol. 2000;30:1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubey J.P. Toxoplasma gondii. Elsevier; London, UK: 2020. The history and life cycle of Toxoplasma gondii; pp. 1–19. [DOI] [Google Scholar]
- 4.Winstanley P. Drug Treatment of Toxoplasmic Encephalitis in Acquired Immunodeficiency Syndrome. Postgrad. Med. J. 1995;71:404–408. doi: 10.1136/pgmj.71.837.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCabe R., Chirurgi V. Issues in Toxoplasmosis. Infect. Dis. Clin. N. Am. 1993;7:587. [PubMed] [Google Scholar]
- 6.Alday P.H., Doggett J.S. Drugs in Development for Toxoplasmosis: Advances, Challenges, and Current Status. Drug Des. Dev. Ther. 2017;11:273–293. doi: 10.2147/DDDT.S60973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanjewar D.N., Surve K.V., Maheshwari M.B., Shenoy B.P., Hira S.K. Toxoplasmosis of the Central Nervous System in the Acquired Immunodeficiency Syndrome. Indian J. Pathol. Microbiol. 1998;41:147–151. [PubMed] [Google Scholar]
- 8.Martins-Duarte E.S., Urbina J.A., de Souza W., Vommaro R.C. Antiproliferative Activities of Two Novel Quinuclidine Inhibitors against Toxoplasma gondii Tachyzoites In Vitro. J. Antimicrob. Chemother. 2006;58:59–65. doi: 10.1093/jac/dkl180. [DOI] [PubMed] [Google Scholar]
- 9.Liu Q., Singla L.D., Zhou H. Vaccines against Toxoplasma gondii: Status, Challenges and Future Directions. Hum. Vaccines Immunother. 2012;8:1305–1308. doi: 10.4161/hv.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angus C., Klivington-Evans D., Dubey J., Kovacs J.A. Immunization with a DNA Plasmid Encoding the SAG1 (P30) Protein of Toxoplasma gondii is Immunogenic and Protective in Rodents. J. Infect. Dis. 2000;181:317–324. doi: 10.1086/315186. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Cao A., Li Y., Li X., Cong H., He S., Zhou H. Immunization with a DNA Vaccine Encoding Toxoplasma gondii Superoxide Dismutase (TgSOD) Induces Partial Immune Protection Against Acute Toxoplasmosis in BALB/C Mice. BMC Infect. Dis. 2017;17:403. doi: 10.1186/s12879-017-2507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parmley S., Slifer T., Araujo F. Protective Effects of Immunization with a Recombinant Cyst Antigen in Mouse Models of Infection with Toxoplasma gondii Tissue Cysts. J. Infect. Dis. 2002;185:S90–S95. doi: 10.1086/338464. [DOI] [PubMed] [Google Scholar]
- 13.Cuppari A.F., Sanchez V., Ledesma B., Frank F.M., Goldman A., Angel S.O., Martin V. Toxoplasma gondii Protease Inhibitor-1 (TgPI-1) is a Novel Vaccine Candidate Against Toxoplasmosis. Vaccine. 2008;26:5040–5045. doi: 10.1016/j.vaccine.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Lourenço E.V., Bernardes E.S., Silva N.M., Mineo J.R., Panunto-Castelo A., Roque-Barreira M. Immunization with MIC1 and MIC4 Induces Protective Immunity against Toxoplasma gondii. Microb. Infect. 2006;8:1244–1251. doi: 10.1016/j.micinf.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Xia N., Zhou T., Liang X., Ye S., Zhao P., Yang J., Zhou Y., Zhao J., Shen B. A Lactate Fermentation Mutant of Toxoplasma Stimulates Protective Immunity against Acute and Chronic Toxoplasmosis. Front. Immunol. 2018;9:1814. doi: 10.3389/fimmu.2018.01814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gigley J.P., Fox B.A., Bzik D.J. Long-Term Immunity to Lethal Acute Or Chronic Type II Toxoplasma gondii Infection is Effectively Induced in Genetically Susceptible C57BL/6 Mice by Immunization with an Attenuated Type I Vaccine Strain. Infect. Immun. 2009;77:5380–5388. doi: 10.1128/IAI.00649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang H., Lee S., Chu K., Lee D., Quan F. Virus-Like Particles Expressing Toxoplasma gondii Rhoptry Protein 18 Induces Better Protection Than Rhoptry Protein 4 against, T. gondii Infection. Korean J. Parasitol. 2018;56:429. doi: 10.3347/kjp.2018.56.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S.H., Chu K.B., Kang H.J., Quan F.S. Virus-Like Particles Containing Multiple Antigenic Proteins of Toxoplasma gondii Induce Memory T Cell and B Cell Responses. PLoS ONE. 2019;14:e0220865. doi: 10.1371/journal.pone.0220865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S., Kang H., Lee D., Kang S., Quan F. Virus-Like Particle Vaccines Expressing Toxoplasma gondii Rhoptry Protein 18 and Microneme Protein 8 Provide Enhanced Protection. Vaccine. 2018;36:5692–5700. doi: 10.1016/j.vaccine.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Lee S., Kang H., Lee D., Quan F. Protective Immunity Induced by Incorporating Multiple Antigenic Proteins of Toxoplasma gondii into Influenza Virus-Like Particles. Front. Immunol. 2018;9:3073. doi: 10.3389/fimmu.2018.03073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T., Wu J., Zhu S., Zang G., Li S., Lv X., Yue W., Qiao Y., Cui J., Shao Y. A Novel C Type CpG Oligodeoxynucleotide Exhibits Immunostimulatory Activity in Vitro and Enhances Antitumor Effect in Vivo. Front. Immunol. 2020;11:8. doi: 10.3389/fphar.2020.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vollmer J., Weeratna R., Payette P., Jurk M., Schetter C., Laucht M., Wader T., Tluk S., Liu M., Davis H.L. Characterization of Three CpG Oligodeoxynucleotide Classes with Distinct Immunostimulatory Activities. Eur. J. Immunol. 2004;34:251–262. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- 23.Lee D., Kim A., Lee S., Quan F. Cross-Protection Induced by Toxoplasma gondii Virus-Like Particle Vaccine upon Intraperitoneal Route Challenge. Acta Trop. 2016;164:77–83. doi: 10.1016/j.actatropica.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Lee D.H., Lee S.H., Kim A.R., Quan F.S. Virus-Like Nanoparticle Vaccine Confers Protection against Toxoplasma gondii. PLoS ONE. 2016;11:e0161231. doi: 10.1371/journal.pone.0161231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang H., Chu K., Lee S., Kim M., Park H., Jin H., Moon E., Quan F. Toxoplasma gondii Virus-Like Particle Vaccination Alleviates Inflammatory Response in the Brain upon T. gondii Infection. Parasite Immunol. 2020;42:e12716. doi: 10.1111/pim.12716. [DOI] [PubMed] [Google Scholar]
- 26.Kang H., Lee S., Kim M., Chu K., Lee D., Chopra M., Choi H., Park H., Jin H., Quan F. Influenza Virus-Like Particles Presenting both Toxoplasma gondii ROP4 and ROP13 Enhance Protection against, T. gondii Infection. Pharmaceutics. 2019;11:342. doi: 10.3390/pharmaceutics11070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S., Nguyen M.T. Recent Advances of Vaccine Adjuvants for Infectious Diseases. Immune Netw. 2015;15:51–57. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasquale A.D., Preiss S., Silva F.T.D., Garçon N. Vaccine Adjuvants: From 1920 to 2015 and Beyond. Vaccines. 2015;3:320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang H.J., Chu K.B., Lee S.H., Kim M.J., Park H., Jin H., Quan F.S. Virus-Like Particle Vaccine Containing Toxoplasma gondii Rhoptry Protein 13 Induces Protection against, T. gondii ME49 Infection in Mice. Korean J. Parasitol. 2019;57:543–547. doi: 10.3347/kjp.2019.57.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathur S., Walley K.R., Boyd J.H. The Toll-Like Receptor 9 Ligand CPG-C Attenuates Acute Inflammatory Cardiac Dysfunction. Shock. 2011;36:478–483. doi: 10.1097/SHK.0b013e31822d6442. [DOI] [PubMed] [Google Scholar]
- 31.Klinman D.M. CpG DNA as a Vaccine Adjuvant. Expert Rev. Vaccines. 2003;2:305–315. doi: 10.1586/14760584.2.2.305. [DOI] [PubMed] [Google Scholar]
- 32.Shi S., Zhu H., Xia X., Liang Z., Ma X., Sun B. Vaccine Adjuvants: Understanding the Structure and Mechanism of Adjuvanticity. Vaccine. 2019;37:3167–3178. doi: 10.1016/j.vaccine.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 33.Jongert E., Roberts C.W., Gargano N., Förster-Waldl E., Petersen E. Vaccines Against Toxoplasma gondii: Challenges and Opportunities. Memórias do Instituto Oswaldo Cruz. 2009;104:252–266. doi: 10.1590/S0074-02762009000200019. [DOI] [PubMed] [Google Scholar]
- 34.Spencer J.A., Smith B.F., Guarino A.J., Blagburn B.L., Baker H.J. The use of CpG as an Adjuvant to Toxoplasma gondii Vaccination. Parasitol. Res. 2004;92:313–316. doi: 10.1007/s00436-003-1039-7. [DOI] [PubMed] [Google Scholar]
- 35.EL-Malky M.A., Al-Harthi S.A., Mohamed R.T., Bali M.A.E., Saudy N.S. Vaccination with Toxoplasma Lysate Antigen and CpG Oligodeoxynucleotides: Comparison of Immune Responses in Intranasal Versus Intramuscular Administrations. Parasitol. Res. 2014;113:2277–2284. doi: 10.1007/s00436-014-3882-0. [DOI] [PubMed] [Google Scholar]
- 36.Liu S., Shi L., Cheng Y., Fan G., Ren H., Yuan Y. Evaluation of Protective Effect of Multi-Epitope DNA Vaccine Encoding Six Antigen Segments of Toxoplasma gondii in Mice. Parasitol. Res. 2009;105:267. doi: 10.1007/s00436-009-1393-1. [DOI] [PubMed] [Google Scholar]
- 37.Anis E., Moerman L., Ginsberg G., Karakis I., Slater P.E., Warshavsky B., Gosinov R., Grotto I., Marva E. Did Two Booster Doses for Schoolchildren Change the Epidemiology of Pertussis in Israel? J. Public Health Policy. 2018;39:304–317. doi: 10.1057/s41271-018-0130-3. [DOI] [PubMed] [Google Scholar]
- 38.Ogra P.L., Faden H., Welliver R.C. Vaccination Strategies for Mucosal Immune Responses. Clin. Microbiol. Rev. 2001;14:430–445. doi: 10.1128/CMR.14.2.430-445.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen S., Denkers E. Border Maneuvers: Deployment of Mucosal Immune Defenses Against Toxoplasma gondii. Mucosal Immunol. 2014;7:744–752. doi: 10.1038/mi.2014.25. [DOI] [PubMed] [Google Scholar]
- 40.Innes E.A., Hamilton C., Garcia J.L., Chryssafidis A., Smith D. A One Health Approach to Vaccines Against Toxoplasma gondii. Food Waterborne Parasitol. 2019;15:e00053. doi: 10.1016/j.fawpar.2019.e00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kodama S., Abe N., Hirano T., Suzuki M. Safety and Efficacy of Nasal Application of CpG Oligodeoxynucleotide as a Mucosal Adjuvant. Laryngoscope. 2006;116:331–335. doi: 10.1097/01.mlg.0000194222.93067.f7. [DOI] [PubMed] [Google Scholar]
- 42.Krieg A.M., Yi A., Matson S., Waldschmidt T.J., Bishop G.A., Teasdale R., Koretzky G.A., Klinman D.M. CpG Motifs in Bacterial DNA Trigger Direct B-Cell Activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 43.Gallichan W.S., Woolstencroft R.N., Guarasci T., McCluskie M.J., Davis H.L., Rosenthal K.L. Intranasal Immunization with CpG Oligodeoxynucleotides as an Adjuvant Dramatically Increases IgA and Protection Against Herpes Simplex Virus-2 in the Genital Tract. J. Immunol. 2001;166:3451–3457. doi: 10.4049/jimmunol.166.5.3451. [DOI] [PubMed] [Google Scholar]
- 44.McCluskie M.J., Weeratna R.D., Payette P.J., Davis H.L. Parenteral and Mucosal Prime-Boost Immunization Strategies in Mice with Hepatitis B Surface Antigen and CpG DNA. FEMS Immunol. Med. Microbiol. 2002;32:179–185. doi: 10.1111/j.1574-695X.2002.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 45.Meek B., Klaren V.N., van Haeringen N.J., Kijlstra A., Peek R. IgA Antibodies to Toxoplasma gondii in Human Tears. Invest. Ophthalmol. Vis. Sci. 2000;41:2584–2590. [PubMed] [Google Scholar]
- 46.Jenum P.A., Stray-Pedersen B. Development of Specific Immunoglobulins G, M, and A Following Primary Toxoplasma gondii Infection in Pregnant Women. J. Clin. Microbiol. 1998;36:2907–2913. doi: 10.1128/JCM.36.10.2907-2913.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruddle N.H., Akirav E.M. Secondary Lymphoid Organs: Responding to Genetic and Environmental Cues in Ontogeny and the Immune Response. J. Immunol. 2009;183:2205–2212. doi: 10.4049/jimmunol.0804324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Estato V., Stipursky J., Gomes F., Mergener T.C., Frazão-Teixeira E., Allodi S., Tibiriçá E., Barbosa H.S., Adesse D. The Neurotropic Parasite Toxoplasma gondii Induces Sustained Neuroinflammation with Microvascular Dysfunction in Infected Mice. Am. J. Pathol. 2018;188:2674–2687. doi: 10.1016/j.ajpath.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Hwang Y.S., Shin J., Yang J., Jung B., Lee S.H., Shin E. Characteristics of Infection Immunity Regulated by Toxoplasma gondii to Maintain Chronic Infection in the Brain. Front. Immunol. 2018;9:158. doi: 10.3389/fimmu.2018.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]