Abstract
Background and Purpose
Perampanel is a newly approved anticonvulsant uniquely targeting AMPA receptors, which mediate the most abundant form of excitatory synaptic transmission in the brain. However, the network mechanism underlying the anti‐epileptic effect of the AMPAergic inhibition remains to be explored.
Experimental Approach
The mechanism of perampanel action was studied with the basolateral amygdala network containing pyramidal–inhibitory neuronal resonators in seizure models of 4‐aminopyridine (4‐AP) and electrical kindling.
Key Results
Application of either 4‐AP or electrical kindling to the basolateral amygdala readily induces AMPAergic transmission‐dependent reverberating activities between pyramidal–inhibitory neuronal resonators, which are chiefly characterized by burst discharges in inhibitory neurons and corresponding recurrent inhibitory postsynaptic potentials in pyramidal neurons. Perampanel reduces post‐kindling “paroxysmal depolarizing shift” especially in pyramidal neurons and, counterintuitively, eliminates burst activities in inhibitory neurons and inhibitory synaptic inputs onto excitatory pyramidal neurons to result in prevention of epileptiform discharges and seizure behaviours. Intriguingly, similar effects can be obtained with not only the AMPA receptor antagonist CNQX but also the GABAA receptor antagonist bicuculline, which is usually considered as a proconvulsant.
Conclusion and Implications
Ictogenesis depends on the AMPA receptor‐dependent recruitment of pyramidal–inhibitory neuronal network oscillations tuned by dynamic glutamatergic and GABAergic transmission. The anticonvulsant effect of perampanel then stems from disruption of the coordinated network activities rather than simply decreased neuronal excitability or excitatory transmission. Positive or negative modulation of epileptic network reverberations may be pro‐ictogenic or anti‐ictogenic, respectively, constituting a more applicable rationale for the therapy against seizures.
Keywords: AMPA receptors, epileptic seizures, GABAergic transmission, glutamatergic transmission, perampanel
Abbreviations
- AP‐5
d ‐(−)‐2‐amino‐5‐phosphonopentanoic acid
- BLA
basolateral amygdala
- CNQX
6‐cyano‐7‐nitroquinoxaline‐2,3‐dione
- GYKI‐53655
1‐(4‐aminophenyl)‐3‐methylcarbamyl‐4‐methyl‐3,4‐dihydro‐7,8‐methylenedioxy‐5H‐2,3‐benzodiazepine hydrochloride
- IN
interneuron
- ISI
interspike interval
- NBQX
2,3‐dioxo‐6‐nitro‐1,2,3,4‐tetrahydrobenzo[f]quinoxaline‐7‐sulfonamide
- PN
pyramidal neuron
What is already known
Perampanel is a novel anticonvulsant that non‐competitively inhibits AMPA receptors.
What this study adds
Epileptic seizure stems from network reverberations tuned by dynamic AMPAergic and GABAergic transmissions.
Perampanel and the selective AMPA antagonist CNQX eliminate seizures by interruption of network reverberations.
What is the clinical significance
Epileptic seizures originate from resonating activities between excitatory and inhibitory neurons rather than excitation/inhibition “imbalance”.
Drugs that promote and disrupt network oscillations may be potentially pro‐ictogenic and anti‐ictogenic respectively.
1. INTRODUCTION
Epilepsy is a heterogeneous neurological disorder defined by self‐repetitive, excessive and synchronized neural network activities (French, Gernandt, & Livingston, 1956; McCormick & Contreras, 2001; Penfield & Jasper, 1954). In the cellular level, it is characterized by neuronal oscillating burst discharges (McCormick & Contreras, 2001; Timofeev & Steriade, 2004). In theory, the self‐repetitiveness could stem from the activities of “oscillator” neurons, which fire spontaneous pacemaking‐like discharges or a group of “resonator” neurons that are synaptically interconnected and collaboratively make a network with “circulating” burst discharges (Buzsáki & Draguhn, 2004; Eggers, 2007; Llinás, 1988). The primary origin of the self‐repetitiveness is an unresolved but a fundamental issue in the generation and/or spreading of epileptic seizures.
Glutamate is the most abundant excitatory neurotransmitter in the mammalian brain and chiefly binds to AMPA, kainate and NMDA ionotropic receptors. Perampanel is an anticonvulsant with a unique pharmacological profile, namely, it is a non‐competitive antagonist of AMPA receptors (Hanada et al., 2011; French et al., 2012; Krauss et al., 2012; Potschka & Trinka, 2019). Perampanel has been demonstrated to inhibit the AMPA receptor‐mediated current in single neurons (Barygin, 2016; Ceolin et al., 2012; Chen, Matt, Hell, & Rogawski, 2014), reduce in vitro epileptiform discharges with extracellular recording in human neocortical slices (Augustin et al., 2018) and decrease seizure behaviours in experimental models (Citraro et al., 2017; Hanada et al., 2011; Russmann, Salvamoser, Rettenbeck, Komori, & Potschka, 2016; Twele, Bankstahl, Klein, Römermann, & Löscher, 2015; Wu, Nagaya, & Hanada, 2014). Although the molecular action of perampanel on AMPA receptors and the ameliorating effect of perampanel on seizure discharges or behaviours have been separately documented and epileptiform discharges can be inhibited by the other AMPA antagonists (Avoli et al., 1996; Gean, 1990), it remains elusive how the anti‐epileptic effect of perampanel is achieved by inhibition of AMPAergic transmission at the neural circuit level. If the occurrence of seizures is simplistically based on increased excitation or decreased inhibition, for instance, then block of AMPAergic transmission onto an excitatory neuron and onto an inhibitory neuron would theoretically lead to an anti‐seizure and pro‐seizure effect respectively. In addition, it was reported that light‐triggered excitatory (Krook‐Magnuson, Armstrong, Oijala, & Soltesz, 2013; Tønnesen, Sørensen, Deisseroth, Lundberg, & Kokaia, 2009; Wagner, Truccolo, Wang, & Nurmikko, 2015) or inhibitory (Krook‐Magnuson et al., 2013; Lu et al., 2016; Shiri, Manseau, Lévesque, Williams, & Avoli, 2016) neuronal activities may induce, reduce or have no apparent effect on seizure discharges. The dichotomous increased‐excitation/decreased‐inhibition model thus does not seem to give a reasonable explanation for these phenomena.
The basolateral amygdala contains excitatory (glutamatergic) pyramidal neurons and inhibitory (GABAergic) interneurons and is one of the most common epileptogenic foci (French et al., 1956; Penfield & Jasper, 1954), as well as the most investigated structure for temporal lobe epilepsy (McIntyre & Gilby, 2008). Basolateral amygdala pyramidal neurons send divergent output to other pyramidal neurons and interneurons (Sah, Faber, Lopez de Armentia, & Power, 2003). Interneurons are connected by gap junctions and collectively send strong inhibitory output to pyramidal neurons (McDonald & Betette, 2001; Muller, Mascagni, & McDonald, 2005). Interneurons are also more prone to show burst discharges (Rainnie, 1999; Rainnie, Mania, Mascagni, & McDonald, 2006), which may in turn pre‐condition pyramidal neurons for burst discharges in the next moment. The basolateral amygdala therefore constitutes an ideal pyramidal–inhibitory neuronal resonating network for the study of the epileptiform or reverberating burst discharges. With in vivo and in vitro seizure models of 4‐aminopyridine (4‐AP) and electrical kindling, we found that pyramidal neurons recruit follower pyramidal neurons and interneurons into network oscillations with AMPAergic transmission. The pyramidal–inhibitory neuronal reverberating activities are chiefly characterized by burst discharges in interneurons that result in inhibitory postsynaptic potentials (IPSPs) in pyramidal neurons. Perampanel decreases the duration and height of “paroxysmal depolarizing shift” (PDS) (Goldensohn & Purpura, 1963) especially in pyramidal neurons and, most intriguingly and counterintuitively, completely inhibits recurrent IPSPs in excitatory pyramidal neurons and burst activities in interneurons to inhibit ictogenesis and seizure behaviours. The initiation and termination of epileptic seizures therefore should involve dynamic constructive and destructive aspect of network reverberations, which are collectively tuned by the ongoing strength of glutamatergic and GABAergic transmission in the system.
2. METHODS
2.1. Animal care and acute brain slice preparation
All experiments were done with the approval of the Chang Gung University Animal Care and Use Committees (Approval Number: CGU106‐081, CGU107‐277, and CGU108‐195). Animal studies are reported in compliance with the ARRIVE guidelines (Percie du Sert et al., 2020) and with the recommendations made by the British Journal of Pharmacology (Lilley et al., 2020). All efforts were made to minimize animal suffering. The animals not included in experiments due to technical reasons (e.g. post‐surgical complications, morbidity, malfunction of implanted cannula or failure of meeting epileptic criteria) were killed with carbon dioxide. Animals were raised in a vivarium with controlled 12‐h dark/light cycle with water and food ad libitum. Wistar rats and C57BL/6 mice were purchased from BioLASCO Taiwan Co. Ltd., Taiwan. For acute brain slice preparation, the brain was obtained from C57BL/6 mice of both sexes (aged p17–25) under isoflurane anaesthesia and put in ice‐cold oxygenated (95% O2/5% CO2) choline cutting solution (containing [in mM] 87 NaCl, 37.5 choline chloride, 25 NaHCO3, 25 glucose, 2.5 KCl, 1.25 NaH2PO4, 7 MgCl2, and 0.5 CaCl2). Coronal slices containing basolateral amygdala (270 μm in thickness, Niittykoski, Nissinen, Penttonen, & Pitkänen, 2004) were then obtained on a vibratome (Leica VT1200S, Germany) and brought to incubation in the oxygenated choline cutting solution for 20 min at 30°C and then in oxygenated saline (containing [in mM] 125 NaCl, 26 NaHCO3, 25 glucose, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2 and 2 CaCl2) for 15 min at 30°C.
2.2. Electrophysiological and morphological identification of basolateral amygdala pyramidal neurons and interneurons
We first conducted whole‐cell current‐clamp recordings to electrophysiologically identify the neurons. Immediately after membrane breakthrough, a suprathreshold depolarizing step current of sufficient magnitude (Mahanty & Sah, 1998) was injected at membrane potential of −60 mV to determine the firing properties and compare this with the literature. Unlike pyramidal, which are larger and show spike adaptation, interneurons possess smaller diameter and show little adaptation with depolarization (Mahanty & Sah, 1998; Sah et al., 2003; Washburn & Moises, 1992). For additional morphological experiments, we filled the recording pipette with red fluorescent dye sulforhodamine 101 (50 nM, Kang et al., 2010) in K+‐based solution to visualize basolateral amygdala neurons on a fluorescence microscope. The photos were captured by Canon EOS D70 digital camera and edited by ImageJ software (National Institutes of Health, USA, RRID:SCR_003070). With depolarizing step current, neurons with a diameter around 10 μm and showing minimal spikes adaptation were identified as interneurons, while neurons with a diameter greater than 12 μm and firing spike with adaptation were identified as pyramidal neurons.
2.3. Electrophysiological recordings
The fresh basolateral amygdala slice was put in a recording chamber with oxygenated saline perfused by a peristaltic pump (Gilson Medical Electric, USA) at a rate of ~5 ml·min−1 and visualized with a ×4 objective, with individual neurons seen with a ×60 water immersion objective on an upright microscope (Olympus BX51WI, Japan). The neurons were patched in whole‐cell current‐clamp mode at room temperature with 3‐ to 6‐MΩ pipettes filled with K+‐based solution (in mM, 116 KMeSO4, 6 KCl, 2 NaCl, 20 HEPES, 0.5 EGTA, 4 MgATP, 0.3 NaGTP, 10 NaPO4 creatine and pH 7.25 adjusted with KOH). The patch pipettes were made from borosilicate capillaries (Harvard Apparatus, USA; 1.65‐mm outer diameter and 1.28‐mm inner diameter) and by a micropipette puller (DMZ‐Zeitz‐Puller, Germany). In vivo kindling denotes a process of repeated focal stimulation of a forebrain structure such as the amygdala to induce seizures in behaving animals (Goddard, McIntyre, & Leech, 1969; McIntyre & Gilby, 2008). In basolateral amygdala slices, focal electrical stimulation that mimics in vivo kindling stimulation was applied to study the discharge profiles. Modified from the protocols in the literatures (Fujiwara‐Tsukamoto, Isomura, Imanishi, Fukai, & Takada, 2007; Stasheff, Bragdon, & Wilson, 1985), the “kindling‐mimicking in vitro stimulation” is a 1‐s train of electrical stimuli at an intensity of 400 μA with frequency of 60 Hz, with each stimulus set as a monophasic rectangular pulse with 0.4‐ms pulse width. The stimulation was delivered at basolateral amygdala via a stimulus isolator (World Precision Instruments A356, USA) with two glass electrodes filled with 0.9% NaCl, one electrode placed into the basolateral amygdala and the other placed right above basolateral amygdala without touching the tissue. The post‐stimulation epileptiform discharges usually lasted no more than 1 min, and we always waited for at least 4 min before delivery of the next “kindling‐mimicking” stimulation. “Pair recording” denotes simultaneous recording of a pair of neurons. Data were collected with a Multiclamp 700B amplifier (MDS Analytical Technologies, USA), sampled at 20 kHz, filtered at 5 kHz, and digitized at 10–20 kHz with a Digidata‐1440 analogue/digital interface and pCLAMP software (MDS Analytical Technologies, USA, RRID:SCR_011323); 10 μM of d‐(−)‐2‐amino‐5‐phosphonopentanoic acid (AP‐5) and 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (CNQX) were used to ensure sufficient inhibition of NMDA and AMPA receptors respectively (Johansen, Drejer, Wätjen, & Nielsen, 1993; Lenz et al., 2016; Lodge et al., 1988; Tateno & Robinson, 2007). The dead space in recording chamber was limited to 1 ml. The electrophysiological recordings usually would last at least 60 min in decent conditions.
2.4. Materials
All drugs were from Tocris Bioscience (UK) or Sigma‐Aldrich (USA), dissolved in dimethylsulfoxide (Sigma‐Aldrich, USA) or distilled water as stocks, and diluted 1:1,000 into the bath solution for desired concentration before conducting the experiments. The final concentration of dimethylsulfoxide (0.1%) did not have a significant effect on the experimental results. The anaesthetic tiletamine and zolazepam (Zoletil®) were purchased from Virbac (France), and isoflurane was purchased from Aesica Queenborough Ltd. (UK). All chemical salts in this study were purchased from Sigma‐Aldrich (USA).
2.5. The concentration of perampanel used for slice recording
In clinical studies of therapeutic drug monitoring for perampanel, the commonly prescribed dosage range is ~0.05–0.2 mg·kg−1, which results in serum concentrations of ~1–5 μM (Gidal, Ferry, Majid, & Hussein, 2013; Patsalos, Gougoulaki, & Sander, 2016). With 95% protein binding, the highest free therapeutic concentration of perampanel in the CSF is therefore ~250 nM (Hibi et al., 2012). The binding rate (~1.5 × 105 M−1·s−1) and the unbinding rate (~0.6 s−1) of perampanel are relatively slow (Chen et al., 2014). Therefore, it is difficult to obtain the local concentration (e.g. in synaptic clefts) of ~250 nM and its effect within a reasonable period of time. This is probably part of the reason why the estimate of IC50 values for perampanel is quite variable in different studies and ~1–10 μM of perampanel were used in brain slices in most cases (e.g., Barygin, 2016; Ceolin et al., 2012; Chen et al., 2014; Palovcik & Phillips, 1986; Zwart et al., 2014). We therefore used 5 μM of perampanel for brain slice recording to ensure sufficient drug penetration and binding to target receptors with the continuous perfusion system. We have, nevertheless, endeavoured to explore the effect of 250 nM of perampanel in slices and obtained similar results after stable perfusion for ~30 min (Figure S1). 5 μM thus is used to ensure sufficient action of the drug within a realistic period of time to avoid jeopardizing the integrity of the slice with prolonged recordings.
2.6. Implantation of microinjection cannula
Under anaesthesia by a mixture of tiletamine and zolazepam (Zoletil®)/xylazine (Zoletil® 20 to 40 mg·kg−1] and xylazine [5 to 10 mg·kg−1]. A stereotactic frame (Stoelting Co., USA) was used to mount the rats with skull exposed following the sterilization procedures. Then a hole above basolateral amygdala was drilled (AP, −2.8 mm; ML, 5 mm from bregma; and DV, 8 mm from dura). A stainless‐steel cannula held by plastic rod was then slowly inserted to the level of basolateral amygdala and the plastic rod was secured with dental cements and screws fixed on the skull. The scalp wound was then closed. Analgesia was provided by subcutaneous injection of bupivacaine (0.25 mg in 50 μl) around the wound. We also provided secure housing with temperature control by an infrared heat lamp. Sunflower seeds and soft diets were provided as post‐surgical nutritional supports. The behaviour studies were conducted at least 7 days after surgery.
2.7. 4‐AP seizure model and microinjection of pharmacological agents for animal behaviour study
The experiments were performed at a fixed time of the day (afternoon) in an open‐field test arena measuring 45 × 45 × 40 cm and made of Plexiglas and accompanied with horizontal and vertical video recording systems. Before the test session, the rat was connected to an inner insertion cannula with a micro‐syringe and then a microinjection pump (Harvard Apparatus) on polyethylene tube. After microinjection of pharmacological agents (0.5 μl·min−1), the rat was transferred to the centre of the open‐field arena. The testing session lasted for 90 min. Two sessions per day were conducted on the same rat, with the first being 4‐AP (2 mM, 2 μl) alone and the last being 4‐AP with either CNQX (200 μM, 2 μl) or perampanel (80 μM, 2 μl). To assure a complete action of perampanel (Chen et al., 2014), perampanel was pre‐injected 2 h before the experimental session having perampanel. The maximal injected volume did not exceed 8 μl·day−1. The behavioural events are counted according to the modified Racine's stages (Racine, 1972):‐ Stage 0, normal behaviour; Stage 1, facial clonus and mouth clonus; Stage 2, head nodding; Stage 3, unilateral forelimb clonus; Stage 4, bilateral forelimb clonus with rearing; Stage 5, rearing and falling; and Stage 6, wild running and jumping. As almost all seizure behaviours occurred within the first 30 min after microinjection, the number of behavioural events corresponding to specific Racine's stages was documented within 60 min after microinjection. On the day of experiments, when an animal showed seizures Stage 4 or above following microinjection of 4‐AP, it was considered qualified for subsequent experiments with 4‐AP plus CNQX or 4‐AP plus perampanel on the same day. The rats would receive at most only two microinjections on 1 day (4‐AP, and 4‐AP plus either CNQX or perampanel). The experiments of CNQX and perampanel were never conducted on the same day in the same rat. The qualified animals would consistently show seizures of at least Stage 4 upon consecutive microinjection of 4‐AP on the same day. Rats not meeting the qualification criteria were discarded. Immediately after microinjection of 4‐AP, usually all seizure behaviours occurred within the first 30 min. We always waited for at least 4 h for full recovery before proceeding to the experimental sessions of 4‐AP plus CNQX or 4‐AP plus perampanel. The design of intraday experiments ensures that only animals showing the strongest seizure activities are tested for a reliable assessment of seizure amelioration by drugs. Upon completion of one set of behavioural experiments on the day, we would let the rats rest for at least 7 days before conducting the next set of experiments. In Figures 1 and 3, out of seven rats completed the two sets of experiments, namely, 4‐AP and 4‐AP plus CNQX on 1 day and 4‐AP and 4‐AP plus perampanel on another day (so that Rat Nos. 1 to 3 in Figure 1 are of the same three rats). We have another two rats that completed only 4‐AP and 4‐AP plus CNQX experiments (Rat Nos. 4 and 5 in Figure 1), and another two rats completed only 4‐AP and 4‐AP plus perampanel (Rat Nos. 6 and 7 in Figure 1). For a better assessment of the reduction of seizure by CNQX and perampanel, the data with the most severe seizure activity induced by 4‐AP were used for analysis. All behavioural experiments were performed in daytime from 8:00 a.m. to 8:00 p.m. No statistical analysis was performed for the behavioural data, which were always compared for different treatments in the same rats (Medina‐Ceja, Cordero‐Romero, & Morales‐Villagrán, 2008).
FIGURE 1.
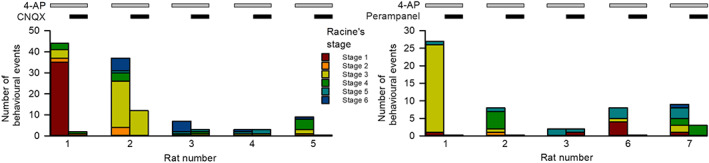
AMPA receptor antagonists ameliorate 4‐aminopyridine (4‐AP)‐induced seizure behaviours in free‐moving rats. Unilateral microinjection of 4‐AP (2 mM) directly into the basolateral amygdala (BLA) in free‐moving rats results in seizure behaviours, which are defined by the modified Racine's stages (see Section 2). The number of behavioural events corresponding to specific Racine's stages was counted within 60 min immediately after microinjection. Co‐injection of 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (CNQX) (200 μM, n = 5 rats) or perampanel (80 μM, n = 5 rats) readily reduces both the occurrence and the severity of seizure behaviours. Rat Numbers 1–3 in the analyses of CNQX and perampanel are of the same three rats
FIGURE 3.
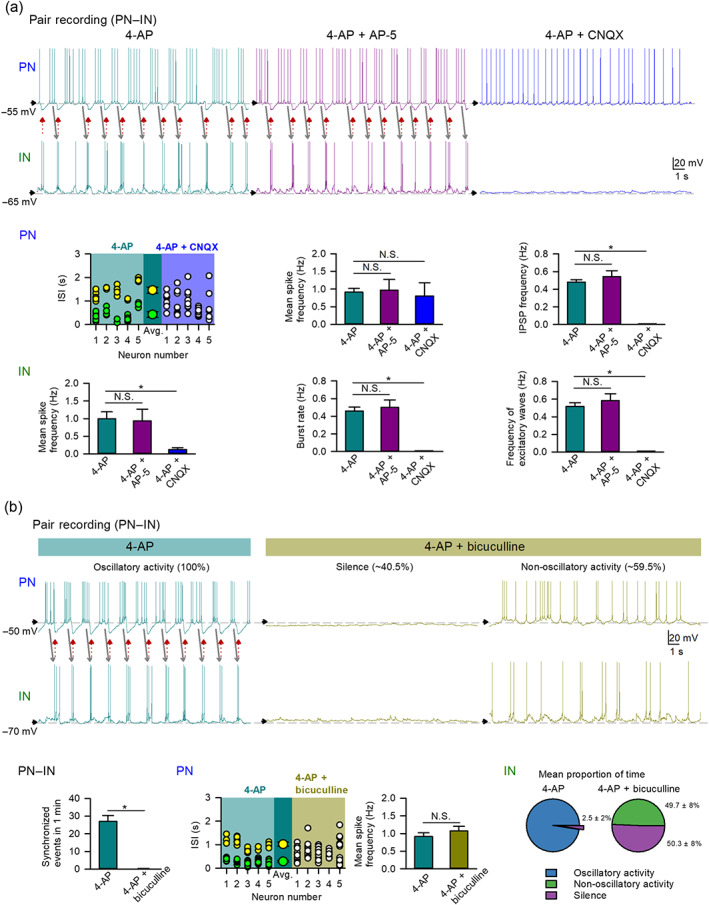
4‐Aminopyridine (4‐AP)‐induced network oscillation requires AMPAergic and GABAergic synaptic transmission. (a) AMPA antagonist 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (CNQX) but not NMDA receptor antagonist AP‐5 blocks 4‐AP‐induced oscillatory activities in pair recording of a pyramidal neuron (PN) and an interneuron (IN) (see red and grey arrows for reverberant and synchronous activities). 4‐AP‐triggered spikes, burst discharges, and excitatory postsynaptic potentials (EPSPs) in INs are abolished by CNQX (10 μM, n = 6) but not AP‐5 (10 μM, n = 7). In PNs, CNQX (10 μM) completely inhibits the recurrent inhibitory postsynaptic potentials (IPSPs) (n = 9) but preserves spikes induced by 4‐AP (n = 7, only those having baseline membrane potential more depolarized than −60 mV used in the analyses of mean spike frequency), while AP‐5 (10 μM, n = 6) has little effect on the recurrent IPSPs and mean spike frequency. Plots of interspike interval (ISI) in 10‐s recording session of PNs (n = 5) show 4‐AP‐induced two clusters of ISIs (yellow and green for long and short ISIs, respectively) eliminated by co‐application of CNQX (10 μM, n = 5, white; only those having baseline membrane potential more depolarized than −55 mV were included in ISI analyses). The two larger symbols represent the mean of long ISIs and that of short ISIs. N.S. P > 0.05; * P < 0.05, Kruskal–Wallis test followed by pairwise Mann–Whitney U test. Except for the analysis of ISI, the analyses of 4‐AP groups are of the same PNs (n = 26–28) and INs (n = 18) in Figure 2. (b) GABAA receptor antagonist bicuculline reduces 4‐AP‐induced reverberating activities. A representative PN–IN pair recording shows recurrent and synchronous burst discharges in INs and IPSPs and clustered spikes in PNs induced by 4‐AP (grey and red arrows). We then define the “oscillatory activity” of INs by having excitatory waves (burst discharges or EPSPs) synchronized with IPSPs in PNs. With co‐application of bicuculline (10 μM), the IN would either show no spikes and no bursts (with the paired PN also firing no spikes, i.e. the “silence”) or exhibit discharges (with the paired PN also firing spikes that are not regulated by the IPSP, i.e. the “non‐oscillatory activity”). The proportion of time staying in different discharge modes is indicated at the top of the representative recordings. Analyses of continuous recording (200 s) of IN activities from the same PN–IN pairs (n = 5 pairs) reveal that co‐application of bicuculline completely eliminates the initial 4‐AP‐induced persistent oscillatory activity (~97.5 ± 2% of the time) and results in either silence (~49.7 ± 8% of the time) or non‐oscillatory activity (~50.3% of the time) of INs (the pie charts). Co‐application of bicuculline eliminates all synchronous events in PN–IN network (n = 5 PN–IN pairs) but preserves the mean spike frequency of PN. The analyses of the PN mean spike frequency in the 4‐AP group are of the same PNs (n = 26) in Figure 2, and in the 4‐AP plus bicuculline (n = 5) are from traces of PNs when the paired INs show non‐oscillatory activity. Plots of interspike interval (ISI) in 10‐s recording session of PNs (n = 5) show 4‐AP‐induced two clusters of ISIs (yellow and green for long and short ISIs, respectively) eliminated by co‐application of bicuculline (10 μM, n = 5, white). N.S. P > 0.05; * P < 0.05, Wilcoxon signed‐rank test for synchronized events, and Mann–Whitney U test for the mean spike frequency
2.8. Data analysis and statistics
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). Slice recording data were analysed with pCLAMP 10 (MDS Analytical Technologies), Excel 2013 (Microsoft) and SigmaPlot 12 (Systat Software Inc., RRID:SCR_003210) softwares. The spikes were detected using the Threshold Search Tool in pCLAMP 10. A burst is defined by the presence of at least one spike on top of rapidly summating high‐frequency EPSPs (modified from Rainnie, 1999). EPSPs and IPSPs with amplitude larger than 3 mV were included. Events of excitatory waves include events of burst discharges and EPSPs. Analyses of cPSP (composite postsynaptic potentials) include burst discharges, EPSPs and IPSPs. In Figure 3b, the “oscillatory activity” of interneuron is defined by having at least two excitatory waves (burst discharges and EPSPs) in a 5‐s time frame that are synchronized with IPSPs in pyramidal neurons. The “silence” of interneuron refers to time period of at least 5 s without any occurrence of burst discharge or EPSPs. The “non‐oscillatory activity” of interneuron is defined as a time period in which interneuron shows rhythmic burst discharges or EPSPs that are not synchronized with postsynaptic events in pyramidal neurons. The analysis of interspike interval was based on a 10‐s stable recording of pyramidal neuron with baseline membrane potential more positive than −55 mV. The interspike intervals separating two spikes with IPSP in between are defined as the long interspike intervals for calculation of the long interspike interval. The interspike intervals separating two spikes without IPSP in between are defined as short interspike intervals for calculation of the mean short interspike interval. Synchronized events are defined as two postsynaptic events, one from each neuron in a paired recording, with the difference in onset less than 50 ms. For the pre‐stimulation and post‐stimulation parameters, each analysis was based on the data averaged from a 45‐s continuous stable recording just before and after the stimuli respectively. The stimulation‐induced plateau height is defined as the difference between the peak baseline potential and the pre‐stimulation baseline potential. The pre‐stimulation baseline potential is calculated as the average membrane potential in 1‐s immediately before stimulation. The stimulation‐induced plateau duration is defined as the time for returning to the pre‐stimulation baseline potential after stimulation. Group size is denoted as n, where in slice recording, n number defines the number of neurons (no more than two neurons per animal) received the treatments, and in behavioural experiments, n number denotes the number of rats received the treatments. The group size in each experiment was not estimated prospectively. The behavioural data are shown individually for each rat. For brain slice recording, the neurons may receive 4‐AP or kindling‐mimicking electrical stimulation in saline as a control, followed by different treatments. In these cases, the control group is presented as an average of the controls of different treatments for simplicity and thus has a larger group size. Most analyses of parameters from the same group of neurons in the same experimental settings have the same group sizes (analyses of spike frequency, burst and excitatory waves in interneurons in Figures 2b and 3a, and the analyses of stimulation‐induced plateaus in both interneurons and pyramidal neurons in Figures 4b and 5c). In Figures 2b, 3a and 4b, the group sizes for analysis of mean spike frequency of PN are different from analyses of other parameters because only the pyramidal neurons with baseline membrane potential more depolarized than −60 mV were included in the analysis of the mean spike frequency. The group sizes for analyses of interspike interval are different from those of other analyses because only pyramidal neurons with baseline membrane potential more depolarized than −55 mV were analysed (scattered plots in Figures 2b and 3a,b). In Figure 4b, the group size for burst analysis differs from analyses of spikes and excitatory waves because only interneurons having burst discharges are included in the burst analysis. The data collection and evaluation were inevitably performed in an unblinded fashion because the experimenters must be aware of the experimental conditions for proper execution of the experiments. However, the same criteria were always applied when evaluating the responses of different drugs in the same experimental settings. All slices and animals were randomly selected for different experimental sessions before performing the experiments. All statistical comparisons were performed on data having group size of at least five (n ≥ 5). The group size is the number of independent values and statistical analysis was done using these independent values. No approach was performed for data transformation or reduction of unwanted data variation and outliers. Non‐parametric two‐tailed Wilcoxon signed‐rank test was used to evaluate matched data (Figure 3b, synchronized events, and Figure S1b). In the plots of multiple groups, non‐parametric statistical comparison was evaluated by Kruskal–Wallis test followed by pairwise Mann–Whitney U test for two independent groups (Figures 2b, 3a, 4b, and 5c; Kodama et al., 1999; Snyder et al., 2003; Picard et al., 2019) or by Friedman test followed by pairwise Wilcoxon signed‐rank test for matched data (Figure S1a). Post hoc tests were only conduced when the Kruskal–Wallis test or Friedman test is significant (P < 0.05). Statistical tests performed are reported in figure legends. All data are shown as means ± SEM. All tests are two sided and P values <0.05 are indicative of statistical significance for all comparisons.
FIGURE 2.
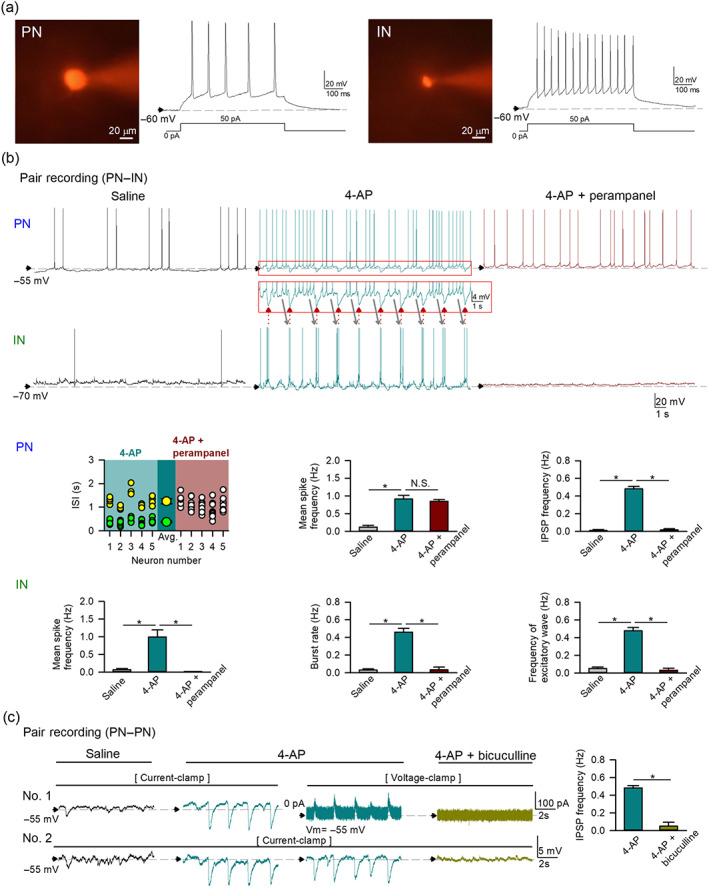
Perampanel completely inhibits 4‐aminopyridine (4‐AP)‐induced recurrent inhibitory postsynaptic potentials (IPSPs) in pyramidal neurons (PNs) of basolateral amygdala (BLA). (a) Morphological and electrophysiological identification of PNs and interneurons (INs) in the BLA (see Section 2 for details). The photos show intracellular labelling of a BLA PN (left) and an IN (right) by sulforhodamine 101 in the recording pipette. The longest diameter of a PN (usually >20 μm) would in general exceed that of an IN (~10 μm, Asprodini, Moises, 1992, Rainnie, Shinnick‐Gallagher & Washburn, 1993). In response to suprathreshold depolarizing current injection, PNs usually show low‐frequency spikes with adaptation, while INs exhibit high‐frequency spikes with little adaptation. (b) Pair recording of a PN and an IN shows reverberating activities between the two types of neurons induced by 4‐AP (10 μM, top rows). In the presence of 4‐AP, the PN fires clusters of spikes that are followed by IN burst discharges (grey arrows). Recurrent IN burst discharges are synchronized with IPSPs in the PN (red arrows) that apparently interrupt PN spikes (traces in the red box are magnified to show the reverberant and synchronous activities). n = 26 and 28 PNs for analyses of spike and IPSP frequency, respectively (only those having baseline membrane potential more depolarized than −60 mV used in the analyses of mean spike frequency). INs: n = 18. Co‐application of perampanel (5 μM, n = 6 and 5 for PNs and INs, respectively) completely inhibits all IPSPs in PNs and burst discharges in INs but preserves PN spikes. In the study of perampanel effect, the analysis of the interspike interval (ISI) is also performed for the recordings with baseline membrane potential more positive than −55 mV. The plot of ISIs in 10‐s recording session of PNs (n = 5) demonstrates two clusters of ISIs, namely, a short ISI cluster (green) and a long ISI cluster (yellow), in the presence of 4‐AP. The two larger symbols represent the mean of long ISIs and that of short ISIs. Co‐application of perampanel abolishes the clustered ISIs so that ISIs are evenly spread (white). Events of burst discharges and excitatory postsynaptic potentials (EPSPs) are also summed as events of “excitatory waves”. N.S. P > 0.05; * P < 0.05, Kruskal–Wallis test followed by pairwise Mann–Whitney U test. (c) A PN–PN pair recording shows that application of 4‐AP (10 μM, n = 28) produces recurrent and coupled IPSPs in the two PNs in the current‐clamp recording at first and then synchronized IPSPs in a PN (No. 2, staying in current clamp) and inhibitory postsynaptic currents (IPSCs) in the other PN (No. 1, switched to the voltage‐clamp mode and held at −55 mV). Both IPSPs and IPSCs are abolished by GABAA receptor antagonist bicuculline (10 μM, n = 5). The IPSP frequency in 4‐AP is from the same group of PNs in (b). * P < 0.05, Mann–Whitney U test
FIGURE 4.
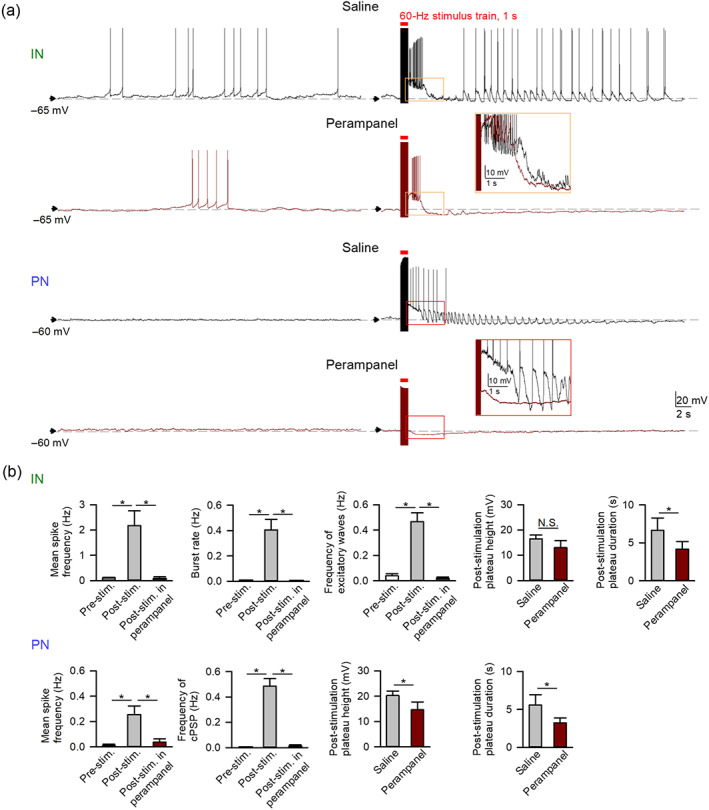
Perampanel reduces electrical stimulation‐induced paroxysmal depolarizing shift and activities in pyramidal neurons (PNs) and interneurons (INs) of basolateral amygdala (BLA). In the representative recording (a) of an IN (top) and a PN (bottom), delivery of kindling‐mimicking stimulation causes large depolarization plateaus (orange and red boxes for IN and PN, respectively). The plateau is followed by an increase in spikes, burst discharges, and/or excitatory waves in all INs (n = 18 for analyses of burst discharges and n = 21 for analyses of spikes and excitatory waves) and also an increase in spikes and/or composite postynaptic potentials or “cPSP” (which include burst discharges, excitatory postsynaptic potentials [EPSPs], and inhibitory postsynaptic potentials [IPSPs]) in some PNs (n = 13; see analyses in b). See Section 2 for details. Application of perampanel (5 μM) reduces both the height and duration of the post‐stimulation depolarization plateau in PNs (see magnified overlaying traces in the red box, n = 8) and the duration of the plateau in INs (see magnified overlaying traces in the orange box, n = 6). Note that all post‐stimulation activities in both INs (n = 6) and PNs (n = 5 and 8 for the mean spike frequency and cPSP frequency, respectively, only those having pre‐stimulation baseline membrane potential more depolarized than −60 mV used in the analyses of mean spike frequency) are abolished by perampanel. N.S. P > 0.05; * P < 0.05, Kruskal–Wallis test followed by pairwise Mann–Whitney U test for post‐stimulation activities and Mann–Whitney U test for post‐stimulation plateau
FIGURE 5.
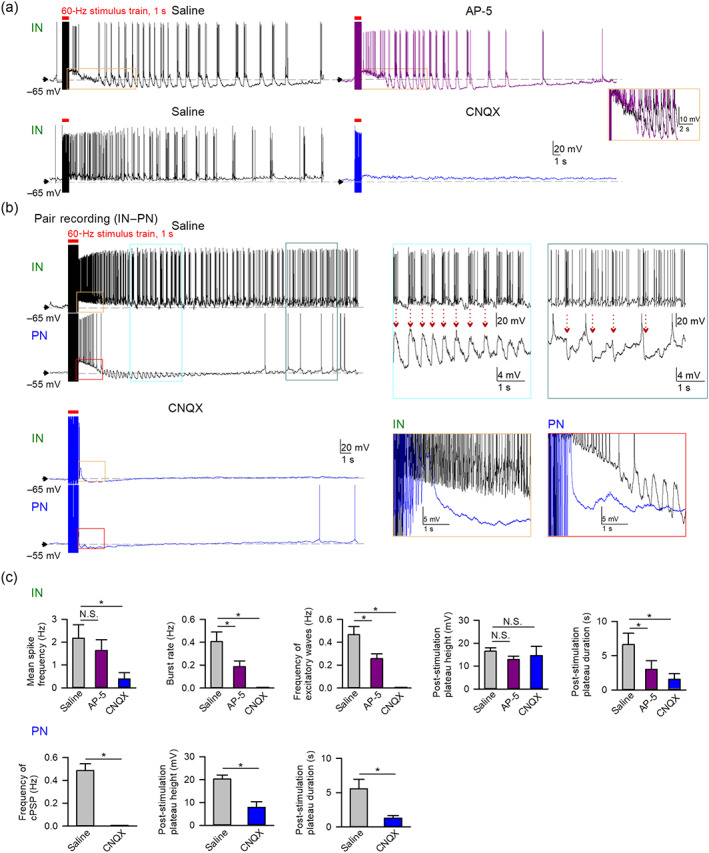
Electrical stimulation‐induced pyramidal neuron (PN)–interneuron (IN) reverberating activities require AMPAergic transmission. (a) Representative recordings show that application of AMPA antagonist 6‐cyano‐7‐nitroquinoxaline‐2,3‐dione (CNQX) (10 μM, bottom traces) rather than NMDA antagonist AP‐5 (10 μM, top traces) effectively abolishes the kindling‐mimicking stimulation‐induced recurrent burst discharges in INs. Orange box: magnified overlaying traces showing that AP‐5 does not significantly reduce the post‐stimulation plateau height. (b) A pair recording of an IN and a PN shows that delivery of kindling‐mimicking stimulation induces large depolarizing plateaus in both neurons, followed by repetitive IN burst discharges, which are synchronized with the repetitive excitatory postsynaptic potentials (EPSPs) and then inhibitory postsynaptic potentials (IPSPs) in the PN (indicated by red dashed arrows in partially magnified traces, blue and teal boxes). Application of CNQX (10 μM) reduces the post‐stimulation plateau and completely inhibits all other post‐stimulation activities. Orange and red boxes: magnified overlaying traces showing the effect of CNQX on the post‐stimulation plateau in IN and PN, respectively. In PNs, CNQX dramatically reduces both the height and duration of post‐stimulation plateau (the red box). In INs, CNQX shortens the post‐stimulation plateau duration (the orange box). (c) Quantitative analyses for the effect of AP‐5 (n = 10) and CNQX (n = 5 for INs and n = 8 for PNs) on the post‐stimulation events in INs (top row) and PNs (bottom row). N.S. P > 0.05; * P < 0.05, Kruskal–Wallis test followed by pairwise Mann–Whitney U test for INs and Mann–Whitney U test for PNs. The analyses in control (saline) were from the same group of PNs (n = 13) and INs (n = 18–21) in Figure 4
2.9. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3. RESULTS
3.1. Perampanel and specific AMPA antagonist CNQX have a similar effect on seizure behaviours induced by topical application of 4‐AP into basolateral amygdala
Application of proconvulsant 4‐AP into the basolateral amygdala is a well‐established experimental seizure model of limbic epilepsy for decades (Avoli, Louvel, Pumain, & Köhling, 2005; Benini, D'Antuono, Pralong, & Avoli, 2003). Microinjection of 4‐AP into the unilateral basolateral amygdala of a free‐moving rat induces vivid seizure behaviours of different severities (Figure 1). Consistent with a selective action of perampanel on AMPA receptors (Barygin, 2016; Ceolin et al., 2012; Chen et al., 2014), co‐injection of either the selective AMPA antagonist CNQX (five rats, numbered 1–5) or perampanel (five rats, numbered 1–3, 6, and 7) into the basolateral amygdala effectively reduces both the occurrence and severity of seizures induced by topical 4‐AP (Figure 1 and Videos S1 and S2 for the representative animal behaviours ~10 min after microinjection of drugs into the basolateral amygdala in two consecutive sessions, with the first being 4‐AP alone and the second being 4‐AP plus perampanel or CNQX in the same rat [see Section 2 for details]. The first three rats in perampanel and CNQX experiments are of the same three rats). In other words, there is a very similar focal action of perampanel as well as CNQX on seizure behaviours initiated from the unilateral basolateral amygdala. The behavioural effect of topical 4‐AP and perampanel/CNQX suggests that inhibition of AMPAergic transmission could hinder neuronal recruitment in a local network and thus preclude the progress into a larger‐scale temporospatial oscillation.
3.2. Perampanel attenuates recurrent discharges in interneurons and thus IPSPs in pyramidal neurons induced by 4‐AP
We then examined the cellular and network mechanisms underlying the action of perampanel in the basolateral amygdala circuitry. Glutamatergic pyramidal neurons and GABAergic interneurons in basolateral amygdala slices are identified according to their electrophysiological and morphological characteristics (Figure 2a; Washburn & Moises, 1992; Mahanty & Sah, 1998; Sah et al., 2003). Simultaneous recordings from a pyramidal neuron and an interneuron demonstrate that application of 4‐AP induces repetitive burst discharges in interneurons and corresponding IPSPs followed by spikes in pyramidal neurons (Figure 2b). The 4‐AP‐induced rhythmic IPSPs in pyramidal neurons result in rhythmic suppression followed by rebound increase of spikes in pyramidal neurons. The burst discharges in interneurons are tightly synchronized with IPSPs in pyramidal neurons, and thus there are alternating activities between interneurons and pyramidal neurons. Perampanel completely abolishes 4‐AP‐triggered burst discharges in interneurons. In contrast, perampanel tends to preserve spontaneous spikes and does not significantly alter the mean spike frequency in pyramidal neurons, although it completely inhibits all IPSPs in pyramidal neurons induced by 4‐AP. We performed further analysis for the interspike intervals of pyramidal neurons in the presence of 4‐AP and found that the 4‐AP‐induced rhythmic IPSPs separate the interspike intervals into two clusters (see the interspike interval analysis in Figure 2b). The shorter interspike intervals come from spikes “between two consecutive IPSPs” (during inter‐IPSP interval) and the longer interspike intervals come from the two consecutive spikes with one IPSP “in between” (so that interspike interval is longer because of the IPSP). Application of perampanel abolishes clustering of interspike intervals induced by 4‐AP. The oscillatory alternating activities between interneurons and pyramidal neurons with 4‐AP therefore are markedly reduced or even eliminated by perampanel (Figure 2b; also see Figure S1a). With paired recordings from two pyramidal neurons (Figure 2c), we further demonstrated that 4‐AP induces rhythmic and highly synchronous bicuculline (a GABAA receptor antagonist)‐sensitive IPSPs/inhibitory postsynaptic currents (IPSCs) in different pyramidal neurons. The same interneurons thus may project to different pyramidal neurons, whose activities would then be synchronized by the (burst) discharges of the interneurons.
3.3. CNQX but not AP‐5 attenuates recurrent discharges in interneurons in a way very similar to perampanel
Figure 3 characterizes the contributions of different glutamatergic drives from pyramidal neurons to interneurons. The AMPA receptor antagonist CNQX but not the NMDA receptor antagonist AP‐5 well mimics the actions of perampanel in Figure 2 and completely eliminates 4‐AP‐induced burst discharges in interneurons (Figure 3a). A closer look reveals that CNQX but not AP‐5 completely inhibits 4‐AP‐induced IPSPs but does not significantly alter the mean spike frequency in pyramidal neurons (Figure 3a). AMPA antagonists (perampanel and CNQX, Figures 2b and 3a) consistently wipe out the activities of interneurons, indicating that glutamatergic input is crucial for the generation of (burst) discharges in interneurons. On the other hand, the mean spike frequency in pyramidal neurons is not altered (although the intervening IPSPs provided by interneurons is abolished) by AMPA antagonists, suggesting that pyramidal neurons exhibit activities even without glutamatergic input. Despite unaltered mean spike frequency, application of CNQX, again, abolishes clustering of interspike intervals induced by 4‐AP. It is counterintuitive that a proconvulsant (e.g. 4‐AP) would promote, but an anticonvulsant (e.g. perampanel and CNQX) would abolish repetitive inhibitory synaptic inputs (IPSPs) in excitatory pyramidal neurons. It is plausible that the proconvulsant or anticonvulsant effect should not be simplistically ascribed to the altered excitability in the network (e.g. increased excitability by the K+ channel inhibitor 4‐AP or decreased excitability by the AMPA antagonists perampanel or CNQX). Our findings indicate that the primary effect of 4‐AP on pyramidal neurons is most likely an increase in rhythmic GABAergic input provided by the enhanced rhythmic burst discharges of interneurons. The apparently paradoxical inhibition of IPSPs by AMPA antagonists (perampanel and CNQX) would further suggest that the increased IPSPs in pyramidal neurons are consequences of glutamate‐dependent burst discharges in interneurons triggered by 4‐AP. In other words, it seems that glutamatergic input is especially important for the genesis of burst discharges in interneurons (and subsequent IPSPs in pyramidal neurons) in the basolateral amygdala network. Perampanel inhibition of AMPAergic transmission would reduce burst discharges in interneurons and then the reverberating oscillations between interneurons and pyramidal neurons and thus an anti‐epileptic effect. Consistent with the view of reverberating interneuron–pyramidal neuron discharges but not increased excitation/decreased inhibition for ictogenesis, addition of another common proconvulsant, the GABAA antagonist bicuculline (Curtis, Duggan, Felix, & Johnston, 1970; Fisher, 1989), abolishes rather than exacerbates 4‐AP‐induced oscillatory activities between interneurons and pyramidal neurons (Figure 3b). In the presence of both 4‐AP and bicuculline, the simultaneous block of K+ and Cl− channels may result in more chances of depolarization block of all membrane activities. Therefore, interneuron and pyramidal neuron pair recordings show either silence of both interneuron and pyramidal neuron (due to depolarization block) or activities in the pyramidal neuron not regulated by the IPSP (Figure 3b). In the latter case, IPSPs and consequently the rhythmic or oscillating discharges in interneurons and pyramidal neurons are abolished, but the mean spike frequency in pyramidal neurons is not significantly altered by bicuculline, similar to the case with AMPR antagonists (Figures 2b and 3a). These findings suggest that the discharge patterns may be more crucial than the spike counts. Thus, bicuculline disrupts 4‐AP‐induced oscillatory activities between interneurons and pyramidal neurons, resulting in either silence of both interneurons and pyramidal neurons or apparently uncoordinated activities in interneurons and pyramidal neurons, consistent with inhibition rather than induction of epileptiform discharges reported in the literature (Brückner, Stenkamp, Meierkord, & Heinemann, 2000).
3.4. Perampanel reduces post‐stimulation plateau depolarization and after discharges in both pyramidal neurons and interneurons
The telencephalic structures, especially the amygdala, are particularly susceptible to kindling (McIntyre & Gilby, 2008), which turns normal brain epileptic by focal repetitive electrical stimulation without introducing apparent structural or chemical derangements to the circuits (Goddard et al., 1969). We explored the discharge profiles of basolateral amygdala neurons in acute brain slices following focal electrical stimulation that mimics in vivo kindling (Figure 4; see Section 2 for stimulation protocols). Immediately after delivery of the kindling‐mimicking stimuli in vitro, long‐lasting membrane depolarization (presented as “post‐stimulation plateau” below for simplicity) could be observed in both interneurons and pyramidal neurons (Figure 4a). The post‐stimulation plateau is reminiscent of the paroxysmal depolarizing shift commonly seen in the earliest part of epileptiform discharges, characterizing ictal onset in experimental seizures (Goldensohn & Purpura, 1963; Matsumoto & Marsan, 1964). Perampanel markedly inhibits the post‐stimulation plateau in pyramidal neurons and, to a lesser extent, also in interneurons. On the other hand, burst discharges (i.e., grouped spikes on top of a depolarization plateau) are markedly increased especially in interneurons in response to in vitro kindling‐mimicking stimulations (Figure 4a,b). The markedly increased burst and spike discharges or excitatory waves (a sum of burst discharges and EPSPs) in interneurons after kindling‐mimicking stimuli are inhibited by perampanel. There are also increased spikes and EPSPs or even burst discharges in pyramidal neurons after stimulation. Perampanel likewise abolishes most stimulation‐induced activities in pyramidal neurons. Lower concentration of perampanel also provides similar findings with a slower action (Figure S1b). These findings suggest that glutamatergic synaptic input may play a major role in the generation of paroxysmal depolarizing shift and perampanel may prohibit seizures by inhibition of depolarization shift and subsequent excitatory waves.
3.5. CNQX but not AP‐5 recapitulates the effect of perampanel on plateau depolarization and after discharges
Similar to perampanel, the AMPA antagonist CNQX completely inhibits stimulation‐induced burst discharges and firing activities in interneurons, whereas the NMDA antagonist AP5 has only a mild effect (Figure 5a). Paired recordings of a pyramidal neuron and an interneuron demonstrate that kindling‐mimicking stimulation induces not only post‐stimulation plateau depolarization followed by increased activities in interneurons and pyramidal neurons but also alternate reverberating activities between interneurons and pyramidal neurons (Figure 5b). Once again, interneurons show a stronger propensity of generation of burst discharges in response to kindling‐mimicking stimulation. Stimulation‐induced interneuron burst discharges are synchronized with stimulation‐induced EPSPs in pyramidal neurons in the early phase, and then synchronized with stimulation‐induced IPSPs in pyramidal neurons in the late phase (Figure 5b), reminiscent of 4‐AP‐induced persistent alternating activities of interneurons and pyramidal neurons (Figures 2 and 3). These findings suggest that the AMPAergic drive provided by pyramidal neurons substantially contributes to the post‐stimulation plateau depolarization as well as early‐phase burst discharges in interneurons and EPSPs in pyramidal neurons. In the late phase, the synchronized burst discharges presumably spread to involve more interneurons. Larger IPSPs would therefore be triggered in pyramidal neurons, enabling more rebound pyramidal neuronal activities and thus apparently alternating activities between interneurons and pyramidal neurons. Consistently, application of CNQX completely abolishes all of the aforementioned stimulation‐induced activities (burst discharges, EPSPs, and IPSPs) in both interneurons and pyramidal neurons (Figure 5b,c), repeating the effect of perampanel in Figure 4. It is especially of note that the actions of perampanel and CNQX are so similar in many important aspects. For example, the inhibitory effect on the stimulation‐induced depolarization shift tends to be more complete in pyramidal neurons than interneurons, with a residual plateau in interneurons but not in pyramidal neurons for both compounds. These findings not only indicate differential contributions of AMPA and NMDAergic transmissions in the process of electrical kindling‐mimicking stimulation but also demonstrate very similar actions of perampanel and CNQX on stimulation‐induced cellular activities, lending a strong support that the anticonvulsant effect of perampanel could be reasonably ascribed to the inhibition of AMPAergic transmission.
4. DISCUSSION
4.1. Epileptic seizures are not simplistic consequences of imbalance between excitation and inhibition in a network
Epileptic discharges are in common considered as abnormally decreased inhibition and/or increased excitation in a telencephalic network such as the basolateral amygdala. Accordingly, it seems conceivable that inhibition of excitatory glutamatergic transmission by perampanel shall have an anticonvulsant effect. However, there are clinical and pharmaco‐therapeutic controversies over the roles of pyramidal neurons and interneurons in epileptogenesis and seizure spreading (Engel, 1996; Weiss et al., 2019). For example, both human and animal studies demonstrated that seizure begins with increased interneuron firing and strong inhibitory postsynaptic events in pyramidal neurons (Elahian et al., 2018; Fujiwara‐Tsukamoto et al., 2010; Librizzi et al., 2017; Truccolo et al., 2011; Ziburkus, Cressman, Barreto, & Schiff, 2006), whereas other reports showed that seizure discharges occur first in pyramidal neurons (Avoli, Psarropoulou, Tancredi, & Fueta, 1993; Borck & Jefferys, 1999). Also, interneuron activities have been shown to be decreased (Misra, Long, Sperling, Sharan, & Moxon, 2018) or increased (Liou et al., 2018) during seizure spreading. In addition, optogenetic stimulation of just pyramidal neurons could induce (Osawa et al., 2013; Wagner et al., 2015) or suppress (Chiang, Ladas, Gonzalez‐Reyes, & Durand, 2014) seizures, whereas photoinhibition of pyramidal neurons consistently reduces seizures (Krook‐Magnuson et al., 2013; Tønnesen et al., 2009; Wykes et al., 2012). Optogenetic stimulation of just interneurons may induce (Sessolo et al., 2015; Shiri et al., 2016) or reduce (Krook‐Magnuson et al., 2013; Sessolo et al., 2015) seizures, while optical inhibition of interneurons would tend to reduce seizures (Krook‐Magnuson, Szabo, Armstrong, Oijala, & Soltesz, 2014; Lu et al., 2016) or have no effect (Krook‐Magnuson et al., 2014; Lu et al., 2016) on seizures. Moreover, bicuculline is a widely used proconvulsant in vitro and in vivo (Curtis et al., 1970; Fisher, 1989), but similar concentrations of bicuculline in similar brain areas have been reported to reduce seizure activities in many in vitro and in vivo studies (Fujiwara‐Tsukamoto et al., 2007; Higashima, Kinoshita, Yamaguchi, & Koshino, 1996; Stasheff, Mott, & Wilson, 1993; Turski et al., 1989; Weng & Rosenberg, 1992). Also, although perampanel could ameliorate seizures, worsening of seizures could happen and epileptic seizures are common in the patients of anti‐AMPA receptor encephalitis (Bien & Holtkamp, 2017; Biró et al., 2015; De Liso et al., 2016). The occurrence of seizures thus should not be envisaged simplistically by increased excitation (e.g. inreased pyramidal neuron activities) or decreased inhibition (e.g. decreased interneuron activities). To reconcile these apparently contradictory findings, we have demonstrated that pyramidal neurons and interneurons may have to coordinate their activities to initiate and maintain the reverberating epileptic oscillations. In this regard, interruption of the coordinating activities between pyramidal neurons and interneurons, rather than simplistic inhibition of excitatory pyramidal neurons and/or excitation of inhibitory interneurons, in a network could be the key to reduce or terminate seizures. Inhibition of both burst activities in interneurons and consequently IPSPs in pyramidal neurons by AMPA receptor antagonists such as perampanel or even inhibition of GABAergic transmission directly by GABA receptor antagonists, that are generally considered as pro‐convulsants, could therefore decrease or prevent epileptic oscillations (Figures 2, 3, 4). In this regard, it is of note that perampanel markedly reduces the plateau depolarization immediately after kindling‐like stimulation in both interneurons and pyramidal neurons (Figure 4). The plateau depolarization is in fact the earliest form of epileptic discharges in many different seizures (De Curtis & Avanzini, 2001; Dreier et al., 2012; Staley, Hellier, & Dudek, 2005). The different reduction of the post‐stimulation plateau in pyramidal neurons and in interneurons by perampanel would further imply that more glutamatergic inputs converge on interneurons than on pyramidal neurons for the post‐stimulation plateau depolarization (Sah et al., 2003; Spampanato, Polepalli, & Sah, 2011). In any case, reduction of the plateau depolarization in both pyramidal neurons and interneurons would signal a decrease in convergent drive upon the initiation of network reverberations, once again implicating the mechanistic significance of disruption of coordinating network activities underlying the anticonvulsant effect of perampanel.
4.2. Perampanel works as modifier of reverberating discharges from the perspective of neural circuitry
Perampanel is a new‐generation and widely prescribed anticonvulsant and is well known for its non‐competitive antagonist action at AMPA receptors (Hanada et al., 2011; Potschka & Trinka, 2019). Epilepsy is characterized by excessive central neuronal discharges and thus most likely excessive glutamate release. For instance, human ictal transition and spread have been reported to rely critically on population burst discharges and synchronous glutamatergic signalling (Huberfeld et al., 2011). A competitive AMPA receptor antagonist such as CNQX would become less effective with more release of the natural agonist glutamate. In contrast, a non‐competitive antagonist such as perampanel presumably would have a more consistent effect with excessive glutamate release during seizures. Indeed, the anti‐seizure effect of a non‐competitive AMPA antagonist 1‐(4‐aminophenyl)‐3‐methylcarbamyl‐4‐methyl‐3, 4‐dihydro‐7,8‐methylenedioxy‐5H‐2,3‐benzodiazepine hydrochloride (GYKI‐53655), which shares the binding site of perampanel (Hanada et al., 2011), was found to be superior to that of the competitive AMPA antagonist 2,3‐dioxo‐6‐nitro‐1,2,3,4‐tetrahydrobenzo[f]quinoxaline‐7‐sulfonamide (NBQX) in experimental seizure models (Yamaguchi, Donevan, & Rogawski, 1993). On the other hand, the binding affinity of perampanel could be much lower than that of CNQX (Chen et al., 2014; Honoré, Drejer, Nielsen, & Nielsen, 1989; Johansen et al., 1993). An anticonvulsant ideally should have a selective inhibitory effect on seizure but spare normal neural function. AMPA receptor‐mediated glutamate transmission is ubiquitous in the mammalian brain. The low affinity therefore could be mandatory for an AMPA receptor antagonist such as perampanel to be a usable medication against seizures in clinical practice. In this study, we had two distinct seizure models for investigation of the action of AMPA receptor antagonists. In the 4‐AP model, there is presumably a diffuse increase in glutamate and GABA release, as well as cellular excitability (lowered threshold for firing an action potential with delayed repolarization). On the other hand, electrical kindling is characterized by focal accentuation of neurotransmission, followed by local extension and distant spreading in order. The two models shall therefore have wide‐scope coverage of the very much heterogeneous epileptogenesis and ictogenesis. It is interesting that perampanel and CNQX show very similar actions in behavioural and electrophysiological assessments and from network to cellular actions in the two experimental models. Most importantly, perampanel and CNQX both abolish the burst discharges in interneurons and corresponding IPSPs and post‐inhibitory rebound spikes in pyramidal neurons (Figures 2 and 3) and markedly reduce the post‐stimulation depolarization shift as well as after discharges (Figures 4 and 5). These are all potentially key actions relevant to the anticonvulsant effect. The overlapping anticonvulsant actions of perampanel in two different seizure models and the similar anti‐epileptic effects of perampanel and CNQX may broaden the clinical implication of the AMPAergic inhibition in seizure networks. Perampanel is a broad‐spectrum anticonvulsant and has been reported effective against many different types of seizures, including focal seizures, generalized tonic–clonic seizures, status epilepticus or seizures of heterogeneous aetiologies such as tumour‐induced seizures and epileptic syndromes including Lennox–Gastaut and Dravet syndromes in children and adolescents (Biró et al., 2015; De Liso et al., 2016; French et al., 2012; French et al., 2015; Krauss et al., 2012; Redecker, Wittstock, Benecke, & Rösche, 2015; Vecht et al., 2017). We have seen that seizure‐related burst discharges happen much more frequently in interneurons than pyramidal neurons. Also, the generation of burst discharges in interneurons is critically dependent on AMPAergic transmission. The anticonvulsant effect of perampanel may at least partly rely on preventing the initiation of burst discharges in interneurons or its downstream postsynaptic effect on network reverberating discharges. For instance, channelopathy‐induced interneuronopathy has been implicated to play a pathogenic role (e.g. altered excitability and firing pattern of inhibitory neurons, Liautard et al., 2013; Tai, Abe, Westenbroek, Scheuer, & Catterall, 2014; Spillane, Kullmann, & Hanna, 2016) in Dravet syndrome, where perampanel could counter the seizure‐related abnormal firing in interneurons by AMPAergic transmission inhibition. In any case, instead of a simplistic view of decrease in excitability because of glutamatergic inhibition, perampanel inhibits seizures by ablation of the reverberating discharge patterns between interneurons and pyramidal neurons. In other words, the alternate synchronized activities in interneurons and pyramidal neurons are critically dependent on the pyramidal neuron‐to‐interneuron AMPAergic neurotransmission, which is inhibited by perampanel. It is interesting that the notorious proconvulsant bicuculline could also reduce the alternating and epileptic discharges in the pyramidal neuron‐interneuron networks by eliminating the necessary GABAergic drive in pyramidal neurons from interneurons (Brückner et al., 2000; Stasheff et al., 1993). Because perampanel does not work simplistically as “reduction in excitability” and has an anticonvulsant effect chiefly on interneurons, it could be ineffective against seizures characterized by excessive activities of pyramidal neurons, which does not require or rely too much on the conditioning by IPSPs and the post‐IPSP rebound discharges. Perampanel could even exacerbate seizures in certain clinical settings or at certain doses (Hsu et al., 2013). Such a “net” proconvulsant effect presumably could be envisaged if a general increase in pyramidal neuron activities happens (because of the ablation of IPSPs) and outweighs the modulation action on circuitry reverberations.
AUTHOR CONTRIBUTIONS
Y.‐C.Y. and G.‐H.W. designed the experiments. G.‐H.W., A.‐Y.C. and S.‐W.H. conducted the experiments. Y.‐C.Y., G.‐H.W. and A.‐Y.C. analysed the data. Y.‐C.Y. and G.‐H.W. wrote the manuscript. Y.‐C.Y. conceived and supervised the study. Y.‐C.Y., G.‐H.W., A.‐Y.C. and S.‐W.H. approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no financial conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis and Animal Experimentation and as recommended by funding agencies, publishers and other organizations engaged with supporting research.
Supporting information
Figure S1. 250 nM of perampanel slowly inhibits 4‐AP as well as electrical stimulation‐induced activities. Representative recordings show that low concentration of perampanel (250 nM) exerts similar inhibitory effect on the 4‐AP‐induced (a, n = 5 for both interneurons (INS) and pyramidal neurons (PNs)) or electrical stimulation induced (b, n = 6 for PNs, see blue arrows, and n = 5 for INs) discharges after stable perfusion of ~30 minutes. The inhibitory effect is less prominent in 10 minutes of perfusion of perampanel (250 nM). Events of burst discharges and EPSPs are summed as events of “excitatory waves” in interneurons (Ins). “cPSPs” include burst discharges, EPSPs and IPSPs in PNs. ~10 minutes treatment of perampanel (250 nM) is used for the analysis in b. N.S. P > 0.05; *P < 0.05, Friedman test followed by pairwise Wilcoxon signed rank test in a, and Wilcoxon signed rank test in b.
Video S1. Amelioration of 4‐AP‐induced seizure behaviours by CNQX. Split screen video showing the seizure behaviours in two consecutive experimental sessions ~10 minutes after microinjection of 4‐AP (2 mM, 2 μl, left screen) and then 4‐AP (2 mM, 2 μl) plus CNQX (200 μM, 2 μl) (right screen) into basolateral amygdala (BLA) in the same rat.
Video S2. Amelioration of 4‐AP‐induced seizure behaviours by perampanel. Split screen video showing the seizure behaviours in two consecutive experimental sessions ~10 minutes after microinjection of 4‐AP (2 mM, 2 μl, left screen) and then 4‐AP (2 mM, 2 μl) plus perampanel (80 μM, 2 μl) (right screen) into basolateral amygdala (BLA) in the same rat.
ACKNOWLEDGEMENTS
This work was supported by Grants MOST107‐2311‐B‐182‐004, MOST108‐2311‐B‐182‐001, MOST 108‐2321‐B‐007‐003‐MY2 and MOST109‐2320‐B‐182‐006 from the Ministry of Science and Technology, Taiwan and Grant CMRPD1H0091‐3 from the Chang Gung Medical Foundation, Taiwan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors are grateful to the Neuroscience Research Center of Chang Gung Memorial Hospital, Linkou, Taiwan.
Yang Y, Wang G, Chuang A, Hsueh S. Perampanel reduces paroxysmal depolarizing shift and inhibitory synaptic input in excitatory neurons to inhibit epileptic network oscillations. Br J Pharmacol. 2020;177:5177–5194. 10.1111/bph.15253
Present address Guan‐Hsun Wang, Department of Neurology, Chang Gung Memorial Hospital, Linkou Medical Center, Tao‐Yuan, Taiwan.
Contributor Information
Ya‐Chin Yang, Email: ycyang@mail.cgu.edu.tw.
Guan‐Hsun Wang, Email: b0102106@cgmh.org.tw.
REFERENCES
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … Collaborators, C. G. T. P. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176 Issue, S1, S142–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin, K. , Williams, S. , Cunningham, M. , Devlin, A. M. , Friedrich, M. , Jayasekera, A. , … Chen, P. E. (2018). Perampanel and decanoic acid show synergistic action against AMPA receptors and seizures. Epilepsia, 59, e172–e178. 10.1111/epi.14578 [DOI] [PubMed] [Google Scholar]
- Avoli, M. , Barbarosie, M. , Lücke, A. , Nagao, T. , Lopantsev, V. , & Köhling, R. (1996). Synchronous GABA‐mediated potentials and epileptiform discharges in the rat limbic system in vitro. Journal of Neuroscience, 16, 3912–3924. 10.1523/JNEUROSCI.16-12-03912.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli, M. , Louvel, J. , Pumain, R. , & Köhling, R. (2005). Cellular and molecular mechanisms of epilepsy in the human brain. Progress in Neurobiology, 77, 166–200. 10.1016/j.pneurobio.2005.09.006 [DOI] [PubMed] [Google Scholar]
- Avoli, M. , Psarropoulou, C. , Tancredi, V. , & Fueta, Y. (1993). On the synchronous activity induced by 4‐aminopyridine in the CA3 subfield of juvenile rat hippocampus. Journal of Neurophysiology, 70, 1018–1029. 10.1152/jn.1993.70.3.1018 [DOI] [PubMed] [Google Scholar]
- Barygin, O. I. (2016). Inhibition of calcium‐permeable and calcium‐impermeable AMPA receptors by perampanel in rat brain neurons. Neuroscience Letters, 633, 146–151. 10.1016/j.neulet.2016.09.028 [DOI] [PubMed] [Google Scholar]
- Benini, R. , D'Antuono, M. , Pralong, E. , & Avoli, M. (2003). Involvement of amygdala networks in epileptiform synchronization in vitro. Neuroscience, 120, 75–84. 10.1016/S0306-4522(03)00262-8 [DOI] [PubMed] [Google Scholar]
- Bien, C. G. , & Holtkamp, M. (2017). “Autoimmune epilepsy”: Encephalitis with autoantibodies for epileptologists. Epilepsy Currents, 17, 134–141. 10.5698/1535-7511.17.3.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biró, A. , Stephani, U. , Tarallo, T. , Bast, T. , Schlachter, K. , Fleger, M. , … Wolff, M. (2015). Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies: First experiences. Neuropediatrics, 46, 110–115. [DOI] [PubMed] [Google Scholar]
- Borck, C. , & Jefferys, J. G. (1999). Seizure‐like events in disinhibited ventral slices of adult rat hippocampus. Journal of Neurophysiology, 82, 2130–2142. 10.1152/jn.1999.82.5.2130 [DOI] [PubMed] [Google Scholar]
- Brückner, C. , Stenkamp, K. , Meierkord, H. , & Heinemann, U. (2000). Effects of bicuculline and different glutamate receptor antagonists on 4‐aminopyridine‐induced epileptiform discharges in rat hippocampal‐entorhinal cortex slices. Neuroscience Research Communications, 26, 41–49. [DOI] [Google Scholar]
- Buzsáki, G. , & Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science, 304, 1926–1929. 10.1126/science.1099745 [DOI] [PubMed] [Google Scholar]
- Ceolin, L. , Bortolotto, Z. A. , Bannister, N. , Collingridge, G. L. , Lodge, D. , & Volianskis, A. (2012). A novel anti‐epileptic agent, perampanel, selectively inhibits AMPA receptor‐mediated synaptic transmission in the hippocampus. Neurochemistry International, 61, 517–522. 10.1016/j.neuint.2012.02.035 [DOI] [PubMed] [Google Scholar]
- Chen, C. Y. , Matt, L. , Hell, J. W. , & Rogawski, M. A. (2014). Perampanel inhibition of AMPA receptor currents in cultured hippocampal neurons. PLoS ONE, 9, e108021 10.1371/journal.pone.0108021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, C. C. , Ladas, T. P. , Gonzalez‐Reyes, L. E. , & Durand, D. M. (2014). Seizure suppression by high frequency optogenetic stimulation using in vitro and in vivo animal models of epilepsy. Brain Stimulation, 7, 890–899. 10.1016/j.brs.2014.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citraro, R. , Leo, A. , Franco, V. , Marchiselli, R. , Perucca, E. , De Sarro, G. , & Russo, E. (2017). Perampanel effects in the WAG/Rij rat model of epileptogenesis, absence epilepsy, and comorbid depressive‐like behavior. Epilepsia, 58, 231–238. 10.1111/epi.13629 [DOI] [PubMed] [Google Scholar]
- Curtis, D. R. , Duggan, A. W. , Felix, D. , & Johnston, G. A. R. (1970). GABA, bicuculline and central inhibition. Nature, 226, 1222–1224. 10.1038/2261222a0 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … MacEwan, D. J. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Curtis, M. , & Avanzini, G. (2001). Interictal spikes in focal epileptogenesis. Progress in Neurobiology, 63, 541–567. 10.1016/S0301-0082(00)00026-5 [DOI] [PubMed] [Google Scholar]
- De Liso, P. , Vigevano, F. , Specchio, N. , De Palma, L. , Bonanni, P. , Osanni, E. , … Spalice, A. (2016). Effectiveness and tolerability of perampanel in children and adolescents with refractory epilepsies—An Italian observational multicenter study. Epilepsy Research, 127, 93–100. 10.1016/j.eplepsyres.2016.08.021 [DOI] [PubMed] [Google Scholar]
- Dreier, J. P. , Major, S. , Pannek, H. W. , Woitzik, J. , Scheel, M. , Wiesenthal, D. , … Speckmann, E. J. (2012). Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain, 135, 259–275. 10.1093/brain/awr303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers, A. E. (2007). Temporal lobe epilepsy is a disease of faulty neuronal resonators rather than oscillators, and all seizures are provoked, usually by stress. Medical Hypotheses, 69, 1284–1289. 10.1016/j.mehy.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Elahian, B. , Lado, N. E. , Mankin, E. , Vangala, S. , Misra, A. , Moxon, K. , … Staba, R. (2018). Low‐voltage fast seizures in humans begin with increased interneuron firing. Annals of Neurology, 84, 588–600. 10.1002/ana.25325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, J. (1996). Excitation and inhibition in epilepsy. Canadian Journal of Neurological Sciences, 23, 167–174. 10.1017/S0317167100038464 [DOI] [PubMed] [Google Scholar]
- Fisher, R. S. (1989). Animal models of the epilepsies. Brain Research Reviews, 14, 245–278. 10.1016/0165-0173(89)90003-9 [DOI] [PubMed] [Google Scholar]
- French, J. A. , Krauss, G. L. , Biton, V. , Squillacote, D. , Yang, H. , Laurenza, A. , … Rogawski, M. A. (2012). Adjunctive perampanel for refractory partial‐onset seizures: Randomized phase III study 304. Neurology, 79, 589–596. 10.1212/WNL.0b013e3182635735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French, J. A. , Krauss, G. L. , Wechsler, R. T. , Wang, X. F. , DiVentura, B. , Brandt, C. , … Bibbiani, F. (2015). Perampanel for tonic‐clonic seizures in idiopathic generalized epilepsy: A randomized trial. Neurology, 85, 950–957. 10.1212/WNL.0000000000001930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French, J. D. , Gernandt, B. E. , & Livingston, R. B. (1956). Regional differences in seizure susceptibility in monkey cortex. AMA Archives of Neurology and Psychiatry, 75, 260–274. 10.1001/archneurpsyc.1956.02330210040005 [DOI] [PubMed] [Google Scholar]
- Fujiwara‐Tsukamoto, Y. , Isomura, Y. , Imanishi, M. , Fukai, T. , & Takada, M. (2007). Distinct types of ionic modulation of GABA actions in pyramidal cells and interneurons during electrical induction of hippocampal seizure‐like network activity. European Journal of Neuroscience, 25, 2713–2725. 10.1111/j.1460-9568.2007.05543.x [DOI] [PubMed] [Google Scholar]
- Fujiwara‐Tsukamoto, Y. , Isomura, Y. , Imanishi, M. , Ninomiya, T. , Tsukada, M. , Yanagawa, Y. , … Takada, M. (2010). Prototypic seizure activity driven by mature hippocampal fast‐spiking interneurons. Journal of Neuroscience, 30, 13679–13689. 10.1523/JNEUROSCI.1523-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gean, P. W. (1990). The epileptiform activity induced by 4‐aminopyridine in rat amygdala slices: Antagonism by non‐N‐methyl‐d‐aspartate receptor anatagonists. Brain Research, 530, 251–256. 10.1016/0006-8993(90)91291-N [DOI] [PubMed] [Google Scholar]
- Gidal, B. E. , Ferry, J. , Majid, O. , & Hussein, Z. (2013). Concentration–effect relationships with perampanel in patients with pharmacoresistant partial‐onset seizures. Epilepsia, 54, 1490–1497. 10.1111/epi.12240 [DOI] [PubMed] [Google Scholar]
- Goddard, G. V. , McIntyre, D. C. , & Leech, C. K. (1969). A permanent change in brain function resulting from daily electrical stimulation. Experimental Neurology, 25, 295–330. 10.1016/0014-4886(69)90128-9 [DOI] [PubMed] [Google Scholar]
- Goldensohn, E. S. , & Purpura, D. P. (1963). Intracellular potentials of cortical neurons during focal epileptogenic discharges. Science, 139, 840–842. 10.1126/science.139.3557.840 [DOI] [PubMed] [Google Scholar]
- Hanada, T. , Hashizume, Y. , Tokuhara, N. , Takenaka, O. , Kohmura, N. , Ogasawara, A. , … Nishizawa, Y. (2011). Perampanel: A novel, orally active, noncompetitive AMPA‐receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia, 52, 1331–1340. 10.1111/j.1528-1167.2011.03109.x [DOI] [PubMed] [Google Scholar]
- Hibi, S. , Ueno, K. , Nagato, S. , Kawano, K. , Ito, K. , Norimine, Y. , … Yonaga, M. (2012). Discovery of 2‐(2‐oxo‐1‐phenyl‐5‐pyridin‐2‐yl‐1, 2‐dihydropyridin‐3‐yl)benzonitrile (perampanel): A novel, noncompetitive α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropanoic acid (AMPA) receptor antagonist. Journal of Medicinal Chemistry, 55, 10584–10600. 10.1021/jm301268u [DOI] [PubMed] [Google Scholar]
- Higashima, M. , Kinoshita, H. , Yamaguchi, N. , & Koshino, Y. (1996). Activation of GABAergic function necessary for afterdischarge generation in rat hippocampal slices. Neuroscience Letters, 207, 101–104. 10.1016/0304-3940(96)12496-4 [DOI] [PubMed] [Google Scholar]
- Honoré, T. , Drejer, J. , Nielsen, E. Ø. , & Nielsen, M. (1989). Non‐NMDA glutamate receptor antagonist 3H‐CNQX binds with equal affinity to two agonist states of quisqualate receptors. Biochemical Pharmacology, 38, 3207–3212. 10.1016/0006-2952(89)90615-1 [DOI] [PubMed] [Google Scholar]
- Hsu, W. W. , Sing, C. W. , He, Y. , Worsley, A. J. , Wong, I. C. , & Chan, E. W. (2013). Systematic review and meta‐analysis of the efficacy and safety of perampanel in the treatment of partial‐onset epilepsy. CNS Drugs, 27, 817–827. 10.1007/s40263-013-0091-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld, G. , de La Prida, L. M. , Pallud, J. , Cohen, I. , Le Van Quyen, M. , Adam, C. , … Miles, R. (2011). Glutamatergic pre‐ictal discharges emerge at the transition to seizure in human epilepsy. Nature Neuroscience, 14, 627–634. 10.1038/nn.2790 [DOI] [PubMed] [Google Scholar]
- Johansen, T. H. , Drejer, J. , Wätjen, F. , & Nielsen, E. Ø. (1993). A novel non‐NMDA receptor antagonist shows selective displacement of low‐affinity [3H]kainate binding. European Journal of Pharmacology: Molecular Pharmacology, 246, 195–204. 10.1016/0922-4106(93)90031-4 [DOI] [PubMed] [Google Scholar]
- Kang, J. , Kang, N. , Yu, Y. , Zhang, J. , Petersen, N. , Tian, G. F. , & Nedergaard, M. (2010). Sulforhodamine 101 induces long‐term potentiation of intrinsic excitability and synaptic efficacy in hippocampal CA1 pyramidal neurons. Neuroscience, 169, 1601–1609. 10.1016/j.neuroscience.2010.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama, M. , Yamada, N. , Sato, K. , Kitamura, Y. , Koyama, F. , Sato, T. , … Kuroda, S. (1999). Effects of YM90K, a selective AMPA receptor antagonist, on amygdala‐kindling and long‐term hippocampal potentiation in the rat. European Journal of Pharmacology, 374, 11–19. 10.1016/S0014-2999(99)00295-2 [DOI] [PubMed] [Google Scholar]
- Krauss, G. L. , Serratosa, J. M. , Villanueva, V. , Endziniene, M. , Hong, Z. , French, J. , … Laurenza, A. (2012). Randomized phase III study 306: Adjunctive perampanel for refractory partial‐onset seizures. Neurology, 78, 1408–1415. 10.1212/WNL.0b013e318254473a [DOI] [PubMed] [Google Scholar]
- Krook‐Magnuson, E. , Armstrong, C. , Oijala, M. , & Soltesz, I. (2013). On‐demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nature Communications, 4, 1376 10.1038/ncomms2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook‐Magnuson, E. , Szabo, G. G. , Armstrong, C. , Oijala, M. , & Soltesz, I. (2014). Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. Eneuro, 1(1), ENEURO.0005–ENEU14.2014. 10.1523/ENEURO.0005-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz, M. , Galanis, C. , Müller‐Dahlhaus, F. , Opitz, A. , Wierenga, C. J. , Szabó, G. , … Vlachos, A. (2016). Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nature Communications, 7, 10020 10.1038/ncomms10020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liautard, C. , Scalmani, P. , Carriero, G. , De Curtis, M. , Franceschetti, S. , & Mantegazza, M. (2013). Hippocampal hyperexcitability and specific epileptiform activity in a mouse model of Dravet syndrome. Epilepsia, 54, 1251–1261. 10.1111/epi.12213 [DOI] [PubMed] [Google Scholar]
- Librizzi, L. , Losi, G. , Marcon, I. , Sessolo, M. , Scalmani, P. , Carmignoto, G. , & De Curtis, M. (2017). Interneuronal network activity at the onset of seizure‐like events in entorhinal cortex slices. Journal of Neuroscience, 37, 10398–10407. 10.1523/JNEUROSCI.3906-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley, E. , Stanford, S. C. , Kendall, D. E. , Alexander, S. P. , Cirino, G. , Docherty, J. R. , … Ahluwalia, A. (2020). ARRIVE 2.0 and the British Journal of Pharmacology: Updated guidance for 2020. British Journal of Pharmacology. 10.1111/bph.15178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou, J. Y. , Ma, H. , Wenzel, M. , Zhao, M. , Baird‐Daniel, E. , Smith, E. H. , … Schevon, C. A. (2018). Role of inhibitory control in modulating focal seizure spread. Brain, 141, 2083–2097. 10.1093/brain/awy116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás, R. R. (1988). The intrinsic electrophysiological properties of mammalian neurons: Insights into central nervous system function. Science, 242, 1654–1664. 10.1126/science.3059497 [DOI] [PubMed] [Google Scholar]
- Lodge, D. , Davies, S. N. , Jones, M. G. , Millar, J. , Manallack, D. T. , Ornstein, P. L. , … Beart, P. M. (1988). A comparison between the in vivo and in vitro activity of five potent and competitive NMDA antagonists. British Journal of Pharmacology, 95, 957–965. 10.1111/j.1476-5381.1988.tb11726.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Y. , Zhong, C. , Wang, L. , Wei, P. , He, W. , Huang, K. , … Wang, L. (2016). Optogenetic dissection of ictal propagation in the hippocampal–entorhinal cortex structures. Nature Communications, 7, 10962 10.1038/ncomms10962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty, N. K. , & Sah, P. (1998). Calcium‐permeable AMPA receptors mediate long‐term potentiation in interneurons in the amygdala. Nature, 394, 683–687. 10.1038/29312 [DOI] [PubMed] [Google Scholar]
- Matsumoto, H. , & Marsan, C. A. (1964). Cortical cellular phenomena in experimental epilepsy: Ictal manifestations. Experimental Neurology, 9, 305–326. 10.1016/0014-4886(64)90026-3 [DOI] [PubMed] [Google Scholar]
- McCormick, D. A. , & Contreras, D. (2001). On the cellular and network bases of epileptic seizures. Annual Review of Physiology, 63, 815–846. 10.1146/annurev.physiol.63.1.815 [DOI] [PubMed] [Google Scholar]
- McDonald, A. J. , & Betette, R. L. (2001). Parvalbumin‐containing neurons in the rat basolateral amygdala: Morphology and co‐localization of Calbindin‐D28k . Neuroscience, 102, 413–425. 10.1016/S0306-4522(00)00481-4 [DOI] [PubMed] [Google Scholar]
- McIntyre, D. C. , & Gilby, K. L. (2008). Mapping seizure pathways in the temporal lobe. Epilepsia, 49, 23–30. 10.1111/j.1528-1167.2008.01507.x [DOI] [PubMed] [Google Scholar]
- Medina‐Ceja, L. , Cordero‐Romero, A. , & Morales‐Villagrán, A. (2008). Antiepileptic effect of carbenoxolone on seizures induced by 4‐aminopyridine: A study in the rat hippocampus and entorhinal cortex. Brain Research, 1187, 74–81. 10.1016/j.brainres.2007.10.040 [DOI] [PubMed] [Google Scholar]
- Misra, A. , Long, X. , Sperling, M. R. , Sharan, A. D. , & Moxon, K. A. (2018). Increased neuronal synchrony prepares mesial temporal networks for seizures of neocortical origin. Epilepsia, 59, 636–649. 10.1111/epi.14007 [DOI] [PubMed] [Google Scholar]
- Muller, J. F. , Mascagni, F. , & McDonald, A. J. (2005). Coupled networks of parvalbumin‐immunoreactive interneurons in the rat basolateral amygdala. Journal of Neuroscience, 25, 7366–7376. 10.1523/JNEUROSCI.0899-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niittykoski, M. , Nissinen, J. , Penttonen, M. , & Pitkänen, A. (2004). Electrophysiologic changes in the lateral and basal amygdaloid nuclei in temporal lobe epilepsy: An in vitro study in epileptic rats. Neuroscience, 124, 269–281. 10.1016/j.neuroscience.2003.11.027 [DOI] [PubMed] [Google Scholar]
- Osawa, S. I. , Iwasaki, M. , Hosaka, R. , Matsuzaka, Y. , Tomita, H. , Ishizuka, T. , … Tominaga, T. (2013). Optogenetically induced seizure and the longitudinal hippocampal network dynamics. PLoS ONE, 8, e60928 10.1371/journal.pone.0060928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palovcik, R. A. , & Phillips, M. I. (1986). A constant perfusion slice chamber for stable recording during the addition of drugs. Journal of Neuroscience Methods, 17, 129–139. 10.1016/0165-0270(86)90066-X [DOI] [PubMed] [Google Scholar]
- Patsalos, P. N. , Gougoulaki, M. , & Sander, J. W. (2016). Perampanel serum concentrations in adults with epilepsy: Effect of dose, age, sex, and concomitant anti‐epileptic drugs. Therapeutic Drug Monitoring, 38, 358–364. 10.1097/FTD.0000000000000274 [DOI] [PubMed] [Google Scholar]
- Penfield, W. , & Jasper, H. (1954). Epilepsy and the functional anatomy of the human brain (Vol. 47) (p. 704). England: Little, Brown & Co. 10.1097/00007611-195407000-00024 [DOI] [Google Scholar]
- Percie du Sert, N. , Hurst, V. , Ahluwalia, A. , Alam, S. , Avey, M. T. , Baker, M. , … Würbel, H. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biology, 18(7), e3000410 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard, E. , Carvalho, F. A. , Agosti, F. , Bourinet, E. , Ardid, D. , Eschalier, A. , … Mallet, C. (2019). Inhibition of Cav3. 2 calcium channels: A new target for colonic hypersensitivity associated with low‐grade inflammation. British Journal of Pharmacology, 176, 950–963. 10.1111/bph.14608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potschka, H. , & Trinka, E. (2019). Perampanel: Does it have broad‐spectrum potential? Epilepsia, 60, 22–36. 10.1111/epi.14456 [DOI] [PubMed] [Google Scholar]
- Racine, R. J. (1972). Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalography and Clinical Neurophysiology, 32, 281–294. 10.1016/0013-4694(72)90177-0 [DOI] [PubMed] [Google Scholar]
- Rainnie, D. G. (1999). Serotonergic modulation of neurotransmission in the rat basolateral amygdala. Journal of Neurophysiology, 82, 69–85. 10.1152/jn.1999.82.1.69 [DOI] [PubMed] [Google Scholar]
- Rainnie D. G., Asprodini E. K., & Shinnick‐Gallagher P. (1993). Intracellular recordings from morphologically identified neurons of the basolateral amygdala. Journal of Neurophysiology, 69(4), 1350–1362. 10.1152/jn.1993.69.4.1350 [DOI] [PubMed] [Google Scholar]
- Rainnie, D. G. , Mania, I. , Mascagni, F. , & McDonald, A. J. (2006). Physiological and morphological characterization of parvalbumin‐containing interneurons of the rat basolateral amygdala. Journal of Comparative Neurology, 498, 142–161. 10.1002/cne.21049 [DOI] [PubMed] [Google Scholar]
- Redecker, J. , Wittstock, M. , Benecke, R. , & Rösche, J. (2015). Efficacy of perampanel in refractory nonconvulsive status epilepticus and simple partial status epilepticus. Epilepsy & Behavior, 45, 176–179. 10.1016/j.yebeh.2015.01.036 [DOI] [PubMed] [Google Scholar]
- Russmann, V. , Salvamoser, J. D. , Rettenbeck, M. L. , Komori, T. , & Potschka, H. (2016). Synergism of perampanel and zonisamide in the rat amygdala kindling model of temporal lobe epilepsy. Epilepsia, 57, 638–647. 10.1111/epi.13328 [DOI] [PubMed] [Google Scholar]
- Sah, P. , Faber, E. L. , Lopez de Armentia, M. , & Power, J. (2003). The amygdaloid complex: Anatomy and physiology. Physiological Reviews, 83, 803–834. 10.1152/physrev.00002.2003 [DOI] [PubMed] [Google Scholar]
- Sessolo, M. , Marcon, I. , Bovetti, S. , Losi, G. , Cammarota, M. , Ratto, G. M. , … Carmignoto, G. (2015). Parvalbumin‐positive inhibitory interneurons oppose propagation but favor generation of focal epileptiform activity. Journal of Neuroscience, 35, 9544–9557. 10.1523/JNEUROSCI.5117-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri, Z. , Manseau, F. , Lévesque, M. , Williams, S. , & Avoli, M. (2016). Activation of specific neuronal networks leads to different seizure onset types. Annals of Neurology, 79, 354–365. 10.1002/ana.24570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder, G. L. , Galdi, S. , Fienberg, A. A. , Allen, P. , Nairn, A. C. , & Greengard, P. (2003). Regulation of AMPA receptor dephosphorylation by glutamate receptor agonists. Neuropharmacology, 45, 703–713. 10.1016/S0028-3908(03)00319-8 [DOI] [PubMed] [Google Scholar]
- Spampanato, J. , Polepalli, J. , & Sah, P. (2011). Interneurons in the basolateral amygdala. Neuropharmacology, 60, 765–773. 10.1016/j.neuropharm.2010.11.006 [DOI] [PubMed] [Google Scholar]
- Spillane, J. , Kullmann, D. M. , & Hanna, M. G. (2016). Genetic neurological channelopathies: Molecular genetics and clinical phenotypes. Journal of Neurology, Neurosurgery & Psychiatry, 87, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley, K. , Hellier, J. L. , & Dudek, F. E. (2005). Do interictal spikes drive epileptogenesis? The Neuroscientist, 11, 272–276. 10.1177/1073858405278239 [DOI] [PubMed] [Google Scholar]
- Stasheff, S. F. , Bragdon, A. C. , & Wilson, W. A. (1985). Induction of epileptiform activity in hippocampal slices by trains of electrical stimuli. Brain Research, 344, 296–302. 10.1016/0006-8993(85)90807-8 [DOI] [PubMed] [Google Scholar]
- Stasheff, S. F. , Mott, D. D. , & Wilson, W. A. (1993). Axon terminal hyperexcitability associated with epileptogenesis in vitro. II. Pharmacological regulation by NMDA and GABAA receptors. Journal of Neurophysiology, 70, 976–984. [DOI] [PubMed] [Google Scholar]
- Tai, C. , Abe, Y. , Westenbroek, R. E. , Scheuer, T. , & Catterall, W. A. (2014). Impaired excitability of somatostatin‐ and parvalbumin‐expressing cortical interneurons in a mouse model of Dravet syndrome. Proceedings of the National Academy of Sciences, 111, E3139–E3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno, T. , & Robinson, H. P. C. (2007). Phase resetting curves and oscillatory stability in interneurons of rat somatosensory cortex. Biophysical Journal, 92, 683–695. 10.1529/biophysj.106.088021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev, I. , & Steriade, M. (2004). Neocortical seizures: Initiation, development and cessation. Neuroscience, 123, 299–336. 10.1016/j.neuroscience.2003.08.051 [DOI] [PubMed] [Google Scholar]
- Tønnesen, J. , Sørensen, A. T. , Deisseroth, K. , Lundberg, C. , & Kokaia, M. (2009). Optogenetic control of epileptiform activity In Proceedings of the National Academy of Sciences of the United States of America (Vol. 106) (pp. 12162–12167). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo, W. , Donoghue, J. A. , Hochberg, L. R. , Eskandar, E. N. , Madsen, J. R. , Anderson, W. S. , … Cash, S. S. (2011). Single‐neuron dynamics in human focal epilepsy. Nature Neuroscience, 14, 635–641. 10.1038/nn.2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski, L. , Cavalheiro, E. A. , Calderazzo‐Filho, L. S. , Bortolotto, Z. A. , Klockgether, T. , Ikonomidou, C. , & Turski, W. A. (1989). The basal ganglia, the deep prepyriform cortex, and seizure spread: Bicuculline is anticonvulsant in the rat striatum. Proceedings of the National Academy of Sciences, 86, 1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twele, F. , Bankstahl, M. , Klein, S. , Römermann, K. , & Löscher, W. (2015). The AMPA receptor antagonist NBQX exerts anti‐seizure but not antiepileptogenic effects in the intrahippocampal kainate mouse model of mesial temporal lobe epilepsy. Neuropharmacology, 95, 234–242. 10.1016/j.neuropharm.2015.03.014 [DOI] [PubMed] [Google Scholar]
- Vecht, C. , Duran‐Peña, A. , Houillier, C. , Durand, T. , Capelle, L. , & Huberfeld, G. (2017). Seizure response to perampanel in drug‐resistant epilepsy with gliomas: Early observations. Journal of Neuro‐Oncology, 133, 603–607. 10.1007/s11060-017-2473-1 [DOI] [PubMed] [Google Scholar]
- Wagner, F. B. P. , Truccolo, W. , Wang, J. , & Nurmikko, A. (2015). Spatiotemporal dynamics of optogenetically‐induced and spontaneous seizure transitions in primary generalized epilepsy. Journal of Neurophysiology, 113, 2321–2234. 10.1152/jn.01040.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn, M. S. , & Moises, H. C. (1992). Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. Journal of Neuroscience, 12, 4066–4079. 10.1523/JNEUROSCI.12-10-04066.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, S. A. , Staba, R. , Bragin, A. , Moxon, K. , Sperling, M. , Avoli, M. , & Engel, J. Jr. (2019). Interneurons and principal cell firing in human limbic areas at focal seizure onset. Neurobiology of Disease, 124, 183–188. 10.1016/j.nbd.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng, X. , & Rosenberg, H. C. (1992). Infusion of bicuculline methiodide into the tectum: Model specificity of pro‐ and anticonvulsant actions. Epilepsy Research, 12, 1–8. 10.1016/0920-1211(92)90085-8 [DOI] [PubMed] [Google Scholar]
- Wu, T. , Nagaya, Y. , & Hanada, T. (2014). Pharmacodynamic and pharmacokinetic interactions of perampanel and other antiepileptic drugs in a rat amygdala kindling model. Seizure, 23, 732–739. 10.1016/j.seizure.2014.06.001 [DOI] [PubMed] [Google Scholar]
- Wykes, R. C. , Heeroma, J. H. , Mantoan, L. , Zheng, K. , MacDonald, D. C. , Deisseroth, K. , … Kullmann, D. M. (2012). Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Science Translational Medicine, 4, 161ra152–161ra152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, S. I. , Donevan, S. D. , & Rogawski, M. A. (1993). Anticonvulsant activity of AMPA/kainate antagonists: Comparison of GYKI 52466 and NBQX in maximal electroshock and chemoconvulsant seizure models. Epilepsy Research, 15, 179–184. 10.1016/0920-1211(93)90054-B [DOI] [PubMed] [Google Scholar]
- Ziburkus, J. , Cressman, J. R. , Barreto, E. , & Schiff, S. J. (2006). Interneuron and pyramidal cell interplay during in vitro seizure‐like events. Journal of Neurophysiology, 95, 3948–3954. 10.1152/jn.01378.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart, R. , Sher, E. , Ping, X. , Jin, X. , Sims, J. R. , Chappell, A. S. , … Hobbs, J. (2014). Perampanel, an antagonist of α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptors, for the treatment of epilepsy: Studies in human epileptic brain and nonepileptic brain and in rodent models. Journal of Pharmacology and Experimental Therapeutics, 351, 124–133. 10.1124/jpet.114.212779 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. 250 nM of perampanel slowly inhibits 4‐AP as well as electrical stimulation‐induced activities. Representative recordings show that low concentration of perampanel (250 nM) exerts similar inhibitory effect on the 4‐AP‐induced (a, n = 5 for both interneurons (INS) and pyramidal neurons (PNs)) or electrical stimulation induced (b, n = 6 for PNs, see blue arrows, and n = 5 for INs) discharges after stable perfusion of ~30 minutes. The inhibitory effect is less prominent in 10 minutes of perfusion of perampanel (250 nM). Events of burst discharges and EPSPs are summed as events of “excitatory waves” in interneurons (Ins). “cPSPs” include burst discharges, EPSPs and IPSPs in PNs. ~10 minutes treatment of perampanel (250 nM) is used for the analysis in b. N.S. P > 0.05; *P < 0.05, Friedman test followed by pairwise Wilcoxon signed rank test in a, and Wilcoxon signed rank test in b.
Video S1. Amelioration of 4‐AP‐induced seizure behaviours by CNQX. Split screen video showing the seizure behaviours in two consecutive experimental sessions ~10 minutes after microinjection of 4‐AP (2 mM, 2 μl, left screen) and then 4‐AP (2 mM, 2 μl) plus CNQX (200 μM, 2 μl) (right screen) into basolateral amygdala (BLA) in the same rat.
Video S2. Amelioration of 4‐AP‐induced seizure behaviours by perampanel. Split screen video showing the seizure behaviours in two consecutive experimental sessions ~10 minutes after microinjection of 4‐AP (2 mM, 2 μl, left screen) and then 4‐AP (2 mM, 2 μl) plus perampanel (80 μM, 2 μl) (right screen) into basolateral amygdala (BLA) in the same rat.


