Abstract
Prothrombin complex concentrate (PCC) is increasingly being used as a treatment for major bleeding in patients who are not taking anticoagulants. The aim of this systematic review and meta‐analysis is to evaluate the effectiveness of PCC administration for the treatment of bleeding in patients not taking anticoagulants. Studies investigating the effectivity of PCC to treat bleeding in adult patients and providing data on either mortality or blood loss were eligible. Data were pooled using Mantel‐Haenszel random effects meta‐analysis or inverse variance random effects meta‐analysis. From 4668 identified studies, 17 observational studies were included. In all patient groups combined, PCC administration was not associated with mortality (odds ratio = 0.83; 95% confidence interval [CI], 0.66‐1.06; P = .13; I 2 = 0%). However, in trauma patients, PCC administration, in addition to fresh frozen plasma, was associated with reduced mortality (odds ratio = 0.64; CI, 0.46‐0.88; P = .007; I 2 = 0%). PCC administration was associated with a reduction in blood loss in cardiac surgery patients (mean difference: −384; CI, −640 to −128, P = .003, I 2 = 81%) and a decreased need for red blood cell transfusions when compared with standard care across a wide range of bleeding patients not taking anticoagulants (mean difference: −1.80; CI, −3.22 to −0.38; P = .01; I 2 = 92%). In conclusion, PCC administration was not associated with reduced mortality in the whole cohort but did reduce mortality in trauma patients. In bleeding patients, PCC reduced the need for red blood cell transfusions when compared with treatment strategies not involving PCC. In bleeding cardiac surgery patients, PCC administration reduced blood loss.
Keywords: bleeding, blood coagulation factors, hemostasis, meta‐analysis, prothrombin complex concentrate, systematic review
1. INTRODUCTION
Severe bleeding is a major health problem, occurring in different clinical settings such as in trauma or perioperatively. Excessive bleeding often results in coagulopathy, with incidences ranging from 1% to 45% depending on the severity of trauma 1 , 2 , 3 or type of surgery, 4 , 5 and develops through several mechanisms, such as the consumption of clotting factors. Coagulopathy may aggravate bleeding, increase transfusion requirements, and contribute to adverse outcomes. 2
Plasma transfusion is frequently used to correct coagulopathy in bleeding patients because it is believed to replenish a deficit of coagulation factors. European guidelines on the management of bleeding trauma patients recommend transfusion of plasma to correct coagulopathy. 6 , 7 In the operative setting, guidelines state that plasma can be used to treat coagulopathy. 8 , 9 However, the effectiveness of plasma to correct coagulopathy is under debate. 10 , 11 , 12 Because the ability of plasma to restore thrombin generation is limited. 10 This limited ability may be due to the presence of anticoagulant proteins in plasma, which inhibit thrombin generation. 10
An alternative approach for the correction of coagulopathy occurring during bleeding may be the use of factor concentrates, such as prothrombin complex concentrate (PCC). PCC contains either three or four of the vitamin K‐dependent coagulation factors (II, IX, X, and sometimes VII). Also, depending on the type of PCC, small amounts of proteins C, S, Z, unfractionated heparin, or antithrombin are present. Because PCCs have gained approval by the US Food and Drug Administration in 2013, they have become the primary treatment for urgent reversal of oral anticoagulation with vitamin K antagonists and congenital vitamin K‐dependent coagulation factor deficiencies.. 13
In addition to these established indications, PCCs are increasingly used to correct coagulopathy in bleeding patients unrelated to coagulopathy. 14 , 15 Guidelines in both trauma and operative settings support the administration of PCC to bleeding patients to reverse coagulopathy. 7 , 8 , 9 However, this is largely based on expert opinion and is supported by limited evidence. By supplementing coagulation factors, PCCs rapidly correct coagulopathy while having lower risks of complications such as transfusion‐associated lung injury, transfusion‐associated circulatory overload, bacterial contamination, and allergic reactions when compared with plasma transfusion. 16 , 17 , 18 Regarding safety, the risk of thromboembolic complications after administration of 4‐factor PCC, when given in the proper dose, seem to be rather low. 19
There are limited trials on the effectivity of PCC. In trauma, a randomized controlled trial showed that PCC is associated with a reduced need for massive transfusion when compared with plasma. 13 The trial was terminated early for safety reasons because the plasma group showed more adverse outcome compared with the PCC group. However, as all PCC patients also received fibrinogen, it is not clear from this trial whether PCC, fibrinogen, or the combination contributed most to the observed benefit. To date, a summary of the effectiveness of PCC in bleeding patients not taking anticoagulants is not present. The objective of this review is to systematically evaluate the effectivity of PCC in bleeding unrelated to anticoagulation in different clinical scenarios.
2. METHODS
2.1. Search strategy
This systematic review and meta‐analysis was conducted according to the PRISMA methodology. 20 No review protocol is available. To identify all articles investigating the use of PCC for treatment of bleeding, a comprehensive computer‐assisted literature search of MEDLINE, EMBASE, and CINAHL electronic databases was performed including articles between 1952 and April 2020. A broad search strategy was used that included multiple synonyms of PCC combined with terms as: “hemorrhage,” “mortality,” and “bleeding.” The full PubMed, EMBASE, and CINAHL search strategies can be found in the supplement (Appendix S1). In addition, the reference list of the most relevant studies and reviews were hand‐searched for eligible studies not captured in the initial literature search.
2.2. Study selection
The selection process was divided in three stages: title, abstract, and full‐text selection. The title selection process was done by one author (D.B.). Thereafter, two authors (D.B., N.J.) independently performed the abstract and full‐text selection to assess the eligibility of the articles to the predefined criteria. Differences in judgment were resolved by discussion. Inclusion criteria were: (a) patients ≥ 18 years old who received 3‐ or 4‐factor PCC for active bleeding; (b) all patients in the experimental group received PCC; (c) at least one of the following outcomes was reported: mortality, blood product utilization, blood loss or thromboembolic (TE) events; (d) the use of a comparator including: placebo, nothing, usual treatment (eg, fresh frozen plasma [FFP]) or other hemostatic agents (eg, recombinant factor VIIa [rFVIIa]); and (e) language: English. Exclusion criteria were: experimental or preclinical study design; use of activated 4‐factor PCC; PCC administration for anticoagulant reversal; use of a different PCC as comparator; and data of outcome could not be extracted. Types of articles eligible for inclusion were randomized controlled trials, nonrandomized controlled trials, and cohort studies. Case reports and congress abstracts were excluded.
2.3. Data extraction
Using a predefined standard data form, data were collected independently by two authors (D.P., M.W.) for the following data points: study design; number of patients; mean age; study setting; PCC indication; timing of PCC administration; type and dose of PCC; type and dose of comparator; mortality; mortality follow‐up days; thromboembolic (TE) events; number of transfused red blood cell (RBC) units; blood loss (total chest tube output at either 12 or 24 hours postsurgery); other significant outcomes; article authors’ conclusion.
2.4. Outcomes
All‐cause mortality was chosen as the primary outcome. Secondary outcomes were: blood loss, RBC utilization, and TE events. Three separate subgroup analyses were performed, including cause of bleeding (trauma, cardiac surgery, liver surgery, other), comparator (PCC with FFP vs FFP, PCC vs FFP, PCC vs rFVIIa, PCC vs nothing), and PCC dosage (<20, 20‐30, >30 IU/kg).
2.5. Study quality assessment
Study quality assessment of all eligible studies was conducted by two authors (D.P., M.W.) independently. Using the Newcastle‐Ottawa quality assessment scale, the risk of bias of cohort and case control studies was assessed. 21 The maximum score of the Newcastle‐Ottawa scale is 9 points. All studies were ranked either as poor, moderate, or good quality depending on the total score: studies receiving ≤ 5 points received a poor quality score; studies receiving 6 points received a moderate quality score; and studies receiving ≥ 7 points received a good quality score.
2.6. Statistical analysis
Data are reported as mean with standard deviation, counts, or percentages. Data retrieved as median with interquartile range were converted to means as reported before to pool data for meta‐analysis. 22 Meta‐analysis was performed using Review Manager 5.3; statistical heterogeneity across the studies was assessed using the Cochran's Q test and I 2 values. Sensitivity analysis to exclude outliers was performed using Review Manager 5.3. The odds ratios (OR) were pooled using the Mantel‐Haenszel procedure, which assumes a random effects model. Mean differences were pooled using the Inverse variance procedure, which also assumes a random effects model.
3. RESULTS
3.1. Characteristics of included studies
The systematic search identified 4668 studies (Medline: 1554; Embase: 2927; CINAHL: 187; Figure 1). After removing duplicates, 3423 studies remained. Of these, 3297 were excluded based on exclusion criteria, yielding 126 studies. Of these, full text was assessed, leading to the exclusion of another 109 studies, resulting in 17 studies that were included in the analysis. Studies included two prospective studies 23 , 24 and 15 retrospective studies, 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 yielding a total of 3060 patients (Figure 1). Characteristics of the included studies are shown in Table 1. Studies were conducted in patients undergoing cardiothoracic surgery (10 studies with a total of 1678 patients) 23 , 24 , 25 , 28 , 29 , 30 , 35 , 36 , 37 , 39 in trauma patients (four studies with a total of 932 patients), 31 , 32 , 33 , 38 in patients undergoing orthotopic liver transplantation (two studies with a total of 383 patients) 26 , 34 and in patients who were bleeding from various causes (one study with a total of 78 patients). 27
FIGURE 1.
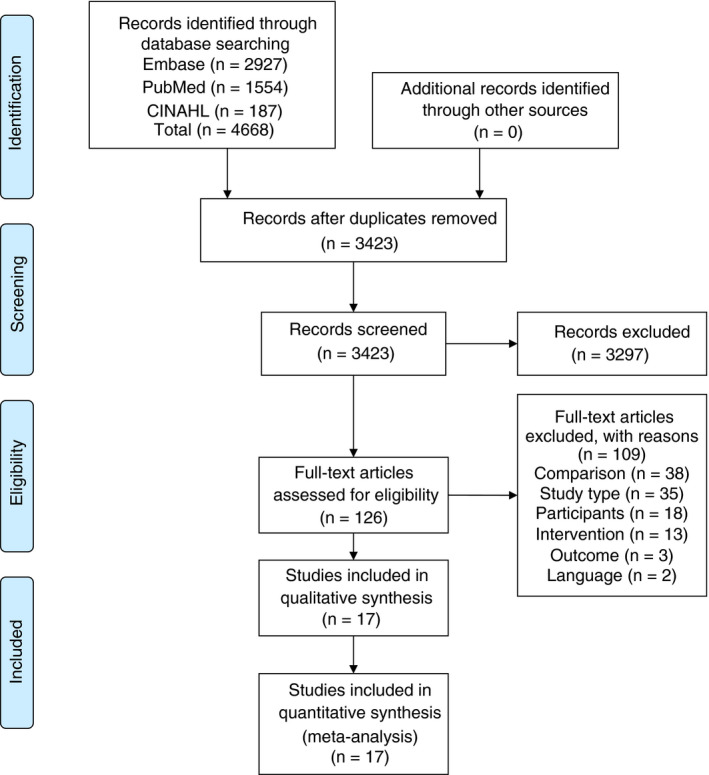
PRISMA flow diagram search strategy
Table 1.
Characteristics of included studies
| Reference Number/Study ID | Design of Study/Study Duration | Setting | Sample Size | Intervention Group | Control Group | Conclusion |
|---|---|---|---|---|---|---|
| Zeeshan et al 2019 38 | Retrospective (propensity matched) | Trauma | 468 | 4‐factor PCC + FFP | FFP | The use of PCC as an adjunct to FFP is associated with improved survival and reduction in transfusion requirements without increasing the risk of TE |
| Jehan et al 2018 31 | Retrospective (propensity matched) | Trauma | 120 | 4‐factor PCC + FFP | FFP | PCC as a component therapy along with FFP is superior to FFP alone in treating coagulopathy |
| Joseph et al 2016 33 | Retrospective (propensity matched) | Trauma | 81 | 3‐factor PCC + FFP | FFP | PCC reduced the time to correct INR and time to intervention compared with patients who received FFP |
| Joseph et al 2014 32 | Retrospective (propensity matched) | Trauma | 252 | 3‐factor PCC + FFP | FFP | PCC as an adjunct to FFP is associated with reduction of blood product requirement and also lowers overall cost |
| DeLoughery et al 2016 27 | Retrospective | Bleeding | 78 | 4‐factor PCC | rFVIIa | PCC was associated with had the shortest LOS among survivors, the rFVIIa group had the lowest mortality |
| Harris et al 2020 30 | Retrospective | Cardiac Surgery | 79 | 4‐factor PCC | Nothing | Among Jehovah's Witness patients undergoing cardiac surgery, 4‐PCC was not associated with a difference in Hb change postoperatively, in the event of excessive blood loss 4pcc may provide a viable option |
| Biancari et al 2019 24 | Prospective multicenter trial (propensity matched) | Cardiac surgery | 202 | Both 3‐ and 4‐factor PCC + FFP | FFP | The use of PCC compared with FFP may reduce the need of blood transfusion after CABG; these results should be considered hypothesis generating |
| Zweng et al 2018 39 | Retrospective (propensity matched) | Cardiac surgery | 160 | 3‐factor PCC + FFP | FFP | PCC is not associated with an increased risk of TE or unfavorable outcomes compared with conventional treatment. PCC may be acceptable for management of severe perioperative bleeding in open heart surgery |
| Fitzgerald et al 2018 28 | Retrospective (propensity matched) | Cardiac surgery | 234 | 4‐factor PCC + FFP | FFP | Use of PCCs as part of a multifaceted coagulation management strategy may have blood‐sparing effects |
| Harper et al 2018 29 | Retrospective (propensity matched) | Cardiac surgery | 106 | 3‐factor PCC | rFVIIa | Use of rFVIIa vs inactive PCCs was associated with renal failure requiring dialysis and increased postoperative bleeding and transfusions |
| Mehringer et al 2018 35 | Retrospective | Cardiac surgery | 129 | 4‐factor PCC | rFVIIa | 4‐factor PCC may be an equally efficacious alternative to rFVIIa for patients experiencing significant bleeding during cardiac surgery |
| Cappabianca et al 2015 23 | Prospective observational (propensity matched) | Cardiac surgery | 450 | 3‐factor PCC | FFP | The use of PCC compared with FFP was associated with decreased postoperative blood loss and RBC transfusion requirements. However, PCC may be associated with a higher risk of AKI |
| Bradford et al 2015 25 | Retrospective | Cardiac surgery | 68 | 3‐factor PCC | Nothing | PCC in LVAD insertion does not appear to be associated with a significant increase in thromboembolic events |
| Ortmann et al 2014 36 | Retrospective (propensity matched) | Cardiac surgery | 100 | 4‐factor PCC | FFP 15 | PCC may be an alternative to FFP in patients who are coagulopathic and bleeding after cardiac surgery |
| Tanaka et al 2013 37 | Retrospective | Cardiac surgery | 150 | 3‐factor PCC | rFVIIa | 3‐factor PCC could be hemostatically effective in dilutional coagulopathy because of its high prothrombin content despite the lower FVII content |
| Colavecchia et al 2017 26 | Retrospective (propensity matched) | Liver surgery | 117 | 4‐factor PCC + FFP | FFP | Use of PCC and fibrinogen concentrate during liver transplantation did not reduce intraoperative blood product requirements |
| Kirchner et al 2014 34 | Retrospective | Liver surgery | 266 | 4‐factor PCC + FFP | FFP | In liver transplantation, ROTEM‐guided treatment with fibrinogen and/or PCC did not increase the occurrence of thrombosis and ischemic events |
Abbreviations: AKI, acute kidney injury; CABG, coronary artery bypass graft; Hb, hemoglobin; INR, International Normalized Ratio; LOS, length of stay; LVAD, left ventricular assist device; ROTEM, rotational thromboelastometry; TE, thromboembolic events.
The most frequent comparator to PCC was FFP with two studies investigating PCC only in comparison to FFP only 23 , 36 and nine studies investigating a combination of PCC and FFP in comparison to FFP only. 24 , 26 , 28 , 31 , 32 , 33 , 34 , 38 , 39 Other comparators included rFVII, which was compared with PCC in four studies. 27 , 29 , 35 , 37 In one study that was conducted in Jehovah's Witness patients, the comparator consisted of not giving any coagulation factor or transfusion product, 30 and in one study the comparator was standard care that was not further specified. 25 Subgroup analyses for comparators for each outcome are available in the supplement (Figures S1‐S4).
The PCC product that was mostly evaluated was 4‐factor PCC (nine studies), 12 , 26 , 27 , 28 , 31 , 34 , 35 , 36 , 38 whereas 3‐factor PCC was evaluated in seven studies. 23 , 25 , 29 , 32 , 33 , 37 , 39 Multiple types of PCC were used in one study. 24 The dose of PCC ranged from 10 to 50 IU/kg. In 13 studies, PCC was administered based on clinical judgment. 24 , 25 , 26 , 27 , 29 , 30 , 31 , 32 , 33 , 35 , 37 , 38 , 39 In four studies, PCC administration was protocolized, usually guided by thromboelastometry. 27 , 29 , 31 , 32 A summary regarding PCC type, dose, and setting can be found in the supplement (Table S1).
3.2. Quality assessment
Of the 17 included studies, 13 were assessed as having a good quality 23 , 24 , 25 , 26 , 28 , 29 , 31 , 32 , 33 , 36 , 37 , 38 , 39 according to the Newcastle‐Ottawa quality assessment scale, 21 one had a fair quality, 30 and three were rated as having a poor quality. 27 , 34 , 35 Details regarding quality assessment can be found in the supplement (Table S2).
3.3. Mortality outcome
Mortality data were available in 14 of the 17 included studies, with a total of 2548 patients. Follow‐up time to mortality ranged from in hospital mortality to 60 days. In all patient groups taken together, PCC administration was not associated with a reduction in mortality (OR 0.83; 95% confidence interval [CI], 0.66‐1.06; P = .13; I 2 = 0%; Figure 2). Subgroup analysis in specific patient populations showed similar results for patients undergoing cardiac surgery (OR 1.04; 95% CI, 0.72‐1.51; P = .83; I 2 = 0%; P for heterogeneity = .92; Figure 2). However, in the trauma subgroup, there was a significant reduction in mortality in patients receiving PCC compared with patients not receiving PCC (OR 0.64; 95% CI, 0.46‐0.88; P = .007; I 2 = 0%; P for heterogeneity = .81; Figure 2). This benefit was observed when PCC was added to FFP compared with FFP alone, but not when PCC as a stand‐alone therapy was compared with FFP (Figure S1). The studies in patients undergoing liver surgery did not report any data on mortality. Subgroup analyses on the dose‐dependent effectiveness of PCC on mortality is available in the supplement (Figure S5). There were no significant differences between lower and higher PCC dosages. A sensitivity analysis excluding outliers did not change results (OR 0.79; 95% CI, 0.62‐1.01; P = .06; I 2 = 0%; P for heterogeneity = .78; Figure S7).
FIGURE 2.
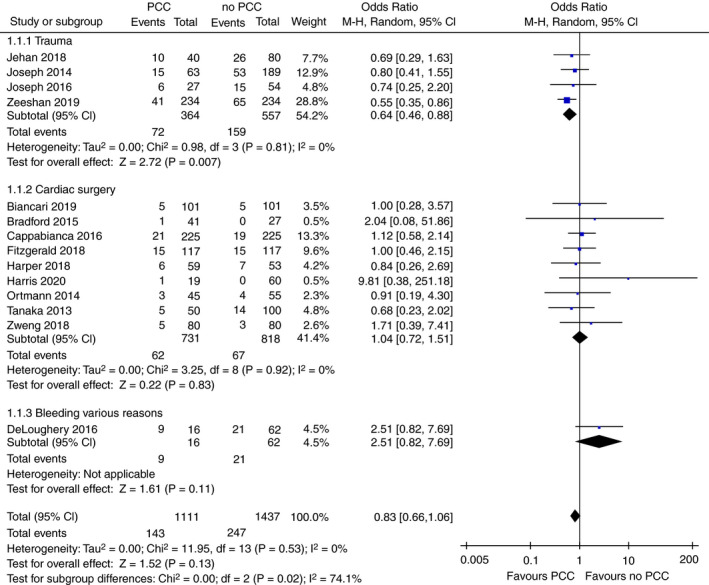
Forest plot comparison of overall and subgroup mortality in patients treated with PCC vs patients not treated with PCC
3.4. Blood loss outcome
Data on blood loss were available in five studies with a total of 875 patients, all of which were performed in patients undergoing cardiac surgery. 23 , 29 , 30 , 36 , 37 The timeframe in which blood loss was recorded was either 12 or 24 hours. Four studies reported blood loss in median values, 29 , 30 , 36 , 37 which were converted into mean values. Mean blood loss ranged from 353 to 1159 mL in patients receiving PCC and 480 to 1644 mL in patients not receiving PCC. Total blood loss was significantly lower in the PCC group (mean difference −293 mL; 95% CI, −546 to −41; P = .02; I 2 = 86%; Figure 3).
FIGURE 3.
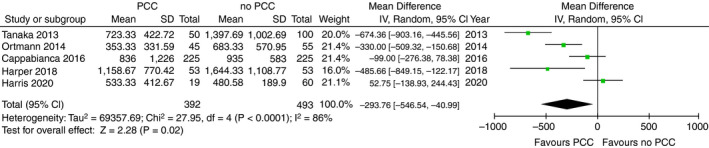
Forest plot comparison of blood loss in cardiac surgery patients treated with PCC vs patients not treated with PCC
3.5. Red blood cell product utilization
Data on the number of RBC units that were transfused was available in 11 of the 17 included studies, ranging across all subpopulations, 23 , 26 , 28 , 29 , 31 , 32 , 33 , 34 , 36 , 37 , 38 with a total of 2294 patients. In four studies, data were reported in median, 28 , 29 , 36 , 37 which were converted into mean. The mean number of RBC units that were transfused ranged from 2.3 to 12.4 in patients receiving PCC and from 1.0 to 10.7 in patients not receiving PCC. The amount of administered RBC products was significantly lower in the PCC group (mean difference −1.61 units; 95% CI, −3.0 to −0.2; P = .02; I 2 = 93%; Figure 4). Also, in the specific patient populations, RBC requirements were lower in the PCC groups. In trauma patients, PCC use resulted in a reduction of 3.0 RBC units compared with patients not receiving PCC (95% CI, −4.1 to −1.9; P < .00001; I 2 = 68%; P for heterogeneity = .02; Figure 4). In cardiac surgery patients, PCC resulted in a reduction of 2.0 units (95% CI, −3.4 to −0.7; P = .003; I 2 = 81%; P for heterogeneity = .0003; Figure 4) compared with those not receiving PCC. Of importance, all analyses showed high heterogeneity. In contrast, patients undergoing liver transplant surgery showed opposite results because patients who received PCC had an increase use of 2.1 RBC units in comparison to patients not receiving PCC (95% CI, 1.2 to −2.8; P = <.00001; I 2 = 0%; P for heterogeneity = .64; Figure 4). Subgroup analyses on the dose‐dependent effectiveness of PCC on RBC utilization is available in the supplement (Figure S6). Dosages < 30 IU/kg showed effects in favor of PCC whereas dosages > 30 IU/kg showed no differences between groups.
FIGURE 4.
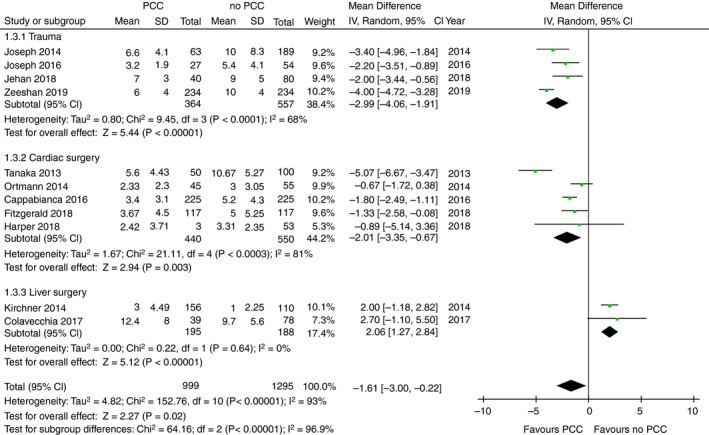
Forest plot comparison of overall and subgroup RBC utilization in patients treated with PCC vs patients not treated with PCC
3.6. Thromboembolic events
Data on TE events was available in 15 of the 17 included studies with a total of 2745 evaluable patients. 23 , 24 , 25 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 38 , 39 The TE rate ranged between 0% and 41% in the PCC group and between 4% and 26% in the no‐PCC group. Overall, PCC administration was not associated with the occurrence of TE (OR 1.11; 95% CI, 0.82‐1.50; P = .49; I 2 = 0%; Figure 5). In subgroup analyses, similar results were found (Figure 5).
FIGURE 5.
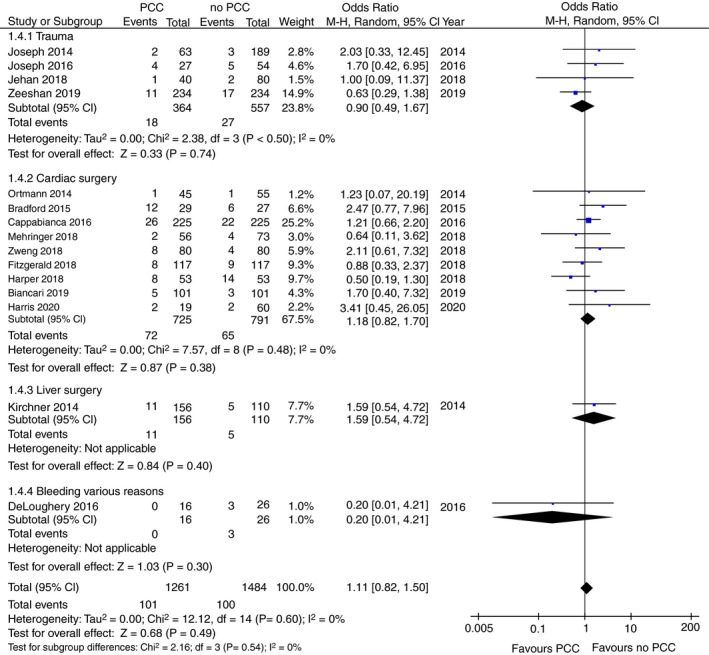
Forest plot comparison of overall and subgroup thromboembolic events in patients treated with PCC vs patients not treated with PCC
4. DISCUSSION
This systematic review analyzes the effectivity of PCC to control bleeding in various patient populations who were no on anticoagulants. Overall, we found that PCC did not reduce mortality in comparison to plasma or to other hemostatic agents. However, in the subgroup analysis, PCC administration when added to FFP was associated with reduced mortality in trauma patients.
An explanation for the difference of an effect between patient groups could be that PCC could play a more important role in patients that are bleeding more excessively, which is more probable in trauma patients compared with the cardiac and liver surgery patients, which may experience more “controlled” blood loss.
Most of the included studies compared PCC in conjunction with FFP to solely FFP. Whereas PCC added to FFP was associated with a reduction in mortality, this effect was not noted in the other comparisons, such as PCC versus FFP or PCC versus no therapy. This implies that PCC is beneficial as an adjunct to standard treatment including FFP rather than a replacement of FFP. This benefit could lie in a more rapid correction of coagulopathy and coagulation factor replacement while not losing the benefit of FFP.
In cardiac surgery patients, a significantly reduced blood loss in favor of the PCC group was found. However, substantial statistical heterogeneity may hamper credibility of results. This heterogeneity is explained by two reasons. First, blood loss was recorded in different time frames (either within 12 or 24 hours postsurgery), yielding a wide range. Second, we extrapolated means from medians in four studies. However, results on amount of blood loss are congruent with the finding of a reduction in RBC utilization in the PCC‐treated patients when compared with the patients not receiving PCC. In most patient categories, results point in the same direction, suggesting that PCC is associated with reduced blood loss.
Of note, liver surgery patients showed opposite results, with more RBC products needed in patients treated with PCC versus a comparator. This may be due to baseline imbalances between groups, with more severe liver injury and coagulopathy in the PCC group. 34 Also, two studies with small sample sizes were included. Therefore, we feel that results in the subgroup of liver failure patients are uncertain.
Regarding safety, the use of PCC did not result in an increase in TE events when compared with patients not receiving PCC. Of note, the CIs of the ORs were very wide, suggesting differential results across studies. However, the large number of patients included in this analysis is a strength. Taken together, PCCs do not seem to come at a cost of increased venous thromboembolic events.
This review has certain limitations because a small number of eligible studies were available with substantial heterogeneity. This heterogeneity is caused by a number of reasons. First, most studies had a retrospective study design, in which PCC was administered based on clinical judgment. This may introduce confounding and bias because PCC may potentially be administered to more severely ill and hemodynamically unstable patients, resulting in underrepresentation of the actual effect. Second, because there is no optimal dose for PCC, there is considerable variety in the type and dose of PCC, as well as the trigger for administration. This could lead to both an under‐ or overrepresentation of the actual effects as certain PCCs might have better effectiveness and safety profiles compared with others. Third, not all included studies had strict inclusion or exclusion criteria of their study population, leading to a heterogeneous study population. Furthermore, we only included articles published in English, which might lead to language bias.
Of interest, our search also yielded multiple studies, including a randomized controlled trial, 40 that used a point‐of‐care coagulation testing‐based protocol in which both PCC and fibrinogen concentrate as were administered as an adjunct to FFP in comparison to a FFP‐based protocol. 15 , 40 , 41 , 42 , 43 Unfortunately, these studies were not included because outcome data from patients receiving PCC could not be separated from those not receiving PCC. Innerhofer et al found that patients receiving PCC and fibrinogen had a decreased need for massive transfusion and rescue therapy when compared with the FFP group. 40 Schöchl et al and Nienaber et al also showed reduced exposure of trauma patients to blood products in the PCC/fibrinogen group when compared with the FFP group. 41 , 43 Görlinger et al also showed reduced blood product utilization after implementing the PCC/fibrinogen based protocol in cardiac surgery patients. 44 , 45 Inclusion of these studies probably would have strengthened the suggestion that a PCC and/or fibrinogen‐based approach may be a reasonable strategy to treat bleeding patients.
5. CONCLUSION
PCC administration in bleeding patients not using anticoagulants had no effect on mortality in the whole cohort of patients. However, in trauma patients, a resuscitation strategy using both PCC and FFP transfusion was associated with reduced mortality when compared to a resuscitation strategy involving solely FFP. Also, PCC reduced the need for RBC transfusions when compared with treatment strategies not involving PCC. In bleeding cardiac surgery patients, PCC administration reduced perioperative blood loss. Risk of TE events was not increased. However, results are subject to considerable heterogeneity and should be interpreted with caution. These data, derived from observational studies, can be used to design trials to further explore the effectivity of PCC in different clinical scenarios of bleeding.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Daan P. van den Brink contributed substantially to the conception, design, acquisition, analysis, and interpretation of data for the work; and drafted and revised the work. Mathijs R Wirtz contributed substantially to the acquisition, analysis, interpretation of data, and revising the intellectual content. A. Serpa Neto made substantial contributions to the interpretation of data and revising the intellectual content. Herbert Schöchl made substantial contributions to the interpretation of data and revising the intellectual content. Victor Viersen made substantial contributions to the interpretation of data and revising the intellectual content. J Binnekade made substantial contributions to the statistical analyses of the data for the work. Nicole P. Juffermans contributed substantially to the conception, design, acquisition, analysis, and interpretation of data for the work; and drafted and revised the work.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
Not applicable.
van den Brink DP, Wirtz MR, Neto AS, et al. Effectiveness of prothrombin complex concentrate for the treatment of bleeding: A systematic review and meta-analysis. J Thromb Haemost. 2020;18:2457–2467. 10.1111/jth.14991
Manuscript handled by: Willem Lijfering
Final decision: Willem Lijfering and 26‐Jun‐2020
Prior presentations: ECISM lives 2019, 1/10/2019, The Cube Berlin
REFERENCES
- 1. MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39‐44. [DOI] [PubMed] [Google Scholar]
- 2. Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127‐1130. [DOI] [PubMed] [Google Scholar]
- 3. Maegele M, Lefering R, Yucel N, et al. Early coagulopathy in multiple injury: an analysis from the German trauma registry on 8724 patients. Injury. 2007;38:298‐304. [DOI] [PubMed] [Google Scholar]
- 4. Bassin L, Stone M, Krol M, Reich DL. Risk factors for intraoperative coagulopathy in cardiac surgery. Heart Lung Circ. 2010;19:506. [Google Scholar]
- 5. Singh SA, Vivekananthan P, Sharma A, Sharma S, Bharathy KG. Retrospective analysis of post‐operative coagulopathy after major hepatic resection at a tertiary care centre in Northern India. Indian J Anaesth. 2017;61:575‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313:471‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rossaint R, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20(1). 10.1186/s13054-016-1265-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lisman T, Porte RJ. Pathogenesis, prevention, and management of bleeding and thrombosis in patients with liver diseases. Res Pract Thromb Haemost. 2017;1:150‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pagano D, Milojevic M, Meesters MI, et al. 2017 EACTS/EACTA guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53:79‐111. [DOI] [PubMed] [Google Scholar]
- 10. Desborough M, Sandu R, Brunskill SJ, et al. Fresh frozen plasma for cardiovascular surgery. Cochrane Database Syst Rev. 2015;10:Cd007614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan S, Davenport R, Raza I, et al. Damage control resuscitation using blood component therapy in standard doses has a limited effect on coagulopathy during trauma hemorrhage. Intensive Care Med. 2015;41:239‐247. [DOI] [PubMed] [Google Scholar]
- 12. Balvers K, van Dieren S, Baksaas‐Aasen K, et al. Combined effect of therapeutic strategies for bleeding injury on early survival, transfusion needs and correction of coagulopathy. Br J Surg. 2017;104:222‐229. [DOI] [PubMed] [Google Scholar]
- 13. Tornkvist M, Smith JG, Labaf A. Current evidence of oral anticoagulant reversal: a systematic review. Thromb Res. 2018;162:22‐31. [DOI] [PubMed] [Google Scholar]
- 14. Tang M, Fenger‐Eriksen C, Wierup P, et al. Rational and timely haemostatic interventions following cardiac surgery ‐ coagulation factor concentrates or blood bank products. Thromb Res. 2017;154:73‐79. [DOI] [PubMed] [Google Scholar]
- 15. Schochl H, Nienaber U, Hofer G, et al. Goal‐directed coagulation management of major trauma patients using thromboelastometry (ROTEM)‐guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14:R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franchini M, Lippi G. Prothrombin complex concentrates: an update. Blood Transfus. 2010;8:149‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li G, Rachmale S, Kojicic M, et al. Incidence and transfusion risk factors for transfusion‐associated circulatory overload among medical intensive care unit patients. Transfusion. 2011;51:338‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52(Suppl 1):65s‐79s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanke AA, Joch C, Gorlinger K. Long‐term safety and efficacy of a pasteurized nanofiltrated prothrombin complex concentrate (Beriplex P/N): a pharmacovigilance study. Br J Anaesth. 2013;110:764‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta‐analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2‐10. [DOI] [PubMed] [Google Scholar]
- 22. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid‐range, and/or mid‐quartile range. Stat Methods Med Res. 2018;27:1785‐1805. [DOI] [PubMed] [Google Scholar]
- 23. Cappabianca G, Mariscalco G, Biancari F, et al. Safety and efficacy of prothrombin complex concentrate as first‐line treatment in bleeding after cardiac surgery. Crit Care. 2016;20:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biancari F, Ruggieri VG, Perrotti A, et al. Comparative analysis of prothrombin complex concentrate and fresh frozen plasma in coronary surgery. Heart Lung Circ. 2019;28:1881‐1887. [DOI] [PubMed] [Google Scholar]
- 25. Bradford CD, Stahovich MJ, Dembitsky WP, Adamson RM, Engelbert JJ, Perreiter AS. Safety of prothombin complex concentrate to control excess bleeding during continuous flow LVAD insertion. ASAIO J. 1992;2015(61):509‐513. [DOI] [PubMed] [Google Scholar]
- 26. Colavecchia AC, Cohen DA, Harris JE, et al. Impact of intraoperative factor concentrates on blood product transfusions during orthotopic liver transplantation. Transfusion. 2017;57:3026‐3034. [DOI] [PubMed] [Google Scholar]
- 27. DeLoughery E, Avery B, DeLoughery TG. Retrospective study of rFVIIa, 4‐factor PCC, and a rFVIIa and 3‐factor PCC combination in improving bleeding outcomes in the warfarin and non‐warfarin patient. Am J Hematol. 2016;91:705‐708. [DOI] [PubMed] [Google Scholar]
- 28. Fitzgerald J, Lenihan M, Callum J, et al. Use of prothrombin complex concentrate for management of coagulopathy after cardiac surgery: a propensity score matched comparison to plasma. Br J Anaesth. 2018;120:928‐934. [DOI] [PubMed] [Google Scholar]
- 29. Harper PC, Smith MM, Brinkman NJ, et al. Outcomes following three‐factor inactive prothrombin complex concentrate versus recombinant activated factor VII administration during cardiac surgery. J Cardiothorac Vasc Anesth. 2018;32:151‐157. [DOI] [PubMed] [Google Scholar]
- 30. Harris JE, Varnado S, Herrera E, Salazar E, Colavecchia AC. Evaluation of postoperative clinical outcomes in Jehovah's Witness patients who receive prothrombin complex concentrate during cardiac surgery. J Card Surg. 2020;35(4):801‐809. [DOI] [PubMed] [Google Scholar]
- 31. Jehan F, Aziz H, O'Keeffe T, et al. The role of four‐factor prothrombin complex concentrate in coagulopathy of trauma: a propensity matched analysis. J Trauma Acute Care Surg. 2018;85:18‐24. [DOI] [PubMed] [Google Scholar]
- 32. Joseph B, Aziz H, Pandit V, et al. Prothrombin complex concentrate versus fresh‐frozen plasma for reversal of coagulopathy of trauma: is there a difference? World J Surg. 2014;38:1875‐1881. [DOI] [PubMed] [Google Scholar]
- 33. Joseph B, Khalil M, Harrison C, et al. Assessing the efficacy of prothrombin complex concentrate in multiply injured patients with high‐energy pelvic and extremity fractures. J Orthop Trauma. 2016;30:653‐658. [DOI] [PubMed] [Google Scholar]
- 34. Kirchner C, Dirkmann D, Treckmann JW, et al. Coagulation management with factor concentrates in liver transplantation: a single‐center experience. Transfusion. 2014;54:2760‐2768. [DOI] [PubMed] [Google Scholar]
- 35. Mehringer SL, Klick Z, Bain J, et al. Activated factor 7 versus 4‐factor prothrombin complex concentrate for critical bleeding post‐cardiac surgery. Ann Pharmacother. 2018;52:533‐537. [DOI] [PubMed] [Google Scholar]
- 36. Ortmann E, Besser MW, Sharples LD, et al. An exploratory cohort study comparing prothrombin complex concentrate and fresh frozen plasma for the treatment of coagulopathy after complex cardiac surgery. Anest Analg. 2015;121:26‐33. [DOI] [PubMed] [Google Scholar]
- 37. Tanaka KA, Mazzeffi MA, Grube M, Ogawa S, Chen EP. Three‐factor prothrombin complex concentrate and hemostasis after high‐risk cardiovascular surgery. Transfusion. 2013;53:920‐921. [DOI] [PubMed] [Google Scholar]
- 38. Zeeshan M, Hamidi M, Feinstein AJ, et al. Four‐factor prothrombin complex concentrate is associated with improved survival in trauma‐related hemorrhage: a nationwide propensity‐matched analysis. J Trauma Acute Care Surg. 2019;87:274‐281. [DOI] [PubMed] [Google Scholar]
- 39. Zweng I, Galvin S, Robbins R, et al. Initial experience of the use of 3‐factor prothrombin complex concentrate and thromboembolic complications after cardiac surgery. Heart Lung Circ. 2019;28:1706‐1713. [DOI] [PubMed] [Google Scholar]
- 40. Innerhofer P, Fries D, Mittermayr M, et al. Reversal of trauma‐induced coagulopathy using first‐line coagulation factor concentrates or fresh frozen plasma (RETIC): a single‐centre, parallel‐group, open‐label, randomised trial. Lancet Haematol. 2017;4:e258‐e271. [DOI] [PubMed] [Google Scholar]
- 41. Schochl H, Forster L, Woidke R, Solomon C, Voelckel W. Use of rotation thromboelastometry (ROTEM) to achieve successful treatment of polytrauma with fibrinogen concentrate and prothrombin complex concentrate. Anaesthesia. 2010;65:199‐203. [DOI] [PubMed] [Google Scholar]
- 42. Innerhofer P, Westermann I, Tauber H, et al. The exclusive use of coagulation factor concentrates enables reversal of coagulopathy and decreases transfusion rates in patients with major blunt trauma. Injury. 2013;44:209‐216. [DOI] [PubMed] [Google Scholar]
- 43. Nienaber U, Innerhofer P, Westermann I, et al. The impact of fresh frozen plasma vs coagulation factor concentrates on morbidity and mortality in trauma‐associated haemorrhage and massive transfusion. Injury. 2011;42:697‐701. [DOI] [PubMed] [Google Scholar]
- 44. Weber CF, Gorlinger K, Meininger D, et al. Point‐of‐care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117:531‐547. [DOI] [PubMed] [Google Scholar]
- 45. Gorlinger K, Dirkmann D, Hanke AA, et al. First‐line therapy with coagulation factor concentrates combined with point‐of‐care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single‐center cohort study. Anesthesiology. 2011;115:1179‐1191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


