Abstract
Key points
Fetal growth restriction induces a haemodynamic response that aims to maintain blood flow to vital organs such as the brain, in the face of chronic hypoxaemia
Maternal sildenafil treatment impairs the hypoxaemia‐driven haemodynamic response and potentially compromises fetal development.
Abstract
Inadequate substrate delivery to a fetus results in hypoxaemia and fetal growth restriction (FGR). In response, fetal cardiovascular adaptations redirect cardiac output to essential organs to maintain oxygen delivery and sustain development. However, FGR infants remain at risk for cardiovascular and neurological sequelae. Sildenafil citrate (SC) has been examined as a clinical therapy for FGR, but also crosses the placenta and may exert direct effects on the fetus. We investigated the effects of maternal SC administration on maternal and fetal cardiovascular physiology in growth‐restricted fetal sheep. Fetal sheep (0.7 gestation) underwent sterile surgery to induce growth restriction by single umbilical artery ligation (SUAL) or sham surgery (control, AG). Fetal catheters and flow probes were implanted to measure carotid and femoral arterial blood flows. Ewes containing SUAL fetuses were randomized to receive either maternal administration of saline or SC (36 mg i.v. per day) beginning 4 days after surgery, and continuing for 20 days. Physiological recordings were obtained throughout the study. Antenatal SC treatment reduced body weight by 32% and oxygenation by 18% in SUAL compared to AG. SC did not alter maternal or fetal heart rate or blood pressure. Femoral blood flow and peripheral oxygen delivery were increased by 49% and 30% respectively in SUALSC compared to SUAL, indicating impaired cardiovascular adaptation to chronic hypoxaemia. Antenatal SC directly impairs the fetal haemodynamic response to chronic hypoxaemia. Consideration of the consequences upon the fetus should be paramount when administering interventions to the mother during pregnancy.
Keywords: antenatal therapy, cardiovascular physiology, fetal growth restriction, sildenafil citrate
Key points
Fetal growth restriction induces a haemodynamic response that aims to maintain blood flow to vital organs such as the brain, in the face of chronic hypoxaemia
Maternal sildenafil treatment impairs the hypoxaemia‐driven haemodynamic response and potentially compromises fetal development.
Introduction
Fetal growth restriction (FGR) complicates ∼5–10% of all pregnancies(MacHado Nardozza et al. 2012) and is defined as the failure of a fetus to reach their genetic growth potential (Nardozza et al. 2017). FGR increases the risk of stillbirth 20‐fold and is a principal cause of perinatal death (Figueras & Gratacós, 2014). Infants born growth‐restricted have an increased risk of both short‐term (Sehgal et al. 2016) and long‐term neurological and cardiovascular sequelae (Sehgal et al. 2013), compared to their appropriately grown (AG) counterparts (Malhotra et al. 2019). FGR most commonly occurs secondary to placental insufficiency, resulting in reduced delivery of nutrients and oxygen to the fetus, which compromises normal fetal growth and organ development (Nardozza et al. 2017).
Impaired oxygen transfer across the placenta causes chronic fetal hypoxaemia and hypoxia. The physiological response of the fetus to chronic hypoxaemia has been well documented, including cardiac output redistribution in an effort to maintain perfusion of key organs (brain, heart, adrenal glands) at the expense of organs such as the gut and periphery; this adaptation is termed ‘brain‐sparing’ (Giussani, 2016). Brain sparing is induced by detection of hypoxaemia within the fetal circulation by chemoreceptors which stimulate an increase in peripheral vasoconstriction (Giussani et al. 1993), concurrent with vasodilatation in the vital organs to maintain adequate oxygen delivery. This process is mediated by the release of adenosine, nitric oxide and prostanoids (Giussani, 2016). However, while brain‐sparing and subsequent growth restriction ensure fetal survival, it does not ensure normal postnatal development, with growth‐restricted newborns being at an increased risk of death as well as cardiovascular and neurological deficits compared to AG infants (Thornton et al. 2004; Walker et al. 2011; Miller et al. 2016; Malhotra et al. 2019).
Placental insufficiency commonly occurs due to abnormally high placental vascular resistance, and thus placental vasodilatation is a key target for therapeutic intervention to increase placental blood flow. Sildenafil citrate (SC) inhibits phosphodiesterase (PDE) 5, causing vasodilatation and increased blood flow (McCullough, 2002). The placenta is rich in PDE5 and there has been considerable interest in targeting this pathway to improve blood flow in pregnancies complicated by placental insufficiency (Dastjerdi et al. 2012; Panda et al. 2014; Perez & Laughon, 2015). Indeed, promising results from animal preclinical and case‐control clinical studies have underpinned the initiation of the multinational, multicentre randomized placebo‐controlled Sildenafil TheRapy in Dismal prognosis Early‐onset fetal growth Restriction (STRIDER) trial (Dilworth et al. 2013; Panda et al. 2014). This set of four trials within the STRIDER Consortium examined the effect of maternal SC (25 mg oral tablets, three times daily) on fetal growth and gestational length (Ganzevoort et al. 2014) in pregnancies complicated by severe placental insufficiency and FGR. Results from the STRIDER trial have been mixed. The UK and New Zealand/Australian arms of the trial demonstrated no positive benefit of antenatal SC on birth weight and gestational length, but also no harm (Sharp et al. 2017; Groom et al. 2019). In contrast, the Netherlands consortium of the STRIDER trial was prematurely halted due to an interim analysis which found evidence of potential harm. In this cohort, SC administration increased rates of persistent pulmonary hypertension and neonatal death (non‐significant) in newborns (Pels et al. 2019). Subsequently, the STRIDER Consortium recommended the cessation of SC treatment for women with placental insufficiency and growth‐restricted fetuses (Groom et al. 2018). The cause of neonatal demise following antenatal SC has not yet been characterized.
SC crosses both the human and the sheep placenta (Russo et al. 2018; Inocencio et al. 2019) and PDE5 receptors are found in the developing fetus (Luong et al. 2011). We have previously shown that SC administration to fetal vessels ex vivo can alter vascular tone (Polglase et al. 2016; Inocencio et al. 2019), and therefore antenatal SC treatment may have direct effects on fetal blood vessels (Francis & Corbin, 2005). We propose that it is important to consider the haemodynamic effects of SC in the developing fetus given its strong vasodilator actions. Accordingly, in this study, we investigated the effects of maternal SC administration on maternal and fetal cardiovascular physiology in growth‐restricted fetal sheep. We hypothesized that antenatal SC administration would induce peripheral vasodilatation in the developing FGR fetus, altering the cardiovascular adaption to chronic hypoxaemia.
Materials and methods
Experiments were approved by the Monash Medical Centre Animal Ethics Committee A (MMCA2016/01) under guidelines established by the National Health and Medical Research Council of Australia code of practice for the care and use of animals for scientific purposes (8th Edition, 2013).
Animals
Singleton bearing Border‐Leicester pregnant ewes were sourced from Monash Animal Research Platform (n = 20). Each ewe underwent sterile surgery on day 105 of pregnancy (term 148–150 days of gestation, d GA) to induce FGR via single umbilical artery ligation (SUAL), as previously described (Alves de Alencar Rocha et al. 2017 b). Briefly, sedation of ewes was initiated via i.v. injection of sodium thiopentone (20ml Pentothal i.v.; Boehringer Ingelheim Australia, North Ryde, NSW, Australia). Ewes were then intubated and anaesthesia was maintained via gaseous isoflurane. (1.5–2.5% in 10/30% O2/N2O; Bomac Animal Health, Hornsby, NSW, Australia). Ewes were randomly allocated for surgery or sham surgery where the umbilical cord of control fetuses was exposed and handled but not ligated (control, AG). SUAL results in placental atrophy, and subsequent disruption of placental function with fetal chronic hypoxaemia, and brain‐sparing (Alves de Alencar Rocha et al. 2017 b).
All fetuses were instrumented with a right femoral artery catheter, threaded 7.5 cm into the artery to enable blood gas sampling and pressure recording from within the descending aorta. Additionally, a jugular vein catheter was also implanted to enable administration of antibiotics [1.52 mm outer diameter (OD), 0.86 mm inner diameter (ID)]. Flow probes (Size 3, Transonic Systems, Ithaca, NY, USA) were placed around the left femoral and right carotid arteries for measurement of arterial blood flow. An amniotic catheter (2.70 mm OD, 1.50 mm ID) was also secured to the hindquarters of the fetus for access to the amniotic cavity for antibiotic administration and to correct fetal pressure recordings. The fetus was returned to the uterus and catheters and flow probes were exteriorized through the right flank of the ewe.
Catheters were inserted into the maternal jugular vein for administration of antibiotics and SC treatment, and the maternal carotid artery for blood sampling and pressure recording (2.70 mm OD, 1.50 mm ID). Fetal, maternal and amniotic catheters were filled with heparinized saline (0.9% NaCl; 25,0000 IU heparin/L).
Experimental procedures
Ewes were placed into mobile cages and, following recovery, were fed twice daily with lucerne chaff and water was available ad libitum. Antibiotics were administered to the ewe (Engemycin 5ml i.v.; Coopers, Macquarie, NSW, Australia) via the maternal jugular vein catheter and the fetus (ampicillin 1 ml: 1 g/5 mL heparinised saline; Austrapenics; CSL, Victoria, Australia) via the jugular vein and an amniotic catheter (ampicillin 4 ml: 1 g/5 ml heparinised saline). Additionally, oral paracetamol (1 g Panadol, GSK, Australia) was administered to the ewe for pain relief 3 days after surgery.
Recording of maternal physiology and fetal in utero physiology
On day 4 after surgery, prior to initiation of SC treatment, fetal and maternal mean arterial pressure (DTX Plus Transducer; Becton Dickinson, Singapore) as well as fetal femoral and carotid blood flows (Transonic Systems) were monitored and recorded (AD Instruments, Sydney, Australia) for 2 h. Fetal and maternal heart rate were derived from the arterial signal. Physiological recordings were conducted from 09.00 to 12.00 h from days 4 to 9 after surgery and thereafter on alternate days until the fetus was 125 d GA. Ewes had constant access to food and water during the recording period.
Maternal sildenafil/saline administration
On day 4 after surgery, ewes with an SUAL fetus were randomized to receive either SC: (36 mg/day) or saline infusion via a pump (CADD‐Legacy 1 Pump; Smiths Medical, Australia) connected to the maternal jugular vein catheter and fastened to the back of the ewe under netting. Thereafter, the groups of interest were: SUAL with SC treatment (SUALSC, n = 7), SUAL with saline (SUAL, n = 7) and appropriately grown with saline (AG, n = 6). SC and saline administration occurred continuously for 20 ± 1 days until post‐mortem (125 d GA). This treatment regime was chosen to reflect the dose of SC in the STRIDER trial (Ganzevoort et al. 2014) where the oral dose has a 50% bioavailability (Jaillard et al. 2006).
Fetal blood samples
Fetal arterial blood samples (∼200 µl) were collected at the beginning of each recording day for the assessment of fetal pH, haematocrit (Hct), oxygen saturation (), the partial pressure of arterial oxygen (), the partial pressure of arterial carbon dioxide (), lactate and glucose. Whole blood was used for analysis and temperature‐corrected (39°C) to account for the ewe's body temperature (ABL800 FLEX, Radiometer, Copenhagen, Denmark).
Post‐mortem
At 125 d GA, ewes and fetuses from all groups were killed via pentobarbital sodium overdose to the ewe (100 mg/kg i.v. Valabarb; Jurox, Rutherford, Australia). Fetuses were exteriorized for measurement of brain, lung and fetal body weight and biparietal diameter, abdominal circumference, and crown rump and lower limb length.
Data analysis
Data are presented as mean ± SD unless otherwise stated.
Mean carotid blood flow was corrected for brain weight and femoral blood flow was corrected for body weight over gestation. Estimates of gestational weights were calculated via the equation:
determined from brain and body weights collected at 80 and 95 d GA (historical data, not shown) and at post‐mortem.
Femoral oxygen delivery was calculated via the following equation: blood flow × mean daily femoral arterial oxygen content. Normality of data was assessed and normalized as required via GraphPad Prism (Prism 7, GraphPad Software, La Jolla, CA, USA). Data analysis was performed using SigmaStat (Systat Software Inc., San Jose, CA, USA). Mean data were analysed in two epochs; weeks 1 (Week 1) and 2 (Week 2) after SC administration. When no difference was seen between treatment weeks, data are presented as total time following SC administration. Fetal weight, biometry and mean physiological parameters were analysed using a one‐way ANOVA. Blood gas parameters and mean daily physiological data over the duration of the experiment were analysed using a two‐way repeated measures ANOVA, and Tukey's post hoc test was used to compare between groups. Significance was accepted at P ≤ 0.05.
Results
Fetal characteristics
Table 1 summarizes the fetal characteristics at post‐mortem. There was no difference in the numbers of males and females per group. At 125 d GA, body weight in the SUAL group was 11% lighter than in AG fetuses (not significant). Treatment with SC following SUAL resulted in a 32% reduction in birthweight compared to the AG group (P = 0.003) and by 23% compared to SUAL (P = 0.01). Brain/body weight ratio was not different between the SUAL and AG groups, but was increased in SUALSC compared to both AG (P = 0.007) and SUAL (P = 0.02). Lung/body weight ratio was not different between groups. Crown–rump length was decreased in SUALSC compared to AG (P = 0.04) but was not different to SUAL. No significant differences were seen in any other measure of fetal biometry (biparietal diameter, crown–rump length, abdominal circumference, lower limb length).
Table 1.
Characteristics of fetal sheep at post‐mortem delivery
| AG | FGR | FGRSC | |
|---|---|---|---|
| Male/total births | 5/6 | 3/7 | 4/7 |
| Birth weight (kg) | 3.4 ± 0.3 | 3.0 ± 0.3 | 2.3 ± 0.5*# |
| Brain/body weight (g/kg) | 14.2 ± 0.8 | 16.0 ± 1.2 | 19.7 ± 3.2*# |
| Lung/body weight(g/kg) | 31.8 ± 4.1 | 32.1 ± 5.1 | 38.1 ± 4.9 |
| Liver/body weight (g/kg) | 35.7 ± 3.1 | 29.1 ± 1.2 | 27.2 ± 2 |
| Biparietal diameter (cm) | 11.1 ± 1.3 | 10.1 ± 0.3 | 10.3 ± 0.9 |
| Crown–rump length (cm) | 43.6 ± 2.9 | 41.1 ± 2.3 | 37.5 ± 4.3* |
| Abdominal circumference (cm) | 33.3 ± 1.9 | 32.5 ± 1.1 | 28.8 ± 4.8 |
| Lower limb length (cm) | 17.4 ± 3.1 | 14.8 ± 3.5 | 13.1 ± 2.9 |
Weights and biometry data are given as are mean ± SD of appropriately grown lambs (AG), fetal growth restricted lambs treated with saline (FGR) and fetal growth restricted lambs treated with sildenafil (FGRSC). *Significant difference to AG; # significant difference to FGR (P = 0.05). Data were analysed using a one‐way ANOVA.
Fetal pH, partial pressure of carbon dioxide and arterial saturation
Fetal pH, partial pressure of CO2 and arterial saturation over the ∼2.5‐week duration of the experiment are shown in Fig. 1. pH (Fig. 1A and B ) was not different between groups throughout the experimental period. Mean over the 2‐week treatment period was greater in SUAL compared to AG and SUALSC (Fig. 1D ; P = 0.001 and 0.01 respectively). Twenty‐four hours after SUAL (106 d GA), was lower in SUAL and SUALSC fetuses (Fig. 1E ; P = 0.004 and P = 0.01) compared to AG fetuses. SUAL and SUALSC fetuses remained hypoxic with lower arterial saturations compared to AG at 112 d GA (Fig. 1E ; P = 0.006 and P = 0.02 respectively) and 117 d GA (Fig. 1E ; P = 0.007 and P = 0.04 respectively). Mean over the 2‐week treatment period was lower in SUAL and SUALSC fetuses compared to AG fetuses (Fig. 1F ; P = 0.001 and 0.01 respectively).
Figure 1. Fetal pH, partial pressure of CO2, arterial saturation and haemoglobin in response to growth restriction and sildenafil treatment.
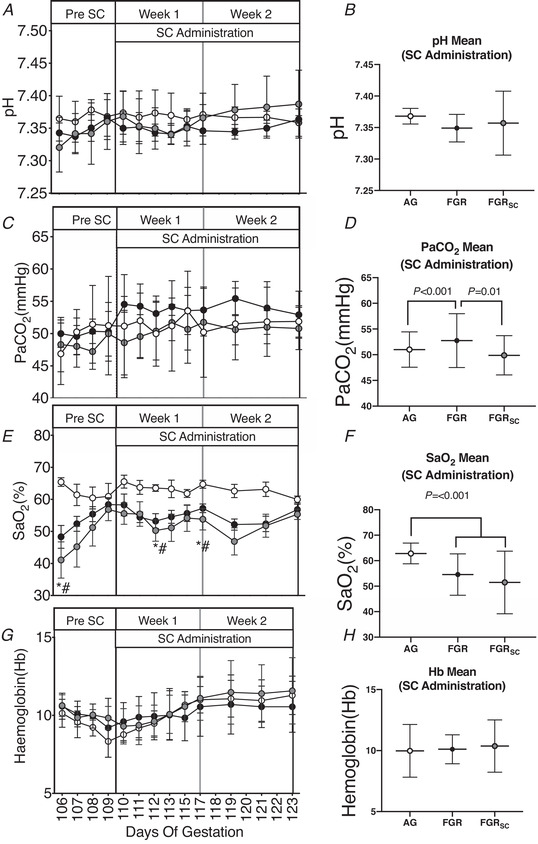
Data are shown as daily mean ± SD. A, C, E and H, pH (A), partial pressure of carbon dioxide (, C), arterial oxygen saturation (, E) and haemoglobin (Hb, G). Total mean values for the duration of the treatment period of all parameters are also shown (right: B, D, F, H). Groups are appropriately grown (AG, white, n = 6), saline‐treated fetal growth‐restricted (FGR, black, n = 7) and sildenafil‐treated fetal growth‐restricted (FGRSC, grey, n = 7) fetuses. Duration of treatment is indicated by the SC administration bar. *Significant difference between FGR and AG; #significant difference between FGRSC and AG. Daily physiological data collected over the duration of the experiment were analysed using a two‐way repeated measures ANOVA. Weekly mean physiological parameters were analysed using a one‐way ANOVA.
Fetal arterial partial pressure of oxygen
The fetal arterial partial pressure of O2 () over the ∼2.5 weeks of the experiment is shown in Fig. 2. Twenty‐four hours after SUAL (106 d GA), was decreased in SUAL and SUALSC fetuses (Fig. 2A : P = 0.05 and 0.02) compared to AG fetuses. recovered to control values in both SUAL groups by the time SC treatment began. At 123 d GA, was lower in SUALSC (Fig. 2A , P = 0.005) compared to AG and SUAL fetuses. Mean after 1 week of saline/SC treatment was lower in both SUAL and SUALSC compared to AG (Fig. 2B ; P = 0.001) and no difference in was seen between SUAL and SUALSC fetuses. After 2 weeks of saline/SC treatment, remained lower in SUAL and SUALSC compared to AG (Fig. 2B ; P = 0. 01), however at this time was lower in SUALSC compared to SUAL (Fig. 2B ; P = 0. 04).
Figure 2. Fetal partial pressure of oxygen in response to growth restriction and sildenafil treatment.
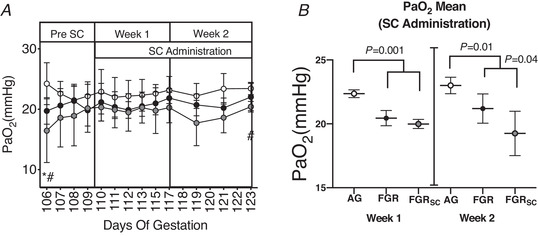
A, individual daily mean ± SD partial pressure of oxygen taken at individual time points (). B, total mean during the first and second week of SC treatment. Groups are appropriately grown (AG, white, n = 6), saline‐treated fetal growth restricted (FGR, black, n = 7) and sildenafil‐treated fetal growth restricted (FGRSC, grey, n = 7) fetuses. Duration of treatment is indicated by the SC administration bar. *Significant difference between FGR and AG; #significant difference between FGRSC and AG. Daily physiological data collected over the duration of the experiment were analysed using a two‐way repeated measures ANOVA. Weekly mean physiological parameters were analysed using a one‐way ANOVA.
Fetal glucose and lactate
SUAL and SUALSC fetuses were hypoglycaemic compared to AG fetuses at 107 and 108 d GA (Fig. 3A ; P < 0.05). SUALSC fetuses were hypoglycaemic at 112 and 121 d GA (Fig. 3A ; P < 0.05) compared to AG, but not SUAL fetuses. Mean fetal glucose over the 2‐week treatment period was not different between SUAL and SUALSC fetuses, but both were lower than AG fetuses (Fig. 3B ; P < 0.0001). Lactate was higher in SUALSC, compared to AG fetuses, at 119 d GA (Fig. 3C ; P = 0.02) and no differences were seen between SUAL and AG at individual timepoints. Mean lactate over the 2‐week treatment period was higher in SUALSC compared to AG fetuses during the SC treatment period (Fig. 3D , P = 0.007) and no difference was seen between AG and SUAL.
Figure 3. Fetal glucose and lactate in response to growth restriction and sildenafil treatment.
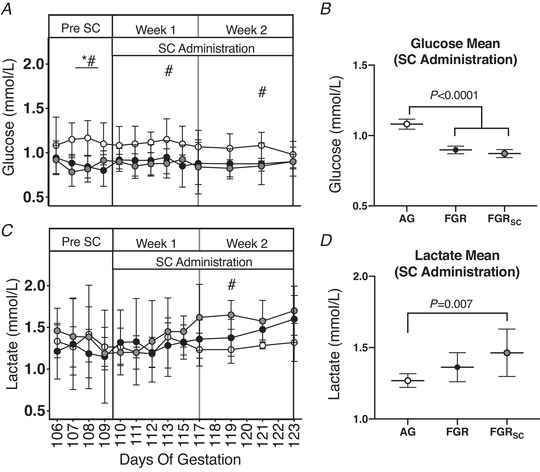
Individual daily mean ± SD glucose (A) and lactate (C). Total mean values for the duration of the treatment period of all parameters are also shown (B and D). Groups are appropriately grown (AG, white, n = 6), saline‐treated fetal growth restricted (FGR, black, n = 7) and sildenafil‐treated fetal growth restricted (FGRSC, grey, n = 7) fetuses. Duration of treatment is indicated by the SC administration bar. *Significant difference between FGR and AG; #significant difference between FGRSC and AG. Daily physiological data collected over the duration of the experiment were analysed using a two‐way repeated measures ANOVA. Weekly mean physiological parameters were analysed using a one‐way ANOVA.
Maternal blood pressure and heart rate response to sildenafil treatment
In all groups, maternal blood pressure decreased throughout the experiment (P = 0.03; Fig. 4A ). Mean maternal blood pressure over the 2‐week treatment period was reduced in ewes carrying SUAL compared to AG fetuses (Fig. 4B , P = 0.03) but no difference was seen compared to SUALSC. Mean maternal diastolic blood pressure over the 2‐week treatment period was reduced in SUAL and SUALSC compared to AG (Fig. 4F , P = 0.002 and P = 0.04 respectively); no difference was seen between SUAL and SUALSC. Maternal systolic blood pressure (Fig. 4E and F ) and maternal heart rate were not different between groups at any time during the study (Fig. 4G and H ).
Figure 4. The maternal cardiovascular response to chronic antenatal sc administration.
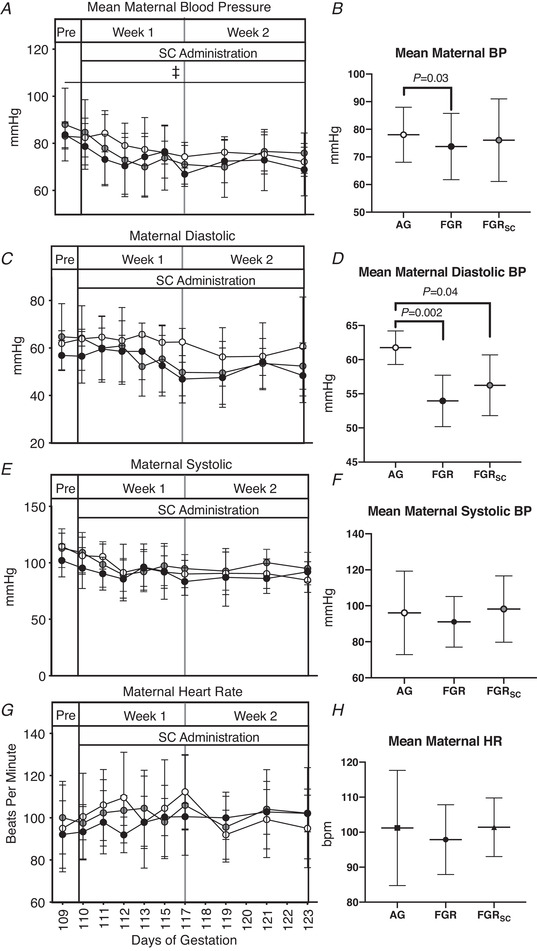
Individual daily mean ± SD maternal blood pressure (BP, A), diastolic blood pressure (C), systolic blood pressure (E) and heat rate (G). Total mean values for the duration of the treatment period of all parameters are also shown (B, D, F, H). Groups are appropriately grown (AG, white, n = 6), saline‐treated fetal growth restricted (FGR, black, n = 7) and sildenafil‐treated fetal growth restricted (FGRSC, grey, n = 7) fetuses. Duration of treatment is indicated by the SC administration bar. ‡Significant difference over time. Daily physiological data collected over the duration of the experiment were analysed using a two‐way repeated measures ANOVA. Weekly mean physiological parameters were analysed using a one‐way ANOVA.
Fetal blood pressure and heart rate response to sildenafil treatment
Mean fetal arterial (Fig. 5B ) and systolic (Fig. 5D ) blood pressure over the 2‐week treatment period were greater in SUAL fetuses compared to AG and SUALSC (Fig. 5B and D , P < 0.05) but no difference was seen between SUALSC and AG. Mean fetal diastolic blood pressure (Fig. 5F ) over the 2‐week treatment period was not different between groups. Mean fetal heart rate was lower in SUALSC fetuses compared to AG and SUAL fetuses (Fig. 5H , P < 0.05) during SC treatment.
Figure 5. The fetal cardiovascular response to chronic antenatal SC administration.
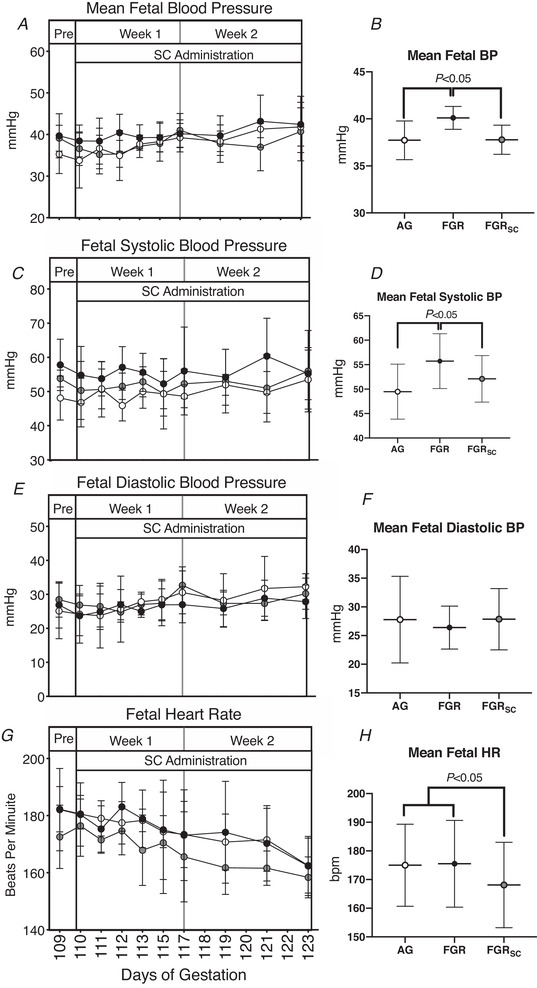
Daily mean ± SD blood pressure (BP) (A), diastolic pressure (C), systolic pressure (E) and heat rate (G). Total means throughout the treatment period of all parameters are also shown (B, D, F, H). Groups are of appropriately grown (AG, white, n = 6), saline‐treated fetal growth restricted (FGR, black, n = 7) and sildenafil‐treated fetal growth restricted (FGRSC, grey, n = 7) fetuses. Duration of treatment is indicated by the SC administration bar. Daily physiological data collected over the duration of the experiment were analysed using a two‐way repeated measures ANOVA. Weekly mean physiological parameters were analysed using a one‐way ANOVA.
Femoral blood flow and oxygen delivery
Mean fetal femoral blood flow over the 2‐week treatment period was lower in SUAL fetuses compared to AG and SUALSC fetuses (Fig. 6D , P = 0.002 and P = <0.001 respectively) but no difference was seen between AG and SUALSC fetuses. Mean fetal femoral oxygen delivery over the 2‐week treatment period was lower in SUAL compared to AG fetuses (Fig. 6F ; P = 0.008) but no difference was seen between AG and SUALSC fetuses.
Figure 6. Fetal carotid and femoral blood flow and oxygen delivery in response to growth restriction and sildenafil treatment.
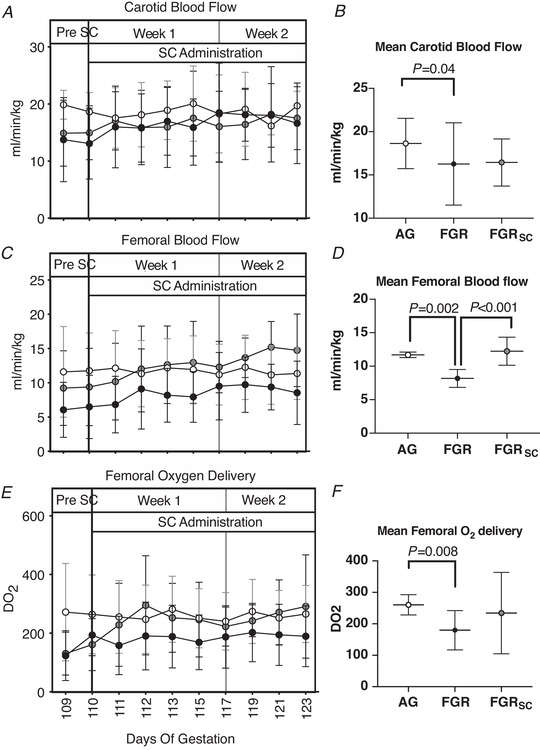
Daily mean ± SD (A, C, E,) carotid blood flow (A), femoral blood flow (C) and femoral oxygen delivery (E). Total weekly means of the treatment period of all parameters are also shown (B, D, F). Groups are appropriately grown (AG, white, n = 6), saline‐treated fetal growth restricted (FGR, black, n = 7) and sildenafil‐treated fetal growth restricted (FGRSC, grey, n = 7) fetuses. Duration of treatment is indicated by the SC administration bar. *Significant difference between FGR and AG. Data are daily physiological data collected over the duration of the experiment were analysed using a two‐way repeated measures ANOVA. Weekly mean physiological parameters were analysed using a one‐way ANOVA.
Discussion
Hypoxaemia and FGR are most commonly caused by impaired placental function resulting in a hypoxic fetal environment. Currently there is no cure or treatment options available for women with suspected FGR. Potential treatments to improve placental function, such as SC (Ganzevoort et al. 2014), are an important area of investigation. The present study aimed to investigate the effect of maternal SC administration on fetal and maternal cardiovascular physiology in ovine pregnancies complicated by placental insufficiency, induced by SUAL. SUAL resulted in reduced femoral blood flow and oxygen delivery consistent with brain‐sparing. Our key finding was that antenatal treatment with SC altered fetal cardiovascular redistribution, coupled with increased fetal distress and hypoxaemia. SC exposure resulted in widespread fetal vasodilatation, which counteracts the normal fetal cardiovascular adaptation (brain‐sparing response) in the setting of chronic hypoxaemia.
Impact of SC on fetal oxygenation and growth
An important finding was the effect of SC on fetal oxygenation and growth. SUAL induces FGR via placental insufficiency and thereby creating a chronic fetal hypoxic environment (Alves de Alencar Rocha et al. 2017 b). A reduction in birthweight was observed in both SUAL groups within this study. Zhang et al. (2015) have discussed the potential for the placental vascular bed to mount a vasodilative compensatory response to placental insufficiency in an attempt to modulate the degree of FGR, and Kitanaka et al. (1989) have shown that uterine artery blood flow increases gradually in pregnant sheep exposed to chronic hypoxia. These studies suggest in adverse pregnancy, the placental vasculature has some vasodilatory ability to increase blood flow and oxygen carrying capacity for the fetus. Accordingly, in the current study we observe a return to normoxia within 4 days of SUAL in saline‐treated SUAL fetuses.
In contrast, SC‐treated SUAL fetuses became progressively more hypoxic, which probably resulted in the reduced growth observed. The increased hypoxaemia in SUALSC fetuses suggests impairment, or failure, to potential placental adaptation to hypoxaemia as described above. We have previously discussed the potential SC to decrease maternal systemic resistance, resulting in a subsequent ‘steal’ of blood from the uteroplacental circulation as it flows to a vascular unit with lower resistance (Miller et al. 2009; Inocencio et al. 2019). It is reasonable to assume the increased fetal hypoxaemia observed in our current study may have also been driven by similar alterations in maternal and/or placental blood flow by SC. Given we also observe effects of SC on the fetal circulation, we suggest that this too may contribute to the increased fetal distress observed.
The normalization of peripheral blood flow in SUAL lambs observed in our current study may be an alternative mechanism behind the exacerbation of fetal hypoxaemia observed. We measured femoral blood flow as an indication of peripheral circulation in our study. As SC crosses the placenta and enters the fetal circulation (Inocencio et al. 2019), the vasodilatory effects of SC are probably global within the fetus. Thereby, in the face of fetal hypoxaemia, global vasodilatation by SC may enable perfusion of all organs, rather than preferential distribution to the brain. This would result in increased oxygen consumption by less important organs at the expense of critical organs such as the heart and brain. We suggest that this may have contributed to the increased hypoxaemia and the subsequent further impairment of growth in SUAL fetuses exposed to SC.
Lactate is a marker of fetal distress and tissue hypoxia (Kastendieck et al. 1988; Borruto et al. 2006). The increased lactate in SUALSC compared to SUAL and AG fetuses we observed is likely to reflect increased fetal stress caused by increased hypoxaemia and nutrient deprivation resulting in anaerobic glycolysis in SUALSC fetuses (Yates et al. 2012). Lactate is the main substrate for brain development (Medina & Tabernero, 2005), and Mann et al. (1971) have shown an increased lactate uptake by the brain following lactate infusion into the fetal circulation. If lactate can act as an energy source for the brain, the increased lactate seen in the growth‐restricted fetuses exposed to SC may have contributed to the increased fetal brain weight relative to the SUAL and AG lambs. As increased brain sparing is associated with worsening placental function (Giussani et al. 2012; Cohen et al. 2016) and adverse fetal outcomes (Cheema et al. 2006), we do not believe that the increased brain weight observed is associated with improved brain development. However, analysis of the brain, which is beyond the scope of this study, will be useful to determine the effects of SC on the developing brain.
Sildenafil citrate impairs cardiovascular adaptations to hypoxaemia
In SUAL fetuses, mean arterial blood pressure increased in response to SUAL, driven by vasoconstriction of the periphery, evidenced by a widening pulse pressure (increased systolic bloob pressure) also shown in our study. It is important to note that several studies using different ovine models of FGR have demonstrates no difference between blood pressure of FGR and control fetuses (Danielson et al. 2005; Bubb et al. 2007; Dyer et al. 2009; Miller et al. 2009) Conversely, studies involving similar models of FGR have demonstrated an increase in fetal blood pressure(Murotsuki et al. 1997; Galan et al. 2005). A potential reason behind these contrasting findings is the severity of hypoxaemia induced in the different models of FGR. Our results suggest that SUAL results in hypoxaemia that is sufficient to drive an increase in fetal blood pressure, and observe effects of SC to blood pressure regulation.
The fetal cardiovascaular response to chronic hypoxaemia is mediated by fetal endocrine and metabolic adaptations and are aimed at maintaining maximal organ perfusion (Morrison, 2008; Giussani et al. 2012; Newby et al. 2015; Giussani, 2016). Peripheral vasoconstriction results in a redistribution of blood flow away from the periphery, as evidenced by a significant reduction in femoral blood flow and oxygen delivery (calculated from oxygen concentration and arterial blood flow) in SUAL lambs, whereas cerebral oxygen delivery was maintained. While brain/body ratio was 12% greater in SUAL lambs compared to control, this difference was not statistically significant. The SUAL procedures surgically induce placental insufficiency and exposes the fetus to a pathological placenta (Emmanouilides et al. 1969). This is evident in our study, with fetal oxygenation decreasing 26% relative to AG fetuses following the SUAL procedure. Our observations suggest that even in the absence of significant brain‐sparing, chronic hypoxaemia in the fetus induces key adaptive mechanisms to facilitate the redistribution of cardiac output from the periphery to the cerebral circulation (Cosmi et al. 2011). Additionally, in another sheep model of FGR, via placental‐restriction, Poudel et al. (2015) have shown a similar physiological response in FGR fetuses whereby blood flow to the brain was similar and femoral blood flow was decreased. Therefore, while SUAL fetuses were not significantly smaller in our current study, the physiological response to placental artery ligation is consistent with previous literature.
However, SC administration normalized mean and systolic fetal blood pressure, and in concert with restoration of femoral blood flow in SUAL fetuses, demonstrates interference by SC to this well‐described cardiovascular adaptation to hypoxaemia. Furthermore, peripheral oxygen delivery was also normalized after SC. SC induces vasodilatation via inhibition of enzyme PDE5, normally responsible for the degradation of cGMP which enables smooth‐muscle vasoconstriction. We have previously shown that femoral arteries of fetal sheep vasodilate in response to SC ex vivo, which demonstrates the presence of PDE5 within this vascular bed (Polglase et al. 2016; Itani et al. 2017). We suggest that SC counteracts the vasoconstrictive adaptation in SUAL fetuses by inducing peripheral vasodilatation, resulting in the normalization of perfusion (BP, resistance) and restoration of femoral oxygen delivery and blood flow. Ruijtenbeek et al. (2000) have shown chronic hypoxaemia increased peripheral sympathetic innervation, which they describe as a key mechanism involved in the maintenance of cardiac output redistribution observed in FGR. It is possible that peripheral vasodilatation and normalization of peripheral oxygen delivery by SC may impair this adaptive peripheral hyperinnervation, resulting in the altered fetal cardiovascular redistribution observed in our study. The consequences of cardiovascular adaptation to placental insufficiency are complex. While redistribution of fetal cardiac output, and subsequent brain‐sparing, is a well‐accepted adaptation to preserve perfusion of key organs and maintain fetal life (Cohen et al. 2015), an increased degree of brain‐sparing is also strongly associated with poorer neonatal outcome (Malhotra et al. 2019). We are unable to determine if the normalization of femoral blood flow observed in our study is detrimental or beneficial to postnatal life. However, in the context of SC, we suggest that benefit is unlikely given the negative effects observed in this study, including further decreases in fetal weight, hypoxaemia and fetal stress evident by higher lactate levels.
Interestingly, despite the potential for widespread vasodilatation, SC treatment did not significantly increase carotid blood flow. As adaptation to hypoxaemia aims to maximally perfuse key organs such as the brain, it is possible that the cerebral circulation is maximally dilated to facilitate maximal oxygen delivery to the brain. The cerebral vasculature may therefore have limited ability to further vasodilate in response to a vasodilator such as SC.
Effects of SC on maternal heart rate and blood pressure
Maternal arterial and diastolic blood pressure decreased following SUAL. Whilst fetal growth restriction is not traditionally associated with decreased blood pressure, Steer et al. (2004) found an association between maternal hypotension and infants that were small for gestational age in their population consisting of >500,000 birth records. As we did not observe a difference between SUAL‐ and SUALSC‐bearing ewes, we suggest that decreased maternal blood pressure in SUAL‐bearing ewes reflects a maternal vascular response to the induction of SUAL.
Limitations
In this study we aimed to mimic the human dose of SC administration used in the recent STRIDER trials. To provide a controlled administration of SC to the pregnant ewe, SC was given as a continuous i.v. infusion to the ewe. In consideration of the different dosing routes, we decreased the dosage of SC to match the bioactivity of the drug when taken orally (i.e. 50% bioactive via oral route). We acknowledge that the pharmacokinetic profile of SC is likely to be different between our study and the women in STRIDER, in which oral administration would result in cyclic (Wang & Craik, 2016) levels of SC, as opposed to our constant SC levels.
Statistically, saline‐treated SUAL fetuses were not growth restricted compared to AG fetuses. However, SUAL fetuses did have a reduction in weight of ∼400g, which represents a reduction of 11.1% in body weight. This is similar to previously reported FGR weights by our group (Malhotra et al. 2018). This difference in body weight probably represents a decrease in growth velocity, which is associated with an increased risk of postnatal complications and stillbirth (MacDonald et al. 2017). Interestingly, SUALSC fetuses had a reduction of 32% in body weight, which highlights the exacerbation of SUAL by maternal SC treatment.
Fetal oxygenation was measured via samples collected within the descending aorta. While blood within the descending aorta eventually supplies the peripheral vasculature, the point of collection is before blood flow through the abdominal organs and the periphery. Therefore, sampling from this vessel limits the ability to account for specific oxygenation differences between the femoral and cerebral vascular beds. Unfortunately, we were limited in our ability to instrument a central vascular bed as we collected the brains for a separate study. It would have been very informative to have obtained an additional blood sample from the carotid artery to allow the determination of carotid oxygen delivery to fully elucidate regional changes in fetal oxygenation during SC administration.
The rationale for the clinical use of SC was undertaken with the expectation that SC would improve placental blood flow and thereby fetal growth (Pels et al. 2017). Although it is possible that the effects of SC on the placenta underlie the decrease in growth and increase in hypoxaemia observed in SUALSC fetuses, we are unable to speculate further on these as they were not investigated in the current study. We have previously shown that SC can acutely impair uterine flow (Miller et al. 2009) and have now shown chronic SC modulates fetal vasodilatation. Future studies could investigate the potential for chronic SC treatment to alter placental and uterine blood flow.
It is also important to note that there are key differences between our model and the human pathology underlying FGR. Furthermore, there are key differences between the aetiology of clinical placental insufficiency and our SUAL model. In contrast to abnormal placental vasodilatation causing a decrease in placental perfusion, SUAL induces placental atrophy to mimic placental insufficiency (Emmanouilides et al. 1969). In our study, indices of hypoxaemia improved over gestation in both SUAL groups, suggesting a potential adaptive response to SUAL. While we are still able to observe detrimental effects of SC, true exacerbation of hypoxaemia may be masked by any adaptation to SUAL. Additional information regarding the mechanisms by which SC impacts fetal growth restriction could be gained by investigating the effects of SC to fetal oxygenation in models where hypoxia and hypoxaemia can be carefully manipulated, such as chronic maternal hypoxia (Allison et al. 2016) and/or umbilicoplacental embolization (Duncan et al. 2000). Furthermore, an increase in umbilical flow may be limited in the SUAL model. Notwithstanding this, the ability of SC to improve placental blood flow is contentious, with some studies demonstrating benefit (Satterfield et al. 2010; Dastjerdi et al. 2012) and others demonstrating impairment (Miller et al. 2009; Inocencio et al. 2019) of placental function.
Conclusions
We demonstrated that chronic administration of SC to SUAL fetal sheep altered the cardiovascular adaptation to chronic hypoxaemia. These adaptations aim to assist with in utero survival, and inhibition of this adaptive mechanism by SC resulted in increased fetal hypoxaemia and exacerbation of growth restriction. This is the first study to demonstrate the decentralization of blood flow in the developing, hypoxic fetus by sildenafil citrate. Whilst the developmental consequences of altered adaptation to FGR are unknown, the increased hypoxaemia, distress and reduced fetal growth suggest outcomes are likely to be worsened.
Importantly, our study highlights the potential of therapies administered to pregnant women to have significant implications upon the developing fetus, and the ability of these treatments to interfere with key survival mechanisms, such as the conservation of oxygen delivery to critical organs during chronic hypoxaemia. Our findings suggest the use of SC in the clinical treatment for placental insufficiency should be approached with caution.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
I.M.I. drafted the manuscript, and was involved in the acquisition, analysis and interpretation of data for the study. B.J.A. was involved in the conception and design of the study, the acquisition and interpretation of the data and review of the manuscript. I.N. was involved in acquisition of the data and review of the manuscript and G.R.P. and S.L.M. were involved in the conception and design of the work, analysis of the data and review of the manuscript.
Funding
NHMRC (1083520).
Supporting information
Statistical Summary Document
Acknowledgements
The authors gratefully acknowledge the technical assistance of Dalibor Stanojkovic, Amy Sutherland and Jamie Mihelakis.
Biography
Ishmael Miguel Inocencio’s research vision is to investigate and develop novel mechanisms for the management and treatment of preterm babies. His academic training and research experience to date has involved the use of large animal models of preterm lung injury and fetal growth restriction, providing an excellent background in fetal development and neonatal management. He has investigated novel therapies for these perinatal conditions, and determined the pulmonary, cardiovascular and cerebral effects of these new therapies. He hopes to translate his extensive basic science experience to the clinical setting. He has a passion for new and cutting‐edge technology and aspires to use recent technological advancements to drive innovative research to develop new and improved methods for perinatal management to improve neonatal care and long‐term outcomes.

Edited by: Kim Barrett & Janna Morrison
Linked articles: This article is highlighted in a Perspectives article by Darby. To read this article, visit https://doi.org/10.1113/JP280444.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Allison BJ, Brain KL, Niu Y, Kane AD, Herrera EA, Thakor AS, Botting KJ, Cross CM, Itani N, Skeffington KL, Beck C & Giussani DA (2016). Fetal in vivo continuous cardiovascular function during chronic hypoxia. J Physiol 594, 1247–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves de Alencar Rocha AK, Allison BJ, Yawno T, Polglase GR, Sutherland AE, Malhotra A, Jenkin G, Castillo‐Melendez M & Miller SL (2017). Early‐ versus late‐onset fetal growth restriction differentially affects the development of the fetal sheep brain. Dev Neurosci 39, 141–155. [DOI] [PubMed] [Google Scholar]
- Borruto F, Comparetto C, Wegher E & Treisser A (2006). Screening of foetal distress by assessment of umbilical cord lactate. Clin Exp Obstet Gynecol 33, 219–222. [PubMed] [Google Scholar]
- Bubb KJ, Cock ML, Black MJ, Dodic M, Boon WM, Parkington HC, Harding R & Tare M (2007). Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J Physiol 578, 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema R, Dubiel M & Gudmundsson S (2006). Fetal brain sparing is strongly related to the degree of increased placental vascular impedance. J Perinat Med 34, 318–322. [DOI] [PubMed] [Google Scholar]
- Cohen E, Baerts W & Van Bel F (2015). Brain‐sparing in intrauterine growth restriction: considerations for the neonatologist. Neonatology 108, 269–276. [DOI] [PubMed] [Google Scholar]
- Cohen E, Wong FY, Horne RSC & Yiallourou SR (2016). Intrauterine growth restriction: Impact on cardiovascular development and function throughout infancy. Pediatr Res 79, 821–830. [DOI] [PubMed] [Google Scholar]
- Cosmi E, Fanelli T, Visentin S, Trevisanuto D & Zanardo V (2011). Consequences in infants that were intrauterine growth restricted. J Pregnancy 2011, 364–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson L, McMillen IC, Dyer JL & Morrison JL (2005). Restriction of placental growth results in greater hypotensive response to α‐adrenergic blockade in fetal sheep during late gestation. J Physiol 563, 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastjerdi MV, Hosseini S & Bayani L (2012). Sildenafil citrate and uteroplacental perfusion in fetal growth restriction. J Res Med Sci 17, 632–636. [PMC free article] [PubMed] [Google Scholar]
- Dilworth MR, Andersson I, Renshall LJ, Cowley E, Baker P, Greenwood S, Sibley CP & Wareing M (2013). Sildenafil citrate increases fetal weight in a mouse model of fetal growth restriction with a normal vascular phenotype. PLoS One 8, e77748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Cock ML, Harding R & Rees SM (2000). Relation between damage to the placenta and the fetal brain after late‐gestation placental embolization and fetal growth restriction in sheep. Am J Obstet Gynecol 183, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Dyer JL, McMillen IC, Warnes KE & Morrison JL (2009). No Evidence for an Enhanced Role of Endothelial Nitric Oxide in the Maintenance of Arterial Blood Pressure in the IUGR Sheep Fetus. Placenta 30, 705–710. [DOI] [PubMed] [Google Scholar]
- Emmanouilides GC, Townsend DE & Bauer RA (1969). Effects of single umbilical artery ligation in the lamb fetus. Obstet Gynecol Surv 24, 1068–1070. [PubMed] [Google Scholar]
- Figueras F & Gratacós E (2014). Update on the diagnosis and classification of fetal growth restriction and proposal of a stage‐based management protocol. Fetal Diagn Ther 36, 86–98. [DOI] [PubMed] [Google Scholar]
- Francis SH & Corbin JD (2005). Sildenafil: efficacy, safety, tolerability and mechanism of action in treating erectile dysfunction. Expert Opin Drug Metab Toxicol 1, 283–293. [DOI] [PubMed] [Google Scholar]
- Galan HL, Anthony R V., Rigano S, Parker TA, De Vrijer B, Ferrazzi E, Wilkening RB & Regnault TRH (2005). Fetal hypertension and abnormal Doppler velocimetry in an ovine model of intrauterine growth restriction. Am J Obstet Gynecol 192, 272–279. [DOI] [PubMed] [Google Scholar]
- Ganzevoort W, Alfirevic Z, von Dadelszen P, Kenny L, Papageorghiou A, van Wassenaer‐Leemhuis A, Gluud C, Mol BW & Baker PN (2014). STRIDER: Sildenafil Therapy In Dismal prognosis Early‐onset intrauterine growth Restriction–a protocol for a systematic review with individual participant data and aggregate data meta‐analysis and trial sequential analysis. Syst Rev 3, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA (2016). The fetal brain sparing response to hypoxia: Physiological mechanisms. J Physiol 594, 1215–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FBP, Cross CM & Herrera EA (2012). Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PLoS One 7, e31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ, Bennet L & Hanson MA (1993). Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol 461, 431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom KM, Ganzevoort W, Alfirevic Z, Lim K, Papageorghiou AT & STRIDER Consortium (2018). Clinicians should stop prescribing sildenafil for fetal growth restriction (FGR): comment from the STRIDER Consortium. Ultrasound Obstet Gynecol 52, 295–296. [DOI] [PubMed] [Google Scholar]
- Groom KM, McCowan LM, Mackay LK, Lee AC, Gardener G, Unterscheider J, Sekar R, Dickinson JE, Muller P, Reid RA, Watson D, Welsh A, Marlow J, Walker SP, Hyett J, Morris J, Stone PR & Baker PN (2019). STRIDER NZAus: a multicentre randomised controlled trial of sildenafil therapy in early‐onset fetal growth restriction. BJOG An Int J Obstet Gynaecol 126, 997–1006. [DOI] [PubMed] [Google Scholar]
- Inocencio IM, Polglase GR, Miller SL, Sehgal A, Sutherland A, Mihelakis J, Li A & Allison BJ (2019). Effects of maternal sildenafil treatment on vascular function in growth‐restricted fetal sheep. Arterioscler Thromb Vasc Biol 39, 27–31. [DOI] [PubMed] [Google Scholar]
- Itani N, Skeffington KL, Beck C & Giussani DA (2017). Sildenafil therapy for fetal cardiovascular dysfunction during hypoxic development: studies in the chick embryo. J Physiol 595, 1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillard S, Larrue B, Deruelle P, Delelis A, Rakza T, Butrous G & Storme L (2006). Effects of phosphodiesterase 5 inhibitor on pulmonary vascular reactivity in the fetal lamb. Ann Thorac Surg 81, 935–942. [DOI] [PubMed] [Google Scholar]
- Kastendieck E, Paulick R & Martius J (1988). Lactate in fetal tissue during hypoxia; correlation to lactate, pH and base deficit in the fetal blood. Eur J Obstet Gynecol Reprod Biol 29, 61–71. [DOI] [PubMed] [Google Scholar]
- Kitanaka T, Gilbert RD & Longo LD (1989). Maternal responses to long‐term hypoxemia in sheep. Am J Physiol Regul Integr Comp Physiol 256, R1340–R1347. [DOI] [PubMed] [Google Scholar]
- Luong C, Rey‐Perra J, Vadivel A, Gilmour G, Sauve Y, Koonen D, Walker D, Todd KG, Gressens P, Kassiri Z, Nadeem K, Morgan B, Eaton F, Dyck JR, Archer SL & Thébaud B (2011). Antenatal sildenafil treatment attenuates pulmonary hypertension in experimental congenital diaphragmatic hernia. Circulation 123, 2120–2131. [DOI] [PubMed] [Google Scholar]
- MacDonald TM, Hui L, Tong S, Robinson AJ, Dane KM, Middleton AL & Walker SP (2017). Reduced growth velocity across the third trimester is associated with placental insufficiency in fetuses born at a normal birthweight: A prospective cohort study. BMC Med 15, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacHado Nardozza LM, Junior EA, Barbosa MM, Rabachini Caetano AC, Re Lee DJ & Moron AF (2012). Fetal growth restriction: current knowledge to the general Obs/Gyn. Arch Gynecol Obstet 286, 1–13. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Allison BJ, Castillo‐Melendez M, Jenkin G, Polglase GR & Miller SL (2019). Neonatal morbidities of fetal growth restriction: pathophysiology and impact. Front Endocrinol (Lausanne) 10, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Castillo‐Melendez M, Allison BJ, Sutherland AE, Nitsos I, Pham Y, Alves de Alencar Rocha AK, Fahey MC, Polglase GR, Jenkin G & Miller SL (2018). Neuropathology as a consequence of neonatal ventilation in premature growth‐restricted lambs. Am J Physiol Integr Comp Physiol 315, R1183–R1194. [DOI] [PubMed] [Google Scholar]
- Mann LI, Solomon G, Carmichael A & Duchin S (1971). The effect of metabolic acidosis on fetal brain function and metabolism. Am J Obstet Gynecol 111, 353–359. [DOI] [PubMed] [Google Scholar]
- McCullough AR (2002). Four‐year review of sildenafil citrate. Rev Urol 3, S26–S38. [PMC free article] [PubMed] [Google Scholar]
- Medina JM & Tabernero A (2005). Lactate utilization by brain cells and its role in CNS development. J Neurosci Res 79, 2–10. [DOI] [PubMed] [Google Scholar]
- Miller SL, Huppi PS & Mallard C (2016). The consequences of fetal growth restriction on brain structure and neurodevelopmental outcome. J Physiol 594, 807–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Loose JM, Jenkin G & Wallace EM (2009). The effects of sildenafil citrate (Viagra) on uterine blood flow and well being in the intrauterine growth‐restricted fetus. Am J Obstet Gynecol 200, 102.e1–102.e7. [DOI] [PubMed] [Google Scholar]
- Morrison JL (2008). Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 35, 730–743. [DOI] [PubMed] [Google Scholar]
- Murotsuki J, Bocking AD & Gagnon R (1997). Fetal heart rate patterns in growth–restricted fetal sheep induced by chronic fetal placental embolization. Am J Obstet Gynecol 176, 282–290. [DOI] [PubMed] [Google Scholar]
- Nardozza LMM, Caetano ACR, Zamarian ACP, Mazzola JB, Silva CP, Marçal VMG, Lobo TF, Peixoto AB & Araujo Júnior E (2017). Fetal growth restriction: current knowledge. Arch Gynecol Obstet 295, 1061–1077. [DOI] [PubMed] [Google Scholar]
- Newby EA, Myers DA & Ducsay CA (2015). Fetal endocrine and metabolic adaptations to hypoxia: The role of the hypothalamic‐pituitary‐adrenal axis. Am J Physiol Endocrinol Metab 309, E429–E439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Das A & Md Nowroz H (2014). Sildenafil citrate in fetal growth restriction. J Reprod Infertil 15, 168–169. [PMC free article] [PubMed] [Google Scholar]
- Pels Anouk, Derks Jan, Elvan‐Taspinar Ayten, Van Drongelen Joris, De Boer Marjon, Duvekot Hans, Van Laar Judith, Van Eyck Jim, Al‐Nasiry Salwan, Sueters Marieke, Morssink Leonard, Onland Wes, Wassenaer‐Leemhuis Aleid Van, Naaktgeboren Christiana, Ganzevoort Wessel (2019) LB 2: Maternal sildenafil for severe early‐onset fetal growth restriction: the Dutch multicentre placebo‐controlled double‐blind STRIDER‐trial. American Journal of Obstetrics and Gynecology, 220, S682–S683. [Google Scholar]
- Pels A, Kenny LC, Alfirevic Z, Baker PN, von Dadelszen P, Gluud C, Kariya CT, Mol BW, Papageorghiou A, van Wassenaer‐Leemhuis AG, Ganzevoort W, Groom KM & the international STRIDER Consortium (2017). STRIDER (Sildenafil TheRapy in dismal prognosis early onset fetal growth restriction): an international consortium of randomised placebo‐controlled trials. BMC Pregnancy Childbirth 17, 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez KM & Laughon M (2015). Sildenafil in term and premature infants: a systematic review. Clin Ther 37, 2598–2607. [DOI] [PubMed] [Google Scholar]
- Polglase GR, Allison BJ, Coia E, Li A, Jenkin G, Malhotra A, Sehgal A, Kluckow M, Gill AW, Hooper SB & Miller SL (2016). Altered cardiovascular function at birth in growth‐restricted preterm lambs. Pediatr Res 80, 538–546. [DOI] [PubMed] [Google Scholar]
- Poudel R, McMillen IC, Dunn SL, Zhang S & Morrison JL (2015). Impact of chronic hypoxemia on blood flow to the brain, heart, and adrenal gland in the late‐gestation IUGR sheep fetus. Am J Physiol Regul Integr Comp Physiol 308, R151–R162. [DOI] [PubMed] [Google Scholar]
- Ruijtenbeek K, Le Noble FAC, Janssen GMJ, Kessels CGA, Fazzi GE, Blanco CE & De Mey JGR (2000). Chronic hypoxia stimulates periarterial sympathetic nerve development in chicken embryo. Circulation 102, 2892–2897. [DOI] [PubMed] [Google Scholar]
- Russo FM, Conings S, Allegaert K, Van Mieghem T, Toelen J, Van Calsteren K, Annaert P & Deprest J (2018). Sildenafil crosses the placenta at therapeutic levels in a dually perfused human cotyledon model. Am J Obstet Gynecol 219, 619.e1–619.e10. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Bazer FW, Spencer TE & Wu G (2010). Sildenafil citrate treatment enhances amino acid availability in the conceptus and fetal growth in an ovine model of intrauterine growth restriction. J Nutr 140, 251–258. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Doctor T & Menahem S (2013). Cardiac function and arterial biophysical properties in small for gestational age infants: postnatal manifestations of fetal programming. J Pediatr 163, 1296–1300. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Skilton MR & Crispi F (2016). Human fetal growth restriction: a cardiovascular journey through to adolescence. J Dev Orig Health Dis 7, 1–10. [DOI] [PubMed] [Google Scholar]
- Sharp A, Cornforth C, Jackson R, Harrold J, Turner MA, Kenny LC, Baker PN, Johnstone ED, Khalil A, Von Dadelszon P, Papageorghiou AT & Alfirevic Z (2017). Maternal sildenafil for severe fetal growth restriction (STRIDER): a multicentre, randomised, placebo‐controlled, double‐blind trial. Lancet Child Adolesc Heal 5, 17–20. [DOI] [PubMed] [Google Scholar]
- Steer PJ, Little MP, Kold‐Jensen T, Chapple J & Elliott P (2004). Maternal blood pressure in pregnancy, birth weight, and perinatal mortality in first births: prospective study. Br Med J 329, 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M & GRIT study group (2004). Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet 364, 513–520. [DOI] [PubMed] [Google Scholar]
- Walker D‐M, Marlow N, Upstone L, Gross H, Hornbuckle J, Vail A, Wolke D & Thornton JG (2011). The Growth Restriction Intervention Trial: long‐term outcomes in a randomized trial of timing of delivery in fetal growth restriction. Am J Obstet Gynecol 204, 34.e1–34.e9. [DOI] [PubMed] [Google Scholar]
- Wang CK & Craik DJ (2016). Cyclic peptide oral bioavailability: lessons from the past. Biopolymers 106, 901–909. [DOI] [PubMed] [Google Scholar]
- Yates DT, MacKo AR, Nearing M, Chen X, Rhoads RP & Limesand SW (2012). Developmental programming in response to intrauterine growth restriction impairs myoblast function and skeletal muscle metabolism. J Pregnancy 2012, 631038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Regnault TRH, Barker PL, Botting KJ, McMillen IC, McMillan CM, Roberts CT & Morrison JL (2015). Placental adaptations in growth restriction. Nutrients 7, 360–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Summary Document
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


