Abstract
Objective
Long‐term information on lifestyle changes among prostate survivors is lacking. In this nationwide, population‐based study we investigated the prevalence of lifestyle changes, factors associated with lifestyle changes and associations between lifestyle changes and general quality of life.
Methods
All men registered in the National Prostate Cancer Register of Sweden diagnosed in 2008 with low‐risk prostate cancer at age 70 years or younger were sent a questionnaire. Logistic regression was used to calculate odds ratios (ORs) with 95% confidence intervals for factors potentially associated with lifestyle change.
Results
Out of 1288, 1720 men (75%) were responded. A total of 279 (22%) reported a positive lifestyle change regarding diet or exercise. Poor functional outcomes after treatment was associated with exercising less (OR 1.6, 95% CI 1.2‐2.1) and less interest in social activities and relationships (OR 1.8, 95% CI 1.5‐2.1). Men who exercised more (OR 7.9, 95% CI 4.4‐14) and men who had an increased interest in relationships and social activities (OR 5.2, 95% CI 2.1‐13) reported higher general quality of life.
Conclusions
A considerable proportion of men reported making positive lifestyle changes after the prostate cancer diagnosis. The time after diagnosis may be a teachable moment that facilitates lifestyle interventions. Poor functional outcomes after treatment may reduce the willingness to engage in positive lifestyle change, which need be considered when supporting men after treatment. Men who made a positive lifestyle change, regardless of whether it was exercise or regarding relationships and social activities more often reported a high level of general quality of life.
Keywords: cancer, lifestyle, oncology, prostate cancer, psycho‐oncology, quality of life, survivorship
1. BACKGROUND
Lifestyle changes after a cancer diagnosis, such as adopting a healthier diet or exercising more, may improve quality of life, as well as reduce the risks of both cancer recurrence and noncancer mortality. 1 , 2 , 3 , 4 , 5 Further, adequate social support may have positive effects on the psychological wellbeing of patients. 6 , 7 Evidence suggest that the emotional impact of a cancer diagnosis can be a teachable moment. 5 Considering the long life expectancy of most men with localized prostate cancer, much can be gained by utilizing the possible teachable moments of a prostate cancer diagnosis to make persisting lifestyle changes.
Results from previous studies indicate that the number of men who change their lifestyle after diagnosis is relatively low. 8 , 9 , 10 However, population‐based information about long‐term lifestyle changes after a prostate cancer diagnosis is lacking, and information about potential barriers to lifestyle changes, such as poor functional outcomes after treatment, lack of social support and trouble with personal finances are scarce. 7 , 8 , 9 , 11 , 12
As there is increasing evidence for that lifestyle changes improve several aspects of life after a cancer diagnosis, both physical and psychological, this nationwide population‐based study was conducted to explore self‐reported long‐term lifestyle changes, and to assess factors potentially associated with lifestyle changes and general quality of life among prostate cancer survivors. We aimed to identify influenceable barriers to lifestyle change and to find a possible association between lifestyle changes and increased quality of life.
2. METHODS
2.1. Study design and participants
All men registered in the National Prostate Cancer Register of Sweden (NPCR) as being diagnosed in 2008 with low‐risk prostate cancer at age 70 years or younger, who had a radical prostatectomy, radiotherapy, or active surveillance as primary treatment, and were still alive in 2015 were included. The capture rate of the NPCR is >96% compared with the national cancer registry, to which physicians are required to report all cancer cases according to Swedish legislation. 13 We selected men diagnosed in 2008 to assess long‐term sustainability of any lifestyle changes.
Low‐risk disease was defined as Gleason score 6, prostate‐specific antigen (PSA) < 10 ng/mL, and clinical stage T1‐2, Nx/N0, Mx/M0.
Participants were invited by a letter, in which we presented the study, its purpose, that it was completely voluntary and that no identifying information would be collected. A study‐specific questionnaire was sent to the participants, with an addressed and stamped envelope included. An individual code to fill out the questionnaire online was also included as an option. Between February and October 2015, 1720 men were invited. A research assistant contacted all men who failed to return the questionnaire via telephone and sent a second questionnaire to those who agreed.
Men who returned the questionnaire was regarded as having provided informed consent. The Regional Ethical Review Board at Uppsala University approved of the study (approval number 2014/278).
2.2. Questionnaire design
Our questionnaire has been previously described. 14 It consisted of 49 study‐specific questions and the Expanded Prostate Cancer Index Composite 26 (EPIC‐26). 15 The study‐specific questions explored mental symptoms, general quality of life, lifestyle changes, overall satisfaction with care at the time of diagnosis and at follow‐up, socio‐demographics, smoking habits, alcohol consumption, physical activity, medical treatments, concurrent diseases (Charlson Comorbidity Index), 16 and if they suffered from depression and/or any other mental disorder (Data S1).
Potential lifestyle changes were assessed by the question: “Has your prostate cancer diagnosis influenced your lifestyle in any way, and if so, in what areas?” Men had the possibility to grade the following areas: “Type of food” as “I eat less healthy,” “Unchanged” or “I eat healthier”; “Exercise” as “I exercise less,” “Unchanged” or “I exercise more”; “Interest in religion/philosophy” and “Interest in social activities/relationships” as “Less,” “Unchanged” or “More.” The question “How has your prostate cancer affected your economic situation?” could be answered as “Impaired,” “Unchanged” or “Improved.”
General quality of life was assessed on a seven‐point visual digital scale by the question “The past four weeks, how was your quality of life?” with answers graded from 1 “No quality of life,” to 7 “Best possible quality of life.” One and two was assessed as low intensity, three to five as moderate, and six and seven as high intensity.
EPIC‐26 is a validated instrument designed to assess health‐related quality of life (HRQoL) specific to functional outcomes after prostate cancer treatment. A score is calculated from several questions specific to five domains of which we use four (urinary incontinence, urinary irritative/obstructive, sexual and bowel function). The score ranges from 0 to 100, with 100 representing the best possible pelvic organ function. An EPIC‐26 score of less than 80 was considered having poor HRQoL in that specific domain (referred to as “poor functional outcomes after treatment” in the abstract, introduction and discussion). 17
2.3. Data collection, analysis and statistical analysis
We assembled the questionnaire responses and cancer characteristics data from the NPCR in a database. Differences between responders and nonresponders were analyzed. Lifestyle changes were divided into four domains; food, exercise, social activities/relationships and religion/philosophy according to our main outcome question. We defined “I eat healthier,” “I exercise more” and an increased interest in social activities/relationships and religion/philosophy as being positive lifestyle changes although this is subjective. Possible factors associated with lifestyle changes and potential confounders were defined using directed acyclic graphs. 18 , 19 We defined factors potentially associated with lifestyle changes as personal finances, treatment, and side effects from treatment. Potential confounders were defined as age, education, marital status and Charlson Comorbidity Index (CCI). Logistic regression and ordinal logistic regression were used for multivariable analysis. We analyzed each domain adjusting for age, education, marital status and CCI. Odds ratios with 95% confidence interval were calculated for the probability of making lifestyle changes, based on the factors described above.
Missing data for covariables were handled using multiple imputations based on the method of chained equations. 20 Five imputation data sets were created. Up to 4 % of the covariables were imputed. No outcomes were imputed.
3. RESULTS
3.1. Patient characteristics
Seventy‐five percent (n = 1288) of the 1720 invited men responded. Mean age at diagnosis was 63 years (range 40‐70). Among responders, 393 (30.5%) had a university education, 1068 (82.9%) were married or had a domestic partner, 1003 (77.9%) were retired and 210 (16.3%) had a CCI of more than two. Curative treatment was reported by 1075 (83.5%) men and high general quality of life by 684 (53.1%) men. A negative impact of the prostate cancer diagnosis on personal finances was reported by 55 men (4.3%) (Table 1).
TABLE 1.
Demographics, potential factors associated with lifestyle changes and quality of life divided by whether the men made a positive lifestyle change or not
| Levels | All | Positive lifestyle change | No lifestyle change | Missing | |
|---|---|---|---|---|---|
| n(%) | 1288 (100.0) | 303 (23.5) | 820 (63.7) | 165 (12.8) | |
| Age, n (range) | 63 (59‐66) | 62 (59‐65) | 63 (59–66) | 64 (60‐67) | |
| Marital status n(%) | Married or domestic partner | 1068 (82.9) | 257 (84.8) | 681 (83.0) | 130 (78.8) |
| Other | 199 (15.5) | 42 (13.9) | 128 (15.6) | 29 (17.6) | |
| Missing | 21 (1.6) | 4 (1.3) | 11 (1.3) | 6 (3.6) | |
| Occupation, n(%) | Not retired | 285 (22.1) | 76 (25.1) | 167 (20.4) | 42 (25.5) |
| Retired | 1003 (77.9) | 227 (74.9) | 653 (79.6) | 123 (74.5) | |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Education level, n(%) | Compulsory school | 351 (27.3) | 63 (20.8) | 235 (28.7) | 53 (32.1) |
| Upper secondary school | 513 (39.8) | 129 (42.6) | 324 (39.5) | 60 (36.4) | |
| University | 393 (30.5) | 106 (35.0) | 246 (30.0) | 41 (24.8) | |
| Missing | 31 (2.4) | 5 (1.7) | 15 (1.8) | 11 (6.7) | |
| Charlson comorbidity index, n(%) | 0 | 411 (31.9) | 75 (24.8) | 276 (33.7) | 60 (36.4) |
| 1 | 438 (34.0) | 121 (39.9) | 279 (34.0) | 38 (23.0) | |
| 2 | 229 (17.8) | 52 (17.2) | 146 (17.8) | 31 (18.8) | |
| >2 | 210 (16.3) | 55 (18.2) | 119 (14.5) | 36 (21.8) | |
| Urinary incontinence, EPIC‐26 score | Median (IQR) | 94 (65‐100) | 97 (61‐100) | 94 (67‐100) | 81 (52‐100) |
| Missing, n(%) | 194 (15.1) | 33 (10.9) | 90 (11.0) | 71 (43.0) | |
| Irritative and obstructive, EPIC‐26 score | Median (IQR) | 88 (75‐100) | 88 (75–100) | 88 (75–100) | 88 (75‐100) |
| Missing, n(%) | 265 (20.6) | 51 (16.8) | 142 (17.3) | 72 (43.6) | |
| Bowel, EPIC‐26 score | Median (IQR) | 100 (88‐100) | 96 (83‐100) | 100 (88–100) | 100 (96‐100) |
| Missing, n(%) | 319 (24.8) | 68 (22.4) | 169 (20.6) | 82 (49.7) | |
| Sexual, EPIC‐26 score | Median (IQR) | 32 (8‐62) | 36 (12‐62) | 30 (8–62) | 32 (7‐57) |
| Missing, n(%) | 300 (23.3) | 58 (19.1) | 172 (21.0) | 70 (42.4) | |
| Financial situation, n(%) | Impaired | 55 (4.3) | 19 (6.3) | 29 (3.5) | 7 (4.2) |
| Unchanged | 1161 (90.1) | 281 (92.7) | 780 (95.1) | 100 (60.6) | |
| Improved | 1 (0.1) | 1 (0.3) | 0 (0.0) | 0 (0.0) | |
| Missing | 71 (5.5) | 2 (0.7) | 11 (1.3) | 58 (35.2) | |
| Treatment, n(%) | Active surveillance | 213 (16.5) | 57 (18.8) | 126 (15.4) | 30 (18.2) |
| Radical prostatectomy | 830 (64.4) | 182 (60.1) | 543 (66.2) | 105 (63.6) | |
| Radiotherapy | 245 (19.0) | 64 (21.1) | 151 (18.4) | 30 (18.2) | |
| Quality of life, n(%) | Low (1–2) | 21 (1.6) | 7 (2.3) | 13 (1.6) | 1 (0.6) |
| Intermediate (3–5) | 533 (41.4) | 120 (39.6) | 335 (40.9) | 78 (47.3) | |
| High (6–7) | 684 (53.1) | 166 (54.8) | 444 (54.1) | 74 (44.8) | |
| Missing | 50 (3.9) | 10 (3.3) | 28 (3.4) | 12 (7.3) |
Abbreviations: EPIC‐26, The Expanded Prostate Cancer Index Composite 26 item short form version; IQR, interquartile range.
Men who responded were on average 1 year older, had a higher T‐stage and higher PSA at diagnosis. They were more likely to have received immediate curative treatment compared to nonresponders (data from the NPCR).
3.2. Lifestyle changes
In all, 303 (24%) stated that they had made some kind of positive lifestyle change; 279 (22%) that they had improved their lifestyle in terms of diet and/or exercise, 184 (14%) reported that they had a healthier diet, and 189 (15%) that they exercised more; 33 (2.6%) reported more interest in religion and philosophy and 26 (2.0%) more interest in social activities and relationships. Negative lifestyle changes were reported by 159 men (12%); 2 (0.2%) reported that they had a less healthy diet, 47 (3.6%) that they exercised less, 23 (1.8%) that they were less interested in religion and philosophy, and 118 (9.2%) that they were less interested in social activities and relationships (Table 2).
TABLE 2.
Lifestyle changes in the domains of food, exercise, social activities/relationships and religion/philosophy
| All | ||
|---|---|---|
| n (%) | 1288 (100.0) | |
| Food, n(%) | I eat less healthy | 2 (0.2) |
| Unchanged | 958 (74.4) | |
| I eat healthier | 184 (14.3) | |
| Missing | 144 (11.2) | |
| Exercise, n(%) | I exercise less | 47 (3.6) |
| Unchanged | 917 (71.2) | |
| I exercise more | 189 (14.7) | |
| Missing | 135 (10.5) | |
| Religion/philosophy, n(%) | Less interested | 23 (1.8) |
| Unchanged | 1091 (84.7) | |
| More interested | 33 (2.6) | |
| Missing | 141 (10.9) | |
| Social activities/relationships, n(%) | Less interested | 118 (9.2) |
| Unchanged | 1022 (79.3) | |
| More interested | 26 (2.0) | |
| Missing | 122 (9.5) |
3.3. Factors associated with lifestyle changes
Poor HRQoL specific to functional outcomes after treatment, regardless of whether it was in the form of urinary incontinence (OR 3.5, 95% CI 1.0‐12), urinary irritative/obstructive symptoms (OR 2.5, 95% CI 1.6‐3.8), sexual (OR 2.2, 95% CI 1.4‐3.3) or bowel related (OR 2.0, 95% CI 1.4‐3.0), were associated with less interest in relationships/social activities (Figure 1).
FIGURE 1.
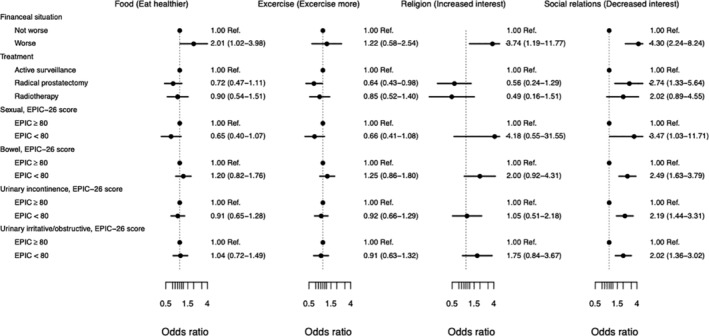
Forest plot illustrating factors potentially associated with lifestyle changes. Odds ratio shows the probability of making a lifestyle change. An EPIC‐26 score < 80 indicate a poor functional outcome after treatment. Adjusted for age, work status, education and Charlson Comorbidity Index. EPIC‐26, The Expanded Prostate Cancer Index Composite 26 item short form version; Ref, reference
Poor HRQoL from two or more domains specific to functional outcomes after treatment was associated with exercising less (OR 1.6, 95% CI 1.2‐2.1) and being less interested in social activities/relationships (OR 1.8, 95% CI 1.5‐2.1) compared to men with changes in one or no domains (data not shown).
Men treated with radical prostatectomy exercised less (OR 0.64, 95% CI 0.43‐0.98) and where more likely to decrease their interest in relationships and social activities (OR 2.74, 95% CI 1.33‐5.64) (Figure 1).
The number of men who changed religious or philosophical views was too small to allow analysis of factors associated with these changes (Table 2).
We found no notable changes in our results when we adjusted for marital status and socioeconomic status (data not shown).
3.4. Lifestyle change and general quality of life
Men who exercised more (OR 7.9, 95% CI 4.4‐14) and men who reported an increased interest in relationships and social activities (OR 5.2, 95% CI 2.1‐13) were more likely to report higher general quality of life.
The relationship between lifestyle changes and general quality of life is shown as a box plot (Figure 2).
FIGURE 2.
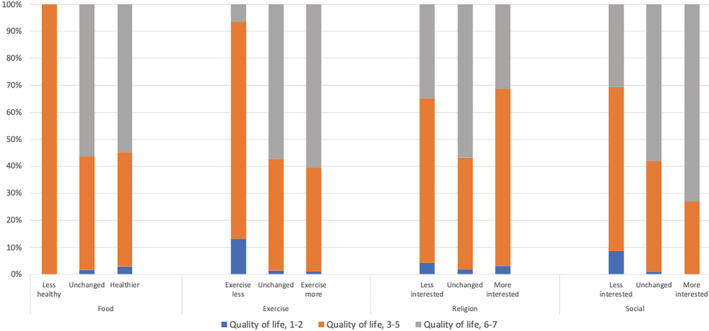
Bar chart illustrating percentage of lifestyle changes among men who rated their quality of life as low (1‐2), moderate (3‐5) or high (6‐7)
4. DISCUSSION
In this population‐based study of men with localized prostate cancer, a considerable proportion, about one fifth, reported lasting, positive, lifestyle changes without the aid of any structured intervention. This indicates that a prostate cancer diagnosis in itself might be a teachable moment, which could enhance the effects of lifestyle interventions. Men who experienced poor functional outcomes after treatment exercised less and were less interested in relationships and social activities which emphasizes the importance of active rehabilitation and support for prostate cancer survivors. Additionally, men who reported making a lasting, positive lifestyle change (more exercise or an increased interested in relationships and social activities) reported a higher general quality of life.
We consider the number of men who reported changing their lifestyle to be of clinical importance. This finding likely mirrors the impact that the diagnosis in itself has as a teachable moment since the study is population‐based and not the result of a structured intervention. Our results are consistent with the results of a recently published study by Hughes et al 8 on diet and exercise among long‐term prostate cancer survivors. We both found that about one fifth of the men reported changing their diet, physical exercise or both. However, they conclude that few men change their habits. Although a modest number of men, we consider the finding to be clinically relevant, particularly as these men changed their lifestyle in the absence of a structured intervention, and that interventions aimed at changing the lifestyle are generally effective. 21 To give perspective, our results can be compared with a large population‐based study of lifestyle changes within 2 years after a type 2 diabetes diagnosis among 888 Australians with a median age of 62 years 22 : With the exception of smoking cessation, no notable lifestyle changes were reported, suggesting that a cancer diagnosis affects lifestyle more than a diagnosis of type 2 diabetes.
A poor functional outcome after treatment for prostate cancer can affect everyday life and erectile dysfunction, urinary leakage and bowel problems are relatively common. 23 Symptoms such as urinary leakage could make exercise or engaging in social activities more difficult, thus working as a barrier toward lifestyle changes. In our population, men with poor functional outcomes after treatment exercised less and were less interested in relationships and social activities. Additionally, men treated with radical prostatectomy also reported exercising less and being less interested in relationships and social activities, likely due to poor functional outcomes in this group. These findings are supported by a recent study by Stone et al 11 showing that men with urinary incontinence after prostate cancer treatment were less likely to start with physical activity. It emphasizes the importance of pelvic floor training before and after a radical prostatectomy to reduce the risk of urinary incontinence. 24 , 25 , 26 Although the possibility of future lifestyle change is not the primary concern when choosing treatment, it is still an important factor to take into consideration and yet another reason for choosing active surveillance when possible.
Previous research indicate that social support may have positive effects on the psychological wellbeing of patients and that men with lower socioeconomic status might suffer more from a prostate cancer diagnosis. 6 , 7 , 27 In our population neither social support in terms of marital status nor socioeconomic status affected whether the men made and sustained a lifestyle change. This might be related to that the vast majority of the men in our study were married and already retired from work.
Hospitalization and absence from work, as well as reduced work capacity after treatment may affect personal finances negatively. It can be speculated that a negative financial impact induces the need to manage costs, for example by managing food expenses and cutting back on social activities. However, in our cohort of men, many of whom were already retired and treated in a mainly public health‐care system, the number of men who reported any impact of their prostate cancer diagnosis on personal finances was small. Among the few men (4%) who did report an impact on personal finances, almost all reported a negative impact and only one man reported an improvement. The number of men who reported a financial impact was too small to allow for any conclusions about the relationship between personal finances and lifestyle changes (Table 1). A British qualitative study on patients with breast, lung or prostate cancer by Timmons et al 12 found that some patients had to make adjustments to cope financially after they received their diagnosis, predominately those who had an employment at the time of diagnosis, lacked social support, and/or had a low income or few savings.
Lifestyle changes may, in addition to reducing cancer recurrence and mortality, improve the general quality of life which further highlights the importance of endorsing lifestyle changes. In our population, men who had made a positive lifestyle change, regardless of whether it was exercise or relationships/social activities more often reported a high level of general quality of life. In our study, the questions about quality of life concerned the past 4 weeks and the question on lifestyle changes the past 7 years, so it is possible that the improvement in general quality of life was a consequence of the lifestyle changes. This finding is supported by Farris et al 28 showing that recreational physical activity in prostate cancer survivors was associated with a higher quality of life. In 2015, Kassianos et al published a systematic review on quality of life and dietary changes among cancer patients. They found evidence linking dietary changes to improved quality of life, however, the results among the reviewed articles were mixed and other important factors influencing quality of life likely plays a significant role. 29 In their study, cancers associated with favorable prognosis, such as prostate cancer, seemed to benefit more from lifestyle changes in terms of improved quality of life.
5. CONCLUSIONS
5.1. Study limitations
The main strengths of this study include the population‐based design, the high response rate for a study of its kind and the study‐specific questionnaire including the direct question on lifestyle change. This was a cross‐sectional study and may therefore be less reliable than a study with a longitudinal survey design would have been. As the questionnaire was distributed 7 years after diagnosis, the men's recollection of their experiences might have changed. Our study is also limited to Swedish men, these findings might not be generalizable to other cultural and health‐care settings. All men in this study had a low‐risk disease. Men diagnosed with low‐risk prostate cancer are generally healthy and might therefor already have a healthy lifestyle, thus a lower incentive to change their lifestyle which might affect our results.
5.2. Clinical implications
A considerable proportion of men make and sustain positive lifestyle changes after a prostate cancer diagnosis. It is notable that most of them do so without any structured intervention. The prostate cancer diagnosis may induce a particularly teachable moment that enhances the effects of specific lifestyle interventions. Considering the increasing evidence for that lifestyle changes improves quality of life, reduce the risk of cancer recurrence, and prolong survival, more efforts should be made to stimulate a healthier lifestyle among men with a recent prostate cancer diagnosis. Our results suggest that poor functional outcomes after treatment reduce the willingness to engage in positive lifestyle change, which need be considered when supporting men after treatment.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Oskar Bergengren had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Oskar Bergengren, Hans Garmo, Ola Bratt, Eva Johansson and Anna Bill‐Axelson contributed to study concept and design. Oskar Bergengren, Eva Johansson and Anna Bill‐Axelson acquired the data and drafted the manuscript. Oskar Bergengren, Anna Pia Enblad, Hans Garmo, Lars Holmberg, Eva Johansson and Anna Bill‐Axelson analyzed and interpreted the data. Oskar Bergengren, Anna Pia Enblad, Ola Bratt, Lars Holmberg, Eva Johansson and Anna Bill‐Axelson critically revised the manuscript for important intellectual content. Hans Garmo contibuted to the statistical analysis. Anna Bill‐Axelson obtained funding and contributed to administrative, technical or material support. Lars Holmberg, Eva Johansson and Anna Bill‐Axelson supervised the manuscript.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGEMENTS
We wish to thank Ellen Kragsterman for her excellent help with data collection during this study. We also wish to thank all of the men who answered the questionnaire. This research was funded by grants from the Swedish Cancer Society. The funder had no role in the design and conduct of the study; collection, management, analysis and interpretation of data; writing of the manuscript; or the decision to submit the manuscript for publication.
Bergengren O, Enblad AP, Garmo H, et al. Changes in lifestyle among prostate cancer survivors: A nationwide population‐based study. Psycho‐Oncology. 2020;29:1713–1719. 10.1002/pon.5513
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta‐analysis. Ann Oncol. 2014;25(7):1293‐1311. [DOI] [PubMed] [Google Scholar]
- 2. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta‐analysis of epidemiological studies. Acta Oncol. 2015;54(5):635‐654. [DOI] [PubMed] [Google Scholar]
- 3. Peisch SF, Van Blarigan EL, Chan JM, Stampfer MJ, Kenfield SA. Prostate cancer progression and mortality: a review of diet and lifestyle factors. World J Urol. 2017;35(6):867‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ligibel J. Lifestyle factors in cancer survivorship. J Clin Oncol. 2012;30(30):3697‐3704. [DOI] [PubMed] [Google Scholar]
- 5. Corbett T, Cheetham T, Muller AM, et al. Exploring cancer survivors' views of health behaviour change: “Where do you start, where do you stop with everything?”. Psychooncology 2018;27(7):1816‐1824. [DOI] [PubMed] [Google Scholar]
- 6. Usta YY. Importance of social support in cancer patients. Asian Pac J Cancer Prev. 2012;13(8):3569‐3572. [DOI] [PubMed] [Google Scholar]
- 7. Korotkin BD, Hoerger M, Voorhees S, Allen CO, Robinson WR, Duberstein PR. Social support in cancer: how do patients want us to help? J Psychosoc Oncol. 2019;37(6):699‐712. [DOI] [PubMed] [Google Scholar]
- 8. Hughes S, Egger S, Carle C, et al. Factors associated with the use of diet and the use of exercise for prostate cancer by long‐term survivors. PloS One. 2019;14(10):e0223407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winkels RM, van Lee L, Beijer S, et al. Adherence to the World Cancer Research Fund/American Institute for Cancer Research lifestyle recommendations in colorectal cancer survivors: results of the PROFILES registry. Cancer Med. 2016;5(9):2587‐2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blanchard CM, Courneya KS, Stein K, American Cancer Society's SCS, II . Cancer survivors' adherence to lifestyle behavior recommendations and associations with health‐related quality of life: results from the American Cancer Society's SCS‐II. J Clin Oncol. 2008;26(13):2198‐2204. [DOI] [PubMed] [Google Scholar]
- 11. Stone CR, Courneya KS, McGregor SE, Li H, Friedenreich CM. Determinants of changes in physical activity from pre‐diagnosis to post‐diagnosis in a cohort of prostate cancer survivors. Support Care Cancer. 2019;27(8):2819‐2828. [DOI] [PubMed] [Google Scholar]
- 12. Timmons A, Gooberman‐Hill R, Sharp L. “It's at a time in your life when you are most vulnerable”: a qualitative exploration of the financial impact of a cancer diagnosis and implications for financial protection in health. PloS One. 2013;8(11):e77549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort profile: the National Prostate Cancer Register of Sweden and prostate cancer data base Sweden 2.0. Int J Epidemiol. 2013;42(4):956‐967. [DOI] [PubMed] [Google Scholar]
- 14. Bergengren O, Garmo H, Bratt O, Holmberg L, Johansson E, Bill‐Axelson A. Satisfaction with care among men with localised prostate cancer: a nationwide population‐based study. Eur Urol Oncol. 2018;1(1):37‐45. [DOI] [PubMed] [Google Scholar]
- 15. Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health‐related quality of life in men with prostate cancer. Urology. 2000;56(6):899‐905. [DOI] [PubMed] [Google Scholar]
- 16. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245‐1251. [DOI] [PubMed] [Google Scholar]
- 17. Laviana AA, Hernandez A, Huang LC, et al. Interpretation of domain scores on the EPIC‐how does the domain score translate into functional outcomes? J Urol. 2019;202(6):1150‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37‐48. [PubMed] [Google Scholar]
- 19. Suttorp MM, Siegerink B, Jager KJ, Zoccali C, Dekker FW. Graphical presentation of confounding in directed acyclic graphs. Nephrol Dial Transplant. 2015;30(9):1418‐1423. [DOI] [PubMed] [Google Scholar]
- 20. Sv B. Flexible Imputation of Missing Data. Boca Raton, FL: CRC Press; 2012. xxv, 316 p. [Google Scholar]
- 21. Afshin A, Babalola D, McLean M, et al. Information technology and lifestyle: a systematic evaluation of internet and mobile interventions for improving diet, physical activity, obesity, tobacco, and alcohol use. J Am Heart Assoc. 2016;5(9):e003058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chong S, Ding D, Byun R, Comino E, Bauman A, Jalaludin B. Lifestyle changes after a diagnosis of type 2 diabetes. Diabetes Spectr. 2017;30(1):43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hamdy FC, Donovan JL, Lane JA, et al. 10‐year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415‐1424. [DOI] [PubMed] [Google Scholar]
- 24. Straczynska A, Weber‐Rajek M, Strojek K, et al. The impact of pelvic floor muscle training on urinary incontinence in men after radical prostatectomy (RP) – a systematic review. Clin Interv Aging. 2019;14:1997‐2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson CA, Omar MI, Campbell SE, Hunter KF, Cody JD, Glazener CM. Conservative management for postprostatectomy urinary incontinence. Cochrane Database Syst Rev. 2015;1:CD001843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goonewardene SS, Gillatt D, Persad R. A systematic review of PFE pre‐prostatectomy. J Robot Surg. 2018;12(3):397‐400. [DOI] [PubMed] [Google Scholar]
- 27. Friberg AS, Rask Moustsen I, Benzon Larsen S, et al. Educational level and the risk of depression after prostate cancer. Acta Oncol. 2019;58(5):722‐729. [DOI] [PubMed] [Google Scholar]
- 28. Farris MS, Kopciuk KA, Courneya KS, McGregor SE, Wang Q, Friedenreich CM. Associations of postdiagnosis physical activity and change from prediagnosis physical activity with quality of life in prostate cancer survivors. Cancer Epidemiol Biomarkers Prev. 2017;26(2):179‐187. [DOI] [PubMed] [Google Scholar]
- 29. Kassianos AP, Raats MM, Gage H, Peacock M. Quality of life and dietary changes among cancer patients: a systematic review. Qual Life Res. 2015;24(3):705‐719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


