Abstract
Objective
To assess the impact of a migraine management program offered as a complimentary service by a company within its corporate well‐being program.
Background
Migraine imposes a substantial burden on patients, families, employers, and societies. As migraine primarily affects working‐age adults, this has important implications for both employees and employers. Workplace educational and well‐being programs positively contribute to employees’ productivity, reduce costs related to absenteeism, and improve the quality of life of the employees living with migraine.
Methods
This was a non‐interventional cohort study, which followed employees and their family members over time. Participants received 1 telemedicine consultation to determine migraine diagnosis or a high probability of having migraine and 6 sessions of individualized telecoaching from a specialized nurse via a specially developed smartphone application to optimize their migraine management leveraging all appropriate medical and lifestyle options. Participants were evaluated during the program and at 3 months after completion through a series of validated questionnaires including Migraine Disability Assessment (MIDAS), Patient Activation Measure (PAM), and satisfaction with the services offered. A cost analysis was also performed to determine the economic benefit of the program considering the number of completers, dropouts, their associated program costs, MIDAS data, average salary of a Swiss employee in the pharma sector, and working days per year.
Results
Of the 141 participants enrolled in the program, 79 completed 6‐month and 42 completed 9‐month assessments. The total MIDAS scores (mean, standard deviation [SD]) significantly improved from baseline by 54% at Month 6 (15.0 [13.6] vs 6.9 [8.2]; mean [SD] reduction: 8.1 [12.9], 95% confidence interval [CI]: 5.6‐10.6; P < .0001) and by 64% at Month 9 (15.4 [14.7] vs 5.6 [6.0]; mean [SD] reduction: 9.8 [14.0], 95% CI: 6.6‐13.0; P < .0001). The PAM scores also significantly improved from baseline by 8% at Month 6 (63.8 [10.9] vs 69.6 [12.8]; mean [SD] increase: 5.8 [12.8], 95% CI: 3.2‐8.4; P = .003) and 11% at Month 9 (63.5 [10.7] vs 71.3 [12.2]; mean [SD] increase: 7.8 [11.0], 95% CI: 4.3‐11.2; P = .003). At Month 6, common coaching lessons and respective action plans focused on progressive muscle relaxation, sleep, hydration, nutrition, general disease education, and stress management. The exit survey showed that the majority of the participants who completed the program had a meaningful and sustained improvement in their overall health and reported a high level of satisfaction with the program. The cost analysis revealed that on average participants gained 10.8 (95% CI: 9.3‐12.3) working days/year that were previously lost due to migraine, resulting in a positive return on investment (ROI) of 490% (95% CI: 410%‐570%), indicating a higher magnitude of savings that could be achieved by the implementation of such program. In addition to ROI and work productivity gained, participants also gained on average 13.6 (95% CI: 9.9‐17.3) migraine‐free days/year for their private and social life.
Conclusion
The employer‐sponsored disease management program provided a better understanding of migraine, promoted methods and approaches to improve management by combining medical and lifestyle options leading to significant improvements in migraine symptoms that sustained beyond the intervention, supporting prolonged effectiveness of such programs. The program also provided a high ROI to the employer, supporting that the systematic inclusion of such programs into corporate well‐being initiatives can be of significant benefit not only to the impacted individuals but to the employers as well.
Keywords: migraine, disease management program, Migraine Disability Assessment, Patient Activation Measure, participants’ satisfaction, return on investment
Introduction
Migraine affected more than 136 million adults across Europe in 2016. 1 Patients with ≥ 4 monthly migraine days account for 10%‐20% of all migraine patients and have a higher associated disability. 2 , 3 The disease disproportionately affects women, with a 2‐ to 3‐fold higher prevalence in women than in men especially after puberty. 4 Despite its high prevalence, migraine remains underdiagnosed and undertreated, with only around 15% of migraine patients in Europe consulting a specialist. 3
Migraine is a leading cause of disability in people during their prime working years (30‐50 years) 5 and imposes an enormous personal and financial burden on the sufferers, their families, and society. Estimated annual costs of migraine in Europe ranged from €18 to €111 billion, about 77%‐93% of which was attributed to productivity loss (one‐third caused by absenteeism). 6 , 7 According to a recent study in European patients with ≥4 migraine days/month, employed migraine sufferers lost an estimated 30.2 workdays/year due to migraine. 8 Estimates from Swiss‐based studies showed that individuals on an average lost 10.2‐31.9 workdays/year due to migraine, highlighting a considerable impact of the disease on both the patients and their employers. 9 , 10
Many employers understand that a healthy workplace fosters the health and well‐being of their employees while enhancing organizational performance. 11 Many employers have initiated workplace wellness programs and one of the programs reported considerable cost savings ranging from £500,000 to £700,000 through improved workplace productivity, emphasizing the benefits of employee engagement programs. 11 Data from population‐based studies indicate that 52% of episodic migraine and 37%‐60% of chronic migraine patients are employed. 12 , 13 Employers are, therefore, well positioned to devise strategies and help employees better manage their migraine. Worksite migraine intervention/education programs are effective in significantly reducing disability, resource utilization, work‐loss, cost of productivity loss, and non‐workplace impairment associated with migraine. 14 , 15 , 16 , 17 , 18 , 19 , 20
Novartis acknowledged the problems surrounding migraine at the workplace and created a unique disease management program called Migraine Care using integrated digital solutions, such as telemedicine and software applications designed in collaboration with patient groups and leading experts in Headache Neurology. The Migraine Care program is an ongoing complimentary migraine management program that is part of Novartis’ corporate well‐being programs called “Energized for Life.” The program was launched by Novartis in June 2018 for all of its Swiss‐based employees and then extended to their immediate family members in February 2019.
The objective of the program was to foster patient empowerment through multidisciplinary approaches in migraine management by combining medical and lifestyle options. Specifically, the program intended to reduce the impact of migraine by creating a migraine‐friendly work organization, by educating all employees and their family members on migraine, and providing them tools to better understand and manage migraine and improve their quality of life. Additionally, a return on investment (ROI) analysis was performed to evaluate the economic benefit of the program from an employer’s perspective. We hypothesized that an employer‐provided multidisciplinary telemedicine disease management program may provide potential health benefits to employees at the workplace as well as high ROI to the employers.
Methods
This was a pre‐planned secondary analysis of previously collected data during the program and is the first study to report the full findings of this program. However, key findings from the study were also presented at various conferences. 21 , 22 , 23 , 24
Migraine Care Program
The program consisted of 3 integrated phases: (1) an educational awareness campaign for all employees, (2) an individualized disease management program for those living with migraine, and (3) data analysis phase (referred to as study hereafter) which assessed the program’s impact among participants’ who consented and enrolled into the study.
The educational awareness campaign was designated to educate all employees (independent of their migraine status) about migraine – to understand the disease, reduce stigma around migraine, and create a migraine‐friendly work environment within the organization. Educational resources used for the campaign included emails, automated teller machine screens and info‐points, a migraine awareness booth, a lecture by a headache neurologist, flyers, newsletters, postcards, brochures, and roll‐up banners.
Following the educational awareness campaign, employees could voluntarily and anonymously register through a Novartis website, after which the independent telemedicine provider (Medgate Tele Clinic) made contact and conducted a call. Subsequently, full participant registration and consent for the program was provided through the Migraine Care Buddy, a special module on the Migraine Buddy smartphone application (e‐diary application by Healint Pte. Ltd.) via phone. After enrollment, participants received a screening call from the telemedical nurse and consultation with a medical doctor to determine if they had a previous migraine diagnosis or a high probability of having migraine based on the ID‐Migraine questionnaire. If the participants were assessed as potentially having migraine, they were referred to a neurologist for assessment and subsequent treatment, as appropriate. If the individual had a prior confirmed diagnosis, the doctor optimized the therapy, as appropriate. Eligible participants then received 6 monthly sessions of individualized telecoaching on migraine management and action plans from the telemedical nurse via a specially developed module (Fig. 1). The module was also used to track progress in the program and to interact with their nurses.
Fig. 1.
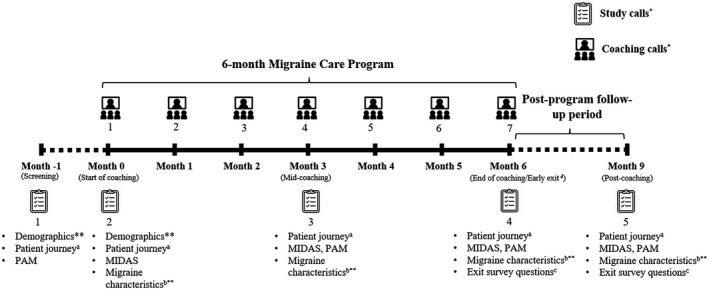
Migraine Care program – design and data collection schedule. Migraine Disability Assessment (MIDAS); Patient Activation Measure (PAM). aPatient journey characteristics assessed were treated by healthcare providers and type of treatments in the past 3 months. bMigraine characteristics assessed were duration of migraine, monthly migraine attacks, monthly migraine days, monthly headache attacks, monthly headache days, and migraine pain severity. cExit survey questions included the number of coaching calls received, type of migraine actions implemented, helpfulness of the program components (coaching calls, action plans, educational content, and the app), net promoter score, whether the program finished early, met expectations, PGIC, progress toward migraine goals, and if the program helped participants to better manage the migraine. dThe early exit survey was performed only if the participant intended to stop the coaching before completing 6 months. The assessments at the early exit call were identical to the assessments at the end of the coaching call. *The day of the call was approximate; data were taken at the designated call number and mapped to the associated day. **Participants self‐entered the data into the e‐diary outside the Migraine Care module. Other assessments were administered and entered into the module by the telemedical nurse.
The individualized coaching lessons offered during the calls included better understanding and managing migraine symptoms, identify individual trigger factors for migraine, management of migraine at work and home, preparation for a healthcare providers (HCP) or neurologist visit, general guidance through a personal migraine diary on the frequency and duration of attacks, and assessing if acute or preventive medication is effective, etc.
The purpose of the action plan was to empower participants to leverage medical and lifestyle options to manage their migraines better. Action plans shared during the call focused on migraine overall (understanding and coping with migraine attacks and taking prophylactic medication to better manage their condition) and lifestyle changes (stress management by progressive muscle relaxation [PMR] technique, acupuncture, and adoption of healthy eating, drinking, and sleeping habits as well as physical activities). In addition, information on managing migraine at the workplace (adopting a work routine and identifying support options), HCP‐related information (confirmation of diagnosis, referral to a neurologist, preparation for an HCP visit, and guidance on specific questions for consultation), and sharing their experiences, was provided. The telemedical nurse provided customized action plans to the participants based on their individual needs. Participants were asked to focus on 1 or 2 actions at a time and were encouraged to provide feedback on each action. If needed, these action plans were updated/modified over time in consultation with the nurse.
The data analysis phase of the program assessed the impact of the program on the burden of migraine and participants’ engagement before and after participation in the program, as well as satisfaction with the support provided. Participation in this phase was voluntary and only participants who consented to the use of their data collected during the program were included.
The study was conducted as per the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee of Northwest and Central Switzerland (Ethikkomission Nordwest‐und Zentralschweiz). Further, the conduct and findings of the study are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. 25 The study protocol is available in Online Appendix‐I.
Participants
Employees (aged ≥ 18 years) of Novartis Pharma AG, Switzerland and their family members who provided consent through the e‐diary application and via phone, were eligible to participate. Additionally, participants were required to have a confirmed diagnosis of migraine or high probability of having migraine (as determined by a score of ≥2 on the ID‐Migraine questionnaire). 26 Furthermore, participants were required to have a baseline and ≥1 post‐baseline assessment available to be included in the analysis.
Participants with no confirmed diagnosis of migraine during the program and those meeting ≥1 SNOOP criterion, ie, at least 1 systemic sign or symptom, neurologic sign or symptom, onset (sudden, eg, thunderclap headaches), older when headaches first appeared, and progression of existing headache disorder, indicating the possibility of a secondary headache disorder were excluded. 27 , 28 Furthermore, participants who did not have any baseline assessments or who withdrew their consent were excluded from the analysis. For patients who were lost to follow‐up due to “no further interest,” “left the company” or “other health reasons,” data until loss to follow‐up were included in the analysis.
Participant Characteristics
Demographic information (age, gender, and participant’s status [whether Novartis employee or their family member]) were collected at baseline. Other parameters evaluated at baseline included time since diagnosis, under‐treatment by an HCP (general physician or specialist), and type of treatments received over the last 3 months.
Outcome Measures
The primary outcome measure was the change in the Migraine Disability Assessment (MIDAS) total score from baseline to Month 6. 29 MIDAS is a 7‐item, self‐administered questionnaire that quantifies headache‐related disability over a 3‐month recall period. 29 The MIDAS score was derived as the sum of responses to 5 questions on missed headache‐related days from work/school, household work and non‐work activities, and days at paid/household work where productivity was reduced by at least half. Higher scores represent more severe disability (range 0‐270) and these scores are categorized into 4 severity grades ranging from Grade I (0‐5, little or no disability) to Grade IV (21+, severe disability). 29 The remaining 2 questions were not used in calculating the score but served as a resource for the clinicians to inquire about the number of days with headache and average pain level associated with the headaches.
Secondary outcomes assessed were changes in the total MIDAS from baseline to Month 3 and Patient Activation Measure (PAM) scores from baseline to Month 3 and Month 6. Activation refers to the patient’s comprehension of their role in managing their own health. 30 The 10‐item PAM questionnaire was used to assess the participants’ ability (knowledge, skills, beliefs, and confidence) to manage their health. 26 The PAM tool is licensed by Insignia Health LLC and hence scoring was performed by them. Participants were asked to respond to each statement on a 4‐point scale with responses ranging from “disagree strongly” to “agree strongly.” Based on their responses, the PAM score (0‐100 scale) was calculated and participants’ were stratified into 4 activation levels, with level 1 indicating poor patient activation and level 4, indicating adequate patient activation. 30
Other secondary outcomes included self‐reported progress in migraine management and engagement with the program at Month 6 using a 5‐point Likert scale (1 = very much so; 5 = none), type of migraine actions implemented through the program, and Patient Global Impression of Change (PGIC) score. 31 The PGIC scale measures the change in the patient’s overall status through a 7‐point rating scale (from “very much improved” to “very much worse”). 31 Engagement with the program, ie, helpfulness of the program components (coaching calls, action plans, educational material, and e‐diary application), was assessed using a 5‐point scale (1 = extremely helpful; 5 = not at all helpful), while the satisfaction score/net promoter score (NPS) was assessed using a 10‐point Likert scale (0 = not likely to recommend; 10 = extremely likely to recommend; respondents were grouped into promoters: those who scored 9‐10, passives: those who scored 7‐8, and detractors: those who scored 0‐6, respectively). The NPS was calculated as the difference between the percentage of promoters and detractors giving a final score between −100 (if all participants were detractors) and 100 (if all participants were promoters). Furthermore, patient‐reported migraine characteristics were assessed at baseline, Month 3 and Month 6 and were reported through the same e‐diary application but outside the Migraine Care module.
Under exploratory objectives, the impact of the program was assessed 3 months after completion (Month 9) through the following measures: changes in the total MIDAS and PAM scores from baseline, PGIC scores, type of migraine actions implemented, and 5‐point Likert response on the questions “Did you make progress toward the goals you had around managing your migraine?” and “Do you feel the program has helped you better manage your migraine?” (1 = very so much; 5 = none).
Subgroup Analyses
Pre‐planned subgroup analyses assessing the changes in the MIDAS and PAM scores from baseline to Month 3, Month 6, and Month 9 among participants with and without a confirmed migraine diagnosis at baseline and those with MIDAS Grade I vs MIDAS Grades II‐IV at baseline were performed. It is important to note that although participants with a high probability of having migraine at baseline were included, only those with a confirmed migraine diagnosis during the course of the program were analyzed in the study.
Return on Investment Analysis
The ROI analysis quantifies the incremental gain or loss of the investment and is calculated by dividing the net benefit of the investment by the total cost of the investment. It is usually expressed as a percentage, with an ROI above 100%, indicating returns exceed costs and an ROI below 100% suggesting that costs outweigh returns. 32
The ROI for the Migraine Care program was calculated using the change in MIDAS scores before and after the program. The data on work‐related absenteeism and presenteeism of the program participants were utilized for the analysis. 6 The data were extrapolated for a year to derive meaningful conclusions. Furthermore, the number of non‐workdays missed (sum of days of household work and days of social activities missed) due to headaches before and after the program was estimated. Other model inputs included in the analysis were the average annual salary of the employees in the Swiss pharma sector (137,670 CHF) and the number of workdays per year (n = 220 workdays/year). The average salary estimate was from the year 2016 and was inflation‐adjusted to the year 2018 using the official Swiss Federal Statistical Office data. 33 , 34 The total costs for the program were estimated by considering participants who completed the program (all 6 monthly sessions) as well as those who dropped out during the course of the program as of February 2020.
Statistical Analysis
This was a non‐interventional cohort study that used data from the Migraine Care program. The power calculation for the study was performed using the interim data analysis of the primary endpoint. All study variables were summarized descriptively. Categorical variables were summarized as counts and percentages, and continuous variables were presented as means, standard deviations (SDs), and 95% confidence interval (CI). MIDAS and PAM scores follow continuous ratio scales and grades were determined by these scores. The normality of distributions was assessed using histograms. A 2‐tailed paired t‐test was used as the same study population was followed‐up at different times during the program. An a priori P value of <.05 was set as the threshold for statistical significance. All data analyses were performed by Healint Pte. Ltd. using Microsoft Excel and Amazon Redshift. An Amazon Redshift database was used for data processing and retrieval of study data.
Results
Between June 2018 and October 2019, 339 participants with a diagnosis or high probability of having migraine registered in the program. Of these, 141 consented to the analysis of their data; 79 participants completed the 6‐month program. Twenty‐eight participants were still in the program and had not completed Month 6 and 5 participants missed a call, while others dropped out due to no further interest (n = 25), lost to follow‐up (n = 12), or other health problems (n = 2); 10 participants re‐entered the study. Of the 79 participants, 42 were re‐evaluated at 3 months after program completion, while the remaining were still between Month 6 and Month 9 (Fig. 2).
Fig. 2.
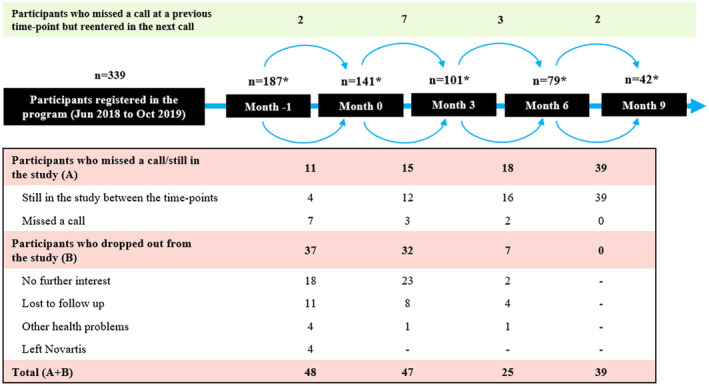
Migraine Care program – Participants flow. *Number of participants at each time‐point = (Number of participants in the previous time‐point – total of participants who dropped out or missed a call or still in the study) + Number of participants who re‐entered the study.
The mean (SD) age of 141 participants at baseline was 41.0 (9.0) years. The majority of participants were female (73.0%; 103/141) and 28.1% (39/139) had no confirmed diagnosis of migraine. Table 1 provides the characteristics of the participants enrolled in the program.
Table 1.
Characteristics of the Participants Enrolled Into the Study
| Baseline (N = 141) | |
|---|---|
| Age, mean (SD) years | 41.0 (9.0) |
| Age group, n (%) | |
| 18‐34 years | 42 (29.8%) |
| 35‐44 years | 59 (41.8%) |
| 45‐54 years | 31 (22.0%) |
| 55‐64 years | 9 (6.4%) |
| ≥65 years | 0 (0.0%) |
| Gender, n (%) | |
| Female | 103 (73.0%) |
| Male | 38 (27.0%) |
| Participant status, n (%) | |
| Employee | 128 (90.8%) |
| Family | 13 (9.2%) |
| How long affected by migraine,† n (%) | |
| <1 year | 1 (0.7%) |
| 1‐5 years | 14 (10.1%) |
| 6‐12 years | 15 (10.8%) |
| 11‐15 years | 22 (15.8%) |
| 16‐20 years | 35 (25.2%) |
| 21+ years | 52 (37.4%) |
| When diagnosed†, n (%) | |
| No diagnosis | 39 (28.1%) |
| In the last 3 months | 1 (0.7%) |
| 3‐6 months | 5 (3.6%) |
| 6‐12 months | 0 (0.0%) |
| 1‐2 years | 10 (7.2%) |
| 2‐5 years | 20 (14.4%) |
| 5‐10 years | 9 (6.5%) |
| 10+ years | 55 (39.5%) |
| Treated by HCP†,‡, n (%) | |
| No | 72 (51.8%) |
| Physician | 42 (30.2%) |
| Past or current treatment by a specialist | 31 (22.3%) |
| Type of treatments over last 3 months†,‡, n (%) | |
| None | 4 (2.9%) |
| OTC pain relievers (without prescription) | 97 (69.8%) |
| Acute treatment prescribed by a doctor | 50 (36.0%) |
| Preventative medication prescribed by a doctor | 13 (9.4%) |
| Preventative device – Cefaly Neuro | 4 (2.9%) |
| Vitamins and supplements | 21 (15.1%) |
| Complementary (homeopathy, massage, ayurveda, etc.) | 15 (10.8%) |
| Other | 12 (8.6%) |
Two participants who completed Month 0 did not complete Month −1 assessments (screening).
Participants could provide more than one response.
HCP = healthcare provider; OTC = over‐the‐counter; SD = standard deviation.
Among participants who completed both the baseline and 3‐month assessments (n = 94), the mean (SD) MIDAS score improved from baseline by 15% at Month 3 (mean [SD] reduction: 2.4 [12.6], 95% CI: 0.5‐5.4; P = .067). Among participants who completed both the baseline and 6 months (n = 73), the mean (SD) MIDAS score significantly improved from baseline by 54% at Month 6 (mean [SD] reduction: 8.1 [12.9], 95% CI: 5.6‐10.6; P < .0001). Among participants who completed both the baseline and Month 9 (n = 41), the mean (SD) MIDAS score significantly improved from baseline by 64% at Month 9 (mean [SD] reduction: 9.8 [14.0], 95% CI: 6.6‐13.0; P < .0001). The presenteeism score decreased from baseline by 23% at Month 3 (mean [SD] reduction: 1.9 [7.2], 95% CI: 0.5‐3.4), 57% at Month 6 (mean [SD] reduction: 4.4 [7.3], 95% CI: 3.1‐5.6), and by 64% at Month 9 (mean [SD] reduction: 5.3 [8.0], 95% CI: 3.5‐7.1). Similarly, the absenteeism score decreased from baseline by 7% at Month 3 (mean [SD] reduction: 0.5 [8.6], 95% CI: −2.4‐1.4), 51% at Month 6 (mean [SD] reduction: 3.8 [7.7], 95% CI: 2.2‐5.3), and 63% at Month 9 (mean [SD] reduction: 4.5 [8.2], 95% CI: 2.6‐6.5) (Fig. 3A). The percentage of employees with MIDAS Grade I increased, whereas those in MIDAS Grades III and IV decreased from baseline at the follow‐ups (Fig. 3B).
Fig. 3.
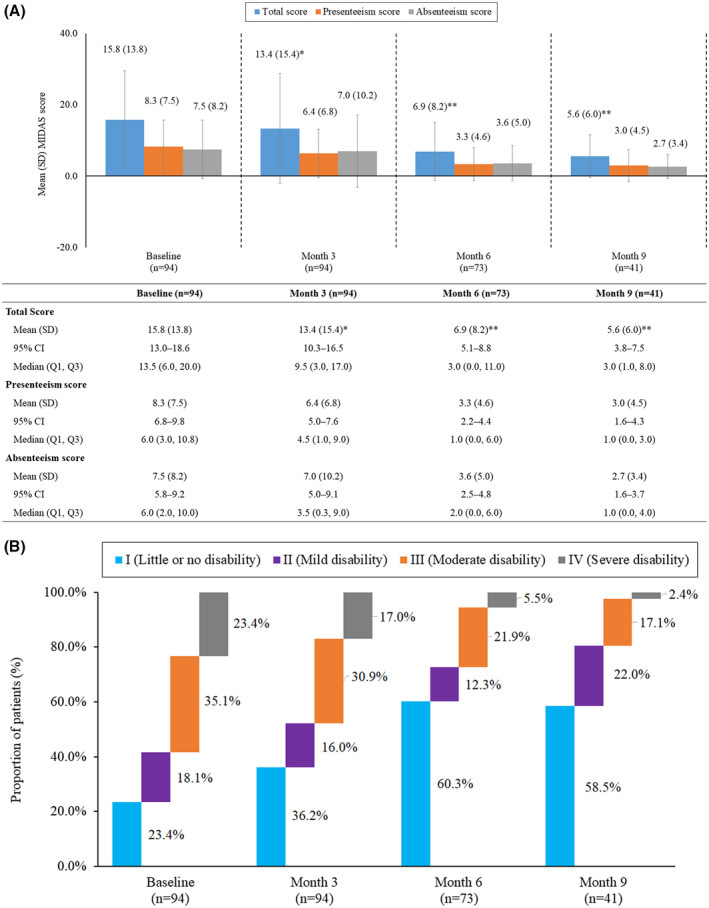
Impact of Migraine Care program on (A) Migraine Disability Assessment (MIDAS) scores and (B) MIDAS grades. (A) MIDAS score at baseline through Month 9. *P = .067 vs baseline; **P < .0001 vs baseline. Data presented in the figure are the mean total MIDAS, presenteeism, and absenteeism scores among participants at different time points throughout the Migraine Care program. The error bars represent standard deviations. Values presented in the table are mean (SD, standard deviation), 95% confidence interval (CI), and median (Q1, Q3). Absenteeism score was calculated as the sum of days of work or school, household work, and social activities missed in the last 3 months because of headaches. The presenteeism score was calculated as the sum of days in the last 3 months during which work productivity and household work was reduced by half due to headaches. (B) Change in MIDAS Grades from baseline to Month 9. Grade I (0‐5): Little or no disability. Grade II (6‐10): Mild disability. Grade III (11‐20): Moderate disability. Grade IV (21+): Severe disability.
Among participants who completed both the baseline and 3 months (n = 100), the mean (SD) PAM score significantly improved from baseline by 6% at Month 3 (64.3 [11.1] vs 68.5 [12.5]; P = .013). In participants who completed both the baseline and 6 months (n = 78), the mean (SD) PAM score significantly improved from baseline by 8% at Month 6 (63.8 [10.9] vs 69.6 [12.8]; P = .003). Among those who completed the post‐program follow‐up (n = 42), the mean (SD) PAM score significantly improved from baseline by 11% at Month 9 (63.5 [10.7] vs 71.3 [12.2]; P = .003) (Fig. 4A). At Month 6, 92.3% (72/78) and Month 9, 90.5% (38/42) of participants were activated (PAM levels 3 and 4), while none had poor activation (PAM level 1) (Fig. 4B). Among participants who completed the 6‐month program (n = 79), common coaching lessons and respective action plans focused on PMR, sleep, hydration, nutrition, general disease education, and stress management (Fig. 5).
Fig. 4.
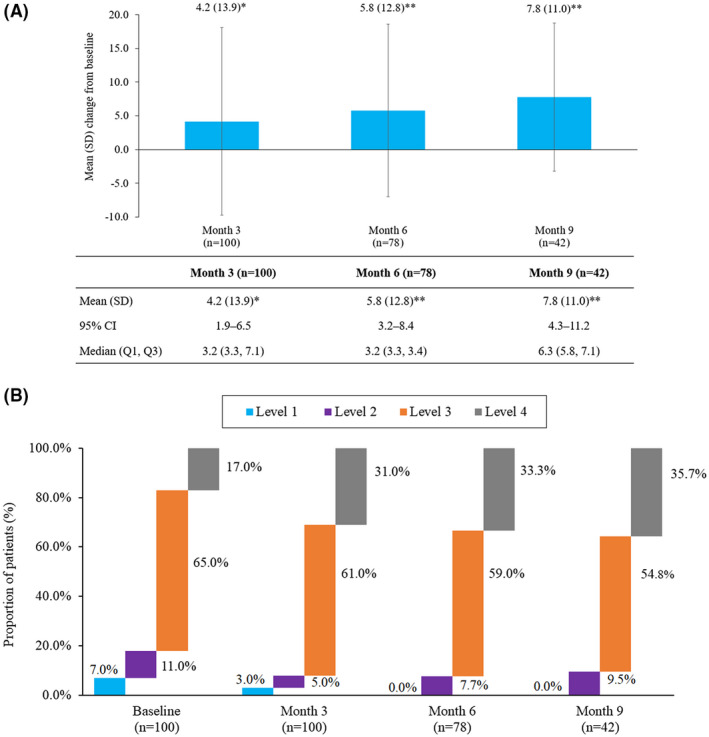
Impact of Migraine Care program on (A) Patient Activation Measure (PAM) scores and (B) PAM levels. (A) Change in PAM score from baseline through Month 9. *P = .013 vs baseline; **P = .003 vs baseline. Data presented in the figure are the mean change from baseline in PAM score among participants. The error bars represent standard deviations. Values presented in the table are mean (SD, standard deviation), 95% confidence interval (CI), and median (Q1, Q3). (B) Change in PAM levels from baseline to Month 9. Patient activation measure (PAM). Level 1 (0.0‐47.0): person does not yet understand their role in healthcare. Level 2 (47.1‐55.1): person does not yet have the knowledge and confidence to take action. Level 3 (55.2‐72.4): person is beginning to engage in positive health behaviors. Level 4 (72.5‐100): person is proactive and engaged in recommended health behaviors.
Fig. 5.
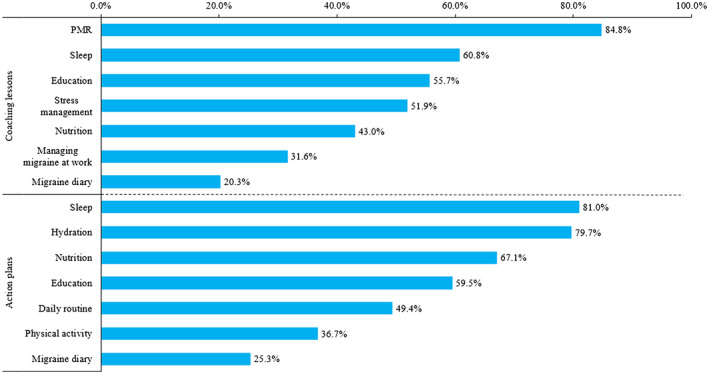
Mostly used coaching lessons and action plans at 6 months (n = 79). Progressive muscle relaxation (PMR).
An exit questionnaire was administered to the participants who completed 6 months to collect feedback and measure their satisfaction level with the services offered. The majority (n = 71; 89.9%) of the 79 participants who completed the study reported feeling improved (under “minimally” “much” and “very much” categories) compared to baseline on the PGIC scale. With 69.6% (55/79) of the participants categorized as promoters and 6.3% (5/79) as detractors, the NPS was estimated at 63.3. While 43.0% (34/79) of the participants reported that the program met their expectations, 32.9% (26/79) and 21.5% (17/79) reported that the program “exceeded” and “greatly exceeded” their expectations, respectively. When prompted for self‐reported progress toward migraine goals and how the program helped them to better manage their migraine, 77.2% (61/79) and 84.8% (67/79) responded in favor of the program, respectively. Participants who were surveyed at 3 months after program completion (n = 42) reported similar satisfaction levels.
Of the 79 participants who completed the 6‐month program, 6 participants finished the program early before completing 6 months (2 stated that they had improved and did not need the program and 1 left the company, while others did not provide any reason).
Patients’ self‐reported data on migraine characteristics were retrieved from the e‐diary application but outside the Migraine Care module. However, these could not be analyzed due to low adherence to this section and reporting inconsistencies.
Subgroup analysis by migraine diagnosis status at baseline showed substantially higher MIDAS scores in participants with a confirmed migraine diagnosis vs those without a confirmed diagnosis (17.8 [SD = 15.6] vs 9.0 [SD = 6.9]). Adherence to the program and reductions in the mean MIDAS score from baseline were relatively higher in participants with a confirmed diagnosis vs those without a confirmed diagnosis (Month 6: 10.2 [SD = 14.7] vs 4.3 [SD = 7.6]; Month 9: 11.4 [SD = 16.2]) vs 7.5 [SD = 6.7]). Further, PAM scores improved markedly among participants with a confirmed migraine diagnosis vs those without a confirmed diagnosis (Month 6: 13.1 [SD = 12.5] vs 0.1 [SD = 10.3]; Month 9: 10.0 [SD = 11.2] vs 2.1 [SD = 13.1]).
Subgroup analysis by the MIDAS grades showed a marginal increase (1‐2 points) in the mean MIDAS score from baseline among participants with MIDAS Grade I, suggesting little perceived benefit in participants with little or no disability. Participants with MIDAS Grades II‐IV at baseline instead reported a significant reduction in migraine disability. The mean (SD) MIDAS score decreased from baseline by 11.7 (12.6) at Month 6 (P < .0001) and by 14.1 (14.1) 3 months after the program (P < .0001). The improvements in the PAM scores at Month 6 were comparable, with MIDAS Grade I participants showing an increase of 8.2% from baseline and those with MIDAS Grades II‐IV showing an increase of 10.2% from baseline. Improvements sustained 3 months after the program, with MIDAS Grade I participants showing an increase of 8.2% and those with MIDAS Grades II‐IV showing an increase of 13.3% from baseline.
Return on Investment Estimation
For participants who completed the 6‐month program as of February 2020 (68.7%), the average cost incurred per participant was CHF 920, while for those who dropped out of the program (31.3%) it was CHF 480. Applying the average cost incurred for each participant (68.7% completers and 31.3% dropouts), the overall cost of the program was calculated as 129,858 CHF.
Overall, participation in the Migraine Care program resulted in a substantial improvement in workplace productivity. The average workdays missed due to headaches in the previous 3 months decreased from 0.9 to 0.5 days (annualized reduction: 1.5, 95% CI: 1.2‐1.7 days). Similarly, the mean number of workdays with reduced work productivity due to headaches in the previous 3 months decreased from 3.9 to 1.6 days (annualized reduction: 9.3, 95% CI: 8.1‐10.6 days). Considering the data for days of work missed (1.5 days) and work productivity reduced by half or more (9.3 days) from the MIDAS questionnaire for study completers, it was calculated that on average participants gained 10.8 (95% CI: 9.3‐12.3) working days/year that were previously lost due to migraine. Translating the work productivity gained into cost benefits among participants who completed the program (68.7%), the Migraine Care program resulted in a positive ROI of 490% (95% CI: 410%‐570%) for the employer.
In addition to the ROI and work productivity gained, participants also gained migraine‐free days for their private and social life. The total non‐work days (days of household work + days of social activities) missed due to headaches in the previous 3 months decreased from 6.5 (95% CI: 4.6‐8.5) days to 3.1 (95% CI: 2.1‐4.2) days, thereby gaining an average of 13.6 (95% CI: 9.9‐17.3) migraine‐free days/year for their private and social life.
Discussion
The results of this study demonstrated that the employer‐sponsored disease management program provided a better understanding of migraine and promoted methods and approaches to improve management by combining multidisciplinary (medical and non‐medical) options leading to significant improvements that were sustained beyond the intervention. In addition to the benefits provided to the employees, the program also provided high ROI to the employer.
Previous employer wellness programs have largely focused on educational aspects of migraine, 14 , 16 , 17 , 19 and only a couple of programs have used an integrated care approach. 15 , 20 However, the Migraine Care program included both disease awareness (the focus was not just to raise awareness among employees living with migraine but among others as well to create a migraine‐friendly work environment) and disease management. The program combined a digital approach (specially designed application to track improvement and follow action plan) with coaching modules (tailored to individual needs), thereby providing direct, cost‐free, and independent support to the employees and their family members living with migraine. The empirical evidence noted in this study supports the effectiveness of the program with the average burden of migraine among study participants reduced by more than half. This reduction was not only significant but was meaningful too. 35 On average, participants gained 10.8 working days/year that was previously lost due to migraine, resulting in a positive ROI of 490%. In addition to the work productivity gained, participants also gained on average 13.6 migraine‐free days/year for their private and social life. The significant improvements observed were maintained beyond the intervention, and hence support the prolonged effectiveness of such programs in migraine and other diseases affecting work productivity.
The program not only reduced migraine burden but participants were also more activated with the support they received through the program, and the high activation levels were maintained beyond the intervention as well. Further, results from the exit survey showed migraine improvement in the majority of the participants who completed the program compared with baseline and reported a high level of satisfaction with the program. Similar results were also observed at 3 months after program completion. Comparison of baseline characteristics of participants who completed the 6‐month program vs those who dropped out showed minimal differences in gender, participant status (employee/family member), under‐treatment of HCPs (physician/specialist), and type of treatments received over last 3 months. Although baseline PAM scores were comparable, MIDAS scores were observed to be slightly higher in dropouts than completers. Given this, it is not likely that dropouts could have biased the estimated effects. Moreover, as dropouts are typical of such worksite‐based intervention/education programs, a complete case analysis approach tends to provide more insights on the impact and usefulness of the program.
It is important to note that migraine is often undiagnosed in most of the employees at a workplace, and therefore benefits of such a program could be maximized if employees with a probable or suspected migraine are also included.
The unusual approach considered for this program proved highly successful based on the study data; however, there were several methodological limitations. Adequate comparison of the results was not possible due to the absence of a control group, although, as stated before, most of the components of the multimodal coaching program are well established. The coaching lessons and actions plans used in the program were pre‐ and well‐defined in line with the recommendations from the Swiss Headache Society. However, they were customized based on individual needs, which might reduce reproducibility and might have introduced bias. This study may be prone to selection bias as it analyzed data from participants who consented to use their data for analysis; characterization of the non‐consenting participants was not possible due to privacy reasons. There is a possibility of a potential reporting bias as the migraine characteristics data (retrieved from outside the module on the e‐diary application) were self‐reported by the participants, and the data could not be validated by the HCPs. Dropout rates from the program may also affect the study results. Although the dropout rate observed in this program is high than the previous worksite intervention/education programs, 14 , 15 , 16 , 17 it is typical of questionnaire‐based studies with no financial incentive; however, it induces a bias in favor of those most invested in the program or those with a greater awareness of migraine. Finally, the ROI estimates measured were based on the cost of the program incurred to Novartis and the average salary of the employees in the Swiss pharma sector, thereby limiting the generalizability to individuals with migraine employed in other sectors of Switzerland or in other countries. Moreover, the costs of providing such programs may be very different in other countries.
Conclusion
In conclusion, this study demonstrated that the employer‐sponsored disease management program offered has the potential to improve the diagnosis and management of migraine and reduce the burden of migraine at the workplace. In addition, the program provided a high ROI to the employer, supporting that the systematic inclusion of such programs into corporate well‐being initiatives can be of significant benefit to the impacted individuals as well as the employers.
Statement of Authorship
Category 1
(a) Conception and Design
Leonhard Schaetz, Timo Rimner, Purnima Pathak, Jelena Mueller
(b) Acquisition of Data
Timo Rimner, Deepak Chandrasekhar
(c) Analysis and Interpretation of Data
Leonhard Schaetz, Timo Rimner, Purnima Pathak, Juanzhi Fang, Deepak Chandrasekhar, Jelena Mueller, Peter S. Sandor, Andreas R. Gantenbein
Category 2
(a) Drafting the Manuscript
Leonhard Schaetz, Timo Rimner, Purnima Pathak, Juanzhi Fang, Deepak Chandrasekhar, Jelena Mueller, Peter S. Sandor, Andreas R. Gantenbein
(b) Revising It for Intellectual Content
Leonhard Schaetz, Timo Rimner, Purnima Pathak, Juanzhi Fang, Deepak Chandrasekhar, Jelena Mueller, Peter S. Sandor, Andreas R. Gantenbein
Category 3
(a) Final Approval of the Completed Manuscript
Leonhard Schaetz, Timo Rimner, Purnima Pathak, Juanzhi Fang, Deepak Chandrasekhar, Jelena Mueller, Peter S. Sandor, Andreas R. Gantenbein
Supporting information
Supplementary Material
Conflict of Interest: None
Disclosures: The funder of this study was involved in study design, data interpretation, and, decision to publish. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
REFERENCES
- 1. GBD 2016 Headache Collaborators . Global, regional, and national burden of migraine and tension‐type headache, 1990‐2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ford JH, Jackson J, Milligan G, Cotton S, Ahl J, Aurora SK. A real‐world analysis of migraine: A cross‐sectional study of disease burden and treatment patterns. Headache. 2017;57:1532‐1544. [DOI] [PubMed] [Google Scholar]
- 3. Katsarava Z, Mania M, Lampl C, Herberhold J, Steiner TJ. Poor medical care for people with migraine in Europe – Evidence from the Eurolight study. J Headache Pain. 2018;19:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vetvik KG, MacGregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. 2017;16:76‐87. [DOI] [PubMed] [Google Scholar]
- 5. Stovner LJ, Zwart JA, Hagen K, Terwindt GM, Pascual J. Epidemiology of headache in Europe. Eur J Neurol. 2006;13:333‐345. [DOI] [PubMed] [Google Scholar]
- 6. Linde M, Gustavsson A, Stovner LJ et al. The cost of headache disorders in Europe: The Eurolight project. Eur J Neurol. 2012;19:703‐711. [DOI] [PubMed] [Google Scholar]
- 7. Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jonsson B. The economic cost of brain disorders in Europe. Eur J Neurol. 2012;19:155‐162. [DOI] [PubMed] [Google Scholar]
- 8. Vo P, Paris N, Bilitou A et al. Burden of migraine in Europe using self‐reported digital diary data from the Migraine Buddy© application. Neurol Ther. 2018;7:321‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paris N, Simsek D, Chandrasekhar D et al. Evaluating the impact of migraine on work productivity in Switzerland using self‐reported data using the Migraine‐Buddy© application. Eur J Neurol. 2018;25:232. [Google Scholar]
- 10. Sokolovic E, Riederer F, Szucs T, Agosti R, Sandor P. Self‐reported headache among the employees of a Swiss university hospital: Prevalence, disability, current treatment, and economic impact. J Headache Pain. 2013;14:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The Work Foundation . Migraine’s impact on employment in Europe. Available at: http://www.theworkfoundation.com/wp‐content/uploads/2019/08/Migraines‐impact‐on‐employment‐in‐Europe‐FINAL‐pub‐vA.pdf. Accessed December 2019. [Google Scholar]
- 12. Buse DC, Manack A, Serrano D, Turkel C, Lipton RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry. 2010;81:428‐432. [DOI] [PubMed] [Google Scholar]
- 13. Shauly O, Gould D, Patel K. The public’s perception of interventions for migraine headache disorders: A crowdsourcing population‐based study. Aesthet Surg J Open Forum. 2019;1:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burton WN, Chen CY, Li X, McCluskey M, Erickson D, Schultz AB. Evaluation of a workplace‐based migraine education program. J Occup Environ Med. 2016;58:790‐795. [DOI] [PubMed] [Google Scholar]
- 15. Cramer H, Hehlke M, Vasmer J et al. Integrated care for migraine and chronic tension‐type headaches: A prospective observational study. Complement Ther Clin Pract. 2019;36:1‐6. [DOI] [PubMed] [Google Scholar]
- 16. Landy S, Brookfield RB, McGinnis J, Hawkes MN. An evaluation of the impact of educational programming on employee‐disability. Headache Pain. 2003;14:156‐160. [Google Scholar]
- 17. Mannix LK, Solomon GD, Kippes CM, Kunkel RS. Impact of headache education program in the workplace. Neurology. 1999;53:868‐871. [DOI] [PubMed] [Google Scholar]
- 18. Mongini F, Ciccone G, Rota E et al. Effectiveness of an educational and physical programme in reducing headache, neck and shoulder pain: A workplace controlled trial. Cephalalgia. 2008;28:541‐552. [DOI] [PubMed] [Google Scholar]
- 19. Page MJ, Paramore LC, Doshi D, Rupnow MF. Evaluation of resource utilization and cost burden before and after an employer‐based migraine education program. J Occup Environ Med. 2009;51:213‐220. [DOI] [PubMed] [Google Scholar]
- 20. Vicente‐Herrero T, Burke TA, Lainez MJ. The impact of a worksite migraine intervention program on work productivity, productivity costs, and non‐workplace impairment among Spanish postal service employees from an employer perspective. Curr Med Res Opin. 2004;20:1805‐1814. [DOI] [PubMed] [Google Scholar]
- 21. Pathak P, Neuckel D, Rimner T, Fang J, Chandrasekhar D, Schaetz L. Return on investment analysis of an employer‐provided migraine disease management program piloted in Switzerland. Value in Health. 202023(Suppl. 1):S265‐S266. [Google Scholar]
- 22. Schaetz L, Rimner T, Pathak P et al. Impact of an employer‐provided migraine coaching program on patient burden and engagement. Eur J Neurol. 2020;27(Suppl. 1):306. [Google Scholar]
- 23. Schaetz L, Rimner T, Pathak P, Fang J, Chandrasekhar D, Mueller J. Impact of an employer‐provided migraine coaching program on burden and patient engagement: Results from interim analysis. Cephalalgia. 2019;39:IHC‐L‐015. [Google Scholar]
- 24. Schaetz L, Rimner T, Pathak P, Fang J, Chandrasekhar D, Mueller J. Impact of an employer‐provided migraine coaching program on burden and patient engagement: Results from interim analysis. Neurology. 2020;94:1126. [Google Scholar]
- 25. Vandenbroucke JP, von Elm E, Altman DG et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int J Surg. 2014;12:1500‐1524. [DOI] [PubMed] [Google Scholar]
- 26. Lipton RB, Dodick D, Sadovsky R et al. A self‐administered screener for migraine in primary care: The ID migraine validation study. Neurology. 2003;61:375‐382. [DOI] [PubMed] [Google Scholar]
- 27. Do TP, Remmers A, Schytz HW et al. Red and orange flags for secondary headaches in clinical practice: SNNOOP10 list. Neurology. 2019;92:134‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dodick DW. Clinical clues and clinical rules: Primary vs secondary headache. Adv Stud Med. 2003;3:S550‐S555. [Google Scholar]
- 29. Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary‐based measure in a population sample of migraine sufferers. Pain. 2000;88:41‐52. [DOI] [PubMed] [Google Scholar]
- 30. Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918‐1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. J Manipulative Physiol Ther. 2004;27:26‐35. [DOI] [PubMed] [Google Scholar]
- 32. Erdogmus H, Favaro J, Strigel W. Return on investment. IEEE Softw. 2004;21:18‐22. [Google Scholar]
- 33. Federal Statistical Office . Wages, Income From Employment and Labour Costs. 2018. Available at: https://www.bfs.admin.ch/bfs/en/home/statistics/work‐income/wages‐income‐employment‐labour‐costs.assetdetail.5126411.html. Accessed April 2020. [Google Scholar]
- 34. Federal Statistical Office . Wages, Income From Employment and Labour Costs. 2018. Available at: https://www.bfs.admin.ch/bfs/en/home/statistics/work‐income/wages‐income‐employment‐labour‐costs/wage‐evolution.html. Accessed April 2020. [Google Scholar]
- 35. Lipton RB, Desai P, Sapra S. How much change in headache‐related disability is clinically meaningful? Estimating minimally important difference (MID) or change in MIDAS using data from the AMPP study. Headache. 2017;57:PF52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


