Abstract
Objectives
To prospectively evaluate quality of life (QoL) and psychosocial outcomes in children with severe acute asthma (SAA) after pediatric intensive care (PICU) admission compared to children with SAA who were admitted to a general ward (GW). In addition, we assessed posttraumatic stress (PTS) and asthma‐related QoL in the parents.
Methods
A preplanned follow‐up of 3 to 9 months of our nationwide prospective multicenter study, in which children with SAA admitted to a Dutch PICU (n = 110) or GW (n = 111) were enrolled between 2016 and 2018. Asthma‐related QoL, PTS symptoms, emotional and behavioral problems, and social impact in children and/or parents were assessed with validated web‐based questionnaires.
Results
We included 100 children after PICU and 103 after GW admission, with a response rate of 50% for the questionnaires. Median time to follow‐up was 5 months (range: 1‐12 months). Time to reach full schooldays after admission was significantly longer in the PICU group (mean of 10 vs 4 days, P = .001). Parents in the PICU group reported more PTS symptoms (intrusion P = .01, avoidance P = .01, arousal P = .02) compared to the GW group.
Conclusion
No significant differences were found between PICU and GW children on self‐reported outcome domains, except for the time to reach full schooldays. PICU parents reported PTS symptoms more often than the GW group. Therefore, monitoring asthma symptoms and psychosocial screening of children and parents after PICU admission should both be part of standard care after SAA. This should identify those who are at risk for developing PTSD, to timely provide appropriate interventions.
Keywords: follow‐up, parents, PICU, status asthmaticus
1. INTRODUCTION
Asthma exacerbations in children can be severe, and even life‐threatening. Admission to a pediatric intensive care unit (PICU) occurs in 5% to 34% of all hospitalizations for severe acute asthma (SAA). 1 , 2 , 3 , 4 Worldwide, PICU admission for SAA increased markedly during the past decade. 1 , 2 , 5 , 6 According to the Dutch national pediatric guideline for SAA, children whose asthma exacerbations do not respond to conventional treatment should receive intravenous (IV) magnesium sulfate. The next step is continuous IV administration of salbutamol followed by immediate transfer to a PICU, regardless of the dosage of IV salbutamol. A PICU stay is known to be a burden, physically and psychologically, for both children and their parents. 7 , 8 Risk factors during PICU admission for poor functional, cognitive, and psychosocial outcomes in children after an unexpected PICU admission in general, not specifically SAA, are longer PICU length of stay (LOS), severity of illness, invasive mechanical ventilation, and exposure to invasive procedures. Besides, parental factors such as a nonintact family structure, parental stress, and anxiety are potential moderators during PICU admissions and after PICU discharge for outcomes in critically ill child. 9 , 10 , 11 , 12 Symptoms of posttraumatic stress disorder (PTSD) among parents after PICU admission (ranging from 3 months to 10 years) of their child have also been reported with almost one‐third of parents fulfilled criteria for PTSD. 7 , 13
Children with SAA admitted to the PICU mostly have single organ failure. We showed 14 that they have a relatively short PICU stay (mean: 3.5 days), low intubation rate (16%), and low mortality (2%) compared to the general PICU population. 15 , 16 , 17 , 18 Nevertheless, a PICU admission might confront children and parents with the perception that asthma, although a chronic and often mild disease, can be fatal. Besides, during their PICU stay parents witness invasive procedures on their child (eg, arterial line, invasive mechanical ventilation). Therefore, children and parents might be at high risk for developing PTSD compared with admission to a general ward (GW).
To our knowledge, there have been no studies on psychosocial outcomes in children (regardless of age) with SAA after PICU admission. In a long term study in adolescents who experienced a life‐threatening asthma‐related event, 20% of these adolescents met criteria for PTSD. 13
We aimed to prospectively assess quality of life (QoL) and psychosocial outcomes in children with SAA who had been admitted to a PICU compared to children with SAA admitted to a GW. We hypothesized that the PICU group would have a lower asthma‐related QoL, more PTS symptoms, worse emotional and behavioral functioning, and more problems with daily activities. Second, we expected more PTS symptoms and PTSD in parents of children admitted to the PICU.
2. METHODS
2.1. Study design and participants
This study was the preplanned follow‐up of our nationwide multicenter prospective study, in which 221 children (aged 2‐18 years) with SAA admitted to a Dutch PICU (n = 110) or GW (n = 111) were enrolled between 2016 and 2018. 14 We prospectively identified all children (2‐18 years old) with SAA admitted to all seven Dutch academic PICUs (N = 110) and the pediatric wards of four participating general hospitals (N = 111). The four general hospitals were recruited based on geographical distribution over the Netherlands and needed to have a staff pediatric pulmonologist. For each group (PICU and GW), patients were recruited until the preplanned sample size of 110 patients per group had been achieved. According to the Dutch national pediatric SAA guideline, 19 an IV bolus magnesium sulfate is administered when the Asthma Score, as developed by Qureshi, 20 is still ≥10 after three consecutive nebulizations with salbutamol plus ipratropium. If continued nebulization and IV magnesium sulfate lead to insufficient response, continuous infusion with salbutamol is started. When IV salbutamol is administered children are transferred to a PICU, regardless of the dosage. 21 Parents, caregivers, or legal guardians (from now on referred to as parents) provided informed consent. Follow‐up was performed within 3 to 9 months after hospital discharge. The study was approved by the Research Ethics Committee of the Erasmus Medical Center Rotterdam (MEC 2015‐709).
2.2. Assessment procedure
As part of standard care, all children were seen by an asthma nurse, a pediatrician, or a pediatric respiratory physician within 4 to 8 weeks after hospital discharge. Thereafter, regular follow‐up visits within 3 to 9 months after hospital discharge were planned based on the level of asthma control. The medical information for our study was collected at the second follow‐up visit after hospital discharge. Psychosocial outcomes in children and parents were assessed through web‐based, validated questionnaires. These questionnaires were sent by email to the parents a week before the regular follow‐up visit, by using LimeSurvey, (https://www.limesurvey.org/ Version 2.06lts, build 160524). After 4 weeks, nonresponders were contacted by email once, and reminded to fill in the web‐based questionnaires.
2.3. Demographical and hospital admission data
Socioeconomic status (SES) was determined by using the highest educational level of the parents (low, middle, or high, according to Statistics Netherlands; statline.cbs.nl). The postal code was used to quantify the neighborhood SES, with a mean of zero. A lower (negative) score is associated with a lower SES. Ethnicity was defined as Caucasian or non‐Caucasian (if African, Turkish, Moroccan, Asian, Latin‐American, Surinamese, or two or more parental races). Hospital admission data included the following outcomes: number of arterial lines, invasive mechanical ventilation, PICU LOS in days, and hospital LOS in days.
2.4. Medical status at time of follow‐up
The following variables for the time period since the index admission were assessed: number of unscheduled emergency department (ED) visits or readmissions due to SAA, number of courses of prednisolone, and medical treatment level according to the Global Initiative for Asthma (GINA). 22 A healthcare professional assessed asthma symptoms during the preceding month reported by parents/children. These asthma symptoms included: nocturnal awakenings, wheezing more than two times per week, bronchodilator use more than two times per week and exercise‐induced symptoms. Asthma was considered well‐controlled if none of these symptoms occurred, partly controlled if one to two of these symptoms occurred and uncontrolled if three to four of these symptoms occurred. In addition, parents/children reported asthma symptom control through the web‐based questionnaire: Childhood Asthma Control Test. 23 A score of less than 20 indicated that asthma was not well‐controlled.
2.5. Quality of life and psychosocial outcomes child
Asthma‐related QoL was assessed with the Paediatric Asthma Quality of Life Questionnaire (PAQLQ), 24 a validated questionnaire with a recall period of 4 weeks. 25 The PAQLQ is completed by children aged 6 to 18 years. Lower scores imply worse QoL. We compared our data with norm data of 52 children with symptomatic asthma. 26 The Visual Analog Scale (VAS), a scale from 0 to 100 validated in somatic conditions, 27 was completed by children between 5 and 18 years on the question: “How much trouble is your asthma giving you right now?” A higher score implies worse QoL (more trouble).
Posttraumatic stress symptoms in children were assessed with the Children's Responses to Trauma Inventory (CRTI), of which the subscales intrusion, avoidance, arousal, and the total score were used. 28 Both a self‐reported version (for children aged 8‐17) and a proxy‐reported version (for parents of children aged 4‐17 years old) were used. A higher score indicated more posttraumatic stress symptoms. 28 We compared our data with normative data of 1440 nonexposed Dutch children. 29
Emotional and behavioral problems in children (aged 11‐18 years old) themselves were assessed with the Youth Self‐report. 30 Parents of children completed the Child Behavior Checklist (CBCL) 1.5 to 5 years 31 or CBCL 6 to 18 years. 30 Internalizing, externalizing, and total emotional and behavioral problem scores were compared with norm data of Dutch children; higher scores indicate more problems.
Social impact of hospital admission for SAA on daily life of the child was assessed through a semi‐structured interview, which we previously developed and now modified for this specific SAA population (Table 3). 32 In the same session, the impact on parents was assessed (Table 4).
Table 3.
Quality of life and psychosocial outcomes child (parent‐reported)
| Questionnaire | N | PICU | N | GW | P valuea | Adjusted P valueb | Norm mean (SD) |
|---|---|---|---|---|---|---|---|
| Posttraumatic stress (CRTI)c | N = 1440 | ||||||
| Intrusion | 34 | 10.3 (4.7) | 34 | 9.7 (3.7) | .48 | .37 | 12.5 (5.4) |
| Avoidance | 34 | 16.6 (8.2) | 34 | 13.8 (4.9) | .09 | .19 | 22.5 (8.2) |
| Arousal | 34 | 10.0 (4.3) | 34 | 9.2 (4.8) | .21 | .53 | 11.3 (4.6) |
| Other child‐specific responses | 34 | 15.7 (5.8) | 34 | 13.9 (5.7) | .09 | .31 | 18.5 (6.9) |
| Total score | 34 | 52.7 (18.7) | 34 | 46.6 (16.4) | .15 | .22 | NA |
| PTSD—intrusion | 34 | 7.8 (3.6) | 34 | 7.2 (2.9) | .46 | .29 | NA |
| PTSD—avoidance | 34 | 11.2 (5.9) | 34 | 9.2 (3.6) | .10 | .16 | NA |
| PTSD—arousal | 34 | 8.5 (3.7) | 34 | 7.7 (4.0) | .21 | .50 | NA |
| PTSD—total score | 34 | 27.5 (11.0) | 34 | 24.1 (8.7) | .23 | .19 | NA |
| PTSD—three times a score of 4 or 5d | 34 | 7 (21) | 34 | 3 (9) | .17 | .14 | NA |
| Emotional and behavioral problems (CBCL)e | N = 1451 | ||||||
| Internalizing | 44 | 8.2 (6.7) | 63 | 7.6 (5.8) | .63 | .64 | 6.64 (5.7) |
| Externalizing | 44 | 9.2 (8.3) | 63 | 11.1 (7.2) | .20 | .63 | 6.32 (5.9) |
| Total problem score | 44 | 30.1 (20.3) | 63 | 30.4 (18.2) | .94 | .60 | 23.91 (16.7) |
| Social impact (semi‐structured interview)f | NA | ||||||
| Absence from school after admission | 43 | 27 (63) | 57 | 28 (49) | .17 | .12 | NA |
| Time to reach full schooldays after admission, in days (range) | 27 | 10 (2‐40) | 28 | 4 (1‐8) | .001 | .001 | NA |
| Limitations of social activities | 43 | 11 (26) | 54 | 14 (26) | .97 | .52 | NA |
| Playing sports | 42 | 28 (67) | 55 | 32 (58) | .39 | .93 | NA |
| Professional help after admission | 45 | 4 (9) | 56 | 4 (7) | 1.00 | NA | NA |
Note: Intrusion between 7 and 49, avoidance 11 and 55, arousal 6 and 30, other child‐specific responses between 10 and 50. High scores imply worse functioning. The overall score of the PTSD CRTI will be between 17 and 85. PTSD Intrusion between 5 and 25, PTSD avoidance 7 and 35, PTSD arousal 5 and 25. Data were not normally distributed for PAQLQ, VAS, and CRTI, and adjusted P values for these outcomes should be considered as indicative only. Bold values are with a P < .05.
Abbreviations: CBCL, Child Behavior Checklist; GW, general ward; PAQLQ, Paediatric Asthma Quality of Life Questionnaire; PICU, pediatric intensive care; PTSD, posttraumatic stress disorder; SD, standard deviation; VAS, Visual Analog Scale.
P values (unadjusted) based on t tests or Mann‐Whitney tests (continuous outcomes) and χ 2 or Fisher exact tests (dichotomous outcomes).
Adjusted P value based on multiple linear or logistic regression adjusted for age, asthma treatment level, and Caucasian/non‐Caucasian.
Data are in mean (SD). The overall score of the Children's Responses to Trauma Inventory (CRTI) will be between 34 and 170.
Data are in number (%). Three times a PTSD score of 4 or 5 implicates that the child needs professional help.
Data are in mean (SD). Child Behavior Checklist (CBCL) = items of these questionnaires were rated on a 3‐point scale (0 = not true; 1 = somewhat or sometimes true; 2 = very true/often true).
Data are in number (%), only time to reach full schooldays is presented as mean (range).
Table 4.
Quality of life and psychosocial outcomes parents
| Questionnaire | N | PICU | N | GW | P valuea | Norm mean (SD) | Adjusted P valueb |
|---|---|---|---|---|---|---|---|
| Asthma‐related quality of life (PACQLQ)c | |||||||
| Activity limitations | 46 | 5.8 (1.8) | 61 | 6.0 (1.5) | .91 | NA | .25 |
| Emotional function | 46 | 5.7 (1.5) | 61 | 6.1 (1.0) | .34 | NA | .07 |
| Trouble given by asthma (VAS) | 52 | 3.0 (2.7) | 66 | 2.8 (2.6) | .76 | NA | .28 |
| Posttraumatic stress (SRIP)d | |||||||
| Intrusion | 39 | 7.6 (2.6) | 51 | 6.6 (1.4) | .03 | 7.1 (2.3) | .01 |
| Avoidance | 39 | 11.5 (3.3) | 51 | 10.1 (2.3) | .04 | 11.9 (4.1) | .01 |
| Arousal | 39 | 10.3 (3.8) | 51 | 8.8 (2.7) | .04 | 10.1 (3.6) | .02 |
| Total score | 39 | 29.4 (9.2) | 51 | 25.4 (5.9) | .03 | 29.0 (8.9) | .02 |
| PTSD | 39 | 1 (3) | 51 | 0 (⋯) | .43 | NA | NA |
| PTSD DSM IV | 39 | 3 (8) | 51 | 1 (2) | .31 | NA | NA |
| Social impact (semi‐structured interview)e | |||||||
| Absence from work | 41 | 16 (39) | 57 | 28 (49) | .32 | NA | .64 |
| Limitations social activities | 41 | 7 (17) | 56 | 13 (23) | .46 | NA | .88 |
| Professional help after admission | 41 | 3 (7) | 56 | 0 (0) | .07 | NA | NA |
Note: Data were not normally distributed for SRIP, PACQLQ, and VAS, and adjusted P values for these outcomes should be considered as indicative only.
Abbreviations: CBCL, Child Behavior Checklist; GW, general ward; PAQLQ, Paediatric Asthma Quality of Life Questionnaire; PICU, pediatric intensive care; PTSD, posttraumatic stress disorder; SD, standard deviation; VAS, Visual Analog Scale. Bold values are with a P < .05.
P values (unadjusted) based on Mann‐Whitney tests (continuous outcomes) and χ 2 or Fisher exact tests (dichotomous outcomes).
Adjusted P value based on multiple linear or logistic regression adjusted for age, asthma treatment level, and Caucasian/non‐Caucasian.
Data are in mean (SD). PACQLQ = overall score between 1 and 7. Low scores imply worse functioning. VAS = score between 0 and 10. High scores imply worse functioning.
Data are in number (%), only PTSD is presented as number (%). SRIP = PTSD if SRIP total score of more than 51. PTSD according to the DSM IV criteria if: at least one score of 3 or higher in intrusion, three scores of 3 or higher in avoidance, and two scores of 3 or higher in arousal.
Data are in number (%).
2.6. Quality of life and psychosocial outcomes parents
Asthma‐related QoL was assessed with the Paediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ), a validated questionnaire with a recall period of 4 weeks. 25 Lower scores imply worse QoL. No norm data for the PACQLQ are available. The VAS was completed by one of the parents with the question: “How much trouble is your child's asthma giving you right now?”. A higher score implies more trouble.
Posttraumatic stress symptoms in parents were assessed with the Self‐Rating Inventory for PTSD (SRIP). 33 , 34 This questionnaire uses a continuous scale where a higher score indicates more symptoms of PTS. Criteria for PTSD are intrusion symptoms, avoidance of reminders of the event, and some symptoms of hyperarousal and/or emotional numbing. PTSD is present if SRIP total score of more than 51. PTSD according to the DSM IV criteria is present if there is at least one score of ≥3 in intrusion, three scores of ≥3 in avoidance, and two scores of ≥3 in arousal. PTSD scores of parents were compared with normative data of the Dutch population (n = 7083).
2.7. Analyses
Data were presented as mean and standard deviation or median and interquartile range. Differences between patients in the PICU and patients in the GW were analyzed using t tests for normally distributed variables, Mann‐Whitney U tests for continuous variables that were not normally distributed, and χ 2 or Fisher's exact tests for categorical variables. Nonnormally distributed data included age and SES scores based on postal code. The linear‐by‐linear χ 2 association test was used for ordinal categories, which included medication steps according to GINA and the number of ED visits. Multivariate analysis was performed for the psychosocial outcomes using linear regression for continuous variables or logistic regression for dichotomous variables to adjust for age, asthma treatment level, and race. All statistical analyses were carried out in SPSS version 25 (Chicago, IL), and a two‐sided significance level of 0.05 was used.
3. RESULTS
We included 100 children after SAA with PICU admission and 103 after GW admission to assess clinical outcomes (Figure 1), with a median time to follow‐up of 5 months (range: 1‐12 months). Baseline characteristics are shown in Table 1. For the web‐based questionnaires, 50% of children and parents were not included due to nonresponse and refusal to participate in this outcome study (reason unknown, Figure 1). Baseline characteristics did not significantly differ from the baseline characteristics of patients who actually completed the questionnaires (Table 1). Among the 101 responders of the web‐based questionnaires, children were significantly younger (median of 6 vs 7 years, P = .002) and more likely to be Caucasian (73% vs 46%, P = .004) than nonresponders. We found no significant differences in asthma symptom control at the follow‐up clinic visit between responders and nonresponders.
Figure 1.
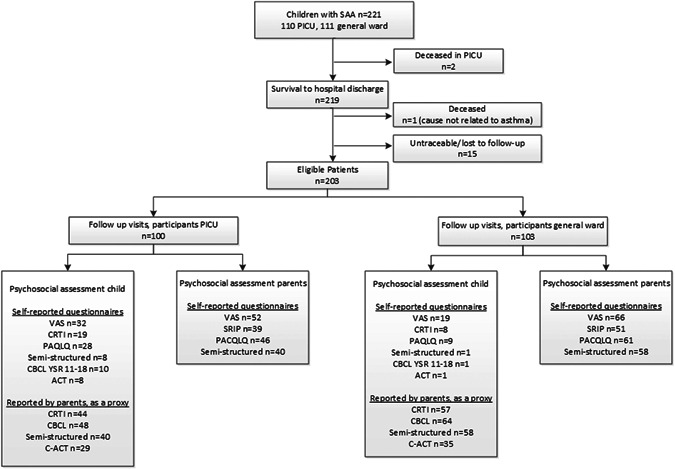
Flowchart study enrollment
Table 1.
Demographic and clinical characteristics
| Responders | Total population | |||
|---|---|---|---|---|
| PICU, N = 50 | GW, N = 62 | PICU, N = 100 | GW, N = 103 | |
| Demographic characteristics at follow‐up | ||||
| Age | 8 (6‐12) | 5 (3‐6)* | 8 (5‐12) | 5 (3‐7)** |
| Male | 31 (62) | 35 (57) | 61 (61) | 63 (61) |
| Caucasian | 29 (58) | 49 (79)* | 56 (56) | 73 (71)* |
| Highest parental education level | ||||
| Low (ISCED 0‐2) | 2 (4) | 0 (0) | 2 (2.6) | 1 (1.2) |
| Middle (ISCED 3‐4) | 18 (39) | 20 (34) | 32 (41.6) | 29 (35.8) |
| High (ISCED 5‐8) | 26 (57) | 39 (66) | 43 (55.8) | 51 (63) |
| Socioeconomic scores postal codea | −0.4 (1.5) | −0.11 (1.3) | −0.36 (1.4) | −0.26 (1.3) |
| One‐caregiver household | 14 (29) | 7 (12)* | 25 (29) | 13 (16)* |
| SAA hospital admission variables | ||||
| Arterial lines | 8 (19) | 0 (0) | 14 (16) | 0 (0) |
| Invasive mechanical ventilation | 5 (10) | 0 (0) | 10 (10) | 0 (0) |
| PICU LOS | 3.7 (1.8) | ⋯ | 3.5 (3) | ⋯ |
| Total hospital LOS | 6 (2) | 3 (1)** | 6 (2) | 3 (1)** |
| Medical status at follow‐up | ||||
| Medication step (GINA) | ||||
| Step 1 | 2 (4) | 3 (5)* | 2 (2) | 7 (7)* |
| Step 2 | 6 (12) | 1 (2) | 11 (11) | 3 (3) |
| Step 3 | 24 (49) | 48 (77) | 51 (52) | 74 (73) |
| Step 4 | 15 (31) | 9 (15) | 31 (31) | 15 (15) |
| Step 5 | 2 (4) | ⋯ | 4 (4) | ⋯ |
| No medication | ⋯ | 1 (2) | ⋯ | 2 (2) |
| Asthma symptom controlb | ||||
| Well‐controlled | 32 (64) | 42 (68) | 62 (64) | 65 (63) |
| Partly controlled | 6 (12) | 13 (21) | 15 (16) | 25 (24) |
| Not controlled | 12 (24) | 7 (11) | 20 (21) | 13 (13) |
| C‐ACT(2‐17 y)c | ||||
| Asthma symptoms well‐controlled | 27 (73) | 27 (77) | 27 (73) | 27 (77) |
| Asthma symptoms not well‐controlled | 10 (27) | 8 (22) | 10 (27) | 8 (22) |
| Readmission | 4 (8) | 10 (16) | 15 (15) | 13 (13) |
| PICU readmission | 2 (4) | 1 (2) | 6 (6) | 1 (1) |
| ED visits | ||||
| None | 37 (74) | 49 (79) | 74 (75) | 87 (85) |
| 1 Visit | 7 (14) | 7 (11) | 13 (13) | 10 (10) |
| >1 Visits | 6 (12) | 6 (10) | 12 (12) | 6 (6) |
Note: Responders = completed the questionnaires during follow‐up. Data presented as number (percentage), only age is presented as median (IQR). *P < .05. ** P < .001.
Abbreviations: C‐ACT, Childhood Asthma Control Test; ED, emergency department; GINA , Global Initiative for Asthma; GW, general ward; IQR, interquartile range; LOS, length of stay; PICU, pediatric intensive care.
Postal code was used to quantify neighborhood socioeconomic status (SES), with a mean of zero. A lower (negative) score is associated with a lower SES.
Asthma symptom control: asthma symptoms during preceding month (nocturnal awakenings, wheezing more than two times per week, bronchodilator use more than two times per week, exercise‐induced symptoms), assessed by a professional. Well‐controlled = no symptoms, partly controlled = one to two symptoms and uncontrolled if three to four of these symptoms.
C‐ACT = a score of less than 20 indicates that asthma was not well‐controlled, reported by the patient and/or parent.
3.1. Medical status at time of follow‐up
Asthma treatment level was significantly higher in the PICU group compared with the GW group at the time of follow‐up, whereas asthma symptoms control was comparable (Table 1). PICU (re)admission after the index admission was more frequent in the PICU group compared with the GW group.
3.2. Quality of life and psychosocial outcomes child in the group of responders
Self‐reported asthma‐related QoL did not significantly differ between PICU and GW children (Table 2). PICU children scored a 1.7 on a VAS scale of 10, compared to a score of 2.3 given by GW children. Activity limitations, emotional functions, and symptoms were comparable between both groups. PICU children had higher scores on the CRTI questionnaire regarding posttraumatic stress, which implies worse functioning.
Table 2.
Quality of life and psychosocial functioning child (self‐reported)
| Questionnaire | N | PICU | N | GW |
|---|---|---|---|---|
| Asthma‐related quality of life (PAQLQ)a | ||||
| Activity limitations | 28 | 6.0 (1.3) | 9 | 5.6 (1.1) |
| Emotional function | 28 | 6.3 (1.2) | 9 | 6.3 (0.7) |
| Symptoms | 28 | 6.1 (1.3) | 9 | 5.8 (1.) |
| Trouble given by asthma (VAS) | 32 | 1.7 (2.2) | 19 | 2.3 (2.3) |
| Posttraumatic stress (CRTI)b | ||||
| Intrusion | 17 | 11.9 (7.6) | 5 | 7.8 (1.8) |
| Avoidance | 17 | 18.7 (12.6) | 5 | 17.2 (6.6) |
| Arousal | 17 | 11.8 (6.7) | 5 | 8.2 (3.2) |
| Other child‐specific responses | 17 | 17.8 (10.0) | 5 | 14.8 (4.8) |
| Total score | 17 | 60.1 (35.5) | 5 | 48 (14.2) |
| PTSD—intrusion | 17 | 9.1 (5.7) | 5 | 5.8 (1.8) |
| PTSD—avoidance | 17 | 12.1 (8.0) | 5 | 12.4 (5.3) |
| PTSD—arousal | 17 | 9.7 (5.6) | 5 | 7.0 (3.1) |
| PTSD—total score | 17 | 30.9 (18.5) | 5 | 8.2 (3.7) |
| PTSD—three times a score of 4 or 5 | 17 | 5 (29) | 5 | 1 (20) |
| Emotional and behavioral problems (YSR)c | ||||
| Internalizing | 10 | 7.3 (5.9) | 1 | 7 (⋯) |
| Externalizing | 10 | 8.1 (4.1) | 1 | 12 (⋯) |
| Total problem score | 10 | 39.4 (14.3) | 1 | 60 (⋯) |
Note: Data are in mean (SD). Intrusion between 7 and 49, avoidance 11 and 55, arousal 6 and 30, other child‐specific responses between 10 and 50. High scores imply worse functioning. The overall score of the PTSD CRTI will be between 17 and 85. PTSD Intrusion between 5 and 25, PTSD avoidance 7 and 35, PTSD arousal 5 and 25. Three times a PTSD score of 4 or 5 implicates that the child needs professional help.
Abbreviations: CBCL, Child Behavior Checklist; GW, general ward; PAQLQ, Paediatric Asthma Quality of Life Questionnaire; PICU, pediatric intensive care; PTSD, posttraumatic stress disorder; SD, standard deviation; VAS, Visual Analog Scale.
PAQLQ = overall score between 1 and 7. Low scores imply worse functioning. VAS = score between 0 and 10. High scores imply worse functioning.
The overall score of the Children's Responses to Trauma Inventory (CRTI) will be between 34 and 170.
CBCL = items of these questionnaires were rated on a 3‐point scale (0 = not true; 1 = somewhat or sometimes true; 2 = very true/often true).
Time to reach full schooldays after hospital discharge was significantly longer in the PICU group, with a mean of 10 days in the PICU group compared to a mean of 4 days in the GW group (Table 3). Parent‐reported PTS symptoms and emotional and behavioral problems in their child after admission to a PICU or GW were comparable between groups (Table 3). In both groups, 26% of responders had limitations in social activities.
3.3. Quality of life and psychosocial outcomes parents in the group of responders
In multivariable analyses, PTS symptom scores for intrusion, avoidance, arousal, and total PTS scores were significantly worse in parents of children admitted to a PICU compared with those admitted to the GW (Table 4). Almost half of all parents reported absence from work in both groups, due to their child's asthma.
4. DISCUSSION
This nationwide multicenter prospective study is the first to investigate asthma‐related QoL and psychosocial outcomes in children with SAA after PICU admission compared with children after GW admission, and parents. No differences were found between the PICU and the GW group in children themselves on the different outcome domains, except for the number of days to reach full schooldays. Parents of children after PICU admission reported more PTS symptoms than the GW group. Furthermore, almost one‐third of all PICU children were readmitted or had unscheduled ED visits soon after their PICU admission, suggesting that these children were still at risk for SAA.
In our study, we found that 3/39 (8%) of the PICU parents met PTSD criteria after PICU admission of their child, compared to 1/51 (2%) of parents of GW children. A study on PTSD in parents after an unexpected PICU treatment of their child showed a similar PTSD rate of 11% after 9 months. 7 Multiple studies, performed in heterogeneous PICU populations, have identified PTSD in approximately 13% to 27% of parents. 7 That parents of children in the PICU group were more likely to report symptoms of PTS, compared to parents of children in the GW group, is in line with previous outcome studies after PICU admission in general. These studies showed that parental PTSD was not strongly related to severity of illness, but more to parents’ perceptions of the threat to their child's life and to acute stress responses during PICU treatment. 7 , 35 , 36 A PICU admission might confront parents with the perception that asthma in their child might be life‐threatening. Avoidance, one of the PTS symptoms, represents an effort to withdraw the attention from certain situations that bring back trauma‐related symptoms. Such a mechanism may induce parents to over‐report well‐controlled asthma, whereas children were clearly at risk for SAA. 37 In line, another study reported on the association between maternal mental wellbeing and a constellation of beliefs and attitudes that may significantly influence adherence to asthma medication and illness management, 37 indicating that psychosocial problems of parents might be a risk factor for poorer asthma control in their child.
As to PTSD in children, a study in the general PICU population (children aged 5‐18 years) showed a PTSD rate in children of 4/19 (21%) compared to 0% of GW children. 36 These results are similar compared to a study in adolescents who experienced a life‐threatening asthma‐related event: 20% of the adolescents met criteria for PTSD. 13 However, some adolescents experienced their last event (PICU admission) up to 10 years previously, which makes causation difficult to prove. In our study, we found a similar number of children (21%) who met PTSD criteria after PICU admission, although parentally reported. This suggests that the child's perception of the life‐threatening event and acute stress response during PICU treatment is determinative for developing PTSD, regardless of the underlying disease.
Psychosocial outcomes such as asthma‐related QoL and emotional and behavioral problems were comparable between SAA children after PICU and GW admission. This could be due to the fact that children with SAA in the Dutch setting had a relatively short average PICU stay due to the low severity of illness. Self‐reported asthma‐related QoL was comparable, but due to the small sample size, this should be considered as indicative only.
5. IMPLICATIONS
To improve psychosocial outcomes after PICU admission, our findings suggest to focus on two domains. First, we need to improve current strategies to optimize asthma control and improve asthma‐related QoL, with the ultimate aim to prevent future (PICU) admissions in asthmatic children. Evidence suggests that improving physician access after hospital admission, with individual discharge plans, is beneficial in preventing hospital readmissions. 38 , 39 Future studies should focus on identifying children at risk for severe asthma symptoms and hospital admission to provide targeted follow‐up and management to prevent hospital admission and morbidity.
In our study a third of all children were readmitted or visited the ED, suggesting we need to monitor these children on a more regular base. For example, a follow‐up visit 1 to 2 weeks after (PICU) admission (instead of 6 weeks), or weekly phone calls, together with education about avoidable risk factors and recognizing early symptoms of SAA might support parents in managing their child's asthma. Furthermore, mobile phone apps, to provide general asthma information, and for tracking medications and symptoms might improve asthma self‐management. 40 , 41
Second, parents in the PICU group showed more posttraumatic stress symptoms. PTS symptoms, and especially PTSD, in parents of PICU children, have been associated with functional impairment, chronicity of symptoms, high psychiatric comorbidity psychiatric, and medical disorders. 7 Psychosocial problems of parents can be a risk factor for poorer asthma control in their child. Therefore, it is essential to identify and address parental psychological problems to facilitate parent‐provider communication and to optimize the child's asthma management and medication adherence. A standardized follow‐up after an acute and unexpected PICU admission due to SAA, for both child and parent, is mandatory to identify those children and parents who might be at risk for developing PTSD. If necessary, child and parents should be referred for interventions to prevent or reduce PTS symptoms. The 2017 Dutch Pediatric Society guideline for standardized follow‐up after acute and unexpected PICU admission, which is currently being implemented, recommends a follow‐up visit 3 to 6 months after PICU discharge by both a pediatrician and psychologist. 42
Psychological support of children and parents during PICU admission might reduce acute stress responses and future PTSD. In a recent meta‐analysis, combined intervention effects significantly reduced parent anxiety and stress but not depression. Interventions included at least one of the following elements: education, emotion regulation, and social or structural support. Coping support interventions can alleviate parents’ psychological distress during children's hospitalization. Limitations included high heterogeneity among included studies and most included studies were conducted at single centers with small sample sizes. 43 More evidence is needed to determine whether such interventions benefit children who are admitted with acute severe asthma.
5.1. Limitations/strengths
To our knowledge, this is the first study on psychosocial outcomes in SAA children after PICU admission. All Dutch PICUs participated, which strengthens external validity. PICU admission criteria were comparable between the PICUs, which facilitated comparisons between PICUs. There are some limitations as well. First, a considerable number of parents did not respond or refused to fill out the web‐based questionnaires. This may have biased the results. Children were significantly younger and more likely to be Caucasian in the responders' group compared to the nonresponders group. There were no differences between levels of asthma control in the two groups at follow‐up, and, therefore, the nonresponse seemed not differential and hence of less concern. Second, we did not collect data on the PICU environment, such as the number of patients in the same room, which might influence illness perception. The PICU is a dynamic environment, full of traumatizing events that influence both child and parents. Evaluating these environmental exposures may help us understand who is at risk for developing PTSD.
Third, asthma‐related school absenteeism might be subject to recall bias. For future studies, assessing school absence more objectively by, for instance, contacting schools, might increase the reliability of this outcome variable. Last, our results might not apply to other countries with different healthcare systems or treatment algorithms, and this limits external validity.
6. CONCLUSION
No significant differences were found in children on the self‐reported outcome domains, except for the time to reach full schooldays. Parents of children after PICU admission reported PTS symptoms more often than the GW group, even though the PICU group had a short PICU stay, a low intervention rate, and low mortality. We recommend that monitoring asthma symptoms and psychosocial screening of children and parents after PICU admission should both be part of standard care after SAA. This should identify those who are at risk for developing PTSD, to timely provide appropriate interventions.
ACKNOWLEDGMENTS
This study was financially supported by the Dutch Foundation for Asthma Prevention (Stichting Astma Bestrijding), Ammodo (Institute of Art and Science), unrestricted grants of Chiesi Pharmaceuticals BV Netherlands and Novartis Pharma, B.V. The Netherlands.
Research consortium SKIC members (Dutch Collaborative PICU Research Network):
Amsterdam University Medical Centers, Amsterdam, The Netherlands: Sabien Heisterkamp, Job van Woensel, Eric Haarman and Berber Kapitein; Wilhelmina Children's Hospital/University Medical Center Utrecht, Utrecht, The Netherlands: Roelie Wösten‐van Asperen; Beatrix Children's Hospital/University Medical Center Groningen, Groningen, The Netherlands: Martin Kneyber; University Medical Center Nijmegen, Nijmegen, The Netherlands: Joris Lemson and Stan Hartman; Maastricht University Medical Center, Maastricht, The Netherlands: Dick van Waardenburg; Leiden University Medical Center, Leiden, The Netherlands: Heleen Bunker and Carole Brouwer; Tergooi Hospital, Blaricum, The Netherlands: Bart van Ewijk; Rijnstate Hospital, Arnhem, The Netherlands: Anneke Landstra; Maasstad Hospital, Rotterdam, The Netherlands: Mariel Verwaal; and Amphia Hospital, Breda, The Netherlands: Anja Vaessen‐Verberne and Sanne Hammer.
Boeschoten SA, Dulfer K, Boehmer ALM, et al. Quality of life and psychosocial outcomes in children with severe acute asthma and their parents. Pediatric Pulmonology. 2020;55:2883–2892. 10.1002/ppul.25034
REFERENCES
- 1. Tse SM, Samson C. Time to asthma‐related readmission in children admitted to the ICU for asthma. Pediatr Crit Care Med. 2017;18(12):1099‐1105. [DOI] [PubMed] [Google Scholar]
- 2. Hartman ME, Linde‐Zwirble WT, Angus DC, Watson RS. Trends in admissions for pediatric status asthmaticus in New Jersey over a 15‐year period. Pediatrics. 2010;126(4):e904‐e911. [DOI] [PubMed] [Google Scholar]
- 3. Bratton SL, Odetola FO, McCollegan J, Cabana MD, Levy FH, Keenan HT. Regional variation in ICU care for pediatric patients with asthma. J Pediatr. 2005;147(3):355‐361. [DOI] [PubMed] [Google Scholar]
- 4. Hasegawa K, Ahn J, Brown MA, et al. MARC‐37 Investigators . Underuse of guideline‐recommended long‐term asthma management in children hospitalized to the intensive care unit: a multicenter observational study. Ann Allergy Asthma Immunol. 2015;115(1):10‐16. e11. [DOI] [PubMed] [Google Scholar]
- 5. Boeschoten SA, Buysse CMP, Merkus P, et al. SKIC Dutch collaborative PICU research network . Children with severe acute asthma admitted to Dutch PICUs: A changing landscape. Pediatr Pulmonol. 2018;53(7):857‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chiang BL, Hsieh CT, Wang LC, et al. Clinical course and outcome of children with status asthmaticus treated in a pediatric intensive care unit: a 15‐year review. J Microbiol Immunol Infect. 2009;42(6):488‐493. [PubMed] [Google Scholar]
- 7. Bronner MB, Peek N, Knoester H, Bos AP, Last BF, Grootenhuis MA. Course and predictors of posttraumatic stress disorder in parents after pediatric intensive care treatment of their child. J Pediatr Psychol. 2010;35(9):966‐974. [DOI] [PubMed] [Google Scholar]
- 8. Bronner MB, Knoester H, Bos AP, Last BF, Grootenhuis MA. Follow‐up after paediatric intensive care treatment: parental posttraumatic stress. Acta Paediatr. 2008;97(2):181‐186. [DOI] [PubMed] [Google Scholar]
- 9. Watson RS, Choong K, Colville G, et al. Life after critical illness in children‐toward an understanding of pediatric post‐intensive care syndrome. J Pediatr. 2018;198:16‐24. [DOI] [PubMed] [Google Scholar]
- 10. Rennick JE, Morin I, Kim D, Johnston CC, Dougherty G, Platt R. Identifying children at high risk for psychological sequelae after pediatric intensive care unit hospitalization. Pediatr Crit Care Med. 2004;5(4):358‐363. [DOI] [PubMed] [Google Scholar]
- 11. Nelson LP, Gold JI. Posttraumatic stress disorder in children and their parents following admission to the pediatric intensive care unit: a review. Pediatr Crit Care Med. 2012;13(3):338‐347. [DOI] [PubMed] [Google Scholar]
- 12. Rennick JE, Dougherty G, Chambers C, et al. Children's psychological and behavioral responses following pediatric intensive care unit hospitalization: the caring intensively study. BMC Pediatr. 2014;14:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kean EM, Kelsay K, Wamboldt F, Wamboldt MZ. Posttraumatic stress in adolescents with asthma and their parents. J Am Acad Child Adolesc Psychiatry. 2006;45(1):78‐86. [DOI] [PubMed] [Google Scholar]
- 14. Boeschoten SA, Boehmer AL, Merkus PJ, et al. Risk factors for intensive care admission in children with severe acute asthma in the Netherlands: a prospective multicentre study. ERJ Open Research. 2020;6(3):00126‐2020 10.1183/23120541.00126-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med. 2017;18(10):915‐923. [DOI] [PubMed] [Google Scholar]
- 16. Newth CJL, Khemani RG, Jouvet PA, Sward KA. Mechanical ventilation and decision support in pediatric intensive care. Pediatr Clin North Am. 2017;64(5):1057‐1070. [DOI] [PubMed] [Google Scholar]
- 17. Derderian SC, Good R, Vuille‐Dit‐Bille RN, Carpenter T, Bensard DD. Central venous lines in critically ill children: thrombosis but not infection is site dependent. J Pediatr Surg. 2019;54(9):1740‐1743. [DOI] [PubMed] [Google Scholar]
- 18. Fivez T, Kerklaan D, Mesotten D, et al. Early versus late parenteral nutrition in critically ill children. N Engl J Med. 2016;374(12):1111‐1122. [DOI] [PubMed] [Google Scholar]
- 19. Nederlandse Vereniging voor Kindergeneeskunde N , https://www.nvk.nl/Portals/0/richtlijnen/acuut%20astma/Methodenacuutastma.pdf. 2012.
- 20. Qureshi F, Pestian J, Davis P, Zaritsky A. Effect of nebulized ipratropium on the hospitalization rates of children with asthma. N Engl J Med. 1998;339(15):1030‐1035. [DOI] [PubMed] [Google Scholar]
- 21. Nederlandse Vereniging voor Kindergeneeskunde, (NVK) , https://www.nvk.nl/
- 22.Global Initiative for Asthma. https://ginasthma.org/gina-reports/. 2019.
- 23. Liu AH, Zeiger R, Sorkness C, et al. Development and cross‐sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119(4):817‐825. [DOI] [PubMed] [Google Scholar]
- 24. Raat H, Bueving HJ, de Jongste JC, Grol MH, Juniper EF, van der Wouden JC. Responsiveness, longitudinal‐ and cross‐sectional construct validity of the Pediatric Asthma Quality of Life Questionnaire (PAQLQ) in Dutch children with asthma. Qual Life Res. 2005;14(1):265‐272. [PubMed] [Google Scholar]
- 25. Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Qual Life Res. 1996;5(1):27‐34. [DOI] [PubMed] [Google Scholar]
- 26. Juniper EF, Guyatt GH, Feeny DH, Griffith LE, Ferrie PJ. Minimum skills required by children to complete health‐related quality of life instruments for asthma: comparison of measurement properties. Eur Respir J. 1997;10(10):2285‐2294. [DOI] [PubMed] [Google Scholar]
- 27. McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18(4):1007‐1019. [DOI] [PubMed] [Google Scholar]
- 28. Alisic E, Kleber RJ. Measuring post‐traumatic stress reactions in children: a preliminary validation of the children's responses to trauma inventory. Journal of Child & Adolescent Trauma. 2010;3:192‐204. [Google Scholar]
- 29. Alisic E, van der Schoot TA, van Ginkel JR, Kleber RJ. Looking beyond posttraumatic stress disorder in children: posttraumatic stress reactions, posttraumatic growth, and quality of life in a general population sample. J Clin Psychiatry. 2008;69(9):1455‐1461. [DOI] [PubMed] [Google Scholar]
- 30. Achenbach TM, Rescorla LA. Manual for the ASEBA school‐age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2001. [Google Scholar]
- 31. Achenbach TM, Rescorla LA. Manual for the ASEBA preschool forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2000. [Google Scholar]
- 32. van Zellem L, Utens EM, Legerstee JS, et al. Cardiac arrest in children: long‐term health status and health‐related quality of life. Pediatr Crit Care Med. 2015;16(8):693‐702. [DOI] [PubMed] [Google Scholar]
- 33. Hovens JE, Bramsen I, van der Ploeg HM. Self‐rating inventory for posttraumatic stress disorder: review of the psychometric properties of a new brief Dutch screening instrument. Percept Mot Skills. 2002;94(3 Pt 1):996‐1008. [DOI] [PubMed] [Google Scholar]
- 34. Hovens JE, van der Ploeg HM, Bramsen I, Reuling IE. Test‐retest reliability of the self‐rating inventory for posttraumatic stress disorder. Psychol Rep. 2000;87(3 Pt 1):735‐737. [DOI] [PubMed] [Google Scholar]
- 35. Bronner MB, Knoester H, Bos AP, Last BF, Grootenhuis MA. Posttraumatic stress disorder (PTSD) in children after paediatric intensive care treatment compared to children who survived a major fire disaster. Child Adolesc Psychiatry Ment Health. 2008;2(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rees G, Gledhill J, Garralda ME, Nadel S. Psychiatric outcome following paediatric intensive care unit (PICU) admission: a cohort study. Intensive Care Med. 2004;30(8):1607‐1614. [DOI] [PubMed] [Google Scholar]
- 37. Bartlett SJ, Krishnan JA, Riekert KA, Butz AM, Malveaux FJ, Rand CS. Maternal depressive symptoms and adherence to therapy in inner‐city children with asthma. Pediatrics. 2004;113(2):229‐237. [DOI] [PubMed] [Google Scholar]
- 38. Auger KA, Kahn RS, Davis MM, Beck AF, Simmons JM. Medical home quality and readmission risk for children hospitalized with asthma exacerbations. Pediatrics. 2013;131(1):64‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hall KK, Petsky HL, Chang AB, O'Grady KF. Caseworker‐assigned discharge plans to prevent hospital readmission for acute exacerbations in children with chronic respiratory illness. Cochrane Database Syst Rev. 2018;11:CD012315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mulvaney SA, Ho YX, Cala CM, et al. Assessing adolescent asthma symptoms and adherence using mobile phones. J Med Internet Res. 2013;15(7):e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Teufel IiRJ, Patel SK, Shuler AB, et al. Smartphones for real‐time assessment of adherence behavior and symptom exacerbation for high‐risk youth with asthma: pilot study. JMIR Pediatr Parent. 2018;1(2):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dutch Pediatric Society . https://www.nvk.nl/Kwaliteit/Richtlijnen-overzicht/Details/articleType/ArticleView/articleId/1831/Follow-up-van-kinderen-na-opname-op-een-intensive-care. 2017.
- 43. Doupnik SK, Hill D, Palakshappa D, et al. Parent coping support interventions during acute pediatric hospitalizations: a meta‐analysis. Pediatrics. 2017;140:3. [DOI] [PMC free article] [PubMed] [Google Scholar]


