Abstract
Targeted biological therapies may achieve maximal therapeutic efficacy at doses below the maximum tolerated dose (MTD); therefore, the search for the MTD in clinical studies may not be ideal for these agents. Emactuzumab is an investigational monoclonal antibody that binds to and inhibits the activation of the cell surface colony‐stimulating factor‐1 receptor. Here, we show how modeling target‐mediated drug disposition coupled with pharmacodynamic end points was used to optimize the dose of emactuzumab without defining an MTD. The model could be used to recommend doses across different disease indications. The approach recommended an optimal biological dose of emactuzumab for dosing every 2 weeks (q2w) ≥ 900 mg, approximately three‐fold lower than the highest dose tested clinically. The model predicted that emactuzumab doses ≥ 900 mg q2w would achieve target saturation in excess of 90% over the entire dosing cycle. Subsequently, a dose of 1,000 mg q2w was used in the extension phase of a phase I study of emactuzumab in patients with advanced solid tumors or diffuse‐type tenosynovial giant cell tumor. Clinical data from this study were consistent with model predictions. The model was also used to predict the optimum dose of emactuzumab for use with dosing every 3 weeks, enabling dosing flexibility with respect to comedications. In summary, this work demonstrates the value of quantitative clinical pharmacology approaches to dose selection in oncology as opposed to traditional MTD methods.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Targeted therapies could achieve maximum efficacy at doses below the maximum tolerated dose (MTD); therefore, the traditional “maximum tolerated dose” approach to determine the recommended phase II dose may not be ideally suited to these agents.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ We established the optimal biological dose of emactuzumab using quantitative clinical pharmacology. We also linked biomarker depletion with the target saturation—allowing us to use target‐mediated drug disposition to provide dose/regimen recommendations.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ Modeling and simulation techniques using all available pharmacokinetic, pharmacodynamic, biomarker, and safety data identified an optimal biological dose that was substantially lower than the MTD.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ Quantitative clinical pharmacology provides a more robust guidance on the selection of dose and regimen for early to late stage clinical investigations with investigational agents. This represents a fundamental shift away from the conventional MTD approach.
Emactuzumab (RG7155) is a humanized monoclonal antibody (mAb) that inhibits the activation of the colony‐stimulating factor‐1 receptor (CSF1R) expressed on the surface of macrophages, monocytes, and other cell types. 1 CSF1R and its ligand CSF1 function to regulate the proliferation, survival, differentiation, and chemotaxis of tissue macrophages. 2 The presence of infiltrating macrophages in solid tumors predicts a poor prognosis in many tumor types. 3 , 4 Specifically, tumor‐associated macrophages (TAMs) of the M2 phenotype promote tumor growth through the synthesis of growth factors and angiogenic factors and the suppression of effector T‐cells. 5 Emactuzumab is designed to inhibit M2 polarized macrophages without affecting granulocyte‐macrophage colony‐stimulating factor dependent tumor‐killing M1 macrophages. Preclinical and clinical data have shown that emactuzumab effectively depletes CSF1R+ macrophages in tumor tissue and achieves objective clinical responses in patients with diffuse‐type tenosynovial giant cell tumor (DTGCT), also known as pigmented villonodular synovitis, a rare locally aggressive, neoplastic disease of the synovia of large joints characterized by overexpression of CSF1. 1 , 6 , 7 , 8
Emactuzumab has been investigated in a phase Ia/Ib dose escalation study with an expansion phase in patients with advanced solid tumors or DTGCT (NCT01494688). 7 , 8 A key initial aim of the study was to establish the maximum tolerated dose (MTD) and recommended phase II dose (RP2D). Although the MTD is useful for establishing the RP2D of cytotoxic drugs—based on the belief that efficacy is maximized with higher drug doses because the therapeutic effect and tolerability are believed to be linked—such an approach may be less useful for the dose optimization of targeted biological therapies. 9 Such agents may achieve maximum efficacy at doses well below the MTD, 10 although typically the highest dose tested is used as the RP2D. An alternative strategy to help guide the selection of the RP2D is to establish the optimal biological dose, defined as a dose that achieves the required drug exposure levels to modulate the target and results in clinical activity with a manageable safety profile. 11
Modeling and simulation of early stage clinical data can maximize the value of the available pharmacokinetic (PK), pharmacodynamic (PD), biomarker, and safety data to better characterize the relationship among drug dose, exposure, target effect, and ultimately clinical activity. 10 , 12 , 13 Such strategies can provide quantitative data to guide dose selection for early phase trials. This approach is particularly well suited to targeted biological therapies for which biomarkers related to the drug’s mechanism of action are usually readily available. PK/PD modeling has successfully been used to support the development of several mAbs 14 , 15 , 16 and model‐based approaches are endorsed by the US Food and Drug Administration (FDA)’s Critical Path Initiative. 17
Therapeutic IgG mAbs often exhibit complex, nonlinear PKs known as target‐mediated drug disposition (TMDD) whereby a significant proportion of the administered mAb interacts with high affinity to its target, which is reflected in its PK characteristics. Rapid elimination of the antibody from the circulation is observed at low doses with clearance decreasing with increasing doses of mAb as the target becomes increasingly saturated. 18 Several authors have successfully incorporated TMDD into population PK models for therapeutic monoclonal antibodies. 18 , 19 , 20 , 21 Here, we describe how we combined PK/PD modeling with a population PK model using data from an ongoing trial coupled with three biomarkers of CSF1R inhibition, CSF1 ligand, TAMs, and skin macrophages 8 to show that: (i) the optimal biological dose of emactuzumab was three times lower than the highest dose tested in the phase I dose escalation study, and (ii) the dosing schedule could be optimized across disease indications based upon this model. In order to thoroughly describe the PK under conditions where CSF1R was not saturated, all patients received a low “run‐in” dose of emactuzumab (translated from nonclinical investigation) 1 week before commencement of therapeutic treatment.
METHODS
Study design and patients
This study used PK/PD data obtained from and open‐label, multicenter phase Ia/Ib study of emactuzumab (NCT01494688) that investigated the safety, PK, and activity of emactuzumab in patients with advanced solid tumors or DTGCT. The results of this study have been reported in full elsewhere. 7 , 8 The study was conducted in two parts between July 26, 2012, and September 25, 2014. Part I followed a traditional 3 + 3 dose‐escalation design with patients administered i.v. emactuzumab every 2 weeks (q2w) as either monotherapy in all solid tumors (except for hepatocellular carcinoma, non‐small cell lung cancer, small cell lung cancer, gastric cancer, malignant melanoma, nonmetastatic, and locally controlled DTGCT; arm A) or in combination with weekly paclitaxel administered according to local practice in patients with locally advanced and/or metastatic ovarian and breast carcinoma (arm B). In both arms, all patients received a 100 mg run‐in dose of emactuzumab followed by doses of 100, 200, 400, 600, 900, 1,000, 1,350, 2,000, or 3,000 mg emactuzumab. Dosing every 3 weeks (q3w) was also investigated.
Study NCT01494688 was conducted in line with the principals of the Declaration of Helsinki. Informed consent was obtained from all patients enrolled to the study. The study was approved by the institutional review board at each study center.
PK data were also obtained from a second study in which patients with locally advanced and/or metastatic triple negative breast cancer, ovarian cancer, bladder cancer, gastric cancer, or soft tissue sarcoma received emactuzumab in combination with atezolizumab, an mAb against programmed cell death‐ligand 1 (NCT02323191). Patients in this dose‐escalation study were treated with i.v. emactuzumab (starting dose 500 mg) plus i.v. atezolizumab (1,200 mg) q3w.
Measurement of serum emactuzumab
Blood samples for PK assessments were taken predose and at the end of infusion on day 1 of all cycles with additional extensive sampling conducted during cycles 1 and 4 (at 1, 2, 3, 4, 5, 6, 24, 72–96, 168, 216, and 264 hours postinfusion start for q2w dosing and at 1, 3, 24, 72, 168, 264, 312, 432, and 480 hours postinfusion start for q3w dosing). Emactuzumab was measured in serum using an in‐house validated enzyme‐linked immunosorbent assay with a lower limit of quantification for emactuzumab of 1.5 ng/mL in 10% human serum.
PD assessments
Tumor and skin biopsies were taken at baseline (pretreatment) and pre‐dose at cycle 2 day 1 (C2D1) and analyzed by immunohistochemistry to quantify the levels of macrophages and CSF1R+ macrophages using antibodies specific for CD68, CD163, and CSF1R, as described previously. 7
General modeling methodologies
Concentration–time data and dosing information (dose, infusion length, infusion rate, and dosing time) were collected. Serum concentration–time profiles were used to estimate the following PK parameters in human using noncompartmental analysis (WinNonlin, version 6.2; Pharsight Corporation, Mountain View, CA): total drug exposure defined as area under the serum concentration–time curve to last (AUClast), total clearance (CLtotal), and observed maximum serum concentration (Cmax). Population PK/PD analysis was conducted using NonMEM version 7 (7.2 or 7.3; ICON, Dublin, Ireland). Data manipulation, graphical analysis, and model diagnostic analysis was conducted using R version 3.1 or greater (R Foundation, Vienna, Austria). Details on the modeling methodologies can be found in Supplementary Material .
Because the target for emactuzumab is a membrane receptor, the nonlinear elimination (NLE) pathway was assumed to be related to removal of drug via internalization of the target receptor. Because the internalization is capacity limited (maximal rate of metabolism (Vmax)), the parameters from the NLE component (TMDD) of the population PK model were used to predict the saturation of the target receptor. Once at capacity (Vmax), the Michaelis‐Menten (Km) component of the elimination pathway can be simplified thus:
At capacity, Vmax = 1 and therefore:
For each individual, the percentage target saturation at steady‐state can be estimated using their Km value and the observed plasma concentration of emactuzumab, assuming that the levels of emactuzumab in the tumor reflect those of the plasma.
Half‐maximal inhibitory concentration – model skin macrophages
A population PD model was created using CSF1R‐expressing or CD163‐expressing macrophages in skin biopsies as a surrogate marker of tumor macrophages. 8 The change in amount of skin CD163+ and CSF1R + macrophages at the time of the dose administered at the start of C2D1 compared with those at the baseline assessment was assessed relative to the levels of emactuzumab in the serum at C2D1.
RESULTS
Clinical pharmacokinetics
The population PK data set comprised 4,158 concentration‐time observations from 177 patients dosed with emactuzumab at doses from 100–3,000 mg. All patients received a single 100 mg run‐in dose of emactuzumab, administered before starting treatment at the cohort dose level, to characterize the anticipated nonlinear PK of the molecule. NCA of the PK data from patients treated in part I of the trial suggested that the PK of emactuzumab was nonlinear at doses of 100–600 mg.
Systemic exposure (AUClast) showed a greater than dose‐proportional increase over this dose range, accompanied by a decline in total clearance with ascending dose (Table 1 ), indicating that the elimination of emactuzumab is predominantly target‐mediated at these exposures. At doses of ≥ 900 mg emactuzumab, exposure increased approximately dose‐proportionally indicating saturation of target‐mediated elimination above this dose level. Systemic exposure of emactuzumab in combination with weekly paclitaxel (arm B) was comparable when emactuzumab was given in monotherapy (data not shown).
Table 1.
Clinical PK of emactuzumab following i.v. infusion as monotherapy or in combination with atezolizumab
| Emactuzumab dose | t 1/2 (days) | AUClast (h•μg/mL)/ dose (dose normalized) | Total Cl (mL/h) | Ctrough (µg/mL) |
|---|---|---|---|---|
| Monotherapy (study NCT01494688) | ||||
| 100 mg q2w (n = 36) a | 1.70 (47.8) | 18 (37.5) | 53.9 (70) | 2.96 (84) d |
| 200 mg q2w b (n = 1) | 5.08 (‐) | 8.0 (‐) | 120 (‐) | 11.30 (‐) |
| 400 mg q2w b (n = 6) | 6.45 (55.1) | 37 (28.6) | 22.9 (47.5) | 21.5 (51) |
| 600 mg q2w b (n = 5) | 6.20 (23.2) | 35 (19.0) | 22.8 (28.5) | 24.19 (54) |
| 900 mg q2w b (n = 6) | 7.88 (39.8) | 50 (26.5) | 12.7 (52.8) | 86.6 (22) |
| 1,350 mg q2w b (n = 3) | 8.04 (55.4) | 44 (36.0) | 17.2 (42.9) | 101 (46) |
| 2,000 mg q2w b (n = 6) | 7.80 (34.7) | 42 (29.4) | 15.1 (42.1) | 128 (33) |
| 3,000 mg q2w b (n = 6) | 7.92 (21.7) | 38 (42.7) | 20.0 (84.6) | 115 (45) |
| Combination with atezolizumab 1,200 mg q3w (study NCT02323191) | ||||
| 500 mg c (n = 5) | 4.79 (57.7) | 29.2 (42.2) | 41.3 (71.1) | 5.95 (45) |
| 1,000 mg c (n = 6) | 9.37 (27.6) | 42.5 (38.3) | 22.0 (45.2) | 33.0 (46) |
| 1,350 mg c (n = 6) | 9.34 (49.9) | 41.8 (30.9) | 22.1 (52.3) | 46.7 (47) |
Data are mean (%CV).
AUClast, area under the serum concentration–time curve to last; Cl, clearance; Ctrough, trough plasma concentration; PK, pharmacokinetic; t1/2, terminal half‐life.
Following single running i.v. dose administration.
Following second dose i.v. administration after the running dose of 100 mg.
Following single i.v. dose administration.
C1D1 since 100 mg was C0.
Graphical analysis of the population‐PK data set suggested that emactuzumab may undergo distribution beyond the central compartment. Furthermore, at lower doses, and during the 100 mg run‐in phase, there was evidence of NLE (TMDD; Figure 1 ). Consequently, a two‐compartmental PK model with both linear and NLE pathways was explored as a starting model. Both proportional (multiplicative) and combined (additive and proportional) error models were tested.
Figure 1.
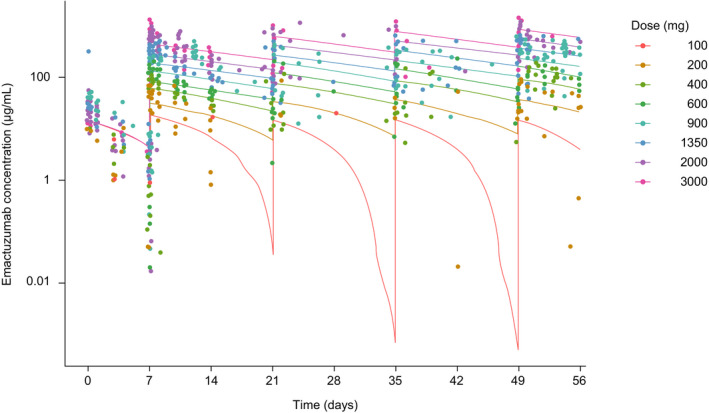
Observed and model‐predicted concentrations of emactuzumab monotherapy following repeated i.v. administration q2w. [Colour figure can be viewed at wileyonlinelibrary.com]
A two‐compartment PK model with both linear and nonlinear clearance pathways and proportional error was shown to best represent the available PK data. The nonlinear clearance pathway may be related to the target levels of receptor at the start of the treatment and could be considered as representing TMDD. The NLE in the two‐compartment model can also be used to predict the saturation of the target‐mediated elimination (a surrogate for target saturation). The observed and predicted PK profiles indicated that the contribution of TMDD to the total clearance begins to reduce at doses of 600 mg and reaches a minimum at doses ≥ 900 mg (Figure 1 ).
CD68 + CD163+ macrophage levels in paired tumor biopsy samples
Decreases (> 10%) in M2‐like TAM levels were observed from the dose escalation cohort (Figure 2a ) following 4 weeks of emactuzumab therapy. Depletion in macrophages appeared to be exposure and dose dependent. Most patients achieving exposure corresponding to a 900 mg dose showed TAM depletion within the range of 70% to 95%.
Figure 2.
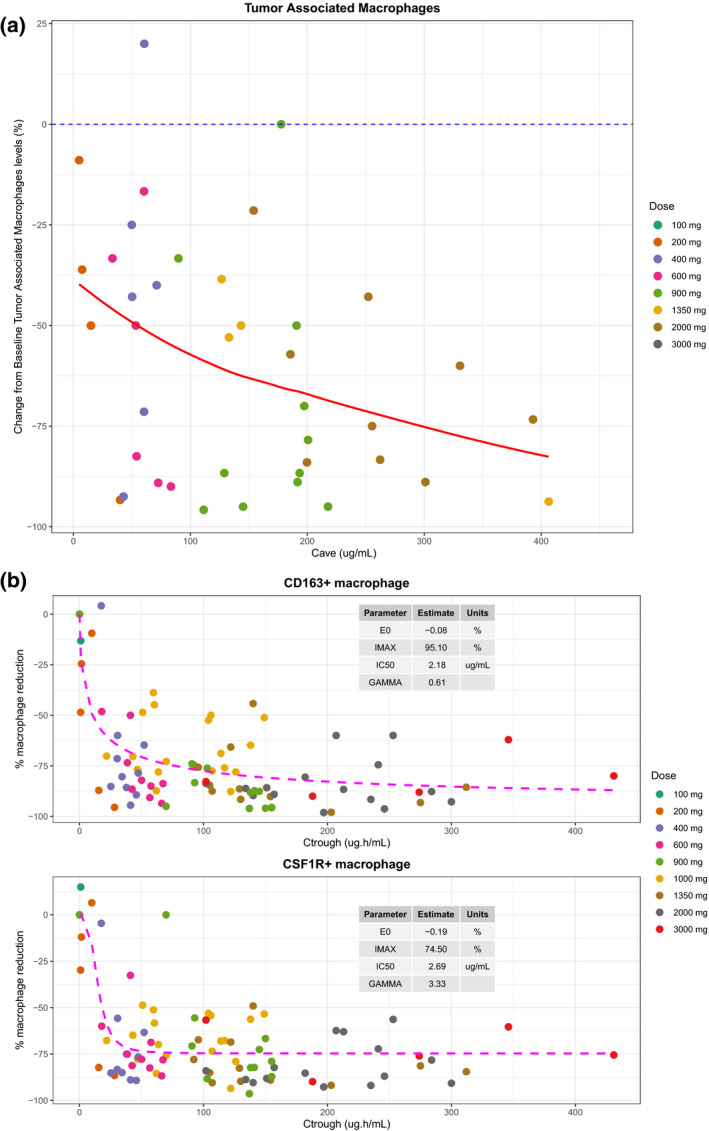
Pharmacodynamic changes in patients administered i.v. emactuzumab q2w according to dose and exposure. (a) Change from baseline in CD68+ CD163+ macrophage levels in paired tumor biopsy samples with exposure. (b) Change from baseline in CD163+ and colony‐stimulating factor‐1 receptor‐positive skin macrophages with exposure. [Colour figure can be viewed at wileyonlinelibrary.com]
CD163+/CSF1R+ skin macrophages
The objective was to estimate the maximal observed reduction in CD163+ and CSF1R+ skin macrophages and to estimate the concentration of emactuzumab in serum required to invoke a 50% depletion (half‐maximal inhibitory concentration).
The CD163 and CSF1R expression data were best represented with an inhibitory sigmoidal maximum effect (Emax) model with a baseline (E0) and a proportional error model, using trough plasma concentration (Ctrough) values (predose on C2D1) as the PK variable. These models predicted that reductions in CD163+ and CSF1R+ macrophages of ~ 50% were possible, with a corresponding half‐maximal inhibitory concentration value of 2.18 µg/mL (Figure 2b ). Data from a cohort of patients dosed with 200 mg and an expansion cohort dosed with 1,000 mg were included in the PD analysis. As can be seen from Figure 2b , the data from these cohorts fit into the concentration‐response curve, and therefore supports the hypothesis that the serum levels of emactuzumab and subsequent target saturation drives the PD response.
The results of the skin macrophages are consistent with the rapid and sustained CSF1 increase in the serum was observed on cycle 1, day 1 after emactuzumab administration, previously reported. 8 Therefore, skin macrophages were considered a robust biomarker to be linked to TMDD.
Linking tissue to PK and target saturation
By calculating the target saturation at time of sample collection (predose on C2D1), we could assess what level of saturation led to optimal reductions in CD163+ and CSF1R+ macrophages in the skin. Examination of the impact of 80%, 85%, 90%, 95%, and 99% saturation on the magnitude of the macrophage reduction revealed that patients reaching ≥ 90% target saturation, no further reduction in high powered field count was observed between baseline and post‐treatment at C2D1 (Figure 3 ). This was not the case when target saturation values were lower than 90% (80 or 85%; data not shown), indicating that ≥ 90% target saturation was optimal to achieve the maximum possible reduction in tumor macrophages.
Figure 3.
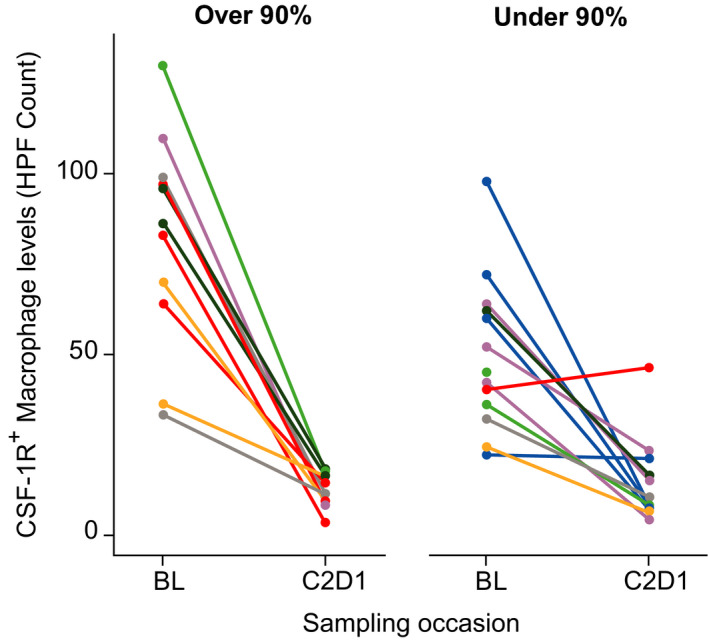
Change in HPF count of colony‐stimulating factor‐1 receptor‐positive skin macrophages at pre‐treatment reading (BL) and HPF count on cycle 2 day 1 in individual patients administered i.v. emactuzumab q2w according to degree of target saturation achieved (< 90% or > 90%). Colored lines represent individual patients. E0, baseline; HPF, high powered field; IC50, half‐maximal inhibitory concentration; Imax, maximum unbound systemic concentration. [Colour figure can be viewed at wileyonlinelibrary.com]
Calculation of target saturation at each time point for each patient suggested that for doses ≥ 900 mg q2w, the levels of saturation would be > 90% for the entire dosing cycle (Figure 4 ), which would lead to optimal reduction of the skin surrogate macrophages. Furthermore, this reflected the changes in AUC and terminal half‐life seen with higher doses following NCA, where doses of ≥ 900 mg displayed linear PK.
Figure 4.
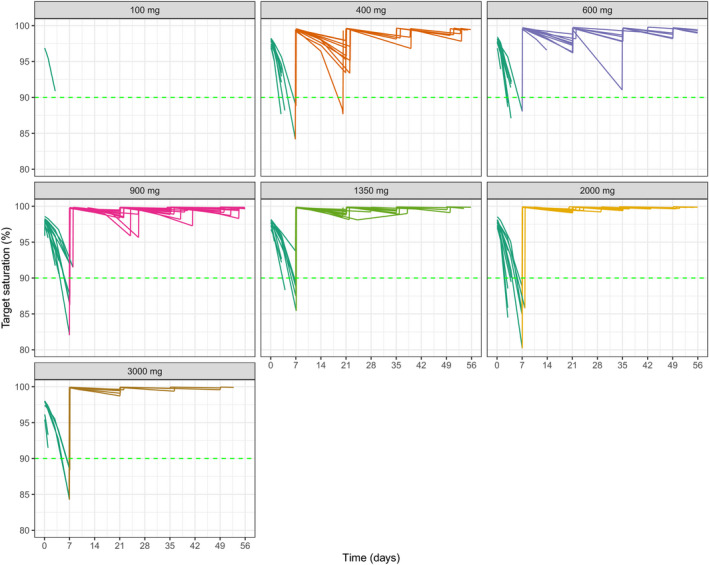
Estimated target saturation at different doses of i.v. emactuzumab (single dose administration). Green line indicates 90% saturation. BL, baseline; CSF, colony‐stimulating factor; HPF, high powered field. [Colour figure can be viewed at wileyonlinelibrary.com]
Application of the PK model to guide dose and schedule administration
As linear PK indicative of target saturation were observed at doses ≥ 900 mg q2w, and this level of emactuzumab achieved and maintained full depletion of tissue macrophages and peripheral biomarkers, the optimal biological dose of emactuzumab was defined as a dose of 1,000 mg q2w. This dose was recommended for administration for part II of the study in monotherapy, and in combination with paclitaxel for all indications, and was threefold lower than the highest dose tested in the dose escalation part of the trial.
In all patients treated to date with emactuzumab 1,000 mg q2w in part II of the study, the observed concentrations were consistent with the model predicted concentrations (Figure 5a ). In addition, the exposure and biomarker relationships from patients treated with 1,000 mg were within the plateau phase.
Figure 5.
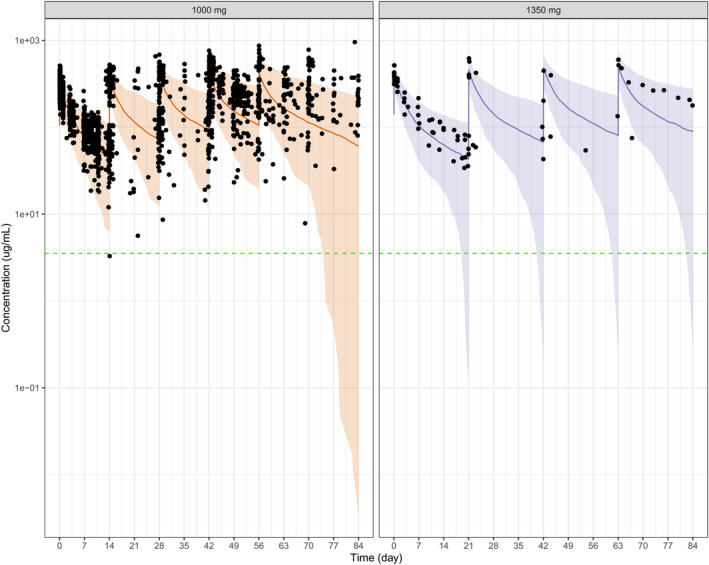
Observed and model predicted concentrations of emactuzumab following repeated i.v. administration of (a) 1,000 mg q2w and (b) 1,350 mg q3w. Green lines indicate median. Grey shaded regions indicate 95% prediction intervals. [Colour figure can be viewed at wileyonlinelibrary.com]
The TMDD model was also used to estimate the emactuzumab dose that would be needed for q3w dosing, consistent with most current standard‐of‐care regimens in the tumor types investigated. The model predicted that an emactuzumab dose of 1,350 mg q3w would show linear PK during the dosing interval for the majority (> 95%) of the patient population and to achieve systemic exposure consistent with ≥ 90% target saturation for the majority of the q3w dosing interval. Again, the emactuzumab concentrations observed in patients subsequently treated with 1,350 mg q3w were consistent with the model predictions indicating that patients will be exposed to systemic levels above the 90% target saturation required (Figure 5b ).
The q3w regimen for emactuzumab was also assessed in combination with atezolizumab (study NCT02323191). In the dose escalation part of this study, emactuzumab doses of 500 mg, 1,000 mg, and 1,350 mg q3w were investigated. Systemic exposure (AUClast) showed a greater than dose‐proportional increase from 500 mg to 1,000 mg accompanied by a decline in total clearance (range: 1,000–510 mL/day; Table 1 ), indicating that the elimination of emactuzumab was predominantly target‐mediated following 500 mg q3w. At doses above 1,000 mg, exposure increased in an approximately dose‐proportional manner, indicating that the target‐mediated elimination was saturated (data not shown). Systemic exposure of emactuzumab following administration of emactuzumab (1,000 mg and 1,350 mg) in combination with atezolizumab was similar to the systemic exposure observed in emactuzumab monotherapy. Data also indicated that a dose of 1,000 mg q3w reached target saturation levels above 95% (Figure 6 ).
Figure 6.

Observed and model predicted emactuzumab concentrations following repeated q3w i.v. administration (500 mg, 1,000 mg, and 1,350 mg) in combination with 1,200 mg q3w of atezolizumab (study NCT02323191). Lines indicate median. Shaded regions indicate 95% prediction intervals. [Colour figure can be viewed at wileyonlinelibrary.com]
DISCUSSION
Using quantitative clinical pharmacology, we showed that the optimal biological dose of emactuzumab for q2w dosing was ≥ 900 mg, approximately threefold lower than the highest dose tested in the trial (in which an MTD was not achieved). All available PK, TMDD, and tissue biomarker data were consistent in this prediction. It was previously reported that peripheral biomarkers, such as CSF1 ligand and CD14BRIGHT CD16DIM monocytes, reached plateau at lower systemic exposures/doses indicating the risk of selecting a suboptimal dose. 8 The exposure response relationship in tumor biopsies was complicated by higher variability observed in the tumor biopsies compared with the skin biopsies, partly due to the limited number of patients and tumor biopsies available per cohort. In addition, in some instances, baseline and post‐treatment tumor biopsies were taken from different tumor lesions due to lesion disappearance following treatment. Therefore, linking the TMDD model with macrophage depletion in surrogate skin enabled the model to be used as a surrogate for PD data, allowing us to also determine the optimal dose for use in q3w regimens with concomitant therapy (1,000 mg q3w).
Our data add to the growing body of evidence that indicates that the MTD approach may not be optimal for establishing the RP2D for targeted agents. The use of modeling and simulation techniques to guide dose selection of targeted therapy represents a paradigm shift in oncology. These techniques maximize the value of all available clinical data, which is particularly important in phase I trials where data are typically limited due to the small number of patients studied. PK/PD models can provide a broader understanding of the relationship between dose and PK/PD efficacy. This generates additional data with which to inform decision making in drug development, enabling predictions for phase II/III investigations to be made with greater confidence.
Any modeling and simulation exercise is an evolving process—models are constantly updated and predictions tested as new clinical data becomes available. 10 Modeling also enables drug development decisions to be made while trials are ongoing, without necessarily waiting for completion of a particular study. This includes making dose recommendations for use in combination therapy extension studies driven by two to three initial cohorts during a monotherapy trial. A novel approach was used in the first entry‐into‐human study where all patients received a single 100 mg run‐in dose of emactuzumab, administered before starting treatment at the cohort dose level, to properly characterize the anticipated nonlinear PK of the molecule. The data from the modeling exercises presented here were used to support the selection of the dose and schedule for the expansion part of the study in patients with advanced solid tumors or DTGCT (NCT01494688). 7 The expansion cohorts of study NCT01494688 used the recommended dose of 1,000 mg emactuzumab. This decision was made in line with all safety, efficacy, and PK/PD data, and after discussion with the investigators. The data from the expansion cohorts were consistent with the model predictions. The dose of 1,000 mg q2w emactuzumab showed remarkable efficacy in patients with DTGCT, providing further confirmation that these modeling exercises helped select an efficacious dose. 7
Using the lowest clinically efficacious dose of a therapeutic agent is clearly desirable. An unnecessarily high dose of a developmental drug may increase the risk of unexpected toxicity, which may only become apparent in later phase studies with larger patient cohorts. Time is also wasted in continuing a dose escalation where the incremental increase will ultimately prove noninformative. Such toxicity contributes to the high rate of dose modifications seen in phase III trials and postmarketing. 9 , 22 Using the optimal efficacious dose is also likely to reduce overall drug costs. No additional clinical benefit with respect to any of the PD biomarkers was seen at emactuzumab doses above 900 mg.
Selecting the biomarker most closely related to clinical efficacy remains an ongoing challenge. This work highlights the limitation of peripheral biomarkers, potentially leading to a suboptimal dose selection. However, the tissue biomarker together with the TMDD and the clinical efficacy observed in pigmented villonodular synovitis (proof of concept indication) indicated that when biomarkers are carefully selected, surrogate approaches, such as TMDD, could guide optimization of dose and schedule.
In conclusion, these data demonstrate the value of a PK/PD approach and TMDD model over the traditional MTD method for guiding dose selection for use in early phase I clinical trials for a novel therapeutic mAb.
Funding
This study and editorial support for the preparation of this manuscript was funded by Roche Diagnostics GmbH, Penzberg, Germany.
Conflict of Interest
All authors are employees of Roche Pharmaceuticals.
Author Contributions
K.S. and G.M.‐L. wrote the manuscript. K.S., D.R., A.P., A.‐C.W., M.B., M.C., and G.M.‐L. designed the research. K.S., A.‐M.B., C.M., C.R., M.C., and G.M.‐L. analyzed the data. K.S. and G.M.‐L. contributed new reagents/analytical tools.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
The authors would like to thank the entire study team at Roche for their assistance in the preparation of this manuscript. We thank Jamie Ashman, PhD, of Prism Ideas for Editorial support in the preparation of this manuscript.
References
- 1. Ries, C.H. et al. Targeting tumor‐associated macrophages with anti‐CSF‐1R antibody reveals a strategy for cancer therapy. Cancer Cell 25, 846–859 (2014). [DOI] [PubMed] [Google Scholar]
- 2. Pixley, F.J. & Stanley, E.R. CSF‐1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 14, 628–638 (2004). [DOI] [PubMed] [Google Scholar]
- 3. Biswas, S.K. , Allavena, P. & Mantovani, A. Tumor‐associated macrophages: functional diversity, clinical significance, and open questions. Semin. Immunopathol. 35, 585–600 (2013). [DOI] [PubMed] [Google Scholar]
- 4. Bingle, L. , Brown, N.J. & Lewis, C.E. The role of tumour‐associated macrophages in tumour progression: implications for new anticancer therapies. J. Pathol. 196, 254–265 (2002). [DOI] [PubMed] [Google Scholar]
- 5. Heusinkveld, M. & van der Burg, S.H. Identification and manipulation of tumor associated macrophages in human cancers. J. Transl. Med. 9, 216 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. West, R.B. et al. A landscape effect in tenosynovial giant‐cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc. Natl. Acad. Sci. USA 103, 690–695 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cassier, P.A. et al. CSF1R inhibition with emactuzumab in locally advanced diffuse‐type tenosynovial giant cell tumours of the soft tissue: a dose‐escalation and dose‐expansion phase 1 study. Lancet Oncol. 16, 949–956 (2015). [DOI] [PubMed] [Google Scholar]
- 8. Gomez‐Roca, C.A. et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2‐like macrophages. Ann. Oncol. 30, 1381–1392 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minasian, L. , Rosen, O. , Auclair, D. , Rahman, A. , Pazdur, R. & Schilsky, R.L. Optimizing dosing of oncology drugs. Clin. Pharmacol. Ther. 96, 572–579 (2014). [DOI] [PubMed] [Google Scholar]
- 10. Barrett, J.S. , Gupta, M. & Mondick, J.T. Model‐based drug development applied to oncology. Expert. Opin. Drug Discov. 2, 185–209 (2007). [DOI] [PubMed] [Google Scholar]
- 11. Adjei, A.A. What is the right dose? The elusive optimal biologic dose in phase I clinical trials. J. Clin. Oncol. 24, 4054–4055 (2006). [DOI] [PubMed] [Google Scholar]
- 12. Miller, R. et al. How modeling and simulation have enhanced decision making in new drug development. J. Pharmacokinet. Pharmacodyn. 32, 185–197 (2005). [DOI] [PubMed] [Google Scholar]
- 13. Tuntland, T. et al. Implementation of pharmacokinetic and pharmacodynamic strategies in early research phases of drug discovery and development at Novartis Institute of Biomedical Research. Front. Pharmacol. 5, 174 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agoram, B.M. , Martin, S.W. & van der Graaf, P.H. The role of mechanism‐based pharmacokinetic‐pharmacodynamic (PK‐PD) modelling in translational research of biologics. Drug Discov. Today 12, 1018–1024 (2007). [DOI] [PubMed] [Google Scholar]
- 15. Jumbe, N.L. et al. Modeling the efficacy of trastuzumab‐DM1, an antibody drug conjugate, in mice. J. Pharmacokinet. Pharmacodyn. 37, 221–242 (2010). [DOI] [PubMed] [Google Scholar]
- 16. Hu, C. , Wasfi, Y. , Zhuang, Y. & Zhou, H. Information contributed by meta‐analysis in exposure‐response modeling: application to phase 2 dose selection of guselkumab in patients with moderate‐to‐severe psoriasis. J. Pharmacokinet. Pharmacodyn. 41, 239–250 (2014). [DOI] [PubMed] [Google Scholar]
- 17. US Food and Drug Administration . FDA's Critical Path Initiative (2012) <http://www.fdagov/ScienceResearch/SpecialTopics/CriticalPathInitiative/ucm076689.htm>.
- 18. Mager, D.E. Target‐mediated drug disposition and dynamics. Biochem. Pharmacol. 72, 1–10 (2006). [DOI] [PubMed] [Google Scholar]
- 19. Mager, D.E. & Jusko, W.J. General pharmacokinetic model for drugs exhibiting target‐mediated drug disposition. J. Pharmacokinet. Pharmacodyn. 28, 507–532 (2001). [DOI] [PubMed] [Google Scholar]
- 20. Cao, Y. & Jusko, W.J. Incorporating target‐mediated drug disposition in a minimal physiologically‐based pharmacokinetic model for monoclonal antibodies. J. Pharmacokinet. Pharmacodyn. 41, 375–387 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luu, K.T. , Bergqvist, S. , Chen, E. , Hu‐Lowe, D. & Kraynov, E. A model‐based approach to predicting the human pharmacokinetics of a monoclonal antibody exhibiting target‐mediated drug disposition. J. Pharmacol. Exp. Ther. 341, 702–708 (2012). [DOI] [PubMed] [Google Scholar]
- 22. Cross, J. , Lee, H. , Westelinck, A. , Nelson, J. , Grudzinskas, C. & Peck, C. Postmarketing drug dosage changes of 499 FDA‐approved new molecular entities, 1980–1999. Pharmacoepidemiol. Drug Saf. 11, 439–446 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


