Abstract
Background
Transnasal Humidified Rapid‐Insufflation Ventilatory Exchange (THRIVE) using high‐flow 100% oxygen during apnoea has gained increased use during difficult airway management and laryngeal surgery due to a slower carbon dioxide rise compared to traditional apnoeic oxygenation. We have previously demonstrated high arterial oxygen partial pressures and an increasing arterial‐alveolar carbon dioxide difference during THRIVE. Primary aim of this study was to characterise lung volume changes measured with electrical impedance tomography during THRIVE compared to mechanical ventilation.
Methods
Thirty adult patients undergoing laryngeal surgery under general anaesthesia were randomised to THRIVE or mechanical ventilation. Subjects were monitored with electrical impedance tomography and repeated blood gas measurement perioperatively. The THRIVE group received 100% oxygen at 70 l min−1 during apnoea. The mechanical ventilation group was intubated and normoventilated with an FiO2 of 0.4.
Results
Mean age were 48.2 (19.9) and 51.3 (12.3) years, and BMI 26.0 (4.5) and 26.0 (3.9) in the THRIVE and mechanical ventilation group respectively. Mean apnoea time in the THRIVE group was 17.9 (4.8) min. Mean apnoea to end‐of‐surgery time was 28.1 (12.8) min in the mechanical ventilation group. No difference in delta End Expiratory Lung Impedance was seen between groups over time. In the THRIVE group all but three subjects were well oxygenated during apnoea. THRIVE was discontinued for the three patients who desaturated.
Conclusions
No difference in lung volume change over time, measured by electrical impedance tomography, was detected when using THRIVE compared to mechanical ventilation during laryngeal surgery.
Editorial Comment.
High‐flow transnasal oxygen insufflation can be used to support lung gas exchange with apnoeic oxygenation during laryngeal surgery. In this trial, lung volumes over time assessed by electrical impedence tomography and oxygenation with this treatment were shown to be similar to those with traditional mechanical positive pressure ventilation.
1. INTRODUCTION
Transnasal Humidified Rapid‐Insufflation Ventilatory Exchange (THRIVE) by high‐flow nasal oxygen is an advanced apnoeic oxygenation technique used under general anaesthesia. THRIVE has gained great interest in the management of difficult airways and to provide gas exchange during shorter laryngeal surgical procedures. 1 , 2 , 3 , 4 With this technique, the safe apnoeic time is prolonged while carbon dioxide rises significantly slower compared to traditional apnoeic oxygenation, which indicates a more effective carbon dioxide wash out. 2 , 4 , 5
Lung volumes during general anaesthesia and mechanical ventilation are reduced in most patients due to decreased functional residual capacity (FRC) and atelectasis formation. 6 The degree of atelectasis formation depends on several factors such as inspired fraction of oxygen and obesity. 7 , 8 The obese have an increased atelectasis formation during anaesthesia due to a lower functional residual capacity compared to individuals with normal BMI 9 and seem more prone to desaturation during apnoeic oxygenation. 10
Notably, little is known about respiratory physiology during apnoeic oxygenation using THRIVE. In a recent study of patients undergoing laryngeal surgery, we observed an increased difference in arterial and end‐tidal carbon dioxide in combination with high arterial oxygen levels during apnoea. 3 On the basis of these observations we hypothesised that there is increased formation of lung atelectasis due to absorption of oxygen and compression atelectasis during THRIVE. 11 Patients in the previous study had an upper BMI limit of 30, hence, we lack information on the effectiveness of THRIVE in patients with higher BMI.
The primary aim of the study was, therefore, to investigate the effect of THRIVE on lung volumes measured using electric impedance tomography compared to mechanical ventilation in patients undergoing laryngeal surgery. The secondary aims were to examine 1) if there were THRIVE‐induced lung volume changes post‐operatively and 2) describe blood gas changes during THRIVE when used in patients with a BMI up to 35.
2. METHODS
This study conforms to the standard of the Declaration of Helsinki and was approved by the Regional Ethics Committee on Human Research at the Karolinska Institutet, Stockholm, Sweden (Dnr 2017/1463‐31, 2017/2245‐32 and 2018/727‐32) and registered prospectively to patient enrolment at the US National Institutes of Health, #NCT03458091, 21st January 2018, principal investigator Malin Jonsson Fagerlund (www.clinicaltrial.gov). The trial was conducted according to Good Clinical Practice and the CONSORT guidelines at the Karolinska University Hospital, Stockholm, Sweden, between January 2018 and October 2018. Patients were included after oral and written informed consent.
Thirty adult patients (>18 years), American Society of Anesthesiologists (ASA) physical status 1 or 2, scheduled to undergo microlaryngoscopy under general anaesthesia planned to last less than 30 minutes, were included in this prospective randomised trial. Participants were sequentially enrolled pre‐operatively by an anaesthetist responsible for data collection in the study. Exclusion criteria were ASA score >2, New York Heart Association class >2, BMI >35, pregnancy, severe gastrointestinal reflux, previous enrolment in the study and neuromuscular disease. The patients were randomised to either THRIVE or mechanical ventilation. Randomisation was concealed and first revealed after inclusion.
Standard vital signs monitoring (Aisys CS, GE Healthcare, United States) was used perioperatively. Changes in lung volumes were assessed by delta End Expiratory Lung Impedance (dEELI) using electrical impedance tomography (EIT) (Pulmovista 500, Dräger, Kista, Sweden), calculating dEELI in four different regions of interests (ROI´s 1‐4). ROI 1 being most ventral, ROI 2 midventral, ROI 3 middorsal and ROI 4 most dorsal. An electrode belt with 16 electrodes was placed around the thorax at intercostal level 6 pre‐operatively with an unaltered position throughout the perioperative period. EIT was continuously monitored during the perioperative period. Measures of dEELI, with T1 set as baseline prior to pre‐oxygenation, were registered in 5 minutes intervals during anaesthesia and emergence from anaesthesia and in 15 minutes intervals in the post‐operative period.
All patients received a radial arterial catheter and two peripheral venous catheters prior to induction of anaesthesia. Arterial blood gases were taken before and after pre‐oxygenation followed by every 5 minutes during anaesthesia and post‐operatively. Blood gases were analysed using an ABL 90 (Radiometer Medical ApS, Brønshøj, Denmark). End‐tidal carbon dioxide (ETCO2) was measured by facemask breathing before pre‐oxygenation and immediately at termination of apnoea in the THRIVE group and continuously in the mechanical ventilation (MV) group. Adductor pollicis train‐of‐four (TOF) ratio or post‐tetanic count (PTC) was used to assess the degree of neuromuscular blockade by stimulation of the ulnar nerve (Aisys CS, GE Healthcare, United States).
On the operating table, patients were placed supine with their head elevated approximately 20 degrees. Patients in the THRIVE group were pre‐oxygenated with Optiflow™ (Fisher & Paykel Healthcare, Auckland, New Zealand) using 100% oxygen at 40 l min−1 for 3 min. Patients in the MV group were pre‐oxygenated with a tight‐fitting face mask, 100% oxygen at 10 l min−1 for 3 minutes (Aisys CS, GE Healthcare, United States). Anaesthesia was induced by using target controlled intravenous infusion (Alaris® PK Syringe pump, Cardinal health, Rolle, Switzerland) using propofol (Propofol‐Lipuro®, B. Braun Melsungen AG, Melsungen, Germany) Cpt 6 µg ml−1 for induction and 3 µg ml−1 for maintenance and remifentanil (Ultiva®, GlaxoSmithKline AB, Solna, Sweden), Cpt 6 ng ml−1 for induction and 3 ng ml−1 for maintenance. Rocuronium (Esmeron®, MSD, Haarlem, Netherlands) 1 mg kg−1 was administered iv to achieve deep neuromuscular blockade.
After anaesthesia induction, at apnoea onset in the THRIVE group, oxygen flow was increased to 70 l min−1 and 100% oxygen, which was maintained at this level throughout the apnoeic period. The airway was kept patent using jaw thrust until a rigid tubular laryngoscope was put in place in full suspension, ensuring a part of the laryngeal inlet open to oxygen flow at all times. The apnoeic period in the THRIVE group was defined as termination of spontaneous breathing monitored by EIT until mask ventilation was started after completion of the procedure, or when discontinuation criteria occurred. THRIVE discontinuation criteria were PaCO2 > 11 kPa, pH < 7.15, SpO2 < 90%, procedure duration >40 min or malignant arrhythmias. If a discontinuation criterion was fulfilled, tracheal intubation was conducted and mechanical ventilation initiated. In the MV group mechanical ventilation was initiated after tracheal intubation with endotracheal tube size 8.0 for men and 7.0 for women. A tidal volume of 6 ml kg−1 (adjusted body weight), respiratory rate of 12 min−1, a positive end expiratory pressure (PEEP) of 5 cm H2O and FiO2 0.4 were used. After completion of the surgical procedure, neuromuscular blockade was reversed by 200 mg of sugammadex iv (Bridion®, MSD, Hertfordshire, Great Britain) to ensure rapid recovery of the adductor pollicis TOF ratio to >90%. In the THRIVE group, mask ventilation was performed until spontaneous breathing reoccurred. In the MV group mechanical ventilation was performed until spontaneous breathing reoccurred, subjects were extubated when fully awake. Supplementary oxygen was administered post‐operatively if SpO2 was below the subject´s preoperative value or <90%. Patients were kept in supine position during the post‐operative period with the head elevated approximately 20 degrees to enable correct bed‐side EIT registration. Participation in the study was concluded when the patient left the post‐anaesthesia care unit (PACU).
2.1. Statistical analysis
In the absence of previous data on changes in lung volume during apnoeic oxygenation using THRIVE, we aimed to detect a 10% change between THRIVE and mechanical ventilation in lung volume at one time point. Based on that, the power analysis with a two‐tailed alpha error of 0.05 and a beta error of 0.2 (power 80%) indicated that 15 patients in each group were needed. Data are presented as mean (SD) or numbers (%) where relevant. For the primary outcome, lung volume changes over time and between groups (THRIVE vs MV) linear mixed effects models were used, as well as in the data with several continuous variables over time and between groups. In the linear mixed effects models, subjects were treated as random factors with regard to their repeated measurements on outcomes over time. The grouping variable (THRIVE vs MV) and time were regarded as fixed factors. The correlation structure of the repeated measurements was modelled using an autocorrelation structure of order 1 (AR1). When comparing differences in variables between groups unpaired t tests were used for continuous variables and Fisher´s exact test for categorical variables. Statistical analysis and graphs were made using Prism 8.0 (GraphPad, Software Inc, La Jolla, CA, USA) and SPSS Statistics 24.0 (IBM, Armonk, New York, USA). A P‐value < .05 was considered statistically significant.
3. RESULTS
3.1. Study population
Thirty patients were enrolled and completed the study protocol (Figure 1). Patient characteristics and procedural data are presented in Tables 1 and 2. There were no significant differences between the groups regarding age, BMI or smoking. The apnoeic time was 17.9 (4.8) min in the THRIVE group and the mean time from intubation to end of surgery was 28.1 (12.8) min in the MV group. In the THRIVE group three subjects reached discontinuation criteria and apnoea was terminated (Table 2). In all other subjects in the THRIVE group, apnoea could continue until end of surgery. Equipment difficulties caused a prolonged anaesthesia time in one mechanically ventilated patient. All subjects remained perioperatively cardiovascular stable, demonstrated in Appendix 1.
Figure 1.
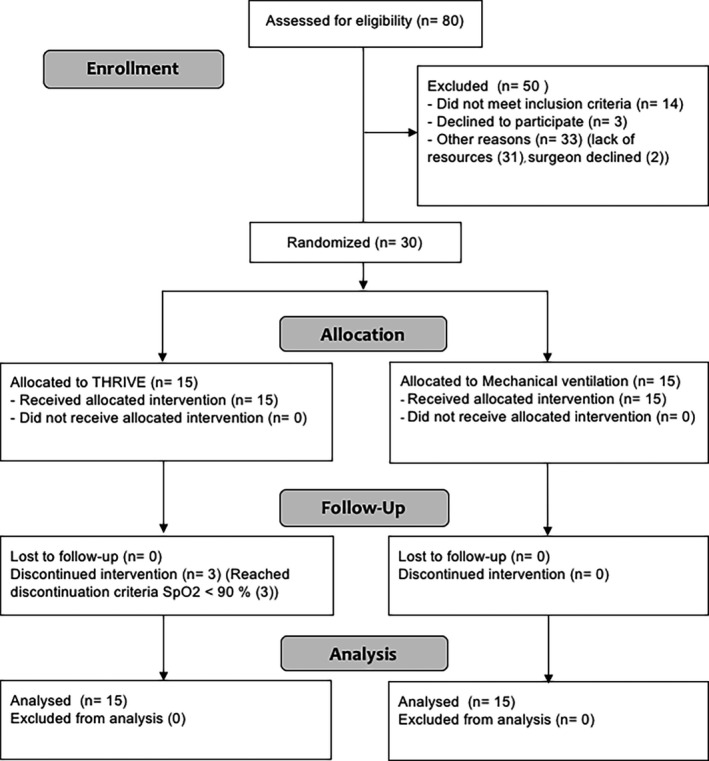
Consort diagram. THRIVE, Transnasal humidified rapid‐insufflation ventilatory exchange
Table 1.
Summary of patient characteristics in the THRIVE group and the Mechanical ventilation group
| THRIVE n = 15 |
Mechanical Ventilation n = 15 |
P‐value | |
|---|---|---|---|
| Female | 7 (46.7%) | 6 (40%) | |
| Male | 8 (53.3%) | 9 (60%) | |
| Age; years | 48.2 (19.9) | 51.3 (12.3) | .61 |
| Length (cm) | 172.8 (8.9) | 174.1 (6.1) | |
| Weight (kg) | 74.7 (18.6) | 78.8 (14.0) | |
| BMI | 25.99 (4.5) | 25.95 (3.9) | .98 |
| ASA 1 | 7 | 11 | |
| ASA 2 | 8 | 4 | |
| Non‐smoker | 10 | 11 | |
| Smoker | 3 | 3 | 1.00 |
| Former smoker | 2 | 1 | |
| Packyears: 1‐10 | 1 | 3 | |
| 11‐ 20 | 0 | 1 | |
| 21‐30 | 1 | 0 | |
| >30 | 3 | 0 | |
| Asthma | 1 | 0 | |
| COPD | 0 | 0 | |
| OSA | 0 | 1 | |
| CVD | 3 | 3 |
Data are presented as Mean (SD) or n = frequency (%) as appropriate. THRIVE: Transnasal humidified rapid insufflation ventilatory exchange; 1 Packyear = 20 cigarettes daily for 1 year; COPD: Chronic Obstructive Pulmonary Disease; OSA: Obstructive Sleep Apnoea; CVD: Cardiovascular disease.
Table 2.
Procedural duration. Oxygenation, carbon dioxide and pH data
|
THRIVE n = 15 |
Mechanical Ventilation n = 15 |
P‐value | |
|---|---|---|---|
| Apnoea duration (min) | 17.9 ± 4.8 | NA | |
| Intubation to EOS (min) | NA | 28.1 ± 12.8 | |
| Discontinuation a | 3 | NA | |
| PaCO2 (kPa) | |||
| At anaesthesia induction | 5.1 (0.9) | 5.1 (0.9) c | .93 |
| Maximum | 9.8 (1.3) | 5.3 (0.7) | .00 |
| PACU admission | 6.2 (1.1) b | 5.8 (0.7) | .20 |
| PACU discharge | 5.6 (0.9) b | 5.2 (0.5) c | .13 |
| PaO2 (kPa) | |||
| At anaesthesia induction | 55.4 (20.8) c | 59.4 (7.8) c | .50 |
| PACU admission | 13.2 (3.3) b | 11.3 (1.4) | .05 |
| PACU discharge | 11.9 (1.7) b | 11.9 (1.6) c | .95 |
| ETCO2 maximum (kPa) | 7.6 (1.1) | 4.7 (0.6) | .00 |
| PaCO2 ‐ETCO2 difference pre (kPa) | 0.41 (0.4) | 0.15 (0.4) | .05 |
| PaCO2 ‐ETCO2 difference post (kPa) | 2.24 (1.1) | 0.95 (1.3) | .007 |
| pH minimum | 7.2 (0.05) | 7.4 (0.04) | .00 |
| SpO2 minimum (%) | 96.1 (4.7) | 97.2 (1.3) | .37 |
Data are presented as mean (SD) or n (%) = frequency as appropriate. THRIVE, Transnasal humidified rapid insufflation ventilatory exchange; MV, Mechanical ventilation; EOS, End of Surgery; NA, Not applicable; PaCO2, arterial carbon dioxide partial pressure; PaO2, arterial oxygen partial pressure; ETCO2, end‐tidal carbon dioxide partial pressure; SpO2, peripheral oxygen saturation.
Discontinued due to SpO2 < 90%.
Discontinued subjects excluded.
n = 14 due to failed sampling.
3.2. Global and regional lung volume changes over time
All patients were monitored perioperatively with EIT. Considering global and regional lung volumes perioperatively, no difference in dEELI over time could be detected over time between the THRIVE group and the MV group (P = .40) (95% CI −0.37 to 0.89) or in any region of interest; dEELI ROI 1 (P = .09) (95% CI −0.02 to 0.23), dEELI ROI 2 (P = .55) (95% CI −0.18 to 0.33), dEELI ROI 3 (P = .87) (95% CI −0.27 to 0.32) and dEELI ROI 4 (P = .44) (95% CI −0.18 to 0.08) (Figure 2).
Figure 2.
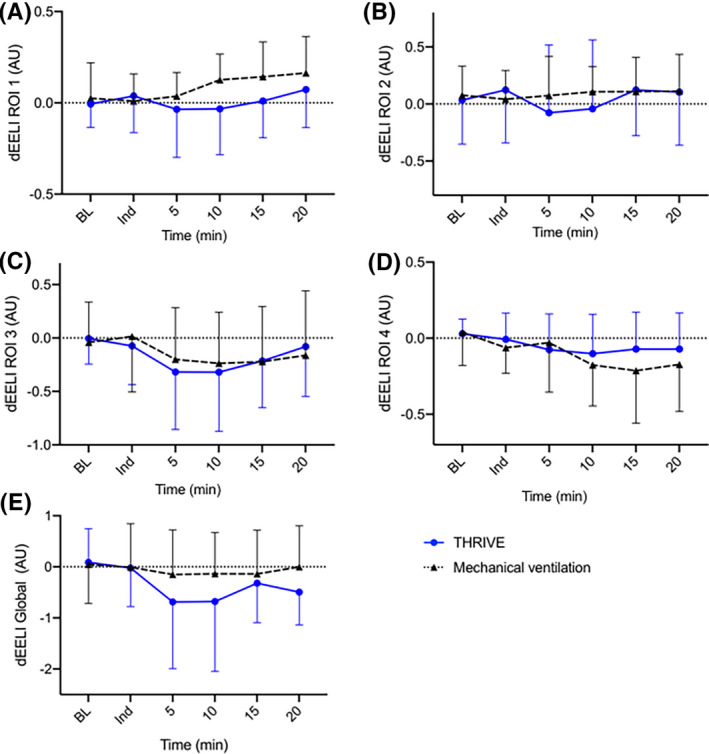
Electrical impedance tomography monitoring perioperatively presented as delta End Expiratory Lung Impedance (dEELI) during THRIVE or mechanical ventilation. Regions of interests (ROI); ROI 1 (A), ROI 2 (B), ROI 3 (C), ROI 4 (D) and global (E). Data are presented as mean (SD) in A‐E. BL, Baseline values before pre‐oxygenation; Ind, at anaesthesia induction; THRIVE, Transnasal humidified rapid‐insufflation ventilatory exchange
Post‐operatively, there was no difference detected in dEELI over time between the THRIVE group and the MV group globally or in any region of interest; dEELI global (P = .36) (95% CI −0.53 to 1.41), dEELI ROI 1 (P = .40) (95% CI −0.14 to 0.33), dEELI ROI 2 (P = .62) (95% CI −0.45 to 0.27), dEELI ROI 3 (P = .94) (95% CI −0.36 to 0.38) and dEELI ROI 4 (P = .77) (95% CI −0.15 to 0.20) (Figure 3).
Figure 3.
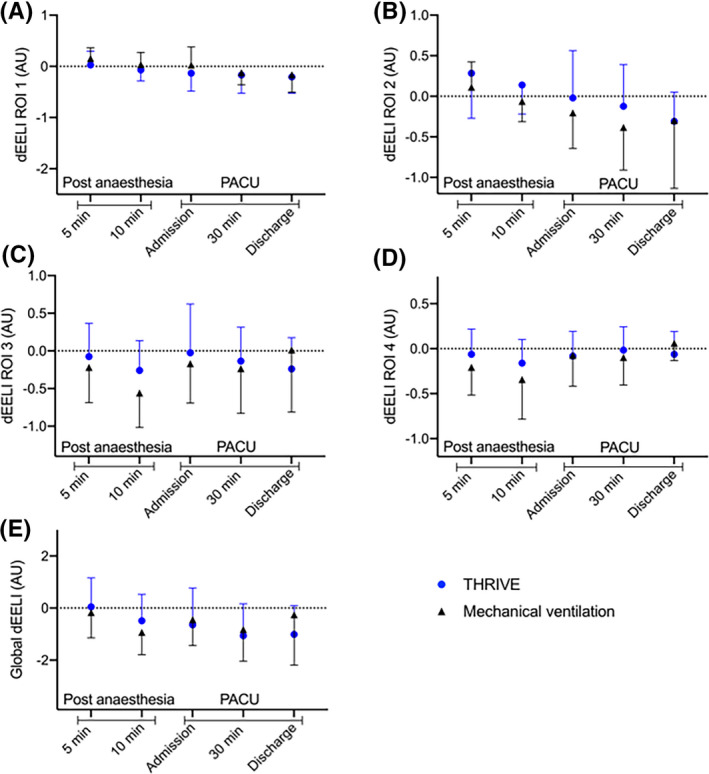
Electrical impedance tomography monitoring post‐operatively presented as delta End Expiratory Lung Impedance (dEELI) during Transnasal humidified rapid‐insufflation ventilatory exchange (THRIVE) or mechanical ventilation. Regions of interests (ROI); ROI 1 (A), ROI 2 (B), ROI 3 (C) and ROI 4 (D) and global (E). Data are presented as mean (SD) in A‐E
Graphs of global delta End Expiratory Lung Impedance (dEELI) during THRIVE (n = 15) using 100% oxygen are presented as subgroups according to BMI, smoking status, gender and discontinuation status in Appendix 2. Statistical analysis was not performed to display differences within the THRIVE group, since the aim of the study was to detect changes in lung volumes between THRIVE and MV.
3.3. Alterations in PaO2, SpO2, PaCO2 and PaCO2‐ETCO2 difference
There was no significant difference in PaO2 (P = .84) or SpO2 (P = .09) over time between the groups (Figure 4A‐B). No difference in PaO2 was seen between the groups at induction (Figure 4A). All but three patients in the THRIVE group were well oxygenated (SpO2 ≥91%) throughout the procedure (Figure 4B). Three patients with a BMI between 29.4 and 32.3 desaturated to SpO2 <90% after 10‐15 min duration of apnoea and were therefore endotracheally intubated in an uncomplicated manner. There was a significant difference in rise of arterial carbon dioxide levels in the THRIVE group compared with the MV group (P < .001). Mean PaCO2 increased in the THRIVE group with 0.28 (0.10) kPa min−1 (Figure 4C). PaCO2 increase was 0.46 (0.14), 0.27 (0.09) and 0.16 (0.11) kPa min−1 between 0‐5 min, 5‐10 min and 10‐15 min, respectively (Figure 4C), in the THRIVE group. ETCO2 increase in the THRIVE group was 0.17 (0.08) kPa min−1 and none in the MV group. The pre‐operative ETCO2 and PaCO2 difference of 0.41 (0.35) kPa and 0.15 (0.36) kPa increased to 2.24 (1.14) kPa and 0.95 (1.26) kPa (P = .007) at the end of the procedure in the THRIVE and MV group respectively. ETCO2 at the end of the procedure is demonstrated in Table 2 (ETCO2 maximum). The PaO2 and PaCO2 during emergence from anaesthesia and post‐operatively are demonstrated in Figure 4D‐E and Table 2. Two patients received supplementary nasal oxygen of 1 l min−1 in the PACU.
Figure 4.
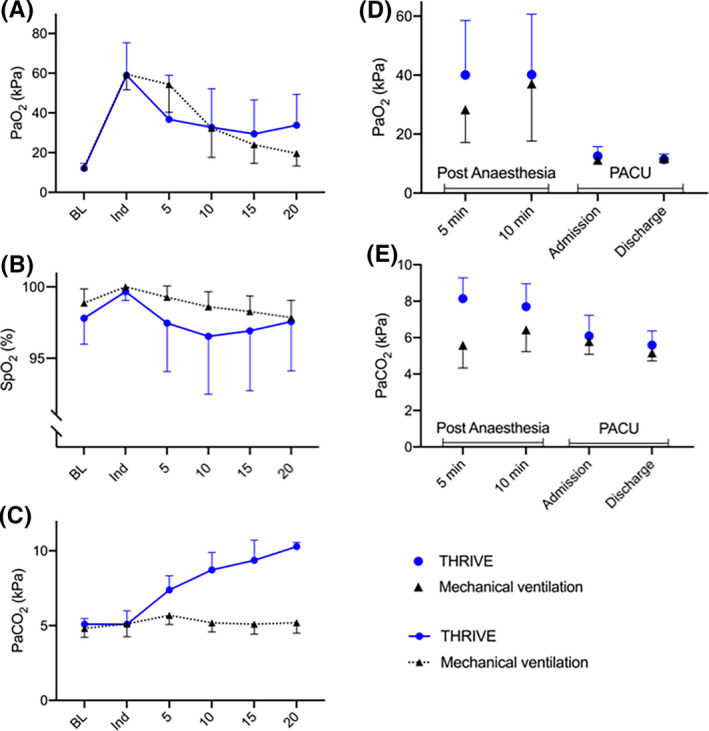
Oxygenation and blood gases during Transnasal humidified rapid‐insufflation ventilatory exchange (THRIVE) and mechanical ventilation peri‐ and post‐operatively. Arterial oxygen partial pressure (PaO2) (A), peripheral oxygen saturation (SpO2) (B) and arterial carbon dioxide partial pressure (PaCO2) (C) during oxygenation with THRIVE or mechanical ventilation. Arterial oxygen partial pressure (PaO2) (D) and arterial carbon dioxide partial pressure (PaCO2) (E) post‐operatively after THRIVE or mechanical ventilation. The Y‐axis in B has been adjusted to display maximum resolution of data. Data are presented as mean (SD) in A‐E. BL, Baseline values before pre‐oxygenation; Ind, at anaesthesia induction
4. DISCUSSION
In this prospective randomised interventional trial, we could not detect a difference in lung volume either perioperatively or post‐operatively as assessed by temporal changes in lung impedance when comparing patients receiving either THRIVE or mechanical ventilation.
There is a lack of data regarding lung volume changes during apnoeic oxygenation using THRIVE. However, in an animal model of traditional apnoeic oxygenation, an increased pulmonary shunt fraction and a lung volume loss with a lower transpulmonary pressure gradient was demonstrated. 12 This, in combination with high PaO2 and an increasing arterial/end‐tidal CO2 difference was the basis for the hypothesis of increased atelectasis formation. In spontaneously breathing individuals an increased end expiratory lung volume and airway pressure has been shown with an increasing flow of oxygen during high‐flow nasal oxygenation. 13 , 14 , 15 Whether THRIVE induces an increased airway pressure and FRC during apnoea has not been established.
EIT displays impedance changes over time bedside and real‐time measurements of dEELI 16 have been validated against delta end expiratory volume (dEELV) changes displayed by computed tomography and spirometry in regional lung volume changes during clinical settings such as decremental PEEP titration, 17 , 18 , 19 lung recruitment manoeuvres 20 and pneumothorax diagnostics. 21 Still, by using EIT, we were unable to detect a decreasing lung volume during THRIVE compared to mechanical ventilation. Since formation of lung atelectasis after induction of general anaesthesia and mechanical ventilation is common, and most presumably also during mechanical ventilation in this cohort, our findings do not exclude some degree of atelectasis formation during THRIVE, based on the absence of differences between groups. Other techniques may therefore be needed to quantify atelectasis formation during THRIVE.
PaCO2 rise during THRIVE was 0.28 kPa min−1 and the lower increase in ETCO2 of 0.17 kPa min−1 are in parallel with recent studies. 2 , 3 The declining PaCO2 increase over time during apnoea in the current study has previously also been described both in humans and in a THRIVE model, yet the underlying mechanisms are not fully understood. 3 , 4 , 5 , 22 , 23
This study confirms an increasing alveolar‐arterial CO2 difference over time, as previously described, indicating an increased ventilation/perfusion mismatch. 2 , 3 , 4 However, the present study does not support the hypothesis that the progressive alveolar‐arterial CO2 difference is caused by increased absorption atelectasis with subsequent shunt and a reduced lung volume with THRIVE compared to mechanical ventilation. It seems unlikely that atelectasis formation presents a clinically significant problem in this relatively healthy cohort during elective day care surgery in general anaesthesia. None of the subjects in our current or earlier trial have desaturated or displayed a respiratory insufficiency in arterial blood gases post‐operatively and were discharged as planned.
Mean PaO2 at induction was equal in both groups and confirms that THRIVE can pre‐oxygenate healthy subjects or subjects with mild systemic disease and a BMI < 35 effectively, as previously demonstrated. 24 , 25 , 26 No difference in SpO2 or PaO2 was seen over time between the groups.
Three subjects in the THRIVE group with a BMI >29 desaturated below SpO2 90% during apnoea within 10‐15 min, which we consider to be an important clinical observation. Two of three of these subjects were smokers with over 40 packyears, and two of three also had hypertension. Endotracheal intubation was rapid and uneventful in these patients but underlines the need for an alternative airway management technique during THRIVE, such as jet ventilation, tracheal intubation or intermittent mask ventilation. A higher BMI, often associated with a lower FRC, shortens the time to desaturation during apnoea 27 and earlier studies of apnoeic oxygenation indicate a less effective oxygenation in subjects with a high BMI and/or a low FRC/weight ratio. 4 , 10 The reasons for this remain unclear, but an increased atelectasis formation in the obese during general anaesthesia may contribute.
To the best of our knowledge, lung impedance monitoring using EIT during THRIVE in comparison to mechanical ventilation has not been described earlier. Simultaneous arterial and end‐tidal CO2 monitoring illustrate their interrelation during THRIVE. This study included subjects with BMI up to 35 allowing evaluation of THRIVE in more compromised patients. Only ASA class 1‐2 were included, therefore, the conclusions may not be applicable to patients with advanced comorbidities. Randomisation was not blinded to the examiner.
The present study demonstrates that lung volume changes over time, monitored by EIT during THRIVE, do not differ from mechanically ventilated patients. The apnoea‐induced rise in PaO2 and PaCO2 during THRIVE was in line with previous data. The increasing arterial/end‐tidal carbon dioxide difference, also previously seen, indicates an increasing V/Q mismatch, and cannot be explained by a reduction in lung volume compared to mechanical ventilation. Additional work is needed to elucidate the mechanism behind this phenomenon. Moreover, the finding of desaturation in three subjects uncovers the need to further explore the efficiency of THRIVE in potential high‐risk patients such as in the obese, in smokers and in subjects with cardiopulmonary disorders. The effect of progressive CO2 increase and acidosis during THRIVE regarding other organs, as well as potential toxic effects of hyperoxia need further evaluation. A proposed mechanism of CO2 removal by cardiac oscillations 28 , 29 in combination with increased turbulent gas flow in the airway during THRIVE 5 would benefit from further examination.
5. CONCLUSION
This prospective randomised trial found no difference of lung volume changes over time when comparing THRIVE to mechanical ventilation during laryngeal surgery. We confirm recent findings of PaCO2 rise and that THRIVE enables oxygenation in healthy patients and patients with mild systemic disease for up to 30 minutes. Still, concerns are raised for patients with a BMI ≥30 due to risk of desaturation during THRIVE. Further studies of THRIVE in compromised patients are therefore recommended.
CONFLICT OF INTEREST
L. I. Eriksson has received lecture fees from MSD Sweden AB, Stockholm, Sweden. M. J. Fagerlund has received travel support and lecture fees from Fisher & Paykel.
AUTHOR CONTRIBUTION
IMF helped with study design, recruited patients, collected, analysed and interpreted the data and wrote the study. JU helped design the study, interpreted the data and reviewed the study. AH helped with collecting data and reviewed the study. ÅL helped with study design, collected and interpreted data and wrote the study. LIE helped with study design, data analysis and interpretation and wrote the study. MJF helped with study supervision, study design, data analysation and interpretation and wrote the study.
PRESENTATION
Preliminary data for this study were presented as an oral presentation at the European Airway Management Society meeting, 5‐7 Dec 2018, Catania, Italy.
Supporting information
Supplementary Material
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the Ear Nose and Throat (ENT) surgeons and staff at the ENT operation ward and the day surgery ward, Karolinska University Hospital, Solna and Huddinge, Sweden, for practical assistance in conduction of the trial, statistician Katarina Selling, Statistikakademin, Uppsala, Sweden, for statistical expertise and Dräger, Sweden, for providing EIT equipment and technical support.
Forsberg I‐M, Ullman J, Hoffman A, Eriksson LI, Lodenius Å, Fagerlund MJ. Lung volume changes in Apnoeic Oxygenation using Transnasal Humidified Rapid‐Insufflation Ventilatory Exchange (THRIVE) compared to mechanical ventilation in adults undergoing laryngeal surgery. Acta Anaesthesiol Scand. 2020;64:1491–1498. 10.1111/aas.13686
Funding information
This work was supported by the departments and institutions involved, Stockholm City Council, Stockholm, Sweden and Gösta Fraenckel Foundation, Karolinska Institutet, Stockholm, Sweden.
REFERENCES
- 1. Badiger S, John M, Fearnley RA, Ahmad I. Optimizing oxygenation and intubation conditions during awake fibre‐optic intubation using a high‐flow nasal oxygen‐delivery system. Br J Anaesth. 2015;115(4):629‐632. [DOI] [PubMed] [Google Scholar]
- 2. Patel A, Nouraei SA. Transnasal Humidified Rapid‐Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015;70(3):323‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gustafsson IM, Lodenius A, Tunelli J, Ullman J, Jonsson FM. Apnoeic oxygenation in adults under general anaesthesia using Transnasal Humidified Rapid‐Insufflation Ventilatory Exchange (THRIVE) ‐ a physiological study. Br J Anaesth. 2017;118(4):610‐617. [DOI] [PubMed] [Google Scholar]
- 4. Lyons C, Callaghan M. Apnoeic oxygenation with high‐flow nasal oxygen for laryngeal surgery: a case series. Anaesthesia. 2017;72(11):1379‐1387. [DOI] [PubMed] [Google Scholar]
- 5. Hermez LA, Spence CJ, Payton MJ, Nouraei SAR, Patel A, Barnes TH. A physiological study to determine the mechanism of carbon dioxide clearance during apnoea when using transnasal humidified rapid insufflation ventilatory exchange (THRIVE). Anaesthesia. 2019;74(4):441‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Strandberg A, Tokics L, Brismar B, Lundquist H, Hedenstierna G. Atelectasis during anaesthesia and in the postoperative period. Acta Anaesthesiol Scand. 1986;30(2):154‐158. [DOI] [PubMed] [Google Scholar]
- 7. Edmark L, Auner U, Enlund M, Ostberg E, Hedenstierna G. Oxygen concentration and characteristics of progressive atelectasis formation during anaesthesia. Acta Anaesthesiol Scand. 2011;55(1):75‐81. [DOI] [PubMed] [Google Scholar]
- 8. Strandberg A, Tokics L, Brismar B, Lundquist H, Hedenstierna G. Constitutional factors promoting development of atelectasis during anaesthesia. Acta Anaesthesiol Scand. 1987;31(1):21‐24. [DOI] [PubMed] [Google Scholar]
- 9. Hedenstierna G, Rothen HU. Atelectasis formation during anesthesia: causes and measures to prevent it. J Clin Monit Comput. 2000;16(5–6):329‐335. [DOI] [PubMed] [Google Scholar]
- 10. Fraioli RL, Sheffer LA, Steffenson JL. Pulmonary and cardiovascular effects of apneic oxygenation in man. Anesthesiology. 1973;39(6):588‐596. [DOI] [PubMed] [Google Scholar]
- 11. Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Hedenstierna G. Atelectasis and pulmonary shunting during induction of general anaesthesia–can they be avoided? Acta Anaesthesiol Scand. 1996;40(5):524‐529. [DOI] [PubMed] [Google Scholar]
- 12. Anthonisen NR. Changes in shunt flow, compliance, and volume of lungs during apneic oxygenation. Am J Physiol. 1964;207:235‐238. [DOI] [PubMed] [Google Scholar]
- 13. Groves N, Tobin A. High flow nasal oxygen generates positive airway pressure in adult volunteers. Australian critical care : official journal of the Confederation of Australian Critical Care Nurses. 2007;20(4):126‐131. [DOI] [PubMed] [Google Scholar]
- 14. Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high‐flow nasal cannulae increase end‐expiratory lung volume and reduce respiratory rate in post‐cardiac surgical patients. Br J Anaesth. 2011;107(6):998‐1004. [DOI] [PubMed] [Google Scholar]
- 15. Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high‐flow oxygen therapy. Respir Care. 2011;56(8):1151‐1155. [DOI] [PubMed] [Google Scholar]
- 16. Lundin S, Stenqvist O. Electrical impedance tomography: potentials and pitfalls. Curr Opin Crit Care. 2012;18(1):35‐41. [DOI] [PubMed] [Google Scholar]
- 17. Costa EL, Borges JB, Melo A, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009;35(6):1132‐1137. [DOI] [PubMed] [Google Scholar]
- 18. Reinartz SD, Imhoff M, Tolba R, et al. EIT monitors valid and robust regional ventilation distribution in pathologic ventilation states in porcine study using differential DualEnergy‐CT (DeltaDECT). Sci Rep. 2019;9(1):9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grivans C, Lundin S, Stenqvist O, Lindgren S. Positive end‐expiratory pressure‐induced changes in end‐expiratory lung volume measured by spirometry and electric impedance tomography. Acta Anaesthesiol Scand. 2011;55(9):1068‐1077. [DOI] [PubMed] [Google Scholar]
- 20. Hinz J, Hahn G, Neumann P, et al. End‐expiratory lung impedance change enables bedside monitoring of end‐expiratory lung volume change. Intensive Care Med. 2003;29(1):37‐43. [DOI] [PubMed] [Google Scholar]
- 21. Costa EL, Chaves CN, Gomes S, et al. Real‐time detection of pneumothorax using electrical impedance tomography. Crit Care Med. 2008;36(4):1230‐1238. [DOI] [PubMed] [Google Scholar]
- 22. Eger EI, Severinghaus JW. The rate of rise of PaCO2 in the apneic anesthetized patient. Anesthesiology. 1961;22:419‐425. [DOI] [PubMed] [Google Scholar]
- 23. Humphreys S, Lee‐Archer P, Reyne G, Long D, Williams T, Schibler A. Transnasal humidified rapid‐insufflation ventilatory exchange (THRIVE) in children: a randomized controlled trial. Br J Anaesth. 2017;118(2):232‐238. [DOI] [PubMed] [Google Scholar]
- 24. Mir F, Patel A, Iqbal R, Cecconi M, Nouraei SA. A randomised controlled trial comparing transnasal humidified rapid insufflation ventilatory exchange (THRIVE) pre‐oxygenation with facemask pre‐oxygenation in patients undergoing rapid sequence induction of anaesthesia. Anaesthesia. 2017;72(4):439‐443. [DOI] [PubMed] [Google Scholar]
- 25. Lodenius A, Piehl J, Ostlund A, Ullman J, Jonsson FM. Transnasal humidified rapid‐insufflation ventilatory exchange (THRIVE) vs. facemask breathing pre‐oxygenation for rapid sequence induction in adults: a prospective randomised non‐blinded clinical trial. Anaesthesia. 2018;73(5):564‐571. [DOI] [PubMed] [Google Scholar]
- 26. Ng I, Krieser R, Mezzavia P, et al. The use of Transnasal Humidified Rapid‐Insufflation Ventilatory Exchange (THRIVE) for pre‐oxygenation in neurosurgical patients: a randomised controlled trial. Anaesth Intensive Care. 2018;46(4):360‐367. [DOI] [PubMed] [Google Scholar]
- 27. Benumof JL, Dagg R, Benumof R. Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine. Anesthesiology. 1997;87(4):979‐982. [DOI] [PubMed] [Google Scholar]
- 28. Slutsky AS. Gas mixing by cardiogenic oscillations: a theoretical quantitative analysis. J Appl Physiol: respiratory, environmental and exercise physiology. 1981;51(5):1287‐1293. [DOI] [PubMed] [Google Scholar]
- 29. Slutsky AS, Brown R. Cardiogenic oscillations: a potential mechanism enhancing oxygenation during apneic respiration. Med Hypotheses. 1982;8(4):393‐400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material


