Abstract
Key points
Cl− and HCO3 − had similar paracellular permeabilities in human airway epithelia.
PCl/PNa of airway epithelia was unaltered by pH 7.4 vs. pH 6.0 solutions.
Under basal conditions, calculated paracellular HCO3 − flux was secretory.
Cytokines that increased airway surface liquid pH decreased or reversed paracellular HCO3 − flux.
HCO3 − flux through the paracellular pathway may counterbalance effects of cellular H+ and HCO3 − secretion.
Abstract
Airway epithelia control the pH of airway surface liquid (ASL), thereby optimizing respiratory defences. Active H+ and HCO3 − secretion by airway epithelial cells produce an ASL that is acidic compared with the interstitial space. The paracellular pathway could provide a route for passive HCO3 − flux that also modifies ASL pH. However, there is limited information about paracellular HCO3 − flux, and it remains uncertain whether an acidic pH produced by loss of cystic fibrosis transmembrane conductance regulator anion channels or proinflammatory cytokines might alter the paracellular pathway function. To investigate paracellular HCO3 − transport, we studied differentiated primary cultures of human cystic fibrosis (CF) and non‐CF airway epithelia. The paracellular pathway was pH‐insensitive at pH 6.0 vs. pH 7.4 and was equally permeable to Cl− and HCO3 −. Under basal conditions at pH ∼6.6, calculated paracellular HCO3 − flux was weakly secretory. Treating epithelia with IL‐17 plus TNFα alkalinized ASL pH to ∼7.0, increased paracellular HCO3 − permeability, and paracellular HCO3 − flux was negligible. Applying IL‐13 increased ASL pH to ∼7.4 without altering paracellular HCO3 − permeability, and calculated paracellular HCO3 − flux was absorptive. These results suggest that HCO3 − flux through the paracellular pathway counterbalances, in part, changes in the ASL pH produced via cellular mechanisms. As the pH of ASL increases towards that of basolateral liquid, paracellular HCO3 − flux becomes absorptive, tempering the alkaline pH generated by transcellular HCO3 − secretion.
Keywords: airway, bicarbonate, epithelia, ion transport
Key points
Cl− and HCO3 − had similar paracellular permeabilities in human airway epithelia.
PCl/PNa of airway epithelia was unaltered by pH 7.4 vs. pH 6.0 solutions.
Under basal conditions, calculated paracellular HCO3 − flux was secretory.
Cytokines that increased airway surface liquid pH decreased or reversed paracellular HCO3 − flux.
HCO3 − flux through the paracellular pathway may counterbalance effects of cellular H+ and HCO3 − secretion.
Introduction
The acid‐base status of airway surface liquid (ASL) is tightly regulated (Fischer & Widdicombe, 2006). Active proton secretion produces an ASL pH that is acidic compared with the interstitial space (Jayaraman et al. 2001a ; Coakley et al. 2003; McShane et al. 2003; Pezzulo et al. 2012; Garland et al. 2013; Abou Alaiwa et al. 2014a ; Schultz et al. 2017; Abou Alaiwa et al. 2018). H+ secretion is neutralized, in part, by HCO3 − secretion by the cystic fibrosis transmembrane conductance regulator (CFTR) anion channel, Ca2+‐activated Cl− channels, and pendrin‐mediated Cl−/HCO3 − exchange (Coakley et al. 2003; Fischer & Widdicombe, 2006; Shah et al. 2016; Lennox et al. 2018; Simonin et al. 2019). In cystic fibrosis (CF), loss of CFTR‐mediated HCO3 − secretion decreases the ASL pH (Pezzulo et al. 2012; Garland et al. 2013; Abou Alaiwa et al. 2014a ; Garnett et al. 2016; Haggie et al. 2016; Shah et al. 2016; Abou Alaiwa et al. 2018; Simonin et al. 2019). The acidic ASL pH impairs at least two host‐defence mechanisms: antimicrobial activity (Pezzulo et al. 2012; Abou Alaiwa et al. 2014b ; Shah et al. 2016; Simonin et al. 2019) and mucociliary transport (Clary‐Meinesz et al. 1998; Hoegger et al. 2014; Tang et al. 2016; Ostedgaard et al. 2017).
In parallel with the cellular pathway, with its channels, transporters and pumps, lies the paracellular pathway, which allows passive ion flux (Diamond, 1978; Anderson & Van Itallie, 2009). The paracellular pathway could provide a route for HCO3 − secretion or absorption, and thus it could modify ASL pH. However, HCO3 − flux through the paracellular pathway is rarely considered, perhaps because the use of the short‐circuit technique negates the influence of the paracellular pathway.
HCO3 − flux through the paracellular pathway might perturb ASL pH in disease. As an example, the ASL of newborn humans with CF, newborn CF pigs, and differentiated cultures of human and pig CF airway epithelia is more acidic than non‐CF ASL (Coakley et al. 2003; Pezzulo et al. 2012; Garland et al. 2013; Abou Alaiwa et al. 2014a ; Garnett et al. 2016; Haggie et al. 2016; Shah et al. 2016; Abou Alaiwa et al. 2018; Simonin et al. 2019). However, over the course of months and years, the in vivo pH of CF ASL alkalinizes (Abou Alaiwa et al. 2014a ; Schultz et al. 2017; Abou Alaiwa et al. 2018). These findings suggest that an in vivo factor might contribute to age‐dependent alkalinization. Airway inflammation develops over a similar time course (Khan et al. 1995; Muhlebach et al. 1999; Dakin et al. 2002; Sly et al. 2009). Consistent with the hypothesis that inflammation may increase ASL pH, previous reports suggest that proinflammatory cytokines alter ASL pH in primary cultures of CF epithelia (Kreindler et al. 2009; Gorrieri et al. 2016; Haggie et al. 2016; Lennox et al. 2018; Scudieri et al. 2018; Kim et al. 2019; Rehman et al. 2020).
Thus, further knowledge of the HCO3 − permeability and flux via the paracellular pathway could aid understanding of how ASL pH is controlled. To assess paracellular HCO3 − permeability, we first tested whether pH alters the paracellular permeability of airway epithelia. We then evaluated the paracellular HCO3 − permeability and calculated the paracellular HCO3 − flux in the absence and presence of cytokines that are associated with airway inflammation.
Methods
Airway epithelial cells were obtained from CF and non‐CF tissue obtained from the Iowa Donor Network with CF donor information supplied in Table 1 and studies were approved by the University of Iowa Institutional Review Board and conform to the principles and regulations of The Journal of Physiology (Grundy, 2015). Epithelial cells were cultured according to a previous protocol (Karp et al. 2002). Briefly, donor tissue was digested with pronase then seeded upon collagen‐coated semi‐permeable membranes (0.33 cm2 polycarbonate filters, Costar #3413) and grown at an air–liquid interface. Cultures were used after complete cellular differentiation (>21 days) and resistances >166 Ω.cm2.
Table 1.
Cystic fibrosis donors used in this study
| Donor | Age | Sex | Cystic fibrosis transmembrane conductance regulator mutations |
|---|---|---|---|
| 1 | 27 | Female | ∆F508/1717‐1G>A |
| 2 | 36 | Female | ∆F508/R347P |
| 3 | 29 | Female | ∆F508/3876delA |
| 4 | 36 | Female | ∆F508/∆F508 |
| 5 | 22 | Male | ∆F508/1717‐1G‐A |
| 6 | 21 | Female | ∆F508/2622 + 1G>A |
| 7 | 36 | Male | ∆F508/R553X |
| 8 | 64 | Male | ∆F508/L1254X |
| 9 | 37 | Male | ∆F508/deletions of exons 2–3 |
| 10 | 38 | Male | ∆F508/∆F508 |
| 11 | 19 | Female | ∆F508/G85E |
| 12 | 31 | Female | ∆F508/∆F508 |
| 13 | 24 | Female | ∆F508/unknown |
| 14 | 31 | Female | ∆F508/G551D |
| 15 | 42 | Female | ∆F508/∆F508 |
| 16 | 34 | Female | ∆F508/3659delC |
| 17 | 54 | Female | ∆F508/I336K |
| 18 | 35 | Female | ∆F508/2184insA |
| 19 | 24 | Female | ∆F508/G551D |
| 20 | 29 | Female | ∆F508/3849 + 10kbC→T |
Solutions
All chemicals were from Sigma‐Aldrich unless otherwise stated. All dilution potential solutions consisted of the same minor salts and glucose in mm; 5 glucose, 1.2 calcium gluconate, 1.2 magnesium gluconate, and were buffered with 5 Hepes (pH 7.4 solutions) or 5 MES (pH 6.0 solutions) and titrated at 37°C to their respective pH value with N‐methyl‐D‐glucamine (NMDG), a cell‐ and junctional‐impermeant sugar, that acts as a strong base (i.e. >99% dissociation) at pH 7.4 and 6.0 (pKa 9.6; 22°C). The [NMDG+] added was < 1 mm and was therefore excluded from ionic strength calculations. All major salts (e.g. NaCl) were made at the following concentrations in mm; 150, 112.5, 75, 37.5 or 18.75 and were gassed with compressed air. All solutions were made to 310 ± 5 mOsm by mannitol addition and verified by a vapour pressure osmometer (Wescor Inc.) each time a solution was made. Solutions were made on the day of each experiment.
For solutions containing HCO3 −, the [HCO3 −] was computed using the Henderson–Hasselbalch equation. 5% CO2 partial pressure was calculated using the average atmospheric pressure of Iowa City (765.5 mmHg; Iowa City Municipal Airport) and correcting for the vapour pressure of water (47.10 mmHg). For dilution potential experiments, NaHCO3 and respective NaCl control solutions were (in mm) 22 or 11. The basolateral solution was 22 mm NaHCO3 or 22 mm NaCl for their respective experiments. The NaCl control solution for NaHCO3 experiments was titrated to the same pH value as its corresponding NaHCO3 solution to control for possible pH‐dependent effects. Calculated pH values for these experiments were pH 7.4 and pH 7.1. However, empirical pH values for these NaHCO3 solutions equilibrated with 5% CO2 were 7.58 (22 mm NaHCO3) and 7.21 (11 mm HCO3), which may be attributed to the increased pKa of HCO3 − (Hastings & Sendroy, 1925) and decreased CO2 solubility (Van Slyke et al. 1928) for low ionic strength solutions. Therefore, control NaCl solutions were titrated to these empirical pH values with NMDG. The paracellular NaCl permeability for pH 7.58 or pH 7.21 low ionic strength experiments did not differ from values obtained from pH 7.4 or pH 6.0 experiments performed at higher ionic strengths. All NaHCO3 solutions were gassed with 5% CO2/21%O2 balanced with nitrogen and all NaCl solutions were gassed with air.
For open‐circuit experiments performed to estimate paracellular HCO3 − current, three different solutions were used. The basolateral solution contained in mm: 5 glucose, 104.8 sodium chloride, 22 sodium bicarbonate, 5.2 potassium chloride, 18.2 sodium gluconate, 1.2 calcium gluconate, 1.2 magnesium gluconate, 2.2 NMDG and 2.2 gluconic acid. Physiological ASL solutions contained 5 glucose, 70 sodium chloride, 20 potassium chloride, 1.2 calcium gluconate, 1.2 magnesium gluconate, 26.3 NMDG, 26.3 gluconic acid and either 22 sodium bicarbonate/13 sodium gluconate (pH 7.4) or 4 sodium bicarbonate/31 sodium gluconate (pH 6.6). These solutions approximate the ionic activity of native human ASL cultured at the air–liquid interface (Knowles et al. 1997; Jayaraman et al. 2001b ; Namkung et al. 2009).
Pharmacological reagents
The following drugs and final concentrations were used in this study: 100 µm amiloride (Sigma‐Aldrich), 100 µm DIDS (Sigma‐Aldrich), 100 µm GlyH‐101 (Cystic Fibrosis Foundation Therapeutics and Robert Bridges), and 1 mm acetazolamide (Sigma‐Aldrich). All drugs were dissolved in DMSO (Thermo Fisher Scientific).
Cytokine treatment
For the cytokine studies, the media was changed every 2 days. For the IL‐13 experiments, 20 ng/ml IL‐13 (R&D Systems) or DMSO vehicle was added to the basolateral compartment of differentiated epithelia, then 20 µl of the basolateral solution was added to the apical surface and experiments were performed 21 days after initial treatment. 20 ng/ml IL‐13 is sufficient to increase goblet cell abundance in many laboratories (Laoukili et al. 2001; Atherton et al. 2003; Zhen et al. 2007; Kanoh et al. 2011; Thavagnanam et al. 2011; Dickinson et al. 2016; Pezzulo et al. 2019). For IL‐17/TNFα experiments, 20 ng/ml IL‐17 (R&D Systems) and 10 ng/ml TNFα (R&D Systems) or DMSO was added to the basolateral media and experiments were performed 2 days later based on preliminary dose–response studies and previous reports (Kao et al. 2004; McAllister et al. 2005; Kreindler et al. 2009; Choy et al. 2015; Lehmann et al. 2018; Pezzulo et al. 2019).
Electrophysiology
Epithelia were assayed in Ussing chambers (Physiologic Instruments) with 3 m KCl agar bridges connected to amplifiers (VCC‐MC8, Physiologic Instruments) recording open‐circuit transepithelial voltage (Vt). A 5 µA bipolar current pulse was applied across the epithelium periodically. The current‐induced change in Vt was used to calculate the transepithelial conductance (Gt). Data were acquired with Acquire & Analyse software (version 2.3.8, Physiologic Instruments). Dilution potentials were generated by perfusing dilutions of the dominant ionic species (e.g. NaCl) into the apical chamber. After the experiment, cells were lysed by distilled water and electrode drift was assessed in the original bilateral solution. Junction potentials induced in the voltage probes by ionic dilutions were then assessed without an epithelium and subtracted from the obtained dilution potentials.
Calculations
Activity coefficients were calculated using the ionic strength of each solution and the extended Debye–Hückel equation (Robinson & Stokes, 1959), which is applicable for physiological concentrations:
| (1) |
Where is the activity coefficient of the ion, z is the ionic charge, μ is the ionic strength of the solution, and is the effective diameter of the hydrated ion taken from Keilland (1937). The constants −0.509 and 3.29 were used because experiments were performed at 37°C (Manov et al. 1943).
Ion activity was calculated by the equation:
| (2) |
Where is the ion activity, is the activity coefficient, and [] is the ion concentration.
Relative permeability (PAnion/PNa) was calculated using the Goldman–Hodgkin–Katz equation (Goldman, 1943; Hodgkin & Katz, 1949):
| (3) |
Approximating the constants at 37°C simplifies to:
| (4) |
Where F is Faraday's constant, R is the gas constant, T is temperature, is the dilution potential, is the ionic activity, and is the relative permeability of anion A− to Na+. When bi‐ionic potentials were measured (potential generated by replacing apical NaCl with an equimolar monovalent chloride salt), we used Eqn 4 and substituted with the substituted cation's activity.
Partial conductance () was computed using the following formula (Schultz, 1980; Sten‐Knudsen, 2002):
| (5) |
Where is the dilution potential, is the paracellular conductance in the corresponding symmetrical salt solution, and and are the computed Nernst potential for the ion during dilution. The value is the partial conductance of the anion (). The quantities and represent the transference numbers for Na+ and its corresponding anion.
Absolute paracellular permeabilities were calculated using the following equation (Schultz, 1980; Sten‐Knudsen, 2002):
| (6) |
Where is the ion's absolute permeability, is the ion's partial conductance, is the ion's charge, is the ion's activity, F is Faraday's constant, R is the gas constant, T is temperature, Vt is the transepithelial voltage and Eion is the ion's Nernst potential. We obtained similar values for using the approach taken by Kimizuka and Koketsu (Kimizuka & Koketsu, 1964), using total G rather than Gion (not shown).
Ionic mobilities () at 37°C were derived from reported limiting equivalent conductivities () at 35°C (Robinson & Stokes, 1959) by the relationship:
| (7) |
Where is the ion's charge and is Faraday's constant. A of 4.11 × 10−4 cm2 sec−1 V−1 was derived by the Nernst–Einstein relationship:
| (8) |
Where is the ion's charge, is Faraday's constant, and is the HCO3 − diffusion coefficient at 35°C, 1.10 × 10−5 cm2 s−1 (Voipio, 1998). We then substitute into Eqn 7 to obtain .
Ionic conductance for non‐symmetrical solutions were obtained by using estimated values, known ionic concentrations, calculated Nernst potentials, and empirical transepithelial voltages substituted into Eqn 6. To estimate for a given paracellular conductance, we used the relationship between donor‐matched PHCO3 estimated from 22 mm NaHCO3 vs. conductance in 150 mm NaCl solution.
With the solved Gion, the ion‐specific current () was calculated using the driving‐force derivation of Ohm's law:
| (9) |
Ion‐specific current was transformed to ion‐specific fluxes using Faraday's constant and were scaled to hours.
Paracellular flux is directly related to paracellular current by IParacellular = zFJParacellular. Therefore, we use the term paracellular flux and paracellular current interchangeably. For the discussion, we refer to the ion concentration, [HCO3 −], and ion activity, αHCO3 −, interchangeably. Ion activities were used for all calculations.
Data analysis
Electrophysiological data were analysed using a custom graphical user interface coded in MATLAB version R2018b (Mathworks). Equations were solved in MATLAB version R2018b (Mathworks) using custom code. All codes were written by Ian M. Thornell and are freely available upon request and the data that support the findings of this study are available from the corresponding authors upon reasonable request.
Statistics
An initial PCl/Na experiment (n = 8 donors) was performed and data were used to perform an a priori power analysis for the remainder of the study using GraphPad Prism 7.0d (GraphPad) and G*Power 3.1 software (Faul et al. 2007). For this initial experiment, relative permeabilities were normally distributed (the Shapiro–Wilk test) and had equal variance (F test). For the obtained effect size (d = 1.64), six donors were used in subsequent experiments to detect a 0.1 change in relative permeability (α = 0.05, β > 0.80). Data were compared using either a paired Student's t test, a one‐way ANOVA with Bonferroni correction, or a Wilcoxon matched‐pairs signed rank test. Linear fits were obtained using the least‐squares method and compared using an extra sum‐of‐squares F test. For all tests, statistical significance was defined as P ≤ 0.05. Any outliers were identified by a Grubb's test, α ≤ 0.01.
Results
Paracellular ion permeability is pH‐insensitive
It is unknown whether physiological changes in apical pH alter the paracellular permeability of airway epithelia. To evaluate paracellular permeability, we first eliminated electrogenic transcellular Na+ transport by adding 100 µm amiloride to the apical solution (Fig. 1A ). To eliminate electrogenic transcellular Cl− transport, we used CF epithelia to eliminate CFTR anion channels and added 100 µm DIDS to the apical solution to inhibit Ca+‐activated Cl− channels (Ousingsawat et al. 2009). This combination resulted in a small apical‐positive transepithelial voltage (Vt), consistent with a previous study (Coakley et al. 2003). We then determined the relative paracellular Cl− to Na+ permeability (PCl/Na) by measuring changes in Vt in response to a series of apical NaCl dilutions. We set pH to 7.4 or 6.0 bilaterally; the pH buffer was Hepes or MES. At both pH 7.4 and 6.0, apical NaCl dilutions depolarized Vt, indicating that Na+ was more permeable than Cl− through the paracellular pathway (Fig 1A and B ). PCl/Na was not altered at pH 6.0 (Fig. 1C ) suggesting that physiological pH does not alter the paracellular ion permeability of airway epithelia. We also estimated the paracellular electrical conductance (Gp) after adding amiloride and DIDS to CF epithelia and before the measurement of dilution potentials. Gp was not affected by the changes in pH (Fig. 1D ). By comparing epithelia from different donors, we found that PCl/Na was independent of Gp (Fig. 1E ), and PNa and PCl increased in parallel with Gp (Fig. 1F ). These data suggest that epithelia with low and high Gp contain similar paracellular permeation pathways.
Figure 1. Human airway epithelia cultured at the air–liquid interface have cation‐selective tight junctions that are not affected by low physiological pH.

Red is pH 6.0, black is pH 7.4, squares are Na+ data and triangles are Cl− and all error bars represent standard deviations of the mean. One donor was omitted because a 11.43 mS cm−2 baseline conductance was identified as an outlier; Grubb's test α ≤ 0.01. A, representative dilution potential experiment not corrected for junction potentials. B, junction potential‐corrected dilution potential summary data for experiments containing identical solutions titrated to pH 7.4 or 6.0; n = 7 donors. The dashed lines represent theoretical dilution potentials calculated by substituting average relative permeability data from panel C into the Goldman–Hodgkin–Katz equation. C, relative paracellular permeability, each circle represents a single human donor. P = 0.54; two‐tailed paired Student's t test; n = 7 donors. D, bilateral 150 mm NaCl paracellular conductance. Each circle represents a single human donor. P = 0.63; paired Student's t test; n = 7 donors. E, relative paracellular permeability vs. paracellular conductance, each circle represents a single human donor. We cannot reject the null hypothesis that the slopes were zero; pH 7.4 P = 0.15, pH 6.0 P = 0.25; F test; n = 7 donors each. F, paracellular ion permeability vs. paracellular conductance. Data were fit with linear regressions, r 2 > 0.98. We cannot reject the null hypothesis that the slopes were equal; P = 0.12; F test; n = 7 donors each ion and pH value.
Paracellular HCO3 − permeability is similar to paracellular Cl− permeability
Because PCl/Na was unaffected by changing from pH 7.4 to pH 6.0 solutions, we were able to assess paracellular HCO3 − permeability with dilution potentials, which at a constant CO2 concentration will impose a concomitant pH change. Decreasing NaHCO3 from 22 mm to 11 mm revealed relative HCO3 − permeabilities, PHCO3/Na, that were similar to pH‐matched reductions in NaCl from 22 mm to 11 mm, PCl/Na (Fig. 2A ). Paracellular HCO3 − permeability was unaffected by inhibiting carbonic anhydrase with 1 mm acetazolamide, indicating that HCO3 − permeated as an ion rather than carbonic anhydrase‐mediated CO2 reconversion reactions (Fig. 2B ).
Figure 2. The paracellular pathway of human airway epithelia is HCO3 − permeable.
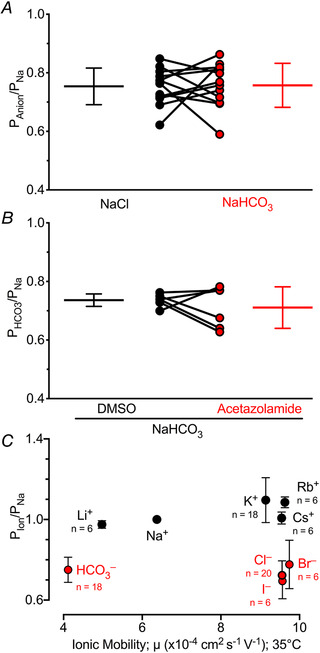
n ≥ 6 donors for all panels and all error bars represent standard deviations of the mean. A, paracellular PNa/Cl vs. PNa/HCO3, each circle represents a single human donor; P = 0.91; two‐tailed paired Student's t test; n = 12 donors. B, paracellular PNa/HCO3 ± 1 mm acetazolamide, each circle is one donor; P = 0.45; two‐tailed paired Student's t test; n = 6 donors. C, relative paracellular permeabilities, Pion/PNa, for several cations and anions. We cannot reject the null hypothesis that the slopes were zero; cations P = 0.15, anions P = 0.76; F test; n values are shown in figure.
To further assess paracellular ion permeability, we tested several monovalent ions of different size. Cations were more permeant than anions (Fig. 2C ). However, ionic size had minimal effects on permeation. Thus, the paracellular pathway was more selective for charge vs. ion size.
Paracellular Cl− and HCO3 − fluxes are determined by PCl and PHCO3, the [Cl−] and [HCO3 −], and Vt. To obtain the data necessary for calculating paracellular HCO3 − flux, open‐circuit Vt and Gt were recorded from human CF and non‐CF epithelia. Figure 3A shows the sequence of additions and timing in a non‐CF epithelium. The relationships between PCl and PHCO3 and Gp are shown in Fig. 3B and C . Epithelia were bathed in solutions with an ASL‐like composition (Knowles et al. 1997; Jayaraman et al. 2001b ; Namkung et al. 2009) titrated to pH 6.6 and also to 7.4. For epithelia from each donor, we determined PCl and PHCO3 (Fig. 3D and G ). The paracellular permeabilities of these two anions were similar. We then used Eqn 6 to calculate the paracellular Cl− and HCO3 − conductances for CF and non‐CF epithelia (Fig. 3E and H ). Because the [HCO3 −] was less than the [Cl−], the paracellular conductance for HCO3 − was less than for Cl−. Raising the apical [HCO3 −] increased the paracellular HCO3 − conductance.
Figure 3. Human airways have small paracellular HCO3 − fluxes.
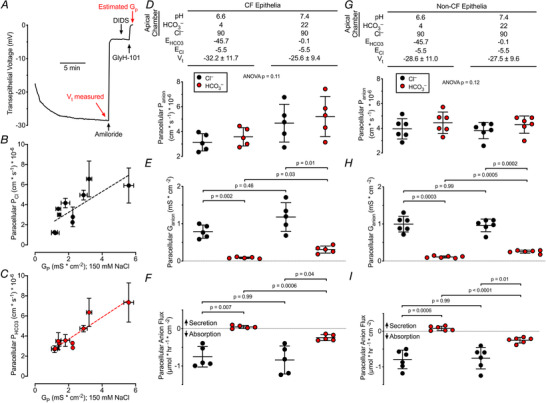
Panels D, E and F; n = 5 cystic fibrosis (CF) donors. 1 of 6 CF donors was excluded from these studies because cultures were unresponsive to amiloride. Black circles represent Cl− data and red circles represent HCO3 − data, each for a single donor. Conditions listed at the top of panel D also apply to aligned data points in panels E and F, and conditions listed at the top of panel G also apply to aligned data points in panels H and I. A, representative transepithelial voltage recording for non‐CF epithelia bathed in symmetrical solutions. Non‐CF summary data are in panels G–I. the same protocol was performed on CF epithelia to generate subsequent panels. B, paracellular PCl vs. donor‐matched 150 mm Gp from Fig. 1 and 2 data, circles represent 11 donors; r 2 = 0.57. C, paracellular PHCO3 from Fig. 2 data vs. donor‐matched 150 mm NaCl Gp from Fig. 1 and 2 data, circles represent 11 donors; r 2 = 0.84. D, paracellular Panion calculated from Gp and either Panel B or Panel C; one‐way ANOVA. Conditions for groups in panels D–F are shown at top. E, Ganion calculated using Eqn 6. Paracellular GHCO3 increased when apical [HCO3 −] increased; Bonferroni‐corrected P values shown, one‐way ANOVA. F, paracellular anion flux calculated using Eqn 9 and Faraday's constant. Minimal paracellular HCO3 − secretion became HCO3 − absorption with increased apical [HCO3 −]; Bonferroni‐corrected P values shown, one‐way ANOVA. G, paracellular Panion calculated from Gp and either Fig. 3B or 3C ; one‐way ANOVA. H, Ganion calculated using Eqn 6. Paracellular GHCO3 increased when apical [HCO3 −] increased; Bonferroni‐corrected P values shown, one‐way ANOVA. I, paracellular anion flux calculated using Eqn 9 and Faraday's constant. Minimal paracellular HCO3 − secretion became HCO3 − absorption with increased apical [HCO3 −]; Bonferroni‐corrected P values shown, one‐way ANOVA.
We also calculated the paracellular Cl− and HCO3 − fluxes from the driving‐force derivation of Ohm's law (Eqn 9) and Faraday's constant (Fig. 3F and I ). These calculations yielded two main observations. First, the paracellular HCO3 − flux was smaller in magnitude than the paracellular Cl− flux. Second, for an apical pH of 6.6, the calculated paracellular fluxes were absorptive for Cl− and slightly secretory for HCO3 −. When we raised the apical pH to 7.4, the calculated HCO3 − flux became absorptive. Thus, at an apical pH of 7.4, the calculated paracellular Cl− and HCO3 − fluxes were in the opposite direction of active transcellular Cl− and HCO3 − secretion observed for airway epithelia.
Proinflammatory cytokines increased ASL pH and eliminated or reversed paracellular HCO3 − secretion
Inflammation involves airway epithelia in many lung diseases. Given the importance of ASL pH to respiratory defences, we asked whether cytokines might change the paracellular HCO3 − permeability of airway epithelia and thereby alter ASL pH. IL‐13 is a cytokine that is an important mediator of allergic diseases and drives the TH2‐high asthma phenotype (Wills‐Karp et al. 1998; Wesolowska‐Andersen & Seibold, 2015; Svenningsen & Nair, 2017). IL‐13 may also play an important role in CF (Hauber et al. 2003). In a previous study, we applied IL‐13 for 21 days to primary cultures of human airway epithelia and induced goblet cell metaplasia (Pezzulo et al. 2019). Here we asked whether IL‐13 changes paracellular anion permeability and calculated transepithelial HCO3 − fluxes. Compared with the vehicle control, IL‐13 increased ASL pH in CF airway epithelia from 6.6 to 7.4 (Table 2). However, IL‐13 did not significantly change Gp or the permeability of HCO3 −, Cl−, Na+ or K+ (Table 2, Fig. 4A ). The calculated paracellular HCO3 − conductance increased with the increase in apical [HCO3 −] (Fig. 4B ). In vehicle‐treated epithelia, we calculated a small secretory HCO3 − flux (Fig. 4C ). IL‐13 reversed that to a small absorptive HCO3 − flux. These results indicate that the IL‐13‐induced ASL alkalinization was not likely due to HCO3 − secretion through the paracellular pathway.
Table 2.
IL‐13 increases airway surface liquid pH of human cystic fibrosis airway epithelia, but does not alter paracellular ion permeabilities
| 21 days vehicle (PBS) | 21 days IL‐13 | P value | |
|---|---|---|---|
| Native airway surface liquid (ASL) experiments; n = 6 | |||
| ASL pH † | 6.58; [6.51, 6.63] | 7.40; [7.20, 7.76] | <0.0001 |
| 22 mm NaCl dilution potential experiments; n = 6 | |||
| GP (mS cm−2) | 0.802 ± 0.355 | 0.755 ± 0.259 | 0.75 |
| PCl/Na | 0.73 ± 0.06 | 0.61 ± 0.16 | 0.06 |
| PNa (x10−6 cm s−1) | 6.87 ± 2.92 | 7.08 ± 1.70 | 0.49 |
| PCl (x10−6 cm s−1) | 5.11 ± 2.39 | 4.48 ± 2.19 | 0.15 |
| 22 mm NaHCO3 dilution potential experiments; n = 5 ‡ | |||
| GP (mS cm−2) | 0.664 ± 0.292 | 0.749 ± 0.158 | 0.51 |
| PHCO3/Na | 0.74 ± 0.07 | 0.61 ± 0.18 | 0.12 |
| PNa (x10−6cm s−1) | 5.44 ± 2.21 | 6.52 ± 1.57 | 0.41 |
| PHCO3 (x10−6 cm s−1) | 4.43 ± 2.14 | 4.61 ± 0.85 | 0.67 |
| 150 mm NaCl/KCl bi‐ionic potential experiments; n = 5 ‡ | |||
| PK/Na | 1.09 ± 0.08 | 1.11 ± 0.12 | 0.67 |
Data reported as means ± standard deviation
P values obtained using two‐tailed paired Student's t test
Statistical analyses, including mean and standard deviation, were performed using [H+]. For presentation, the [H+] were converted to pH, and hence the standard deviations are shown as intervals.
Donor excluded from analysis; Grubb's test α ≤ 0.01
Figure 4. Proinflammatory cytokines increased airway surface liquid pH and induce HCO3 − absorption or minimal HCO3 − flux.
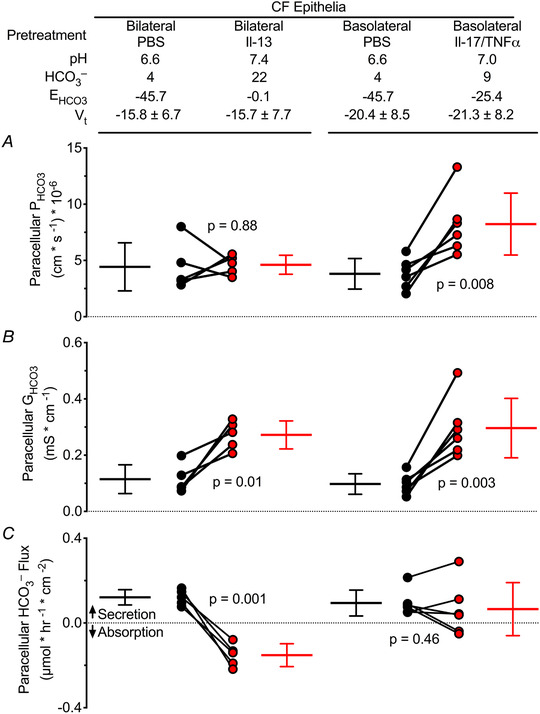
1 of 6 cystic fibrosis (CF) donors was excluded from the IL‐13 analysis because of a high IL‐13‐induced paracellular HCO3 − conductance of 0.868 mS cm−2, Grubb's test α ≤ 0.01. Red circles represent HCO3 − data and black circles represent Cl− data, each for a single donor. A, paracellular PHCO3 reported in Table 2 and Table 3; two‐tailed paired Student's t test, P values shown. B, GHCO3 calculated using Eqn 6, paracellular GHCO3 increased due to increased apical [HCO3 −]; two‐tailed paired Student's t test, P values shown. C, paracellular HCO3 − flux calculated using Eqn 9 and Faraday's constant. Minimal HCO3 − secretion was reduced or absorptive with cytokine treatments; two‐tailed paired Student's t test, P values shown.
We also tested IL‐17 and TNFα because they are commonly elevated in CF airways. They are involved in neutrophil recruitment (Smart & Casale, 1994; Lukacs et al. 1995; Laan et al. 1999; Ferretti et al. 2003; Stoppelenburg et al. 2013; Michel et al. 2014), a prominent feature of CF airway disease (Conese et al. 2003; Cantin et al. 2015; Russell et al. 2016; Liu et al. 2017). We asked whether these cytokines alter paracellular HCO3 − permeability and HCO3 − secretion and thereby contribute, at least in part, to the increased ASL pH. Compared with the vehicle control, IL‐17 /TNFα increased ASL pH (Table 3). These cytokines increased: PNa, PCl and PHCO3 (Table 3, Fig. 4A ). However, the increase was not the result of a non‐specific leak because they decreased PCl/Na and PK/Na, whereas a non‐specific leak would be predicted to increase PCl/Na and PK/Na. Paracellular HCO3 − conductance increased due to the increased PHCO3 and the increased apical [HCO3 −] (Fig. 4B ). With vehicle‐treated control epithelia, the calculated paracellular HCO3 − flux was small and secretory (Fig. 4C ). After IL‐17 and TNFα, the paracellular HCO3 − flux was not different from zero.
Table 3.
IL‐17/TNFα increases airway surface liquid pH of human cystic fibrosis airway epithelia and alters paracellular ion permeabilities
| 48 h vehicle (PBS) | 48 h IL‐17/TNFα | P value | |
|---|---|---|---|
| Native airway surface liquid (ASL) experiments; n = 6 | |||
| ASL pH † | 6.57; [6.54, 6.59] | 7.04; [6.93, 7.19] | <0.0001 |
| 22 mm NaCl dilution potential experiments; n = 6 | |||
| GP (mS cm−2) ‡ | 0.585 ± 0.158 | 1.026 ± 0.472 | 0.03 |
| PCl/Na | 0.79 ± 0.04 | 0.62 ± 0.09 | 0.01 |
| PNa (x10−6 cm s−1) ‡ | 4.85 ± 1.17 | 8.90 ± 3.75 | 0.03 |
| PCl (x10−6 cm s−1) ‡ | 3.83 ± 0.96 | 5.61 ± 2.92 | 0.03 |
| 22 mm NaHCO3 dilution potential experiments; n = 6 | |||
| GP (mS cm−2) | 0.597 ± 0.193 | 1.344 ± 0.390 | 0.002 |
| PHCO3/Na | 0.74 ± 0.08 | 0.69 ± 0.05 | 0.31 |
| PNa (x10−6 cm s−1) | 5.06 ± 1.52 | 11.72 ± 3.07 | 0.0009 |
| PHCO3 (x10−6 cm s−1) | 3.82 ± 1.36 | 8.23 ± 2.75 | 0.007 |
| 150 mm NaCl/KCl bi‐ionic potential experiments; n = 6 | |||
| PK/Na | 1.02 ± 0.04 | 0.87 ± 0.05 | 0.005 |
Data reported as means ± standard deviation
Statistical analyses, including mean and standard deviation, were performed using [H+]. For presentation, the [H+] were converted to pH, and hence the standard deviations are shown as intervals.
P values obtained using two‐tailed paired Student's t test or
Two‐tailed Wilcoxon matched‐pairs ranked sign
Discussion
Our results indicate that human airway epithelia have a paracellular pathway that is as permeable to HCO3 − as to Cl−. The HCO3 − permeability indicates that the paracellular pathway could influence ASL pH. Indeed, under basal conditions, we calculated a small secretory paracellular HCO3 − flux. This flux would tend to counterbalance, in part, the acidic ASL pH produced by H+ secretion (Coakley et al. 2003; Fischer & Widdicombe, 2006; Shah et al. 2016; Lennox et al. 2018; Simonin et al. 2019). After treating epithelia with the proinflammatory cytokines, IL‐13 and IL‐17/TNFα, ASL pH increased. However, at a pH of 7.0, the calculated paracellular HCO3 − fluxes were negligible, and at a pH of 7.4, paracellular HCO3 − fluxes were absorptive. Thus, as the pH of ASL increases towards that of basolateral liquid, paracellular HCO3 − flux becomes absorptive, tempering the alkaline pH generated by transcellular HCO3 − secretion.
We considered the possibility that inflammation could disrupt the barrier function of airway epithelia. Because ASL has a pH and [HCO3 −] that is lower than that of basolateral liquid, barrier disruption might allow basolateral HCO3 − to flow into and alkalinize ASL. Both IL‐13 and IL‐17/TNFα increased ASL pH, whereas IL‐13 did not change Gp or PHCO3, and IL‐17/TNFα increased both. However, both sets of cytokines decreased PCl/Na; the opposite of what would be expected for disruption of the epithelial barrier because the mobility of Cl− in water is approximately 1.5 times that of Na+. Moreover, pH changes in the physiological range did not alter paracellular conductance or PCl/Na, suggesting that effects of cytokines were not secondary to altered pH.
HCO3 − permeated the paracellular pathway as an anion rather than as a CO2 reconversion reaction as indicated by its carbonic anhydrase insensitivity. Moreover, HCO3 − and other tested monovalent anions had similar permeabilities, and all were approximately 3/4 as permeable as Na+, K+, and the other tested monovalent cations. It is possible that the size and charge selectivity arose from the summation of many intercellular spaces with distinct size and charge selectivity. However, these data suggest that the net paracellular transport of airway epithelia can be generalized as weakly cation‐selective with minimal size selectivity.
Our dilution potential experiments revealed that paracellular PCl and PHCO3 were equal. A previous report suggested that PCl was greater than PHCO3 (Coakley et al. 2003). A potential explanation for this difference is that the previous study used bi‐ionic experiments, and the HCO3 − concentration (125 mm HCO3 −, pH 8.15) might result in CO3 2‐ formation and precipitation of extracellular Ca2+, which is required to maintain tight junction integrity (Cereijido et al. 1998; Wang et al. 2000). Disrupted barrier integrity would lead to an inflated PCl because Cl− has a higher ionic mobility in free solution than HCO3 −. However, other culture or technical differences might be responsible.
The proximal tubule, another epithelium with luminal acidification, has a PHCO3 that is less than PCl (Cogan & Alpern, 1984). That arrangement may achieve maximal transepithelial HCO3 − absorption as a result of transcellular HCO3 − absorption with minimal HCO3 − reflux through the paracellular pathway, while allowing paracellular Cl− absorption. The factors that determine the ratio of PHCO3 to PCl in the proximal tubule or in airway epithelia are unknown. However, it may be relevant that absolute paracellular permeabilities in the proximal tubule are much greater than in airway epithelia.
Previous studies have reported that IL‐13 increased (Lennox et al. 2018) and decreased (Haggie et al. 2016) ASL pH. Applying IL‐17 to airway epithelia for two days was also reported to increase ASL pH (Kreindler et al. 2009). We found that treating epithelia for 21 days with IL‐13 or two days with IL‐17/TNFα increased ASL pH. Interestingly, IL‐13 did not alter Gp, and in a separate study, one day of IL‐17/TNFα treatment increased ASL pH but did not alter Gp (Rehman et al. 2020). These differences highlight the likely contribution of time of treatment and identity of cytokine for responses, as has been previously noted (Coyne et al. 2002). In addition to cytokines, second messengers might alter paracellular HCO3 − flux. Previous reports with differentiated airway cell lines suggested that cAMP alters the paracellular conductance (Nilsson et al. 2010; Weiser et al. 2011). Whether or not HCO3 − flux is appreciable under these conditions will depend on PHCO3, Vt, and the apical pH after cAMP stimulation.
Our study has limitations. We used the Goldman–Hodgkin–Katz equation, which assumes a constant electrical field, to obtain ion permeabilities. It is possible that along the lateral space the ion encounters more complex forces than a constant field. We did not consider the unstirred layer effect, which was likely nominal because the osmotic‐induced water permeability of airway epithelia is independent of bath perfusion rates (Folkesson et al. 1996). We did not address paracellular proton permeability, which cannot be measured by electrophysiological methods due to its nanomolar concentration. However, the lumen‐negative transepithelial voltages of airway epithelia predict paracellular proton secretion with inflammatory cytokines, which are not consistent with the alkalinization observed. We performed dilution potential experiments with CF airway epithelia to reduce transcellular Cl− and HCO3 − secretion and extended the permeability values to non‐CF airways. However, LeSimple et al. (LeSimple et al. 2010) found that CFTR expression in immortalized CF epithelial cells increased transepithelial resistance via tight junction assembly. Li et al. (2012) found that CFTR expression in MDCK cells decreased transepithelial resistance. Consistent with a role for CFTR in tight junction assembly, Ruan et al. (2014) found that CFTR co‐localizes with ZO‐1 in the trachea. However, our estimated Gp was similar for CF and non‐CF epithelia.
These data suggest that the paracellular pathway acts as a HCO3 − shunt. Under physiological conditions, the reversal potential for HCO3 − is slightly hyperpolarized relative to Vt and passive HCO3 − flux through the paracellular pathway opposes net transepithelial acidification. Under pathological conditions that increase the ASL pH, such as those modelled by cytokine treatment, the reversal potential for HCO3 − is depolarized relative to the Vt and passive HCO3 − flux opposes net transepithelial alkalinization. This shunting mechanism may help to maintain an optimal ASL pH for antimicrobial activity and mucus rheology.
Additional information
Author contributions
IMT, TR, AAP and MJW conceived and designed the studies. IMT, TR and AAP conducted the experiments and acquired the data. IMT, TR, AAP and MJW analysed the data. IMT and MJW wrote the manuscript. All authors revised the manuscript.
Competing interests
No conflicts of interest, financial or otherwise, are declared by the authors.
Funding
This work was supported by the National Institutes of Health (HL007638) to IMT, HL140261 to AAP, (HL051670 and HL091842) to MJW, and a Cystic Fibrosis Foundation Research Development Program pilot award to TR. IMT is supported by the Gilead Sciences Research Program in Cystic Fibrosis. AAP is supported by the Parker B. Francis Fellowship Program. MJW is an investigator of the Howard Hughes Medical Institute.
Supporting information
Statistical Summary Document
Acknowledgements
GlyH‐101 was a generous gift from the Cystic Fibrosis Foundation Therapeutics and Robert Bridges. We thank the University of Iowa In Vitro Models and Cell Culture Core for their technical assistance. Portions of this work have been published in preliminary form (Thornell et al. 2019a, 2019b).
Biography
Ian Thornell is a Research Assistant Professor of Internal Medicine at the University of Iowa. He trained in ion transport and pH physiology as a graduate student with Mark Bevensee (University of Alabama Birmingham) and as a postdoctoral scholar with Michael Welsh (Howard Hughes Medical Institute and University of Iowa). His lab investigates how transepithelial transport influences physiology and disease using a combination of molecular biology and genetic techniques, animal models, electrophysiology, and optical methods.

Edited by: Peying Fong & Rajini Rao
Linked articles: This article is highlighted in a Perspectives article by Parker. To read this article, visit https://doi.org/10.1113/JP280467.
Contributor Information
Ian M. Thornell, Email: ian-thornell@uiowa.edu.
Michael J. Welsh, Email: michael-welsh@uiowa.edu.
References
- Abou Alaiwa M, Launspach J, Grogan B, Carter S, Zabner J, Stoltz DA, Singh PK, McKone EF & Welsh MJ (2018). Ivacaftor‐induced sweat chloride reductions correlate with increases in airway surface liquid pH in cystic fibrosis. JCI Insight 3, pii: 121468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou Alaiwa MH, Beer AM, Pezzulo AA, Launspach JL, Horan RA, Stoltz DA, Starner TD, Welsh MJ & Zabner J (2014a). Neonates with cystic fibrosis have a reduced nasal liquid pH; A small pilot study. J Cyst Fibros 13, 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, Zabner J & Welsh MJ (2014b). pH modulates the activity and synergism of the airway surface liquid antimicrobials beta‐defensin‐3 and LL‐37. Proc Natl Acad Sci U S A 111, 18703–18708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM & Van Itallie CM (2009). Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1, a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton HC, Jones G & Danahay H (2003). IL‐13‐induced changes in the goblet cell density of human bronchial epithelial cell cultures: MAP kinase and phosphatidylinositol 3‐kinase regulation. Am J Physiol Lung Cell Mol Physiol 285, L730–L739. [DOI] [PubMed] [Google Scholar]
- Cantin AM, Hartl D, Konstan MW & Chmiel JF (2015). Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros 14, 419–430. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Valdes J, Shoshani L & Contreras RG (1998). Role of tight junctions in establishing and maintaining cell polarity. Annu Rev Physiol 60, 161–177. [DOI] [PubMed] [Google Scholar]
- Choy DF, Hart KM, Borthwick LA, Shikotra A, Nagarkar DR, Siddiqui S, Jia G, Ohri CM, Doran E, Vannella KM, Butler CA, Hargadon B, Sciurba JC, Gieseck RL, Thompson RW, White S, Abbas AR, Jackman J, Wu LC, Egen JG, Heaney LG, Ramalingam TR, Arron JR, Wynn TA & Bradding P (2015). TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med 7, 301ra129. [DOI] [PubMed] [Google Scholar]
- Clary‐Meinesz C, Mouroux J, Cosson J, Huitorel P & Blaive B (1998). Influence of external pH on ciliary beat frequency in human bronchi and bronchioles. Eur Respir J 11, 330–333. [DOI] [PubMed] [Google Scholar]
- Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, O'Neal WK & Boucher RC (2003). Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci U S A 100, 16083–16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan MG & Alpern RJ (1984). Regulation of proximal bicarbonate reabsorption. Am J Physiol 247, F387–F395. [DOI] [PubMed] [Google Scholar]
- Conese M, Copreni E, Di Gioia S, De Rinaldis P & Fumarulo R (2003). Neutrophil recruitment and airway epithelial cell involvement in chronic cystic fibrosis lung disease. J Cyst Fibros 2, 129–135. [DOI] [PubMed] [Google Scholar]
- Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC & Johnson LG (2002). Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell 13, 3218–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin CJ, Numa AH, Wang H, Morton JR, Vertzyas CC & Henry RL (2002). Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 165, 904–910. [DOI] [PubMed] [Google Scholar]
- Diamond JM (1978). Channels in epithelial cell membranes and junctions. Fed Proc 37, 2639–2643. [PubMed] [Google Scholar]
- Dickinson JD, Alevy Y, Malvin NP, Patel KK, Gunsten SP, Holtzman MJ, Stappenbeck TS & Brody SL (2016). IL13 activates autophagy to regulate secretion in airway epithelial cells. Autophagy 12, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG & Buchner A (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Ferretti S, Bonneau O, Dubois GR, Jones CE & Trifilieff A (2003). IL‐17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide‐induced airway neutrophilia: IL‐15 as a possible trigger. J Immunol 170, 2106–2112. [DOI] [PubMed] [Google Scholar]
- Fischer H & Widdicombe JH (2006). Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol 211, 139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson HG, Matthay MA, Frigeri A & Verkman AS (1996). Transepithelial water permeability in microperfused distal airways. Evidence for channel‐mediated water transport. J Clin Invest 97, 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland AL, Walton WG, Coakley RD, Tan CD, Gilmore RC, Hobbs CA, Tripathy A, Clunes LA, Bencharit S, Stutts MJ, Betts L, Redinbo MR & Tarran R (2013). Molecular basis for pH‐dependent mucosal dehydration in cystic fibrosis airways. Proc Natl Acad Sci U S A 110, 15973–15978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett JP, Kalsi KK, Sobotta M, Bearham J, Carr G, Powell J, Brodlie M, Ward C, Tarran R & Baines DL (2016). Hyperglycaemia and pseudomonas aeruginosa acidify cystic fibrosis airway surface liquid by elevating epithelial monocarboxylate transporter 2 dependent lactate‐H(+) secretion. Sci Rep 6, 37955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DE (1943). Potential, impedance, and rectification in membranes. J Gen Physiol 27, 37–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrieri G, Scudieri P, Caci E, Schiavon M, Tomati V, Sirci F, Napolitano F, Carrella D, Gianotti A, Musante I, Favia M, Casavola V, Guerra L, Rea F, Ravazzolo R, Di Bernardo D & Galietta LJ (2016). Goblet cell hyperplasia requires high bicarbonate transport to support mucin release. Sci Rep 6, 36016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggie PM, Phuan PW, Tan JA, Zlock L, Finkbeiner WE & Verkman AS (2016). Inhibitors of pendrin anion exchange identified in a small molecule screen increase airway surface liquid volume in cystic fibrosis. FASEB J 30, 2187–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings AB & Sendroy JJ (1925). The effect of variation in ionic strength on the apparent first and second dissociation constants of carbonic acid. J Biol Chem 65, 445–455. [Google Scholar]
- Hauber HP, Gholami D, Koppermann G, Heuer HE, Meyer A & Pforte A (2003). Increased expression of interleukin‐13 but not interleukin‐4 in cystic fibrosis patients. J Cyst Fibros 2, 189–194. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL & Katz B (1949). The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol 108, 37–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, Moninger TO, Michalski AS, Hoffman EA, Zabner J, Stoltz DA & Welsh MJ (2014). Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 345, 818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman S, Song Y & Verkman AS (2001a). Airway surface liquid pH in well‐differentiated airway epithelial cell cultures and mouse trachea. Am J Physiol 281, C1504–C1511. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Song Y, Vetrivel L, Shankar L & Verkman AS (2001b). Noninvasive in vivo fluorescence measurement of airway surface liquid depth, salt concentration, and pH. J Clin Invest 107, 317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh S, Tanabe T & Rubin BK (2011). IL‐13‐induced MUC5AC production and goblet cell differentiation is steroid resistant in human airway cells. Clin Exp Allergy 41, 1747–1756. [DOI] [PubMed] [Google Scholar]
- Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW & Wu R (2004). IL‐17 markedly up‐regulates beta‐defensin‐2 expression in human airway epithelium via JAK and NF‐kappaB signaling pathways. J Immunol 173, 3482–3491. [DOI] [PubMed] [Google Scholar]
- Karp PH, Moninger T, Weber SP, Nesselhauf TS, Launspach J, Zabner J & Welsh MJ (2002). An in vitro model of differentiated human airway epithelia: methods and evaluation of primary cultures In Epithelial Cell Culture Protocols, ed. Wise C, pp. 115–137. Humana Press, Inc., Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ & Riches DW (1995). Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 151, 1075–1082. [DOI] [PubMed] [Google Scholar]
- Kielland J (1937). Individual activity coefficients of ions in aqueous solutions. J Am Chem Soc 59, 1675–1678. [Google Scholar]
- Kim D, Huang J, Billet A, Abu‐Arish A, Goepp J, Matthes E, Tewfik MA, Frenkiel S & Hanrahan JW (2019). Pendrin mediates bicarbonate secretion and enhances CFTR function in airway surface epithelia. Am J Respir Cell Mol Biol 60, 705–716. [DOI] [PubMed] [Google Scholar]
- Kimizuka H & Koketsu K (1964). Ion transport through cell membrane. J Theor Biol 6, 290–305. [DOI] [PubMed] [Google Scholar]
- Knowles MR, Robinson JM, Wood RE, Pue CA, Mentz WM, Wager GC, Gatzy JT & Boucher RC (1997). Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease‐control subjects. J Clin Invest 100, 2588–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreindler JL, Bertrand CA, Lee RJ, Karasic T, Aujla S, Pilewski JM, Frizzell RA & Kolls JK (2009). Interleukin‐17A induces bicarbonate secretion in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 296, L257‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE & Linden A (1999). Neutrophil recruitment by human IL‐17 via C‐X‐C chemokine release in the airways. J Immunol 162, 2347–2352. [PubMed] [Google Scholar]
- Laoukili J, Perret E, Willems T, Minty A, Parthoens E, Houcine O, Coste A, Jorissen M, Marano F, Caput D & Tournier F (2001). IL‐13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest 108, 1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R, Muller MM, Klassert TE, Driesch D, Stock M, Heinrich A, Conrad T, Moore C, Schier UK, Guthke R & Slevogt H (2018). Differential regulation of the transcriptomic and secretomic landscape of sensor and effector functions of human airway epithelial cells. Mucosal Immunol 11, 627–642. [DOI] [PubMed] [Google Scholar]
- Lennox AT, Coburn SL, Leech JA, Heidrich EM, Kleyman TR, Wenzel SE, Pilewski JM, Corcoran TE & Myerburg MM (2018). ATP12A promotes mucus dysfunction during type 2 airway inflammation. Sci Rep 8, 2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSimple P, Liao J, Robert R, Gruenert DC & Hanrahan JW (2010). Cystic fibrosis transmembrane conductance regulator trafficking modulates the barrier function of airway epithelial cell monolayers. J Physiol 588, 1195–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yang W, Mendes F, Amaral MD & Sheppard DN (2012). Impact of the cystic fibrosis mutation F508del‐CFTR on renal cyst formation and growth. Am J Physiol Renal Physiol 303, F1176–F1186. [DOI] [PubMed] [Google Scholar]
- Liu J, Pang Z, Wang G, Guan X, Fang K, Wang Z & Wang F (2017). Advanced role of neutrophils in common respiratory diseases. J Immunol Res 2017, 6710278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs NW, Strieter RM, Chensue SW, Widmer M & Kunkel SL (1995). TNF‐alpha mediates recruitment of neutrophils and eosinophils during airway inflammation. J Immunol 154, 5411–5417. [PubMed] [Google Scholar]
- Manov GG, Bates RG, Hamer WJ & Acree SF (1943). Values of the constants in the Debye—Hückel equation for activity coefficients1. J Am Chem Soc 65, 1765–1767. [Google Scholar]
- McAllister F, Henry A, Kreindler JL, Dubin PJ, Ulrich L, Steele C, Finder JD, Pilewski JM, Carreno BM, Goldman SJ, Pirhonen J & Kolls JK (2005). Role of IL‐17A, IL‐17F, and the IL‐17 receptor in regulating growth‐related oncogene‐alpha and granulocyte colony‐stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol 175, 404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane D, Davies JC, Davies MG, Bush A, Geddes DM & Alton EW (2003). Airway surface pH in subjects with cystic fibrosis. Eur Respir J 21, 37–42. [DOI] [PubMed] [Google Scholar]
- Michel O, Dinh PH, Doyen V & Corazza F (2014). Anti‐TNF inhibits the airways neutrophilic inflammation induced by inhaled endotoxin in human. BMC Pharmacol Toxicol 15, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlebach MS, Stewart PW, Leigh MW & Noah TL (1999). Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med 160, 186–191. [DOI] [PubMed] [Google Scholar]
- Namkung W, Song Y, Mills AD, Padmawar P, Finkbeiner WE & Verkman AS (2009). In situ measurement of airway surface liquid [K+] using a ratioable K+‐sensitive fluorescent dye. J Biol Chem 284, 15916–15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson HE, Dragomir A, Lazorova L, Johannesson M & Roomans GM (2010). CFTR and tight junctions in cultured bronchial epithelial cells. Exp Mol Pathol 88, 118–127. [DOI] [PubMed] [Google Scholar]
- Ostedgaard LS, Moninger TO, McMenimen JD, Sawin NM, Parker CP, Thornell IM, Powers LS, Gansemer ND, Bouzek DC, Cook DP, Meyerholz DK, Abou Alaiwa MH, Stoltz DA & Welsh MJ (2017). Gel‐forming mucins form distinct morphologic structures in airways. Proc Natl Acad Sci U S A 114, 6842–6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD & Kunzelmann K (2009). Loss of TMEM16A causes a defect in epithelial Ca2+‐dependent chloride transport. J Biol Chem 284, 28698–28703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford‐Lenane CL, Haagsman HP, van Eijk M, Banfi B, Horswill AR, Stoltz DA, McCray PB, Jr. , Welsh MJ & Zabner J (2012). Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 487, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzulo AA, Tudas RA, Stewart CG, Buonfiglio LGV, Lindsay BD, Taft PJ, Gansemer ND & Zabner J (2019). HSP90 inhibitor geldanamycin reverts IL‐13‐ and IL‐17‐induced airway goblet cell metaplasia. J Clin Invest 129, 744–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman T, Thornell IM, Pezzulo AA, Thurman AL, Romano Ibarra GS, Karp PH, Tan P, Duffey ME & Welsh MJ (2020). TNFalpha and IL‐17 alkalinize airway surface liquid through CFTR and pendrin. Am J Physiol Cell Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RA & Stokes RH (1959). Electrolye solutions: the measurement and interpretation of conductance, chemical potential, and diffision in solutions of simple electrolytes.
- Ruan YC, Wang Y, Da Silva N, Kim B, Diao RY, Hill E, Brown D, Chan HC & Breton S (2014). CFTR interacts with ZO‐1 to regulate tight junction assembly and epithelial differentiation through the ZONAB pathway. J Cell Sci 127, 4396–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DW, Gaggar A & Solomon GM (2016). Neutrophil fates in bronchiectasis and alpha‐1 antitrypsin deficiency. Ann Am Thorac Soc 13(Suppl 2), S123–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A, Puvvadi R, Borisov SM, Shaw NC, Klimant I, Berry LJ, Montgomery ST, Nguyen T, Kreda SM, Kicic A, Noble PB, Button B & Stick SM (2017). Airway surface liquid pH is not acidic in children with cystic fibrosis. Nat Commun 8, 1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SG (1980). Basic Principles of Membrane Transport. Cambridge University Press, Cambridge. [Google Scholar]
- Scudieri P, Musante I, Caci E, Venturini A, Morelli P, Walter C, Tosi D, Palleschi A, Martin‐Vasallo P, Sermet‐Gaudelus I, Planelles G, Crambert G & Galietta LJ (2018). Increased expression of ATP12A proton pump in cystic fibrosis airways. JCI Insight 3, e123616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah VS, Meyerholz DK, Tang XX, Reznikov L, Abou Alaiwa M, Ernst SE, Karp PH, Wohlford‐Lenane CL, Heilmann KP, Leidinger MR, Allen PD, Zabner J, McCray PBJ, Ostedgaard LS, Stoltz DA, Randak CO & Welsh MJ (2016). Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science 351, 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin J, Bille E, Crambert G, Noel S, Dreano E, Edwards A, Hatton A, Pranke I, Villeret B, Cottart CH, Vrel JP, Urbach V, Baatallah N, Hinzpeter A, Golec A, Touqui L, Nassif X, Galietta LJV, Planelles G, Sallenave JM, Edelman A & Sermet‐Gaudelus I (2019). Airway surface liquid acidification initiates host defense abnormalities in cystic fibrosis. Sci Rep 9, 6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF & Ranganathan SC (2009). Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med 180, 146–152. [DOI] [PubMed] [Google Scholar]
- Smart SJ & Casale TB (1994). Pulmonary epithelial cells facilitate TNF‐alpha‐induced neutrophil chemotaxis. A role for cytokine networking. J Immunol 152, 4087–4094. [PubMed] [Google Scholar]
- Sten‐Knudsen O (2002). Biological Membranes: Theory of Transport, Potentials and Electric Impulses. Cambridge University Press, Cambridge. [Google Scholar]
- Stoppelenburg AJ, Salimi V, Hennus M, Plantinga M, Huis in ’t Veld R, Walk J, Meerding J, Coenjaerts F, Bont L & Boes M (2013). Local IL‐17A potentiates early neutrophil recruitment to the respiratory tract during severe RSV infection. PLoS One 8, e78461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsen S & Nair P (2017). Asthma endotypes and an overview of targeted therapy for asthma. Front Med (Lausanne) 4, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, Choudhury B, Varki A, Stoltz DA & Welsh MJ (2016). Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest 126, 879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thavagnanam S, Parker JC, McBrien ME, Skibinski G, Heaney LG & Shields MD (2011). Effects of IL‐13 on mucociliary differentiation of pediatric asthmatic bronchial epithelial cells. Pediatr Res 69, 95–100. [DOI] [PubMed] [Google Scholar]
- Thornell IM, Rehman T & Welsh MJ (2019a). Inflammatory cytokines IL‐13 or IL‐17/TNFα do not alter the low paracellular bicarbonate permeability of cystic fibrosis airway epithelia. FASEB J 33, 544.15–544.15. [Google Scholar]
- Thornell IM, Rehman T & Welsh MJ (2019b). Paracellular bicarbonate flux is minimal in human cystic fibrosis airway epithelia. Pediatr Pulmonol 54, S164–S164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Slyke DD, Sendroy JJ, Hastings AB & Neill JM (1928). Studies of gas and electrolyte equilibria in blood: X. the solubility of carbon dioxide at 38° in water, salt solution, serum, and blood cells. J Biol Chem 78, 765–799. [Google Scholar]
- Voipio J (1998). Diffusion and buffering aspects of H+, HCO3‐, and CO2 movments in brain tissue In pH and Brain Function, ed. Kaila K. & Ranson BR, pp. 45–66. Wiley‐Liss, New York. [Google Scholar]
- Wang G, Zabner J, Deering C, Launspach J, Shao J, Bodner M, Jolly DJ, Davidson BL & McCray PB, Jr (2000). Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo . Am J Respir Cell Mol Biol 22, 129–138. [DOI] [PubMed] [Google Scholar]
- Weiser N, Molenda N, Urbanova K, Bahler M, Pieper U, Oberleithner H & Schillers H (2011). Paracellular permeability of bronchial epithelium is controlled by CFTR. Cell Physiol Biochem 28, 289–296. [DOI] [PubMed] [Google Scholar]
- Wesolowska‐Andersen A & Seibold MA (2015). Airway molecular endotypes of asthma: dissecting the heterogeneity. Curr Opin Allergy Clin Immunol 15, 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills‐Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL & Donaldson DD (1998). Interleukin‐13: central mediator of allergic asthma. Science 282, 2258–2261. [DOI] [PubMed] [Google Scholar]
- Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC & Erle DJ (2007). IL‐13 and epidermal growth factor receptor have critical but distinct roles in epithelial cell mucin production. Am J Respir Cell Mol Biol 36, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Summary Document


