Abstract
Objective
To assess the safety and efficacy of a novel Wnt pathway modulator, lorecivivint (SM04690), for treating pain and inhibiting structural progression in moderately to severely symptomatic knee osteoarthritis (OA).
Methods
Subjects in this 52‐week, phase IIa, multicenter, randomized, double‐blind, placebo‐controlled, dose‐ranging trial received a single 2‐ml intraarticular injection of lorecivivint (dose of 0.03 mg, 0.07 mg, or 0.23 mg) or placebo. Efficacy was assessed based on change from baseline on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score subscales for pain and function (scale 0–100 for each) and change from baseline in the radiographic medial joint space width (JSW). Baseline‐adjusted analysis of covariance with multiple imputation was performed separately to evaluate efficacy. This proof‐of‐concept study evaluated the intent‐to‐treat population as well as a prespecified group of subjects with unilateral symptoms of knee OA (designated UNI) and an additional post hoc subgroup of subjects with unilateral symptoms but without widespread pain (designated UNI WP−).
Results
In this trial, 455 subjects were randomized to a treatment group. The primary end point, significant improvement in the WOMAC pain score compared with placebo at week 13, was not met by any lorecivivint dose group (mean ± SD change from baseline, −23.3 ± 2.2 in the 0.03 mg group, −23.5 ± 2.1 in the 0.07 mg group, −21.3 ± 2.2 in the 0.23 mg group, and −22.1 ± 2.1 in the placebo group; each P > 0.05 versus placebo). All groups (including placebo) demonstrated clinically meaningful (≥20‐point) improvements from baseline in the WOMAC pain score. The durability of response was evaluated through week 52. In the prespecified UNI group and post hoc UNI WP− group at week 52, treatment with 0.07 mg lorecivivint significantly improved the WOMAC pain score (between‐group difference versus placebo, −8.73, 95% confidence interval [95% CI] −17.44, −0.03 [P = 0.049] and −11.21, 95% CI −20.99, −1.43 [P = 0.025], respectively) and WOMAC function score (between‐group difference versus placebo, −10.26, 95% CI −19.82, −0.69 [P = 0.036] and −13.38, 95% CI −24.33, −2.43 [P = 0.017], respectively). Relative to baseline, the mean change in the medial JSW at week 52 was −0.04 mm in the 0.03 mg cohort, −0.09 mm in the 0.07 mg cohort, −0.16 mm in the 0.23 mg cohort, and −0.14 mm in the placebo cohort; no treatment group achieved a significant change in medial JSW compared with placebo at week 52. In both unilateral symptom subgroups, the 0.07 mg lorecivivint dose significantly increased medial JSW compared with placebo at week 52 (medial JSW 0.39 mm, 95% CI 0.06, 0.72 in the UNI group [P = 0.021] and 0.42 mm, 95% CI 0.04, 0.80 in the UNI WP− group [P = 0.032]). Changes observed in the 0.03 mg and 0.23 mg dose groups were not significantly different from those in the placebo group for any of these measures. Lorecivivint appeared safe and well tolerated.
Conclusion
This phase IIa, proof‐of‐concept trial in patients with symptomatic knee OA did not meet its primary end point. Nevertheless, the study identified a target population in whom to evaluate the potential efficacy of lorecivivint for the treatment of knee OA.
INTRODUCTION
Knee osteoarthritis (OA) is a common, chronic disorder that is characterized by cartilage destruction, subchondral bone thickening, and osteophyte formation, leading to pain, functional limitation, and physical disability 1. The severity of knee OA is assessed by a combination of patient‐reported outcome measures that include assessments of pain and function and objective structural measures such as radiologically assessed joint space narrowing 2. Pharmacologic interventions for knee OA management are symptom‐alleviating treatments, including oral and topical nonsteroidal antiinflammatory drugs (NSAIDs), nonopioid analgesics, and intraarticular (IA) corticosteroid or hyaluronic acid injections 3, 4. However, many of these treatments have limited short‐term and long‐term efficacy 4, 5, 6, 7 and are associated with a high incidence of side effects. There remains a great unmet need for new treatments that provide symptom relief and even more of a need for disease‐modifying OA drugs (DMOADs).
The Wnt pathway is integral for tissue homeostasis and regeneration 8, 9 and is a key regulator of progenitor cell differentiation in the knee joint 10. Cartilage homeostasis requires a balance of Wnt pathway activity. While necessary for chondrocyte differentiation and function 11, aberrant Wnt pathway activity in OA directs progenitor cell differentiation in the joint toward development of osteoblasts instead of chondrocytes 12. Excessive activation of the Wnt pathway is known to increase OA susceptibility in animals and humans 13, 14, 15, 16, whereas excessive inhibition of the Wnt pathway can cause cartilage and bone destruction 17, 18, 19. Therefore, a potential Wnt pathway–targeted DMOAD approach would need to maintain signaling within an optimal range.
Lorecivivint (SM04690) is a small‐molecule Wnt pathway modulator currently in development as a potential DMOAD for the treatment of knee OA 20, 21. Lorecivivint affects Wnt pathway activity via inhibition of 2 intranuclear targets, CDC‐like kinase 2 (CLK2) and dual‐specificity tyrosine phosphorylation‐regulated kinase 1A (DYRK1A), through which it acts both independently and in combination to improve chondrocyte health and function while inhibiting inflammation 22. Preclinical studies, including repeat dosing in rats and dogs, have found the no‐observed‐adverse‐effect level to be ~400 times the planned dose in humans (data on file). In vitro studies demonstrated that lorecivivint modulated Wnt signaling, reduced release of matrix‐degrading enzymes from chondrocytes, demonstrated anabolic activity in chondrocytes, and reduced STAT3 signaling, NF‐κB signaling, and inflammatory cytokine production in synoviocytes 20. In a rat model of anterior cruciate ligament transection and partial medial meniscectomy‐induced knee OA, a single IA injection of lorecivivint protected chondrocytes from catabolic breakdown 20.
In a previous phase I, randomized, placebo‐controlled trial (n = 61), a single IA injection of lorecivivint at a dose of 0.03 mg, 0.07 mg, or 0.23 mg administered into the target knee joint of subjects with moderately to severely symptomatic knee OA appeared safe and well tolerated and showed no evidence of systemic exposure. While all lorecivivint and placebo groups demonstrated improvements from baseline in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and function subscale scores at week 24, the 0.07 mg lorecivivint treatment group demonstrated more favorable reductions in both WOMAC indices as compared with placebo. Treatment with 0.07 mg lorecivivint also resulted in increased radiographic joint space width (JSW) beyond a minimum detectable difference of 0.13 mm 23, thus suggesting that lorecivivint may be a potential DMOAD for use in treating knee OA. Therefore, the objective of this phase IIa trial was to evaluate the safety and efficacy of lorecivivint among subjects with moderately to severely symptomatic knee OA.
SUBJECTS AND METHODS
Study design
This was a 52‐week, phase IIa, multicenter, randomized, double‐blind, placebo‐controlled, dose‐ranging trial of 3 different dose concentrations of lorecivivint injected into the target (most painful) knee joint of subjects with moderately to severely symptomatic knee OA. This study was conducted at 36 clinical sites in the United States between September 2015 and April 2017. Subjects participated in a screening period of up to 21 days and were periodically observed during a 52‐week follow‐up period. Visits were scheduled at screening, treatment visit day 1, and at follow‐up weeks 4, 13, 26, 39, and 52.
On day 1, subjects were randomized to receive a single 2‐ml IA injection of lorecivivint at a dose of 0.03 mg, 0.07 mg, or 0.23 mg or phosphate buffered saline as placebo. These 3 doses of lorecivivint corresponded to the lower, middle, and upper therapeutic ranges that were established previously in preclinical studies (data on file). Randomization was accomplished using Medidata Balance (Medidata Solutions, Inc.) such that eligible subjects were randomized at a ratio of 1:1:1:1 using a permuted block design, with a block size of 8 and stratification by site. An unblinded pharmacist at each site mixed the working dose from a common stock solution bottle, and an unblinded injector performed the injection. Ultrasound guidance and joint aspiration (up to 0.5 cc) were allowed if these were considered part of the site's standard IA injection protocol for joint placement. All unblinded site personnel were instructed to minimize any contact with study subjects and were not allowed to perform any study assessments. All study investigators and subjects were blinded with regard to group assignment, and subject blinding was maintained by not allowing any subject to witness the injection.
This study was conducted in accordance with the ethics principles of the Declaration of Helsinki, the International Conference on Harmonisation Guidelines for Good Clinical Practice, and applicable regulations. The study protocol was approved by each clinic site's independent ethics committee or institutional review board. All subjects provided written informed consent prior to participating in any study‐related procedures.
Subjects
Eligible subjects were ages 40–80 years with an established diagnosis of primary knee OA (established within ≥6 months prior to study start) and fulfilled American College of Rheumatology clinical and radiographic classification criteria 24. Enrolled subjects were required to have Kellgren/Lawrence (K/L) radiographic disease stage 2 or stage 3 OA in their target knee (defined at screening as the knee with greater pain based on the subject's evaluation and the investigator's clinical judgment) 25. Subjects were required to have a pain visual analog scale (VAS) score of 30–80 mm (on 100‐mm VAS) 26 and a WOMAC total score of 72–192 (of 240) 27 for the target knee at screening. There were no limitations on contralateral knee pain. Subjects were considered eligible if they were in good general health and ambulatory; assistive devices (e.g., canes) were allowed if needed <50% of the time, whereas any use of a walker was excluded.
Key exclusion criteria included male subjects with female partners of childbearing potential who refused to use an effective contraceptive method and women who were of childbearing potential, pregnant, or lactating. Further exclusions included body mass index (BMI) >40 kg/m2, history of partial or complete joint replacement in the target knee, previous exposure to lorecivivint, a major surgery (e.g., interventional arthroscopy) in the target knee within 52 weeks prior to study medication injection, and any planned or elective surgery anywhere in the body during the study period. Additional exclusion criteria included having comorbid conditions that could affect pain assessment of the target knee or a history of malignancy (except for in situ cancer or basal or squamous cell skin cancer) <5 years prior to injection. Subjects could not receive any IA injections of glucocorticoids, hyaluronic acid, or other therapeutic agents into either knee during the study or within 2 months, 6 months, or 1 month prior to randomization, respectively. Electrotherapy or acupuncture for knee OA, chiropractic knee adjustments, or planned or elective surgery (e.g., arthroscopy) were also prohibited. Subjects could not take opioid analgesics or oral glucocorticoids during the study, although a stable background regimen of NSAIDs and acetaminophen was allowed provided that they were not taken within 24 hours prior to study visits.
Data collection
Subject characteristics, medical history, weight, and height were collected at screening. Unilateral or bilateral symptomatic knee OA status was designated by investigators at baseline based on the findings from history and physical examination. To assess comorbidity‐related pain and symptoms, subjects completed the fibromyalgia diagnostic questionnaire, Widespread Pain Index (WPI) (total score range 0–19), and Symptom Severity Scale (total score range 0–12) at screening 28.
Efficacy assessments
Efficacy assessments administered at all study visits included the WOMAC questionnaire (version NRS 3.1) to assess pain (subscale range 0–100 [no pain–extreme pain]) and function (subscale range 0–100 [no difficulty with daily activities–extreme difficulty]) and the patient global assessment of disease activity (PtGA) (VAS score range 0–100 mm [“doing very well”–“doing very poorly”]). Fixed‐flexion, posterior‐anterior radiographs of the tibiofemoral compartments were obtained using a QuAP positioner at screening, week 26, and week 52. Quality control assessments of the radiographs were conducted at a central imaging laboratory (Medical Metrics Labs) in blinded manner (blinded with regard to treatment assignment), and medial JSW was measured using a landmark‐based, fixed‐location method.
The primary efficacy end point was the change from baseline in WOMAC pain score in the target knee at week 13 in the 0.07 mg lorecivivint group compared with the placebo group. Key secondary end points included 1) change from baseline in WOMAC pain score in the target knee at week 26, 2) change from baseline in WOMAC function score in the target knee at weeks 13 and 26, 3) change from baseline in the PtGA score at weeks 13 and 26, and 4) change from baseline in medial JSW in the target knee at week 26. Exploratory end points included 1) change from baseline in WOMAC pain and function scores in the target knee at weeks 4, 39, and 52, 2) change from baseline in the PtGA score at weeks 4, 39, and 52, and 3) change from baseline in medial JSW in the target knee at week 52.
Safety
Safety was assessed by evaluating the incidence, severity, and seriousness of treatment‐emergent adverse events (TEAEs) and clinically significant changes in clinical laboratory measures and vital signs; no formal statistical analyses were planned for safety outcomes. Safety measures were summarized for all treatment groups as treated, not as randomized.
Sample size
A sample size of ~445 subjects was planned for this trial based on standard statistical practice to establish an acceptable level of precision with respect to treatment effect estimation 29; no formal calculation was used a priori to determine sample size. However, based on data from phase Ib trials and historical data, a Monte Carlo simulation was conducted to estimate the possible power of using the WOMAC pain score (estimated power of 95.8%) and WOMAC function score (estimated power of 78.5%) to estimate treatment effect given this sample size.
Statistical analysis
Statistical analyses were conducted using SAS version 9.4 (SAS Institute). Baseline characteristics of the subjects in each treatment group are presented as the mean ± SD for continuous variables and as the frequency (proportion of patients) for categorical variables. Efficacy outcome measures were evaluated using analysis of covariance models adjusted for baseline values under the intent‐to‐treat (ITT) analysis set (i.e., comprising all subjects as randomized). Multiple imputation under the missing‐at‐random assumption was performed for efficacy outcomes with missing values. The least‐squares estimate of the difference in change in the outcome from baseline at each time point between each lorecivivint dose group and placebo, adjusted for baseline value, is reported along with the 95% confidence intervals (95% CIs). The familywise error rate for the efficacy analyses was controlled in the strong sense (i.e., regardless of whether the global null hypothesis is true) using the closed, fixed‐sequence testing method 30, 31. Hypothesis tests were evaluated in a prespecified, sequential order that matched clinical inference from prior lorecivivint studies regarding the relative therapeutic benefit of each dose (the first hypothesis test evaluated the change in WOMAC pain score at week 13 between the 0.07 mg lorecivivint group and placebo group). If a hypothesis test did not meet the critical significance level of α = 0.05, all subsequent tests were considered to be exploratory. The fixed sequence is detailed in the statistical analysis plan (see Supplementary Table 1 [http://onlinelibrary.wiley.com/doi/10.1002/art.41315/abstract]).
In support of the primary and secondary end points, interim analyses were conducted after all subjects completed the week 26 visit and prior to completion of the trial at 52 weeks. An exploratory analysis was prespecified for a clinically relevant subject population with unilateral symptomatic knee OA (designated the UNI subgroup), defined on the basis of the history and physical examination identifying unilateral symptomatic knee OA. A second exploratory analysis was prespecified on the basis of the WPI score, but this analysis did not have sufficient data to pursue further. A post hoc exploratory analysis was subsequently completed based on subjects with unilateral symptomatic knee OA but without widespread pain (designated the UNI WP– subgroup), defined as having a WPI score of ≤4 and Symptom Severity Scale question 2 score of ≤2 (disregarding question 3).
A post hoc concordance analysis was conducted to estimate the ability of one outcome to predict another outcome. It employed within‐group logistic regression to estimate the likelihood of baseline‐adjusted changes in medial JSW being associated with positive clinical responses (i.e., achieving both WOMAC pain and WOMAC function score improvements of ≥50% [relative change] and ≥20 points [of 100]). The area under the curve (AUC) of receiver operator characteristic curves represented the concordance between change in medial JSW and clinical response. Concordance was defined as “acceptable” when the AUC was >0.7 and “excellent” when the AUC was >0.8 30; an AUC of 0.5 represents concordance that is no better than statistical chance.
RESULTS
Subject disposition and baseline characteristics
Overall, 1,033 subjects were screened and 455 (44.0%) were randomized; 3 subjects were removed from the study prior to administration of a study drug injection (Figure 1). Cohorts of 112 subjects, 117 subjects, 109 subjects, and 114 subjects were randomized to receive 0.03 mg lorecivivint, 0.07 mg lorecivivint, 0.23 mg lorecivivint, or placebo, respectively. For subjects in the UNI group (n = 164), cohort sizes were 45 subjects, 35 subjects, 45 subjects, and 39 subjects in the 0.03 mg lorecivivint, 0.07 mg lorecivivint, 0.23 mg lorecivivint, and placebo groups, respectively. For subjects in the UNI WP− group (n = 128), cohort sizes were 34 subjects, 29 subjects, 33 subjects, and 32 subjects in the 0.03 mg lorecivivint, 0.07 mg lorecivivint, 0.23 mg lorecivivint, and placebo groups, respectively.
Figure 1.
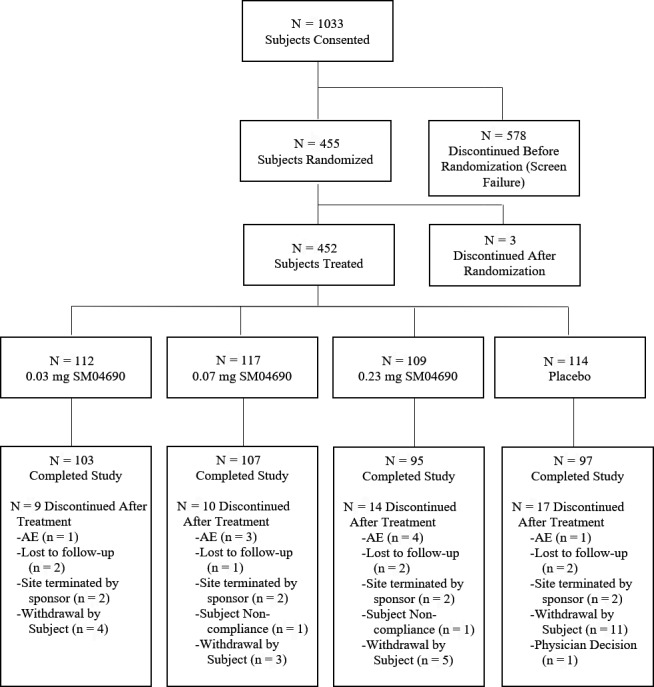
Disposition of the study subjects for the phase II, randomized trial of lorecivivint (SM04690) versus placebo and primary reasons for discontinuation. AE = adverse event.
Among the subjects who completed the study, 103 (92%) were in the 0.03 mg cohort, 107 (91.5%) were in the 0.07 mg cohort, 95 (86.4%) were in the 0.23 mg cohort, and 97 (83.6%) were in the placebo cohort. At enrollment, the mean ± SD age of the subjects was 60.3 ± 8.7 years and the mean ± SD BMI was 29.9 ± 4.6 kg/m2. Overall, 268 (58.9%) of the enrolled subjects were women, 392 (86.2%) were white, 292 (64.2%) had a K/L radiographic OA severity grade of 3 in the target knee, and 164 (36.0%) were classified as having unilateral symptomatic disease (Table 1). Among the 424 subjects who were assigned a K/L radiographic severity grade for the nontarget knee, 386 (91%) had equal or worse radiographic disease in the nontarget knee. In general, the baseline characteristics of the subjects were balanced between the treatment groups.
Table 1.
Demographic and clinical characteristics of the eligible subjects at baseline, by treatment groupa
| Lorecivivint | Placebo (n = 114) | |||
|---|---|---|---|---|
| 0.03 mg (n = 112) | 0.07 mg (n = 117) | 0.23 mg (n = 109) | ||
| Age, mean ± SD years | 59.0 ± 9.0 | 60.0 ± 8.2 | 61.3 ± 8.7 | 60.7 ± 8.9 |
| Body mass index, mean ± SD kg/m2 | 29.77 ± 4.81 | 30.81 ± 4.74 | 29.64 ± 4.45 | 29.17 ± 4.40 |
| Female, no. (%) | 68 (60.7) | 60 (51.3) | 68 (61.8) | 72 (62.1) |
| Race/ethnicity, no. (%) | ||||
| White | 92 (82.1) | 102 (87.2) | 96 (87.3) | 102 (87.9) |
| African American | 18 (16.1) | 14 (12.0) | 12 (10.9) | 10 (8.6) |
| Hispanic or Latino | 20 (17.9) | 23 (19.7) | 17 (15.5) | 21 (18.1) |
| Other | 2 (1.8) | 1 (0.9) | 2 (1.8) | 4 (3.4) |
| K/L grade 3 radiographic OA, no. (%) | 74 (66.1) | 74 (63.2) | 70 (63.6) | 74 (63.8) |
| Unilateral symptomatic knee OA, no. (%) | 45 (40.2) | 35 (29.9) | 45 (40.9) | 39 (33.6) |
| WPI ≤4 and SS Scale score ≤2, no. (%) | 73 (65.2) | 79 (67.5) | 76 (69.1) | 75 (64.7) |
K/L = Kellgren/Lawrence; OA = osteoarthritis; WPI = Widespread Pain Index; SS = Symptom Severity (question 2).
Clinical outcomes
All subjects
The differences in change from baseline in WOMAC pain scores between the lorecivivint dose groups and the placebo group were not statistically significant at week 13 (mean ± SD change from baseline, −23.3 ± 2.2 in the 0.03 mg group, −23.5 ± 2.1 in the 0.07 mg group, −21.3 ± 2.2 in the 0.23 mg group, and −22.1 ± 2.1 in the placebo group; each P > 0.05 versus placebo); thus, the primary end point was not met, and all analyses were considered exploratory. However, subjects in all of the lorecivivint dose groups and the placebo group achieved at least a 20‐point mean improvement from baseline in the WOMAC pain and function subscale scores at week 13 through week 52 and in the PtGA score at all time points postinjection (Figures 2A and 3A, and Supplementary Figure 1A, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41315/abstract). Treatment with 0.07 mg lorecivivint led to numerically larger improvements from baseline in the pain and function scores as compared with treatment with either the 0.03 mg or 0.23 mg dose of lorecivivint. This was apparent starting at 13 weeks postinjection and continued through 52 weeks.
Figure 2.
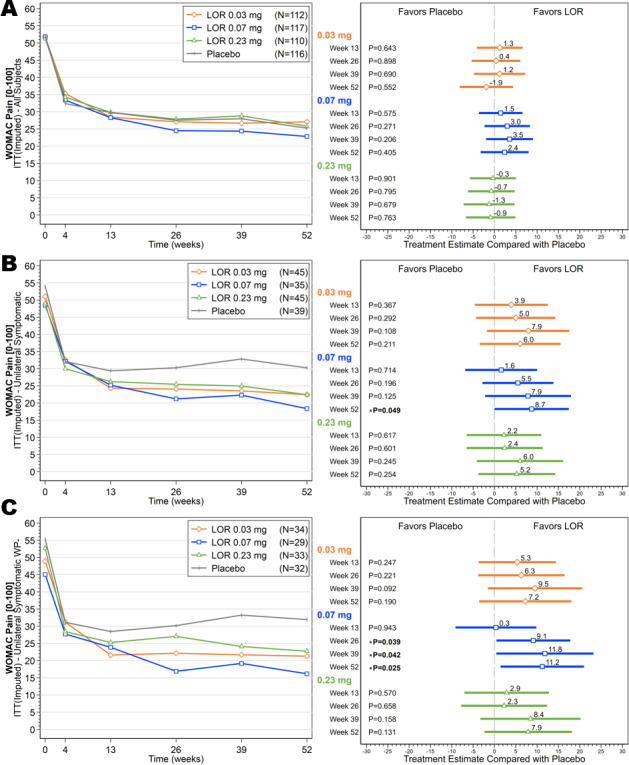
Mean scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale over time (left) and ladder plots (mean and 95% confidence intervals) of baseline‐adjusted change from baseline in the WOMAC pain scores (right), comparing the lorecivivint (LOR) dose groups and the placebo group over time in the intent‐to‐treat (ITT) analysis set (A), subjects with unilateral symptomatic knee osteoarthritis (OA) (B), and subjects with unilateral symptomatic knee OA but without widespread pain (WP–) (C).
Figure 3.
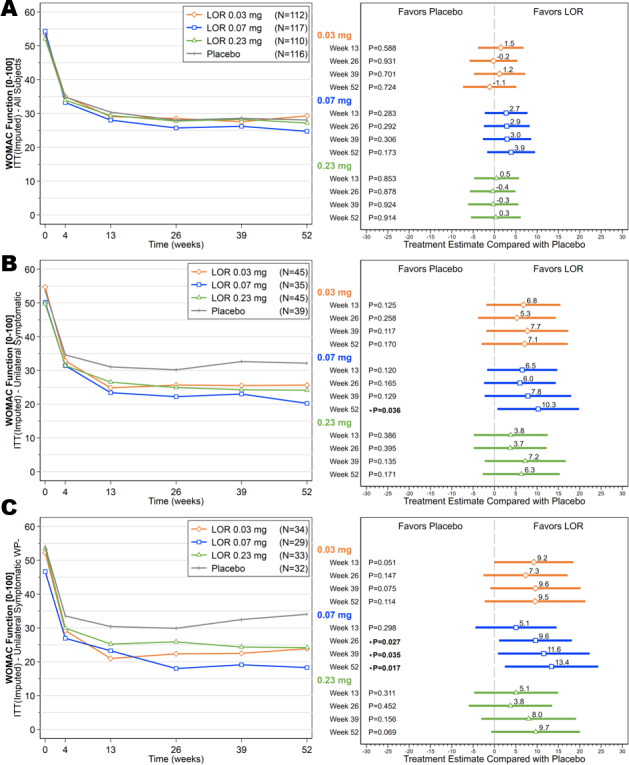
Mean scores on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) function subscale over time (left) and ladder plots (mean and 95% confidence intervals) of baseline‐adjusted change from baseline in the WOMAC function scores (right), comparing the lorecivivint (LOR) dose groups and the placebo group over time in the intent‐to‐treat (ITT) analysis set (A), subjects with unilateral symptomatic knee osteoarthritis (OA) (B), and subjects with unilateral symptomatic knee OA but without widespread pain (WP–) (C).
UNI group
Among the subjects in the UNI subgroup, those receiving the 0.07 mg dose of lorecivivint demonstrated improvements in baseline‐adjusted change in WOMAC pain and function scores compared with placebo at week 13, which continued through week 52 (Figures 2B and 3B). At week 52, the 0.07 mg lorecivivint group had significantly lower scores on the WOMAC pain subscale compared with the placebo group (between‐group difference, −8.73, 95% CI −17.44, −0.03; P = 0.049) and significantly lower scores on the WOMAC function subscale compared with the placebo group (between‐group difference, −10.26, 95% CI −19.82, −0.69; P = 0.036). In this subgroup of patients with unilateral symptomatic knee OA, there were no significant differences in either the WOMAC pain score or WOMAC function score between the 0.03 mg or 0.23 mg lorecivivint dose groups compared with the placebo group (Figures 2B and 3B). The 0.03 mg treatment group showed significant improvement in the PtGA score compared with the placebo group at weeks 13 and 26 (see Supplementary Figure 1B [http://onlinelibrary.wiley.com/doi/10.1002/art.41315/abstract]).
UNI WP− group
Among the subjects in the UNI WP− subgroup, those receiving 0.07 mg lorecivivint demonstrated significant improvements in the WOMAC pain and function scores compared with the placebo group. The between‐group difference in the WOMAC pain score was −9.11 (95% CI −17.75, −0.47) (P = 0.039) at week 26, −11.83 (95% CI −23.23, −0.42) (P = 0.042) at week 39, and −11.21 (95% CI −20.99, −1.43) (P = 0.025) at week 52. The between‐group difference in the WOMAC function score was −9.62 (95% CI −18.14, −1.10) (P =0.027) at week 26, −11.57 (95% CI −22.31, −0.82) (P = 0.035) at week 39, and −13.38 (95% CI −24.33, −2.43) (P = 0.017) at week 52 (Figures 2C and 3C). In this subgroup of patients with unilateral symptomatic knee OA but without widespread pain, there were no significant differences in change in pain or function scores between the 0.03 mg or 0.23 mg lorecivivint dose groups and the placebo group (Figures 2C and 3C). However, the 0.03 mg treatment group showed a significant improvement in the PtGA score compared with the placebo group at week 13, and both the 0.03 mg and 0.07 mg treatment groups showed significant improvements in the PtGA score compared with the placebo group at week 26 in this post hoc analysis (see Supplementary Figure 1C [http://onlinelibrary.wiley.com/doi/10.1002/art.41315/abstract]).
Radiographic outcomes
All subjects
Compared with the values at baseline, the mean change in medial JSW was −0.07 mm at week 26 and −0.04 mm at week 52 in the 0.03 mg cohort, −0.11 mm at week 26 and −0.09 mm at week 52 in the 0.07 mg cohort, −0.02 mm at week 26 and −0.16 mm at week 52 in the 0.23 mg cohort, and −0.20 mm at week 26 and −0.14 mm at week 52 in the placebo cohort (Figure 4A). At week 26, the mean change in medial JSW in those receiving the 0.23 mg dose of lorecivivint was significantly different from that in the placebo group (between‐group difference, 0.19 mm, 95% CI 0.02, 0.36; P = 0.032). At week 52, the mean change in medial JSW in the 0.03 mg and 0.07 mg lorecivivint dose groups was similar to that seen at week 26, whereas the mean change in the 0.23 mg lorecivivint dose group and placebo group had declined (Figure 4A). Among all subjects, both treatment with 0.03 mg lorecivivint (at week 52, change from baseline −0.04 mm) and treatment with 0.07 mg lorecivivint (at week 52, change from baseline −0.09 mm), but not treatment with 0.23 mg lorecivivint, maintained the medial JSW when compared with that in the placebo group (at week 52, change from baseline −0.14 mm); however, the differences were not statistically significant.
Figure 4.
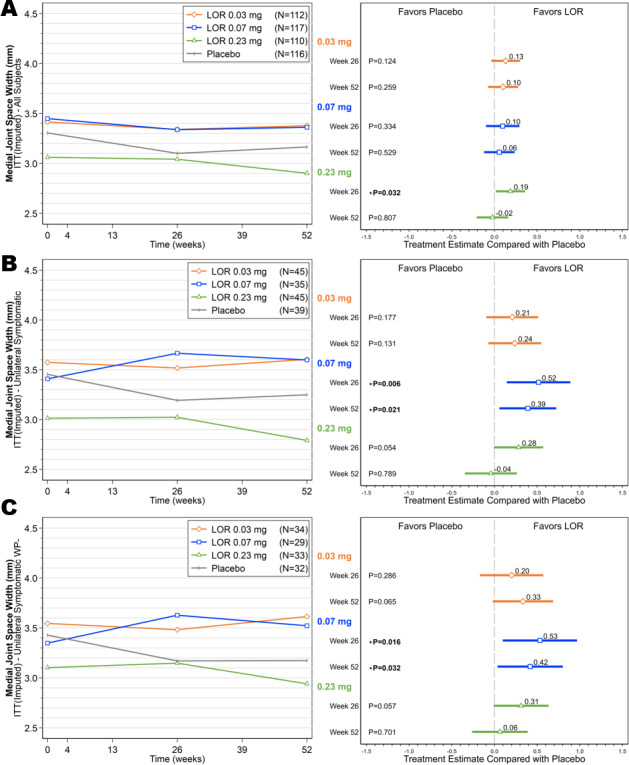
Mean medial joint space width (JSW) measurements over time (left) and ladder plots (mean and 95% confidence intervals) of baseline‐adjusted change from baseline in the medial JSW (right), comparing the lorecivivint (LOR) dose groups and the placebo group over time in the intent‐to‐treat (ITT) analysis set (A), subjects with unilateral symptomatic knee osteoarthritis (OA) (B), and subjects with unilateral symptomatic knee OA but without widespread pain (WP–) (C).
UNI group
Subjects in the UNI subgroup treated with 0.07 mg lorecivivint showed improvements in the medial JSW at weeks 26 and 52 (mean change from baseline 0.26 mm and 0.19 mm, respectively), whereas subjects in the UNI subgroup treated with placebo showed worsening of medial JSW (mean change from baseline −0.26 mm and −0.21 mm, respectively) (Figure 4B). The differences between the 0.07 mg lorecivivint group and the placebo group were significant at both time points; the mean change in medial JSW was 0.52 mm (95% CI 0.15, 0.89) at week 26 (P = 0.006) and 0.39 mm (95% CI 0.06, 0.72) at week 52 (P = 0.021). Among these subjects with unilateral symptomatic knee OA, there were no significant differences in the change in medial JSW when comparing the 0.03 mg or 0.23 mg lorecivivint group with the placebo group (Figure 4B).
UNI WP− group
In the UNI WP− subgroup, the 0.07 mg lorecivivint treatment group demonstrated improved medial JSW at week 26 (mean change from baseline 0.28 mm) and week 52 (mean change from baseline 0.17 mm), whereas the placebo treatment group had worsening of medial JSW at both time points (mean change from baseline −0.26 mm and −0.26 mm, respectively) (Figure 4C). The differences between the 0.07 mg lorecivivint group and the placebo group were significant at both time points (between‐group difference, 0.53 mm, 95% CI 0.10, 0.97 at week 26 [P = 0.016] and 0.42 mm, 95% CI 0.04, 0.80 at week 52 [P = 0.032]). Subjects with unilateral symptomatic OA but without widespread pain in the 0.03 mg or 0.23 lorecivivint treatment groups showed no significant differences in change in medial JSW compared with the placebo group (Figure 4C).
Concordance between change in medial JSW and clinical response
In the all‐subjects analysis, no treatment group achieved an AUC of >0.7 (a measure of concordance between change in medial JSW and clinical response) (see Supplementary Figure 2A, available on the Arthritis & Rheumatology website at http://onlinelibrary.wiley.com/doi/10.1002/art.41315/abstract). Among subjects receiving the 0.07 mg dose of lorecivivint, concordance was “acceptable” (AUC 0.783) in the UNI subgroup and “excellent” (AUC 0.825) in the UNI WP− subgroup (Supplementary Figures 2B and C [http://onlinelibrary.wiley.com/doi/10.1002/art.41315/abstract]). No other doses in either subgroup achieved an AUC >0.7.
Safety
No clinically significant safety concerns with respect to vital signs, clinical laboratory results, or AEs were observed; rates were comparable between the lorecivivint and placebo groups. No deaths were reported during the study. Fifteen subjects incorrectly received a study injection that diverged from that prescribed by the protocol; these subjects are described as “Other” in the safety analysis.
In total, 547 TEAEs were reported by 237 subjects (52.4% of 452 subjects), of which 40 AEs in 32 subjects (7.1%) were deemed related to the study drug by the investigator (Table 2). Among the treatment groups, 142 TEAEs were reported by 61 subjects in the 0.03 mg cohort (55.0% of 111 treated subjects), 147 TEAEs by 65 subjects in the 0.07 mg cohort (57.0% of 114 treated subjects), 107 TEAEs by 47 subjects in the 0.23 mg cohort (45.2% of 104 treated subjects), 117 TEAEs by 53 subjects in the placebo cohort (49.1% of 108 treated subjects), and 34 TEAEs by 11 subjects who were classified in the “Other” dose group (73.3% of 15 subjects). Arthralgia, defined for this study as an exacerbation (increase in frequency, severity, or specificity) of an existing condition, was the most common AE reported across all study cohorts with 61 AEs reported by 49 subjects (10.8% of 452 treated subjects); 38 AEs in 36 subjects (8.0% of the 452 treated subjects) were reported to occur in the target knee. There were 10 AEs in 10 subjects that occurred in the nontarget knee and 12 AEs in 11 subjects that were reported to occur in other (non‐knee) joints (7 hips, 4 elbows, and 1 wrist).
Table 2.
TEAEs with a reported frequency of >1%, by treatment groupa
| Reported TEAEs | Lorecivivint | Placebo (n = 108) | All subjects (n = 452)b | ||
|---|---|---|---|---|---|
| 0.03 mg (n = 111) | 0.07 mg (n = 114) | 0.23 mg (n = 104) | |||
| Total TEAEs | 142/61 (55.0) | 147/65 (57.0) | 107/47 (45.2) | 117/53 (49.1) | 547/237 (52.4) |
| Arthralgia | 16/13 (11.7) | 14/13 (11.4) | 13/9 (8.7) | 12/10 (9.3) | 61/49 (10.8) |
| Back pain | 0/0 (0.0) | 2/2 (1.8) | 1/1 (1.0) | 2/2 (1.9) | 5/5 (1.1) |
| Bronchitis | 2/2 (1.8) | 3/3 (2.6) | 3/2 (1.9) | 1/1 (0.9) | 9/8 (1.8) |
| Bursitis | 2/2 (1.8) | 3/2 (1.8) | 2/2 (1.9) | 0/0 (0.0) | 7/6 (1.3) |
| Contusion | 1/1 (0.9) | 2/2 (1.8) | 3/3 (2.9) | 2/2 (1.9) | 8/8 (1.8) |
| Cystitis | 0/0 (0.0) | 3/3 (2.6) | 2/1 (1.0) | 1/1 (0.9) | 6/5 (1.1) |
| Fall | 2/2 (1.8) | 2/2 (1.8) | 0/0 (0.0) | 1/1 (0.9) | 5/5 (1.1) |
| Gastroenteritis | 3/3 (2.7) | 0/0 (0.0) | 1/1 (1.0) | 1/1 (0.9) | 5/5 (1.1) |
| Headache | 0/0 (0.0) | 6/3 (2.6) | 2/2 (1.9) | 4/4 (3.7) | 13/10 (2.2) |
| Hypertension | 0/0 (0.0) | 4/4 (3.5) | 4/4 (3.8) | 3/3 (2.8) | 11/11 (2.4) |
| Increased AST level | 2/2 (1.8) | 1/1 (0.9) | 0/0 (0.0) | 2/2 (1.9) | 5/5 (1.1) |
| Influenza | 4/4 (3.6) | 0/0 (0.0) | 2/2 (1.9) | 0/0 (0.0) | 6/6 (1.3) |
| Joint effusion | 5/4 (3.6) | 2/2 (1.8) | 1/1 (1.0) | 2/2 (1.9) | 10/9 (2.0) |
| Joint injury | 2/2 (1.8) | 0/0 (0.0) | 1/1 (1.0) | 1/1 (0.9) | 6/6 (1.3) |
| Joint swelling | 5/3 (2.7) | 4/4 (3.5) | 2/2 (1.9) | 6/5 (4.6) | 17/14 (3.1) |
| Meniscus injury | 2/2 (1.8) | 2/2 (1.8) | 0/0 (0.0) | 0/0 (0.0) | 5/5 (1.1) |
| Nasopharyngitis | 4/4 (3.6) | 3/3 (2.6) | 3/3 (2.9) | 0/0 (0.0) | 11/11 (2.4) |
| Nausea | 2/2 (1.8) | 1/1 (0.9) | 2/2 (1.9) | 1/1 (0.9) | 6/6 (1.3) |
| Noncardiac chest pain | 1/1 (0.9) | 2/2 (1.8) | 1/1 (1.0) | 1/1 (0.9) | 6/6 (1.3) |
| Osteoarthritis | 4/3 (2.7) | 2/2 (1.8) | 3/3 (2.9) | 5/3 (2.8) | 14/11 (2.4) |
| Sinusitis | 1/1 (0.9) | 2/2 (1.8) | 1/1 (1.0) | 5/5 (4.6) | 9/9 (2.0) |
| Tendinitis | 3/3 (2.7) | 1/1 (0.9) | 1/1 (1.0) | 1/1 (0.9) | 6/6 (1.3) |
| Upper respiratory tract infection | 5/5 (4.5) | 2/2 (1.8) | 1/1 (1.0) | 3/3 (2.8) | 12/12 (2.7) |
| Urinary tract infection | 2/2 (1.8) | 2/2 (1.8) | 3/2 (1.9) | 3/3 (2.8) | 10/9 (2.0) |
Values are the number of reported treatment‐emergent adverse events (TEAEs)/number of unique subjects reporting the event (% of treatment group). AST = aspartate aminotransferase.
The group of all subjects includes those who received a dose of lorecivivint or placebo that was not specified per protocol (n = 15).
Twenty‐nine serious adverse events (SAEs) were reported by 17 subjects (3.8% of 452 subjects), and all were deemed unrelated to the study drug by the investigator. Seven SAEs were reported by 5 subjects in the 0.03 mg cohort (4.5% of 111 treated subjects), 12 SAEs by 4 subjects in the 0.07 mg cohort (3.5% of 114 treated subjects), 5 SAEs by 4 subjects in the 0.23 mg cohort (3.8% of 104 treated subjects), 3 SAEs by 3 subjects in the placebo cohort (2.8% of 108 treated subjects), and 2 SAEs by 1 subject who received an unidentified dose (6.7% of 15 subjects in the “Other” group). Within the 0.07 mg cohort, 6 cardiovascular SAEs werereported by 1 subject. The other 6 SAEs that occurred in the 0.07 mg cohort were distributed among the other 3 subjects within this cohort. The most common SAEs included infections and cardiac disorders (see Supplementary Table 2 [http://onlinelibrary.wiley.com/doi/10.1002/art.41315/abstract]).
DISCUSSION
In this phase IIa, 52‐week, randomized, placebo‐controlled, proof‐of‐concept clinical trial among subjects with moderately to severely symptomatic knee OA, there was no statistically significant difference in improvement in WOMAC pain scores between treatment groups at week 13. However, IA injection of lorecivivint generally appeared safe and well tolerated. There were no meaningful differences in the incidence of AEs between the lorecivivint dose groups and the placebo group. Moreover, no SAEs were deemed related to the study treatment by the investigators.
Though the primary end point of this study was not met, additional preplanned and post hoc analyses of these data suggest that IA injection of lorecivivint could have potential efficacy in the treatment of knee OA. Even though there were no statistically significant differences (including improvement in WOMAC pain scores, the primary end point) between the placebo and any of the lorecivivint dose groups in the all‐subjects analysis, if a minimal clinically important difference threshold of a 10% (10‐point) change in score 32 were to be applied, both lorecivivint and placebo would be found to produce clinically meaningful improvements from baseline in the WOMAC pain and function subscales.
Analysis of the prespecified UNI subgroup showed greater improvements in WOMAC pain scores, WOMAC function scores, and medial JSW for the 0.07 mg cohort compared with the placebo cohort. These differences appeared to be further enhanced in the post hoc analysis of subjects in the UNI WP− subgroup receiving 0.07 mg lorecivivint. Pain reporting by subjects with bilateral symptomatic knee OA is known to be complicated, not only because of the presence of contralateral knee pain, but also because other joints may be affected by OA 33, 34, nociceptive biomechanical factors may be involved, and other centralized pain conditions (e.g., fibromyalgia) may be present 35. Therefore, the improvements compared with placebo observed in both the UNI and the UNI WP− subgroups versus the all‐subjects group after an IA injection of lorecivivint into the target knee may be attributable to a predominance of subjects with unilateral OA symptoms being able to discriminate their target knee pain from other pain sources. These results inform the design of future lorecivivint trials by identifying a target population in whom potential symptomatic efficacy could be more clearly delineated.
In addition to symptom improvements, inhibition of structural progression is a key goal of disease modification in OA 36; in fact, the slowing of joint space narrowing has been recommended as an appropriate structural end point for DMOAD trials 36. In the all‐subjects analysis, both 0.03 mg lorecivivint (at week 52, change from baseline in medial JSW −0.04 mm) and 0.07 mg lorecivivint (at week 52, change from baseline in medial JSW −0.09 mm) maintained, at least numerically, the medial JSW at week 52, but neither dose achieved a significant difference when compared with placebo (at week 52, change from baseline in medial JSW −0.14 mm). Subjects with unilateral symptomatic OA (i.e., both the UNI and the UNI WP− subgroups) treated with 0.07 mg lorecivivint showed mean medial JSW increases beyond a 0.13‐mm minimum detectable difference 23, whereas subjects who received placebo showed decreases (narrowing) in mean medial JSW from baseline. Joint space narrowing has also been correlated with clinical outcomes, including an increased risk of total knee replacement in those with joint space narrowing of >0.5 mm over 2 years (an outcome indicative of treatment failure) 36, 37. Knee OA is associated with typical joint space narrowing of 0.1–0.3 mm per year 38, 39; although such changes require precise and reproducible measurement methods, such as the positioned, fixed‐flexion radiography technique employed herein, the accuracy of the knee radiographic measurement of medial JSW can range from 0.04 mm to 0.5 mm 23, 40, 41, 42. The relative improvement in medial JSW in the unilateral symptomatic subject subgroups may also be related to a more favorable local biomechanical environment in individuals with unilateral knee pain 43, 44.
A post hoc analysis of both the UNI and the UNI WP− subgroups demonstrated that the radiographic findings (medial JSW) and clinical findings (WOMAC pain and function scores) in the 0.07 mg dose group were concordant (i.e., the change in the former is associated with change in the latter). This suggested a connection between improvement in structural measures and improvement in clinical responses. The 2018 draft guidance from the US Food and Drug Administration on OA structural end points suggests that additional data are needed to support the relationship between structural measurements and clinical outcomes; this analysis sought to contribute to this growing evidence base.
This phase IIa study has several limitations, including no formal, preplanned sample size or power calculation and considerable placebo responses for patient‐reported outcomes, similar to those demonstrated in other OA trials 5, 45. Although trials investigating IA therapies for knee OA commonly use saline as a placebo comparator arm, evidence suggests that IA saline might actually be therapeutic 46. Therefore, further studies of lorecivivint in larger clinical trials with refined inclusion criteria (e.g., focusing on subjects with unilateral symptomatic OA, as lorecivivint is administered into the single most painful knee) are needed to disentangle the active treatment effects from the placebo effects. Although radiographic medial JSW represents an objective measure for assessing structural progression, the evidence supporting the usefulness of this measurement is not definitive and other imaging modalities, such as magnetic resonance imaging, may also be considered. Larger and longer studies are needed to determine the best methods for assessing the disease‐modifying abilities of drugs in knee DMOAD trials.
Finally, the primary statistical analysis Type 1 error control strategy was not achieved, leading to all statistical results being considered exploratory. Since the (prespecified) UNI and (post hoc) UNI WP− groups were small with respect to the number of subjects, the results of these exploratory analyses in both groups are considered to be hypothesis generating, and thus require validation in a prospective trial.
In summary, although the primary end point in all subjects was not met, treatment of subjects with moderately to severely symptomatic knee OA in the UNI and UNI WP− subgroups with an IA injection of 0.07 mg lorecivivint resulted in numerical improvements in pain, function, and medial JSW compared with placebo. Furthermore, treatment with 0.07 mg of lorecivivint demonstrated the greatest improvements in WOMAC pain and function scores and the highest concordance between symptom relief and structural changes. This study identified a target group of subjects with unilateral symptomatic knee OA and a potentially optimal dose of lorecivivint (0.07 mg). The clinical and radiographic outcomes warrant additional studies of the potential of lorecivivint for both analgesia and disease‐modifying activity in knee OA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Yazici had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Yazici, Swearingen, DiFrancesco, Simsek, Tambiah.
Acquisition of data
Yazici, Swearingen, DiFrancesco.
Analysis and interpretation of data
Yazici, McAlindon, Gibofsky, Lane, Clauw, Jones, Swearingen, DiFrancesco, Bergfeld, Simsek, Tambiah, Hochberg.
ROLE OF THE STUDY SPONSOR
Samumed, LLC designed, funded, and monitored the study and also conducted the data management and statistical analysis. The authors independently interpreted the results and had the final decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Samumed, LLC.
Supporting information
Supplementary Figure S1
Supplementary Figure S2
Supplementary Table S1 and S2
Supplementary Figure Legends
ClinicalTrials.gov identifier: NCT02536833.
Supported by Samumed, LLC.
Drs. Yazici, Swearingen, Simsek, and Tambiah and Ms DiFrancesco own stock or stock options in Samumed. Dr. McAlindon has received consulting fees from Samumed, Astellas, Flexion, Pfizer, Regeneron, and Seikugaku (less than $10,000 each) and research support from Samumed. Dr. Gibofsky has received consulting fees, speaking fees, and/or honoraria from AbbVie, Pfizer, Relburn Pharma, Samumed, Flexion, Celgene, and Eli Lilly (less than $10,000 each), owns stock or stock options in Amgen, AbbVie, Pfizer, and Johnson & Johnson, and has served as a paid consultant with investment analysts on behalf of the Gerson Lehrman Group. Dr. Lane has received consulting fees from Samumed, Amgen, Eli Lilly, and Pfizer (less than $10,000 each). Dr. Clauw has received consulting fees from Aptinyx, Daiichi Sankyo, Intec Pharma, Eli Lilly, Samumed, Theravance, Tonix, and Zynerba (less than $10,000 each) and from Pfizer (more than $10,000), received research support from Aptinyx and Pfizer, and has served as an expert witness on behalf of Williams & Connolly, LLP and Nix Patterson, LLP. Dr. Jones has received consulting fees from Samumed (less than $10,000) and receives royalties for the Journal of Bone and Joint Surgery. Dr. Bergfeld has received consulting fees from Samumed (less than $10,000). Dr. Hochberg has received consulting fees, speaking fees, and/or honoraria from Bone Therapeutics, Bristol Myers Squibb, EMD Serono, IBSA, Novartis Pharma AG, Regenosine, Samumed, Symic Bio, Theralogix, TissueGene, Vertex Pharmaceuticals, Vizuri Health Sciences, Zynerva, Covance, Galapagos, ICON, and IQVIA (less than $10,000 each) and from Eli Lilly and Pfizer (more than $10,000 each), owns stock or stock options in BriOri Biotech and Theralogix, and receives royalties from Wolters Kluwer for UpToDate and from Elsevier for Rheumatology 7th Edition
References
- 1. Hochberg MC, Cisternas MG. Osteoarthritis In: The burden of musculoskeletal diseases in the United States (BMUS). 4th ed Rosemont (IL): United States Bone and Joint Initiative; 2019. [Google Scholar]
- 2. Conaghan PG, Hunter DJ, Maillefert JF, Reichmann WM, Losina E. Summary and recommendations of the OARSI FDA Osteoarthritis Assessment of Structural Change working group. Osteoarthritis Cartilage 2011;19:606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–74. [DOI] [PubMed] [Google Scholar]
- 4. McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma‐Zeinstra SM, et al. OARSI guidelines for the non‐surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014; 22:363–88. [DOI] [PubMed] [Google Scholar]
- 5. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta‐analysis. Ann Intern Med 2015;162:46–54. [DOI] [PubMed] [Google Scholar]
- 6. Roberts E, Nunes VD, Buckner S, Latchem S, Constanti M, Miller P, et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheum Dis 2016;75:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Da Costa BR, Reichenbach S, Keller N, Nartey L, Wandel S, Juini P, et al. Effectiveness of nonsteroidal anti‐inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta‐analysis. Lancet 2017;390:e21–33. [DOI] [PubMed] [Google Scholar]
- 8. Lories RJ, Corr M, Lane NE. To Wnt or not to Wnt: the bone and joint health dilemma [review]. Nat Rev Rheumatol 2013;9:328–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thysen S, Luyten FP, Lories RJ. Loss of Frzb and Sfrp1 differentially affects joint homeostasis in instability‐induced osteoarthritis. Osteoarthritis Cartilage 2015;23:275–9. [DOI] [PubMed] [Google Scholar]
- 10. De Boer J, Wang HJ, van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng 2004;393–401. [DOI] [PubMed] [Google Scholar]
- 11. Yano F, Kugimiya F, Ohba S, Ikeda T, Chikuda H, Ogasawara T, et al. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9–dependent manner. Biochem Biophys Res Comm 2005;333:1300–8. [DOI] [PubMed] [Google Scholar]
- 12. Day TF, Guo X, Garrett‐Beal L, Yang Y. Wnt/β‐catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 2005;8:739–50. [DOI] [PubMed] [Google Scholar]
- 13. Lories RJ, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, et al. Articular cartilage and biomechanical properties of the long bones in Frzb‐knockout mice. Arthritis Rheum 2007;56:4095–103. [DOI] [PubMed] [Google Scholar]
- 14. Loughlin J, Dowling B, Chapman K, Marcelline L, Mustafa Z, Southam L, et al. Functional variants within the secreted frizzled‐related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci U S A 2004;9757–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, et al. Activation of β‐catenin signaling in articular chondrocytes leads to osteoarthritis‐like phenotype in adult β‐catenin conditional activation mice. J Bone Miner Res 2009;24:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Usami Y, Gunawardena AT, Iwamoto M, Enomoto‐Iwamoto M. Wnt signaling in cartilage development and diseases: lessons from animal studies. Lab Invest 2016;96:186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. Dickkopf‐1 is a master regulator of joint remodeling. Nat Med 2007;13:156–63. [DOI] [PubMed] [Google Scholar]
- 18. Kronke G, Uderhardt S, Kim K, Stock M, Scholtysek C, Zaiss MM, et al. R‐spondin 1 protects against inflammatory bone damage during murine arthritis by modulating the Wnt pathway. Arthritis Rheum 2010;62:2303–12. [DOI] [PubMed] [Google Scholar]
- 19. Zhu M, Chen M, Zuscik M, Quiquian W, Wang YJ, Rosier RN, et al. Inhibition of β‐catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum 2008;58:2053–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deshmukh V, Hu H, Barroga C, Bossard C, Kc S, Dellamary L, et al. A small‐molecule inhibitor of the Wnt pathway (LOR) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage 2018;26:18–27. [DOI] [PubMed] [Google Scholar]
- 21. Yazici Y, McAlindon TE, Fleischmann R, Gibofsky A, Lane NE, Kivitz AJ, et al. A novel Wnt pathway inhibitor, LOR, for the treatment of moderate to severe osteoarthritis of the knee: results of a 24‐week, randomized, controlled, phase 1 study. Osteoarthritis Cartilage 2017;25:1598–606. [DOI] [PubMed] [Google Scholar]
- 22. Deshmukh V, O'Green AL, Bossard C, Seo T, Lamangan L, Ibanez M, et al. Modulation of the Wnt pathway through inhibition of CLK2 and DYRK1A by lorecivivint as a novel, potentially disease‐modifying approach for knee osteoarthritis treatment. Osteoarthritis Cartilage 2019;27:1347–60. [DOI] [PubMed] [Google Scholar]
- 23. Dupuis DE, Beynnon BD, Richard MJ, Novotny JE, Skelly JM, Cooper SM. Precision and accuracy of joint space width measurements of the medial compartment of the knee using standardized MTP semi‐flexed radiographs. Osteoarthritis Cartilage 2003;11:716–24. [DOI] [PubMed] [Google Scholar]
- 24. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum 1986;29:1039–49. [DOI] [PubMed] [Google Scholar]
- 25. Kellgren JH, Lawrence JS, editors. The epidemiology of chronic rheumatism: atlas of standard radiographs. Vol. II Oxford: Blackwell Scientific; 1962. [Google Scholar]
- 26. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken) 2011;63:S240–52. [DOI] [PubMed] [Google Scholar]
- 27. McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum 2001;45:453–61. [DOI] [PubMed] [Google Scholar]
- 28. Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–10. [DOI] [PubMed] [Google Scholar]
- 29. Piantadosi S, editor. Clinical trials: a methodologic perspective. Hoboken (NJ); Wiley‐Interscience; 1997. [Google Scholar]
- 30. Dmitrienko A, Tamhane AC, Bretz F, editors. Multiple testing problems in pharmaceutical statistics. Boca Raton (FL): CRC Press; 2010. [Google Scholar]
- 31. Hosmer DW, Lemeshow S, editors. Applied logistic regression. 2nd edition New York: John Wiley & Sons; 2000. [Google Scholar]
- 32. Strand V, Boers M, Idzerda L, Kirwan JR, Kvien TK, Tugwell PS, et al. It's good to feel better but it's better to feel good and even better to feel good as soon as possible for as long as possible: response criteria and the importance of change at OMERACT 10. J Rheumatol 2011;38:1720–7. [DOI] [PubMed] [Google Scholar]
- 33. Riddle DL, Stratford PW. Unilateral vs bilateral symptomatic knee osteoarthritis: associations between pain intensity and function. Rheumatology (Oxford) 2013;52:2229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ 2009;339:b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clauw DJ, Hassett AL. The role of centralised pain in osteoarthritis. Clin Exp Rheumatol 2017;35 Suppl 107:79–84. [PubMed] [Google Scholar]
- 36. Raynauld JP, Martel‐Pelletier J, Haraoui B, Choquette D, Dorais M, Wildi LM, et al. Risk factors predictive of joint replacement in a 2‐year multicentre clinical trial in knee osteoarthritis using MRI: results from over 6 years of observation. Ann Rheum Dis 2011;70:1382–8. [DOI] [PubMed] [Google Scholar]
- 37. Bruyere O, Richy F, Reginster JY. Three year joint space narrowing predicts long term incidence of knee surgery in patients with osteoarthritis: an eight year prospective follow up study. Ann Rheum Dis 2005;64:1727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bruyere O, Genant H, Kothari M, Zaim S, White D, Peterfy C, et al. Longitudinal study of magnetic resonance imaging and standard x‐rays to assess disease progression in osteoarthritis. Osteoarthritis Cartilage 2007;15:98–103. [DOI] [PubMed] [Google Scholar]
- 39. Benichou OD, Hunter DJ, Nelson D, Guermazi A, Eckstein F, Kwoh K, et al. One‐year change in radiographic joint space width in patients with unilateral joint space narrowing: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2010;62:924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buckland‐Wright JC, Ward RJ, Peterfy C, Mojcik CF, Leff RL. Reproducibility of the semiflexed (metatarsophalangeal) radiographic knee position and automated measurements of medial tibiofemoral joint space width in a multicenter clinical trial of knee osteoarthritis. J Rheumatol 2004;31:1588–97. [PubMed] [Google Scholar]
- 41. Nevitt MC, Peterfy C, Guermazi A, Felson DT, Duryea J, Woodworth T, et al. Longitudinal performance evaluation and validation of fixed‐flexion radiography of the knee for detection of joint space loss. Arthritis Rheum 2007;56:1512–20. [DOI] [PubMed] [Google Scholar]
- 42. Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Reichmann WM, et al. Responsiveness and reliability of MRI in knee osteoarthritis: a meta‐analysis of published evidence. Osteoarthritis Cartilage 2011;19:589–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Creaby MW, Bennell KL, Hunt MA. Gait differs between unilateral and bilateral knee osteoarthritis. Arch Phys Med Rehabil 2012;93:822–7. [DOI] [PubMed] [Google Scholar]
- 44. Waller C, Hayes D, Block JE, London NJ. Unload it: the key to the treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 2011;19:1823–9. [DOI] [PubMed] [Google Scholar]
- 45. Altman RD, Devji T, Bhandari M, Fierlinger A, Niazi F, Christensen R. Clinical benefit of intraarticular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta‐analysis of randomized trials. Semin Arthritis Rheum 2016;46:151–9. [DOI] [PubMed] [Google Scholar]
- 46. Bannuru RR, McAlindon TE, Sullivan MC, Wong JB, Kent DM, Schmid CH. Effectiveness and implications of alternative placebo treatments: a systematic review and network meta‐analysis of osteoarthritis trials. Ann Intern Med 2015;163:365–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Table S1 and S2
Supplementary Figure Legends


