Abstract
Digital therapeutics is a new approach to treat hypertension via using software programs such as smartphone apps and/or device algorithms. We develop a HERB system—new interactive smartphone app (HERB Mobile) with web‐based patient management console (HERB Console)—to lower blood pressure (BP) based on an algorithm that helps users to promote lifestyle modifications in conjunction with medically validated non‐pharmacological interventions. The app can assess the personalities, behavior characteristics, and hypertension determinants of each patient with hypertension to provide adequate guidance. To demonstrate the efficacy of the system, we designed a randomized, controlled, multicenter, open‐label trial “HERB‐DH1 (HERB digital hypertension 1)” to assess the efficacy of HERB system in patients with essential hypertension. The authors allocate patients to the intervention group (HERB system + standard lifestyle modification) or to the control group (standard lifestyle modification alone). In the intervention group, we provide the HERB Mobile for patients and the HERB Console for their primary physicians for 24 weeks. Both groups are instructed for standard lifestyle modifications based on the current recommendations in the Japanese Society of Hypertension 2019 guideline. The primary outcome is the mean change from baseline to 12 weeks in 24‐hour systolic BP measured by ambulatory BP monitoring. We started this study in December of 2019, and the trial results will be expected in early 2021. We believe that this trial enables us to verify the efficacy of the HERB system in patients with essential hypertension.
Keywords: digital therapeutics, hypertension, lifestyle modification, mobile applications, randomized controlled trials
1. INTRODUCTION
Hypertension is the most considerable but treatable risk factor of cardiovascular disease (CVD) and is thus an enormous economic and social burden. 1 Previous randomized controlled clinical trials using various antihypertensive drugs clearly demonstrated that blood pressure (BP) lowering per se is associated with a reduction of CVD events, independently of the class of antihypertensive drugs. 2 , 3 , 4 Reductions of systolic BP by 10 mmHg and diastolic BP by 5 mmHg have been shown to reduce stroke and heart failure by 25%, and coronary artery disease and total mortality by 15%. 1 , 3 , 4 Both early BP control and lower BP control over 24‐hour are essential to achieve “zero” CVD events. 5 , 6
Despite robust development of various antihypertensive drugs of different classes, and the introduction of community‐based lifestyle initiatives as non‐pharmacological therapy, real‐world BP control rate remains poor. 1 , 7 The BP control rate for the threshold of 140/90 mmHg varies widely from 15% to 70% globally. 5 , 8 Recently, released guidelines for the management of hypertension lowered the target BP to <130/80 mmHg, and thus, the prevalence of uncontrolled hypertension using the new threshold (130/80 mmHg) has been markedly increased. 7
In order to achieve the current targets for BP control, real‐world innovations in the interventions for hypertensive management are urgently needed. 1 , 7 As information and communication technology (ICT) become increasingly central to our daily lives, development of digital health care and medicine has become a “hot” topic. 9 , 10 In the era of digital therapeutics (DTx), various trials using applications for chronic conditions such as nicotine dependence, 11 , 12 substance abuse, 13 or attention deficit hyperactivity disorder 14 have been initiated. In the case of hypertension management, several non‐pharmacological intermittent educational interventions have been well‐validated in previous studies. For example, in our previous 3‐month intervention study, a face‐to‐face approach by professional nutritionists reduced salt intake by 1.8 g/per day, resulting in a significant reduction to 7.3 mmHg of the 24‐hour ambulatory systolic BP. 15 However, non‐pharmacological approaches are not consistently successful across all settings, because their efficacy is highly dependent on the characteristics of individual patients.
DTx is an emerging therapeutic method using software programs such as smartphone apps and/or device algorithms. 16 Here, we describe our development of a new interactive smartphone app, HERB, to lower BP, based on an algorithm that helps users to make lifestyle modifications in conjunction with medically validated non‐pharmacological interventions, such as salt reduction, maintenance of adequate body weight, regular exercise, and alcohol restriction. We incorporated the advance in the field of behavioral science 17 specially adapted for the smartphone app into our antihypertensive program. In addition, the application can assess the personalities, behavioral characteristics, and hypertension determinants of individual patients to provide adequate guidance on an individual level. To demonstrate the efficacy of this smartphone app for personalized hypertension management, we have initiated a randomized intervention trial. We here present the design and rationale of the HERB‐DH1 (HERB digital hypertension 1) trial, which is the first randomized controlled trial (RCT) of digital hypertensive therapeutics.
2. METHODS
2.1. Overall trial design
This is a randomized, controlled, multicenter, open‐label trial. We aim to evaluate the efficacy of a smartphone app, HERB Mobile, for the treatment of essential hypertension in addition to guideline‐based promotion of lifestyle modification. Patients in the intervention group will be provided the HERB smartphone app, and their primary physicians will be provided a web‐based patient management console, HERB Console, for 24 weeks. Figure 1 shows the flowchart of this trial, and Table 1 shows the overall follow‐up schedule.
FIGURE 1.
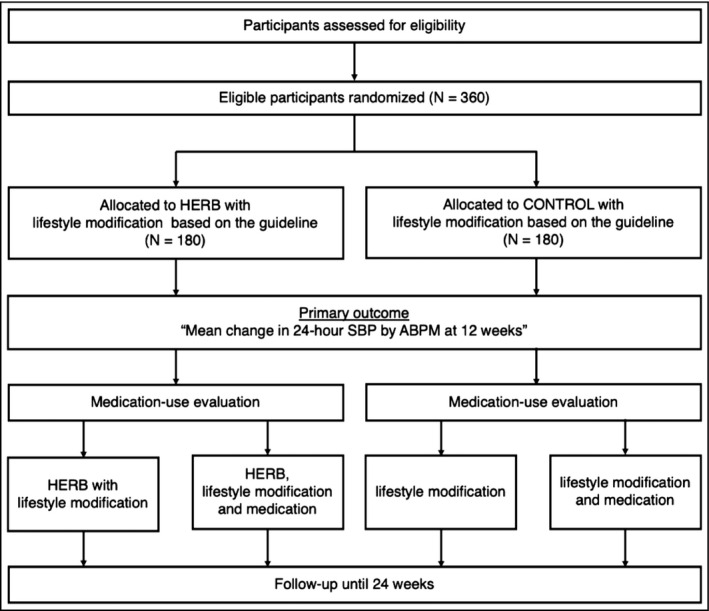
CONSORT flowchart
TABLE 1.
Follow‐up assessment schedule of the trial
|
Assessments ● Mandatory ○ If needed |
Screening | Treatment period | At withdrawal | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 8 | Week 12 | Medication‐use evaluation | Week 16 | Week 20 | Week 24 | ||||
| Day1 | Day 29 | Day 57 | Day 85 | Day 113 | Day 141 | Day 169 | |||||
| Scheduled visits | 28 d before registration | Registration | ±14 d | ±14 d | ±14 d | Within 14 d after ABPM at 12 wk | ±14 d | ±14 d | ±14 d | Within 14 d after withdrawal | |
| Eligibility | ● | ● | |||||||||
| Informed consent | ● | ||||||||||
| Registration & Randomization | ● | ||||||||||
| Usage monitoring of | |||||||||||
| investigational medical device (app) | ● | ● | ● | ○ | ○ | ● | ● | ||||
| Baseline characteristics | ● | ||||||||||
| Examination | Subjective | ● | ● | ● | ● | ● | ● | ○ | ○ | ● | ● |
| Objective | ● | ● | ● | ● | ● | ● | ○ | ○ | ● | ● | |
| Physical | Height | ● | |||||||||
| Weight | ● | ● | ● | ● | ○ | ○ | ● | ● | |||
| BMI | ● | ● | ● | ● | ○ | ○ | ● | ● | |||
| Waist circumference | ● | ● | ● | ● | |||||||
| Physiological functions | ABPM | ● | ● | ● | ● | ||||||
| Home BP and HR (awake and bedtime) | ● | ● | ● | ● | ○ | ○ | ● | ● | |||
| Office BP and HR | ● | ● | ● | ● | ○ | ○ | ● | ● | |||
| 12‐lead ECG | ● | ||||||||||
| Epworth sleepiness scale | ● | ||||||||||
| Laboratory | Blood test | ● | ● | ● | ● | ||||||
| Pregnancy test | ○ | ||||||||||
| Urinalysis | ● | ● | ● | ● | |||||||
| Salt intake check sheet | ● | ● | ● | ● | |||||||
| Medication usage | ● | ○ | ○ | ● | ○ | ||||||
| Concomitant medication & treatment | ● | ● | ● | ||||||||
| Adverse events | ● | ● | |||||||||
This trial will be conducted in compliance with the Declaration of Helsinki, Medical Device Good Clinical Practice, and all other applicable laws and guidelines in Japan. This trial protocol was also approved by the Institutional Review Board at Jinbo Orthopedics (Tokyo), Jichi Medical University Hospital (Tochigi, Japan) and affiliate institutions and was registered at the Japan Registry of Clinical Trials (jRCT2032190148).
2.2. HERB: Digital therapeutic system for hypertension
The “HERB” system is investigational software as a medical device (SaMD) that consists of a smartphone app for patients (HERB Mobile), a web application for health care providers (HERB Console), and a data server (Figure 2). The HERB smartphone app was developed at CureApp Inc. under the supervision of the Division of Cardiovascular Medicine, Department of Medicine, Jichi Medical University School of Medicine. The app runs on both iOS and Android platforms. The app's prescription code is provided to the patients in the intervention group at the timing of randomization via the electric data capture (EDC) system (DATATRAK ONE®; DATATRAK International). Patients download the app through their smartphones, activate the app by entering the prescription code, and input their personal baseline characteristics, including age, sex, lifestyle, social background, and behavior patterns. These data are efficiently gathered through conversations with a virtual nurse in the app.
FIGURE 2.

Overview of HERB system. The HERB system consists of “HERB Mobile” smartphone application for patients and “HERB Console” web‐based patient management console
The HERB Mobile will retrieve BP data measured by home BP monitoring (HBPM) via a Bluetooth® connection. The patient data will then be securely transferred to the data server and analyzed based on an algorithm developed with the assistance of health professionals in order to generate a personalized program of lifestyle modifications to lower BP. The patient data, including the BP record, various daily activities, and progress on the proposed program, will be simultaneously shown to health care providers through the HERB Console. Based on the personal data, the physicians will support patients to promote daily app usage including watching educational videos in the app etc. and will educate them in regard to BP and its measurement.
The HERB Mobile has the following 3 stages to ensure the lifestyle modification: Step 1) input and education; Step 2) app‐initiated experiments; and Step 3) self‐planning and evaluation (Figure 3). First, in Step 1, the app provides each user a personalized educational program including lectures and advice via a virtual nurse to enhance their lifestyle modifications for the control of essential hypertension. Next, in Step 2, the app instructs users non‐pharmacological interventions, for example, reduced salt intake, exercise or smoking cessation, to lower their BP based on the knowledge and techniques given in Step 1. Finally, in Step 3, the app encourages users to create their own strategies to lower their BP by combining some of the non‐pharmacological interventions learned in Step 2. Users will also be encouraged to evaluate and review the process and results of their individually devised plans. Through this process, the app will allow the lifestyle plans devised by users to take root and realize the necessary behavioral changes to reduce BP.
FIGURE 3.

App‐supported lifestyle modification in HERB Mobile. The HERB Mobile uses a 3‐step approach to ensure lifestyle modification: input and education, app‐initiated experiments, and self‐planning and evaluation. Through this process, the app users can change their behavior to reduce their blood pressure
2.3. Outcomes
The primary outcome is the mean change from baseline to 12 weeks in 24‐hour systolic BP (SBP) measured by ambulatory BP monitoring (ABPM). We will also evaluate secondary outcomes at 12 and 24 weeks (compared with baseline) as shown in Table 2. ABPM will be taken at the baseline, at 12 weeks, and at 24 weeks after registration for the primary outcome. In this trial, adverse event is defined as any unfavorable medical occurrences for all participants (both the intervention and control groups). Participants suffering from adverse events may be considered termination from the trial at their physicians' discretion.
TABLE 2.
Primary and secondary outcomes
| Primary outcome |
| Mean change in 24‐h SBP by ABPM at 12 wk |
| Secondary outcomes |
| [Evaluated at both 12 and 24 wk] |
| 1) Management rate (goal 1, home SBP < 135 mmHg and DBP < 85 mmHg; goal 2, home SBP < 125 mmHg and DBP < 75 mmHg) |
| 2) Efficacy rate (percentage of patients whose mean change in 24‐h SBP is equal to or more than 5 mmHg) |
| 3) Mean changes by ABPM in 24‐h DBP |
| 4) Daytime/nighttime SBPs and DBPs, 24‐h pulse pressure, 24‐h/daytime/nighttime HRs, and 24‐h SBPs and DBPs in the extreme‐dipper/dipper/non‐dipper/riser groups |
| 5) Coefficient of variations in 24‐h/daytime/nighttime SBPs and DBPs |
| 6) Mean changes in home awake and nighttime SBP, DBP, and HR |
| 7) Coefficient of variations in home awake and bedtime SBP and DBP |
| 8) Mean changes in office SBP, DBP, and HR |
| 9) Mean changes in weight, BMI, and waist circumference |
| 10) Mean change in points obtained by salt intake check sheet |
| 11) Changes in each variable by blood tests and urinalysis |
| 12) Smartphone app‐related variables (app usage rate, progress of app educational programs, etc.) |
| 13) Rate of home BP measurement, rate of behavior modification, and rate of self‐monitoring |
| 14) Any adverse events including device‐related adverse events |
| 15) Malfunctions of the investigational medical device |
| [Evaluated at 24 wk] |
| 16) Mean change in 24‐h SBP by ABPM at 24 wk |
| 17) Percentage of medication users; mean changes between 12 and 24 wk by ABPM in 24‐h/daytime/nighttime SBPs and DBPs |
| 18) Mean changes between 12 and 24 wk in home awake/bedtime SBPs and DBPs |
Abbreviations: ABPM, ambulatory blood pressure monitoring; BMI, body mass index; DBP, diastolic blood pressure;HP, heart rate; SBP, systolic blood pressure.
2.4. Patient recruitment and randomization
We will recruit patients with essential hypertension from December 2019 to May 2020 or until the recruitment process completes; we plan to follow them until November 2020. Participants who meet all inclusion criteria will be included in the trial (Table 3) and those meeting any of the exclusion criteria will be excluded from the trial (Table 4). We will obtain written informed consent from all the trial participants. We will randomize eligible patients to the intervention group (HERB system + standard lifestyle modification by the doctor) or the control group (standard lifestyle modification alone) using an independent web‐based block randomization system, with stratification by centres, history of antihypertensive medications, and 24‐hour SBP in ABPM at registration (≥145 mmHg or <145 mmHg). The EDC system will automatically allocate patients to either the intervention or control group.
TABLE 3.
Inclusion criteria
| We will include patients who meet all the following criteria: |
| 1) Age ≥20 y and <65 y |
| 2) Diagnosed with essential hypertension (Office SBP 140‐179 mmHg, and/or DBP 90‐109 mmHg) |
| 3) 24‐h mean BP ≥130 mmHg by ABPM at screening |
| 4) Antihypertensive medication‐naïve for more than 3 mo before registration |
| 5) Can use a smartphone daily (operating system: Android 6.0 and above or iOS 11.0 or above) |
| 6) Agree to receive ABPM at both 12 and 24 wk after randomization |
| 7) Investigators or clinical trial physicians assesses it is reasonable for the patient to receive 12‐wk lifestyle modification without antihypertensive medications |
Abbreviations: ABPM, ambulatory blood pressure monitoring; DBP, diastolic blood pressure; SBP, systolic blood pressure.
TABLE 4.
Exclusion criteria
| We will exclude patients who meet any of the following criteria: |
| 1) Office SBP ≥180 mmHg and/or DBP ≥110 mmHg |
| 2) Suspected secondary hypertensions |
| 3) Use of contraindicated medications |
| 4) Recommended immediate medication therapy by medical history and/or comorbidities. |
| 5) Female with pregnancy or expecting |
| 6) History of renal denervation therapy |
| 7) Do not have or use a smartphone daily |
| 8) Participating in ongoing clinical trials, or participated in the previous HERB pilot study |
| 9) Relatives or cohabitant partners who have already participated in this trial |
| 10) Judged by the investigator or clinical trial physicians to be unsuitable for participation in this trial for any other reason |
Abbreviations are the same as in Table 3.
Patients in the intervention group will be instructed to connect the HBPM device to the HERB Mobile via Bluetooth and forward the BP values to the HERB Mobile. The patients in the control group will be instructed to connect the HBPM device using specific software available at the study site and provide the data stored in the HBPM device at their planned visit. Both groups will receive instruction on lifestyle modifications as a standard regimen for essential hypertension based on the current recommendations in JSH 2019 Guideline. 1 The principal investigators or sub‐investigators will give patients relevant instructions with the help of a brochure on lifestyle modifications. At each study visit, patients in both groups will check the progress of lifestyle modification on a specific check sheet, and patients in the intervention group will also discuss with their physicians on what to focus until next clinic visit with reference to their progress of the program.
2.5. Follow‐up
During the follow‐up (Table 1), the following interventions will be prohibited due to their potential to affect BP: (a) antihypertensive medications except when indicated based on the ambulatory BP levels taken at 12 weeks after registration; (b) the use of application software that measures BP, stores measured BP data or aims to reduce BPs; (c) intake of any foods approved by the Consumer Affairs Agency as “food for specified health uses”; (d) use of medications or Chinese herbal medicines known to cause drug‐induced hypertension (eg, non‐steroidal anti‐inflammatory drugs, licorice, glucocorticoid, cyclosporine, erythropoietin, oral contraceptives, and sympathomimetic agents [topical product as ophthalmic solution or ointments are excluded]); and (e) use of sodium‐glucose transporter 2 inhibitors, which have been shown to lower home and ambulatory BPs. 18 , 19 Antihypertensive or other medications can be used after 12 weeks of randomization according to the JSH 2019 Guideline and the discretion of physicians 1 .
2.6. Blood pressure measurement
2.6.1. Ambulatory BP
Ambulatory BP will be taken using the validated TM‐2441 (A&D Co.) device 20 based on the JSH Guidelines and HOPE Asia Network recommendations for the clinical use of 24‐hour ABPM. 21 , 22 , 23 In brief, we will first check whether the discrepancy of BPs between office BP and ABPM is equal to or <5 mmHg. The total measurement duration should exceed 25 hours, and the first 1 hours will be removed from the analysis. The measurement intervals are 30 minutes in both daytime and nighttime. Daytime and nighttime are defined from the participants' diaries. SBP ≤70 or ≥250, DBP ≤30 or ≥130, and pulse pressure ≤20 or ≥160 will be considered outliers and removed from the analysis. The minimum required rate of measurement will be 70% of successful readings. The mean 24‐hour BP will be calculated as an arithmetic mean by averaging all successful readings.
All patients will record the times that they fall asleep or wake up in their diaries. All individuals will be instructed to rest or sleep during the nighttime and to maintain their usual activities during the daytime. Nighttime readings will be those ranging from the time of falling asleep to the time of waking up based on the diary entries, and the remaining readings will be defined as daytime readings.
The night/day SBP ratio will be calculated, and the patients will be classified into the following four dipping groups on the basis of the baseline nocturnal BP fall: extreme dippers, with a nocturnal BP fall of 20% or more; dippers, with a fall between 10% and 20%; non‐dippers, with a fall between 0% and 10%; and risers, with a nocturnal increase of SBP.
2.6.2. Home BP
Patients will be instructed to measure their BP at home based on the instructions of the JSH 2019 Guideline 1 and the recommendations of the HOPE Asia Network 24 , 25 using the validated HBPM device (UA‐651BLE; A&D Co.) twice in the morning and twice at bedtime for 7 days (or a minimum of 5 days) before the planned study visit. The morning measurements are taken within 1 hour after waking up, after urination, before taking any medications, prior to eating breakfast, and after resting 1‐2 minutes while in a seated position in a quiet room without talking. The cuff position should be maintained at the heart level. The bedtime measurements are to be taken before going to bed and after 1‐2 minutes rest in a seated position.
2.6.3. Office BP
Office BPs are measured twice based on the JSH 2019 Guideline, 1 and the average of the stable values ( 5 mmHg difference) will be recorded. Office BP measurements are to be taken after resting 1‐2 minutes while in a seated position in a quiet room at an appropriate temperature without talking. The center of the cuff is positioned at the heart level. The interval between the two measurements should be 1‐2 minutes. If these BP values differ significantly, additional BP measurements will be taken.
2.7. Data monitoring
On‐site data monitoring will be conducted by an independent monitoring staff. Moreover, the staff will review the trial database to confirm that the principal investigator and sub‐investigators at each institution conducted the clinical trial according to the research protocol and standard operation procedures. If necessary, data queries will also be made.
2.8. Statistics
2.8.1. Sample size
The mean change from baseline to 12 weeks in 24‐hour SBP by ABPM is the primary outcome of this trial. In our HERB pilot study (University Hospital Medical Information Network Clinical Trial Registry [UMIN‐CTR] ID: UMIN000033311), the difference in the mean change from baseline to 16 weeks in 24‐hour SBP by ABPM between the HERB system and Control groups was 7.6 mmHg, and the SD of the HERB group was 11.5 mmHg in 59 medication‐naïve patients between the ages of 20 and 65. Thus, we set the estimated difference in the mean change in 24‐hour SBP between the intervention and control groups at 5 mmHg with an SD of 14 mmHg. In the sample size calculation, we determined that the number of patients required per group was 165 at a two‐tailed alpha level of 5% and 90% power. Assuming a dropout rate of 10%, we will need a total of 180 patients per group for the trial.
2.8.2. Statistical analysis
All analysis will be based on the intention‐to‐treat principle. The baseline characteristics will be described as the mean and standard deviation, or median and quantiles (for continuous variables), or proportion (for categorical variables). We will compare the primary and secondary outcomes between the intervention group and controls. For the analyses, a two‐tailed P value less than 0.05 will be considered as statistically significant. We will calculate 95% confidence intervals (CIs). Analysis of the primary endpoint will be performed using the analysis of covariance (ANCOVA) including centers, history of antihypertensive medications, and 24‐hour SBP in ABPM at registration. Moreover, we will compare secondary outcomes between the groups at each defined period using ANCOVA, or logistic regression adjusted by appropriate covariates. In addition, we will perform a sensitivity analysis based on the Per‐Protocol Set for these analyses. A statistical analysis plan will be completed prior to locking the database. SAS statistical software version 9.4 or higher (SAS Institute Inc.) will be used for the analyses.
3. DISCUSSION
The HERB‐DH1 pivotal study is the first clinical trial designed to evaluate the efficacy of the ICT‐based, BP‐lowering DTx synergistically facilitating the medically well‐validated non‐pharmacological interventions at the individual level in patients with essential hypertension.
3.1. Primary outcome
The primary outcome of the HERB‐DH1 trial is the reduction of 24‐hour SBP measured by ABPM at 12 weeks. Recently, released guidelines or recommendations for the management of hypertension have recommended the use of out‐of‐office BP evaluated by ABPM and/or home BP monitoring. 1 , 5 , 7 , 23 , 26 , 27 This is because all the previous population‐based and clinical studies clearly demonstrated that out‐of‐office BP was more closely associated with organ damages and CVD prognosis than office BP. 28 ABPM, which can monitor BP through the day, in particular allows for an increased number of objective measurements. Thus, all the recent clinical trials of non‐pharmacological devices for hypertension treatment have assigned the mean change in 24‐hour SBP measured by ABPM as the primary outcome. 29 , 30 A reduction of 5 mmHg or more of 24‐hour SBP will support the evidence of a reduction of CVD events by 20%. 31
3.2. Validated non‐pharmacological interventions of HERB
HERB Mobile targets multiple lifestyle modification components, such as reduction of salt intake, body weight control, exercise, sleep conditions, stress management, and cessation of smoking and drinking alcohol (Figure 2). Antihypertensive effects of several lifestyle modifications have been shown in meta‐analyses, for example, reducing salt intake by 4.6 g/d lowered SBP/DBP by 4.96 ± 0.40/2.73 ± 0.24 mmHg 32 , reducing body weight by 4.0 kg in 6 months contributed to SBP/DBP reduction of 4.5/3.2 mmHg, 33 30‐60 minutes/d of exercise reduced SBP/DBP by 4.6/2.4 mmHg, 34 and reducing alcohol intake by 76% lowered SBP/DBP by 3.31/2.04 mmHg. 35 Sleep conditions, especially short sleep duration and psychological stress, are also associated with the development of hypertension. 36 , 37 HERB Mobile will provide guidance based on a combination of several components of intervention to maximize the potential BP‐lowering effect, since the outcome from multiple components is better than that from a single component. The JSH2019 Guideline mentioned the synergistic effects of multiple components of intervention: the DASH (Dietary Approach for Stop Hypertension) diet, DASH diet plus reduction of salt intake, 38 and body weight control plus reduction of salt intake, 39 indicated to potentiate the effect of BP‐lowering.
3.3. Personalized intervention of HERB
HERB Mobile will provide the personalized antihypertensive guidance including multiple components of the lifestyle modification interventions. The response to each intervention may vary according to the patient's background, because the effectiveness of each component depends not only on the pressor mechanism of each individual patient, but also on the patient's motivation and preference for each intervention. However, previous non‐pharmacological intervention trials have been conducted in the same standardized manner without considering the motivations and preferences of individual patients. On the other hand, this app will take a personalized approach to assess the personal data and provide suitable directions for each individuals; thus, the BP‐lowering effect of this app may be sustainable for a longer period of time than other standard non‐pharmacological interventions. This smartphone app will enable us to introduce personalized medicine into the clinical practice of BP management. If the influencing factors of each component and “the responders,” which are characterized specific sensitivity of each intervention, are revealed, we will finally be able to categorize hypertensive patients based on their sensitivity to each lifestyle modification component.
3.4. Rationale of study patients
The target patients of the HERB‐DH1 trial are non‐medicated younger adults (30‐64 years of age) with mild‐to‐moderate hypertension (140‐159/90‐99 mmHg). All of the major guidelines recommend that patients with mild‐to‐moderate hypertension receive non‐pharmacological intervention starting at the time of diagnosis of hypertension before antihypertensive medication is initiated. 1 , 7 , 26 In addition, the predominant cause of systolic hypertension in the elderly is increased arterial stiffness, and we speculate that the BP reduction, especially 24‐hour SBP, may take a longer time in elderly hypertensives 40 . In the perspective of the DTx, younger people are generally more comfortable with the use of smartphones and thus may find it easier to use a new app than the elderly. We will therefore target younger adults in the early stage of hypertension to evaluate the BP‐lowering effect within a relatively short period of time in this study.
3.5. Digital therapeutics
Digital therapeutics can provide evidence‐based intervention to prevent, manage, or treat diseases independently or in combination with existing therapies. 16 For example, the randomized controlled trial of a novel DTx smartphone app, CureApp‐SC™, demonstrated the significant clinical efficacy of the app when used in conjunction with standard‐of‐care (SoC) compared with SoC alone for nicotine dependence. 41 Today, nearly 80% of the general population in Japan use smartphones, which far exceeds the usage rate for personal computers. 42 We expect that our HERB system will be a highly promising DTx to significantly lower BP for essential hypertension when used together with SoC by continuously promoting behavioral changes in users via daily use of a smartphone.
3.6. Study limitation
This is an open‐label study because the control group will not use the application, patients will thus recognize whether they have been allocated to the application group or control group. In addition, both groups will equally receive doctors' instructions based on JSH 2019 Guidelines, 1 in regard to non‐pharmacological interventions in their clinical practice. Such “face‐to‐face” instruction from the doctors may overwhelm the BP‐lowering effect of the application over the relatively short time period of the study. Moreover, the TASMINH4, an unmasked randomized controlled trial, demonstrated that self‐BP monitoring, with or without tele‐monitoring, to titrate antihypertensive medication in individuals with poorly controlled blood pressure, leads to significantly lower BP than titration guided by clinic readings. 43 In the settings of our study, patients in both the groups will manage their BP by self‐monitoring although tele‐monitoring is only applied for the app group. Therefore, a certain amount of BP‐lowering effect may occur in both these groups by just conducting HBPM and by doctors' instruction based on JSH2019 Guidelines.
4. CONCLUSIONS AND PERSPECTIVES
This trial will allow us to verify the hypothesis that HERB Mobile, which is treatment using a smartphone application, is effective in reducing BP and also has no harm in the management of hypertension. If the efficacy is validated, this will be a first step toward the practical use in application‐based personalized medicine for the management of hypertension. We started this study in December of 2019, and the trial results will be disseminated in early 2021.
CONFLICT OF INTEREST
KK received research grants from A&D Co. (Tokyo) and from Omron Healthcare (Kyoto, Japan). KK and AN received consulting fees from CureApp Inc. AN and KS are founders of the CureApp Institute. TT, RS, and KN are employees of CureApp Inc. KS and SS are founders and shareholders of CureApp Inc. and patent holders of the HERB system. EH has a consultation contract as a biostatistician with CureApp Inc. The other authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
KK, AN, TT, AO, and NH wrote the draft manuscript. KK, AN, TT, RS, KN, SS, KS, and EH conceptualized and designed the trial. KK, SS, and KS developed the HERB system. All authors agreed to the final version of the protocol.
ACKNOWLEDGMENT
We would like to express our gratitude to all the trial participants and staff. We also thank Mr. Hiroshi Mamada, Ms. Tomoko Shiga, Ms. Chiharu Saito, and Ms. Tomoko Morimoto for their study coordination and data management.
Kario K, Nomura A, Harada N, et al. A multicenter clinical trial to assess the efficacy of the digital therapeutics for essential hypertension: Rationale and design of the HERB‐DH1 trial. J Clin Hypertens. 2020;22:1713–1722. 10.1111/jch.13993
Funding information
This trial is supported by CureApp Inc. CureApp Inc. provided funding and contributed to the development of the HERB system and the design of the trial.
REFERENCES
- 1. Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235‐1481. [DOI] [PubMed] [Google Scholar]
- 2. Fujiyoshi A, Ohkubo T, Miura K, et al. Blood pressure categories and long‐term risk of cardiovascular disease according to age group in Japanese men and women. Hypertens Res. 2012;35(9):947‐953. [DOI] [PubMed] [Google Scholar]
- 3. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blood Pressure Lowering Treatment Trialists' Collaboration . Blood Pressure Lowering Treatment Trialists C. Blood pressure‐lowering treatment based on cardiovascular risk: a meta‐analysis of individual patient data. Lancet. 2014;384(9943):591‐598. [DOI] [PubMed] [Google Scholar]
- 5. Kario K. Global impact of 2017 American Heart Association/American College of Cardiology Hypertension Guidelines: a perspective from Japan. Circulation. 2018;137(6):543‐545. [DOI] [PubMed] [Google Scholar]
- 6. Kario K. Essential Manual on Perfect 24‐hour Blood Pressure Management from Morning to Nocturnal Hypertension: Up‐to‐date for Anticipation Medicine. Tokyo, Japan: Wiley; 2018:1–309. [Google Scholar]
- 7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e426‐e483. [DOI] [PubMed] [Google Scholar]
- 8. Kario K, Wang JG. Could 130/80 mm Hg be adopted as the diagnostic threshold and management goal of hypertension in consideration of the characteristics of Asian populations? Hypertension. 2018;71(6):979‐984. [DOI] [PubMed] [Google Scholar]
- 9. Steinhubl SR, Muse ED, Topol EJ. The emerging field of mobile health. Sci Transl Med. 2015;7(283):283rv3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Topol EJ. A decade of digital medicine innovation. Sci Transl Med. 2019;11(498):eaaw7610. [DOI] [PubMed] [Google Scholar]
- 11. Masaki K, Tateno H, Kameyama N, et al. Impact of a novel smartphone app (CureApp smoking cessation) on nicotine dependence: prospective single‐arm interventional pilot study. JMIR Mhealth Uhealth. 2019;7(2):e12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nomura A, Tateno H, Masaki K, et al. A novel smoking cessation smartphone app integrated with a mobile carbon monoxide checker for smoking cessation treatment: protocol for a randomized controlled trial. JMIR Res Protoc. 2019;8:e12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell AN, Nunes EV, Matthews AG, et al. Internet‐delivered treatment for substance abuse: a multisite randomized controlled trial. Am J Psychiatry. 2014;171(6):683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koliins SH, DeLoss DJ, Canadas E, et al. A novel digital intervention for actively reducing severity of paediatric ADHD (STARS‐ADHD): a randomised controlled trial. Lancet Digital Health. 2020;2(4):E168‐E178. [DOI] [PubMed] [Google Scholar]
- 15. Nakano M, Eguchi K, Sato T, Onoguchi A, Hoshide S, Kario K. Effect of intensive salt‐restriction education on clinic, home, and ambulatory blood pressure levels in treated hypertensive patients during a 3‐month education period. J Clin Hypertens (Greenwich). 2016;18(5):385‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Digital therapeutics alliance. https://dtxalliance.org. Accessed 26 March, 2020.
- 17. Wendel S. Designing for Behavior Change. USA: O'Reilly Media, Inc.; 2014. [Google Scholar]
- 18. Kario K, Okada K, Kato M, et al. 24‐hour blood pressure‐lowering effect of an SGLT‐2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the randomized placebo‐controlled SACRA study. Circulation. 2018;139(18):2089‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kario K, Hoshide S, Okawara Y, et al. Effect of canagliflozin on nocturnal home blood pressure in Japanese patients with type 2 diabetes mellitus: The SHIFT‐J study. J Clin Hypertens (Greenwich). 2018;20(10):1527‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kario K, Hoshide S, Saito K, et al. Validation of the TM‐2441 ambulatory blood pressure measurement device according to the ISO 81060–2: 2013 standard. Blood Press Monit. 2019;24(1):38‐41. [DOI] [PubMed] [Google Scholar]
- 21. JCS Joint Working Group . Guidelines for the clinical use of 24 hour ambulatory blood pressure monitoring (ABPM) (JCS 2010): ‐ digest version. Circ J. 2012;76(2):508‐519. [DOI] [PubMed] [Google Scholar]
- 22. Shin J, Kario K, Chia YC, et al. Current status of ambulatory blood pressure monitoring in Asian countries: a report from the HOPE Asia Network. J Clin Hypertens (Greenwich). 2020;22(3):384‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kario K, Shin J, Chen CH, et al. Expert panel consensus recommendations for ambulatory blood pressure monitoring in Asia: the HOPE Asia Network. J Clin Hypertens (Greenwich). 2019;21(9):1250‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park S, Buranakitjaroen P, Chen CH, et al. Expert panel consensus recommendations for home blood pressure monitoring in Asia: the Hope Asia Network. J Hum Hypertens. 2018;32(4):249‐258. [DOI] [PubMed] [Google Scholar]
- 25. Kario K, Park S, Buranakitjaroen P, et al. Guidance on home blood pressure monitoring: a statement of the HOPE Asia Network. J Clin Hypertens (Greenwich). 2018;20(3):456‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 27. Kario K, Shimbo D, Hoshide S, et al. Emergence of home blood pressure‐guided management of hypertension based on global evidence. Hypertension. 2019;74:229‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kario K, Thijs L, Staessen JA. Blood pressure measurement and treatment decisions. Circ Res. 2019;124(7):990‐1008. [DOI] [PubMed] [Google Scholar]
- 29. Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter‐based renal denervation in the absence of antihypertensive medications (SPYRAL HTN‐OFF MED Pivotal): a multicentre, randomised, sham‐controlled trial. Lancet. 2020;395(10234):1444‐1451. [DOI] [PubMed] [Google Scholar]
- 30. Kandzari DE, Bohm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6‐month efficacy and safety results from the SPYRAL HTN‐ON MED proof‐of‐concept randomised trial. Lancet. 2018;391(10137):2346‐2355. [DOI] [PubMed] [Google Scholar]
- 31. Kario K, Okura A, Okawara Y, Tomitani N, Ikemoto T, Hoshide S. Impact of introducing catheter‐based renal denervation into Japan for hypertension management: estimation of number of target patients and clinical relevance of ambulatory blood pressure reduction. Curr Hypertens Rev. 2016;12(2):156‐163. [DOI] [PubMed] [Google Scholar]
- 32. He FJ, MacGregor GA. Effect of modest salt reduction on blood pressure: a meta‐analysis of randomized trials. Implications for public health. J Hum Hypertens. 2002;16(11):761‐770. [DOI] [PubMed] [Google Scholar]
- 33. Semlitsch T, Jeitler K, Berghold A, et al. Long‐term effects of weight‐reducing diets in people with hypertension. Cochrane Database Syst Rev. 2016;(3):CD008274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dickinson HO, Mason JM, Nicolson DJ, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24(2):215‐233. [DOI] [PubMed] [Google Scholar]
- 35. Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta‐analysis of randomized controlled trials. Hypertension. 2001;38(5):1112‐1117. [DOI] [PubMed] [Google Scholar]
- 36. Hoshide S, Nishizawa M, Okawara Y, et al. Salt intake and risk of disaster hypertension among evacuees in a shelter after the Great East Japan earthquake. Hypertension. 2019;74(3):564‐571. [DOI] [PubMed] [Google Scholar]
- 37. St‐Onge MP, Grandner MA, Brown D, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367‐e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH‐Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 39. Espeland MA, TONE Cooperative Research Group . Predictors and mediators of successful long‐term withdrawal from antihypertensive medications. Arch Fam Med. 1999;8:228‐236. [DOI] [PubMed] [Google Scholar]
- 40. Kario K, Chirinos JA, Townsend RR, et al. Systemic hemodynamic atherothrombotic syndrome (SHATS) ‐ Coupling vascular disease and blood pressure variability: Proposed concept from pulse of Asia. Prog Cardiovasc Dis. 2020;63(1):22‐32. [DOI] [PubMed] [Google Scholar]
- 41. Masaki K, Tateno H, Nomura A, et al. A randomized controlled trial of a smoking cessation smartphone application with a carbon monoxide checker. NPJ Digit Med. 2020;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ministry of Internal Affairs and Communications Japan . Information and communications in Japan: white Paper 2019. https://www.soumu.go.jp/johotsusintokei/whitepaper/eng/WP2019/2019-index.html. Accessed 25 March, 2020.
- 43. McManus R, Mant J, Franssen M, et al. Efficacy of self‐monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391(3):949‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]


