Abstract
Objectives
To characterise the clinical features and to discover predictive factors of adult males with nocturnal enuresis (NE).
Patients and Methods
A total of 43 eligible adult male patients (mean age was 57.8 years) were recruited prospectively over a 2‐year period. After documentation of medical history, lower urinary tract symptoms (LUTS) were assessed using the International Consultation on Incontinence Modular Questionnaire‐male LUTS (ICIQ‐MLUTS), and a 3‐day ICIQ‐bladder diary (ICIQ‐BD). Video‐urodynamic studies (VUDS) were conducted conforming to the International Continence Society standards. Univariate and multivariate linear regressions were performed to determine potential predictive factors.
Results
Patients with NE had a variety of LUTS and had a high incidence of obesity and comorbidities. On the ICIQ‐BD, NE was associated with nocturnal polyuria (NP), reduced nocturnal bladder capacity (NBC), or a combination of both. Subgroup analysis indicated that patients with more frequent NE had: higher body mass index (BMI); more comorbidities; reduced daytime urinary frequency and urgency quality of life (QoL) sub‐scores; and increased stress urinary incontinence (SUI) and nocturnal bedwetting sub‐scores. Patients with reduced NBC only, had fewer NE episodes, while patients with NP, or with both NP and reduced NBC were more likely have frequent NE. Multivariate analysis confirmed that: BMI; neurogenic causes; sub‐scores of SUI QoL and bedwetting domain; the presence of reduced NBC, and both NP and reduced NBC; and bladder outlet obstruction, were all independent predictive factors for the severity of NE.
Conclusions
NE n the adult male should be systemically assessed and treated, as obesity, neurogenic disorders, excessive urine production, bladder storage and emptying dysfunctions are risk factors. Bladder diaries and VUDS provide valuable information on potential pathophysiological causes, which could assist clinical evaluation and selection of focussed treatment.
Keywords: nocturnal enuresis, nocturia, nocturnal polyuria, nocturnal bladder capacity, bladder diary, video‐urodynamic studies, lower urinary tract symptoms
Introduction
The definition of nocturnal enuresis (NE), standardised by the International Continence Society (ICS) is the complaint of intermittent urinary incontinence (UI) that occurs during periods of sleep [1]. The key feature which differentiates NE from other forms of nocturnal UI is that the patient has to be asleep when bedwetting happens. NE is a common problem among children and adolescents, and the prevalence of NE has been reported as 10%, 3.1% and 1.3% at the ages of 7, 11–12, and 16–17 years, respectively [2]. When it comes to adults, the overall prevalence was estimated to be 0.5–2.3% based on two epidemiological studies [3, 4].
In adults, NE is associated with multiple risk factors and has significant negative impacts on quality of life (QoL) [5]. NE can be either primary, which is further stratified into persistent (never dry for >6 months) and recurrent (dry for > 6 months before adulthood recurrence) subtypes based on symptom continuity; or secondary to lower urinary tract (LUT) dysfunctions, neurogenic conditions, psychiatric disorders, sleep apnoea, metabolic disturbance, and certain medication consumption [6, 7, 8]. Although studies have investigated the symptoms and urodynamic features of NE in adults, some key evaluation outcomes have been missing, and in particular, the bladder diary, which is a useful method in assessing LUTS, as recommended by the guidelines [9]. In order to understand the associations between clinical evaluation indices and adult onset NE, a more systematic assessment appeared necessary, encompassing medical history, comorbidities, subjective symptom appraisal, bladder diaries and video‐urodynamic studies (VUDS). Therefore, the present study aimed to assess the value of comprehensive investigations on clinical features of NE in adult males, and to determine whether there are any predictive factors that could improve our current assessment, understanding, and management of this clinical condition.
Patients and Methods
The study received ethical approval from local Ethics Committee boards. Males aged ≥18 years, presenting with NE were recruited and investigated prospectively since September 2017 over a 2‐year period. All patients were screened and evaluated by a single urological specialist (Q.X.S) to ensure the highest level of consistency. A verbal consent was obtained from each patient prior to performing the tests. A thorough clinical history was taken to confidently distinguish NE from other causes of nocturnal UI. We used the following criteria when recruiting our patients: (i) the complain of unintended voiding during night‐time sleep (in other words, the bedwetting occurs due to patients failing to wake up to void); (ii) NE occurring at least once per week; (iii) and with a symptom duration of ≥3 months [1, 6, 10]. Urine analysis was done to exclude concurrent UTI, and physical examination was performed to exclude palpable mass, potential malignancies, and anatomical abnormalities of the urinary tract. Any serious medical conditions, such as heart and kidney failure, recent cerebrovascular accident, etc., were carefully screened out. To eliminate the effects of non‐standard sleep patterns, nightshift workers were also excluded.
The validated International Consultation on Incontinence Modular Questionnaire‐male lower urinary tract symptoms (ICIQ‐MLUTS) long form was used to assess subjective LUTS and QoL [11]. Assistance was available while the patients were completing the questionnaires to ensure full comprehension of each question. To better analyse different groups of symptoms, we divided the questionnaire into seven domains: frequency and post‐voiding domains, in which each item scores from 0 to 8 for degree and from 0 to 20 for bother; urgency, urgency UI (UUI), stress UI (SUI) and nocturnal bedwetting, in which each item scores from 0 to 4 for degree and from 0 to 10 for bother; and voiding domain, with scores from 0 to 29 for degree and from 0 to 60 for bother.
Patients were asked to complete a bladder diary for 3 consecutive days using the validated ICIQ‐bladder diary (ICIQ‐BD) [12]. From each diary, mean voided volume, maximum voided volume, 24‐h frequency, nocturnal frequency, 24‐h urine volume, and nocturnal urine volume were calculated in accordance with the ICS definitions [1, 13, 14]. Increased 24‐h polyuria was defined as 24‐h urine volume of >40 mL/kg [1]. The Nocturnal Polyuria Index (NPi) was defined as the nocturnal urine volume divided by the 24‐h urine volume [15]. As previously described, a NPi of ≥20% for age <35 years or an NPi of ≥33% for age >35 years was indicative of nocturnal polyuria (NP) [16]. The Nocturnal Bladder Capacity Index (NBCi) was calculated as actual number of night voids minus the predicted number of night voids (nocturnal voided volume divided by maximum voided volume minus 1) [15, 17]. Patients with a NBCi of >1.3 were considered to have reduced nocturnal bladder capacity (NBC) [17]. In addition to completing the ICIQ‐BD, NE episodes during 7 continuous days were recorded separately and were not included for the calculation of numbers of night voids and NBCi.
Uroflowmetry was performed in each patient twice with a minimum voided volume of 150 mL. Subsequently, transabdominal ultrasonography was used to measure the post‐void residual urine volume (PVR) and prostate volume calculated as: length × width × height × π/6. Multi‐channel VUDS (Triton; Laborie Medical Technologies, Mississauga, ON, Canada) were carried out in compliance with the ICS Good Urodynamic Practice Guidelines [18], using a water‐filled pressure conduction system. A 7‐F double‐lumen transurethral catheter and a 7‐F transrectal balloon catheter were used for pressure recording. The bladder was filled with radiographic contrast at a medium rate of 30–50 mL/min. The Bladder Outlet Obstruction Index (BOOI) and Bladder Contractility Index (BCI) were calculated as detrusor pressure at maximum flow rate (PdetQmax) − 2Qmax and PdetQmax + 5Qmax, respectively [19].
Data were analysed using the Statistical Package for the Social Sciences (SPSS®; version 25, SPSS Inc., IBM Corp., Armonk, NY, USA) by an investigator blinded to the medical status of the patients. Quantitative variables were presented as mean ± standard deviation (SD) if normally distributed and were compared using two‐sample t‐tests. The median (interquartile range [IQR]) was used to describe non‐normally distributed variables, which were compared via Mann–Whitney U‐tests. Nominal variables were shown as number of cases (%) and significance was detected using Pearson’s chi‐squared tests or Fisher’s exact tests. Univariate and multivariate linear regression was performed to determine the risk factors for NE episodes. The variables with a P ≤ 0.3 in univariate analysis were included in the multivariate analysis and processed with a stepwise selection approach. A P < 0.05 was considered to indicate statistical significance.
Results
During the 2‐year period of enrolment, we identified 51 eligible patients. Among them, seven were excluded due to either lack of pressure–flow study data or bladder diaries. One patient with poor cognitive ability was also excluded, which resulted in 43 patients left for the subsequent analysis.
As shown in Table 1 and Appendix S1, over half of our recruited patients were aged 50–70 years (53.5%) and had a body mass index (BMI) >24 kg/m2 (53.5%). In all, 39.5% and 18.6% reported smoking and alcohol consumption on ≥10 occasions within the last 30 days, respectively. Comorbidities were presented in 88.4% of the patients, of which hypertension, hyperlipidaemia, diabetes mellitus, hyperuricaemia, and heart diseases were the most common conditions. LUTS medications, namely α‐blockers, 5α‐reductase inhibitors, and anti‐muscarinics, were taken by 79.1% of the cohort patients either alone or in combination. Despite the high drug‐taking rate, surprisingly, we found nearly half (21 out 43 men) had never consulted a urology specialist for their bedwetting symptom, probably due to either embarrassment or lack of knowledge that it is a treatable condition, or assuming that their prescribed medications should treat their NE. Within the 62.8% of patients with primary onset NE, we identified one primary persistent and 26 primary recurrent cases. The recruited patients with secondary NE were due to neurogenic deficiencies (16.3%, including cerebral infarction, Parkinson’s disease, Alzheimer’s disease, encephalitis and cerebellar atrophy), usage of antipsychotic drugs (9.3%), or surgical procedures, including radical prostatectomy (7.0%) and TURP (4.6%).
Table 1.
Demographic data of the adult male patients with NE.
| Variable | Value |
|---|---|
| Number of patients | 43 |
| Age, years, mean (SD) | 57.8 (15.6) |
| BMI, kg/m2, mean (SD) | 23.9 (3.4) |
| Prostate volume, ml, mean (SD) (n = 40) | 41.8 (11.8) |
| Comorbidities, n (%) | |
| 0 | 5 (11.6) |
| 1 | 11 (25.6) |
| 2 | 9 (20.9) |
| ≥3 | 18 (41.9) |
| Frequency of each comorbid condition, n (%) | |
| Hypertension | 26 (60.5) |
| Hyperlipidaemia | 17 (39.5) |
| Diabetes mellitus | 10 (23.3) |
| Hyperuricaemia | 10 (23.3) |
| Heart disease | 9 (20.9) |
| Neurogenic condition | 8 (18.6) |
| Chronic kidney disease | 5 (11.6) |
| Sleep apnoea | 2 (4.7) |
| COPD | 1 (2.3) |
| Hepatitis B | 1 (2.3) |
| LUTS medications, n (%) | |
| None | 9 (20.9) |
| α‐blocker | 22 (51.2) |
| 5α‐reductase inhibitor | 15 (34.9) |
| Anti‐muscarinics | 14 (32.6) |
| β3 agonist | 2 (4.7) |
| Monotherapy | 17 (39.5) |
| Combined therapy | 17 (39.5) |
| Enuresis classification, n (%) | |
| Primary persistent | 1 (2.3) |
| Primary recurrent | 26 (60.5) |
| Secondary (neurogenic) | 7 (16.3) |
| Secondary (drug related) | 4 (9.3) |
| Secondary (surgery related) | 5 (11.6) |
COPD, chronic obstructive pulmonary disease.
The prevalence of each LUTS is shown in Figure 1A. Storage, voiding and post‐voiding symptoms were reported in 97.7%, 86.0% and 69.8% of the patients, with significant symptom overlap. Nocturia, defined as the individual waking up one or more times to void, was the most prevalent storage symptom, followed by frequency and urgency [1, 14]. A detailed analysis of nocturia episodes showed that >90% of patients reported a nocturnal frequency of ≥2 times (Figure 1B). Overall, we found that 41.9% of the cohort patients had NE co‐existing with either UUI, SUI, or mixed UI. There were also a small number of patients who reported orchidalgia (7.0%), painful voiding (14.0%), bladder region pain (14.0%) and haematuria (7.0%). The ICIQ‐MLUTS scores, ICIQ‐BD and VUDS parameters are shown in Table 2 and Appendix S1. We found that the enrolled patients with NE had either NP alone (14.0%), reduced NBC alone (34.9%), both (32.5%) or neither (18.6%): NP and NBC were calculated from the bladder diaries. VUDS demonstrated six (14.0%) completely normal patients, three (7.0%) patients with urodynamic SUI (post prostatectomy or cerebral infarction), and five (11.6%) patients had opened bladder neck during filling (either after prostatic procedures or cerebral infarction). Increased bladder sensation (first sensation at <150 mL) and detrusor overactivity (DO) were identified in 30 (69.8%) and 15 (34.9%) patients, separately. In all, 10 (23.3%) patients were obstructed and 13 (30.2%) had detrusor underactivity during voiding.
Fig. 1.
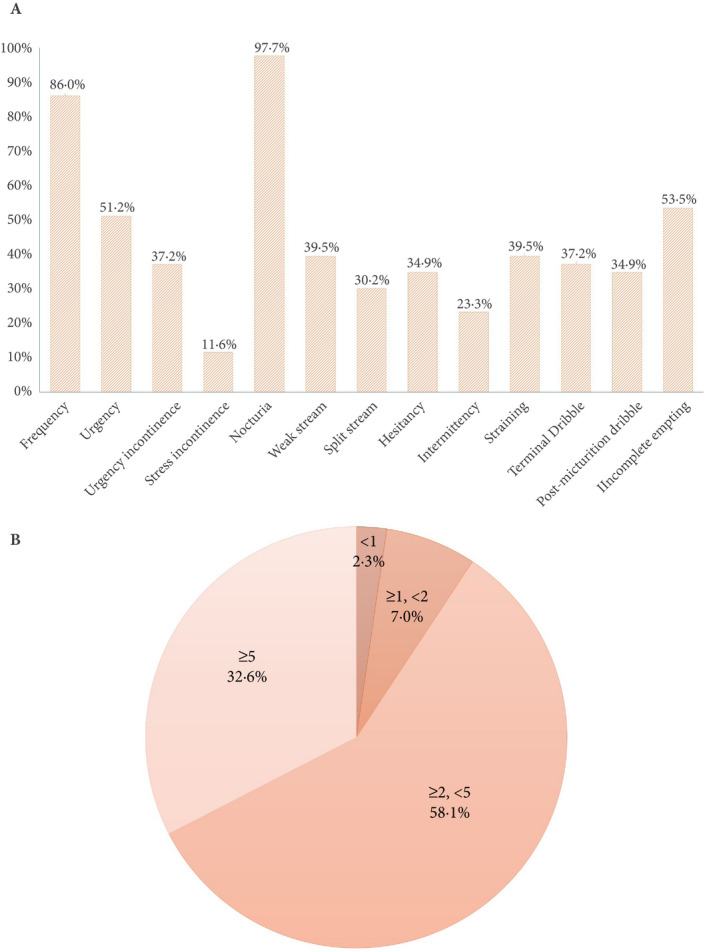
The prevalence of each LUTS (A) and distribution of nocturia episodes (B).
Table 2.
ICIQ‐MULTS score, bladder diary and urodynamic variables.
| Variable | Value |
|---|---|
| ICIQ‐MLUTS score | |
| Frequency, median (IQR) | 6.0 (5.0–7.0) |
| Frequency QoL, mean (SD, range) | 14.3 (4.0, 6.0–20.0) |
| Urgency, median (IQR) | 3.0 (1.0–4.0) |
| Urgency QoL, median (IQR) | 6.0 (3.0–9.0) |
| UUI, median (IQR) | 3.0 (1.0–3.0) |
| UUI QoL, median (IQR) | 5.0 (1.0–9.0) |
| SUI, median (IQR) | 0.0 (0.0–1.0) |
| SUI QoL, median (IQR) | 0.0 (0.0–2.0) |
| Voiding, mean (SD, range) | 10.9 (5.3, 2.0–24.0) |
| Voiding QoL, mean (SD, range) | 19.0 (12.8, 0.0–51.0) |
| Post‐voiding, mean (SD, range) | 3.0 (1.9, 0.0–8.0) |
| Post‐voiding QoL, mean (SD, range) | 7.2 (5.0, 0.0–20.0) |
| Nocturnal bedwetting, median (IQR) | 3.0 (2.0–3.0) |
| Nocturnal bedwetting QoL, median (IQR) | 7.0 (5.0–9.0) |
| Bladder diary | |
| Mean voided volume, mL, mean (SD, range) | 167.7 (48.5, 62.4–245.4) |
| Maximum voided volume, mL, mean (SD, range) | 265.7 (75.5, 150–500) |
| 24‐h frequency, voids, median (IQR) | 12.6 (11.1–14.4) |
| Nocturnal frequency, voids, median (IQR) | 4.0 (3.0–5.0) |
| 24‐h urine volume, mL, mean (SD, range) | 2159.3 (416.7, 1377.6–2928.2) |
| 24‐h polyuria, n (%) | 11 (25.9) |
| Nocturnal urine volume, mL, median (IQR) | 708.9 (450.4–794.2) |
| NP, n (%) | 20 (46.5) |
| Reduced NBC, n (%) | 30 (69.8) |
| NP only, n (%) | 6 (14.0) |
| Reduced NBC only, n (%) | 15 (34.9) |
| NP + reduced NBC, n (%) | 14 (32.5) |
| NE episodes in a week, median (IQR) | 3.0 (2.0–5.0) |
| Uroflowmetry | |
| Qmax, mL/s, mean (SD, range) | 19.6 (9.5, 4.1–46.1) |
| Voided volume, mL, median (IQR) | 278.9 (193.7–347.1) |
| PVR, mL, median (IQR) | 32.5 (0.0–100.0) |
| VUDS | |
| First sensation, mL, median (IQR) | 117.4 (80.2–166.7) |
| Cystometric capacity, mL, mean (SD, range) | 267.8 (93.0, 127.0–553.9) |
| Compliance, mL/cmH2O, median (IQR) | 51.4 (32.4–86.6) |
| PdetQmax, cmH2O, median (IQR) | 46.5 (36.7–62.3) |
| Qmax, mL/s, mean (SD, range) | 13.2 (6.0, 4.4–29.4) |
| DO, n (%) | 15 (34.9) |
| BOOI, median (IQR) | 21.1 (10.2–38.6) |
| BCI, mean (SD, range) | 120.4 (31.1, 50.6–176.5) |
As shown in Table 3 and Appendix S2, we performed subgroups analysis stratified by age, BMI, NP, reduced NBC, and NE episodes. Not surprisingly, older men had larger prostate volumes, more voiding symptoms, worse QoL scores, and nocturnal frequency, but without any significant difference in NE‐related parameters. It was noted that obese patients (BMI ≥24 kg/m2) had significantly higher nocturnal bedwetting sub‐scores, more NE episodes/week, along with higher incidence rates of reduced NBC and comorbidities. Patients with NP manifested significantly more NE episodes/week in comparison with those without, but this was not found when comparing patients with and without reduced NBC. It is worth mentioning that in patients with fewer NE episodes (<4/week): the BMI was significantly lower; there were fewer comorbidities; and higher frequency, worse urgency QoL, and lower bedwetting sub‐scores. From the ICIQ‐BD data, those patients were as prone to have significantly smaller maximum voided volume, and a higher proportion of pure reduced NBC, which was consistent with the ICIQ‐MLUTS recorded symptoms. For those with more severe NE (≥4/week), compared to patients with mild symptoms, significantly increased SUI sub‐score; and higher percentages of NP and combined NP plus reduced NBC were found.
Table 3.
Subgroup analysis.
| NP | Reduced NBC | NE episodes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No (n = 23) | Yes (n = 20) | P | No (n = 14) | Yes (n = 29) | P | NE < 4/week (n = 23) | NE ≥ 4/week (n = 20) | P | |
| Age, years, mean (SD) | 61.4 (11.4) | 55.7 (19.0) | 0.25 | 63.2 (14.4) | 56.6 (15.8) | 0.09 | 57.0 (14.7) | 60.8 (16.5) | 0.43 |
| BMI, kg/m2, mean (SD) | 23.3 (2.9) | 24.6 (4.0) | 0.24 | 22.6 (2.6) | 24.5 (3.6) | 0.03* | 22.4 (3.2) | 25.6 (2.9) | <0.01* |
| Prostate volume, mL, mean (SD) (n = 40) | 42.4 (10.4) | 41.1 (13.4) | 0.74 | 45.4 (12.3) | 40.3 (11.4) | 0.23 | 40.0 (10.4) | 45.6 (12.7) | 0.09 |
| Comorbid conditions, n (%) | |||||||||
| 0 | 3 (13.0) | 2 (10.0) | 0.76 | 1 (7.1) | 4 (13.8) | 0.23 | 4 (17.4) | 1 (5.0) | 0.21 |
| 1 | 8 (34.8) | 3 (15.0) | 0.08 | 6 (42.9) | 5 (17.2) | 0.52 | 10 (43.5) | 1 (5.0) | <0.01* |
| 2 | 6 (26.1) | 3 (15.0) | 2 (14.3) | 7 (24.2) | 5 (21.7) | 4 (20.0) | |||
| ≥3 | 6 (26.1) | 12 (60.0) | 5 (35.7) | 13 (44.8) | 4 (17.4) | 14 (70.0) | |||
| ICIQ‐MLUTS | |||||||||
| Frequency, mean (SD) | 5.7 (1.5) | 5.7 (1.6) | 0.86 | 5.5 (1.7) | 5.8 (1.5) | 0.57 | 6.3 (1.4) | 5.0 (1.5) | <0.01* |
| Frequency QoL, mean (SD) | 14.6 (4.0) | 13.9 (4.1) | 0.59 | 13.1 (3.8) | 14.8 (4.1) | 0.21 | 15.8 (3.6) | 12.5 (3.8) | <0.01* |
| Urgency, median (IQR) | 3.0 (2.0) | 2.0 (2.0) | 0.24 | 2.0 (2.0) | 3.0 (2.0) | 0.02* | 3.0 (2.0) | 2.0 (2.0) | 0.14 |
| Urgency QoL, median (IQR) | 8.0 (7.0) | 4.0 (4.0) | 0.10 | 3.5 (5.3) | 8.0 (6.0) | 0.02* | 8.0 (7.0) | 4.5 (4.5) | 0.04* |
| UUI, median (IQR) | 3.0 (2.0) | 1.0 (2.0) | 0.14 | 1.0 (2.3) | 3.0 (3.0) | 0.04* | 3.0 (3.0) | 1.5 (2.0) | 0.17 |
| UUI QoL, median (IQR) | 6.0 (9.0) | 5.0 (6.5) | 0.18 | 2.5 (7.8) | 5.8 (6.5) | 0.14 | 6.0 (9.0) | 5.0 (6.5) | 0.31 |
| SUI, median (IQR) | 0.0 (0.0) | 0.0 (1.8) | 0.07 | 0.0 (1.0) | 0.0 (1.0) | 0.72 | 0.0 (0.0) | 0.5 (1.8) | 0.02* |
| SUI QoL, median (IQR) | 0.0 (0.0) | 0.0 (2.8) | 0.07 | 0.0 (3.0) | 0.0 (0.5) | 0.33 | 0.0 (0.0) | 0.0 (6.8) | 0.05 |
| Voiding, mean (SD) | 11.7 (5.0) | 10.1 (5.7) | 0.33 | 9.9 (4.6) | 11.4 (5.7) | 0.41 | 10.9 (4.6) | 11.0 (6.2) | 0.96 |
| Voiding QoL, mean (SD) | 19.4 (12.1) | 18.4 (13.9) | 0.80 | 14.6 (11.1) | 21.1 (13.2) | 0.12 | 19.1 (10.7) | 18.8 (15.2) | 0.94 |
| Post‐voiding, mean (SD) | 2.7 (1.4) | 3.2 (2.2) | 0.35 | 2.1 (1.0) | 3.3 (2.0) | 0.01* | 2.9 (1.0) | 3.0 (2.2) | 0.89 |
| Post‐voiding QoL, mean (SD) | 6.4 (4.2) | 8.0 (5.7) | 0.34 | 4.9 (3.3) | 8.2 (5.4) | 0.02* | 7.1 (4.1) | 7.2 (6.0) | 0.99 |
| Nocturnal bedwetting, median (IQR) | 2.0 (2.0) | 3.0 (2.0) | 0.12 | 2.5 (1.0) | 3.0 (1.0) | 0.82 | 2.0 (2.0) | 3.0 (2.0) | <0.01* |
| Nocturnal bedwetting QoL, median (IQR) | 6.0 (5.0) | 8.0 (4.0) | 0.25 | 5.5 (7.0) | 8.0 (3.0) | 0.19 | 5.0 (4.0) | 8.5 (3.0) | <0.01* |
| Bladder diary | |||||||||
| Mean voided volume, mL, | 165.8 (47.9) | 170.0 (50.4) | 0.78 | 195.3 (44.1) | 154.4 (45.5) | 0.01* | 159.0 (53.4) | 177.8 (41.3) | 0.20 |
| Maximum voided volume, mL, mean (SD) | 251.5 (58.9) | 282.0 (89.8) | 0.20 | 269.5 (59.9) | 263.8 (82.9) | 0.80 | 241.7 (65.9) | 293.2 (78.0) | 0.03* |
| 24‐h frequency, voids, median (IQR) | 12.7 (4.3) | 12.5 (3.0) | 0.90 | 11.2 (2.5) | 13.4 (4.4) | <0.01* | 12.7 (4.3) | 12.9 (3.8) | 0.74 |
| Nocturnal frequency, voids, median (IQR) | 4.0 (2.0) | 4.0 (2.0) | 0.14 | 3.0 (2.0) | 4.0 (2.0) | <0.01* | 4.0 (2.0) | 4.0 (2.0) | 0.52 |
| 24‐h polyuria, n (%) | 7 (30.4) | 4 (20.0) | 0.43 | 3 (21.4) | 8 (27.6) | 0.21 | 4 (17.4) | 7 (35.0) | 0.19 |
| Nocturnal urine volume, mL, mean (SD) | 518.6 (211.0) | 742.4 (138.5) | <0.01* | 573.4 (253.3) | 646.5 (188.5) | 0.35 | 577.7 (183.4) | 674.4 (233.9) | 0.14 |
| NE episodes in a week, median (IQR) | 3.0 (2.0) | 5.0 (4.0) | 0.02* | 2.5 (3.0) | 4.0 (3.0) | 0.52 | 2.0 (2.0) | 5.0 (2.0) | <0.01* |
| NP, n (%) | 0 (0.0) | 20 (100.0) | <0.01* | 6 (42.9) | 14 (48.3) | 0.74 | 6 (26.1) | 14 (70.0) | <0.01* |
| Reduced NBC, n (%) | 15 (65.2) | 14 (70.0) | 0.74 | 0 (0.0) | 29 (100.0) | <0.01* | 16 (69.6) | 13 (65.0) | 0.75 |
| Pure NP, n (%) | 0 (0.0) | 6 (30.0) | 0.01* | 6 (42.9) | 0 (0.0) | <0.01* | 2 (8.7) | 4 (20.0) | 0.29 |
| Pure reduced NBC, n (%) | 15 (65.2) | 0 (0.0) | <0.01* | 0 (0.0) | 15 (51.7) | <0.01* | 12 (52.2) | 3 (15.0) | 0.01* |
| NP + reduced NBC, n (%) | 0 (0.0) | 14 (70.0) | <0.01* | 0 (0.0) | 14 (48.3) | <0.01* | 4 (17.4) | 10 (50.0) | 0.02* |
| Uroflowmetry | |||||||||
| Qmax, mL/s, mean (SD) | 19.8 (7.8) | 19.3 (11.3) | 0.88 | 24.7 (9.1) | 17.1 (8.7) | 0.02* | 19.4 (8.3) | 19.8 (10.9) | 0.87 |
| Voided volume, mL, median (IQR) | 303.6 (168.0) | 245.5 (140.5) | 0.19 | 303.2 (184.5) | 278.4 (143.6) | 0.47 | 292.8 (151.4) | 255.8 (172.0) | 0.43 |
| PVR, mL, median (IQR) | 20.0 (99.0) | 50.0 (100.0) | 0.71 | 11.0 (130.0) | 50.0 (97.5) | 0.60 | 25.0 (78.0) | 37.5 (143.8) | 0.69 |
| VUDS | |||||||||
| First sensation, mL, median (IQR) | 129.0 (87.5) | 105.6 (90.7) | 0.47 | 123.8 (61.2) | 105.6 (104.5) | 0.58 | 130.3 (88.4) | 95.7 (70.9) | 0.08 |
| Cystometric capacity, mL, mean (SD) | 273.6 (86.7) | 260.9 (101.6) | 0.66 | 274.5 (90.7) | 264.6 (95.5) | 0.75 | 271.5 (82.4) | 263.7 (106.0) | 0.79 |
| Compliance, mL/cmH2O, median (IQR) | 46.4 (42.4) | 60.2 (67.1) | 0.35 | 86.3 (128.7) | 42.8 (38.8) | 0.01* | 50.5 (54.9) | 54.3 (69.4) | 0.64 |
| <40, n (%) | 8 (34.8) | 7 (35.0) | 0.99 | 2 (14.3) | 13 (44.8) | 0.05 | 9 (39.1) | 6 (30.0) | 0.53 |
| BOOI, median (IQR) | 16.8 (27.7) | 33.5 (41.2) | 0.77 | 13.6 (32.8) | 24.6 (34.8) | 0.33 | 30.0 (27.6) | 15.0 (37.0) | 0.23 |
| BCI, mean (SD) | 114.4 (27.2) | 124.1 (34.2) | 0.31 | 116.3 (28.4) | 120.1 (32.1) | 0.70 | 119.9 (29.0) | 117.8 (33.2) | 0.83 |
*Indicates statistically significant difference between groups.
Univariate linear regression indicated several significant predictors for NE episodes, including: BMI; high number of comorbidities; neurogenic NE; the existence of SUI and mixed UI symptoms; high frequency, SUI and nocturnal bedwetting sub‐scores; increased PVR, maximum voided volume, 24‐h and nocturnal urine volume; and the presence of 24‐h polyuria, NP, and combined NP plus reduced NBC. Within the 32 variables incorporated in the multivariate analysis, we discovered that BMI; a neurogenic cause; SUI QoL and nocturnal bedwetting sub‐scores; the presence of reduced NBC and combination of NP plus reduced NBC; and BOO were independent predictors of NE severity (Table 4).
Table 4.
Potential risk factors associated with NE severity.
| Univariate linear regression | Multivariate linear regression | |||||
|---|---|---|---|---|---|---|
| B | β | P | B | β | P | |
| Age | 0.02 | 0.17 | 0.30 | ‐ | ‐ | 0.61 |
| BMI | 0.25 | 0.48 | <0.01 | 0.12 | 0.20 | 0.02 |
| Smoking status | 0.70 | 0.30 | 0.05 | ‐ | ‐ | 0.50 |
| Alcohol consumption | −0.19 | −0.04 | 0.82 | ‐ | ‐ | ‐ |
| Prostate volume | 0.05 | 0.29 | 0.07 | ‐ | ‐ | 0.93 |
| The presence of comorbid conditions | 1.48 | 0.27 | 0.09 | ‐ | ‐ | 0.72 |
| Numbers of comorbid conditions | 0.92 | 0.55 | <0.01 | ‐ | ‐ | 0.93 |
| Whether secondary NE | 1.75 | 0.42 | 0.01 | ‐ | ‐ | 0.13 |
| Neurogenic condition related | 2.64 | 0.48 | <0.01 | 3.40 | 0.63 | <0.01 |
| Drug related | 0.39 | 0.06 | 0.73 | ‐ | ‐ | ‐ |
| Surgery related | 0.17 | 0.03 | 0.87 | ‐ | ‐ | ‐ |
| The presence of UUI | −0.54 | −0.13 | 0.41 | ‐ | ‐ | ‐ |
| The presence of SUI | 2.39 | 0.43 | <0.01 | ‐ | ‐ | 0.23 |
| The presence of MUI | 3.05 | 0.44 | <0.01 | ‐ | ‐ | 0.58 |
| ICIQ‐MLUTS | ||||||
| Frequency sub‐score | −0.42 | −0.37 | 0.02 | ‐ | ‐ | 0.45 |
| Frequency QoL sub‐score | −0.17 | −0.38 | 0.01 | ‐ | ‐ | 0.09 |
| Urgency sub‐score | −0.31 | −0.20 | 0.21 | ‐ | ‐ | 0.23 |
| Urgency QoL sub‐score | −0.12 | −0.21 | 0.18 | ‐ | ‐ | 0.62 |
| UUI sub‐score | −0.25 | −0.19 | 0.22 | ‐ | ‐ | 0.24 |
| UUI QoL sub‐score | −0.03 | −0.06 | 0.71 | ‐ | ‐ | ‐ |
| SUI sub‐score | 0.60 | 0.34 | 0.03 | ‐ | ‐ | 0.69 |
| SUI QoL sub‐score | 0.20 | 0.33 | 0.03 | −0.29 | −0.36 | <0.01 |
| Voiding sub‐score | −0.02 | −0.05 | 0.74 | ‐ | ‐ | ‐ |
| Voiding QoL sub‐score | −0.01 | −0.05 | 0.77 | ‐ | ‐ | ‐ |
| Post‐voiding sub‐score | −0.05 | −0.05 | 0.77 | ‐ | ‐ | ‐ |
| Post‐voiding QoL sub‐score | −0.01 | −0.03 | 0.85 | ‐ | ‐ | ‐ |
| Nocturnal bedwetting sub‐score | 1.20 | 0.60 | <0.01 | 0.79 | 0.39 | <0.01 |
| Nocturnal bedwetting QoL sub‐score | 0.43 | 0.54 | <0.01 | ‐ | ‐ | 0.35 |
| Bladder diary | ||||||
| Mean voided volume | 0.01 | 0.27 | 0.09 | ‐ | ‐ | 0.22 |
| Maximum voided volume | 0.01 | 0.35 | 0.02 | ‐ | ‐ | 0.30 |
| 24‐h frequency | −0.07 | −0.16 | 0.33 | ‐ | ‐ | ‐ |
| Nocturnal frequency | −0.18 | −0.16 | 0.30 | ‐ | ‐ | 0.55 |
| 24‐h urine volume | 0.00 | 0.35 | 0.03 | ‐ | ‐ | 0.43 |
| Nocturnal urine volume | 0.00 | 0.35 | 0.02 | ‐ | ‐ | 0.13 |
| The presence of 24‐h polyuria | 1.29 | 0.32 | 0.04 | ‐ | ‐ | 0.18 |
| The presence of NP | 1.59 | 0.39 | 0.01 | ‐ | ‐ | 0.70 |
| The presence of reduced NBC | −0.64 | −0.17 | 0.29 | −1.99 | −0.45 | <0.01 |
| The presence of NP + reduced NBC | 1.60 | 0.36 | 0.02 | 2.17 | 0.50 | <0.01 |
| Uroflowmetry | ||||||
| Qmax | 0.01 | 0.05 | 0.75 | ‐ | ‐ | ‐ |
| PVR | 0.01 | 0.37 | 0.02 | ‐ | ‐ | 0.89 |
| VUDS | ||||||
| Cystometric capacity | 0.00 | −0.02 | 0.93 | ‐ | ‐ | ‐ |
| The presence of reduced compliance | −0.54 | −0.14 | 0.38 | ‐ | ‐ | ‐ |
| The presence of hypersensitive bladder | 1.04 | 0.24 | 0.13 | ‐ | ‐ | 0.15 |
| The presence of DO | −0.05 | −0.01 | 0.93 | ‐ | ‐ | ‐ |
| The presence of urodynamic SUI | 1.62 | 0.23 | 0.14 | ‐ | ‐ | 0.40 |
| The presence of BOO | 0.98 | 0.20 | 0.19 | 1.17 | 0.24 | <0.01 |
| The presence of detrusor underactivity | −0.07 | −0.02 | 0.92 | ‐ | ‐ | ‐ |
Thirty‐two parameters (P ≤ 0.3 in univariate analysis) were included in the multivariable analysis. The Durbrin‐Watson test value was 1.725, R = 0.934, R 2 = 0.872 and the adjusted R 2 = 0.844.
Discussion
Although a relatively rare condition in adults, NE poses a considerable threat to an individual’s QoL and mental health, due to disturbed sleep, lack of personal hygiene, increased financial burden, and by lowering of self‐esteem and in particular embarrassment. [4, 5]. Although insufficient arousal mechanisms during sleep are an essential part of NE, NE may be caused by multifactorial aetiologies, such as LUT dysfunctions, neurogenic diseases, psychiatric medications, and sleep disorders just to name a few [6]. Apart from the lack of attention and available research data, published studies have focussed on either clinical symptoms or urodynamic characteristics, without providing comprehensive evaluations on quantified symptoms, bladder function, and comorbid conditions. Therefore, in the present study we attempted to fill this gap. With strict screening criteria, the present study recruited 43 adult males who sought a medical consultation for NE by a single urologist during a 2‐year enrolment period. Most of our present cohort had a high BMI, which was an independent predictor of NE frequency, as evidenced by the multivariate analysis. This is consistent with the finding that high BMI is associated with high prevalence of NE in studies based on women and children [20, 21]. In our secondary NE subtype, there was a strong association between neurogenic factors and NE episodes. However, this was not identified in those on psychiatric medications, or with prostate surgeries. It is well known that neurogenic conditions may lead to a variety of VUDS abnormalities, such as opened bladder neck during filling, reduced compliance, SUI and chronic urinary retention, which could jeopardise the night‐time continence mechanisms.
In the present study, nocturia was the most prevalent storage symptom, followed by frequency (86.1%), urgency (51.2%) and UI (41.9%). In contrast, Yeung et al. [4] have reported a lower prevalence of these symptoms in one epidemiological study, possibly due to the younger age (16–40 years) of those investigated. The incidence of UI has been previously found to be as high as 82% and 92% in women with NE [20, 22]. Considering that UI is a more pronounced symptom in the female general population, the much lower overall UI rate in male patients with NE could be due to the gender difference [23, 24]. It is worth mention that the existing results were not consistent in respect to patients’ symptoms as predictors of the presence of NE [4, 20, 22, 25]. Although Campbell et al. [20, 25] indicated that the symptoms of overactive bladder, UI and nocturia were independent predictors in women, and the improvement of NE was associated with the alleviation of UI by transvaginal tape procedures, other studies failed to show a significant correlation regarding SUI symptoms in women [20, 25]. Unlike previous studies, we found that the presence of SUI and mixed UI symptoms, both frequency and SUI sub‐scores, but not urgency and UUI sub‐scores and nocturia episodes, were significantly associated with NE episodes in the univariate analysis; however, only the SUI QoL sub‐scores met the criteria as an independently predictive factor during multivariate analysis. It is worth mentioning that all patients who reported SUI had secondary NE, either due to neurogenic disorders or prostate surgery. As alluded to above, the divergence among studies could be attributed to different inclusion criteria and symptom assessment approaches.
Bladder diaries have been authenticated as a valuable tool in the clinical evaluation of LUTS, especially in patients with nocturia, and allow nocturia to be separated into four categories, namely due to, 24‐h polyuria, NP, reduced NBC and both NP plus reduced NBC, based on the calculation formulas from the diaries [13, 15, 26]. As yet, few publications have looked into these parameters in detail in adult patients with NE. Hofmeester et al. [27] suggested that amongst patients with NE aged 11–42 years, 42% and 96% had small maximum voided volume and NP, respectively. In addition to previous knowledge, our present study has shown that men with NE can possess both or either increased urine production and bladder function problems, with reduced NBC, and NP with reduced NBC, to be independent predictors of NE episodes. We might also infer that the presence of reduced NBC, as shown by bladder diaries, implies that the patients may experience fewer NE episodes, but increased nocturia episodes. However, on the contrary, the detection of NP plus reduced NBC, predicts a greater severity of NE. Therefore, it is clear that the bladder diary is of great value in providing evidence on the potential pathophysiological causative mechanisms, which could guide the selection of treatment for NE, e.g. by targeting reduced bladder capacity or excessive urine production during the night.
The uroflowmetry studies showed that, over half of the men had a normal Q max and <30% presented a PVR of >100 mL. This seems to be widely divergent with the published data, in which the average Q max and PVR was 8.5 mL/s and 350 mL, respectively [28]. The discrepancy could be explained by the enrolment of patients with more severe LUTS with proven obstruction in the previous study, as evidenced by a high proportion of vesicoureteral reflux, bladder diverticulum and reduced compliance [28]. During VUDS, DO was found in more than one‐third of the patients, in agreement with one of the previous studies (32%), but significantly lower compared with other reports (56–93%), which included a substantial number of female patients [20, 29, 30]. It should be noted that, although existing studies substantiated that DO is more common in women with secondary NE compared to the control population, we did not find that the presence of DO was not an independent predictor of NE episodes in male patients with NE [20]. Instead, BOO, caused by an enlarged prostate, was the only significant urodynamic parameter associated with the frequency of NE episodes, echoing with the previous outcomes that BOO may herald adult onset NE, which can be resolved by TURP [28].
Our present study has some limitations. To begin with, the relatively small sample size, which is due to the rarity of this disease and the stringent recruitment criteria. Nevertheless, our present study is the largest non‐retrospective and non‐epidemiological study of male patients with NE. Our present study provides insight into the clinical features and predictive features of NE, especially data from bladder diaries, the use of which has been previously neglected. Additionally, there is a lack of continued medical records for those who may have had abnormal childhood bladder function and their NE status, which has been previously found to be associated with adolescence or adult onset NE and LUTS [31, 32]. Likewise, we could not trace the information on other potential aetiologies, such as psychological disorders, genetic and hereditary aspects.
In conclusion, NE in adult men can be characterised by high prevalence of obesity and comorbidities, and with a variety of LUTS, and may be caused by neurogenic conditions, urine overproduction, bladder hypersensitivity, DO, reduced capacity and/or BOO. Of considerable importance, we have also shown that there are independent predictive factors of NE severity: BMI, the onset of neurogenic disease (especially, cerebral infarction, neurodegenerative diseases and encephalitis), ICIQ‐MLUTS SUI QoL and nocturnal bedwetting sub‐scores, the presence of reduced NBC and combination of NP plus reduce NBC, and BOO. Bladder diaries and VUDS, provide valuable information on the pathophysiological mechanisms causing NE, and should be considered during the assessment of men with NE, so that focussed treatment can be delivered.
Funding
National Natural Science Foundation of China (81500579).
Translational Medicine Research Project of Naval Medical University (2017JZ44).
Conflict of Interest
Paul Abrams is a consultant for Recordati, Astellas, and Ipsen, and a lecturer for Astellas, Pfizer, and Sun Pharma. Other authors have no real or potential conflicts of interest with respect to the trial, authorship, and/or publication of this article.
Abbreviations
- (S)(U)UI
(stress) (urgency) urinary incontinence
- BCI
Bladder Contractility Index
- BMI
body mass index
- BOOI
Bladder Outlet Obstruction Index
- DO
detrusor overactivity
- ICIQ(‐BD)(‐MLUTS)
International Consultation on Incontinence Modular Questionnaire(‐bladder diary) (‐male lower urinary tract symptoms)
- IQR
interquartile range
- LUT
lower urinary tract
- NBC
nocturnal bladder capacity
- NBCi
Nocturnal Bladder Capacity Index
- NE
nocturnal enuresis
- NPi
Nocturnal Polyuria Index
- PdetQmax
detrusor pressure at maximum urinary flow
- PVR
post‐void residual urine volume
- Qmax
maximum urinary flow rate
- QoL
quality of life
- VUDS
video‐urodynamic studies
Supporting information
Appendix S1. Additional demographic and urodynamic data.
Appendix S2. Supplementary data of subgroup analysis stratified by age and BMI.
Acknowledgements
We gratefully appreciate Prof. Xiaofei Ye for his expertise on statistics, as well as Mr Aiguo Wang and Ms Chan Wu for their urodynamic technical support. Dr Qi‐Xiang Song would like to express his sincerely gratitude to Ms Carol Chen for taking meticulous care of Timo during the novel coronavirus pandemic while this paper was written.
References
- 1. Hashim H, Blanker MH, Drake MJ et al. International Continence Society (ICS) report on the terminology for nocturia and nocturnal lower urinary tract function. Neurourol Urodyn 2019; 38: 499–508 [DOI] [PubMed] [Google Scholar]
- 2. Buckley BS, Lapitan MC. Epidemiology Committee of the Fourth International Consultation on Incontinence P. Prevalence of urinary incontinence in men, women, and children–current evidence: findings of the Fourth International Consultation on Incontinence. Urology 2010; 76: 265–70 [DOI] [PubMed] [Google Scholar]
- 3. Hirasing RA, van Leerdam FJ, Bolk‐Bennink L, Janknegt RA. Enuresis nocturna in adults. Scand J Urol Nephrol 1997; 31: 533–6 [DOI] [PubMed] [Google Scholar]
- 4. Yeung CK, Sihoe JD, Sit FK, Bower W, Sreedhar B, Lau J. Characteristics of primary nocturnal enuresis in adults: an epidemiological study. BJU Int 2004; 93: 341–5 [DOI] [PubMed] [Google Scholar]
- 5. Lee D, Dillon BE, Lemack GE. Adult onset nocturnal enuresis: identifying causes, cofactors and impact on quality of life. Low Urin Tract Symptoms 2018; 10: 292–6 [DOI] [PubMed] [Google Scholar]
- 6. Akhavizadegan H, Locke JA, Stothers L, Kavanagh A. A comprehensive review of adult enuresis. Can Urol Assoc J 2018; 13: 282–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harrison‐Woolrych M, Skegg K, Ashton J, Herbison P, Skegg DC. Nocturnal enuresis in patients taking clozapine, risperidone, olanzapine and quetiapine: comparative cohort study. Br J Psychiatry 2011; 199: 140–4 [DOI] [PubMed] [Google Scholar]
- 8. Chen JH, Huang R, Luo JM, Xiao Y, Zhang Y. Adult Monosymptomatic Nocturnal Enuresis with Obstructive Sleep Apnea Syndrome. Chin Med J (Engl) 2016; 129: 2011–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gravas S, Cornu JN, Gacci M et al. EAU Guidelines on Management of Non‐Neurogenic Male Lower Urinary Tract Symptoms (LUTS), incl. Benign Prostatic Obstruction (BPO). Eur Assoc Urol 2019. Available at: https://uroweb.org/wp‐content/uploads/EAU‐Guidelines‐on‐the‐Management‐of‐Non‐Neurogenic‐Male‐LUTS‐2019.pdf. Accessed June 2020. [Google Scholar]
- 10. Ekinci O, Celik T, Unal S, Oktay G, Toros F, Ozer C. Nocturnal enuresis in sickle cell disease and thalassemia major: associated factors in a clinical sample. Int J Hematol 2013; 98: 430–6 [DOI] [PubMed] [Google Scholar]
- 11. Huang W, Wang Q, Chen J, Wu P. Development and validation of the International Consultation on Incontinence Modular Questionnaire for Male Lower Urinary Tract Symptoms (ICIQ‐MLUTS) and the ICIQ‐MLUTS Long Form in Chinese population. Low Urin Tract Symptoms 2019; 11: 189–94 [DOI] [PubMed] [Google Scholar]
- 12. Bright E, Cotterill N, Drake M, Abrams P. Developing and validating the International Consultation on Incontinence Questionnaire bladder diary. Eur Urol 2014; 66: 294–300 [DOI] [PubMed] [Google Scholar]
- 13. Hashim H, Drake MJ. Basic concepts in nocturia, based on international continence society standards in nocturnal lower urinary tract function. Neurourol Urodyn 2018; 37: S20–S24 [DOI] [PubMed] [Google Scholar]
- 14. van Kerrebroeck P, Abrams P, Chaikin D et al. The standardisation of terminology in nocturia: report from the Standardisation Sub‐committee of the International Continence Society. Neurourol Urodyn 2002; 21: 179–83 [DOI] [PubMed] [Google Scholar]
- 15. Weiss JP. Nocturia: "do the math". J Urol 2006; 175: S16–S18 [DOI] [PubMed] [Google Scholar]
- 16. Drangsholt S, Ruiz MJA, Peyronnet B, Rosenblum N, Nitti V, Brucker B. Diagnosis and management of nocturia in current clinical practice: who are nocturia patients, and how do we treat them? World J Urol 2019; 37: 1389–94 [DOI] [PubMed] [Google Scholar]
- 17. Burton C, Weiss JP, Parsons M, Blaivas JG, Coats AC. Reference values for the Nocturnal Bladder Capacity Index. Neurourol Urodyn 2011; 30: 52–7 [DOI] [PubMed] [Google Scholar]
- 18. Rosier P, Schaefer W, Lose G et al. International Continence Society Good Urodynamic Practices and Terms 2016: Urodynamics, uroflowmetry, cystometry, and pressure‐flow study. Neurourol Urodyn 2017; 36: 1243–60 [DOI] [PubMed] [Google Scholar]
- 19. Abrams P. Bladder outlet obstruction index, bladder contractility index and bladder voiding efficiency: three simple indices to define bladder voiding function. BJU Int 1999; 84: 14–5 [DOI] [PubMed] [Google Scholar]
- 20. Madhu CK, Hashim H, Enki D, Drake MJ. Risk factors and functional abnormalities associated with adult onset secondary nocturnal enuresis in women. Neurourol Urodyn 2017; 36: 188–91 [DOI] [PubMed] [Google Scholar]
- 21. Weintraub Y, Singer S, Alexander D et al. Enuresis–an unattended comorbidity of childhood obesity. Int J Obes (Lond) 2013; 37: 75–8 [DOI] [PubMed] [Google Scholar]
- 22. Abdelwahab HA, Soltan EM, Metawee MA, Sherief MH, Metwally AH. Nocturnal enuresis in women and its relation to urinary incontinence. Arab J Urol 2015; 13: 199–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coyne KS, Sexton CC, Thompson CL et al. The prevalence of lower urinary tract symptoms (LUTS) in the USA, the UK and Sweden: results from the Epidemiology of LUTS (EpiLUTS) study. BJU Int 2009; 104: 352–60 [DOI] [PubMed] [Google Scholar]
- 24. Irwin DE, Milsom I, Hunskaar S et al. Population‐based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol 2006; 50: 1306–15 [DOI] [PubMed] [Google Scholar]
- 25. Campbell P, Li W, Money‐Taylor J, Davies J, Gray T, Radley S. Nocturnal enuresis: prevalence and associated LUTS in adult women attending a urogynaecology clinic. Int Urogynecol J 2017; 28: 315–20 [DOI] [PubMed] [Google Scholar]
- 26. Epstein MR, Monaghan T, Weiss JP. Etiology of nocturia response in men with diminished bladder capacity. Neurourol Urodyn 2019; 38: 215–22 [DOI] [PubMed] [Google Scholar]
- 27. Hofmeester I, Brinker AE, Steffens MG et al. Reference values for frequency volume chart and uroflowmetry parameters in adolescent and adult enuresis patients. Neurourol Urodyn 2017; 36: 463–8 [DOI] [PubMed] [Google Scholar]
- 28. Sakamoto K, Blaivas JG. Adult onset nocturnal enuresis. J Urol 2001; 165: 1914–7 [DOI] [PubMed] [Google Scholar]
- 29. Wadie BS. Primary nocturnal enuresis persistent to adulthood, functional evaluation. Neurourol Urodyn 2004; 23: 54–7 [DOI] [PubMed] [Google Scholar]
- 30. Yeung CK, Sihoe JD, Sit FK, Diao M, Yew SY. Urodynamic findings in adults with primary nocturnal enuresis. J Urol 2004; 171: 2595–8 [DOI] [PubMed] [Google Scholar]
- 31. Bower WF, Sit FK, Yeung CK. Nocturnal enuresis in adolescents and adults is associated with childhood elimination symptoms. J Urol 2006; 176: 1771–5 [DOI] [PubMed] [Google Scholar]
- 32. Yucel S, Kutlu O, Kukul E, Baykara M. Impact of urodynamics in treatment of primary nocturnal enuresis persisting into adulthood. Urology 2004; 64: 1020–5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Additional demographic and urodynamic data.
Appendix S2. Supplementary data of subgroup analysis stratified by age and BMI.


