Abstract
Immune checkpoint inhibitors (ICIs) improved the prognosis of patients with advanced lung cancers. The combination therapy of cytotoxic drugs and ICI is approved as first-line chemotherapy in non-small-cell lung cancer (NSCLC) and extensive disease small-cell lung cancer (ED-SCLC). It has been reported various immune-related adverse events (irAEs). We herein report a 65-year-old man with NSCLC who developed hepatitis and pancreatitis simultaneously during the combination immunochemotherapy. In the treatment of hepatitis and pancreatitis, the clinical course was different. In this report, the importance of accurate diagnosis through detailed examination and treatment priority depending on the severity of the symptoms is indicated.
Keywords: Immune checkpoint inhibitors (ICIs), Combination immunochemotherapy, Immune-related adverse events (irAEs), Hepatitis, Pancreatitis
Highlights
-
•
The side effects of the combination therapy of cytotoxic drugs and immune checkpoint inhibitor (ICI) is said to be addition of cytotoxic drug and ICI, not be multiplication.
-
•
When corticosteroids are resistant irAE hepatitis, addition of immuonosuppressive drugs is a treatment option.
-
•
In case of coexistence of multiple irAEs, the treatment priority should be given depending on the severity of the symptoms.
1. Introduction
Immune checkpoint inhibitors (ICIs) have brought a breakthrough to a treatment for various types of cancer. In lung cancer, anti-programmed death-1 (PD-1) antibodies (nivolumab and pembrolizumab) and anti-PD-1 ligand 1 (PD-L1) antibodies (atezolizumab and durvalumab) were approved. The first line combination therapy of cytotoxic drugs and ICI have improved the progression-free and overall survival of non-small-cell lung cancer (NSCLC) and extensive disease small-cell lung cancer (ED-SCLC) patients regardless of PD-L1 status [[1], [2], [3], [4], [5]]. The side effects of the combination therapy was said to be addition of cytotoxic drug and ICI, not be multiplication. There has been some reports of cases with multiple immune-related adverse events (irAEs), however no reports of simultaneous occurrence of irAE hepatitis and irAE pancreatitis. We herein report the first case of a patient with lung squamous cell carcinoma who developed simultaneously hepatitis and pancreatitis following the administration of combination immunochemotherapy (carboplatin (CBDCA), nab-paclitaxel (nab-PTX), pembrolizumab).
2. Case report
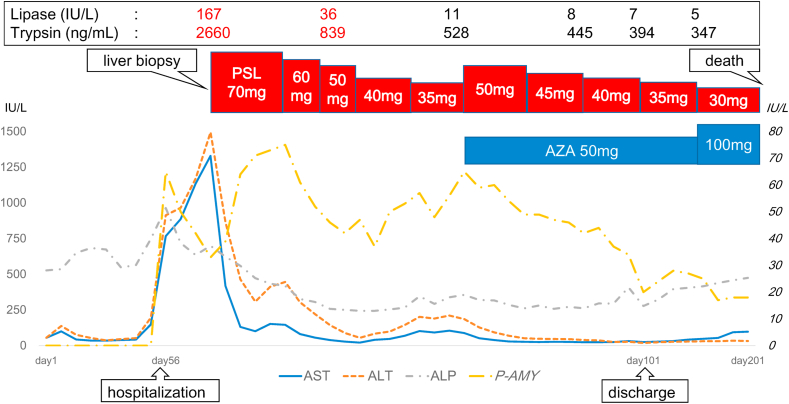
A 65-year-old man was referred to our hospital because of an abnormal shadow in the right upper lung field (Fig. 1). He had no significant medical history and reported that he was a smoker (30 pack-years). He was diagnosed with lung squamous cell carcinoma in the right upper lobe. The lung cancer was staged as T4N2M1a StageⅣA. Driver mutations (epidermal growth factor receptor (EGFR), echinoderm mictotubule-associated protein-like 4-anaplastic lymphoma kinase fusion gene (ALK), c-ros oncogene 1 (ROS-1), v-raf murine sarcoma viral oncogene homolog B1 (BRAF) was negative, however, a tumor proportion score of PD-L1) was 1–49%. His Eastern Cooperative Oncology Group (ECOG) performance-status (PS) was 1. A combination immunochemotherapy (carboplatin (CBDCA) AUC6 day1, nab-paclitaxel (nab-PTX) 100mg/m2 day1,8,15, pembrolizumab 200mg/body day1, every 3 weeks) was chosen as first-line therapy. After 2 cycles of the therapy, tumor shrinked as partial response (PR) (Response Evaluation Criteria in Solid Tumors: RECIST version 1.1) judging from CT. However, laboratory findings revealed elevated liver enzymes (aspartate aminotransferase (AST) 148 U/L, alanine transaminase (ALT) 196 U/L) and elevated biliary enzymes (alkaline phosphatase (ALP) 748 U/L, gamma-glutamyl transpeptidase (γ-GTP) 246U/L) before administration of 3 cycles. He had no abdominal symptoms. Administration of 3rd cycle was postponed and we introduced him to the gastroenterology department. Ursodeoxycholic acid (UDCA) was raised from 300mg/day to 600mg/day. He was followed-up because he had no subjective symptoms. After a week, liver enzymes and biliary enzymes were further elevated, so he was admitted to our hospital urgently. On physical examination, he had mild tenderness but no rebound pain around epigastric region. Laboratory findings revealed elevated pancreatic enzymes in addition to abnormal hepatobiliary enzymes. Neither a viral infection (hepatitis B and C virus, Epstein-Barr virus and Cytomegalovirus) nor autoimmune origin (antinuclear antibodies, antimitochondrial antibodies) were proven (Table 1). Diffuse pancreatic swelling was detected on enhanced abdominal computed tomography (CT), although gallstone and common bile duct stones were not observed (Fig. 2). He was not heavy drinker and did not take new drugs except for CBDCA, nab-PTX and pembrolizumab. Therefore we suspected his condition of drug induced liver injury and pancreatic injury caused by the combination immunochemotherapy. The patient had mild abdominal pain around epigastric region and pancreatic enzymes (pancreatic amylase, lipase and trypsin) elevated at admission. CT showed diffuse pancreatic enlargement. In clinical judgement, he was diagnosed with irAE pancreatitis, grade 2 (Common Terminology Criteria for Adverse Events (CTCAE) v5.0). First, we decided to limit oral intake and watch him. Pancreatic enzymes (amylase, trypsin and lipase) decreased and diffuse pancreatic swelling improved on CT (Fig. 2). However, liver enzymes gradually increased (maximum AST/ALT = 1329 IU/L/1495 IU/L). Pathological examination in liver biopsy revealed severe inflammation in the central venous region and no inflammation and no fibrotic changes in portal region. Immunostaining showed that the inflammatory infiltrate was composed predominantly of CD8+ lymphocytes (Fig. 3). Based on these findings, we diagnosed as irAE hepatitis, grade 3 (CTCAE v5.0). We began prednisolone (PSL) 70mg (1mg/kg)/day administration. Transaminases improved after the administration of PSL. However, liver enzymes began to increase when PSL reduced to 35mg/day. We thought that the administration of the PSL only was not enough and additional immunosuppressive drug was needed for the treatment of the irAE hepatitis. PSL was raised to 50mg/day and azathioprine (AZA) 50mg/day was added on. Liver enzymes decreased after the increase of PSL and the addition of AZA (Fig. 4). He was discharged on the 101th hospital day. However, we decided on best supportive care in accordance with his treatment policy and in consideration of his general condition. The lung tumor progressed gradually and he eventually died 201 days following the first administration of a combination immunochemotherapy.
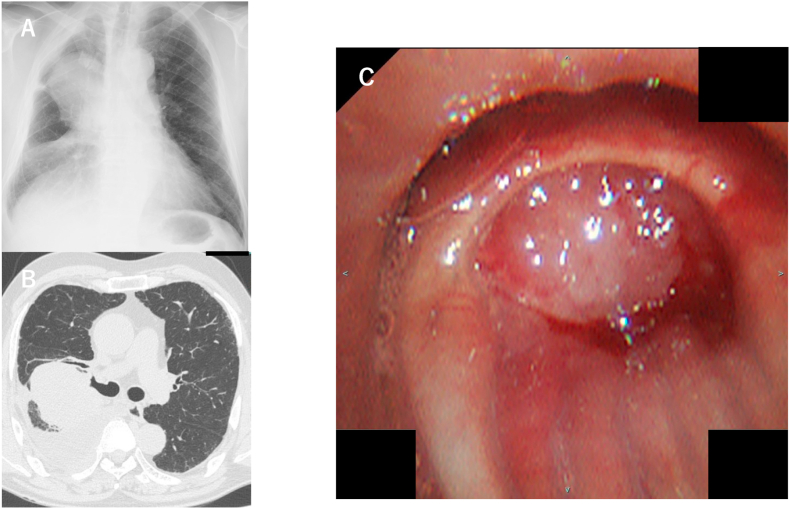
Fig. 1.
A: Chest X-ray showed a tumor shadow in the right upper lung field and lower permeability in the right lower lung field.
B: Chest CT showed a tumor in the right S2 and pleural effusion in the right lung field.
C: Flexible bronchoscopy showed obstruction of laryngeal tumor in the right B2 bronchus.
Table 1.
Laboratory findings on admission.
| Hemoglobin (g/dL) | 12.1 |
| Platelet ( × 104) | 24.6 |
| White blood cell (/μL) | 5300 |
| Alkaline phosphatase (IU/L) | 964 |
| Aspartate aminotransferase (IU/L) | 767 |
| Alanine aminotransferase (IU/L) | 913 |
| Alkaline phosphatase (IU/L) | 964 |
| γ- Glutamyltranspeptidase (IU/L) | 249 |
| Lactate Dehydrogenase (IU/L) | 477 |
| Blood urea nitrogen (mg/dL) | 12.0 |
| Creatinine (mg/dL) | 0.75 |
| Amylase (IU/L) | 153 |
| Pancreas amylase (IU/L) | 70 |
| Lipase (IU/L) | 167 |
| Trypsin (ng/mL) | 2660 |
| Ammonia (μg/dL) | 91 |
| C-reactive protein (mg/dL) | 12.38 |
| Feritine (ng/mL) | 10,110 |
| Prothorombin time (%) | 69.3 |
| Prothorombin time-international normalized ratio | 1.2 |
| Immunoglobulin G (mg/dL) | 1001 |
| Immunoglobulin G4 (mg/dL) | 85.4 |
| Antinuclear antibody | < × 40 |
| Antimitochondria M2 antibody | (−) |
| Antiacetylcholine receptor binding antibody | (−) |
| Epstein-Barr virus-Immunoglobulin G | × 160 |
| Epstein-Barr virus-Immunoglobulin A | < × 10 |
| Epstein-Barr virus-Immunoglobulin M | < × 10 |
| Epstein-Barr virus-nuclear antigen | < × 40 |
| Cytomegalovirus antigenaemia |
(−) |
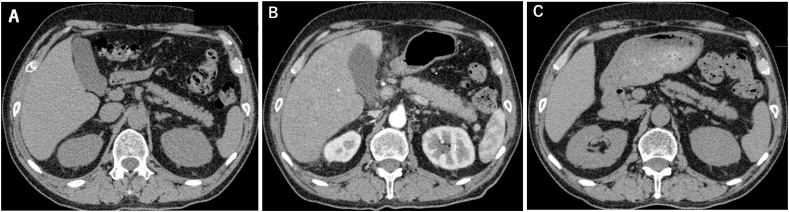
Fig. 2.
A–C: Changes in pancreatic enlargement from a combination immunochemotherapy.
A: The normal pancreas before administration of a combination immunochemotherapy.
B: The pancreatic enlargement at diagnosis of irAE hepatitis and pancreatitis.
C: The improvement of the pancreatic enlargement after the treatment of PSL and AZA.
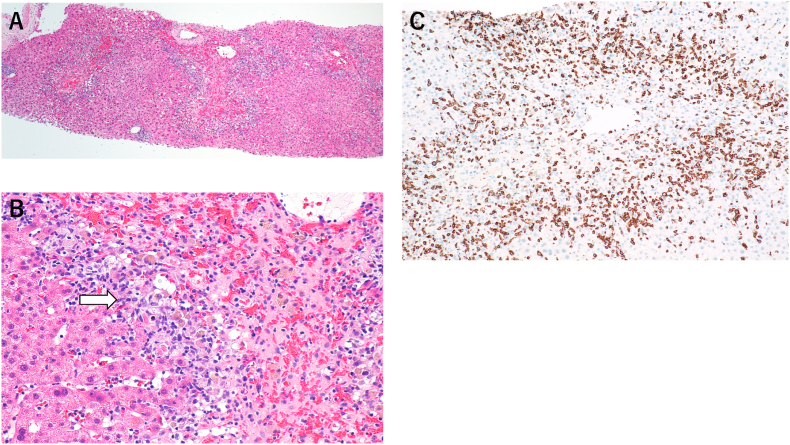
Fig. 3.
A,B: Histopathology of the liver biopsy showed centrilobular necrosis and inflammation (white arrow) (A: Hematoxylin and Eosin staining × 40, B: × 200).
C: Immunohistochemical staining showed that infiltration due to inflammatory cells was composed predominantly of CD8+ lymphocytes (CD8 staining by monoclonal anti-human CD8 mouse antibodies × 400).
Fig. 4.
Clinical cource in our case.
3. Discussion
The irAEs can affect various organ systems and range from mild symptoms to severe fatal events. The mainly affected organ systems are gastrointestinal, hapatic, pulmonary, dermatologic, and endocrine systems.
IrAE-hepatitis has been reported to occur in 2%–10.0% of patients treated with ICI monotherapy [6] and 9.7%–24.7% of patients with combination therapy of cytotoxic drugs and ICI [[1], [2], [3], [4], [5]]. The most common time of onset is 6–12 weeks after the initiation of the treatment [7]. However early and delayed events can be occurred. Most cases are asymptomatic and occasionally patients have an associated fever and abdominal discomfort. Radiographic findings are not specific findings, however it may show mild hepatomegaly, periportal edema, or periportal lymphadenopathy by computed tomography (CT). Liver biopsy is needed for correct diagnosis. The differential diagnosis are drug-induced liver injury, autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), acute viral hepatitis, and acute alcoholic liver disease. Distinguishing between AIH and irAE hepatitis is often challenging, as these two conditions have similar pathogenesis related to immunological processes. However, liver histopathological findings can be helpful to discriminate irAE from other conditions including AIH. The histological tissue in irAE hepatitis predominantly presents panlobular hepatitis and infiltrating cells are mainly lymphocytes and occasional eosinophils [8]. In addition to this, immunostaining reveals a large number of CD8+ lymphocytes and a markedly smaller number of CD4+ lymphocytes infiltration [9]. In the treatment of irAE hepatitis, ICI should be discontinued at grade 2 or greater (CTCAE v5.0) irAE hepatitis and corticosteroids therapy should be considered. Corticosteroids dose ranges 0.5–2 mg/kg/day depending on the grade. When the effects of corticosteroids therapy are unresponsive, immunosuppressive drugs (azathioprine (AZA), mycophenolate mofetil (MMF)) are required. The TNF-α blocker Infliximab is not recommended considering the liver toxicity [6].
On the other hand, pancreatitis caused irAE has been reported to occur in 0.9–3% for anti-CTLA-4 monotherapy, 1.2–2.1% for combination therapy of anti-CTLA-4 and anti-PD-1 and 0.5–1.6% for anti-PD-1 monotherapy according to a meta-analysis [10]. The incidence of the irAE pancreatitis caused by combination therapy of cytotoxic drugs and ICI was 0–1.4% [[1], [2], [3], [4], [5]]. Typical clinical symptoms of acute pancreatitis are epigastric discomfort and pain, fever, diarrhea, dyspnea, nausea and vomiting. Typical radiographic imaging findings by CT are focal and diffuse parenchymal enlargement, indistinct pancreatic margins, surrounding retroperitoneal fat stranding. In the treatment of irAE pancreatitis, ICI should be discontinued at grade 2 or greater irAE pancreatitis and initial treatments are fasting and intravenous fluids. Corticosteroids (1–2mg/kg/day) are often considered.
In this case, hepatobiliary enzymes began to elevate on the 7 weeks after the first administration of a combination immunochemotherapy and it was typical course in irAE hepatitis. CT showed no common bile duct stone and no tumor in hepatobiliary tract. Other viral and other autoimmune causes were excluded. In pathological findings of liver biopsy, centrilobular necrosis and inflammation revealed centrilobular acute liver injury. Infiltrating lymphocytes was predominantly CD8+ lymphocytes in immunostaining. This is considered to be one feature of irAE hepatitis. Based on these findings, the patient was diagnosed irAE hepatitis grade 3 (CTCAE v5.0). In addition to this, the patient had abdominal symptom (mild abdominal pain around epigastric region) and pancreatic enzymes (pancreatic amylase, lipase and trypsin) elevated at admission. CT showed diffuse pancreatic enlargement. Patholoatigical examination could not be performed, however, he was diagnosed with irAE pancreatitis grade 2 (CTCAE v 5.0) based on clinical findings. IrAE pancreatitis improved only by fasting and intravenous fluids. In irAE hepatitis, liver enzymes improved after the administration of PSL (70mg/day), however, liver enzymes began to increase when PSL reduced to 35mg/day. We thought that the administration of the PSL only was not enough and additional immunosuppressive drug was needed for the treatment of the irAE hepatitis. The American Association for the Study of Liver Diseases (AASLD) guidelines for treatment of AIH and the Japanese medical AIH guidelines recommend AZA as the first choice for steroid refractory cases, and MMF is considered as an alternative treatment for patients who did not previously tolerate AZA [11,12]. In recent years, it is recommended to a NUDT15 R139C heterozygosity before administration of AZA. It has been reported that NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia [13]. In this case, we checked if the patient had a NUDT15 R139C heterozygosity before the administration of AZA. He did not have a NUDT15 R139C heterozygosity, so he was administrated AZA. Liver enzymes decreased after the increase of PSL and the addition of AZA. No hair loss or leukopenia occurred after administration of AZA.
In our case, irAE hepatitis and pancreatitis simultaneously occurred and each treatment course was different. Judging from immunohistological findings, hepatitis is thought to be due to irAE. No comprehensive reports on irAE pancreatitis have been published. According to post market survey in Japan, very few cases (6 cases) of pancreatitis has been reported with nab-PTX. On the other hand, there have been relatively many cases (44 cases) of pancreatitis with pembrolizumab. Pancreatitis in this case was presumed to be irAE based on incidence frequency of post market survey and medical history. Further research is needed in order to further understand irAE pancreatitis. Some reports of cases with multiple irAEs has been published, however, there has been no reports of simultaneous occurrence of irAE hepatitis and irAE pancreatitis. We report the first case of irAE hepatitis and irAE pancreatitis at the same time. This case report demonstrates the importance of treatment priority according to the severity of the symptoms in case of simultaneous occurrence of irAEs.
Declaration of competing interest
The authors declare that they have no conflicts of interest (COI).
References
- 1.Gandhi L., Rodríguez-Abreu D., Gadgeel S. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 2.Paz-Ares L., Luft A., Vicente D. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 3.West H., McCleod M., Hussein M. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 4.Horn L., Mansfield A.S., Szczęsna A. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N. Engl. J. Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 5.Socinski M.A., Jotte R.M., Cappuzzo F. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 2018;378:2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 6.Brahmer Julie R., Lacchetti Christina, Schneider Bryan J. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical Oncology clinical practice guideline. J. Clin. Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziemer M., Koukoulioti E., Simon J.C. Managing immune checkpoint-inhibitor-induced severe autoimmune-like hepatitis by liver-directed topical steroids. J. Hepatol. 2017;66:657–659. doi: 10.1016/j.jhep.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Zen Y., Yeh M.M. Checkpoint inhibitor-induced liver injury: a novel form of liver disease emerging in the era of cancer immunotherapy. Semin. Diagn. Pathol. 2019;36:434–440. doi: 10.1053/j.semdp.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Zen Yoh, Yeh Matthew M. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod. Pathol. 2018;31:965–973. doi: 10.1038/s41379-018-0013-y. [DOI] [PubMed] [Google Scholar]
- 10.Su Qiang, Zhang Xiao-chen, Zhang Chen-guang. Risk of immune-related pancreatitis in patients with Solid tumors treated with immune checkpoint inhibitors: systematic assessment with meta-analysis. J Immunol Res. 2018;1027323:1–9. doi: 10.1155/2018/1027323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennes Elke, Ye Oo, Schramm Christoph. Mycophenolate mofetil as second line therapy in autoimmune hepatitis? Am. J. Gastroenterol. 2008;103:3063–3070. doi: 10.1111/j.1572-0241.2008.02180.x. [DOI] [PubMed] [Google Scholar]
- 12.Gleeson Dermot, Heneghan Michael A. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2011;60:1611–1629. doi: 10.1136/gut.2010.235259. [DOI] [PubMed] [Google Scholar]
- 13.Yang Suk-Kyun, Hong Myunghee, Baek Jiwon. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat. Genet. 2014;46:1017–1020. doi: 10.1038/ng.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]