Abstract
Thousand and one kinases (TAOKs) are members of the MAP kinase kinase kinase (MAP3K) family. Three members of this subfamily, TAOK1, 2, and 3, have been identified in mammals. It has been shown that TAOK1, 2 and 3 regulate the p38 MAPK and Hippo signaling pathways, while TAOK 1 and 2 modulate the SAPK/JNK cascade. Furthermore, TAOKs are involved in additional interactions with other cellular proteins and all of these pathways modulate vital physiological and pathophysiological responses in cells and tissues. Dysregulation of TAOK-related pathways is implicated in the development of diseases including inflammatory and immune disorders, cancer and drug resistance, and autism and Alzheimer’s diseases. This review collates current knowledge concerning the roles of TAOKs in protein–protein interaction, signal transduction, physiological regulation, and pathogenesis and summarizes the recent development of TAOK-specific inhibitors that have the potential to ameliorate TAOKs’ effects in pathological situations.
Keywords: thousand and one kinase, p38 MAPK, SAPK/JNK, Hippo, TAOK
1. Introduction
Kinases are enzymes that catalyze the transfer of phosphate groups from a phosphate-donating molecule (such as ATP) to their substrate molecule, which can be a protein, lipid, or carbohydrate. The phosphorylation state of a substrate can affect its activity and ability to bind to and regulate other molecules. Mitogen-activated protein kinases (MAPKs) phosphorylate serine/threonine residues of their substrates, resulting in activation or de-activation of the downstream targets [1]. MAP kinases are evolutionarily conserved and ubiquitously expressed in eukaryotes. The sterile 20 (STE20) kinase of Saccharomyces cerevisiae functions downstream of a heterotrimeric G protein but upstream of MAP kinases in the MAPK cascade of the yeast pheromone response pathway [2,3]. While searching for the STE20 kinase ortholog in mammals, the TAO kinases were identified. The first “thousand and one” kinase, TAOK1, was identified in the rat by fishing the cDNA library with a degenerate STE20 kinase probe [4]. It is named “thousand and one” because 1001 amino acids are encoded by the TAOK1 gene. A second TAO kinase with closely related sequences, TAOK2, was also identified and later characterized [4,5]. The third TAO kinase, human TAOK3, was identified by its association with EPS8 (EGFR kinase substrate 8) protein in an expression library assay [6]. Human TAO kinase orthologs (TAOK1, TAOK2, and TAOK3) were identified subsequently [7,8]. TAOKs are involved in various cellular signaling pathways including the p38/MAPK14 stress-activated MAPK cascade, the JNK/SAPK cascade, and the Salvador–Warts–Hippo cascade. In addition, TAOKs are found to interact with other cellular proteins both dependent and independent of their kinase activity. Through these interactions, TAOKs regulate the DNA damage responses, cytoskeleton stability, apoptosis, and other physiological and pathophysiological responses.
MAPK pathways participate in the transduction of extracellular changes into coordinated and integrated intracellular adaptive responses. MAPKs respond to diverse stimuli, including mitogens, osmotic stress, heat shock, and inflammatory cytokines, and regulate vital cellular processes such as mitosis, proliferation, differentiation, apoptosis, stress, and immune responses [9]. Activation of a MAPK cascade occurs via the multi-tiered, consecutive phosphorylation of downstream targets. When triggered by stimuli, the first kinase in the cascade, the MAP3K (MAP kinase kinase kinase) is phosphorylated and activated by protein kinases downstream of surface G protein-coupled receptors [10]. In a classical MAPK cascade, the MAP3K then activates a downstream MAP2K (MAP kinase kinase or MKK), which, in turn, activates a MAPK [9]. Upon activation, the MAPK phosphorylates various targets in the cytosol and nucleus to alter protein function or gene expression appropriate biological responses. Three classical MAPK pathways are known in mammalian cells: the extracellular signal-regulated kinase 1/2 (ERK1/2), the p38 MAPK, and the c-JUN N-terminal kinase (JNK) pathways. ERK1/2 is activated in response primarily to hormones, growth factors, and proinflammatory stimuli, while p38 MAPKs and JNKs respond to cellular and environmental stresses, such as inflammatory cytokines, oxidative stress, DNA damage, ultraviolet irradiation, heat, and osmotic shock. Among these three classical MAPK pathways, TAOKs are currently known to be involved in the regulation of the p38 MAPK and JNK cascades.
The Hippo signaling pathway, also known as the Salvador–Warts–Hippo (SWH) pathway, controls organ size through regulation of cell proliferation and apoptosis [11]. This pathway also regulates the self-renewal and expansion of stem cell and tissue-specific progenitor cells. The Hippo pathway is also a kinase cascade, wherein a series of activated kinases phosphorylate the transcription co-activators YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ-binding motif) and inhibit their nuclear translocation. Lack of the transcriptional activator YAP/TAZ results in the downregulation of genes that support cell proliferation and inhibit apoptosis. Given that the Hippo cascade is involved in controlling cell proliferation and modulating apoptosis, dysfunction of this pathway could play an important role in the development of human cancer [12]. TAOKs have also been found to be regulators of the Hippo signaling pathway, in addition to their involvement in the MAPK cascades.
In this review, we summarize current knowledge concerning the signaling pathways regulated by TAOKs and their correlation to physiological regulation and disease progression. Finally, we discuss the progress toward the development of a selective and potent TAOK inhibitor, which may be of benefit in combating TAOK-associated pathogenesis.
2. Structure and Function of TAO Kinases
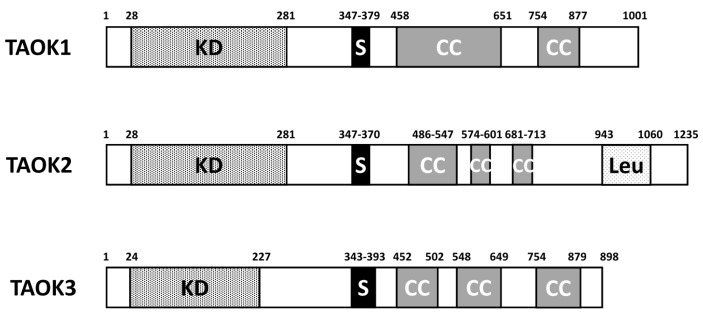
There are three members in the TAO kinase family: TAOK1, TAOK2, and TAOK3. In humans, TAOK1 is located on chromosome 17p at position 11.2, while TAOK2 is on 16p11.2 and TAOK3 is on 12q24.23. Several splice variants are noted for all three TAOKs. They share similar domain structures despite the difference in the amino acid length. TAOKs are serine/threonine-protein kinases noted for their N-terminal positioning of the kinase domain (Figure 1). A serine-rich domain is located around 350–380 a. a. Two to three coiled coil regions are located in the C-terminal half of TAOKs. In TAOK2, there is a leucine-rich repeat situated close to the C-terminal end. The human TAOK2 protein kinase domain displays 89.8% amino acid identity to TAOK1 [5], while the homology of the kinase domain of TAOK3 to TAOK1 and TAOK2 is 88.6% and 82.7%, respectively [8]. Generally, TAOKs are ubiquitously expressed in most tissues, with the highest levels in the testes and brain [6,8]. One exception is that TAOK3 is highly expressed in peripheral blood leukocytes, spleen, and thymus, while TAOK1 and TAOK2 are low in these tissues. The high expression of TAOK1/2 in the brain and TAOK3 in myeloid/lymphoid tissues may reflect the tissue-specific functions of individual TAOKs.
Figure 1.
Diagram of domain structure of human TAOK1/2/3. KD: kinase domain; S: serine-rich domain; CC: coiled coil regions; Leu: leucine-rich repeat.
3. Signaling Pathways and Cellular Physiologies Regulate by TAO Kinases
TAOKs have been reported to regulate the p38/MAPK, JNK/SAPK, and Hippo signaling pathways. In addition to these signaling pathways, TAOKs also interact with other proteins and regulate several additional cellular physiological functions. These findings are highlighted in the following sections.
3.1. TAO Kinases Regulate the p38/MAPK Pathway
p38 MAPKs are activated by stress stimuli and involved in cell differentiation, apoptosis, and autophagy. Four members of this family, p38 MAPK α (MAPK14), β (MAPK11), δ (MAPK12/ERK6), and γ (MAPK13/SAPK4), have been identified. As with typical MAPK cascades, the first component is a MAP3K that phosphorylates and activates MKK3/6 (the MAP2Ks), and MKK3/6 then phosphorylates and activates the p38 MAPK. p38 MAPK is involved in the regulation of several cytosolic cofactors and nuclear transcription factors.
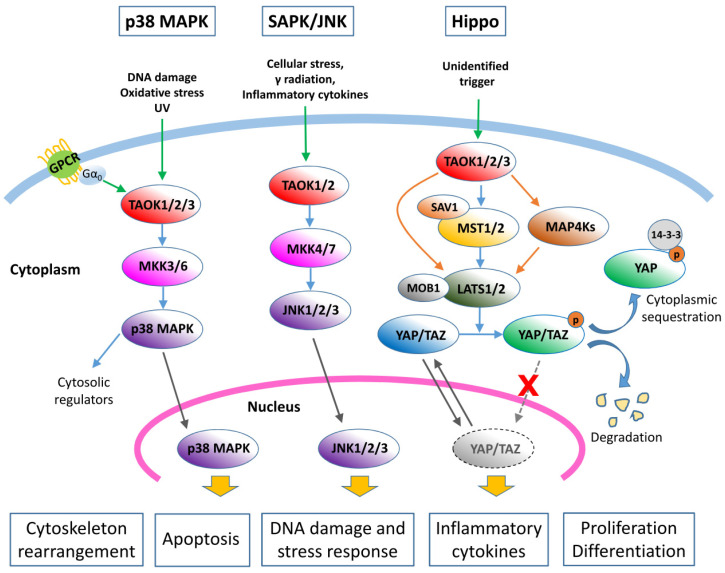
TAOKs, through their activity as MAP3Ks, were found to activate the p38 MAPK pathways (Figure 2) [4,5,8]. TAOKs are activated intensely by ionizing and ultraviolet radiation, indicating a primary function in response to DNA damage via p38 activation. Under these conditions, Raman et al. [13] reported that TAOKs are activated by ATM (ataxia telangiectasia mutated) phosphorylation to regulate p38-mediated DNA damage responses. In addition, the heterotrimeric G protein Gα0 was also found to activate TAOK2 and the downstream p38 cascades [14]. It has therefore been suggested that TAOKs are the intermediates that link specific G protein-coupled receptors (GPCRs) to the p38 MAPK pathway (Figure 2) [14]. Activated TAOKs phosphorylate MKK3/6, which then phosphorylate p38 kinases [4,5,15] (Figure 2). Chen et al. [5,15] confirmed that TAOK2 docks to MKK3 through a region (314–451 a.a.) adjacent to the TAOK kinase domain. Taken together, these findings reveal that TAOKs play the intermediate MAP3K roles that link environmental stimuli to the p38 MAPK signaling pathway (Figure 2).
Figure 2.
Kinase cascades regulated by TAOK1/2/3. TAO kinases are involved in p38 MAPK, SAPK/JNK, and Hippo signaling pathways. GPCR: G protein-coupled receptor; SAV1: Salvador 1. Green arrows: upstream stimuli; blue arrows: typical/canonical pathways; orange arrows: alternative pathways in the Hippo cascades; grey arrows: nuclear translocation/exportation.
3.2. TAO Kinases Regulate the SAPK/JNK Pathway
Stress-activated protein kinases (SAPK)/c-Jun N-terminal kinases (JNK) belong to the MAPK family and are activated by a variety of environmental stresses. JNK1, 2, and 3 respond to stimuli including cytokines, ultraviolet irradiation, heat, and osmotic shock. Their activation is carried out by two MAP2Ks, MKK4 and MKK7, which are activated by upstream MAP3Ks. Activated JNK translocates to the nucleus, where it regulates the activity of multiple transcription factors [16]. JNKs are involved in cell proliferation, differentiation, apoptosis, neurodegeneration, and inflammatory responses [17].
In addition to MKK3/6 in the p38 MAPK cascade, TAOK1 and TAOK2 were found to phosphorylate MKK4/MKK7 and activate the JNK signaling cascade (Figure 2) [5,7,18,19]. Treatment with the apoptosis-inducing agents paclitaxel and staurosporine activated endogenous TAOK 1 and 2 and JNK pathways [18,19]. Overexpression of TAOK2 also activated the endogenous JNK/SAPK cascade in HEK293 cells [15]. The involvement of TAOK3 in the SAPK/JNK pathway is somewhat controversial. While an initial study by Tassi et al. [6] showed that TAOK3 inhibits the basal activity of SAPK/JNK and diminishes its activation in response to human epidermal growth factor in COS7 cells, a later report by Zhang et al. [20] indicated that TAOK3 activated SAPK/JNK when transfected in NIH3T3 cells. A more recent study by MacKeigan et al. [21] demonstrated that downregulation of TAOK3 resulted in rapid activation of JNK1/2 and caspase-9, and PARP cleavage, which led to apoptosis in HeLa cells, whilst a study by Kapfhamer et al. [22] showed that the level of activated phosphor-JNK was higher in the brain of the TAOK3-disrupted mouse as compared to the control mouse, which suggests that TAOK3 is a negative regulator of the SAPK/JNK cascade. The inconsistency of JNK activation by TAOK3 could be a consequence of differences in cellular context. Additional studies are required to validate the role of TAOK3 in the JNK pathway. Currently, it is generally believed that TAOK 1 and 2 are activators of the SAPK/JNK pathway while TAOK3 is not.
3.3. TAO Kinases Regulate the Hippo Signaling Pathway
The Hippo pathway is also a kinase cascade, wherein MST1/2 kinases (mammalian STE20-like 1/2; the ortholog of Drosophila Hippo) and SAV1 (Salvador 1) form a complex to phosphorylate and activate LATS1/2 (large tumor suppressor 1/2; the ortholog of Drosophila Warts). LATS1/2 sequentially phosphorylate the transcription co-activators YAP and TAZ and inhibit their nuclear translocation by retaining them in the cytosol or targeting them for degradation. Non-phosphorylated YAP/TAZ translocate into the nucleus and interact with TEAD (transcriptional enhanced associate domain) and other transcription factors to induce genes that support cell proliferation and inhibit apoptosis. YAP/TAZ can also reprogram cancer cells into cells with stem-like traits [23]. In this cascade, genes involved in the phosphorylation of YAP/TAZ are identified as tumor suppressors, whereas YAP/TAZ are recognized as oncogenes [24].
Intriguingly, it was found that loss of TAO1 (TAOK1 ortholog in Drosophila) upregulated Hippo signaling targets (Figure 2) [25]. TAO1 was shown to phosphorylate Hippo to activate the pathway, which then functions to restrict cell proliferation in Drosophila [26]. A study of human TAOK1 in 293T cells also showed that TAOK1 induced substantial phosphorylation of the Hippo ortholog MST2 [26]. By using a TAOK1/2/3-knockout model of HEK293A cells, Plouffe et al. [27] showed that TAOKs act not only upstream of MST1/2, but also in parallel to directly activate LATS1/2, and that eliminating TAOKs significantly decreased phosphorylation of YAP/TAZ and their cytoplasmic retention. In addition, Meng et al. [28] found that knockout of MAP4K4, 6, and 7 significantly blocked TAOK1-induced YAP phosphorylation, indicating that TAOK1 may also act through MAP4K4/6/7 to activate LATS1/2. Collectively, TAOKs are regulators of the Hippo signaling pathway and their activation suppresses YAP/TAZ transactivation ability (Figure 2). Although proteins in the Hippo signaling pathway that decrease the YAP/TAZ activity are regarded as potential tumor suppressors [24,26], currently there is no direct evidence indicating that TAOKs play specific roles in tumor suppression via the Hippo cascade.
3.4. Cytoskeleton Regulations by TAO Kinases
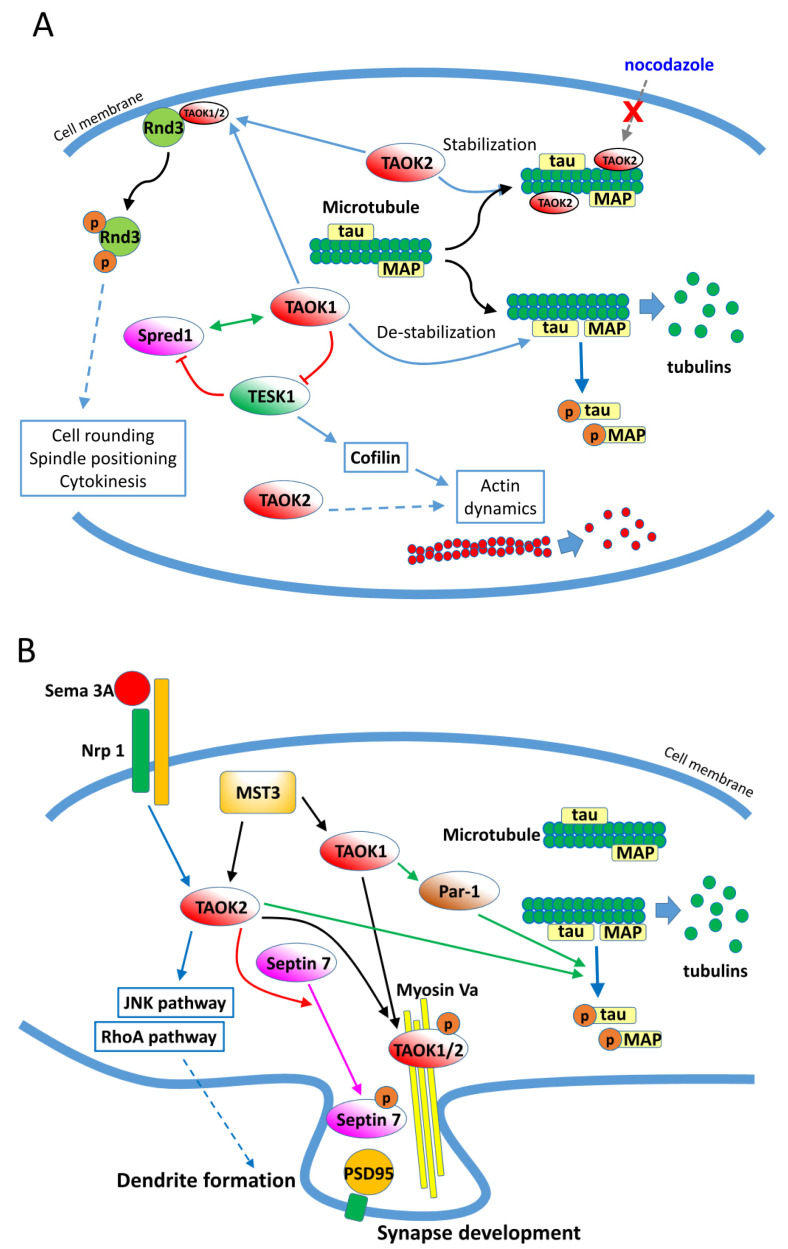
In addition to the aforementioned signaling pathways, TAOKs are reported to interact with other cytosolic proteins and be involved in additional physiological processes. TAOKs modulate the dynamics and organization of several cytoskeleton components [29,30,31]. TAOKs are activated catalytically during mitosis and neuritogenesis [32,33,34,35,36]. By phosphorylation of microtubule-binding proteins including tau, TAOK1 induces microtubule instability by causing dissociation of tau from microtubules, which results in their disassembly [30,31,37] (Figure 3A). Conversely, TAOK2 binds directly to microtubules through its C-terminal region (amino acids 745–1235) and stabilizes microtubules at the perinuclear regions. Furthermore, the TAOK2-stabilized microtubules are resistant to nocodazole-induced depolymerization [29]. TAOK2 was found to increase the levels of acetylated α-tubulin when associated with microtubules and also to bind and phosphorylate α- and β-tubulin in vitro [29]. It was demonstrated that in mitotic cells, activated TAOK1 localizes to the cytoplasm while TAOK2 localizes to the centrosomes, and both TAOKs are required for spindle positioning and mitotic cell rounding [33,35]. Garg et al. [38] further showed that TAOK1 and 2 bind and phosphorylate the atypical Rho family protein Rnd3 and elicit the translocation of Rnd3 from the plasma membrane to the cytosol, which contributes to spindle positioning, mitotic cell rounding, and cytokinesis (Figure 3A). Additionally, TAOKs may regulate the dynamics of actin. Cells overexpressing TAOK2 are found to be rounded, have fewer processes, and show a marked reduction in actin stress fibers. The alteration in cell morphology and actin organization by TAOK2 was dependent on both its C-terminal actin-binding motif and kinase activity [7]. It was also found that TAOK1, Sprouty-related protein with EVH-1 domain1 (Spred1), and testis-specific protein kinase (TESK1) form a three-way interacting network that modulates the dynamics of both microtubules and actin in CHO cells [39] (Figure 3A). In Drosophila, TAO1 controls the dynamic interplay between microtubule plus ends and the actin cortex in the regulation of cell morphology [31]. Collectively, these studies showed that TAOKs regulate the dynamics and organization of cytoskeleton proteins, particularly during mitosis.
Figure 3.
Regulation of cytoskeleton components by TAOKs. (A) Regulation of microtubules and actin filaments by TAOK1 and TAOK2 in normal cells. (B) Signaling pathways modulated by TAOK1 and TAOK2 in neurons during development and dendrite/synapse formation. MAP: microtubule-binding proteins.
3.5. TAO Kinases Regulate Neuron Development, Neuritogenesis, and Homeostasis
In addition to mitotic cell morphology regulation, TAOKs are involved in neuron development, neuritogenesis, and maintaining a homeostatic neural network via modulating cytoskeleton components. As described above, TAOK1 phosphorylates tau and induces microtubule disassembly. In neurons, TAOK1 activates microtubule affinity-regulating kinase Par1, which then phosphorylates tau and allows the rearrangement of microtubules and development of neurites during neuron differentiation [30] (Figure 3B). TAOK2 acts downstream of Sema3A (Semaphorin 3A) and Nrp1 (Neuropilin 1) and modulates basal dendrite formation and axon elongation during neuron development through activation of the JNK cascade [34]. TAOK1 and 2 are phosphorylated by MST3 (mammalian STE20-like kinase 3), bind with Myosin Va, and relocate to the dendrites to regulate synapse development in neurons [40]. More recently, it was found that TAOK2 phosphorylates the cytoskeletal GTPase Septin7 and participates in maturation of the dendritic spine via stabilization of PSD95 (postsynaptic density protein 95) [41]. Additionally, TAOK2 regulates neurodevelopment and synapse formation via activation of the RhoA signaling pathway [42]. These results indicate that TAOKs regulate the development and differentiation of neurons and formation of synapses through modulating the cytoskeleton components (Figure 3B). Homophilic binding of extracellular arcadlin (rat ortholog of human protocadherin 8/PCDH8) domains activates TAOK2β, a splice variant of TAOK2, which then activates p38 MAPK. The activation of p38 MAPK then positively feeds back to TAOK2β, phosphorylating an essential C-terminal serine 1038 required for endocytosis of the N-cadherin-arcadlin complex at the synapse in hippocampal neurons [43]. It is suggested that PCDH8-mediated N-cadherin endocytosis via TAOK2β signaling is an event within the recovery phase after synaptic stimulation and provides a homeostatic mechanism for maintaining the complexity of the neural network.
3.6. Regulation of Inflammation, Immunity, Apoptosis, and other Cellular Pathways by TAOKs
TAOKs are implicated in the regulation of inflammation and immunity. Zhang et al. [44] showed that TAOK1 is a negative regulator of interleukin-17 (IL-17)-mediated signal transduction via preventing the formation of the IL-17 receptor and Act1 (nuclear factor activator 1) complex, thus inhibiting IL-17-associated inflammation and potentially modulating autoimmune progression. TAOK1 was also found to increase the lipopolysaccharide (LPS)-induced production of pro-inflammatory cytokines, including IL-6, TNF-α (tumor necrosis factor-α), and IL12p40, in macrophages [45]. It was found that TAOK1 enhances the LPS-induced activation of ERK1/2 by interacting with TRAF6 (TNF receptor-associated factor 6) and TPL2 (MAP3K8). In this situation, TAOK1 is a positive regulator of the toll-like receptor 4-induced inflammatory responses in macrophages [45]. During positive selection in the spleen, B cell receptor and Notch signaling induces surface expression of ADAM10 in type 1 transitional B cells in a TAOK3-dependent manner, and cells expressing ADAM10 are then committed to marginal zone B in the spleen [46]. As a result, TAOK3 is involved in B cell maturation and fate determination in the spleen. TAOK3 is also reported to be required for canonical T-cell receptor (TCR) signaling through the modulation of SHP-1-dependent LCK (lymphocyte-specific protein tyrosine kinase) inactivation [47]. It was proposed that TAOK3 acts as a binding partner for LCK, diminishing its availability for SHP-1-mediated inactivation. TAOK3 deficiency impairs TCR signaling in human T cells and weakens primary T cell responses [47]. A homozygous missense variant of TAOK2 (c.2098C > T; p.R700C) was found to associate with impaired T cell activation [48], although the underlying mechanism is not known.
TAOKs are involved in apoptosis regulation. Upon treatment of apoptosis-inducing agents including staurosporine and paclitaxel, endogenous TAOK1 and TAOK2 are activated. Activation of TAOK1 in the non-small-cell lung carcinoma cell line H1299 induces cell contraction, membrane blebbing, cleavage of Rho kinase 1 and caspase 3, and activates the JNK pathway for induction of apoptosis [18]. Zihni et al. [19] have also shown that apoptosis-inducing agents stimulate TAOK2-induced JNK and caspase activation and subsequent cleavage and nuclear localization of the N-terminal domain of TAOK2 for apoptosis induction. Interestingly, TAOKs activate caspases, and TAOK1 and 2 have been found to be the substrates of activated caspases [18,19]. TAOKs therefore regulate apoptotic morphological change by reorganizing cytoskeleton proteins via activation of downstream targets including JNK and caspases.
TAOKs are reported to interact with other cytosolic proteins and be involved in additional physiological processes. TAOK2 is found to interact with another MAP3K member, TAK1, and inhibit TAK1-mediated activation of NF-κB by preventing the interaction of TAK1 with IKK (IκB kinase) [49]. Interestingly, the TAK1–TAOK2 complex can still activate JNK, while the TAK1-mediated activation of NF-κB is abolished. This unique regulation is suggested to be a specific cellular response to osmotic stress [49]. Drosophila TAO1 has also been shown to affect the migration of cells during embryonic development [50]. All in all, these results reflect the complexity of TAOK-associated regulation of cell physiology, and the mechanisms we know so far may only represent a small part of the complete story of the TAOK family.
4. Role of TAO Kinases in Cancers
Perturbation of kinase signaling resulting from dysregulated expression or activity is often associated with malignant transformation. As described above, studies have shown that TAOKs regulate the activation of several signaling pathways that could be linked to cancer development. However, to date, reports of TAOKs’ correlation with clinical manifestation are few. In cell line studies, TAOKs are reported to be involved in the DNA damage checkpoint of the G2/M transition via p38 MAPK activation. Knockdown of TAOKs not only diminished p38 activation but also impaired the DNA damage response of the G2/M checkpoint [13]. In addition, knockdown of TAOK1 induced various mitotic abnormalities and resulted in chromosome loss in cells [51]. Moreover, TAOK1 and TAOK2 are activated in response to apoptosis-inducing agents and act as regulators of apoptosis by modulating morphological changes including membrane blebbing and the formation of apoptotic bodies via activation of the JNK pathway [18,19]. These studies indicate that dysregulation of TAOKs could be involved in tumorigenesis. In the following sections, we summarize current study results implicating TAOKs in tumor development.
4.1. TAO Kinases in Breast Cancer
In a study by Capra et al. [52] using in situ hybridization on tissue microarrays to search for alterations in the expression of serine/threonine kinases in human cancers, TAOK1 was found to be overexpressed in breast cancer tissues compared to normal breast tissues. In a prognostic analysis using a public database (Kaplan–Meier Plotter breast module, probe ID: 220761_s_at; http://kmplot.com/analysis/index.php?p=service&cancer=breast; for details, see [53]), high TAOK3 expression was correlated with poor recurrence-free survival in breast cancer patients who received adjuvant chemotherapy. This study also revealed that TAOK3 enhanced microtubule-targeted drug (paclitaxel, eribulin, and vinorelbine) resistance via the NF-κB signaling pathway in breast cancer cell lines [53] and suggested that disrupting the interaction between TAOK3 and NF-κB signaling may have beneficial therapeutic effects for breast cancer patients treated with anti-microtubule agents. Additionally, in a genomic study, TAOK1-PCGF2 was among the nine fusion genes identified in the breast cancer cell line ZR-75-30 [54].
4.2. TAO Kinases in Colorectal Cancer
In the study by Capra et al. [52], TAOK1 was also found to be overexpressed in colorectal cancer tissues compared to normal colon tissues. Conversely, an assay of the kinome profile in colon cancer revealed that TAOK3 is downregulated in adenocarcinoma compared to the normal colon [55]. These results suggest that TAOK1 and TAOK3 have opposite effects in colorectal tumorigenesis, though the underlying mechanism is not clear.
4.3. TAO Kinases in Lung Cancer
TAOK1 was found to be overexpressed in lung cancer tissues compared to normal lung tissues in a study using in situ hybridization to search for alterations of serine/threonine kinase expression in cancers [52]. However, in a transcriptome assay of lung adenocarcinoma, TAOK2 was found to be downregulated in tumor tissues compared to the normal lung [56]. Such results may indicate the opposite roles of TAOK1 and TAOK2 in lung tumorigenesis.
4.4. TAO Kinases in Pancreatic Cancer
In a pancreatic cancer cell study by Bian et al. [57], expression of TAOK3 was required to support the cancer stem cell-enriching spheroid growth, and knockdown of TAOK3 decreased expression of stem cell traits, spheroid formation, and sensitized cells to gemcitabine treatment. In this study, the authors identified an ITK inhibitor, NCGC00188382, which could inhibit the activity of TAOK3, aurora B kinase, and cyclin-dependent kinase 7 in cancer cells and suppress the stemness traits and growth of pancreatic tumors.
4.5. TAO Kinases in Prostate Cancer
The androgen pathway is an important modulator in prostate cancer progression. In the study by Romanuik et al. [58], TAOK3 was found to be an androgen response gene in prostate cancer cells. Furthermore, a later study by Bii et al. [59] confirmed that TAOK3 is a prostate cancer progression-associated gene and expression of TAOK3 can predict the risk of recurrence after androgen deprivation therapy in prostate cancer.
4.6. TAO Kinases in Melanoma
In a study by Sharma et al. [60], several kinases including TAOK2 were found to increase ATP uptake in BRAF inhibitor-resistant melanoma cells. In these resistant cells, the activity of TAOK2 is increased and activation of the JNK pathway by TAOK2 is thought to contribute to BRAF inhibitor (vemurafenib) resistance.
4.7. TAO Kinases in Larynx Cancer and Leukemia
In the study by Capra et al. [52], TAOK1 was found to be overexpressed in larynx cancer tissues compared to the normal larynx. In acute B lymphoblastic leukemia cells with PAX5 alterations, TAOK1 was found to be a novel fusion partner and the fusion protein PAX5–TAOK1 was proposed to be a competitive inhibitor of wild-type PAX5 for its transactivation activity [61].
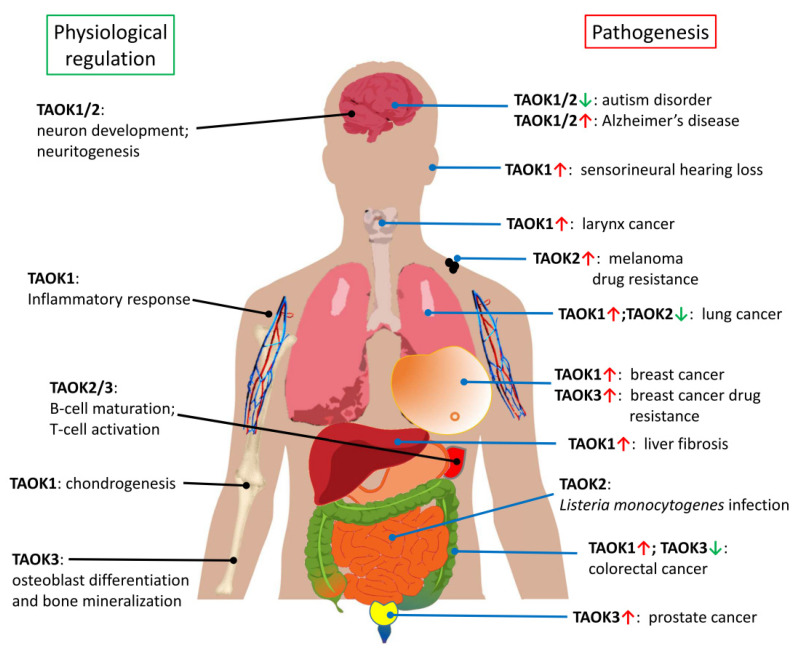
Currently, most TAOK studies seem to show the tumor-promoting characteristics of TAOKs. However, as aforementioned, TAOKs have also been suggested to act as potential tumor suppressor genes due to their involvement in the Hippo signaling pathway [26] (Figure 2). Consistent with this hypothesis, downregulation of TAOK2 and TAOK3 was noted in lung and colorectal cancer tissues, respectively. The role of each TAOK as a tumor promoter or suppressor under the effect of different cellular contexts or extracellular stimuli remains to be explored (Figure 4).
Figure 4.
Current knowledge of TAOKs in physiological regulation and pathogenesis. The red “UP” arrows indicate increased expression of TAOK; the green “DOWN” arrows indicate decreased expression of TAOK. This figure contains elements (liver, stomach, and intestine) downloaded from the public domain (see “Acknowledgements” for details).
5. TAO Kinases in Cognitive Disorders and Neurodegenerative Diseases
Given that TAOKs are involved in neuron development via modulation of cytoskeleton components, it is not surprising that dysregulation of TAOKs is found to be associated with the development of neural diseases and cognitive disorders. We highlight recent reports in the following sections.
5.1. TAO Kinases in Autism Spectrum Disorder
Microdeletion or microduplication of chromosome 16p11.2 has been linked to susceptibility to autism disease [62]. One of the genes from the affected region is TAOK2. TAOK2 downregulation was reported to impair basal dendrite formation while not affecting apical dendrites [34]. This study found that Sema3A and Nrp1 transduce signals via the TAOK2–JNK pathway to regulate basal dendrite development. Low expression of TAOK2 may therefore affect the development of basal dendrites and lead to autism spectrum disorder [34]. Richter et al. [42] subsequently showed that loss of TAOK2 activity causes a reduction in RhoA activation, which then affects F-actin stability in developing neurons and results in reduced brain development and impaired neural connectivity. This study indicates that reduced TAOK2 activity during neural development is associated with autism-related neurodevelopmental and cognitive abnormalities. A study by Dulovic-Mahlow et al. [63] also identified loss-of-function, de novo variants of TAOK1 which correlated with neurodevelopmental disorders. Collectively, these results indicate that abnormal reduction of TAOK1/2 activity during neuron development may result in cognitive abnormalities and lead to the development of autism.
5.2. TAO Kinases in Alzheimer’s Disease
As aforementioned, TAOK1 phosphorylates the microtubule-associated protein tau and allows the rearrangement of microtubules and development of neurites during neuron differentiation [30]. However, in Alzheimer’s disease (AD), tau is atypically phosphorylated and aggregates into the characteristic intraneuronal neurofibrillary tangles. Both TAOK1 and TAOK2 have been shown to phosphorylate tau in domains (amino acids 244–368) that are known to regulate the tau–microtubule interactions [30,37] (Figure 3B). The colocalization of catalytically active TAOKs and phosphorylated tau in the AD brain with tangle-bearing neurons has been reported. Such observations suggest that TAOKs participate in the development of dementia and, more specifically, AD by dysregulating tau phosphorylation [32,37].
5.3. TAO Kinases in Parkinson’s Disease
In a study of dominant mutation of LRRK2 (leucine-rich repeat kinase 2) in a Parkinson’s disease (PD) model, TAOK3, serine/threonine kinase 3 (STK3), STK24, and STK25 were identified to be novel LRRK2 substrates that may be involved in LRRK2-induced synaptic dysfunction and neurite fragmentation [64]. The kinase cascade from LRRK2 to TAOK, PAR-1, and finally to tau phosphorylation has been proposed as a pathological transformation in tauopathy and axonal pathology in the brains of LRRK2-overexpressing mice and in human PD patients [39,64,65].
5.4. TAO Kinases in Cerebral Ischemic Stroke
Although abnormal activity of TAOKs may lead to defects in neuron development, Li et al. [66] have shown that overexpression of TAOK1 ameliorates oxygen glucose deprivation-induced cell injury in neurons and protects rats from induced cerebral ischemic stroke by decreasing pro-inflammatory factors and reducing apoptosis through the PI3K/AKT and MAPK signaling pathways. Taken together, these results support the homeostatic and protective role of TAOKs in the central nervous system under physiological conditions, whereas their dysregulation leads to cognitive disorders and neurodegenerative diseases (Figure 4).
6. TAO Kinases in Other Physiological Processes and Diseases
In addition to the aforementioned physiological and pathological roles, TAOKs have been found to regulate additional processes (Figure 4). TAOK3 is reported to function as an upstream activator of the JNK pathway in osteoblasts and its deficiency induces a marked decrease in osteoblast differentiation and defective mineralization [67]. Oxidative stress was found to induce α-SMA (α-smooth muscle actin), PKCα (Protein kinase Cα), and TAOK1 expression during liver fibrogenesis. MicroRNA miR-706 directly inhibits PKCα and TAOK1 expression via binding to their 3′-untranslated regions, thus preventing the epithelial to mesenchymal transition and alleviating hepatic fibrosis [68]. Jing et al. [69] showed that miR-381-3p promoted chondrogenesis in umbilical cord mesenchymal stem cells through direct suppression of TAOK1 and the downstream Hippo signaling pathway, indicating that TAOK1 may regulate chondrogenesis via the Hippo cascade. In an acoustic trauma study by Patel et al. [70], the downregulation of miRNA-183 and upregulation of TAOK1 were observed in noise-traumatized cochlear cells in rats, indicating that the miR-183/TAOK1 pathway is likely to play a role in sensorineural hearing loss.
There are other physiological processes or pathogeneses reported to be TAOK-associated, with mechanisms yet to be defined. In a differentially expressed microarray assay, TAOK1 was found to be highly expressed in patients with coronary artery disease [71]. A missense variant in the kinase domain of TAOK2 (pV244M) was reported in natural killer cell proliferative disorder [72]. A homozygous missense variant of TAOK2 (c.2098C > T; p.R700C) was also found to be associated with impaired T cell activation [48]. Interestingly, TAOK2 was found to be recruited to the internalization vacuole containing intracellular bacteria in Listeria monocytogenes infected cells and involved in regulation of vacuolar rupture and cytoplasmic access of these bacteria [73]. Genetic variations in the TAOK3 locus rs795484 were reported to be associated with increased morphine requirement in children of European Caucasian ancestry and with increased acute postoperative pain in both European Caucasian and African American subjects [74,75], although the underlying mechanism is unknown and requires further determination [76]. It has been proposed that TAOK3 may act as a pharmacogene that affects the response to analgesic treatment [77]. Mice with a heterozygous disrupted allele of TAOK3 were resistant to the acute sedative effects of ethanol [22], and those with conditional ablation of TAOK2 were found to recover quickly from ethanol-induced ataxia and consumed increased amounts of ethanol compared with control animals [78]. In a genome-wide methylation analysis, lower methylation of CpG loci within TAOK3 was associated with childhood obesity [79]. TAOK3 was found to be the host kinase that phosphorylates the herpes virus inhibitors methylenecyclopropane nucleosides, instead of viral thymidine kinase [80]. Interestingly, it has also been shown that TAOK3 interacts with the herpes simplex virus structural tegument protein pUL37 [81].
7. Current Development of TAOK Inhibitors
Given that TAOKs are involved in many pathological processes and diseases, including apoptosis, inflammation and immune regulation, cancer and drug resistance, autism disorder, and Alzheimer’s disease (Figure 4), the development of specific inhibitors targeting TAOK-related pathways may provide a way to ameliorate their effects on disease progression.
Staurosporine is a broad-range protein kinase inhibitor isolated from Streptomyces species. Staurosporine inhibits TAOK2 with an IC50 of 3 μM [82]; however, staurosporine also inhibits a number of other serine/threonine protein kinases with high potency, and this lack of specificity has precluded its use in the clinic. The MST1 inhibitor 9E1 suppresses TAOK2 activity with an IC50 value of 0.3 μmol/L [83]; yet it has the same specificity issue as staurosporine. Recently, two TAOK inhibitors, compounds 43 and 63, were isolated with high specificity to TAOK1, 2, and 3 [33]. Both compounds are ATP-competitive inhibitors of TAOK activity. Compound 43 (N-[2-oxo-2-(1,2,3,4-tetrahydro-naphthalen-1-ylamino)ethyl]biphenyl-4-carboxamide) was found to target and inhibit cancer cells selectively while not affecting non-tumor cells [33]. Compound 43 prolongs the duration of mitosis, reduces the percentage of cells exiting mitosis, and increases mitotic cell death in cancer cells, while non-malignant MCF-10A breast cells continue to proliferate normally [33]. It has also been shown that reducing TAOK expression enhances the sensitivity to γ-radiation in colony survival assays [13], and knockdown of TAOK3 abolishes the drug resistance to microtubule-targeted drugs in breast cancer cells [53]. Therefore, inhibition of TAOK activity by compound 43 may sensitize tumor cells to anticancer treatments. Compound 43 has been demonstrated to decrease tau phosphorylation in murine and human neural cell models of tauopathy. Giacomini et al. [32] have shown that abnormal TAOK activity is present in tauopathies and TAOK inhibition effectively reduces tau phosphorylation on pathological sites. Therefore, compound 43 has the potential to be an effective and specific TAOK inhibitor for further evaluation of its clinical value in cancer, drug resistance, neurodegeneration, and inflammation/autoimmune disorders. In addition, a previously identified ITK inhibitor (NCGC00188382) was shown to inhibit the activity of TAOK3, aurora B kinase, and cyclin-dependent kinase 7 in pancreatic cancer cells and suppress the stemness traits and growth of tumor spheroids [57]. However, given that this ITK inhibitor is a multikinase inhibitor, it is not clear how much of the inhibition is TAOK3-related. Recently, a high-throughput screen of a 200 k compound library identified two additional compounds, SW034538 and SW083688, that showed significant inhibition activity to TAOK2 (IC50 values = 300 nmol/L and 1.3 μmol/L, respectively) [84]. However, the detailed characteristics of these two molecules are currently unknown and remain to be evaluated. The characteristics of currently available TAOK inhibitors are summarized in Table 1.
Table 1.
Characteristics of currently available TAOK inhibitors.
| Compound Name | IUPAC Name | Cell-Based Inhibition Assay (IC50) (Assay Methodology) * | Kinase Inhibition Assay (IC50) (Assay Methodology) * | Reference |
|---|---|---|---|---|
| Compound 43 | N-[2-oxo-2-(1,2,3,4-tetrahydro-naphthalen-1-ylamino)ethyl]biphenyl-4-carboxamide | NA/showed growth inhibition to SK-BR-3 but not MCF-10A cells | TAOK1: 11 ± 0.44 nmol/L (MBPp) TAOK2: 15 ± 1.63 nmol/L (MBPp) Selectively inhibits TAOK1, 2, 3 while showing low inhibition for other 19 kinases |
[33] |
| Compound 63 | N-{3-[(2-{[6-methoxy-1,3-benzothiazol-2yl]amino}-2-oxoethyl)amino]-3-oxo-1-phenylpropyl}benzamide | NA | TAOK1: 19 ± 1.87 nmol/L (MBPp) TAOK2: 39 ± 6.43 nmol/L (MBPp) Selectively inhibits TAOK1, 2, 3 while showing low inhibition for other 19 kinases |
[33] |
| NCGC00188382 | N-[5-[(3,3-dimethylbutan-2-ylamino)methyl]-1-(2-hydroxy-2-methylpropyl)benzimidazol-2-yl]-5-(1H-pyrazol-4-yl)thiophene-2-carboxamide | 25 to 300 nmol/L in 24 pancreatic cancer cell lines (FA6 cell: ~25 nmol/L; MDA-Panc-28: ~300 nmol/L) (Cytotoxicity assay) | NA/ showed inhibition to the activity of TAOK3, aurora B kinase, and cyclin-dependent kinase 7 in pancreatic cancer cells | [57] |
| SW034538 | N-(2-((2,5-dimethoxyphenyl)amino)-4’-methyl-[4,5’-bithiazol]-2’-yl)propionamide | NA | TAOK2: 300 nmol/L (MBPp) | [84] |
| SW083688 | N-((2,3-dihydrobenzo[b][1,4]dioxin-2-yl)methyl)-3-(3-ethoxypropyl)-4-oxo-2-thioxo-1,2,3,4-tetrahydroquinazoline-7-carboxamide | NA | TAOK2: 1.3 μmol/L (MBPp) | [84] |
* Abbreviations: MBPp: inhibition assay of myelin basic protein phosphorylation; NA: not available.
8. Concluding Remarks and Future Perspectives
TAO kinases are members of the MAP kinase cascade whose activators and regulation are only beginning to be uncovered compared to other well-characterized members in this family. In addition to the p38/MAPK and SAPK/JNK cascades, studies have shown that TAOKs are also involved in the Hippo signaling pathway and interact with other cytosolic targets to regulate cellular physiology. However, their upstream activator is largely unknown, and the modulation of TAOK activity under normal physiology and pathogenic conditions remains to be fully deciphered. Although studies are beginning to show that TAOKs regulate immune responses in B and T cells and modulate tissue inflammatory responses via cytokine regulation, further investigation is required to unveil their diverse roles in immune modulation. High expression of TAOK3 in immune cells, and TAOK1/2 in neural cells, may indicate their tissue-specific regulation in the corresponding cells. Based on current knowledge, it seems that certain cellular pathways regulated by TAOK3 are different from those regulated by TAOK1/2. For example, TAOK1 and 2 are involved in cytoskeleton regulation (Figure 3), while the role of TAOK3 in this process is unclear, although a recent study showed that TAOK3 confers resistance to microtubule-targeted drugs in breast cancer cells [53]. Whether this result indicates the involvement of TAOK3 in cytoskeleton modulation in healthy cells requires further investigation. Hence, their tissue-specific expression and diversity in pathway regulations are worth further investigation.
As aforementioned, TAOKs are overexpressed in some tumor tissues while being downregulated in others; however, recent tumorigenic studies of TAOKs were mostly carried out in cell lines and their links to clinical manifestation remain to be fully elucidated. The diversity of TAOKs in modulating the p38/JNK and Hippo pathways is especially worth in-depth investigation, given that they could lead to tumor promoting or inhibiting consequences. Since TAOKs respond to stress conditions, their expression seems to correlate with drug resistance, as demonstrated in breast and melanoma cell studies [53,60]. The underlying mechanisms of drug resistance induced by TAOKs are also of great value for further study. Dysregulation of TAOKs also plays an important role in the tauopathy associated with Alzheimer’s disease and Parkinson’s disease and is implicated in autism-related neurodevelopmental disorders.
The development of TAOK-specific inhibitors seems to be a promising area of research. By targeting their kinase domains, highly specific inhibitors may be selected which do not interfere with other related MAP kinases. However, the efficacy and toxicity of these candidates will need to be evaluated in vivo. Well-planned animal studies are urgently required to evaluate the potency of TAOK inhibitors in disease models before they can proceed to human clinical evaluation. In addition to kinase domain interaction, TAOK2 binds to microtubules and actin through its C-terminal region [7,29]. The development of inhibitors that disrupt the TAOK2 C-terminal interaction may have potential use in the clinic if the interaction is involved in disease pathology. Recently, various kinase inhibitor candidates have been developed by structure-guided, kinase domain virtual screening [85,86]. This is usually achieved by searching a compound that binds to a target through the quantitative structure–activity relationship model derived from existing molecule datasets [85]. Via computer-aided molecular docking, optimal binding modes of ligands for a given binding pocket can be generated, thus facilitating the discovery of specific inhibitors. Since the crystal structures of rat TAOK2 (PDB ID: 1U5Q/1U5R) [87] and human TAOK3 (PDB ID: 6BDN; http://dx.doi.org/10.2210/pdb6bdn/pdb) have been revealed, the application of these methodologies to assist in the development of TAOK-specific inhibitors is achievable and highly anticipated. Given that TAOKs are implicated in many important diseases (summarized in Table 2), the development of a TAOK inhibitor with high specificity and potency could provide a successful treatment for TAOK-associated malignancy and pathogenesis.
Table 2.
The role of TAO kinases in tumorigenesis, inflammation, cognitive/neurodegenerative disorders, and other diseases.
| Disease | Sample and Experimental Approach * | Results | Reference |
|---|---|---|---|
| Cancers | |||
| Breast cancer | Biopsy/ISH; cell line/KD and pathway assay | Upregulation of TAOK1 in tumor tissue; TAOK3 enhances microtubule-targeted drug resistance | [52,53] |
| Colorectal cancer | Biopsy/ISH and kinome profiling | Upregulation of TAOK1 in tumor tissue; downregulation of TAOK3 in adenocarcinoma | [52,55] |
| Lung cancer | Biopsy/ISH and transcriptome assay | Upregulation of TAOK1 in tumor tissue; downregulation of TAOK2 in tumor tissue | [52,56] |
| Pancreatic cancer | Cell line/OE and KD, mouse xenograft model | TAOK3 supports the stemness traits and growth of tumor spheroids | [57] |
| Prostate cancer | Cell line/transcriptome; MGS-PCR | TAOK3 is a prostate cancer progression gene and its expression can predict the risk of recurrence after androgen deprivation therapy | [58,59] |
| Melanoma | Cell line/activity-based protein profiling | TAOK2 activates JNK and contributes to the BRAF inhibitor (vemurafenib) resistance | [60] |
| Larynx cancer | Biopsy/ISH | Upregulation of TAOK1 in tumor tissue | [52] |
| B cell leukemia | Cell line/rolling-circle amplification of cDNA ends | PAX5–TAOK1 fusion protein may be a competitive inhibitor of wild-type PAX5 | [61] |
| Neurodegenerative disease | |||
| Autism | Cell line; mouse model; patient DNA sample/KD and ectopic expression; TAOK2-KO mouse; genotyping | Downregulation of TAOK1/2 activity during neuron development results in cognitive abnormalities and autism | [34,42,62,63] |
| Alzheimer’s disease | Cell line; mouse model; human biopsy/ OE in cell; IHC; inhibitor assay | TAOK1/2 dysregulate tau phosphorylation and participate in the development of dementia and AD | [32,37] |
| Parkinson’s disease | Cell line/protein array assay | TAOK3 is a novel LRRK2 substrate and involved in LRRK2-induced PD | [64] |
| Cerebral ischemic stroke | Mouse model/induced cerebral ischemic stroke | TAOK1 ameliorates induced cerebral ischemic stroke by decreasing pro-inflammatory factors and reducing apoptosis | [66] |
| Inflammation | |||
| IL-17-associated | Cell line/KD and OE; TAOK1 KO-mouse model | TAOK1 inhibits IL-17-mediated signal transduction and inflammation | [44] |
| LPS-induced | KO-mouse model of TAOK1 in myeloid cells | TAOK1 enhances LPS-induced activation of ERK1/2 and positively regulates the TLR4-induced inflammatory response | [45] |
| Other diseases | |||
| Liver fibrosis | Mouse model of liver fibrosis | miR-706 inhibits PKCα and TAOK1 expression, thus prevents liver fibrosis | [68] |
| Listeria infection | Bacteria and cell line/siRNA microscopy screening | TAOK2 regulates vacuolar rupture and cytoplasmic access of Listeria | [73] |
| Coronary artery disease | Expression database analysis | TAOK1 highly expressed in patients with coronary artery disease | [71] |
| Sensorineural hearing loss | Noise-traumatized rat model/ miRNA expression analysis | Downregulation of miRNA-183 and upregulation of TAOK1 may be involved in sensorineural hearing loss | [70] |
* Abbreviations: AD: Alzheimer’s disease; EMT: epithelial–mesenchymal transition; IHC: immunohistochemistry; ISH: in situ hybridization; KD: knockdown; KO: knockout; LPS: lipopolysaccharide; MGS-PCR: modified genomic sequencing PCR; OE: overexpression; PD: Parkinson’s disease; TLR4: toll-like receptor 4.
Acknowledgments
Figure 4 contains figure elements (liver, stomach and intestine (Author: LadyofHats/Mariana Ruiz, Jmarchn, and Mikael Häggström), which have been released into the public domain. These elements were downloaded from Wikimedia Commons (https://commons.wikimedia.org).
Author Contributions
Preparation of original draft by C.-Y.F. and T.-C.L., C.-Y.F., Y.-C.C., and M.H. participated in the conceptualization. Y.-C.C. and M.H. contributed to critical editing of manuscript. Funding was provided by M.H. Data acquisition was done by C.-Y.F. and T.-C.L. Supervision, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Academia Sinica (AS-SUMMIT-108), (AS-SUMMIT-109), (ASKPQ-109-BioMed), and Ministry of Science and Technology, Taiwan (MOST-107–2320-B-001–016-MY3), (MOST-108–3114-Y-001–002) to Michael Hsiao.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Pearson G., Robinson F., Gibson T.B., Xu B.E., Karandikar M., Berman K., Cobb M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001;22:153–183. doi: 10.1210/er.22.2.153. [DOI] [PubMed] [Google Scholar]
- 2.Leberer E., Dignard D., Harcus D., Thomas D.Y., Whiteway M. The Protein-Kinase Homolog Ste20p Is Required to Link the Yeast Pheromone Response G-Protein Beta-Gamma Subunits to Downstream Signaling Components. Embo J. 1992;11:4815–4824. doi: 10.1002/j.1460-2075.1992.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramer S.W., Davis R.W. A Dominant Truncation Allele Identifies a Gene, Ste20, That Encodes a Putative Protein-Kinase Necessary for Mating in Saccharomyces-Cerevisiae. Proc. Natl. Acad. Sci. USA. 1993;90:452–456. doi: 10.1073/pnas.90.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutchison M., Berman K.S., Cobb M.H. Isolation of TAO1, a protein kinase that activates MEKs in stress-activated protein kinase cascades. J. Biol. Chem. 1998;273:28625–28632. doi: 10.1074/jbc.273.44.28625. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z., Hutchison M., Cobb M.H. Isolation of the protein kinase TAO2 and identification of its mitogen-activated protein kinase/extracellular signal-regulated kinase kinase binding domain. J. Biol. Chem. 1999;274:28803–28807. doi: 10.1074/jbc.274.40.28803. [DOI] [PubMed] [Google Scholar]
- 6.Tassi E., Biesova Z., Di Fiore P.P., Gutkind J.S., Wong W.T. Human JIK, a novel member of the STE20 kinase family that inhibits JNK and is negatively regulated by epidermal growth factor. J. Biol. Chem. 1999;274:33287–33295. doi: 10.1074/jbc.274.47.33287. [DOI] [PubMed] [Google Scholar]
- 7.Moore T.M., Garg R., Johnson C., Coptcoat M.J., Ridley A.J., Morris J.D.H. PSK, a novel STE20-like kinase derived from prostatic carcinoma that activates the c-Jun N-terminal kinase mitogen-activated protein kinase pathway and regulates actin cytoskeletal organization. J. Biol. Chem. 2000;275:4311–4322. doi: 10.1074/jbc.275.6.4311. [DOI] [PubMed] [Google Scholar]
- 8.Yustein J.T., Xia L., Kahlenburg J.M., Robinson D., Templeton D., Kung H.J. Comparative studies of a new subfamily of human Ste20-like kinases: Homodimerization, subcellular localization, and selective activation of MKK3 and p38. Oncogene. 2003;22:6129–6141. doi: 10.1038/sj.onc.1206605. [DOI] [PubMed] [Google Scholar]
- 9.Morrison D.K. MAP Kinase Pathways. Csh Perspect Biol. 2012;4:a011254. doi: 10.1101/cshperspect.a011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuevas B.D., Abell A.N., Johnson G.L. Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene. 2007;26:3159–3171. doi: 10.1038/sj.onc.1210409. [DOI] [PubMed] [Google Scholar]
- 11.Yu F.X., Zhao B., Guan K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saucedo L.J., Edgar B.A. Filling out the Hippo pathway. Nat. Rev. Mol. Cell Biol. 2007;8:613–621. doi: 10.1038/nrm2221. [DOI] [PubMed] [Google Scholar]
- 13.Raman M., Earnest S., Zhang K., Zhao Y.M., Cobb M.H. TAO kinases mediate activation of p38 in response to DNA damage. Embo J. 2007;26:2005–2014. doi: 10.1038/sj.emboj.7601668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z., Raman M., Chen L., Lee S.F., Gilman A.G., Cobb M.H. TAO (thousand-and-one amino acid) protein kinases mediate signaling from carbachol to p38 mitogen-activated protein kinase and ternary complex factors. J. Biol. Chem. 2003;278:22278–22283. doi: 10.1074/jbc.M301173200. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z., Cobb M.H. Regulation of stress-responsive mitogen-activated protein (MAP) kinase pathways by TAO2. J. Biol. Chem. 2001;276:16070–16075. doi: 10.1074/jbc.M100681200. [DOI] [PubMed] [Google Scholar]
- 16.Weston C.R., Davis R.J. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Bogoyevitch M.A., Ngoei K.R.W., Zhao T.T., Yeap Y.Y.C., Ng D.C.H. c-Jun N-terminal kinase (JNK) signaling: Recent advances and challenges. Bba-Proteins. Proteom. 2010;1804:463–475. doi: 10.1016/j.bbapap.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Zihni C., Mitsopoulos C., Tavares I.A., Ridley A.J., Morris J.D.H. Prostate-derived sterile 20-like kinase 2 (PSK2) regulates apoptotic morphology via C-jun N-terminal kinase and Rho kinase-1. J. Biol. Chem. 2006;281:7317–7323. doi: 10.1074/jbc.M513769200. [DOI] [PubMed] [Google Scholar]
- 19.Zihni C., Mitsopoulos C., Tavares I.A., Baum B., Ridley A.J., Morris J.D.H. Prostate-derived sterile 20-like kinase 1-alpha induces apoptosis—JNK- and caspase-dependent nuclear localization is a requirement for membrane blebbing. J. Biol. Chem. 2007;282:6484–6493. doi: 10.1074/jbc.M608336200. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W.P., Chen T.Y., Wan T., He L., Li N., Yuan Z.L., Cao X.T. Cloning of DPK, a novel dendritic cell-derived protein kinase activating the ERK1/ERK2 and JNK/SAPK pathways. Biochem. Bioph. Res. Commun. 2000;274:872–879. doi: 10.1006/bbrc.2000.3244. [DOI] [PubMed] [Google Scholar]
- 21.MacKeigan J.P., Murphy L.O., Blenis J. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat. Cell Biol. 2005;7:591–600. doi: 10.1038/ncb1258. [DOI] [PubMed] [Google Scholar]
- 22.Kapfhamer D., King I., Zou M.E., Lim J.P., Heberlein U., Wolf F.W. JNK Pathway Activation Is Controlled by Tao/TAOK3 to Modulate Ethanol Sensitivity. PLoS ONE. 2012;7:e50594. doi: 10.1371/journal.pone.0050594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccolo S., Dupont S., Cordenonsi M. The Biology of Yap/Taz: Hippo Signaling and Beyond. Physiol. Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 24.Yu F.X., Guan K.L. The Hippo pathway: Regulators and regulations. Gene Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boggiano J.C., Vanderzalm P.J., Fehon R.G. Tao-1 Phosphorylates Hippo/MST Kinases to Regulate the Hippo-Salvador-Warts Tumor Suppressor Pathway. Dev. Cell. 2011;21:888–895. doi: 10.1016/j.devcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poon C.L.C., Lin J.I., Zhang X.M., Harvey K.F. The Sterile 20-like Kinase Tao-1 Controls Tissue Growth by Regulating the Salvador-Warts-Hippo Pathway. Dev. Cell. 2011;21:896–906. doi: 10.1016/j.devcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Plouffe S.W., Meng Z.P., Lin K.C., Lin B.A., Hong A.W., Chun J.V., Guan K.L. Characterization of Hippo Pathway Components by Gene Inactivation. Mol. Cell. 2016;64:993–1008. doi: 10.1016/j.molcel.2016.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng Z., Moroishi T., Mottier-Pavie V., Plouffe S.W., Hansen C.G., Hong A.W., Park H.W., Mo J.S., Lu W., Lu S., et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat. Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitsopoulos C., Zihni C., Garg R., Ridley A.J., Morris J.D. The prostate-derived sterile 20-like kinase (PSK) regulates microtubule organization and stability. J. Biol. Chem. 2003;278:18085–18091. doi: 10.1074/jbc.M213064200. [DOI] [PubMed] [Google Scholar]
- 30.Timm T., Li X.Y., Biernat J., Jiao J., Mandelkow E., Vandekerckhove J., Mandelkow E.M. MARKK, a Ste20-like kinase, activates the polarity-inducing kinase MARK/PAR-1. Embo J. 2003;22:5090–5101. doi: 10.1093/emboj/cdg447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu T., Rohn J.L., Picone R., Kunda P., Baum B. Tao-1 is a negative regulator of microtubule plus-end growth. J. Cell Sci. 2010;123:2708–2716. doi: 10.1242/jcs.068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giacomini C., Koo C.Y., Yankova N., Tavares I.A., Wray S., Noble W., Hanger D.P., Morris J.D.H. A new TAO kinase inhibitor reduces tau phosphorylation at sites associated with neurodegeneration in human tauopathies. Acta Neuropathol. Commun. 2018;6:37. doi: 10.1186/s40478-018-0539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koo C.Y., Giacomini C., Reyes-Corral M., Olmos Y., Tavares I.A., Marson C.M., Linardopoulos S., Tutt A.N., Morris J.D.H. Targeting TAO Kinases Using a New Inhibitor Compound Delays Mitosis and Induces Mitotic Cell Death in Centrosome Amplified Breast Cancer Cells. Mol. Cancer Ther. 2017;16:2410–2421. doi: 10.1158/1535-7163.MCT-17-0077. [DOI] [PubMed] [Google Scholar]
- 34.de Anda F.C., Rosario A.L., Durak O., Tran T., Graff J., Meletis K., Rei D., Soda T., Madabhushi R., Ginty D.D., et al. Autism spectrum disorder susceptibility gene TAOK2 affects basal dendrite formation in the neocortex. Nat. Neurosci. 2012;15:1022–1031. doi: 10.1038/nn.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wojtala R.L., Tavares I.A., Morton P.E., Valderrama F., Thomas N.S.B., Morris J.D.H. Prostate-derived Sterile 20-like Kinases (PSKs/TAOKs) Are Activated in Mitosis and Contribute to Mitotic Cell Rounding and Spindle Positioning. J. Biol. Chem. 2011;286:30161–30170. doi: 10.1074/jbc.M111.228320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shrestha R.L., Tamura N., Fries A., Levin N., Clark J., Draviam V.M. TAO1 kinase maintains chromosomal stability by facilitating proper congression of chromosomes. Open Biol. 2014;4:130108. doi: 10.1098/rsob.130108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tavares I.A., Touma D., Lynham S., Troakes C., Schober M., Causevic M., Garg R., Noble W., Killick R., Bodi I., et al. Prostate-derived Sterile 20-like Kinases (PSKs/TAOKs) Phosphorylate Tau Protein and Are Activated in Tangle-bearing Neurons in Alzheimer Disease. J. Biol. Chem. 2013;288:15418–15429. doi: 10.1074/jbc.M112.448183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garg R., Koo C.Y., Infante E., Giacomini C., Ridley A.J., Morris J.D.H. Rnd3 interacts with TAO kinases and contributes to mitotic cell rounding and spindle positioning. J. Cell Sci. 2020;133 doi: 10.1242/jcs.235895. [DOI] [PubMed] [Google Scholar]
- 39.Johne C., Matenia D., Li X.Y., Timm T., Balusamy K., Mandelkow E.M. Spred1 and TESK1--two new interaction partners of the kinase MARKK/TAO1 that link the microtubule and actin cytoskeleton. Mol. Biol. Cell. 2008;19:1391–1403. doi: 10.1091/mbc.e07-07-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ultanir S.K., Yadav S., Hertz N.T., Oses-Prieto J.A., Claxton S., Burlingame A.L., Shokat K.M., Jan L.Y., Jan Y.N. MST3 kinase phosphorylates TAO1/2 to enable Myosin Va function in promoting spine synapse development. Neuron. 2014;84:968–982. doi: 10.1016/j.neuron.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yadav S., Oses-Prieto J.A., Peters C.J., Zhou J., Pleasure S.J., Burlingame A.L., Jan L.Y., Jan Y.N. TAOK2 Kinase Mediates PSD95 Stability and Dendritic Spine Maturation through Septin7 Phosphorylation. Neuron. 2017;93:379–393. doi: 10.1016/j.neuron.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richter M., Murtaza N., Scharrenberg R., White S.H., Johanns O., Walker S., Yuen R.K.C., Schwanke B., Bedurftig B., Henis M., et al. Altered TAOK2 activity causes autism-related neurodevelopmental and cognitive abnormalities through RhoA signaling. Mol. Psychiatry. 2019;24:1329–1350. doi: 10.1038/s41380-018-0025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasuda S., Tanaka H., Sugiura H., Okamura K., Sakaguchi T., Tran U., Takemiya T., Mizoguchi A., Yagita Y., Sakurai T., et al. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2 beta and p38 MAP kinases. Neuron. 2007;56:456–471. doi: 10.1016/j.neuron.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z.R., Tang Z., Ma X.W., Sun K., Fan L.P., Fang J., Pan J.P., Wang X.J., An H.Z., Zhou J. TAOK1 negatively regulates IL-17-mediated signaling and inflammation. Cell Mol. Immunol. 2018;15:794–802. doi: 10.1038/cmi.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu L., Yu Q., Gao P., Liu Q., Luo X., Jiang G., Ji R., Yang R., Ma X., Xu J., et al. TAOK1 positively regulates TLR4-induced inflammatory responses by promoting ERK1/2 activation in macrophages. Mol. Immunol. 2020;122:124–131. doi: 10.1016/j.molimm.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Hammad H., Vanderkerken M., Pouliot P., Deswarte K., Toussaint W., Vergote K., Vandersarren L., Janssens S., Ramou I., Savvides S.N., et al. Transitional B cells commit to marginal zone B cell fate by Taok3-mediated surface expression of ADAM10. Nat. Immunol. 2017;18:313–320. doi: 10.1038/ni.3657. [DOI] [PubMed] [Google Scholar]
- 47.Ormonde J.V.S., Li Z.G., Stegen C., Madrenas J. TAOK3 Regulates Canonical TCR Signaling by Preventing Early SHP-1-Mediated Inactivation of LCK. J. Immunol. 2018;201:3431–3442. doi: 10.4049/jimmunol.1800284. [DOI] [PubMed] [Google Scholar]
- 48.Molho-Pessach V., Ramot Y., Mogilevsky M., Cohen-Daniel L., Eisenstein E.M., Abu-Libdeh A., Siam I., Berger M., Karni R., Zlotogorski A. Generalized verrucosis and abnormal T cell activation due to homozygous TAOK2 mutation. J. Dermatol. Sci. 2017;87:123–129. doi: 10.1016/j.jdermsci.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 49.HuangFu W.C., Omori E., Akira S., Matsumoto K., Ninomiya-Tsuji J. Osmotic stress activates the TAK1-JNK pathway while blocking TAK1-mediated NF-kappa B activation—TAO2 regulates TAK1 pathways. J. Biol. Chem. 2006;281:28802–28810. doi: 10.1074/jbc.M603627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pflanz R., Voigt A., Yakulov T., Jackle H. Drosophila gene tao-1 encodes proteins with and without a Ste20 kinase domain that affect cytoskeletal architecture and cell migration differently. Open Biol. 2015;5:140161. doi: 10.1098/rsob.140161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liskovykh M., Goncharov N.V., Petrov N., Aksenova V., Pegoraro G., Ozbun L.L., Reinhold W.C., Varma S., Dasso M., Kumeiko V., et al. A novel assay to screen siRNA libraries identifies protein kinases required for chromosome transmission. Genome Res. 2019;29:1719–1732. doi: 10.1101/gr.254276.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Capra M., Nuciforo P.G., Confalonieri S., Quarto M., Bianchi M., Nebuloni M., Boldorini R., Pallotti F., Viale G., Gishizky M.L., et al. Frequent alterations in the expression of serine/threonine kinases in human cancers. Cancer Res. 2006;66:8147–8154. doi: 10.1158/0008-5472.Can-05-3489. [DOI] [PubMed] [Google Scholar]
- 53.Lai T.C., Fang C.Y., Jan Y.H., Hsieh H.L., Yang Y.F., Liu C.Y., Chang P.M.H., Hsiao M. Kinase shRNA screening reveals that TAOK3 enhances microtubule-targeted drug resistance of breast cancer cells via the NF-κB signaling pathway. Cell Commun. Signal. 2020 doi: 10.1186/s12964-020-00600-2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulte I., Batty E.M., Pole J.C., Blood K.A., Mo S., Cooke S.L., Ng C., Howe K.L., Chin S.F., Brenton J.D., et al. Structural analysis of the genome of breast cancer cell line ZR-75–30 identifies twelve expressed fusion genes. BMC Genom. 2012;13:719. doi: 10.1186/1471-2164-13-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hennig E.E., Mikula M., Rubel T., Dadlez M., Ostrowski J. Comparative kinome analysis to identify putative colon tumor biomarkers. J. Mol. Med. 2012;90:447–456. doi: 10.1007/s00109-011-0831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y., He C.L., Li W.X., Zhang R.X., Duan Y. Transcriptome analysis reveals gender-specific differences in overall metabolic response of male and female patients in lung adenocarcinoma. PLoS ONE. 2020;15:e0230796. doi: 10.1371/journal.pone.0230796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bian Y., Teper Y., Mathews Griner L.A., Aiken T.J., Shukla V., Guha R., Shinn P., Xin H.W., Pflicke H., Powers A.S., et al. Target Deconvolution of a Multikinase Inhibitor with Antimetastatic Properties Identifies TAOK3 as a Key Contributor to a Cancer Stem Cell-Like Phenotype. Mol. Cancer Ther. 2019;18:2097–2110. doi: 10.1158/1535-7163.MCT-18-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romanuik T.L., Wang G., Holt R.A., Jones S.J.M., Marra M.A., Sadar M.D. Identification of novel androgen-responsive genes by sequencing of LongSAGE libraries. BMC Genom. 2009;10:476. doi: 10.1186/1471-2164-10-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bii V.M., Collins C.P., Hocum J.D., Trobridge G.D. Replication-incompetent gammaretroviral and lentiviral vector-based insertional mutagenesis screens identify prostate cancer progression genes. Oncotarget. 2018;9:15451–15463. doi: 10.18632/oncotarget.24503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma R., Fedorenko I., Spence P.T., Sondak V.K., Smalley K.S.M., Koomen J.M. Activity-Based Protein Profiling Shows Heterogeneous Signaling Adaptations to BRAF Inhibition. J. Proteome Res. 2016;15:4476–4489. doi: 10.1021/acs.jproteome.6b00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coyaud E., Struski S., Prade N., Familiades J., Eichner R., Quelen C., Bousquet M., Mugneret F., Talmant P., Pages M.P., et al. Wide diversity of PAX5 alterations in B-ALL: A Groupe Francophone de Cytogenetique Hematologique study. Blood. 2010;115:3089–3097. doi: 10.1182/blood-2009-07-234229. [DOI] [PubMed] [Google Scholar]
- 62.Weiss L.A., Shen Y.P., Korn J.M., Arking D.E., Miller D.T., Fossdal R., Saemundsen E., Stefansson H., Ferreira M.A.R., Green T., et al. Association between microdeletion and microduplication at 16p11.2 and autism. N. Engl. J. Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 63.Dulovic-Mahlow M., Trinh J., Kandaswamy K.K., Braathen G.J., Di Donato N., Rahikkala E., Beblo S., Werber M., Krajka V., Busk O.L., et al. De Novo Variants in TAOK1 Cause Neurodevelopmental Disorders. Am. J. Hum. Genet. 2019;105:213–220. doi: 10.1016/j.ajhg.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zach S., Felk S., Gillardon F. Signal Transduction Protein Array Analysis Links LRRK2 to Ste20 Kinases and PKC Zeta That Modulate Neuronal Plasticity. PLoS ONE. 2010;5:e13191. doi: 10.1371/journal.pone.0013191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taymans J.M., Cookson M.R. Mechanisms in dominant parkinsonism: The toxic triangle of LRRK2, alpha-synuclein, and tau. Bioessays. 2010;32:227–235. doi: 10.1002/bies.200900163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J.H., Liu Z.J., Wang L.L., Xu H.Y., Wang Y.L. Thousand and one kinase 1 protects MCAO-induced cerebral ischemic stroke in rats by decreasing apoptosis and pro-inflammatory factors. Biosci. Rep. 2019;39 doi: 10.1042/BSR20190749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Z., Oh H., Cung M., Marquez S.J., Sun J., Hammad H., Janssens S., Pouliot P., Lambrecht B.N., Yang Y.S., et al. TAOK3 is a MAP3K contributing to osteoblast differentiation and skeletal mineralization. Biochem. Biophys. Res. Commun. 2020 doi: 10.1016/j.bbrc.2020.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin R., Guo D., Zhang S., Zhang X. miR-706 inhibits the oxidative stress-induced activation of PKCalpha/TAOK1 in liver fibrogenesis. Sci. Rep. 2016;6:37509. doi: 10.1038/srep37509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jing H., Zhang X.Y., Luo K., Luo Q.C., Yin M., Wang W., Zhu Z.Q., Zheng J.H., He X.M. miR-381-abundant small extracellular vesicles derived from kartogenin-preconditioned mesenchymal stem cells promote chondrogenesis of MSCs by targeting TAOK1. Biomaterials. 2020;231 doi: 10.1016/j.biomaterials.2019.119682. [DOI] [PubMed] [Google Scholar]
- 70.Patel M., Cai Q.F., Ding D.L., Salvi R., Hu Z.H., Hu B.H. The miR-183/Taok1 Target Pair Is Implicated in Cochlear Responses to Acoustic Trauma. PLoS ONE. 2013;8:e58471. doi: 10.1371/journal.pone.0058471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balashanmugam M.V., Shivanandappa T.B., Nagarethinam S., Vastrad B., Vastrad C. Analysis of Differentially Expressed Genes in Coronary Artery Disease by Integrated Microarray Analysis. Biomolecules. 2019;10:35. doi: 10.3390/biom10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gasparini V.R., Binatti A., Coppe A., Teramo A., Vicenzetto C., Calabretto G., Barila G., Barizza A., Giussani E., Facco M., et al. A high definition picture of somatic mutations in chronic lymphoproliferative disorder of natural killer cells. Blood Cancer J. 2020;10:42. doi: 10.1038/s41408-020-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Quereda J.J., Morel C., Lopez-Montero N., Ziveri J., Rolland S., Grenier T., Aulner N., Danckaert A., Charbit A., Enninga J., et al. A role for Taok2 in Listeria monocytogenes vacuolar escape. J. Infect. Dis. 2020;10:1–11. doi: 10.1093/infdis/jiaa367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cook-Sather S.D., Li J., Goebel T.K., Sussman E.M., Rehman M.A., Hakonarson H. TAOK3, a novel genome-wide association study locus associated with morphine requirement and postoperative pain in a retrospective pediatric day surgery population. Pain. 2014;155:1773–1783. doi: 10.1016/j.pain.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gutteridge T., Kumaran M., Ghosh S., Fainsinger R., Klepstad P., Tarumi Y., Damaraju S., Baracos V.E. Single-Nucleotide Polymorphisms in TAOK3 Are Associated With High Opioid Requirement for Pain Management in Patients With Advanced Cancer Admitted to a Tertiary Palliative Care Unit. J. Pain Symptom Manag. 2018;56:560–566. doi: 10.1016/j.jpainsymman.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 76.Sadhasivam S., Zhang X., Prows C.A., Kaufman K.M., Martin L.J. Challenges and cautions with small and retrospective postoperative pain genome-wide association studies with TAOK3. Pain. 2014;155:2434–2435. doi: 10.1016/j.pain.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 77.Matic M., van den Bosch G.E., de Wildt S.N., Tibboel D., van Schaik R.H.N. Pain versus analgesia: TAOK3 as a pharmacogene Reply. Pain. 2017;158:1622–1623. doi: 10.1097/j.pain.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 78.Kapfhamer D., Taylor S., Zou M.E., Lim J.P., Kharazia V., Heberlein U. Taok2 controls behavioral response to ethanol in mice. Genes Brain Behav. 2013;12:87–97. doi: 10.1111/j.1601-183X.2012.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang R.C., Garratt E.S., Pan H., Wu Y., Davis E.A., Barton S.J., Burdge G.C., Godfrey K.M., Holbrook J.D., Lillycrop K.A. Genome-wide methylation analysis identifies differentially methylated CpG loci associated with severe obesity in childhood. Epigenetics Us. 2015;10:995–1005. doi: 10.1080/15592294.2015.1080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Komazin-Meredith G., Cardinale S.C., Comeau K., Magalhaes K.J., Hartline C.B., Williams J.D., Opperman T.J., Prichard M.N., Bowlin T.L. TAOK3 phosphorylates the methylenecyclopropane nucleoside MBX 2168 to its monophosphate. Antivir. Res. 2015;119:23–27. doi: 10.1016/j.antiviral.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelly B.J., Diefenbach E., Fraefel C., Diefenbach R.J. Identification of host cell proteins which interact with herpes simplex virus type 1 tegument protein pUL37. Biochem. Bioph. Res. Commun. 2012;417:961–965. doi: 10.1016/j.bbrc.2011.12.044. [DOI] [PubMed] [Google Scholar]
- 82.Zhou T.J., Sun L.G., Gao Y., Goldsmith E.J. Crystal structure of the MAP3K TAO2 kinase domain bound by an inhibitor staurosporine. Acta Biochimica et Biophysica Sinica. 2006;38:385–392. doi: 10.1111/j.1745-7270.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 83.Anand R., Maksimoska J., Pagano N., Wong E.Y., Gimotty P.A., Diamond S.L., Meggers E., Marmorstein R. Toward the Development of a Potent and Selective Organoruthenium Mammalian Sterile 20 Kinase Inhibitor. J. Med. Chem. 2009;52:1602–1611. doi: 10.1021/jm8005806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piala A.T., Akella R., Potts M.B., Dudics-Giagnocavo S.A., He H.X., Wei S.G., White M.A., Posner B.A., Goldsmith E.J. Discovery of novel TAOK2 inhibitor scaffolds from high-throughput screening. Bioorganic Med. Chem. Lett. 2016;26:3923–3927. doi: 10.1016/j.bmcl.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 85.Chen X., Liu H., Xie W., Yang Y., Wang Y., Fan Y., Hua Y., Zhu L., Zhao J., Lu T., et al. Investigation of crystal structures in structure-based virtual screening for protein kinase inhibitors. J. Chem. Inf. Model. 2019;59:5244–5262. doi: 10.1021/acs.jcim.9b00684. [DOI] [PubMed] [Google Scholar]
- 86.Liu J., Wen Y., Gao L., Gao L., He F., Zhou J., Wang J., Dai R., Chen X., Kang D., et al. Design, synthesis and biological evaluation of novel 1h-1,2,4-triazole, benzothiazole and indazole-based derivatives as potent fgfr1 inhibitors viafragment-based virtual screening. J. Enzyme Inhib. Med. Chem. 2020;35:72–84. doi: 10.1080/14756366.2019.1673745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou T., Raman M., Gao Y., Earnest S., Chen Z., Machius M., Cobb M.H., Goldsmith E.J. Crystal structure of the tao2 kinase domain: Activation and specificity of a ste20p map3k. Structure. 2004;12:1891–1900. doi: 10.1016/j.str.2004.07.021. [DOI] [PubMed] [Google Scholar]