Abstract
The identification of the human cannabinoid receptors and their roles in health and disease, has been one of the most significant biochemical and pharmacological advancements to have occurred in the past few decades. In spite of the major strides made in furthering endocannabinoid research, therapeutic exploitation of the endocannabinoid system has often been a challenging task. An impaired endocannabinoid tone often manifests as changes in expression and/or functions of type 1 and/or type 2 cannabinoid receptors. It becomes important to understand how alterations in cannabinoid receptor cellular signaling can lead to disruptions in major physiological and biological functions, as they are often associated with the pathogenesis of several neurological, cardiovascular, metabolic, and inflammatory diseases. This review focusses mostly on the pathophysiological roles of type 1 and type 2 cannabinoid receptors, and it attempts to integrate both cellular and physiological functions of the cannabinoid receptors. Apart from an updated review of pre-clinical and clinical studies, the adequacy/inadequacy of cannabinoid-based therapeutics in various pathological conditions is also highlighted. Finally, alternative strategies to modulate endocannabinoid tone, and future directions are also emphasized.
Keywords: endocannabinoid system, neurological diseases, cardiac functions, metabolic diseases, immune functions
1. Introduction
Discovery of the Endocannabinoid System
The therapeutic potential of cannabis, commonly known as marijuana, has been a subject of great interest for several centuries. Its anxiolytic and euphoric properties were acknowledged in religious scriptures that date back to several millennia [1]. Many cultures and civilizations have used cannabis preparations to treat a variety of ailments, ranging from rheumatism, inflammation and malaria for several millennia [2]. While evidence of therapeutic utility of cannabis was known in Asia and Africa, cannabis was relatively unknown to the western world until the 19th century [3]. The first scientific report on cannabis was published by the Irish physician, William ‘O Shaughnessy, which marked the earliest traces of cannabis globalization. By providing evidence of its therapeutic efficacy and safety for pathological conditions such as infantile convulsions and cholera, he was instrumental in laying the foundation for cannabis research [3]. Pioneering works from the groups of Todd, Adams, and Mechoulam, in the 20th century, led to a better understanding of the chemical makeup of cannabis [4,5,6,7].
The first report of the existence of the brain cannabinoid receptor, termed the cannabinoid receptor type 1 (CB1R), was reported by Howlett’s group in the late 1980s [8]. The discovery of the CB1R was followed by the identification of the second cannabinoid receptor, termed the peripheral cannabinoid receptor (due to the lack of expression in brain) or cannabinoid receptor type 2 (CB2R) by Abu-Shaar’s group [9], and their two endogenous ligands, anandamide and 2-arachidonoylglycerol (2-AG) [10,11,12], by Mechoulam’s and Waku’s groups. The identification of biosynthetic and degradative pathways of endocannabinoids in the following years, make up the classical endocannabinoid system [13,14,15,16,17]. These seminal discoveries laid the foundations for endocannabinoid research. For a detailed account on cannabinoid and endocannabinoid history, please refer to the excellent reviews by Di Marzo [3] and Pertwee [18].
2. Cannabinoid Receptors
2.1. Molecular Architecture
The CB1 gene (CNR1), which was first cloned by Matsuda et al. [19], encodes for the CB1R. The human CNR1 is located on chromosome 6 (6q15, HGNC:2159) [20], and comprises of four exons, with exon 4 described as the main coding exon [21,22]. The mouse and rat CNR1, localized on chromosome 4 and 5 respectively, comprises of a single promoter [23]. The CB1R, a 53 kDa protein, is glycosylated post-translationally resulting in a 64 kDa glycosylated form [24]. The glycosylated fraction supersedes the non-glycosylated fraction in abundance [24]. The CB1R belongs to the Class A G protein coupled receptor (GPCR) subfamily, often regarded as one the most diverse subfamilies of GPCRs in humans [25]. Similar to the other GPCRs in this family, the CB1R comprises of a seven-transmembrane helical domain, extracellular and intracellular loops, an extracellular N terminus and an intracellular carboxy terminal tail [25,26]. The extracellular N terminal region is an important factor in conferring receptor stability and trafficking [27,28]. Although the N-terminal region has been reported to have a minor role in orthosteric binding, the membrane-proximal region of the tail is implicated in the modulation of the binding affinity of allosteric ligands [27,29]. Published crystal structure studies of inverse agonist- and antagonist-bound CB1R further helped to characterize the ligand binding pocket and provided insight into the importance of the extracellular loop, the N-terminal region, and the specific transmembrane helices for docking of agonists [30,31]. Similar to the other GPCRs, the second and the third intracellular loops have been demonstrated to have an important role in both receptor activation and desensitization [32,33], while the carboxy terminal tail mostly regulates desensitization and internal sorting [34,35]. Phosphorylation of serine and threonine residues located in the carboxy terminal region has been shown to regulate receptor desensitization and internalization via interaction with the CB1R interacting protein (CR1P) 1a [36], G protein coupled receptor kinases [34] and arrestins [37]. Interestingly, the carboxy terminal region has also been reported to adopt two amphipathic helical domains, which are functionally capable of affecting receptor signaling, polarity and surface expression [38,39,40,41].
The CB2 gene (CNR2), which was first cloned by Munro et al. [9], encodes for the CB2R. The human CNR2 is located on chromosome 1 (1p36.11, HGNC ID: 2160). Unlike CNR1, both human and mouse CNR2 have been reported to comprise of two separate promoters [42]. The CB2R has a similar structure to the other GPCRs in this class. It comprises of 7 transmembrane domains, N terminus and C terminus, 3 extracellular and intracellular loops, and also an amphipathic cytoplasmic helix [43,44,45]. The CB2R shares 44% of overall sequence identity, and 68% of transmembrane sequence identity, although the sequence identity has been reported to be lower in TM1, TM4 and TM5 [9,46]. Unlike the CB1R, the CB2R does not have a long N-terminal region. Other key differences include an aromatic-rich environment in the TM5 of CB2R, and a lack of phosphorylation site for PKC in the third intracellular loop in CB2R, the latter of which is present in CB1R [46]. The second intracellular loop in combination with the carboxy terminal region plays a pivotal role in CB2R-mediated signal transduction [47].
2.2. Ligands
2.2.1. Endogenous Cannabinoids
Both CB1Rs and CB2Rs are class A, lipid-like GPCRs that are activated by endogenously produced lipophilic ligands [48]. The prototypical endogenous cannabinoids or endocannabinoids are 2-AG and anandamide. 2-AG and anandamide are eicosanoids that are synthesized on-demand from arachidonic acid-containing phospholipids, such as phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylethanolamine (PE), respectively. These ligands have complementary as well as divergent functions [49]. While 2-AG is a full agonist at both CB1Rs and CB2Rs, anandamide is a partial agonist for both receptors. Other lesser-known endocannabinoids or non-classical eicosanoids include, N-acyl dopamine (NADA) and 2-arachidonyl glyceryl ether (noladin ether), both of which bind strongly to the CB1R [50,51]. Additionally, virodhamine was identified to be a full agonist at the CB2R, and have antagonistic activity at the CB1R [52]. Apart from endogenous orthosteric ligands, endogenous allosteric modulators for the CB1R and the CB2R have also been identified. Lipoxin A4 and pepcan 12 are both reported to be positive allosteric modulators of CB1Rs and CB2Rs, respectively [53,54].
2.2.2. Exogenous Cannabinoids
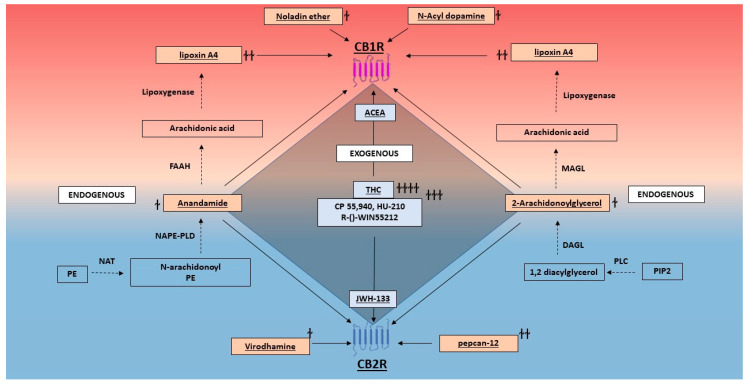
Exogenous cannabinoids comprise of both naturally occurring phytocannabinoids, such as Δ9- tetrahydrocannabinol (THC), and synthetic cannabinoids. THC has a high affinity for both the CB1R and the CB2R. Synthetic cannabinoids such as HU-210, R-()-WIN55212 and CP55940 also display high affinity for both receptors. ACEA, noladin ether, and arachidonylcyclopropylamide display higher affinity for the CB1R when compared to the CB2R, while JWH-133, HU-308, and JWH-133 display higher affinity for the CB2R when compared to the CB1R [55]. For the classification based on chemical structure, please refer to the report by the International Union of Basic and Clinical Pharmacology (IUPHAR) [55]. The various components of the endocannabinoid signaling system, along with endogenous cannabinoid modulators and the exogenous cannabinoid receptor ligands (phytocannabinoids and synthetic cannabinoids) are shown in Figure 1. While this review focusses on mostly the CB1R and the CB2R, it is important to understand that endogenous and exogenous cannabinoids are also capable of interacting with an array of different receptors, channels, and proteins [56,57].
Figure 1.
Overview of endogenous and exogenous cannabinoids that activate/modulate CB1R and CB2R. An overview of CB1R activators and modulators are shown in this figure. This includes endogenous (ɫ,ɫɫ), and exogenous (ɫɫɫ,ɫɫɫɫ) ligands. Examples of endogenous ligands listed here are the endocannabinoids (2-AG, anandamide, N-acyl dopamine, noladin ether and virodhamine (ɫ)); endogenous positive allosteric modulators (lipoxin A4 and pepcan-12 (ɫɫ)); synthetic exogenous cannabinoids for CB1R (ACEA (ɫɫɫ)), CB2R (JWH133 (ɫɫɫ)) and both (CP 55,940, HU-210 and R-()-WIN55212 (ɫɫɫ)); and phytocannabinoids (Δ9-tetrahydrocannabinol (ɫɫɫɫ)). (PE: phosphatidylethanolamine; NAT: N-acetyltransferase; NAPE-PLD: N-acyl phosphatidylethanolamine phospholipase D; FAAH: fatty acid amide hydrolase; PIP2: phosphatidylinositol 4,5-bisphosphate; PLC: phospholipase C; DAGL: diacylglycerol lipase; MAGL: monoacylglycerol lipase; ACEA: arachidonyl-2′-chloroethylamide; THC: Δ9- tetrahydrocannabinol).
2.3. Tissue and Cellular Distribution
Cells of the central and the peripheral nervous system: The CB1R is highly expressed in most regions of the central nervous system (CNS), with densities that rival other neurotransmitter and neuromodulatory receptors [58]. Moderate to high expression of CB1Rs have been observed in the cerebral cortex (cingulate gyrus, prefrontal cortex, and hippocampus), basal ganglia (globus pallidus, substantia nigra), periaqueductal gray, hypothalamus, amygdala, and cerebellum [58]. The CB1R expression is relatively sparse in the medullary respiratory control centers of the brainstem. However, the CB1R is highly expressed in brainstem medullary nuclei, such as the nucleus of the solitary tract and area postrema, serving as the primary integrative centers for the cardiovascular system and emesis, respectively. Moderate CB1R expression was also confirmed in the spinal cord (dorsal horn and lamina I, III, and X) [58,59,60]. More recently, dense CB1R positive fibers were identified in the ventral horn [61]. Apart from the CNS, CB1R expression was reported in the somatic, sympathetic, parasympathetic, and the enteric nervous systems [61,62,63,64,65,66,67,68,69,70].
Neuroanatomical studies identified the CB1R to be primarily located in the presynaptic terminals of GABAergic (amygdala and cerebellum) [71], glutamatergic (cortex, hippocampus and amygdala) [71,72,73], dopaminergic [71], GABAergic interneurons [74,75], cholinergic neurons [76], noradrenergic [77], and serotonergic neurons [78]. Low levels of presynaptic CB1R were also detected in the nociceptive primary afferent fibers in the spinal cord [79]. In addition to neuronal cells, CB1Rs were also identified in the perivascular and perisynaptic astroglial processes [72,80] and oligodendrocytes [81]. Apart from astrocytes, the CB1R was also identified in other cells of the blood brain barrier (BBB) such as the brain endothelial cells [82], pericytes, and vascular cells [83]. Apart from the glial cells in the CNS, the CB1R was also observed in myelinating Schwann cells of the peripheral nervous system [61]. The CB2R on the other hand is expressed at very low levels in the CNS under physiological conditions. However, pathological conditions characterized by a neuroinflammatory state resulted in an upregulation of CB2R levels in glial cells, such as microglia [84,85,86,87].
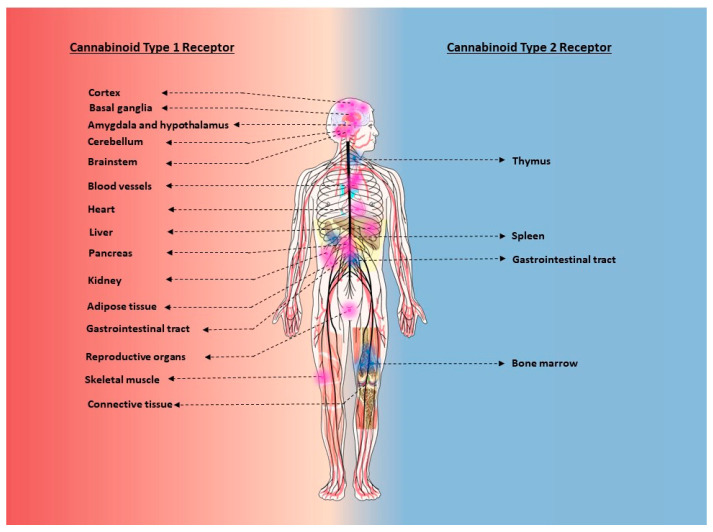
Non-neuronal cells: The CB1R has also been reported in the peripheral organ systems of the body, albeit at lower, but functional levels [88,89]. Functional CB1R have been reported in the liver [90], muscle [91], adipose tissue [92], vasculature [93,94], heart [95,96], pancreatic beta cells [97], reproductive organs [98], and alveolar cells [99]. The CB2R however is expressed in high levels in immune cells and in lymphoid tissues. Cells that participate in both innate and adaptive immune response, such as the spleen, thymus, and peripheral blood mononuclear cells, are known to express high levels of CB2R [45,100]. Interestingly, under pathological conditions, various peripheral cell types have also been shown to express detectable levels of the CB2R. This includes activated hepatic stellate cells [101] and renal cells from fibrotic kidney [102], and lung tissue from rats subjected to chemical-induced lung injury [103]. However, there have been reports of detectable levels of the CB2R under normal physiological conditions in pancreatic acinar cells [104], adipocytes [105], skeletal muscle cells [106], cardiomyocytes, and endothelial cells [107]. Additionally, both CB1R and CB2R have been detected in connective tissue such as fascial fibroblasts and osteoclast cells [108,109]. An overview of tissue distribution of the CB1R and the CB2R in healthy conditions is shown in Figure 2.
Figure 2.
Tissue distribution of CB1R and CB2R under normal physiological conditions. The figure shows tissue expression of CB1R and CB2R under healthy conditions. Although CB1R protein or transcripts have been identified in several non-neural tissues, they are predominantly localized in the central nervous system (CNS). On the other hand, the CB2R is predominantly found in tissues involved in immune regulation. It is sparsely distributed in the CNS under normal physiological conditions.
2.4. Signaling
2.4.1. Canonical
Inhibitory G protein-coupled and β-arrestin signaling: Classically, the CB1R couples to the pertussis toxin (PTX)-sensitive G-protein (Gαi/o), which results in the inhibition of forskolin-stimulated cAMP, activation of G-protein coupled inwardly rectifying potassium channels (GIRKs), and an inhibition of several calcium channels [110,111,112,113]. G protein-mediated signaling in the CB1R is followed by the recruitment of β-arrestins 1 and 2, which play a role in receptor internalization and signaling [114,115]. G-protein-coupled receptor kinases along with β-arrestin 2 have been shown to trigger receptor desensitization, internalization, and G-protein signal termination, while β-arrestin 1 is involved in activation of mitogen-activated protein kinases (MAPKs) and also regulation of gene expression [116,117,118,119]. Both β-arrestin 1 and 2 have also been implicated in ERK1/2 activation [120]. MAPKs such as ERK1/2, p38, and c-Jun N-terminal kinase (JNK) were shown to be activated in response to CB1R agonists in a variety of cell types [121,122,123]. Additionally, the CB1R activation was also shown to activate the PI3K/AKT pathway resulting in the regulation of neuronal survival [124]. Activation of MAPK, JNK, and PI3K/AKT pathways via the CB1R have all been linked to induction of several transcription factors such as Krox-24 [121], CREBH [122], and BDNF [124]. For a detailed review on the CB1R cell signaling, please refer to the review by Turu and Hunyady [125].
Similar to the CB1R, the CB2R is also a GPCR that couples to the inhibitory G-protein. Stimulation of CB2R has also been shown to activate MAPKs and subsequent induction of Krox-24 expression [126]. Additionally, the CB2R has also been shown to promote neuronal survival through the activation of the PI3K/AKT and JNK pathway [127]. Also, CB2R activation results in significant β-arrestin 2 recruitment and β-arrestin 1-mediated MAPK activation [128,129].
2.4.2. Non-Canonical
Stimulatory G protein coupled signaling: While the CB1R has been reported to preferentially couple to Gαi/o type G proteins, some endogenous and synthetic cannabinoids, have been observed to enhance CB1R-mediated coupling to stimulatory G proteins such as Gαq/11 and Gαs [130,131]. Interestingly, stimulated CB1R was shown to access two distinct G protein pools, resulting in biphasic ERK 1/2 activation in primary neuronal cultures [132]. Additionally, the exogenous synthetic cannabinoid WIN55,212-2, has also been reported to enhance calcium levels via a CB1R-Gq-PLC pathway in both HEK293 cells and in cultured hippocampal neurons [130]. Furthermore, endocannabinoids have also been observed to elevate calcium levels and glutamate release via activation of astrocytic CB1Rs [133]. As a result, calcium spikes in response to astroglial CB1R stimulation can have profound consequences on the modulation of synaptic strength. The role of astroglial CB1R in the regulation of synaptic activity is described in a later section. A biphasic ERK 1/2 activation [123], and an elevation in calcium levels via a Gi independent pathway [134], were also observed in other cell types.
Similar to the CB1R, there have been reports on the ability of the CB2R to elevate calcium levels via a PLC pathway in endothelial cells [135], and insulinoma beta-cells [134], suggesting a Gi-independent coupling route. Intracellular administration of CB2R agonists, in CB2R-transfected U2OS cells, triggered dose-dependent activation of calcium responses which was Gq-mediated [136].
Crosstalk: Evidence of crosstalk between the CB1R and other GPCRs was identified by multiple studies. Co-stimulation of the CB1R and dopamine type 2 receptor (D2R) resulted in extensive colocalization, dimerization, and an increase in cAMP levels, suggesting a potential change in signal transduction profile [137]. Heterodimers between CB1Rs and D2Rs, independent of receptor occupancy, was also observed in transiently transfected cells [138]. Further evidence for an interplay between CB1Rs and D2Rs was demonstrated in striatal neurons, whereby a D2R antagonist was able to hinder CB1R agonist-mediated phosphorylation of ERK1/2 [139]. Additionally, the existence of dimers between the CB1R and the Adenosine (A2A) receptors [140], and also heterooligomers with CB1R- A2A-D2R [141], were also reported in transfected cells. At a functional level, blockade of A2A receptors was shown to attenuate the CB1R-dependent motor depressant effects, suggesting possible interdependency between the two receptors [140]. Similar functional interdependency was observed between the CB1Rs and the orexin receptors, whereby their co-expression in a heterologous cell line resulted in potentiation of orexin-mediated MAPK activity [142]. However, functional antagonism between the CB1R and other GPCRs has also been reported. Co-stimulation of the CB1R and the μ opioid receptors resulted in attenuation of STAT3 phosphorylation and neuritogenesis in Neuro-2A cells [143]. Reciprocal inhibition has also been reported in the hippocampus between CB1Rs and GABA-b receptors [144]. This type of reciprocal inhibition was also observed in astrocytes from our laboratory, whereby co-stimulation of astrocytes with Angiotensin (Ang) II and the CB1R agonist, resulted in significantly reduced MAPK activation when compared to Ang II alone [123]. However, the results presented in this study differ from another where potential heterodimerization between AT1R and CB1R was observed [145]. In this study, co-treatment of Ang II with the CB1R agonist, HU-210, led to an increase in AT1R-mediated activation of ERK1/2 in a neuroblastoma cell line, suggestive of a possible synergism between the two receptors [145]. In the brain, there is evidence of CB1R involvement in AT1R-mediated elevation in blood pressure. An increase in mean arterial pressure from the administration of Ang II into paraventricular nucleus (PVN) of Wistar Kyoto (WKY) rats was blunted by simultaneous infusion of the CB1R inverse agonist, AM251 [146]. This however is indicative of a potentiation of AT1R-mediated effects by CB1R in the CNS. A detailed understanding of the molecular signaling of central and peripheral AT1Rs in pathophysiology and possible crosstalk with CB1R, is beyond the scope of this review. Please refer to reviews from Haspula and Clark [147] and Forrester et al. [148] for a better understanding of the AT1R signaling mechanisms.
The CB2R has also been reported to heterodimerize with the CB1R in co-transfected neuronal cells, and co-stimulation was demonstrated to result in negative AKT phosphorylation and neurite outgrowth [149]. They also exhibited cross-antagonism, whereby CB2R antagonists were demonstrated to block the CB1R-mediated effects, and vice-versa [149]. Additionally, the CB2R has been reported to crosstalk with other receptors. The CB2R-selective ligand, SR 144528, was demonstrated to inhibit downstream signaling of the insulin and lysophosphatidic acid receptors in Chinese hamster ovary (CHO) cells transfected with recombinant CB2R [150]. Additionally, evidence of the CB2R heterodimerizing with the C-X-C Motif Chemokine Receptor 4 (CXCR4) has been observed in metastatic cancer cell lines, resulting in diminished functions of CXCR4 receptor and limited tumor cell migration and progression [151,152].
Transactivation: The interaction between the CB1R and other GPCRs is not limited to receptor dimerization. The concept of paracrine transactivation of the CB1R by Gq GPCRs, was identified in non-neuronal cells by the Hunyady group. In CHO and other commercial cell lines, the CB1R was demonstrated to be transactivated, both in an autocrine and paracrine fashion, by Ang II via the AT1R-Gq-DAGL axis [153,154]. Interestingly, the CB1R could also be transactivated by other Gq GPCRs as well [154]. Studies from the same group also showed an augmentation of AT1R-mediated vasoconstriction by Ang II in the presence of a CB1R antagonist [89]. Interestingly, cannabinoids have also been shown to transactivate other receptors, such as epidermal growth factor receptor (EGFR) and tropomyosin receptor kinase B, resulting in cancer cell proliferation [155], interneuron migration and cortical development [156], and corneal epithelial cell proliferation [157]. For a comprehensive understanding of paracrine transactivation of CB1R by other GPCRs, please refer to the review by Gyombolai [146].
Biased signaling: Biased signaling refers to the propensity of a ligand to preferentially activate a distinct signaling pathway over others. This was observed for both exogenous and endogenous cannabinoids at multiple downstream steps following CB1R stimulation; that is at the level of G protein signaling, cAMP signaling, MAPK activation, and calcium mobilization. For instance, anandamide demonstrated a greater ability to inhibit cAMP and activate ERK1/2 signaling when compared to 2-AG [158]. The endogenous cannabinoid N-arachidonoyl dopamine, which acts as an agonist for both the CB1R and the transient receptor potential cation channel subfamily V member 1 (TRPV1) [159], displayed high functional selectivity for calcium mobilization via Gq protein signaling, while remaining relatively ineffective at activating other canonical signaling pathways [160]. Exogenous cannabinoids such as CP55940, THC, and HU-210 displayed a greater ability, when compared to WIN55,212-2, to inhibit cAMP over ERK1/2 activation [158]. The ability of both endogenous and exogenous cannabinoids to activate distinct signaling pathways and cellular processes could also be attributed to a selection bias for either G-protein or β-arrestin-induced signaling. Neuronal CB1R stimulation by exogenous cannabinoids, THC and CP55,940, triggered significant β-arrestin 2 recruitment, while WIN55,212-2 showed greater preference for Gαi/o and Gβγ signaling [131]. Among the endocannabinoids tested in the same study, 2-AG showed greater preference toward β-arrestin 2 binding, when compared to anandamide [131]. In a cell culture model of Huntington’s disease, 2-AG and AEA demonstrated greater preference to G-protein signaling, while CP55,940 and THC had greater preference for β-arrestin 1 recruitment and reduced CB1R protein levels [161]. Another study observed a high dependence of β-arrestin 1 on the activity of CP55940, but not THC, in both in vitro and in vivo assays [162]. This suggests that functional selectivity of the ligands may be dependent on the cell type and pathophysiological alterations.
Similar to the CB1R, functional selectivity at the CB2R for both endogenous and exogenous cannabinoids was also demonstrated by multiple studies. 2-AG via the CB2R induces a greater degree of ERK1/2 activation, when compared to noladin ether as well as CP55,940 [163]. However, noladin ether and CP55,940 demonstrated greater potency in the inhibition of adenylyl cyclase, when compared to 2-AG [163]. Exogenous cannabinoids such as CP55,940 and WIN55,212-2 exhibited differences with regards to CB2R internalization and inhibition of voltage-gated calcium channels, in spite of ERK1/2 phosphorylation and β-arrestin 2 recruitment [128]. Additionally, in a study profiling functional selectivity of a wide range of CB2R agonists, THC and 2-AG were found to display a strong signaling bias for the CB2R, displaying a significantly greater preference for the activation of their most-preferred pathway [164]. Additionally, SR144528, which is an antagonist for the CB2R, displayed greater efficacy at blocking cAMP signaling when compared to other signal transduction pathways [164]. For a detailed overview of functional selectivity of CB2R signaling, please refer to the review by Dhopeshwarkar and Mackie [165].
Constitutive activity: A constitutively active receptor assumes an active state, initiating downstream signaling, even in the absence of ligand stimulation. High basal signal for the CB1R was not only observed in cells expressing the recombinant form of the receptor, but also seen in cells expressing the native receptor [166,167,168,169,170]. Further evidence supporting this view comes from the subcellular localization of CB1R under baseline conditions. High levels of CB1Rs have been observed to be localized intracellularly in various subcellular compartments, including lysosomes and endosomes [171,172,173]. Considering that a constitutively active CB1R may be involved in maintaining homeostasis, utilizing neutral antagonists over inverse agonists were found to be beneficial in preclinical studies [174,175].
Apart from the CB1R, the CB2R was also reported to exhibit constitutive activity in cells expressing recombinant as well as native receptors [150,176,177]. CB2R has been observed to be located intracellularly in neuronal cells from rat prefrontal cortex [178], and in endolysosomes in CB2R-transfected U2OS cells [136].
Heterologous desensitization: Mackie’s group identified that PKC activators can trigger phosphorylation of CB1Rs at single serine residue (S317) in the third intracellular loop, resulting in the attenuation of receptor activity [179]. Since Gq GPCRs can activate PKC, our laboratory investigated whether other Gq GPCRs, such as the Ang AT1R, could trigger an increase in CB1R phosphorylation at the exact same residue. We observed that Ang II via the AT1R is capable of inducing phosphorylation of CB1Rs at the third intracellular loop, thereby potentially triggering desensitization of the receptor [123]. This was most prominent in the cerebellum [123]. It would be interesting to see if other Gq GPCRs could also trigger phosphorylation of the CB1R via this mechanism.
Intracellular signaling: While both CB1Rs and CB2Rs are predominantly located in the cell membrane, there is evidence of intracellular localization and signaling of CB1R in various organelles such as endocytes, mitochondria, and the nucleus as mentioned previously [171,172,173,180]. Since endocannabinoids are lipophilic, they can traverse the cell membrane and interact with their receptors that are localized on various intracellular organelles and compartments.
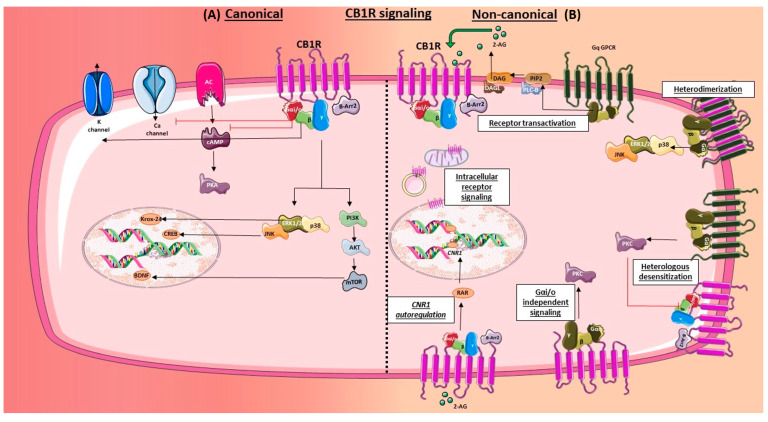
Autoinduction: GPCRs usually undergo receptor downregulation in response to persistent activation of the receptor by ligands. The CB1R is similar in this aspect and induction of cannabinoid tolerance is often a biological consequence of exogenous cannabinoid induced CB1R downregulation. However, 2-AG was demonstrated to increase CB1R transcription via CB1R-mediated retinoic acid receptor activation in hepatocytes [181]. Interestingly, acute treatment of both exogenous and endogenous cannabinoids has also been implicated in elevating CB1R mRNA in a variety of cell types and tissues [182,183]. Endocannabinoids released from astrocytes were also elevated by the activation of the astroglial CB1R by cannabinoids [184]. Interestingly, cannabinoids have also been shown to increase the CB1R gene expression via CB2R [185]. An overview of the canonical and the non-canonical signaling is described as a schematic in Figure 3.
Figure 3.
Canonical and non-canonical signaling of CB1R. (A) Canonically, CB1R activation results in coupling of pertussis toxin (PTX)-sensitive G-protein (Gαi/o), and the activation of GIRK and inhibition of calcium channels. CB1Rs also activate MAPKs, such as ERK1/2 and JNK, which result in the subsequent induction of Krox-24 and CREB respectively. In addition, CB1R stimulation also leads to the downstream activation of PI3K/AKT/mTOR pathway, which further results in the transcription of BDNF. (B) The CB1R is also involved in non-canonical signaling. Gαq/11 GPCR-mediated-mobilization of endocannabinoids, and subsequent activation of the CB1R in an autocrine or paracrine fashion (Receptor transactivation). The CB1R has also been shown to dimerize with other GPCRs resulting in a change in CB1R-mediated signaling, such as changes in MAPK activation patterns (Heterodimerization). Additionally, GPCRs that activate PKC, such as Gαq/11 GPCRs, have also been shown to phosphorylate CB1R and potentially dampen its activity in certain cell types (heterologous desensitization). Certain cannabinoids have been shown to couple to Gαq/11 and Gα(s) proteins, and effectively activate calcium channels (Gαi/o independent signaling). In addition to being membrane-bound, functional CB1R has also been reported to localize intracellularly, such as the nucleus and the mitochondria, where they are capable of signaling (intracellular signaling). Finally, cannabinoids have been shown to trigger an induction of CNR1 by the activation of CB1R in various cell types (autoinduction). (AC: adenylyl cyclase; cAMP: cyclic adenosine monophosphate; PKA: protein kinase A; β-Arr2: Beta-arrestin-2; ERK1/2: extracellular signal-regulated kinases; JNK: c-Jun N-terminal kinases; PI3K: phosphoinositide 3-kinases; AKT: protein kinase B; mTOR: mammalian target of rapamycin; PIP2: phosphatidylinositol 4,5-bisphosphate; PLC-β: phospholipase C beta; DAG: diacylglycerol; DAGL: diacylglycerol lipase; 2-AG: 2-arachidonoylglycerol; RAR: retinoic acid receptor; CNR1: cannabinoid receptor 1 gene).
3. Biological Role of the Cannabinoid Receptors
The previous sections described the molecular architecture of the cannabinoid receptors, their localization and distribution, and their cellular signaling mechanisms. The ubiquitous nature of cannabinoid receptors lends itself to regulate a variety of cellular and physiological processes. From regulation of cellular functions, such as neuromodulation, to orchestrating complex metabolic and immune responses, cannabinoid receptors serve essential role in both physiological and pathological conditions. Since the endocannabinoid system is vital for homeostasis of several biological processes, several pathological conditions pertaining to cardiovascular, neurological, metabolic and immunological diseases, are often associated with alterations of endocannabinoid tone. This section describes the roles of cannabinoid receptors in various biological processes, and the pathological conditions associated with changes in endocannabinoid tone. This includes results from both pre-clinical and clinical studies. For a complete list of recently published clinical studies (2010–2020), pertaining to the aforementioned pathological conditions, please refer to Table 1.
Table 1.
Systematic review of recent * clinical studies reporting cannabinoid receptors as biomarkers, and/or investigating the efficacy of cannabinoids in pathological conditions.
| Subjects and Measurement Indices | Study Design | CB receptors as Biomarkers | Outcome of Pharmacological Intervention | Reference |
|---|---|---|---|---|
| Measurement of CB1R density in brown adipose tissue in lean and obese healthy males | Non-randomized, crossover clinical trial | CB1R upregulation in obese individuals | CB1R blockade increased lipolysis | [186] |
| Determination of the effect of dronabinol in gut transit in irritable bowel syndrome with diarrhea. | double-blind, randomized, placebo-controlled, parallel-group study | CNR1 rs806378 CT/TT is associated with a delay in colonic transit compared with CC | Dronabinol delays transit in individuals with CNR1 rs806378 CT/TT | [187] |
| Impact of Sativex on CNR1 and CNR2 expression in peripheral blood mononuclear cells (PBMCs) in patients with multiple sclerosis (MS) secondary progressive (MSS-SP). | Controlled clinical trial | - | Significant decrease in CNR2 expression | [188] |
| Measurement of CB1R density in the brains of schizophrenic individuals with or without antipsychotic medication | prospective study | Increased CB1R binding in mesocorticolimbic regions of individuals with schizophrenia | - | [189] |
| Measurement of CB1R density in the brains of pre- Huntington disease mutation carriers | prospective study | Decrease in CB1R density in prefrontal cortex compared to controls | - | [190] |
| Determination of whether hypocaloric diet and/or aerobic exercise alters subcutaneous adipose tissue CB1R and FAAH expression in obese women | Randomized clinical trial | Caloric restriction alone lowered gluteal CB1R and FAAH, while both caloric restriction plus aerobic exercise reduced abdominal adipose tissue FAAH gene expression | - | [191] |
| Determination of whether exercise training resulted in changes in muscle CB1R and TRPV1 expression in heart failure patients | Randomized controlled trial | Exercise training significantly increased gene expression of the TRPV1 receptor and the CB1R | - | [192] |
| Impact of single nucleotide polymorphism rs3123554 in CNR2 on metabolic and adiposity parameters in obese induvial on two hypocaloric diets | Randomized controlled trial | Individuals that are carriers for CNR2 genetic variant loose less body weight. | - | [193] |
| Determination of whether CNR2 gene variation rs35761398 (Q63R) is significantly associated with chronic idiopathic thrombocytopenic purpura (ITP) in children | Case-control association study | CNR2 gene variation is significantly associated with childhood chronic ITP | - | [194] |
| Impact of interferon therapy on CB1R and CB2R gene expression in immune cells from patients on interferon therapy | Controlled trial | Reduction in both CB1R and CB2R after interferon therapy | - | [195] |
| Impact of Sativex on the clinical improvement of motor, cognitive and psychiatric measures in patients with Huntington’s Disease. | double-blind, randomized, cross-over, placebo-controlled, pilot trial | - | No improvement in motor, cognitive, and behavioral functional scores when compared to the placebo | [196] |
| Impact of pirfenidone on CB1 and CB2 gene expression in liver biopsies, in individuals with chronic hepatitis C | Open-label, non-controlled, and non-randomized clinical trial | - | Significant upregulation of CNR2, while no statistical difference in CNR1. | [197] |
| Determination of the effects of isocaloric low and high-fat diets on endocannabinoid system in obese individuals | randomized cross-over study | Reductions in skeletal muscle CB1R in high fat diet group | - | [198] |
| Measurement of VEGF and cytokines in sera of obese PCOS women in response to rimonabant. | Randomized open-labelled parallel study | - | CB1R blockade raises VEGF and the pro-inflammatory cytokine IL-8 in obese women with PCOS | [199] |
| Measurement of insulin-like growth factor I levels in women with anorexia nervosa (AN) in response to dronabinol | Prospective, double-blind randomized crossover study | - | Dronabinol affected neither the concentration nor the activity of the circulating IGF-system in women with severe and chronic AN. | [200] |
| Measurement of skin conductance response to determine whether dronabinol facilitates fear extinction learning in healthy individuals | Randomized, double-blind, placebo-controlled trial | - | Dronabinol facilitates extinction of conditioned fear in humans | [201] |
| Measurement of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) responses during oral glucose tolerance test (OGTT) in the plasma of lean and obese participants | Randomized, double-blind, crossover, placebo-controlled trial | - | CBR agonist increased circulating GIP levels, but not GLP levels, in the fasting (nonstimulated) state. | [202] |
| Measurement of body weight and HbA1c in obese and overweight individuals with and without diabetes in response to CB1R antagonist, CP-945,598. | double-blind, placebo-controlled trial | - | CP-945,598 resulted in a reduction in body weight and better glycemic control. | [203] |
| Impact of smoked medicinal cannabis on appetite hormones ghrelin, leptin and PYY in individuals with HIV-associated neuropathic pain | double-blind cross-over, placebo-controlled trial | - | Medical cannabis resulted in significant increases in plasma levels of ghrelin and leptin, and decreases in PYY | [204] |
| Impact of rimonabant on improving the risk of cardiovascular death, myocardial infarction, or stroke in individuals with cardiovascular risk factors or prior cardiovascular events. | Randomized, double-blind, placebo-controlled trial | - | Termination of trial due to neuropsychiatric effects | [205] |
| Impact of CB1R inverse agonist, taranabant, for maintenance of prior weight loss achieved on a low-calorie diet | Randomized, double-blind, placebo-controlled trial | - | Improvement in weight loss with taranabant, compared to maintenance therapy alone | [206] |
| Impact of CB1R inverse agonist, taranabant, on weight loss in obese and overweight patients | Randomized, double-blind, placebo-controlled trial | - | Taranabant treatment resulted in statistically significant weight loss | [207] |
| Impact of CB1 neutral antagonist tetrahydrocannabivarin on resting state functional connectivity in key brain regions relevant to development of obesity, in healthy individuals | Randomized, within-subject, double-blind, placebo-controlled trial | - | Alteration in resting state functional connectivity without significant effects on mood. | [208] |
| Impact of CB1 neutral antagonist tetrahydrocannabivarin on activation of brain regions involved in food aversion in healthy individuals | Randomized, double-blind, placebo-controlled trial | - | Treatment increased neural responses to aversive stimuli | [209] |
| Impact of CB1/CB2 receptor agonist KN38-7271 on survival rates following head injury | Randomized, double-blind, placebo-controlled phase II trial | - | Improved survival rates in the early phase of the comatose patient after a head injury | [210] |
| Impact of peripherally acting CB1/CB2 receptor agonist, AZD 1940, on capsaicin-evoked pain and hyperalgesia | Randomized, double-blind, placebo-controlled | - | No evidence of analgesic efficacy in the human capsaicin pain model. | [211] |
| Impact of oral dronabinol on the progression of primary and secondary progressive multiple sclerosis in patients with prior diagnosis | Randomized, double-blind, placebo-controlled study | - | No significant effect on the progression of multiple sclerosis in the progressive phase | [212] |
| Impact of oral dronabinol on altering pain threshold in individuals diagnosed with functional chest pain (FCP) | Randomized, double-blind, placebo-controlled study | - | Pain threshold was increased, and the frequency and intensity of pain was reduced, in FCP | [213] |
| Impact of rimonabant on changes in liver fat in individuals with metabolic syndrome | Randomized, double-blind, placebo-controlled trial | - | Reduction in liver fat is proportional to weight loss | [214] |
| Impact of rimonabant on carotid atherosclerosis in obese individuals | Randomized, double-blind, placebo-controlled trial | - | No significant effect on the progression of atherosclerosis | [215] |
| Impact of rimonabant on body weight in obese patients with binge eating disorders | randomized, double-blind, placebo-controlled study | - | Reduction in body weight when compared to placebo group | [216] |
| Impact of rimonabant on neurocognitive impairments in individuals with schizophrenia | randomized, double-blind, placebo-controlled study | - | Improvement on a probabilistic learning task, with no improvement in global cognitive functioning | [217] |
| Impact of rimonabant on fatty acid and triglyceride metabolism and insulin sensitivity after controlling for metabolic effects of weight loss in obese women | Randomized controlled trial | - | Increased lipolysis and fatty acid oxidation without any effect on insulin sensitivity | [218] |
| Impact of rimonabant on insulin regulation of free fatty acid and glucose metabolism, after controlling for weight loss, in obese, metabolic syndrome individuals | randomized, double-blind, placebo-controlled study | - | Improvement in insulin regulation of free fatty acids and glucose metabolism was due to weight loss | [219] |
| Treatment of dementia-related neuropsychiatric symptoms (NPS) in response to low-dose oral THC | Prospective study | - | low-dose THC does not significantly reduce NPS | [220] |
* Clinical trials pertaining to the endocannabinoid system in cardiovascular, neurological and metabolic disorders from 2010–2020, are listed in this table.
3.1. Cellular Morphogenesis and Developmental Roles
3.1.1. Embryogenesis
Embryogenesis refers to the formation of a multicellular embryo from a single cell zygote via series of processes that include division, differentiation, and cell fate specification. Although evidence of endocannabinoid synthesis can be traced back to unicellular organisms [221], the origins of the CB1R closely parallels the evolution of multicellular organisms [222]. This could well be indicative of their importance in cell differentiation and specialization of functions. Both the CB1R and the CB2R were detected prior to the blastocyst stage, and cannabinoid agonists were demonstrated to arrest the growth of an embryo in culture [223]. Elevated levels of the CB1R were also observed in the blastocyst, particularly in the trophectoderm [223,224], and implantation was shown to be mediated by the CB1R and not the CB2R [225]. Furthermore, a greater mRNA expression of CB1R when compared to the CB2R highlights the importance of CB1R signaling in embryogenesis [226]. Additionally, there have been a few reports of a possible involvement of the CB2R during various stages of embryonic development. While both CB1R and CB2R transcripts were identified in the human trophoblast during the first trimester, in vitro experiments demonstrated that anandamide prevented the proliferation of BeWo trophoblast cells via the CB2R [227]. In a recent study using zebrafish embryos, it was demonstrated that blocking CB2Rs during early development resulted in a greater inhibition of heart rate than that observed with a CB1R blocker [228]. Additionally, both CB1Rs and CB2Rs were also reported in embryonic stem cells (ESC) [229,230]. They were also implicated in increasing cell viability in embryoid bodies (EB) [231]. Increased survival of EB serves as a critical preliminary step for ESC differentiation.
3.1.2. Neurodevelopment
Functional CB1Rs were detected in the fetal rat brain as early as gestational day (GD)-14 [232]. Additionally, mRNA transcripts for CB1Rs were also reported in the subventricular zone at GD-21, highlighting its importance in neuronal and glial cell generation in the developing CNS [232]. Apart from critical roles in early neurodevelopment, the CB1R was also shown to regulate neuronal differentiation from embryonic cortical neuron progenitor cells, and regulation of neurogenesis in adult hippocampus [233]. Knockout studies further confirmed the vital role of CB1Rs in adult neurogenesis [234] and neurosphere formation [235]. HU210, a synthetic cannabinoid, was also shown to promote neurogenesis in the hippocampal dentate gyrus [236]. Apart from neurogenesis and neuronal differentiation, the CB1R was also reported to play critical roles in synaptogenesis, pathfinding, and network formation [237,238,239], possibly because of elevated expression in axonal growth cones. The CB1R also regulates neural progenitor cell fate decisions, as evidenced by its involvement in the differentiation of neural progenitor cells toward an astroglial lineage [240]. Interestingly, both CB1Rs and CB2Rs have been shown to regulate oligodendrocyte progenitor cells function, via a PI3K/AKT pathway, whereby their activation exerts a protective role [81,241]. Although the role of CB2R in CNS development is not as well studied, there is evidence of its involvement in neural progenitor cell proliferation, and possibly in neurogenesis [242].
3.1.3. Implications of a Dysregulated Endocannabinoid Tone in Neurodevelopmental Disorders
Cognitive defects associated with developmental abnormalities: Multiple studies have linked alteration in cannabinoid signaling during early developmental stages to various cognitive defects, suggesting a link between defective endocannabinoid signaling and various developmental disorders [243,244,245]. Dysregulated CB1R signaling was reported in a mouse model of fragile X syndrome (fmr1 knockout), a disorder characterized by intellectual and developmental disabilities [246]. Although another study reported unchanged levels of CB1Rs in mice lacking fmr1, an increased glutamate receptor-mediated mobilization of endocannabinoids was reported [247]. Additionally, genetic and pharmacological blockade of CB1Rs in mice lacking fmr1 was demonstrated to improve cognitive impairment [246,247,248]. Apart from protein expression, CNR1 was also reported to be significantly downregulated in postmortem brains of individuals with autism [249]. Interestingly, indirect lines of evidence highlight CNR1 variants to be a key genetic factor in contributing to differences in striatal response to various emotions [250,251].
ADHD: Genetic variants of CNR1 were also associated with neurobehavioral disorders such as attention deficit hyperactivity disorder (ADHD) [252]. In vitro experiments demonstrated that cannabinoid administration resulted in a reduction in hyperactivity in juvenile spontaneously hypertensive rats (SHRs), a rat model of ADHD [253,254]. Reduced expression of CB1Rs was also observed in the prefrontal cortex of SHRs, suggesting potential hypoactivation of the CB1R [254]. Evidence from case studies also suggests an improvement in ADHD symptoms in response to cannabis and cannabinoids consumption [255,256].
Schizophrenia: Several studies found a strong association of cannabis use to schizophrenia [257,258]. Additionally, evidence of altered emotional status and impulsive behavior in adolescents, due to cannabinoid exposure, has also been reported [259,260]. Intriguingly, by employing radiotracer binding and mRNA assays, altered CB1R expression was also reported in postmortem brains of schizophrenic subjects, that were independent of cannabis use [261,262,263,264]. Quantitative PET imaging techniques also revealed a strong association between changes in CB1R levels in specific brain regions and schizophrenia symptoms [265]. Additionally, in vitro experiments revealed that the temporal changes in CB1R expression in adults coincided with the onset of schizophrenia symptoms, further highlighting the involvement of the endocannabinoid system in schizophrenia [266]. Preclinical studies also established the utility of CB1R blockade as a stand-alone therapy, or in conjunction with an established antipsychotic in the treatment of cognitive defects induced by psychotomimetics in rats [267,268]. Considering that rimonabant, a CB1R antagonist, is discontinued because of neuropsychiatric adverse effects, alternative strategies should be devised to reduce endocannabinoid tone in the treatment of neurodevelopmental disorders such as schizophrenia.
3.2. Neurological
3.2.1. Neuromodulation
The ability of the CB1R to fine-tune and regulate GABAergic synaptic transmission via retrograde signaling [269,270,271], known as depolarization induced suppression of inhibition (DSI), remains one of the cornerstones in endocannabinoid research. DSI is the retrograde transmission of messenger signals towards the pre-synaptic cleft resulting in GABAergic synaptic inhibition [272]. Excessive postsynaptic receptor activation results in mobilization of endocannabinoids into the synaptic cleft. They can then traverse the synaptic cleft, from the postsynaptic neuron to the presynaptic neuron, where they activate the CB1R. Activation of CB1Rs attenuates neurotransmitter release into the synaptic cleft, resulting in dampened synaptic activity. This ‘circuit-breaker’ like functionality is a crucial modus operandi of the CB1R by which it influences synaptic plasticity [273]. In addition to dampening the activity of inhibitory neurons, the CB1R is abundantly expressed on presynaptic glutamatergic neurons [71,72]. Hence it can also suppress excitatory neuronal activity [274], thereby affecting the two arms of short-term plasticity. Activation of metabotropic glutamate receptors results in mobilization of endocannabinoids and inhibition of synaptic transmission [275,276]. Additionally, CB1R activation regulates the induction of long-term synaptic depression (LTD) in the nucleus accumbens [277], cerebellum [278], and prefrontal cortex [279]. It also regulates long term potentiation (LTP) in the hippocampus [280]. However, the action of endocannabinoids on LTP is controversial since there has been reports showing that they restrict LTP in the hippocampus [281,282,283], as well as have a dual role on LTP strengthening [284]. CB1R-mediated activation of mTOR and subsequent induction of the protein synthesis machinery is attributed to its modulation of long-term memory [285]. While CB1R-mediated transient response is attributed to inhibiting the activity of calcium channels and activating GIRK, changes in reduction of cAMP/PKA signaling and RIM1α, along with changes in protein synthesis are some of the mechanistic changes linked to a sustained and long term modulation of synaptic activity [273,286,287,288]. For a comprehensive overview of the various mechanisms that govern CB1R-mediated induction of long-term synaptic plasticity, please refer to the review by Heifets and Castillo [289]. CB1R’s widespread distribution in the brain, in conjunction with the ability to affect long term changes in synaptic strengthening, makes the CB1R a critical player in cognition, memory, behavior, and learning.
Relevant to neuronal plasticity, is the ability of astrocytes to communicate with neurons through mobilization of calcium and neurotransmitters [290]. The concept of tripartite synapse first introduced by Araque et al. introduces astroglia as a potential third signaling cell together with the pre- and post-synaptic neuronal cells [291]. Endocannabinoids released from neurons could then activate distant neurons by activating the astroglial CB1R, thereby serving an unidentified role in neuromodulation [133,292]. Endocannabinoid-mediated bidirectional communication between astrocytes and neurons has been demonstrated to significantly impact synaptic plasticity [293,294] and memory formation [295], further underpinning the importance of the astroglial endocannabinoid system in regulating physiological functions that were earlier believed to be exclusively neuronal. This suggests that both the neuronal and the astroglial CB1R could have an important role to play in neuromodulation. For a detailed overview of the molecular mechanisms of the astroglial CB1R in the regulation of short, spike-time-dependent-, and long-term synaptic plasticity, please refer to the reviews by Catillo et al. [296], Navarrete et al. [292], Oliveira da Cruz et al. [297] and Guerra-Gomes et al. [298].
There is conflicting evidence for the existence of the CB2R in the brain. While several studies have identified CB2R expression in both neuronal and microglial cells [299,300], the lack of specificity of several commercially available CB2R antibodies in combination with low CB2R expression in quiescent microglia [85,301,302,303,304], has resulted in inconclusive evidence to prove the existence of the CB2R in healthy brain cells. But by employing knock out mice, several studies revealed the existence of CB2Rs in various regions of the brain [305,306]. Unlike the CB1R, the CB2R was also observed to be located on the post synaptic terminals [307]. Additionally, both glutamatergic and GABAergic neurons in the hippocampus were reported to express CB2R mRNA [304]. Recently, CB2R agonists were reported to reduce dopaminergic neuronal excitability in the ventral tegmental area (VTA) through cAMP reduction [308].
3.2.2. Neuroinflammation and Neuroprotection
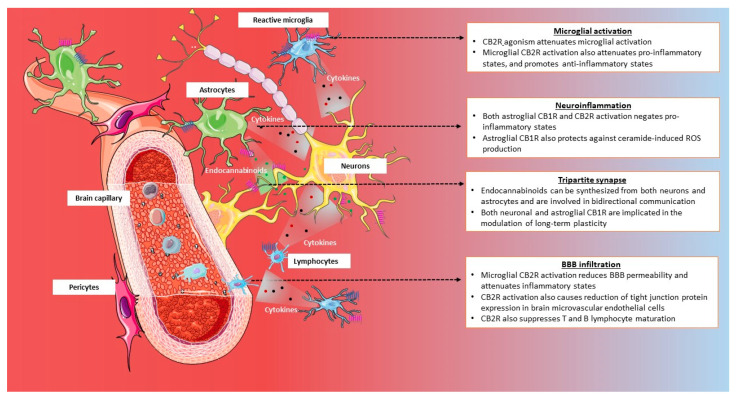
Cannabinoids, both endogenous and exogenous, have been demonstrated to have anti-inflammatory, anti-oxidant, and neuroprotective effects in the CNS [309,310,311,312,313]. Glial cells, both astrocytes and microglia, are well-known to have potent immunomodulatory roles in the brain [314,315,316]. Activation of the glial CB1R and the CB2R has been demonstrated to promote an anti-inflammatory state by elevating anti-inflammatory cytokines and also lowering the levels of pro-inflammatory cytokines [317,318,319]. Activation of the astroglial CB1R has also been implicated in conferring protection to astrocytes against ceramide-induced elevation in free radicals and apoptosis [320,321]. However, CB1R antagonists were also reported to effectively attenuate various inducers of neurotoxicity, suggesting a far more complex mechanism by which CB1Rs confer neuroprotection [322,323]. Interestingly, both CB1Rs and CB2Rs are involved in abolishing lipopolysaccharide (LPS)-mediated pro inflammatory effects in astroglial cell culture [324], as well as in activated microglial cells [325,326,327,328]. The CB2R has also been shown to be upregulated in microglial cells when stimulated with inflammatory cytokines, and thereby has a significant role in the molecular underpinnings of pathological conditions characterized by neuroinflammatory states [85]. CB2R agonists have been shown to attenuate microglial activation when exposed to neuroinflammatory triggers [329], and also limit inflammatory responses at the BBB by reduction of tight junction protein expression in brain microvascular endothelial cells [330]. CB2R activation was also demonstrated to dampen inflammatory markers and reduce BBB permeability in post-traumatic brain injury [331,332,333]. The ability of the CB2R to modulate various aspects of neuroinflammation, involving both microglial activation and infiltration of peripheral immune cells, lends itself to be a valuable therapeutic target in neuroinflammatory diseases. For a comprehensive overview on the mechanisms of CB2R-mediated regulation of peripheral and central inflammatory states, please refer to the review by Cabral and Griffin-Thomas [334]. The role of CB2R in the regulation of peripheral immune cells is discussed in a later section. Schematic describing the role of astroglial, microglial, and lymphocytic cannabinoid receptors in the regulation of neuroinflammatory states and synaptic strengthening is illustrated in Figure 4.
Figure 4.
Regulation of synaptic activity and neuroinflammatory states by cannabinoid receptors. CB2R located on both microglial and astroglial cells are involved in the attenuation of inflammatory states. They do so by inhibition of microglial activation, reduced secretion of pro-inflammatory cytokines, and increased secretion of anti-inflammatory cytokines, and limiting the infiltration of peripheral immune cells. The CB2R is also expressed in the brain microvascular endothelial cells whereby they regulate expression of tight junctions, and further limit chemotaxis and transmigration of peripheral immune cells into the CNS. The CB2R also limits T- and B-cell proliferation and immunomodulation. Astroglial CB1R also promotes an anti-inflammatory state while simultaneously lowering levels of pro-inflammatory cytokines. Additionally, both neuronal and astroglial cells secrete endocannabinoids which are involved in modulation of synaptic strengthening by LTP and LTD. Additionally, astroglial CB1R activation has also been demonstrated to protect against excitotoxic neuronal damage and some forms of neurotoxic damage.
3.2.3. Implications of Dysregulated Endocannabinoid Tone in Neurological Disorders
Epilepsy: The fine tuning of excitatory and inhibitory synapses has important implications in epileptogenic activity. Evidence of THC demonstrating considerable anticonvulsant properties was studied prior to the identification of the endocannabinoid system [335,336]. After the discovery of the endocannabinoid system, the anticonvulsant properties of cannabinoids were shown to be associated with the brain CB1R activation [337]. CB1R activation has also been reported by multiple groups to mediate seizure termination and protection against excitotoxicity, gliosis, and brain damage [338,339,340,341,342,343]. In agreement with the above, SR141716A, a CB1R blocker, exhibited significant acute proconvulsant properties in various models of epilepsy [245,339,344]. Changes in CB1R expression in various forms of epilepsy have also been reported. An augmentation in CB1R expression in status epilepticus and febrile seizures [338,345], while a downregulation of CB1R protein and mRNA expression in excitatory axon terminals in hippocampal samples from patients with intractable temporal lobe epilepsy, has been reported [346]. Furthermore, in vivo PET human imaging further identified reorganization in CB1R availability in different brain regions following seizure onset [347]. CB1R expression was not only reported to be reorganized at various stages following status epilepticus [348,349], but its activation has been linked to region-specific differences in modulating synchrony [350].
However, the role of CB1R in regulating seizure activity is still unclear, and several factors such as age of onset may have a crucial role in determining the utility of employing an agonist versus an antagonist for treatment [351,352]. Opposing results from several studies have further complicated its role. CB1R-mediated protection against seizure activity was shown to be potentiated as well as neutralized by GABA-A modulators and agonists, suggesting a far more complex interaction with GABAergic system [351,352,353]. Contrasting with studies that reported the anticonvulsant properties of cannabinoids, both exogenous and endogenous cannabinoids have been reported to exhibit proconvulsant properties in various models of epilepsy [354,355,356]. While CB1R antagonism was shown to induce epileptogenic activity in a cell culture model of status epilepticus and in individuals with a history of epilepsy [357,358], CB1R blockade was shown to be efficacious in decreasing long term consequences of febrile seizures, suggesting potential benefits of preventing seizure-induced changes in endocannabinoid signaling [344]. Endocannabinoid signaling plasticity and the dual role of CB1R modulation in altering disease progression is discussed in detail elsewhere [359].
Apart from ambiguity over the CB1R’s role in the prevention of epileptic seizures, and the potential to induce psychological adverse effects, there is also some concern over the effectiveness of targeting CB1R solely as a therapeutic strategy [360,361]. By employing animal and cell culture models of epilepsy, several studies have demonstrated that CB2R is involved in conferring a protective phenotype to a variety of seizure types [362,363,364]. CB2R, which is primarily expressed on activated microglial cells, induced astroglial cell proliferation and survival in an astrocyte model of epilepsy [363]. Additionally, it was elevated in hippocampal neurons post pilocarpine-induced status epilepticus [365]. It could be that the CB2R could confer a neuroprotective phenotype by not only negating neuroinflammatory states, but also protecting neuronal and astroglial cells from toxic insults at various stages of epilepsy.
Parkinson’s disease: As mentioned earlier CB1R and other components of the endocannabinoid system are highly localized in the basal ganglia. Basal ganglia structures, such as the dorsal striatum, globus pallidus, and substantia nigra, play key roles in motor function. Failure to properly initiate or terminate synaptic activity in the basal ganglia neuronal circuitry has been linked to movement disorders such as Parkinson’s disease (PD) and Huntington’s disease (HD), which are characterized by hypo-and hyper--kinesia respectively. An augmented endocannabinoid tone, characterized by a significant increase in endocannabinoid and CB1R levels, were identified in the basal ganglia of animal models, and post-mortem brains of individuals with PD [366,367,368]. Higher levels of endocannabinoids were observed in the cerebral spinal fluid of untreated PD patients, which were normalized with dopaminergic treatment [369,370]. While promising results were observed in preclinical studies with SR141716A when administered with quinpirole [367], clinical studies with SR141716A failed to show any improvement in motor disability in PD patients [371]. CB1R agonists also showed mixed results. A pilot study demonstrated the efficacy of nabilone, a mixed CB1R/CB2R agonist, in alleviating symptoms in PD patients experiencing levodopa-induced dyskinesia [372]. On the other hand, a larger clinical trial demonstrated that cannabis was ineffective in ameliorating symptoms of levodopa-induced dyskinesia [373]. Biphasic changes in CB1Rs at early versus later stages of disease progression, in combination with brain structure-specific changes in CB1R expression, suggests a far more complex association of the endocannabinoid system with the progression of PD [368,374]. Interestingly, an open label observational study determined a significant improvement in motor symptoms in PD patients treated with medical marijuana [375]. This may suggest that medicinal marijuana, containing several cannabinoids, may offer greater therapeutic benefit in the treatment of PD, rather than a single CB1R agonist therapy. While “entourage effect” could be the most probable explanation, the involvement of CB1R-independent mechanisms in the progression of the disease should not be disregarded. HU-308, a CB2R agonist, has been previously shown to provide mild neuroprotection in rats with unilateral lesions of dopaminergic neurons [376]. Activation of CB2R in a MPTP mouse model of PD attenuated inflammatory markers and BBB damage, thereby protecting nigrostriatal neurons [377]. Targeting cannabinoid receptors other than CB1Rs can offer alternative routes to amelioration of symptoms in PD individuals [378].
Huntington’s disease: While PD is characterized by an augmented endocannabinoid tone, a significant reduction in CB1R expression, and also in endocannabinoid levels, has been reported in pre-clinical and clinical studies in HD [379,380,381,382,383,384]. In fact, a recent study reported that pre- frontal CB1R expression was reduced at very early stages of the disorder in individuals that carry the HD mutation, even prior to the onset of motor symptoms, suggesting that CB1R dysregulation may be an important factor in cognitive symptoms associated with HD [190]. Since cannabinoids can also counteract neuroinflammatory and pro-oxidant processes, apart from offering neuroprotection via negating excitotoxicity, employing cannabinoid-based therapeutics that target both CB1Rs and CB2Rs were viewed as viable strategies to limit neuronal damage often seen in HD. A pilot study examining the effect of nabilone in HD, reported an improvement in non-motor functions such as neuropsychiatric symptoms [385]. CB2R activation alone was also demonstrated to offer considerable neuroprotection, which was achieved by a reduction in the levels of pro-inflammatory markers in a rat model of HD [386]. However, a randomized, placebo-controlled, pilot trial examining the effects of Sativex, which comprises of THC and cannabidiol, did not show any improvement in motor, cognitive and behavioral functional scores when compared to the placebo [196]. More studies are needed to assess the efficacy of cannabinoid therapeutics in HD.
Alzheimer’s disease: Several groups have also investigated the utility of targeting the cannabinoid receptors in Alzheimer’s disease (AD) due to their role in neuroprotection. However, contradictory findings of changes in CB1R expression [387,388,389,390,391,392], and a lack of correlation between the CB1R and AD molecular markers or cognition [393], highlight a need to research non-CB1R components of the endocannabinoid system for AD. Brain CB2R have been reported to show significant correlation with AD markers, Aβ(42) levels, and plaque score [393]. CB2Rs are observed in activated microglia, the latter a prominent feature in inflammatory-related neurodegenerative diseases [393,394]. Apart from dampening of microglial activation [86], CB2R agonists have also been shown to stimulate Aβ clearance [395,396]. Given that CB2R agonists negate AD-related pathological outcomes via multiple mechanisms, and their relatively selective expression in activated microglia, they offer a potential alternative in the treatment of AD. This is however highly dependent on whether the preclinical outcomes of CB2R agonists hold true in a clinical setting. A novel CB2R agonist, NTRX-07, which has been shown to ameliorate neuroinflammatory states, is currently under Phase 1 trials for the treatment of AD [397].
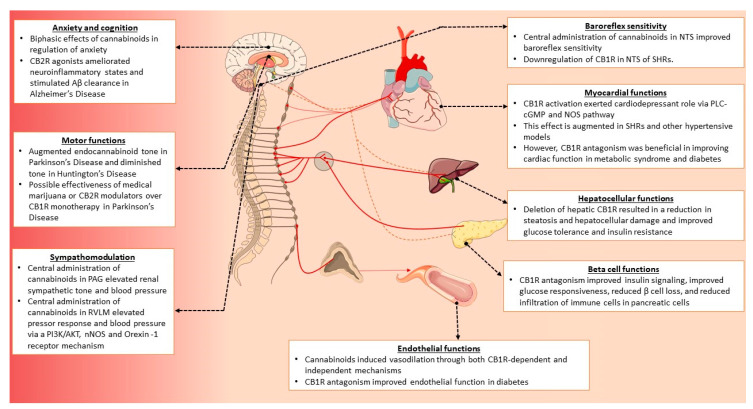
Anxiety: Since CB1Rs are present in high abundance in prefrontal cortex, amygdala and hippocampus, the so called “emotional circuitry,” the effect of CB1R modulation has been investigated in reducing anxiety-like behavior. The CB1R has been demonstrated to play a role in controlling behavioral responses related to altered emotional states [398]. Cannabinoid agonists were reported to have a biphasic effect on modulating anxiety and stress, whereby low doses attenuate anxiety and higher doses bring about anxiogenic effects [399,400]. The molecular underpinnings of differential CB1R-mediated responses are diverse. These include contradictory functions of different brain regions in modulating anxiety [401], preferential activation of distinct CB1R populations on either GABAergic or glutamatergic neurons [402], and the potential activation of non-CB1R related mechanisms [403,404]. Additionally, environmental factors, such as stress attenuating GABA response to CB1R agonists, are also involved [405]. Apart from GABAergic and glutamatergic neurons, CB1Rs are also expressed in the raphe nuclei, whereby they can modulate functions of serotonergic neurons. Mice with serotonergic neurons deficient in CB1Rs, were shown to have heightened anxiety and reduced sociability [406]. Additionally, neuroinflammatory states have been also ascribed in the pathogenesis of anxiety and depression [407,408]. Since both the CB1R and the CB2R can reduce neuroinflammatory states, cannabinoid receptors provide multiple routes to induce anxiolytic effects. Evidence of CB2R polymorphisms in Japanese subjects with depression was reported, suggesting a link between the CB2R and behavior [409]. Additionally, in the same study, administration of antisense oligonucleotide targeting the CB2R mRNA expression in mice resulted in a reduction of anxiety-like behaviors [409]. By employing CB2R-over expressed mice and also spontaneously anxious mice, it was observed that the CB2R could alter anxiety-like behaviors via GABA receptors [410,411]. Deletion of the dopaminergic CB2R in the VTA of mice was also demonstrated to alter anxiety, depression, and psychomotor behavior [412]. Since CB1R antagonism by rimonabant has been associated with neurological adverse effects, alternate therapeutic avenues involving CB2R modulation may be a viable option.
Stroke: Cannabinoids have been demonstrated to exhibit neuroprotective functions against ischemic injuries via both the CB1R and the CB2R [413,414]. Cerebral ischemia-induced brain damage was observed to be more severe in CB1R knockout mice than the wild type mice, suggesting that the CB1R has neuroprotective roles post ischemia [414]. The protective role of CB1Rs in stroke is linked to not just the neuronal CB1R, but also involves the glial CB1R, whereby it has been shown to reduce glial cell reactivity and regulate LTD [415,416]. However, there is some ambiguity over CB1R’s neuroprotective role in cerebral ischemia [417]. In two separate studies, CB1R antagonists SR141716A and AM251 were shown to have beneficial effects in attenuating neuronal damage caused by cerebral ischemia [418,419]. Interestingly, CB2R agonists have also been shown to mitigate inflammatory states and promote neurogenesis in post stroke and post intracerebral hemorrhage [420,421]. The possibility of employing a dual CB1R antagonist/CB2R agonist strategy to attain effective coverage in attenuating the severity of ischemic injury, could be a viable therapeutic strategy based on the available pre-clinical data [422,423].
Neuropathic and inflammatory pain: Cannabinoids have been established to be potent analgesics [424,425]. Several studies have revealed that cannabinoids produce antinociception in diverse pathologies through both spinally and supraspinally located CB1Rs [426,427,428,429,430,431,432,433]. In pain models of inflammation, both CB1R and CB2R agonists showed considerable effectiveness in demonstrating analgesic effects [434,435]. Owing to the ability of CB2Rs to suppress microglial activation and neuroinflammatory states, CB2R agonists were also investigated as a potential therapeutic strategy to treat neuropathic pain. CB2R knockout mice showed enhanced interferon (IFN)-γ production by glial cells [436]. Consistent with this, the CB2R exhibited potent hyperanalgesic effects through both central and peripheral mechanisms [437,438,439,440,441]. Interestingly, CB2Rs were reported to be upregulated within the spinal cord in a neuropathic, but not an inflammatory, pain model [301]. However, CB2R agonists were reported to reduce thermal hyperalgesia associated with inflammation [442,443]. A recently performed meta-analysis of randomized controlled trials revealed cannabinoids produced a small, yet significant, improvement in pain reduction in individuals with neuropathic and non-neuropathic pain [444].
3.3. Metabolic
3.3.1. Hypophagia
CB1R activation has been well-established to promote a feeding response. Reduced food intake was reported in rats administered CB1R antagonists and also in CB1R knockout mice, which suggests that tonically activated CB1R has a critical role in modulating food intake [445,446]. CB1Rs exert this effect through a multi-level regulation of appetite [447]. The primary level of regulation is at the hypothalamic nuclei, where it modulates the release of orexigenic and anorexigenic neuropeptides [448,449]. The CB1R has been shown to be essential for both leptin’s and ghrelin’s effects on food intake [446,450]. Apart from the hypothalamic nuclei, the CB1R is also reported to regulate food intake and energy homeostasis at the level of the brainstem nuclei [451]. Lastly in the mesolimibic reward system, whereby CB1R agonism promotes reward effects by elevating dopamine release [452]. However, the role of CB1R-mediated hypophagic action is far more complex, since factors such as the current prandial state and fluctuations in endocannabinoid levels and/or CB1R-mediated signaling, also impact its effect on appetite regulation [453].
3.3.2. Peripheral Energy Regulation
While the central CB1R has well-established roles in the regulation of appetite by rewiring neuronal circuitry to facilitate rewarding behaviors, peripheral CB1Rs play a key role in the homeostatic control of metabolites. Importantly, the CB1R has been implicated in the development of obesity [454]. CB1R antagonism improved insulin signaling in pancreatic cells, improved β-cell proliferation and improved glucose responsiveness by increasing glucokinase and glucose transporter 2 (GLUT-2) expression in pancreatic β cells [455,456]. Ibipinabant, a selective CB1R antagonist, exhibited significant antidiabetic effects, such as attenuation of β-cell loss in male Zucker diabetic fatty rats which was independent of its effects on weight loss [457]. Additionally, β-cell-specific CB1R ablation has been shown to reduce a heightened inflammatory state in the pancreatic cells in response to a high fat/sugar diet [458]. Although multiple lines of evidence have shown the importance of the pancreatic CB1R in preventing β-cell loss and improving function, activation of the CB1R expressed on infiltrating macrophages was shown to be associated with pancreatic β-cell failure [459]. For a detailed mechanistic overview on the role of pancreatic CB1R in diabetes, please refer to the review by Jourdan et al. [460].
Apart from the pancreas, functional CB1Rs were reported in various insulin-sensitive tissues such as liver, adipose, and skeletal muscle. Similar to the pancreatic CB1R, hepatic CB1R deletion was associated with a positive outcome; whereby a reduction in steatosis and hepatocellular damage, and an improvement in glucose tolerance and insulin resistance was observed [90]. In the case of adipocytes, CB1R activation was associated with altered metabolic profiles such as augmented lipogenesis and surges in plasma triglycerides and cholesterol in vivo [461,462]. CB1R activation was also reported to stimulate peroxisome proliferator-activated receptor gamma (PPAR-γ) and decreased adiponectin in adipocytes [463], while blockade was shown to elevate Slc2a4/GLUT4 expression in adipocytes [464]. Peripheral CB1R blockade also improved insulin resistance in adipose tissue in diet-induced obese mice by mitigating an inflammatory response [465]. Additionally, CB1R blockade was shown to improve mitochondrial biogenesis and elevated nNOS expression in cultured white adipocytes, while agonism impaired mitochondrial biogenesis and decreased AMPK phosphorylation [466,467]. CB1R antagonism was also reported to recalibrate the abdominal muscle glycolytic and TCA cycle pathways in response to a hypercaloric diet [468]. In the case of skeletal muscles, CB1R antagonism was shown to upregulate GLUT-4 and improve intermittent hypoxia-induced insulin resistance [469], while CB1R agonism was shown to interfere with insulin signaling pathways [470,471]. Additionally, CB1R antagonism was shown to affect mRNA expression of genes involved in skeletal muscle oxidation in myotubules in obese individuals [472]. This suggests that the CB1R is well placed to have important modulatory effects on peripheral energy homeostasis.
3.3.3. Implications of a Dysregulated Endocannabinoid Tone in Metabolic Disorders
Metabolic disorders and associated comorbidities: Since both central and peripheral CB1Rs are involved in the regulation of appetite and metabolic processes, CB1R antagonism was investigated as a potential therapeutic opportunity for obesity and other metabolic disorders. While multiple trials confirmed the efficacy of rimonabant in weight loss and improvement of metabolic risk factors, an elevated risk of developing neuropsychiatric adverse effects has led to the discontinuation of this drug [473,474,475,476]. The association of neurological complications with rimonabant treatment, is a major impediment for the use of CB1R antagonists as a therapeutic strategy [205,477]. Since the peripherally selective CB1 antagonist TM38837 was reported to elicit minimal CNS effects in humans [478], peripheral CB1R blockade may well be a viable therapeutic option for targeting metabolic disorders. In addition to promoting weight loss [479,480,481,482,483], peripherally selective CB1R antagonist/inverse agonist also demonstrated improvements in metabolic profiles [465,484,485] and liver function [486] in preclinical studies, thereby further highlighting the therapeutic utility of targeting the peripheral CB1R in metabolic syndromes. Interestingly, a dual therapy comprising of a peripheral CB1R antagonist with a CB2R agonist, was shown to be a highly effective strategy in the prevention of diabetes-related comorbidities in streptozocin-induced diabetic rats [487]. By combining CB1R antagonists with drugs that have complimentary roles, peripheral cannabinoid therapeutics could be an attractive approach in the treatment of metabolic disorders. It should be noted that evidence of beneficial effects of endocannabinoid activation in metabolic syndrome has also been reported. Analysis of NHANES data revealed a lower incidence of metabolic syndrome in individuals with marijuana use [488]. Since marijuana comprises of several types of phytocannabinoids, mechanisms other than CB1R activation cannot be discounted in that study.
Cachexia: Cachexia, one of the more debilitating consequence of wasting syndrome, is characterized by a loss of fat and muscle tissues. In addition to a decrease in food intake, metabolic abnormalities such as a shift from anabolic to catabolic processes are believed to result in tissue degeneration [489,490]. As cannabinoids have a profound effect on appetite and metabolism, they have been widely employed to stimulate appetite in pathological conditions such as cachexia. In this regard, THC and nabilone have been approved for the treatment of cachexia [491]. The CB1R is believed to have a critical role in cachexia, because of its ability to stimulate appetite, induce lipogenesis, fat storage, and induce weight gain [492]. Although anecdotal evidence for the utility of cannabinoids in cachexia is abundant, direct evidence of cannabinoid related improvement of quality of life in individuals with cachexia surprisingly is still not definitive [493,494].
3.4. Cardiovascular
3.4.1. Triple/Triphasic Response of Cannabinoids
Since the CB1R is expressed in the heart, blood vessels, and the cardiovascular centers of the brainstem and hypothalamus, cannabinoids can exert an influence on blood pressure regulation through multiple mechanisms [474,495]. Intravenous administration of cannabinoids, both exogenous and endogenous, triggers an immediate fall in blood pressure which is vagally mediated (Phase I), followed by a brief pressor effect (Phase II), and this gives way to a prolonged hypotensive effect (Phase III) [496,497]. While TRPV-1 is implicated in mediating cannabinoids-induced reflex bradycardia (phase I), the prolonged hypotensive effect (phase III) is mediated via peripherally located CB1R, although central effects cannot be disregarded [498,499,500,501]. For a comprehensive review of cannabinoid-mediated triphasic effect, please refer to the review by Malinowska et al. [495].
3.4.2. Central Mechanisms of Blood Pressure Regulation
The cardioregulatory centers in the medulla are implicated in the short and long-term regulation of blood pressure. The peripherally located baroreceptors senses increases in blood pressure and relay the information to the critical command center, NTS. This in turn activates the parasympathetic tone, and inhibits the pressor center, RVLM. Thus, a decrease in baroreflex sensitivity, often results in a reduced buffering capacity to a spike in blood pressure. Over time this results in baroreflex resetting; often an essential pathophysiological alteration in essential hypertension [502]. For an overview of general central mechanisms that regulate blood pressure, please refer to detailed reviews on this subject by Fisher [503,504] and Haspula and Clark [505]. Central administration of cannabinoids has been shown to elicit either sympathoinhibition or sympathoexcitation based on the site of microinjection. For instance, microinjection of cannabinoids into the NTS of rats can enhance baroreflex responses and triggers sympathoinhibition [506,507,508,509,510], partially by altering GABA release and baroreflex sensitivity [506,510]. However, microinjection of cannabinoids into the midbrain periaqueductal gray and also the RVLM of rats resulted in increased sympathetic activity, and increased blood pressure [511,512,513]. The intracellular signaling kinases PI3K and ERK1/2, as well as nNOS have been implicated as potential mechanisms in cannabinoid-induced pressor responses in the RVLM [514,515]. Additionally, enhanced orexin receptor 1 signaling has been described as a priming event for the central CB1R-mediated pressor response [516].
3.4.3. Peripheral Mechanisms of Blood Pressure Regulation
Although the CB1R can modulate heart rate via central mechanisms, cardiac CB1R activation was shown to elicit negative chronotropic and ionotropic effects, that were potentially independent of the CNS [95,517,518]. Mechanistic studies revealed that the PLC-cGMP and NOS pathways were crucial signaling pathways for mediating the contractile responses of CB1R [519]. CB1R activation was also shown to prevent cardiac remodeling in cultured rat cardiomyocytes by the suppression of phosphorylation of MAPKs and EGFR [520]. Interestingly, this CB1R-dependent cardiodepressant effect is augmented only in individuals with pathological conditions, and not in healthy individuals [96,518,521]. This was evident in CB1R knockout mice, where blood pressure and heart rate remained normal suggesting a lack of tonic control over these cardiovascular indices [522]. But in a mouse model of congestive heart failure and also acute heart failure, CB1R deficiency was associated with an increase in mortality rate [520,523]. Regarding the cardiodepressant mechanism, a possible involvement of peripheral TRPV1 channels cannot be disregarded [524]. Apart from direct cardiodepressant effects, several studies have reported vasodilatory effects of cannabinoids in the aorta and coronary arteries through both CB1R-dependent and independent mechanisms [525,526,527,528,529,530]. Anandamide was demonstrated to activate two distinct signaling streams via the CB1R and the endothelial atypical receptor or GPR55 based on the activation and clustering of integrins in human endothelial cells [531]. This involved CB1R-mediated translocation of NFκB via spleen tyrosine kinase, and alternatively GPR55-mediated calcium mobilization via PI3K-PLC pathway [531]. Interestingly, anandamide was also shown to decrease endothelin-1 production and increase nitric oxide levels via a non-CB1R mechanism in human endothelial cells [532]. For a comprehensive review on the effects of cannabinoids through non-CB receptor mechanisms in the vasculature, please refer to the review by Bondarenko [533].
3.4.4. Implications of a Dysregulated Endocannabinoid Tone in Cardiovascular Disorders
Hypertension: The use of cannabinoids as potential anti-hypertensive medications has mixed results in a clinical setting. Studies in the 1970s reported hypotensive effects of chronic cannabinoid use in humans. Prolonged use, either by marijuana inhalation, or by THC consumption, resulted in a significant fall in heart rate and blood pressure [534,535]. THC was also demonstrated to have a potent hypotensive effect in hypertensive individuals when compared to normotensive individuals [536]. Multiple studies have also reported evidence of central sympathoinhibition in response to cannabinoids [88,537]. However more recently, mixed results were reported on the modulation of cannabinoid receptor activity. While the cross-sectional analysis of NHANES identified a lower prevalence of metabolic syndrome in marijuana users, a higher occurrence of elevated systolic blood pressure was reported [488]. Interestingly, no differences in blood pressure were noted when rimonabant-treated groups were compared with the control groups over a period of 2 years [473].
By employing SHRs and other rat models of hypertension, several studies in the late 1990s were able to elucidate the mechanisms by which cannabinoids alter cardiovascular function in hypertension. Systemic administration of cannabinoids was observed to have a mild hypotensive effect on blood pressure regulation in normotensive rat models. However, in hypertensive rat models, a greater reduction in blood pressure when compared to the normotensive models in response to cannabinoids was observed [96]. This enhancement of basal endocannabinoid tone resulted in improved cardiovascular parameters, such as heart rate and vascular resistance, in SHRs and other models of hypertension [96]. Interestingly, while the myocardial and endothelial CB1Rs were elevated in SHRs [96], a reduced density of CB1Rs was reported by multiple groups in SHR brains [254,538,539], and a dampened endocannabinoid tone was reported in the CNS [538]. Centrally administered cannabinoids had a marked sympathoinhibitory effect in normotensive rat models such as WKY and Sprague Dawley rats, but not in SHRs [538]. Results from the aforementioned pre-clinical studies suggest a differential alteration in CB1R functionality in hypertensive conditions at the level of the peripheral organs, when compared to the CNS. In this regard, endocannabinoid hyperactivity in the periphery could be an adaptive or a compensatory mechanism in response to an elevation in blood pressure triggered by sympathetic augmentation. Hypofunctional endocannabinoid system in the NTS resulting in an elevated sympathetic activity in SHRs could in fact be a crucial mechanism early on in the development of hypertension. This is a plausible theory since sympathetic hyperactivity is theorized to be a critical cardioregulatory modification that occurs at earlier stages of hypertension [505,540]. Although several studies have also reported a blood pressure-lowering effect on cannabinoid receptor activation, sympathoexcitatory effects of endocannabinoid system activation have also been observed [511,541]. In this case, microinjection of the CB1R antagonist into the NTS was shown to improve baroreflex sensitivity and reduce systolic blood pressure in (mRen2)27 rats [542,543]. The apparent discrepancy in results in clinical and pre-clinical settings suggests a more complex relationship between CB1Rs and blood pressure regulation in hypertension. Some of the reasons could be due to the pleiotropic effects of cannabinoids, and differences in experimental design such as the use of unanesthetized versus anaesthetized animals, and the use of monogenic or polygenic rat models of hypertension.
Cardiac, endothelial, and vascular dysfunction: Since CB1R activation exerts cardiodepressant and protective roles, cardiac CB1Rs could be potentially modulated for improving cardiac function. Although CB1R agonism has been shown to have beneficial effects in animal models of hypertension and heart failure [96,520,523], CB1R antagonism was shown to improve cardiac function after experimental myocardial infarction and metabolic syndrome [544]. Additionally, both pharmacological and genetic inhibition of CB1R improved cardiac function and resulted in an attenuation of oxidative stress, inflammation, and fibrosis in a rat model of diabetes [545]. CB1R inhibition in apolipoprotein E-deficient mice on a cholesterol-rich diet resulted in improvement of endothelial parameters and function, such as a decreased AT1R expression and ROS production [546]. This is in accordance with previous studies on the beneficial effects of rimonabant on improvement of cardiovascular and metabolic parameters in metabolic syndrome. However, Gq/11-mediated vascular endocannabinoid synthesis, and the resultant CB1R activation in vascular smooth muscle cells was shown to attenuate Ang II-mediated vasoconstriction [89,547]. It could be that therapeutically activating or inhibiting cardiac and vascular CB1R is dependent on whether cardiac dysfunction is present with comorbidities such as hypertension or diabetes. However, the use of synthetic cannabinoids for cardiovascular diseases needs to be researched further, since they have been associated with various cardiac events in adults [548]. Some of the most important biological functions of the cannabinoid receptors discussed in this review thus far, are shown as a schematic in Figure 5.
Figure 5.
Functional overview of cannabinoid receptors in the CNS and peripheral tissues. CB1R is densely expressed in regions of the brain involved in the regulation of anxiety and cognition such as prefrontal cortex, amygdala, and hippocampus. Activation of the CB1R has been shown to have both anxiogenic and anxiolytic effects. Endocannabinoid tone is also altered in basal ganglia disorders such as Parkinson’s and Huntington’s Diseases. In neurological disorders characterized by neuroinflammation, CB2R-based therapies could be an attractive alternative to targeting CB1R. In the case of cardiac and metabolic diseases, both central and peripheral CB1Rs are known to play a key role in their etiology. Central administration of cannabinoids into the PAG and RVLM triggers a sympathoexcitatory response, while administration in the NTS is known to improve baroreflex sensitivity and facilitates inhibition of pressor responses. Interestingly, CB1Rs were downregulated in the NTS of SHRs. However, myocardial CB1R was augmented in SHRs, and is implicated in mediating cardiodepressant effects. Stimulation of endothelial CB1R was also implicated in mediating vasodilation. Although some studies reported beneficial effects of CB1R agonism in hypertension, CB1R antagonism was demonstrated to improve both cardiovascular and endothelial function in metabolic syndrome. CB1R antagonism also improved functions of insulin-sensitive tissues such as liver and pancreas. Blockade of CB1R was shown to attenuate hepatocellular damage and beta cell loss, and also improve insulin signaling.
3.5. Modulation of Immune System
3.5.1. Immune Cell Development and Differentiation
The CB2R, which is present primarily on immune cells, plays an integral role in the regulation of both humoral and cell-mediated immunity. Cannabinoids are well-known to possess potent immunosuppressive properties [319,549]. Cannabinoids via CB2Rs have been demonstrated to suppress T- and B-cell proliferation and function, and induce apoptosis of splenocytes and thymocytes [550,551,552]. Deficiency of CB2Rs has been demonstrated to cause an augmentation of inflammatory state and chemotactic functioning [553]. However, CB2R deficiency was also shown to reduce T- and B-cell population [554], and also resulted in an impaired B-cell retention and function [555]. In support of this, lower concentrations of synthetic cannabinoids were shown to also enhance proliferation and maturation of B lymphocytes potentially via the CB2R [556], suggesting a more complex role for the CB2R in immunomodulation [557]. Varying levels of CB2Rs were also reported among the various immune cell subpopulations [100,558,559]. Additionally, CB2R expression is regulated by the type of stimulus and the activation status of immune cells [559]. Stimulation of CB2R in activated macrophages was shown to attenuate LPS/IFNγ-induced interleukin (IL)-12p40 release, and elevated IL-10 release [560].
3.5.2. Implications of a Dysregulated Endocannabinoid Tone in Immunological Disorders
Rheumatoid arthritis: Rheumatoid arthritis (RA) is an inflammatory disease that is characterized by an augmented infiltration of immune cells in the synovial cavity. In addition to regulating various aspects of T- and B-cell functions, the CB2R is also found in various other cell types involved in RA pathology, such as synovial tissue [561], osteoclasts [562], and chondrocytes [563]. Interestingly, the CB2R was upregulated in fibroblast-like synoviocytes in response to pro-inflammatory triggers such as IL1-β and TNFα [564]. Furthermore, a CB2R agonist negated IL1-β and TNFα-induced elevation in proinflammatory cytokines and matrix metalloproteases in RA [564,565]. Additionally, activation of the CB2R was also shown to negate several proinflammatory mediators and inhibit RA-induced bone damage in collagen-induced activation in mice [566]. While the anti-inflammatory effects observed with CB2R agonists were most likely mediated via the CB2R, involvement of other mechanisms or receptors, such as the glucocorticoid receptor, cannot be ruled out [567].
Autism spectrum disorder-mediated immune impairment: Clinical and pre-clinical studies have routinely reported immune dysfunction in autistic individuals [568,569]. While the CB1R was unchanged in peripheral blood mononuclear cells in children with autism, both CB2R protein and mRNA were significantly upregulated [570]. In a follow-up study, Gc protein-derived macrophage-activating factor treatment, which was shown to have beneficial effects in the treatment of autism, was able to reduce CB2R expression in bone-marrow-derived macrophage cells from autistic individuals, and reduce macrophage activation [571]. Although these results are promising, further studies are required to ascertain whether the CB2R can be therapeutically targeted for normalizing immune functions in autistic individuals.
Multiple sclerosis: Multiple sclerosis (MS) is an autoimmune disorder characterized by demyelination of nerve fibers in the brain and spinal cord. Evidence of a role for cannabinoid receptors in the pathogenesis of MS comes from multiple in vitro studies that employed an experimental autoimmune encephalomyelitis (EAE) model of MS. Both CB1Rs and CB2Rs were altered in immune cells isolated from primary-progressive and relapsing–remitting MS patients [195,572]. Additionally, both CB1Rs and CB2Rs were also involved in neuroprotection against excitotoxicity in a chronic model of MS [573]. Postmortem brain samples taken from MS individuals showed high CB1R and CB2R immunoreactivity in non-neuronal cells, such as oligodendrocytes, microglia, and macrophages, from active plaques [574]. Since glial inflammation and infiltration of inflammatory cells is a key factor of MS, targeting only CB2R to attenuate neuroinflammatory states would be devoid of CB1R-related psychological adverse effects [575]. Interestingly the CB2R, but not the CB1R, was reported to be upregulated in activated microglial cells and peripheral macrophages in EAE model of MS [85], and was demonstrated to have critical roles in inhibiting leukocyte/endothelial interactions and attenuating infiltration of inflammatory cells [576]. Additionally, CB2R-selective agonists were shown to exert potent anti-inflammatory and anti-proliferative effects on immune cells isolated from MS patients [577,578]. WIN-55,212-2 was reported to reduce inflammatory infiltrates in spinal cord and also induced apoptosis of encephalitogenic T-cell populations, partially via the CB2R in EAE model [579]. However, CB1R-related therapies cannot be disregarded since the CB1R has also been reported to treat various symptoms of MS, such as spasticity [580,581]. Interestingly, CB1Rs expressed on neuronal cells, but not on T cells, were also shown to have a critical role in cannabinoid-mediated attenuation of EAE pathology [582].
Atherosclerosis: Since atherosclerosis is a chronic inflammatory disease characterized by macrophage infiltration, the potential of targeting cannabinoid receptors was investigated using animal models of atherosclerosis. Both phytocannabinoids and synthetic cannabinoids have been demonstrated to exert potent anti-inflammatory and anti-atherosclerotic effects via the CB2R in preclinical studies [583,584]. CB2R knockout in mice with hyperlipidemia-induced atherogenesis displayed greater macrophage and matrix metalloproteinase content when compared to their controls [585]. Apart from cannabinoid treatments altering metabolic and lipid levels, the CB2R agonism was also shown to have potent anti-chemotactic effects via alteration of chemokine receptor and adhesion molecule expression in monocytes [586].
Pain: Exacerbated inflammatory responses are known to be associated with both neurogenic and non-neurogenic pain states [587,588]. The role of central CB1R, and both central and peripheral CB2R inducing anti-nociceptive actions in neuropathic and inflammatory models of pain has been described in earlier (Section 3.2.2 and Section 3.2.3). Additionally, recently conducted retrospective and meta-analysis revealed the utility of cannabinoids in the treatment of postoperative pain and other forms of acute pain states [589,590]. Interestingly, the peripheral CB1R has also been implicated in inducing anti-nociception and resolving associated inflammatory states in various animal models of pain [591,592,593,594]. Since THC has been shown to have tremendous anti-inflammatory potential, which includes its actions on prostaglandin synthesis [595,596], designing peripherally-restricted cannabinoid therapies for the treatment of pain can be a potential alternative to opioid-based therapies [597].
4. Therapeutic Strategies
Strategies to alter endocannabinoid tone in pathological conditions, with minimal-to-no adverse effect profiles, are highly desirable. This is especially true, since targeting central CB1R has been associated with severe neurological adverse effects. This has to be achieved by either employing a direct strategy, which involves either activating or inactivating cannabinoid receptors using mono- or combination-drug therapies, orindirectly, by inhibiting degradative enzymes of endocannabinoids resulting in enhanced cannabinoid receptor activation [359,598,599,600].
4.1. Multi-Drug Strategy
Factors that have been implicated in the pathogenesis of cardiovascular, metabolic, autoimmune, and neurological disorders, such as Ang II and inflammatory cytokines, have all been implicated to intrinsically modulate the endocannabinoid tone by altering cannabinoid receptor expression or activity, through receptor crosstalk mechanisms [89,123,139,153,539]. As a result, employing low dose partial CB1R agonists/ neutral antagonists as adjuvant therapies to existing drug therapies could potentially limit the neuropsychiatric adverse effects that are often linked to high dose of CB1R monotherapies. Combination therapies were suggested by numerous studies based on strong preclinical data [542,601]. In addition to dual drug therapy, the ability of the CB1R to dimerize with other receptors can also be leveraged to design novel therapies. The CB1R has a high tendency to crosstalk with several different GPCRs, including but not limited to the AT1R and the opioid receptors [141,145,602,603,604]. Since receptor heteromers are selectively expressed in only distinct pathological conditions [605,606], targeting heteromeric complexes has been discussed as a viable therapeutic strategy [607]. Additionally, targeting CB1R heteromeric complexes using divalent ligands would result in selective targeting of tissues or brain regions expressing dimers, without altering CB1R-based signaling in other regions. The design and synthesis of divalent ligands for CB1R-orexin receptor and CB1R-D2R heteromer have already been reported [608,609].
4.2. Peripheral CB1R Antagonists
Another strategy is to employ peripherally restrictive CB1R antagonists that would considerably enhance the safety profile of the drugs. Second and third generation CB1R antagonists, that have enhanced peripheral selectiveness, have already demonstrated significant therapeutic potential in preclinical studies [610]. Although peripheral CB1R modulators may possess greater benefit-to-risk ratio than those that cross the BBB, the former may be lacking in effectiveness for the treatment of neurological disorders characterized by a dysregulated endocannabinoid tone.
4.3. Allosteric Modulators
Nonselective activation/deactivation by orthosteric ligands are known to elicit both therapeutic and unwanted effects of CB1R. The utility of allosteric modulators of the CB1R or neutral CB1R antagonists, as an alternative for CB1R orthosteric ligands and inverse agonists respectively, could be useful to circumvent the neuropsychiatric adverse effects associated with CB1R-based therapies [611,612]. Studies on the negative CB1R allosteric modulators, Org27569 and PSNCBAM-1, have already been reported [118,158,613]. The design of the newer generation negative allosteric modulator, such as GAT100, may open up unprecedented avenues for CB1R drug discovery since it demonstrated enhanced potency with negligible inverse agonism properties in functional cell assays [614].
4.4. CB2R Modulators
Considering that the CB2R does not participate in normal brain and peripheral tissue function under healthy conditions, and it is only present in pathological states, targeting the CB2R in neurological disorders characterized by neuroinflammation could be a viable therapeutic strategy to leverage neuroprotective effects of the endocannabinoid system [615]. Additionally, the CB2R displays a reduced adverse effect profile, as CB1R-mediated tolerance and physical dependence with repeated doses is circumvented [616]. In addition to employing multiple drugs, more recently, “dual-mechanism” drugs, such as TV-6–41, which possess both CB2R agonism and CB1R neutral antagonist properties have displayed a reduced adverse effects profile when compared to rimonabant in preclinical studies [617]. Such drugs may be valuable in increasing the therapeutic effectiveness of cannabinoid receptors while significantly attenuating CB1R-mediated unwanted effects. While CB2R-based therapies have shown promise in animal studies, their inability to translate effectiveness from preclinical to clinical studies remains a major hurdle [616,618,619]. Although several reasons have been previously highlighted, one important factor could be the lack of specific commercially available antibodies for the CB2R which results in an over-reliance on gene expression data to develop therapeutics. Since a lack of significantly high correlations between gene and protein expression exists [620], inferences drawn solely from CB2R gene expression studies in preclinical studies need to be confirmed with other additional functional assays.
4.5. Inhibitors of Degradative Enzymes
Because of the adverse effects on the CNS, the adverse effect profile of exogenous cannabinoids is a limiting factor. To circumvent this issue of dose-dependent neurological adverse effects, inhibitors of endocannabinoid degradative enzymes, monoacylglycerol lipase (MAGL) and FAAH, were employed to augment the endocannabinoid tone. ABD-1970, a potent MAGL inhibitor, was demonstrated to exhibit significant antinociceptive effects without the behavioral effects that would be observed with exogenous cannabinoids [621]. PF-04457845, also a FAAH inhibitor, was well-tolerated with limited efficacy in osteoarthritis [622,623]. However, phase-1 clinical trials employing BIA 10-2474 (another FAAH inhibitor) resulted in lethal adverse effects with the use of the highest dose of the drug [624]. This suggests that further research on the safety and efficacy of optimal dosing of indirect cannabinoid therapies is needed.
5. Conclusions
Since the discovery of the endocannabinoid system, there has been a major surge in publications on medical cannabis, cannabinoid-based therapeutics, and cannabinoid receptor pharmacology [625,626]. These studies have aided greatly in deciphering the endocannabinoid system in the molecular and the physiological realms [18,627]. Recent studies have brought to light the versatility and malleability of context-dependent CB1R signaling [628,629,630]. Identifying factors and conditions that could regulate CB1R expression and CNR1 gene expression could therefore help circumvent the need for direct CB1R agonists/antagonists. Since overcoming the neuropsychiatric adverse effects of CB1R-directed agonists/antagonists remains a challenge, CB2R-based therapeutics could be the path forward. Not only do both the CB1R and the CB2R have complimentary roles in various pathological conditions, but they also exhibit contrasting/unique roles in several disease states. The utility of CB2R-based therapeutics could also be greatly enhanced by combining them with pre-existing treatment options instead of a stand-alone therapeutic strategy [631]. Additionally, indirect modulators of endocannabinoid tone may be another promising avenue. Regardless of the promising therapeutic efficacy demonstrated by the aforementioned strategies, safety profiles of newer cannabinoid therapeutics remain an ever-present concern [624]. A multidisciplinary approach could also be utilized in personalizing cannabinoid therapeutics based on genetic variables associated with basal endocannabinoid tone [632,633]. As we embark into the fourth decade of endocannabinoid research, the future of cannabinoid-based therapeutics still holds a lot of promise
Acknowledgments
Aspects of the figures was made using artwork obtained from Servier Medical Art (www.servier.com).
Author Contributions
M.A.C. and D.H. contributed to all aspects of the manuscript, including review of literature, writing, editing, and compilation of figures. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was made possible by a Nova Southeastern University President’s Faculty Research and Development Grant (335309), and a Nova Southeastern University Health Professions Division Grant (335585).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Touw M. The Religious and Medicinal Uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs. 1981;13:23–34. doi: 10.1080/02791072.1981.10471447. [DOI] [PubMed] [Google Scholar]
- 2.Zuardi A.W. History of cannabis as a medicine: A review. Rev. Bras. Psiquiatr. 2006;28:153–157. doi: 10.1590/S1516-44462006000200015. [DOI] [PubMed] [Google Scholar]
- 3.Di Marzo V. A brief history of cannabinoid and endocannabinoid pharmacology as inspired by the work of British scientists. Trends Pharmacol. Sci. 2006;27:134–140. doi: 10.1016/j.tips.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Jacob A., Todd A.R. Cannabis indica. Part II. Isolation of cannabidiol from Egyptian hashish. Observations on the structure of Cannabinol. J. Chem. Soc. 1940:649–653. doi: 10.1039/jr9400000649. [DOI] [Google Scholar]
- 5.Adams R., Baker B.R., Wearn R.B. Structure of Cannabinol. III. Synthesis of Cannabinol, 1-Hydroxy-3-n-amyl-6,6,9-trimethyl-6-dibenzopyran. J. Am. Chem. Soc. 1940;62:2204–2207. doi: 10.1021/ja01865a083. [DOI] [Google Scholar]
- 6.Gaoni Y., Mechoulam R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964;86:1646–1647. doi: 10.1021/ja01062a046. [DOI] [Google Scholar]
- 7.Gaoni Y., Mechoulam R. The Isolation and Structure of Δ-Tetrahydrocannabinol and Other Neutral Cannabinoids from Hashish. J. Am. Chem. Soc. 1971;93:217–224. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- 8.Devane W.A., Dysarz F.A., Johnson M.R., Melvin L.S., Howlett A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- 9.Munro S., Thomas K.L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 10.Devane W.A., Hanus L., Breuer A., Pertwee R.G., Stevenson L.A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 11.Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N.E., Schatz A.R., Gopher A., Almog S., Martin B.R., Compton D.R., et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-D. [DOI] [PubMed] [Google Scholar]
- 12.Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 13.Di Marzo V., Fontana A., Cadas H., Schinelli S., Cimino G., Schwartz J.C., Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 14.Cadas H., Gaillet S., Beltramo M., Venance L., Piomelli D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J. Neurosci. 1996;16:3934–3942. doi: 10.1523/JNEUROSCI.16-12-03934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cravatt B.F., Giang D.K., Mayfield S.P., Boger D.L., Lerner R.A., Gilula N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 16.Dinh T.P., Carpenter D., Leslie F.M., Freund T.F., Katona I., Sensi S.L., Kathuria S., Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bisogno T., Howell F., Williams G., Minassi A., Cascio M.G., Ligresti A., Matias I., Schiano-Moriello A., Paul P., Williams E.J., et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J. Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pertwee R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2009;147:S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., Bonner T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 20.Hoehe M.R., Caenazzo L., Martinez M.M., Hsieh W.T., Modi W.S., Gershon E.S., Bonner T.I. Genetic and physical mapping of the human cannabinoid receptor gene to chromosome 6q14-q15. New Biol. 1991;3:880–885. [PubMed] [Google Scholar]
- 21.Zhang P.W., Ishiguro H., Ohtsuki T., Hess J., Carillo F., Walther D., Onaivi E.S., Arinami T., Uhl G.R. Human cannabinoid receptor 1: 5′ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Mol. Psychiatry. 2004;9:916–931. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]
- 22.González-Mariscal I., Krzysik-Walker S.M., Doyle M.E., Liu Q.R., Cimbro R., Santa-Cruz Calvo S., Ghosh S., Cieala A., Moaddel R., Carlson O.D., et al. Human CB1 Receptor Isoforms, present in Hepatocytes and β-cells, are Involved in Regulating Metabolism. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q.R., Huang N.S., Qu H., O’Connell J.F., Gonzalez-Mariscal I., Santa-Cruz-Calvo S., Doyle M.E., Xi Z.X., Wang Y., Onaivi E.S., et al. Identification of novel mouse and rat CB1R isoforms and in silico modeling of human CB1R for peripheral cannabinoid therapeutics. Acta Pharmacol. Sin. 2019;40:387–397. doi: 10.1038/s41401-018-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song C., Howlett A.C. Rat brain cannabinoid receptors are N-linked glycosylated proteins. Life Sci. 1995;56:1983–1989. doi: 10.1016/0024-3205(95)00179-A. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Q., Yang D., Wu M., Guo Y., Guo W., Zhong L., Cai X., Dai A., Jang W., Shakhnovich E., et al. Common activation mechanism of class a GPCRs. Elife. 2019;8 doi: 10.7554/eLife.50279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Zoubi R., Morales P., Reggio P.H. Structural Insights into CB1 Receptor Biased Signaling. Int. J. Mol. Sci. 2019;20:1837. doi: 10.3390/ijms20081837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson H., D’Antona A.M., Kendall D.A., Von Heijne G., Chin C.-N. Membrane assembly of the cannabinoid receptor 1: Impact of a long N-terminal tail. Mol. Pharmacol. 2003;64:570–577. doi: 10.1124/mol.64.3.570. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher-Jones A., Hildick K.L., Evans A.J., Nakamura Y., Henley J.M., Wilkinson K.A. Protein Interactors and Trafficking Pathways That Regulate the Cannabinoid Type 1 Receptor (CB1R) Front. Mol. Neurosci. 2020;13:108. doi: 10.3389/fnmol.2020.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fay J.F., Farrens D.L. The membrane proximal region of the cannabinoid receptor CB1 N-terminus can allosterically modulate ligand affinity. Biochemistry. 2013;52:8286–8294. doi: 10.1021/bi400842k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hua T., Vemuri K., Pu M., Qu L., Han G.W., Wu Y., Zhao S., Shui W., Li S., Korde A., et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell. 2016;167:750–762. doi: 10.1016/j.cell.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao Z., Yin J., Chapman K., Grzemska M., Clark L., Wang J., Rosenbaum D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature. 2016;540:602–606. doi: 10.1038/nature20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulfers A.L., McMurry J.L., Miller A., Wang L., Kendall D.A., Mierke D.F. Cannabinoid receptor-G protein interactions: Gαi1-bound structures of IC3 and a mutant with altered G protein specificity. Protein Sci. 2009;11:2526–2531. doi: 10.1110/ps.0218402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X., Yang W., Fan Y., Luo J., Hong K., Wang Z., Yan J., Chen X., Lu J., Benovic J., et al. Structural determinants in the second intracellular loop of the human cannabinoid CB 1 receptor mediate selective coupling to G s and G i. Br. J. Pharmacol. 2010;161:1817–1834. doi: 10.1111/j.1476-5381.2010.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin W., Brown S., Roche J.P., Hsieh C., Celver J.P., Kovoor A., Chavkin C., Mackie K. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J. Neurosci. 1999;19:3773–3780. doi: 10.1523/JNEUROSCI.19-10-03773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abadji V., Lucas-Lenard J.M., Chin C., Kendall D.A. Involvement of the Carboxyl Terminus of the Third Intracellular Loop of the Cannabinoid CB1 Receptor in Constitutive Activation of Gs. J. Neurochem. 2008;72:2032–2038. doi: 10.1046/j.1471-4159.1999.0722032.x. [DOI] [PubMed] [Google Scholar]
- 36.Niehaus J.L., Liu Y., Wallis K.T., Egertová M., Bhartur S.G., Mukhopadhyay S., Shi S., He H., Selley D.E., Howlett A.C., et al. CB1 cannabinoid receptor activity is modulated by the cannabinoid receptor interacting protein CRIP 1a. Mol. Pharmacol. 2007;72:1557–1566. doi: 10.1124/mol.107.039263. [DOI] [PubMed] [Google Scholar]
- 37.Bakshi K., Mercier R.W., Pavlopoulos S. Interaction of a fragment of the cannabinoid CB1 receptor C-terminus with arrestin-2. Febs Lett. 2007;581:5009–5016. doi: 10.1016/j.febslet.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nie J., Lewis D.L. The proximal and distal C-terminal tail domains of the CB1 cannabinoid receptor mediate G protein coupling. Neuroscience. 2001;107:161–167. doi: 10.1016/S0306-4522(01)00335-9. [DOI] [PubMed] [Google Scholar]
- 39.Ahn K.H., Pellegrini M., Tsomaia N., Yatawara A.K., Kendall D.A., Mierke D.F. Structural analysis of the human cannabinoid receptor one carboxyl-terminus identifies two amphipathic helices. Biopolymers. 2009;91:565–573. doi: 10.1002/bip.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn K.H., Nishiyama A., Mierke D.F., Kendall D.A. Hydrophobic residues in helix 8 of cannabinoid receptor 1 are critical for structural and functional Properties. Biochemistry. 2010;49:502–511. doi: 10.1021/bi901619r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fletcher-Jones A., Hildick K.L., Evans A.J., Nakamura Y., Wilkinson K.A., Henley J.M. The C-Terminal helix 9 motif in rat cannabinoid receptor type 1 regulates axonal trafficking and surface expression. Elife. 2019;8 doi: 10.7554/eLife.44252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Q.R., Pan C.H., Hishimoto A., Li C.Y., Xi Z.X., Llorente-Berzal A., Viveros M.P., Ishiguro H., Arinami T., Onaivi E.S., et al. Species differences in cannabinoid receptor 2 (CNR2 gene): Identification of novel human and rodent CB2 isoforms, differential tissue expression and regulation by cannabinoid receptor ligands. Genesbrain Behav. 2009;8:519–530. doi: 10.1111/j.1601-183X.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H.Y., Bi G.H., Li X., Li J., Qu H., Zhang S.J., Li C.Y., Onaivi E.S., Gardner E.L., Xi Z.X., et al. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology. 2015;40:1037–1051. doi: 10.1038/npp.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie X.-Q., Chen J.-Z., Billings E.M. 3D structural model of the G-protein-coupled cannabinoid CB2 receptor. Proteins Struct. Funct. Genet. 2003;53:307–319. doi: 10.1002/prot.10511. [DOI] [PubMed] [Google Scholar]
- 45.Li X., Hua T., Vemuri K., Ho J.H., Wu Y., Wu L., Popov P., Benchama O., Zvonok N., Locke K., et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell. 2019;176:459–467. doi: 10.1016/j.cell.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montero C., Campillo N.E., Goya P., Ṕez J.A. Homology models of the cannabinoid CB1 and CB2 receptors. A docking analysis study. Eur. J. Med. Chem. 2005;40:75–83. doi: 10.1016/j.ejmech.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Zheng C., Chen L., Chen X., He X., Yang J., Shi Y., Zhou N. The Second Intracellular Loop of the Human Cannabinoid CB2 Receptor Governs G Protein Coupling in Coordination with the Carboxyl Terminal Domain. PLoS ONE. 2013;8:e63262. doi: 10.1371/journal.pone.0063262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basith S., Cui M., Macalino S.J.Y., Park J., Clavio N.A.B., Kang S., Choi S. Exploring G protein-coupled receptors (GPCRs) ligand space via cheminformatics approaches: Impact on rational drug design. Front. Pharmacol. 2018;9:128. doi: 10.3389/fphar.2018.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Marzo V., De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. B Biol. Sci. 2012;367:3216–3228. doi: 10.1098/rstb.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bisogno T., Melck D., Bobrov M.Y., Gretskaya N.M., Bezuglov V.V., De Petrocellis L., Di Marzo V. N-acyl-dopamines: Novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity In Vitro and In Vivo. Biochem. J. 2000;351:817–824. doi: 10.1042/bj3510817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanus L., Abu-Lafi S., Fride E., Breuer A., Vogel Z., Shalev D.E., Kustanovich I., Mechoulam R. 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. USA. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Porter A.C., Sauer J.M., Knierman M.D., Becker G.W., Berna M.J., Bao J., Nomikos G.G., Carter P., Bymaster F.P., Leese A.B., et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- 53.Pamplona F.A., Ferreira J., De Lima O.M., Duarte F.S., Bento A.F., Forner S., Villarinho J.G., Bellochio L., Wotjak C.T., Lerner R., et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc. Natl. Acad. Sci. USA. 2012;109:21134–21139. doi: 10.1073/pnas.1202906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrucci V., Chicca A., Glasmacher S., Paloczi J., Cao Z., Pacher P., Gertsch J. Pepcan-12 (RVD-hemopressin) is a CB2 receptor positive allosteric modulator constitutively secreted by adrenals and in liver upon tissue damage. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-09808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pertwee R.G., Howlett A.C., Abood M.E., Alexander S.P.H., Di Marzo V., Elphick M.R., Greasley P.J., Hansen H.S., Kunos G., Mackie K., et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morales P., Reggio P.H. An Update on Non-CB 1, Non-CB 2 Cannabinoid Related G-Protein-Coupled Receptors. Cannabis Cannabinoid Res. 2017;2:265–273. doi: 10.1089/can.2017.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller C., Morales P., Reggio P.H. Cannabinoid ligands targeting TRP channels. Front. Mol. Neurosci. 2019;11:487. doi: 10.3389/fnmol.2018.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herkenham M., Lynn A.B., Johnson M.R., Melvin L.S., de Costa B.R., Rice K.C. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsou K., Brown S., Sañudo-Peña M.C., Mackie K., Walker J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/S0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 60.Farquhar-Smith W.P., Egertová M., Bradbury E.J., McMahon S.B., Rice A.S.C., Elphick M.R. Cannabinoid CB1 Receptor Expression in Rat Spinal Cord. Mol. Cell. Neurosci. 2000;15:510–521. doi: 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- 61.Freundt-Revilla J., Kegler K., Baumgärtner W., Tipold A. Spatial distribution of cannabinoid receptor type 1 (CB1) in normal canine central and peripheral nervous system. PLoS ONE. 2017;12:e0181064. doi: 10.1371/journal.pone.0181064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishac E.J.N., Jiang L., Lake K.D., Varga K., Abood M.E., Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coutts A.A., Pertwee R.G. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br. J. Pharmacol. 1997;121:1557–1566. doi: 10.1038/sj.bjp.0701301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Croci T., Manara L., Aureggi G., Guagnini F., Rinaldi-Carmona M., Maffrand J.-P., Fur G., Mukenge S., Ferla G. In Vitro functional evidence of neuronal cannabinoid CB 1 receptors in human ileum. Br. J. Pharmacol. 1998;125:1393–1395. doi: 10.1038/sj.bjp.0702190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hohmann A.G., Herkenham M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 1999;92:1171–1175. doi: 10.1016/S0306-4522(99)00220-1. [DOI] [PubMed] [Google Scholar]
- 66.Kulkarni-Narla A., Brown D.R. Localization of CB1 -cannabinoid receptor immunoreactivity in the porcine enteric nervous system. Cell Tissue Res. 2000;302:73–80. doi: 10.1007/s004410000261. [DOI] [PubMed] [Google Scholar]
- 67.Coutts A.A., Irving A.J., Mackie K., Pertwee R.G., Anavi-Goffer S. Localisation of cannabinoid CB(1) receptor immunoreactivity in the guinea pig and rat myenteric plexus. J. Comp. Neurol. 2002;448:410–422. doi: 10.1002/cne.10270. [DOI] [PubMed] [Google Scholar]
- 68.Gómez R., Navarro M., Ferrer B., Trigo J.M., Bilbao A., Del Arco I., Cippitelli A., Nava F., Piomelli D., Rodríguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J. Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coutts A.A., Izzo A.A. The gastrointestinal pharmacology of cannabinoids: An update. Curr. Opin. Pharmacol. 2004;4:572–579. doi: 10.1016/j.coph.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 70.Vianna C.R., Donato J., Rossi J., Scott M., Economides K., Gautron L., Pierpont S., Elias C.F., Elmquist J.K. Cannabinoid Receptor 1 in the Vagus Nerve Is Dispensable for Body Weight Homeostasis But Required for Normal Gastrointestinal Motility. J. Neurosci. 2012;32:10331–10337. doi: 10.1523/JNEUROSCI.4507-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ong W.Y., Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience. 1999;92:1177–1191. doi: 10.1016/S0306-4522(99)00025-1. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez J.J., Mackie K., Pickel V.M. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J. Neurosci. 2001;21:823–833. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawamura Y., Fukaya M., Maejima T., Yoshida T., Miura E., Watanabe M., Ohno-Shosaku T., Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J. Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tsou K., Mackie K., Sañudo-Peña M.C., Walker J.M. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93:969–975. doi: 10.1016/S0306-4522(99)00086-X. [DOI] [PubMed] [Google Scholar]
- 75.Katona I., Sperlágh B., Sík A., Käfalvi A., Vizi E.S., Mackie K., Freund T.F. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gessa G.L., Casu M.A., Carta G., Mascia M.S. Cannabinoids decrease acetylcholine release in the medial-prefrontal cortex and hippocampus, reversal by SR 141716A. Eur. J. Pharmacol. 1998;355:119–124. doi: 10.1016/S0014-2999(98)00486-5. [DOI] [PubMed] [Google Scholar]
- 77.Oropeza V.C., Mackie K., Van Bockstaele E.J. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007;1127:36–44. doi: 10.1016/j.brainres.2006.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hermann H., Marsicano G., Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–460. doi: 10.1016/S0306-4522(01)00509-7. [DOI] [PubMed] [Google Scholar]
- 79.Nyilas R., Gregg L.C., Mackie K., Watanabe M., Zimmer A., Hohmann A.G., Katona I. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur. J. Neurosci. 2009;29:1964–1978. doi: 10.1111/j.1460-9568.2009.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moldrich G., Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. doi: 10.1016/S0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- 81.Molina-Holgado E., Vela J.M., Arévalo-Martín A., Almazán G., Molina-Holgado F., Borrell J., Guaza C. Cannabinoids promote oligodendrocyte progenitor survival: Involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J. Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Golech S.A., McCarron R.M., Chen Y., Bembry J., Lenz F., Mechoulam R., Shohami E., Spatz M. Human brain endothelium: Coexpression and function of vanilloid and endocannabinoid receptors. Mol. Brain Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 83.Zhang H., Hilton D.A., Hanemann C.O., Zajicek J. Cannabinoid Receptor and N-acyl Phosphatidylethanolamine Phospholipase D-Evidence for Altered Expression in Multiple Sclerosis. Brain Pathol. 2011;21 doi: 10.1111/j.1750-3639.2011.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carlisle S.J., Marciano-Cabral F., Staab A., Ludwick C., Cabral G.A. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int. Immunopharmacol. 2002;2:69–82. doi: 10.1016/S1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- 85.Maresz K., Carrier E.J., Ponomarev E.D., Hillard C.J., Dittel B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- 86.Ehrhart J., Obregon D., Mori T., Hou H., Sun N., Bai Y., Klein T., Fernandez F., Tan J., Shytle D. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J. Neuroinflammation. 2005;2 doi: 10.1186/1742-2094-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pacher P., Bátkai S., Kunos G. Cardiovascular pharmacology of cannabinoids. Handb. Exp. Pharmacol. 2005;168:599–625. doi: 10.1007/3-540-26573-2_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szekeres M., Nádasy G.L., Turu G., Soltész-Katona E., Tóth Z.E., Balla A., Catt K.J., Hunyady L. Angiotensin II induces vascular endocannabinoid release, which attenuates its vasoconstrictor effect via CB1 cannabinoid receptors. J. Biol. Chem. 2012;287:31540–31550. doi: 10.1074/jbc.M112.346296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Osei-Hyiaman D., DePetrillo M., Pacher P., Liu J., Radaeva S., Bátkai S., Harvey-White J., Mackie K., Offertáler L., Wang L., et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Investig. 2005;115:1298–1305. doi: 10.1172/JCI200523057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pagotto U., Marsicano G., Cota D., Lutz B., Pasquali R. The Emerging Role of the Endocannabinoid System in Endocrine Regulation and Energy Balance. Endocr. Rev. 2006;27:73–100. doi: 10.1210/er.2005-0009. [DOI] [PubMed] [Google Scholar]
- 92.Bensaid M., Gary-Bobo M., Esclangon A., Maffrand J.P., Le Fur G., Oury-Donat F., Soubrié P. The Cannabinoid CB 1 Receptor Antagonist SR141716 Increases Acrp30 mRNA Expression in Adipose Tissue of Obese fa/fa Rats and in Cultured Adipocyte Cells. Mol. Pharmacol. 2003;63:908–914. doi: 10.1124/mol.63.4.908. [DOI] [PubMed] [Google Scholar]
- 93.Gebremedhin D., Lange A.R., Campbell W.B., Hillard C.J., Harder D.R. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am. J. Physiol. 1999;276:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- 94.Liu J., Gao B., Mirshahi F., Sanyal A.J., Khanolkar A.D., Makriyannis A., Kunos G. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem. J. 2000;346(Pt 3):835–840. doi: 10.1042/bj3460835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bonz A., Laser M., Küllmer S., Kniesch S., Babin-Ebell J., Popp V., Ertl G., Wagner J.A. Cannabinoids Acting on CB1 Receptors Decrease Contractile Performance in Human Atrial Muscle. J. Cardiovasc. Pharmacol. 2003;41:657–664. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 96.Bátkai S., Pacher P., Osei-Hyiaman D., Radaeva S., Liu J., Harvey-White J., Offertáler L., Mackie K., Rudd M.A., Bukoski R.D., et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakata M., Yada T. Cannabinoids inhibit insulin secretion and cytosolic Ca2+ oscillation in islet β-cells via CB1 receptors. Regul. Pept. 2008;145:49–53. doi: 10.1016/j.regpep.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 98.Meccariello R., Battista N., Bradshaw H.B., Wang H. Updates in reproduction coming from the endocannabinoid system. Int. J. Endocrinol. 2014;2014:412354. doi: 10.1155/2014/412354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rice W., Shannon J.M., Burton F., Fiedeldey D. Expression of a brain-type cannabinoid receptor (CB1) in alveolar Type II cells in the lung: Regulation by hydrocortisone. Eur. J. Pharmacol. 1997;327:227–232. doi: 10.1016/S0014-2999(97)89665-3. [DOI] [PubMed] [Google Scholar]
- 100.Galiègue S., Mary S., Marchand J., Dussossoy D., Carrière D., Carayon P., Bouaboula M., Shire D., Le Fur G., Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 101.Julien B., Grenard P., Teixeira-Clerc F., Van Nhieu J.T., Li L., Karsak M., Zimmer A., Mallat A., Lotersztajn S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology. 2005;128:742–755. doi: 10.1053/j.gastro.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 102.Zhou L., Zhou S., Yang P., Tian Y., Feng Z., Xie X.Q., Liu Y. Targeted inhibition of the type 2 cannabinoid receptor is a novel approach to reduce renal fibrosis. Kidney Int. 2018;94:756–772. doi: 10.1016/j.kint.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dinis-Oliveira R.J., Duarte J.A., Sánchez-Navarro A., Remião F., Bastos M.L., Carvalho F. Paraquat Poisonings: Mechanisms of Lung Toxicity, Clinical Features, and Treatment. Crit. Rev. Toxicol. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 104.Michler T., Storr M., Kramer J., Ochs S., Malo A., Reu S., Göke B., Schäfer C. Activation of cannabinoid receptor 2 reduces inflammation in acute experimental pancreatitis via intra-acinar activation of p38 and MK2-dependent mechanisms. Am. J. Physiol. Liver Physiol. 2013;304:G181–G192. doi: 10.1152/ajpgi.00133.2012. [DOI] [PubMed] [Google Scholar]
- 105.Roche R., Hoareau L., Bes-Houtmann S., Gonthier M.-P., Laborde C., Baron J.-F., Haffaf Y., Cesari M., Festy F. Presence of the cannabinoid receptors, CB1 and CB2, in human omental and subcutaneous adipocytes. Histochem. Cell Biol. 2006;126:177–187. doi: 10.1007/s00418-005-0127-4. [DOI] [PubMed] [Google Scholar]
- 106.Yu T.S., Cheng Z.H., Li L.Q., Zhao R., Fan Y.Y., Du Y., Ma W.X., Guan D.W. The cannabinoid receptor type 2 is time-dependently expressed during skeletal muscle wound healing in rats. Int. J. Leg. Med. 2010;124:397–404. doi: 10.1007/s00414-010-0465-1. [DOI] [PubMed] [Google Scholar]
- 107.Lépicier P., Lagneux C., Sirois M.G., Lamontagne D. Endothelial CB1-receptors limit infarct size through NO formation in rat isolated hearts. Life Sci. 2007;81:1373–1380. doi: 10.1016/j.lfs.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 108.Fede C., Albertin G., Petrelli L., Sfriso M.M., Biz C., De Caro R., Stecco C. Expression of the endocannabinoid receptors in human fascial tissue. Eur. J. Histochem. 2016;60:130–134. doi: 10.4081/ejh.2016.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Whyte L.S., Ford L., Ridge S.A., Cameron G.A., Rogers M.J., Ross R.A. Cannabinoids and bone: Endocannabinoids modulate human osteoclast function In Vitro. Br. J. Pharmacol. 2012;165:2584–2597. doi: 10.1111/j.1476-5381.2011.01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Howlett A.C., Fleming R.M. Cannabinoid Inhibition of Adenylate Cyclase. Pharmacology of the Response in Neuroblastoma Cell Membranes-PubMed. Mol Pharm. 1984;26:532–538. [PubMed] [Google Scholar]
- 111.Howlett A., Qualy J.M., L K.L. Involvement of Gi in the Inhibition of Adenylate Cyclase by Cannabimimetic Drugs-PubMed. Mol Pharm. 1986;29:307–313. [PubMed] [Google Scholar]
- 112.Henry D.J., Chavkin C. Activation of inwardly rectifying potassium channels (GIRK1) by co-expressed rat brain cannabinoid receptors in Xenopus oocytes. Neurosci. Lett. 1995;186:91–94. doi: 10.1016/0304-3940(95)11289-9. [DOI] [PubMed] [Google Scholar]
- 113.Mackie K., Lai Y., Westenbroek R., Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J. Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Flores-Otero J., Ahn K.H., Delgado-Peraza F., Mackie K., Kendall D.A., Yudowski G.A. Ligand-specific endocytic dwell times control functional selectivity of the cannabinoid receptor 1. Nat. Commun. 2014;5 doi: 10.1038/ncomms5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nogueras-Ortiz C., Yudowski G.A. The multiple waves of cannabinoid 1 receptor signaling. Mol. Pharmacol. 2016;90:620–626. doi: 10.1124/mol.116.104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nguyen P.T., Schmid C.L., Raehal K.M., Selley D.E., Bohn L.M., Sim-Selley L.J. β-Arrestin2 regulates cannabinoid CB 1 receptor signaling and adaptation in a central nervous system region-dependent manner. Biol. Psychiatry. 2012;71:714–724. doi: 10.1016/j.biopsych.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gyombolai P., Boros E., Hunyady L., Turu G. Differential β-arrestin 2 requirements for constitutive and agonist-induced internalization of the CB1 cannabinoid receptor. Mol. Cell. Endocrinol. 2013;372:116–127. doi: 10.1016/j.mce.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 118.Ahn K.H., Mahmoud M.M., Shim J.-Y., Kendall D.A. Distinct roles of β-arrestin 1 and β-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1) J. Biol. Chem. 2013;288:9790–9800. doi: 10.1074/jbc.M112.438804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Delgado-Peraza F., Ahn K.H., Nogueras-Ortiz C., Mungrue I.N., Mackie K., Kendall D.A., Yudowski G.A. Mechanisms of Biased β-Arrestin-Mediated Signaling Downstream from the Cannabinoid 1 Receptor. Mol. Pharmacol. 2016;89:618–629. doi: 10.1124/mol.115.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mahavadi S., Sriwai W., Huang J., Grider J.R., Murthy K.S. Inhibitory signaling by CB1 receptors in smooth muscle mediated by GRK5/β-arrestin activation of ERK1/2 and Src kinase. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306:G535. doi: 10.1152/ajpgi.00397.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Graham E.S., Ball N., Scotter E.L., Narayan P., Dragunow M., Glass M. Induction of Krox-24 by endogenous cannabinoid type 1 receptors in neuro2a cells is mediated by the MEK-ERK MAPK pathway and is suppressed by the phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 2006;281:29085–29095. doi: 10.1074/jbc.M602516200. [DOI] [PubMed] [Google Scholar]
- 122.Chanda D., Kim D.K., Li T., Kim Y.H., Koo S.H., Lee C.H., Chiang J.Y.L., Choi H.S. Cannabinoid Receptor Type 1 (CB1R) signaling regulates hepatic gluconeogenesis via induction of endoplasmic reticulum-bound transcription factor cAMP-responsive element-binding protein H (CREBH) in primary hepatocytes. J. Biol. Chem. 2011;286:27971–27979. doi: 10.1074/jbc.M111.224352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haspula D., Clark M.A. MAPK activation patterns of AT1R and CB1R in SHR versus Wistar astrocytes: Evidence of CB1R hypofunction and crosstalk between AT1R and CB1R. Cell. Signal. 2017;40:81–90. doi: 10.1016/j.cellsig.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 124.Blázquez C., Chiarlone A., Bellocchio L., Resel E., Pruunsild P., García-Rincón D., Sendtner M., Timmusk T., Lutz B., Galve-Roperh I., et al. The CB1 cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death Differ. 2015;22:1618–1629. doi: 10.1038/cdd.2015.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Turu G., Hunyady L. Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 2010;44:75–85. doi: 10.1677/JME-08-0190. [DOI] [PubMed] [Google Scholar]
- 126.Bouaboula M., Poinot-Chazel C., Marchand J., Canat X., Bourrié B., Rinaldi-Carmona M., Calandra B., Le Fur G., Casellas P. Signaling pathway associated with stimulation of CB2 peripheral cannabinoid receptor: Involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. Eur. J. Biochem. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- 127.Viscomi M.T., Oddi S., Latini L., Pasquariello N., Florenzano F., Bernardi G., Molinari M., Maccarrone M. Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J. Neurosci. 2009;29:4564–4570. doi: 10.1523/JNEUROSCI.0786-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Atwood B.K., Wager-Miller J., Haskins C., Straiker A., Mackie K. Functional selectivity in CB 2 cannabinoid receptor signaling and regulation: Implications for the therapeutic potential of CB 2 ligands. Mol. Pharmacol. 2012;81:250–263. doi: 10.1124/mol.111.074013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nogueras-Ortiz C., Roman-Vendrell C., Mateo-Semidey G.E., Liao Y.H., Kendall D.A., Yudowski G.A. Retromer stops beta-arrestin 1-mediated signaling from internalized cannabinoid 2 receptors. Mol. Biol. Cell. 2017;28:3554–3561. doi: 10.1091/mbc.e17-03-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lauckner J.E., Hille B., Mackie K. The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc. Natl. Acad. Sci. USA. 2005;102:19144–19149. doi: 10.1073/pnas.0509588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Laprairie R.B., Bagher A.M., Kelly M.E.M., Dupré D.J., Denovan-Wright E.M. Type 1 cannabinoid receptor ligands display functional selectivity in a cell culture model of striatal medium spiny projection neurons. J. Biol. Chem. 2014;289:24845–24862. doi: 10.1074/jbc.M114.557025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Asimaki O., Mangoura D. Cannabinoid receptor 1 induces a biphasic ERK activation via multiprotein signaling complex formation of proximal kinases PKCε, Src, and Fyn in primary neurons. Neurochem. Int. 2011;58:135–144. doi: 10.1016/j.neuint.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 133.Navarrete M., Araque A. Endocannabinoids mediate neuron-astrocyte communication. Neuron. 2008;57:883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 134.De Petrocellis L., Marini P., Matias I., Moriello A.S., Starowicz K., Cristino L., Nigam S., Di Marzo V. Mechanisms for the coupling of cannabinoid receptors to intracellular calcium mobilization in rat insulinoma β-cells. Exp. Cell Res. 2007;313:2993–3004. doi: 10.1016/j.yexcr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 135.Zoratti C., Kipmen-Korgun D., Osibow K., Malli R., Graier W.F. Anandamide initiates Ca2+ signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Br. J. Pharmacol. 2003;140:1351–1362. doi: 10.1038/sj.bjp.0705529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brailoiu G.C., Deliu E., Marcu J., Hoffman N.E., Console-Bram L., Zhao P., Madesh M., Abood M.E., Brailoiu E. Differential activation of intracellular versus plasmalemmal CB2 Cannabinoid receptors. Biochemistry. 2014;53:4990–4999. doi: 10.1021/bi500632a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kearn C.S., Blake-Palmer K., Daniel E., Mackie K., Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: A mechanism for receptor cross-talk? Mol. Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- 138.Marcellino D., Carriba P., Filip M., Borgkvist A., Frankowska M., Bellido I., Tanganelli S., Müller C.E., Fisone G., Lluis C., et al. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology. 2008;54:815–823. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 139.Chiang Y.-C., Lo Y.-N., Chen J.-C. Crosstalk between dopamine D2 receptors and cannabinoid CB1 receptors regulates CNR1 promoter activity via ERK1/2 signaling. J. Neurochem. 2013;127:163–176. doi: 10.1111/jnc.12399. [DOI] [PubMed] [Google Scholar]
- 140.Carriba P., Ortiz O., Patkar K., Justinova Z., Stroik J., Themann A., Müller C., Woods A.S., Hope B.T., Ciruela F., et al. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology. 2007;32:2249–2259. doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- 141.Navarro G., Carriba P., Gandí J., Ciruela F., Casadó V., Cortés A., Mallol J., Canela E.I., Lluis C., Franco R. Detection of Heteromers Formed by Cannabinoid CB 1, Dopamine D 2, and Adenosine A 2A G-Protein-Coupled Receptors by Combining Bimolecular Fluorescence Complementation and Bioluminescence Energy Transfer. Sci. World J. 2008;8:1088–1097. doi: 10.1100/tsw.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hilairet S., Bouaboula M., Carrière D., Le Fur G., Casellas P. Hypersensitization of the Orexin 1 Receptor by the CB1 Receptor. J. Biol. Chem. 2003;278:23731–23737. doi: 10.1074/jbc.M212369200. [DOI] [PubMed] [Google Scholar]
- 143.Rios C., Gomes I., Devi L.A. μ opioid and CB1 cannabinoid receptor interactions: Reciprocal inhibition of receptor signaling and neuritogenesis. Br. J. Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cinar R., Freund T.F., Katona I., Mackie K., Szucs M. Reciprocal inhibition of G-protein signaling is induced by CB1 cannabinoid and GABAB receptor interactions in rat hippocampal membranes. Neurochem. Int. 2008;52:1402–1409. doi: 10.1016/j.neuint.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 145.Rozenfeld R., Gupta A., Gagnidze K., Lim M.P., Gomes I., Lee-Ramos D., Nieto N., Devi L.A. AT1R-CB1R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. Embo J. 2011;30:2350–2363. doi: 10.1038/emboj.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gyombolai P., Pap D., Turu G., Catt K.J., Bagdy G., Hunyady L. Regulation of endocannabinoid release by G proteins: A paracrine mechanism of G protein-coupled receptor action. Mol. Cell. Endocrinol. 2012;353:29–36. doi: 10.1016/j.mce.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Haspula D., Clark M.A. Molecular Basis of the Brain Renin Angiotensin System in Cardiovascular and Neurologic Disorders: Uncovering a Key Role for the Astroglial Angiotensin Type 1 Receptor AT1R. J. Pharmacol. Exp. Ther. 2018;366:251–264. doi: 10.1124/jpet.118.248831. [DOI] [PubMed] [Google Scholar]
- 148.Forrester S.J., Booz G.W., Sigmund C.D., Coffman T.M., Kawai T., Rizzo V., Scalia R., Eguchi S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018;98:1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Callén L., Moreno E., Barroso-Chinea P., Moreno-Delgado D., Cortés A., Mallol J., Casadó V., Lanciego J.L., Franco R., Lluis C., et al. Cannabinoid receptors CB 1 and CB 2 form functional heteromers in brain. J. Biol. Chem. 2012;287:20851–20865. doi: 10.1074/jbc.M111.335273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bouaboula M., Desnoyer N., Carayon P., Combes T., Casellas P. G(i) protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: Implication for intracellular signalization cross-regulation. Mol. Pharmacol. 1999;55:473–480. [PubMed] [Google Scholar]
- 151.Coke C.J., Scarlett K.A., Chetram M.A., Jones K.J., Sandifer B.J., Davis A.S., Marcus A.I., Hinton C.V. Simultaneous activation of induced heterodimerization between CXCR4 chemokine receptor and cannabinoid receptor 2 (CB2) reveals a mechanism for regulation of tumor progression. J. Biol. Chem. 2016;291:9991–10005. doi: 10.1074/jbc.M115.712661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Scarlett K.A., White E.S.Z., Coke C.J., Carter J.R., Bryant L.K., Hinton C.V. Agonist-induced CXCR4 and CB2 heterodimerization inhibits Ga13/RhoA-mediated migration. Mol. Cancer Res. 2018;16:728–739. doi: 10.1158/1541-7786.MCR-16-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Turu G., Simon A., Gyombolai P., Szidonya L., Bagdy G., Lenkei Z., Hunyady L. The Role of Diacylglycerol Lipase in Constitutive and Angiotensin AT1 Receptor-stimulated Cannabinoid CB1 Receptor Activity. J. Biol. Chem. 2007;282:7753–7757. doi: 10.1074/jbc.C600318200. [DOI] [PubMed] [Google Scholar]
- 154.Turu G., Várnai P., Gyombolai P., Szidonya L., Offertaler L., Bagdy G., Kunos G., Hunyady L. Paracrine transactivation of the CB1 cannabinoid receptor by AT1 angiotensin and other Gq/11 protein-coupled receptors. J. Biol. Chem. 2009;284:16914–16921. doi: 10.1074/jbc.M109.003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hart S., Fischer O.M., Ullrich A. Cannabinoids Induce Cancer Cell Proliferation via Tumor Necrosis Factor α-Converting Enzyme (TACE/ADAM17)-Mediated Transactivation of the Epidermal Growth Factor Receptor. Cancer Res. 2004;64:1943–1950. doi: 10.1158/0008-5472.CAN-03-3720. [DOI] [PubMed] [Google Scholar]
- 156.Berghuis P., Dobszay M.B., Wang X., Spano S., Ledda F., Sousa K.M., Schulte G., Ernfors P., Mackie K., Paratcha G., et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc. Natl. Acad. Sci. USA. 2005;102:19115–19120. doi: 10.1073/pnas.0509494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang H., Wang Z., Capó-Aponte J.E., Zhang F., Pan Z., Reinach P.S. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp. Eye Res. 2010;91:462–471. doi: 10.1016/j.exer.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Khajehali E., Malone D.T., Glass M., Sexton P.M., Christopoulos A., Leach K. Biased Agonism and Biased Allosteric Modulation at the CB1 Cannabinoid Receptor. Mol. Pharmacol. 2015;88:368–379. doi: 10.1124/mol.115.099192. [DOI] [PubMed] [Google Scholar]
- 159.Grabiec U., Dehghani F. N-Arachidonoyl Dopamine: A Novel Endocannabinoid and Endovanilloid with Widespread Physiological and Pharmacological Activities. Cannabis Cannabinoid Res. 2017;2:183–196. doi: 10.1089/can.2017.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Redmond W.J., Cawston E.E., Grimsey N.L., Stuart J., Edington A.R., Glass M., Connor M. Identification of N-arachidonoyl dopamine as a highly biased ligand at cannabinoid CB1 receptors. Br. J. Pharmacol. 2016;173:115–127. doi: 10.1111/bph.13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Laprairie R.B., Bagher A.M., Kelly M.E.M., Denovan-Wright E.M. Biased Type 1 Cannabinoid Receptor Signaling Influences Neuronal Viability in a Cell Culture Model of Huntington Disease. Mol. Pharmacol. 2016;89:364–375. doi: 10.1124/mol.115.101980. [DOI] [PubMed] [Google Scholar]
- 162.Breivogel C.S., Vaghela M.S. The effects of beta-arrestin1 deletion on acute cannabinoid activity, brain cannabinoid receptors and tolerance to cannabinoids in mice. J. Recept. Signal Transduct. Res. 2015;35:98–106. doi: 10.3109/10799893.2014.1003659. [DOI] [PubMed] [Google Scholar]
- 163.Shoemaker J.L., Ruckle M.B., Mayeux P.R., Prather P.L. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J. Pharmacol. Exp. Ther. 2005;315:828–838. doi: 10.1124/jpet.105.089474. [DOI] [PubMed] [Google Scholar]
- 164.Soethoudt M., Grether U., Fingerle J., Grim T.W., Fezza F., De Petrocellis L., Ullmer C., Rothenhäusler B., Perret C., Van Gils N., et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat. Commun. 2017;8:1–14. doi: 10.1038/ncomms13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dhopeshwarkar A., Mackie K. Functional selectivity of CB2 cannabinoid receptor ligands at a canonical and noncanonical pathways. J. Pharmacol. Exp. Ther. 2016;358:342–351. doi: 10.1124/jpet.116.232561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bouaboula M., Perrachon S., Milligan L., Canat X., Rinaldi-Carmona M., Portier M., Barth F., Calandra B., Pecceu F., Lupker J., et al. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J. Biol. Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- 167.Pan X., Ikeda S.R., Lewis D.L. SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoid receptor activity. Mol. Pharmacol. 1998;54:1064–1072. doi: 10.1124/mol.54.6.1064. [DOI] [PubMed] [Google Scholar]
- 168.Kearn C.S., Greenberg M.J., DiCamelli R., Kurzawa K., Hillard C.J. Relationships between ligand affinities for the cerebellar cannabinoid receptor CB1 and the induction of GDP/GTP exchange. J. Neurochem. 1999;72:2379–2387. doi: 10.1046/j.1471-4159.1999.0722379.x. [DOI] [PubMed] [Google Scholar]
- 169.Hillard C.J., Muthian S., Kearn C.S. Effects of CB1 cannabinoid receptor activation on cerebellar granule cell nitric oxide synthase activity. FEBS Lett. 1999;459:277–281. doi: 10.1016/S0014-5793(99)01253-3. [DOI] [PubMed] [Google Scholar]
- 170.Vásquez C., Lewis D.L. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J. Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McIntosh H.H., Song C., Howlett A.C. CB1 cannabinoid receptor: Cellular regulation and distribution in N18TG2 neuroblastoma cells. Mol. Brain Res. 1998;53:163–173. doi: 10.1016/S0169-328X(97)00294-5. [DOI] [PubMed] [Google Scholar]
- 172.Leterrier C., Bonnard D., Carrel D., Rossier J., Lenkei Z. Constitutive endocytic cycle of the CB1 cannabinoid receptor. J. Biol. Chem. 2004;279:36013–36021. doi: 10.1074/jbc.M403990200. [DOI] [PubMed] [Google Scholar]
- 173.Rozenfeld R., Devi L.A. Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. FASEB J. 2008;22:2311–2322. doi: 10.1096/fj.07-102731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Meye F.J., Trezza V., Vanderschuren L.J.M.J., Ramakers G.M.J., Adan R.A.H. Neutral antagonism at the cannabinoid 1 receptor: A safer treatment for obesity. Mol. Psychiatry. 2013;18:1294–1301. doi: 10.1038/mp.2012.145. [DOI] [PubMed] [Google Scholar]
- 175.Meye F.J., Ramakers G.M.J., Adan R.A.H. The vital role of constitutive GPCR activity in the mesolimbic dopamine system. Transl. Psychiatry. 2014;4:e361. doi: 10.1038/tp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mancini I., Brusa R., Quadrato G., Foglia C., Scandroglio P., Silverman L., Tulshian D., Reggiani A., Beltramo M. Constitutive activity of cannabinoid-2 (CB 2) receptors plays an essential role in the protean agonism of (+)AM1241 and L768242. Br. J. Pharmacol. 2009;158:382–391. doi: 10.1111/j.1476-5381.2009.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Salort G., Álvaro-Bartolomé M., García-Sevilla J.A. Regulation of cannabinoid CB2 receptor constitutive activity In Vivo: Repeated treatments with inverse agonists reverse the acute activation of JNK and associated apoptotic signaling in mouse brain. Psychopharmacol. Berl. 2017;234:925–941. doi: 10.1007/s00213-017-4537-5. [DOI] [PubMed] [Google Scholar]
- 178.Den Boon F.S., Chameau P., Schaafsma-Zhao Q., Van Aken W., Bari M., Oddi S., Kruse C.G., Maccarrone M., Wadman W.J., Werkmana T.R. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc. Natl. Acad. Sci. USA. 2012;109:3534–3539. doi: 10.1073/pnas.1118167109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Garcia D.E., Brown S., Hille B., Mackie K. Protein kinase C disrupts cannabinoid actions by phosphorylation of the CB1 cannabinoid receptor. J. Neurosci. 1998;18:2834–2841. doi: 10.1523/JNEUROSCI.18-08-02834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bénard G., Massa F., Puente N., Lourenço J., Bellocchio L., Soria-Gómez E., Matias I., Delamarre A., Metna-Laurent M., Cannich A., et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism-Supplementary information. Nat. Neurosci. 2012;15:558–564. doi: 10.1038/nn.3053. [DOI] [PubMed] [Google Scholar]
- 181.Mukhopadhyay B., Liu J., Osei-Hyiaman D., Godlewski G., Mukhopadhyay P., Wang L., Jeong W.-I., Gao B., Duester G., Mackie K., et al. Transcriptional regulation of cannabinoid receptor-1 expression in the liver by retinoic acid acting via retinoic acid receptor-gamma. J. Biol. Chem. 2010;285:19002–19011. doi: 10.1074/jbc.M109.068460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Miller L.K., Devi L.A. The highs and lows of cannabinoid receptor expression in disease: Mechanisms and their therapeutic implications. Pharmacol. Rev. 2011;63:461–470. doi: 10.1124/pr.110.003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Laprairie R.B., Kelly M.E.M., Denovan-Wright E.M. The dynamic nature of type 1 cannabinoid receptor CB1 transcription. Br. J. Pharmacol. 2012;167:1583–1595. doi: 10.1111/j.1476-5381.2012.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hegyi Z., Oláh T., Koszeghy Á., Pisticelli F., Holló K., Pál B., Csernoch L., Di Marzo V., Antal M. CB1 receptor activation induces intracellular Ca2+ mobilization and 2-arachidonoylglycerol release in rodent spinal cord astrocytes. Sci. Rep. 2018;8:1–16. doi: 10.1038/s41598-018-28763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Börner C., Höllt V., Sebald W., Kraus J. Transcriptional regulation of the cannabinoid receptor type 1 gene in T cells by cannabinoids. J. Leukoc. Biol. 2007;81:336–343. doi: 10.1189/jlb.0306224. [DOI] [PubMed] [Google Scholar]
- 186.Lahesmaa M., Eriksson O., Gnad T., Oikonen V., Bucci M., Hirvonen J., Koskensalo K., Teuho J., Niemi T., Taittonen M., et al. Cannabinoid type 1 receptors are upregulated during acute activation of brown adipose tissue. Diabetes. 2018;67:1226–1236. doi: 10.2337/db17-1366. [DOI] [PubMed] [Google Scholar]
- 187.Wong B.S., Camilleri M., Eckert D., Carlson P., Ryks M., Burton D., Zinsmeister A.R. Randomized pharmacodynamic and pharmacogenetic trial of dronabinol effects on colon transit in irritable bowel syndrome-diarrhea. Neurogastroenterol. Motil. 2012;24 doi: 10.1111/j.1365-2982.2011.01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Santoro M., Mirabella M., De Fino C., Bianco A., Lucchini M., Losavio F., Sabino A., Nociti V. Sativex® effects on promoter methylation and on CNR1/CNR2 expression in peripheral blood mononuclear cells of progressive multiple sclerosis patients. J. Neurol. Sci. 2017;379:298–303. doi: 10.1016/j.jns.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 189.Ceccarini J., De Hert M., Van Winkel R., Peuskens J., Bormans G., Kranaster L., Enning F., Koethe D., Leweke F.M., Van Laere K. Increased ventral striatal CB1 receptor binding is related to negative symptoms in drug-free patients with schizophrenia. Neuroimage. 2013;79:304–312. doi: 10.1016/j.neuroimage.2013.04.052. [DOI] [PubMed] [Google Scholar]
- 190.Ceccarini J., Ahmad R., Van De Vliet L., Casteels C., Vandenbulcke M., Vandenberghe W., Van Laere K. Behavioral symptoms in premanifest Huntington disease correlate with reduced frontal CB 1 R levels. J. Nucl. Med. 2019;60:115–121. doi: 10.2967/jnumed.118.210393. [DOI] [PubMed] [Google Scholar]
- 191.You T., Disanzo B.L., Wang X., Yang R., Gong D. Adipose tissue endocannabinoid system gene expression: Depot differences and effects of diet and exercise. Lipids Health Dis. 2011;10 doi: 10.1186/1476-511X-10-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Antunes-Correa L.M., Nobre T.S., Groehs R.V., Alves M.J.N.N., Fernandes T., Couto G.K., Rondon M.U.P.B., Oliveira P., Lima M., Mathias W., et al. Molecular basis for the improvement in muscle metaboreflex and mechanoreflex control in exercise-trained humans with chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H1655–H1666. doi: 10.1152/ajpheart.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.de Luis D.A., Mulero I., Primo D., Izaola O., Aller R. Effects of polymorphism rs3123554 in the cannabinoid receptor gene type 2 (CB2R) on metabolic and adiposity parameters after weight loss with two hypocaloric diets. Diabetes Res. Clin. Pract. 2018;139:339–347. doi: 10.1016/j.diabres.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 194.Rossi F., Mancusi S., Bellini G., Roberti D., Punzo F., Vetrella S., Matarese S.M.R., Nobili B., Maione S., Perrotta S. CNR2 functional variant (Q63R) influences childhood immune thrombocytopenic purpura. Haematologica. 2011;96:1883–1885. doi: 10.3324/haematol.2011.045732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sánchez López A.J., Román-Vega L., Ramil Tojeiro E., Giuffrida A., García-Merino A. Regulation of cannabinoid receptor gene expression and endocannabinoid levels in lymphocyte subsets by interferon-β: A longitudinal study in multiple sclerosis patients. Clin. Exp. Immunol. 2015;179:119–127. doi: 10.1111/cei.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.López-Sendón Moreno J.L., García Caldentey J., Trigo Cubillo P., Ruiz Romero C., García Ribas G., Alonso Arias M.A.A., García de Yébenes M.J., Tolón R.M., Galve-Roperh I., Sagredo O., et al. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington’s disease. J. Neurol. 2016;263:1390–1400. doi: 10.1007/s00415-016-8145-9. [DOI] [PubMed] [Google Scholar]
- 197.Flores-Contreras L., Sandoval-Rodríguez A.S., Mena-Enriquez M.G., Lucano-Landeros S., Arellano-Olivera I., Álvarez-Álvarez A., Sanchez-Parada M.G., Armendáriz-Borunda J. Treatment with pirfenidone for two years decreases fibrosis, cytokine levels and enhances CB2 gene expression in patients with chronic hepatitis C. Bmc Gastroenterol. 2014;14 doi: 10.1186/1471-230X-14-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Engeli S., Lehmann A.C., Kaminski J., Haas V., Janke J., Zoerner A.A., Luft F.C., Tsikas D., Jordan J. Influence of dietary fat intake on the endocannabinoid system in lean and obese subjects. Obesity. 2014;22 doi: 10.1002/oby.20728. [DOI] [PubMed] [Google Scholar]
- 199.Sathyapalan T., Javed Z., Kilpatrick E.S., Coady A.M., Atkin S.L. Endocannabinoid receptor blockade increases vascular endothelial growth factor and inflammatory markers in obese women with polycystic ovary syndrome. Clin. Endocrinol. Oxf. 2017;86:384–387. doi: 10.1111/cen.13239. [DOI] [PubMed] [Google Scholar]
- 200.Andries A., Frystyk J., Flyvbjerg A., Støving R.K. Changes in IGF-I, urinary free cortisol and adipokines during dronabinol therapy in anorexia nervosa: Results from a randomised, controlled trial. Growth Horm. Igf Res. 2015;25:247–252. doi: 10.1016/j.ghir.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 201.Rabinak C.A., Angstadt M., Sripada C.S., Abelson J.L., Liberzon I., Milad M.R., Phan K.L. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64:396–402. doi: 10.1016/j.neuropharm.2012.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chia C.W., Carlson O.D., Liu D.D., González-Mariscal I., Santa-Cruz Calvo S., Egan J.M. Incretin secretion in humans is under the influence of cannabinoid receptors. Am. J. Physiol. Endocrinol. Metab. 2017;313:E359–E366. doi: 10.1152/ajpendo.00080.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Aronne L.J., Finer N., Hollander P.A., England R.D., Klioze S.S., Chew R.D., Fountaine R.J., Powell C.M., Obourn J.D. Efficacy and safety of CP-945,598, a selective cannabinoid CB1 receptor antagonist, on weight loss and maintenance. Obesity. 2011;19:1404–1414. doi: 10.1038/oby.2010.352. [DOI] [PubMed] [Google Scholar]
- 204.Riggs P.K., Vaida F., Rossi S.S., Sorkin L.S., Gouaux B., Grant I., Ellis R.J. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012;1431:46–52. doi: 10.1016/j.brainres.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Topol E.J., Bousser M.-G., Fox K.A., Creager M.A., Despres J.-P., Easton J.D., Hamm C.W., Montalescot G., Steg P.G., Pearson T.A., et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): A randomised, multicentre, placebo-controlled trial. Lancet. 2010;376:517–523. doi: 10.1016/S0140-6736(10)60935-X. [DOI] [PubMed] [Google Scholar]
- 206.Wadden T.A., Fujioka K., Toubro S., Gantz I., Erondu N.E., Chen M., Suryawanshi S., Carofano W., Johnson-Levonas A.O., Shapiro D.R., et al. A randomized trial of lifestyle modification and taranabant for maintaining weight loss achieved with a low-calorie diet. Obesity. 2010;18:2301–2310. doi: 10.1038/oby.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Proietto J., Rissanen A., Harp J.B., Erondu N., Yu Q., Suryawanshi S., Jones M.E., Johnson-Levonas A.O., Heymsfield S.B., Kaufman K.D., et al. A clinical trial assessing the safety and efficacy of the CB1R inverse agonist taranabant in obese and overweight patients: Low-dose study. Int. J. Obes. 2010;34:1243–1254. doi: 10.1038/ijo.2010.38. [DOI] [PubMed] [Google Scholar]
- 208.Rzepa E., Tudge L., McCabe C. The CB1 Neutral Antagonist Tetrahydrocannabivarin Reduces Default Mode Network and Increases Executive Control Network Resting State Functional Connectivity in Healthy Volunteers. Int. J. Neuropsychopharmacol. 2015;19:pyv092. doi: 10.1093/ijnp/pyv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tudge L., Williams C., Cowen P.J., McCabe C. Neural Effects of Cannabinoid CB1 Neutral Antagonist Tetrahydrocannabivarin on Food Reward and Aversion in Healthy Volunteers. Int. J. Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Firsching R., Piek J., Skalej M., Rohde V., Schmidt U., Striggow F. Early survival of comatose patients after severe traumatic brain injury with the dual cannabinoid CB1/CB2 receptor agonist KN38-7271: A randomized, double-blind, placebo-controlled phase II trial. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2012;73:204–216. doi: 10.1055/s-0032-1304815. [DOI] [PubMed] [Google Scholar]
- 211.Kalliomäki J., Annas P., Huizar K., Clarke C., Zettergren A., Karlsten R., Segerdahl M. Evaluation of the analgesic efficacy and psychoactive effects of AZD1940, a novel peripherally acting cannabinoid agonist, in human capsaicin-induced pain and hyperalgesia. Clin. Exp. Pharmacol. Physiol. 2013;40:212–218. doi: 10.1111/1440-1681.12051. [DOI] [PubMed] [Google Scholar]
- 212.Zajicek J., Ball S., Wright D., Vickery J., Nunn A., Miller D., Cano M.G., McManus D., Mallik S., Hobart J. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): A randomised, placebo-controlled trial. Lancet Neurol. 2013;12:857–865. doi: 10.1016/S1474-4422(13)70159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Malik Z., Bayman L., Valestin J., Rizvi-Toner A., Hashmi S., Schey R. Dronabinol increases pain threshold in patients with functional chest pain: A pilot double-blind placebo-controlled trial. Dis. Esophagus. 2017;30 doi: 10.1111/dote.12455. [DOI] [PubMed] [Google Scholar]
- 214.Bergholm R., Sevastianova K., Santos A., Kotronen A., Urjansson M., Hakkarainen A., Lundbom J., Tiikkainen M., Rissanen A., Lundbom N., et al. CB 1 blockade-induced weight loss over 48 weeks decreases liver fat in proportion to weight loss in humans. Int. J. Obes. 2013;37:699–703. doi: 10.1038/ijo.2012.116. [DOI] [PubMed] [Google Scholar]
- 215.O’Leary D.H., Reuwer A.Q., Nissen S.E., Després J.P., Deanfield J.E., Brown M.W., Zhou R., Zabbatino S.M., Job B., Kastelein J.J.P., et al. Effect of rimonabant on carotid intimaemedia thickness (CIMT) progression in patients with abdominal obesity and metabolic syndrome: The AUDITOR Trial. Heart. 2011;97:1143–1150. doi: 10.1136/hrt.2011.223446. [DOI] [PubMed] [Google Scholar]
- 216.Pataky Z., Gasteyger C., Ziegler O., Rissanen A., Hanotin C., Golay A. Efficacy of rimonabant in obese patients with binge eating disorder. Exp. Clin. Endocrinol. Diabetes. 2013;121:20–26. doi: 10.1055/s-0032-1329957. [DOI] [PubMed] [Google Scholar]
- 217.Boggs D.L., Kelly D.L., McMahon R.P., Gold J.M., Gorelick D.A., Linthicum J., Conley R.R., Liu F., Waltz J., Huestis M.A., et al. Rimonabant for neurocognition in schizophrenia: A 16-week double blind randomized placebo controlled trial. Schizophr. Res. 2012;134:207–210. doi: 10.1016/j.schres.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Backhouse K., Sarac I., Shojaee-Moradie F., Stolinski M., Robertson M.D., Frost G.S., Bell J.D., Thomas E.L., Wright J., Russell-Jones D., et al. Fatty acid flux and oxidation are increased by rimonabant in obese women. Metabolism. 2012;61:1220–1223. doi: 10.1016/j.metabol.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 219.Triay J., Mundi M., Klein S., Toledo F.G., Smith S.R., Abu-Lebdeh H., Jensen M. Does rimonabant independently affect free fatty acid and glucose metabolism? J. Clin. Endocrinol. Metab. 2012;97:819–827. doi: 10.1210/jc.2011-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Van Den Elsen G.A.H., Ahmed A.I.A., Verkes R.J., Kramers C., Feuth T., Rosenberg P.B., Van Der Marck M.A., Olde Rikkert M.G.M. Tetrahydrocannabinol for neuropsychiatric symptoms in dementia: A randomized controlled trial. Neurology. 2015;84:2338–2346. doi: 10.1212/WNL.0000000000001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Elphick M.R. The evolution and comparative neurobiology of endocannabinoid signalling. Philos. Trans. R. Soc. B Biol. Sci. 2012;367:3201–3215. doi: 10.1098/rstb.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Elphick M.R., Egertová M. The phylogenetic distribution and evolutionary origins of endocannabinoid signalling. Handb. Exp. Pharmacol. 2005;168:283–297. doi: 10.1007/3-540-26573-2_9. [DOI] [PubMed] [Google Scholar]
- 223.Paria B.C., Das S.K., Dey S.K. The preimplantation mouse embryo is a target for cannabinoid ligand-receptor signaling. Proc. Natl. Acad. Sci. USA. 1995;92:9460–9464. doi: 10.1073/pnas.92.21.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yang Z.M., Paria B.C., Dey S.K. Activation of brain-type cannabinoid receptors interferes with preimplantation mouse embryo development. Biol. Reprod. 1996;55:756–761. doi: 10.1095/biolreprod55.4.756. [DOI] [PubMed] [Google Scholar]
- 225.Paria B.C., Ma W., Andrenyak D.M., Schmid P.C., Schmid H.H., Moody D.E., Deng H., Makriyannis A., Dey S.K. Effects of cannabinoids on preimplantation mouse embryo development and implantation are mediated by brain-type cannabinoid receptors. Biol. Reprod. 1998;58:1490–1495. doi: 10.1095/biolreprod58.6.1490. [DOI] [PubMed] [Google Scholar]
- 226.Buckley N.E., Hansson S., Harta G., Mezey É. Expression of the CB1 and CB2 receptor messenger RNAs during embryonic development in the rat. Neuroscience. 1997;82:1131–1149. doi: 10.1016/S0306-4522(97)00348-5. [DOI] [PubMed] [Google Scholar]
- 227.Habayeb O.M.H., Taylor A.H., Bell S.C., Taylor D.J., Konje J.C. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology. 2008;149:5052–5060. doi: 10.1210/en.2007-1799. [DOI] [PubMed] [Google Scholar]
- 228.Sufian M.S., Amin M.R., Kanyo R., Ted Allison W., Ali D.W. CB1 and CB2 receptors play differential roles in early zebrafish locomotor development. J. Exp. Biol. 2019;222 doi: 10.1242/jeb.206680. [DOI] [PubMed] [Google Scholar]
- 229.Jiang S., Fu Y., Williams J., Wood J., Pandarinathan L., Avraham S., Makriyannis A., Avraham S., Avraham H.K. Expression and function of cannabinoid receptors CB1 and CB2 and their cognate cannabinoid ligands in murine embryonic stem cells. PLoS ONE. 2007;2:e641. doi: 10.1371/journal.pone.0000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bari M., Tedesco M., Battista N., Pasquariello N., Pucci M., Gasperi V., Scaldaferri M.L., Farini D., De Felici M., Maccarrone M. Characterization of the endocannabinoid system in mouse embryonic stem cells. Stem Cells Dev. 2011;20:139–147. doi: 10.1089/scd.2009.0515. [DOI] [PubMed] [Google Scholar]
- 231.Nones J., Spohr T.C., Furtado D.R., Sartore R.C., Paulsen B.S., Guimarães M.Z., Rehen S.K. Cannabinoids modulate cell survival in embryoid bodies. Cell Biol. Int. 2010;34:399–408. doi: 10.1042/CBI20090036. [DOI] [PubMed] [Google Scholar]
- 232.Berrendero F., García-Gil L., Hernández M.L., Romero J., Cebeira M., De Miguel R., Ramos J.A., Fernández-Ruiz J.J. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179–3188. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- 233.Rueda D., Navarro B., Martinez-Serrano A., Guzman M., Galve-Roperh I. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J. Biol. Chem. 2002;277:46645–46650. doi: 10.1074/jbc.M206590200. [DOI] [PubMed] [Google Scholar]
- 234.Jin K., Xie L., Kim S.H., Parmentier-Batteur S., Sun Y., Mao X.O., Childs J., Greenberg D.A. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol. Pharmacol. 2004;66:204–208. doi: 10.1124/mol.66.2.204. [DOI] [PubMed] [Google Scholar]
- 235.Aguado T., Monory K., Palazuelos J., Stella N., Cravatt B., Lutz B., Marsicano G., Kokaia Z., Guzmán M., Galve-Roperh I. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- 236.Jiang W., Zhang Y., Xiao L., Van Cleemput J., Ji S.-P., Bai G., Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J. Clin. Investig. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Berghuis P., Rajnicek A.M., Morozov Y.M., Ross R.A., Mulder J., Urbán G.M., Monory K., Marsicano G., Matteoli M., Canty A., et al. Hardwiring the brain: Endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- 238.Mulder J., Aguado T., Keimpema E., Barabás K., Ballester Rosado C.J., Nguyen L., Monory K., Marsicano G., Di Marzo V., Hurd Y.L., et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc. Natl. Acad. Sci. USA. 2008;105:8760–8765. doi: 10.1073/pnas.0803545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wu C.-S., Zhu J., Wager-Miller J., Wang S., O’Leary D., Monory K., Lutz B., Mackie K., Lu H.-C. Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. Eur. J. Neurosci. 2010;32:693–706. doi: 10.1111/j.1460-9568.2010.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Aguado T., Palazuelos J., Monory K., Stella N., Cravatt B., Lutz B., Marsicano G., Kokaia Z., Guzmán M., Galve-Roperh I. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J. Neurosci. 2006;26:1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ilyasov A.A., Milligan C.E., Pharr E.P., Howlett A.C. The Endocannabinoid System and Oligodendrocytes in Health and Disease. Front. Neurosci. 2018;12:733. doi: 10.3389/fnins.2018.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Palazuelos J., Ortega Z., Díaz-Alonso J., Guzmán M., Galve-Roperh I. CB 2 cannabinoid receptors promote neural progenitor cell proliferation via mTORC1 signaling. J. Biol. Chem. 2012;287:1198–1209. doi: 10.1074/jbc.M111.291294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mereu G., Fà M., Ferraro L., Cagiano R., Antonelli T., Tattoli M., Ghiglieri V., Tanganelli S., Gessa G.L., Cuomo V. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc. Natl. Acad. Sci. USA. 2003;100:4915–4920. doi: 10.1073/pnas.0537849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Antonelli T., Tomasini M.C., Tattoli M., Cassano T., Tanganelli S., Finetti S., Mazzoni E., Trabace L., Steardo L., Cuomo V., et al. Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb. Cortex. 2005;15:2013–2020. doi: 10.1093/cercor/bhi076. [DOI] [PubMed] [Google Scholar]
- 245.Bernard C., Milh M., Morozov Y.M., Ben-Ari Y., Freund T.F., Gozlan H. Altering cannabinoid signaling during development disrupts neuronal activity. Proc. Natl. Acad. Sci. USA. 2005;102:9388–9393. doi: 10.1073/pnas.0409641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.MacCarrone M., Rossi S., Bari M., De Chiara V., Rapino C., Musella A., Bernardi G., Bagni C., Centonze D. Abnormal mGlu 5 receptor/endocannabinoid coupling in mice lacking FMRP and BC1 RNA. Neuropsychopharmacology. 2010;35:1500–1509. doi: 10.1038/npp.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zhang L., Alger B.E. Enhanced Endocannabinoid Signaling Elevates Neuronal Excitability in Fragile X Syndrome. J. Neurosci. 2010;30:5724–5729. doi: 10.1523/JNEUROSCI.0795-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Busquets-Garcia A., Gomis-González M., Guegan T., Agustín-Pavón C., Pastor A., Mato S., Pérez-Samartín A., Matute C., de la Torre R., Dierssen M., et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat. Med. 2013;19:603–607. doi: 10.1038/nm.3127. [DOI] [PubMed] [Google Scholar]
- 249.Purcell A.E., Jeon O.H., Zimmerman A.W., Blue M.E., Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/WNL.57.9.1618. [DOI] [PubMed] [Google Scholar]
- 250.Chakrabarti B., Baron-Cohen S. Variation in the human cannabinoid receptor CNR1 gene modulates gaze duration for happy faces. Mol. Autism. 2011;2:10. doi: 10.1186/2040-2392-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chakrabarti B., Persico A., Battista N., Maccarrone M. Endocannabinoid Signaling in Autism. Neurotherapeutics. 2015;12:837–847. doi: 10.1007/s13311-015-0371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lu A.T., Ogdie M.N., Järvelin M.R., Moilanen I.K., Loo S.K., McCracken J.T., McGough J.J., Yang M.H., Peltonen L., Nelson S.F., et al. Association of the cannabinoid receptor gene (CNR1) with ADHD and post-traumatic stress disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2008;147:1488–1494. doi: 10.1002/ajmg.b.30693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Beltramo M., Rodríguez De Fonseca F., Navarro M., Calignano A., Gorriti M.A., Grammatikopoulos G., Sadile A.G., Giuffrida A., Piomelli D. Reversal of dopamine D2 receptor responses by an anandamide transport inhibitor. J. Neurosci. 2000;20:3401–3407. doi: 10.1523/JNEUROSCI.20-09-03401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Adriani W., Caprioli A., Granstrem O., Carli M., Laviola G. The spontaneously hypertensive-rat as an animal model of ADHD: Evidence for impulsive and non-impulsive subpopulations. Neurosci. Biobehav. Rev. 2003;27:639–651. doi: 10.1016/j.neubiorev.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 255.Strohbeck-Kuehner P., Skopp G., Mattern R. Cannabis improves symptoms of ADHD. Cannabinoids. 2008;3:1–3. [Google Scholar]
- 256.Hupli A.M.M. Medical Cannabis for Adult Attention Deficit Hyperactivity Disorder: Sociological Patient Case Report of Cannabinoid Therapeutics in Finland. Med. Cannabis Cannabinoids. 2018;1:112–118. doi: 10.1159/000495307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Saito A., Ballinger M.D.L., Pletnikov M.V., Wong D.F., Kamiya A. Endocannabinoid system: Potential novel targets for treatment of schizophrenia. Neurobiol. Dis. 2013;53:10–17. doi: 10.1016/j.nbd.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Marconi A., Di Forti M., Lewis C.M., Murray R.M., Vassos E. Meta-Analysis of the association between the level of cannabis use and risk of psychosis. Schizophr. Bull. 2016;42:1262–1269. doi: 10.1093/schbul/sbw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Renard J., Krebs M.O., Le Pen G., Jay T.M. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front. Neurosci. 2014;8 doi: 10.3389/fnins.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rubino T., Parolaro D. The impact of exposure to cannabinoids in adolescence: Insights from animal models. Biol. Psychiatry. 2016;79:578–585. doi: 10.1016/j.biopsych.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 261.Dean B., Sundram S., Bradbury R., Scarr E., Copolov D.D. Studies on [3H]CP-55940 binding in the human central nervous system: Regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103:9–15. doi: 10.1016/S0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- 262.Newell K.A., Deng C., Huang X.F. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp. Brain Res. 2006;172:556–560. doi: 10.1007/s00221-006-0503-x. [DOI] [PubMed] [Google Scholar]
- 263.Eggan S.M., Hashimoto T., Lewis D.A. Reduced cortical cannabinoid 1 receptor messenger RNA and protein expression in schizophrenia. Arch. Gen. Psychiatry. 2008;65:772–784. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Eggan S.M., Stoyak S.R., Verrico C.D., Lewis D.A. Cannabinoid CB1 receptor immunoreactivity in the prefrontal cortex: Comparison of schizophrenia and major depressive disorder. Neuropsychopharmacology. 2010;35:2060–2071. doi: 10.1038/npp.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wong D.F., Kuwabara H., Horti A.G., Raymont V., Brasic J., Guevara M., Ye W., Dannals R.F., Ravert H.T., Nandi A., et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. Neuroimage. 2010;52:1505–1513. doi: 10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Gomes F.V., Edelson J.R., Volk D.W., Grace A.A. Altered brain cannabinoid 1 receptor mRNA expression across postnatal development in the MAM model of schizophrenia. Schizophr. Res. 2018;201:254–260. doi: 10.1016/j.schres.2018.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Black M.D., Stevens R.J., Rogacki N., Featherstone R.E., Senyah Y., Giardino O., Borowsky B., Stemmelin J., Cohen C., Pichat P., et al. AVE1625, a cannabinoid CB1 receptor antagonist, as a co-treatment with antipsychotics for schizophrenia: Improvement in cognitive function and reduction of antipsychotic-side effects in rodents. Psychopharmacol. Berl. 2011;215:149–163. doi: 10.1007/s00213-010-2124-0. [DOI] [PubMed] [Google Scholar]
- 268.Kruk-Slomka M., Budzynska B., Slomka T., Banaszkiewicz I., Biala G. The Influence of the CB1 Receptor Ligands on the Schizophrenia-Like Effects in Mice Induced by MK-801. Neurotox. Res. 2016;30:658–676. doi: 10.1007/s12640-016-9662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wilson R.I., Nicoll R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 270.Ohno-Shosaku T., Maejima T., Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/S0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 271.Alger B.E. Retrograde signaling in the regulation of synaptic transmission: Focus on endocannabinoids. Prog. Neurobiol. 2002;68:247–286. doi: 10.1016/S0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 272.Pitler T.A., Alger B.E. Depolarization-induced suppression of GABAergic inhibition in rat hippocampal pyramidal cells: G protein involvement in a presynaptic mechanism. Neuron. 1994;13:1447–1455. doi: 10.1016/0896-6273(94)90430-8. [DOI] [PubMed] [Google Scholar]
- 273.Katona I., Freund T.F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 2008;14:923–930. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- 274.Kreitzer A.C., Regehr W.G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/S0896-6273(01)00246-X. [DOI] [PubMed] [Google Scholar]
- 275.Maejima T., Hashimoto K., Yoshida T., Aiba A., Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/S0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- 276.Galante M., Diana M.A. Group I metabotropic glutamate receptors inhibit GABA release at interneuron-Purkinje cell synapses through endocannabinoid production. J. Neurosci. 2004;24:4865–4874. doi: 10.1523/JNEUROSCI.0403-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Robbe D., Kopf M., Remaury A., Bockaert J., Manzoni O.J. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. USA. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Safo P.K., Regehr W.G. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48:647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 279.Auclair N., Otani S., Soubrie P., Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J. Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- 280.Carlson G., Wang Y., Alger B.E. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat. Neurosci. 2002;5:723–724. doi: 10.1038/nn879. [DOI] [PubMed] [Google Scholar]
- 281.Bohme G.A., Laville M., Ledent C., Parmentier M., Imperato A. Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience. 1999;95:5–7. doi: 10.1016/S0306-4522(99)00483-2. [DOI] [PubMed] [Google Scholar]
- 282.Slanina K.A., Roberto M., Schweitzer P. Endocannabinoids restrict hippocampal long-term potentiation via CB1. Neuropharmacology. 2005;49:660–668. doi: 10.1016/j.neuropharm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 283.Basavarajappa B.S., Nagre N.N., Xie S., Subbanna S. Elevation of endogenous anandamide impairs LTP, learning, and memory through CB1 receptor signaling in mice. Hippocampus. 2014;24:808–818. doi: 10.1002/hipo.22272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Silva-Cruz A., Carlström M., Ribeiro J.A., Sebastião A.M. Dual influence of endocannabinoids on long-term potentiation of synaptic transmission. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Puighermanal E., Marsicano G., Busquets-Garcia A., Lutz B., Maldonado R., Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat. Neurosci. 2009;12:1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- 286.Wilson R.I., Kunos G., Nicoll R.A. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/S0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- 287.Chevaleyre V., Heifets B.D., Kaeser P.S., Südhof T.C., Castillo P.E. Endocannabinoid-Mediated Long-Term Plasticity Requires cAMP/PKA Signaling and RIM1α. Neuron. 2007;55:169. doi: 10.1016/j.neuron.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Yin H.H., Davis M.I., Ronesi J.A., Lovinger D.M. The role of protein synthesis in striatal long-term depression. J. Neurosci. 2006;26:11811–11820. doi: 10.1523/JNEUROSCI.3196-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Heifets B.D., Castillo P.E. Endocannabinoid Signaling and Long-Term Synaptic Plasticity. Annu. Rev. Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Fellin T. Communication between neurons and astrocytes: Relevance to the modulation of synaptic and network activity. J. Neurochem. 2009;108:533–544. doi: 10.1111/j.1471-4159.2008.05830.x. [DOI] [PubMed] [Google Scholar]
- 291.Araque A., Parpura V., Sanzgiri R.P., Haydon P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/S0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 292.Navarrete M., Diez A., Araque A. Astrocytes in endocannabinoid signalling. Philos. Trans. R. Soc. B Biol. Sci. 2014;369:20130599. doi: 10.1098/rstb.2013.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Navarrete M., Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68:113–126. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 294.Gómez-Gonzalo M., Navarrete M., Perea G., Covelo A., Martín-Fernández M., Shigemoto R., Luján R., Araque A. Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cereb. Cortex. 2015;25:3699–3712. doi: 10.1093/cercor/bhu231. [DOI] [PubMed] [Google Scholar]
- 295.Han J., Kesner P., Metna-Laurent M., Duan T., Xu L., Georges F., Koehl M., Abrous D.N., Mendizabal-Zubiaga J., Grandes P., et al. Acute Cannabinoids Impair Working Memory through Astroglial CB1 Receptor Modulation of Hippocampal LTD. Cell. 2012;148:1039–1050. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 296.Castillo P.E., Younts T.J., Chávez A.E., Hashimotodani Y. Endocannabinoid Signaling and Synaptic Function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Oliveira da Cruz J.F., Robin L.M., Drago F., Marsicano G., Metna-Laurent M. Astroglial type-1 cannabinoid receptor (CB1): A new player in the tripartite synapse. Neuroscience. 2016;323:35–42. doi: 10.1016/j.neuroscience.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 298.Guerra-Gomes S., Sousa N., Pinto L., Oliveira J.F. Functional Roles of Astrocyte Calcium Elevations: From Synapses to Behavior. Front. Cell. Neurosci. 2018;11:427. doi: 10.3389/fncel.2017.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Gong J.P., Onaivi E.S., Ishiguro H., Liu Q.R., Tagliaferro P.A., Brusco A., Uhl G.R. Cannabinoid CB2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 300.Onaivi E.S. Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology. 2007;54:231–246. doi: 10.1159/000100778. [DOI] [PubMed] [Google Scholar]
- 301.Zhang J., Hoffert C., Vu H.K., Groblewski T., Ahmad S., O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- 302.Ashton J.C. The use of knockout mice to test the specificity of antibodies for cannabinoid receptors. Hippocampus. 2012;22:643–644. doi: 10.1002/hipo.20946. [DOI] [PubMed] [Google Scholar]
- 303.Cécyre B., Thomas S., Ptito M., Casanova C., Bouchard J.F. Evaluation of the specificity of antibodies raised against cannabinoid receptor type 2 in the mouse retina. Naunyn. Schmiedebergs. Arch. Pharmacol. 2014;387:175–184. doi: 10.1007/s00210-013-0930-8. [DOI] [PubMed] [Google Scholar]
- 304.Li Y., Kim J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience. 2015;311:253–267. doi: 10.1016/j.neuroscience.2015.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Van Sickle M.D., Duncan M., Kingsley P.J., Mouihate A., Urbani P., Mackie K., Stella N., Makriyannis A., Piomelli D., Davison J.S., et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 306.Zhang H.Y., Gao M., Liu Q.R., Bi G.H., Li X., Yang H.J., Gardner E.L., Wu J., Xi Z.X. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc. Natl. Acad. Sci. USA. 2014;111:E5007–E5015. doi: 10.1073/pnas.1413210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Onaivi E.S., Ishiguro H., Gong J.P., Patel S., Meozzi P.A., Myers L., Perchuk A., Mora Z., Tagliaferro P.A., Gardner E., et al. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: From mice to human subjects. PLoS ONE. 2008;3:e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Ma Z., Gao F., Larsen B., Gao M., Luo Z., Chen D., Ma X., Qiu S., Zhou Y., Xie J., et al. Mechanisms of cannabinoid CB 2 receptor-mediated reduction of dopamine neuronal excitability in mouse ventral tegmental area. EBioMedicine. 2019;42:225–237. doi: 10.1016/j.ebiom.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Marchalant Y., Cerbai F., Brothers H.M., Wenk G.L. Cannabinoid receptor stimulation is anti-inflammatory and improves memory in old rats. Neurobiol. Aging. 2008;29:1894–1901. doi: 10.1016/j.neurobiolaging.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Sánchez-Blázquez P., Rodríguez-Muñoz M., Vicente-Sánchez A., Garzón J. Cannabinoid receptors couple to NMDA receptors to reduce the production of NO and the mobilization of zinc induced by glutamate. Antioxid. Redox Signal. 2013;19:1766–1782. doi: 10.1089/ars.2012.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Jia J., Ma L., Wu M., Zhang L., Zhang X., Zhai Q., Jiang T., Wang Q., Xiong L. Anandamide protects HT22 cells exposed to hydrogen peroxide by inhibiting CB1 receptor-mediated type 2 NADPH oxidase. Oxid. Med. Cell. Longev. 2014;2014 doi: 10.1155/2014/893516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Braun M., Khan Z.T., Khan M.B., Kumar M., Ward A., Achyut B.R., Arbab A.S., Hess D.C., Hoda M.N., Baban B., et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain. Behav. Immun. 2018;68:224–237. doi: 10.1016/j.bbi.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Sahu P., Mudgal J., Arora D., Kinra M., Mallik S.B., Rao C.M., Pai K.S.R., Nampoothiri M. Cannabinoid receptor 2 activation mitigates lipopolysaccharide-induced neuroinflammation and sickness behavior in mice. Psychopharmacol. Berl. 2019;236:1829–1838. doi: 10.1007/s00213-019-5166-y. [DOI] [PubMed] [Google Scholar]
- 314.Streit W.J., Mrak R.E., Griffin W.S.T. Microglia and neuroinflammation: A pathological perspective. J. Neuroinflammation. 2004;1 doi: 10.1186/1742-2094-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Frank-Cannon T.C., Alto L.T., McAlpine F.E., Tansey M.G. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol. Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Bélanger M., Magistretti P.J. The role of astroglia in neuroprotection. Dialogues Clin. Neurosci. 2009;11:281–295. doi: 10.31887/DCNS.2009.11.3/mbelanger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Molina-Holgado F., Pinteaux E., Moore J.D., Molina-Holgado E., Guaza C., Gibson R.M., Rothwell N.J. Endogenous interleukin-1 receptor antagonist mediates anti-inflammatory and neuroprotective actions of cannabinoids in neurons and glia. J. Neurosci. 2003;23:6470–6474. doi: 10.1523/JNEUROSCI.23-16-06470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sheng W.S., Hu S., Min X., Cabral G.A., Lokensgard J.R., Peterson P.K. Synthetic cannabinoid WIN55,212-2 inhibits generation of inflammatory mediators by IL-1beta-stimulated human astrocytes. Glia. 2005;49:211–219. doi: 10.1002/glia.20108. [DOI] [PubMed] [Google Scholar]
- 319.Nagarkatti P., Pandey R., Rieder S.A., Hegde V.L., Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009;1:1333–1349. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Gómez Del Pulgar T., De Ceballos M.L., Guzmán M., Velasco G. Cannabinoids protect astrocytes from ceramide-induced apoptosis through the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 2002;277:36527–36533. doi: 10.1074/jbc.M205797200. [DOI] [PubMed] [Google Scholar]
- 321.Carracedo A., Geelen M.J.H., Diez M., Hanada K., Guzmán M., Velasco G. Ceramide sensitizes astrocytes to oxidative stress: Protective role of cannabinoids. Biochem. J. 2004;380:435–440. doi: 10.1042/bj20031714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Chen Y., Luo X., Liu S., Shen Y. Neuroprotective effect of cannabinoid receptor 1 antagonist in the MNU-induced retinal degeneration model. Exp. Eye Res. 2018;167:145–151. doi: 10.1016/j.exer.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 323.Zhang D., Liu X., Dong X., Zhu R., Jiang J., Ye Y., Jiang Y. Cannabinoid 1 Receptor Antagonists Play a Neuroprotective Role in Chronic Alcoholic Hippocampal Injury Related to Pyroptosis Pathway. Alcohol. Clin. Exp. Res. 2020:14391. doi: 10.1111/acer.14391. [DOI] [PubMed] [Google Scholar]
- 324.Molina-Holgado F., Molina-Holgado E., Guaza C., Rothwell N.J. Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide-induced nitric oxide release in astrocyte cultures. J. Neurosci. Res. 2002;67:829–836. doi: 10.1002/jnr.10165. [DOI] [PubMed] [Google Scholar]
- 325.Waksman Y., Olson J.M., Carlisle S.J., Cabral G.A. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J. Pharmacol. Exp. Ther. 1999;288:1357–1366. [PubMed] [Google Scholar]
- 326.Correa F., Hernangómez M., Mestre L., Loría F., Spagnolo A., Docagne F., Di Marzo V., Guaza C. Anandamide enhances IL-10 production in activated microglia by targeting CB2 receptors: Roles of ERK1/2, JNK, and NF-κB. Glia. 2010;58:135–147. doi: 10.1002/glia.20907. [DOI] [PubMed] [Google Scholar]
- 327.Merighi S., Gessi S., Varani K., Simioni C., Fazzi D., Mirandola P., Borea P.A. Cannabinoid CB2 receptors modulate ERK-1/2 kinase signalling and NO release in microglial cells stimulated with bacterial lipopolysaccharide. Br. J. Pharmacol. 2012;165:1773–1788. doi: 10.1111/j.1476-5381.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 328.Malek N., Popiolek-Barczyk K., Mika J., Przewlocka B., Starowicz K. Anandamide, Acting via CB2 Receptors, Alleviates LPS-Induced Neuroinflammation in Rat Primary Microglial Cultures. Neural Plast. 2015;2015:130639. doi: 10.1155/2015/130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ma L., Jia J., Liu X., Bai F., Wang Q., Xiong L. Activation of murine microglial N9 cells is attenuated through cannabinoid receptor CB2 signaling. Biochem. Biophys. Res. Commun. 2015;458:92–97. doi: 10.1016/j.bbrc.2015.01.073. [DOI] [PubMed] [Google Scholar]
- 330.Ramirez S.H., Haskó J., Skuba A., Fan S., Dykstra H., McCormick R., Reichenbach N., Krizbai I., Mahadevan A., Zhang M., et al. Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions. J. Neurosci. 2012;32:4004–4016. doi: 10.1523/JNEUROSCI.4628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Elliott M.B., Tuma R.F., Amenta P.S., Barbe M.F., Jallo J.I. Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a model of traumatic brain injury. J. Neurotrauma. 2011;28:973–981. doi: 10.1089/neu.2010.1672. [DOI] [PubMed] [Google Scholar]
- 332.Amenta P.S., Jallo J.I., Tuma R.F., Elliott M.B. A cannabinoid type 2 receptor agonist attenuates blood-brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 2012;90:2293–2305. doi: 10.1002/jnr.23114. [DOI] [PubMed] [Google Scholar]
- 333.Amenta P.S., Jallo J.I., Tuma R.F., Craig Hooper D., Elliott M.B. Cannabinoid receptor type-2 stimulation, blockade, and deletion alter the vascular inflammatory responses to traumatic brain injury. J. Neuroinflammation. 2014;11:191. doi: 10.1186/s12974-014-0191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Cabral G.A., Griffin-Thomas L.T. Emerging role of the cannabinoid receptor CB 2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 2009;11 doi: 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Wada J.A., Wake A., Sato M., Corcoran M.E. Antiepileptic and prophylactic effects of tetrahydrocannabinols in amygdaloid kindled cats. Epilepsia. 1975;16:503–510. doi: 10.1111/j.1528-1157.1975.tb06080.x. [DOI] [PubMed] [Google Scholar]
- 336.Karler R., Turkanis S.A. Subacute cannabinoid treatment: Anticonvulsant activity and withdrawal excitability in mice. Br. J. Pharmacol. 1980;68:479–484. doi: 10.1111/j.1476-5381.1980.tb14562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Wallace M.J., Wiley J.L., Martin B.R., DeLorenzo R.J. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur. J. Pharmacol. 2001;428:51–57. doi: 10.1016/S0014-2999(01)01243-2. [DOI] [PubMed] [Google Scholar]
- 338.Wallace M.J., Blair R.E., Falenski K.W., Martin B.R., DeLorenzo R.J. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 2003;307:129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- 339.Marsicano G., Goodenough S., Monory K., Hermann H., Eder M., Cannich A., Azad S.C., Cascio M.G., Ortega-Gutiérrez S., Van der Stelt M., et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- 340.Monory K., Massa F., Egertová M., Eder M., Blaudzun H., Westenbroek R., Kelsch W., Jacob W., Marsch R., Ekker M., et al. The Endocannabinoid System Controls Key Epileptogenic Circuits in the Hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Karanian D.A., Karim S.L., Wood J.T., Williams J.S., Lin S., Makriyannis A., Bahr B.A. Endocannabinoid enhancement protects against kainic acid-induced seizures and associated brain damage. J. Pharmacol. Exp. Ther. 2007;322:1059–1066. doi: 10.1124/jpet.107.120147. [DOI] [PubMed] [Google Scholar]
- 342.Guggenhuber S., Monory K., Lutz B., Klugmann M. AAV vector-mediated overexpression of CB1 cannabinoid receptor in pyramidal neurons of the hippocampus protects against seizure-induced excitoxicity. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0015707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Bhaskaran M.D., Smith B.N. Cannabinoid-mediated inhibition of recurrent excitatoryCircuitry in the dentate gyrus in a mouse model of temporal lobe epilepsy. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0010683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Chen K., Neu A., Howard A.L., Földy C., Echegoyen J., Hilgenberg L., Smith M., Mackie K., Soltesz I. Prevention of plasticity of endocannabinoid signaling inhibits persistent limbic hyperexcitability caused by developmental seizures. J. Neurosci. 2007;27:46–58. doi: 10.1523/JNEUROSCI.3966-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Chen K., Ratzliff A., Hilgenberg L., Gulyás A., Freund T.F., Smith M., Dinh T.P., Piomelli D., Mackie K., Soltesz I. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39:599–611. doi: 10.1016/S0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- 346.Ludányi A., Eross L., Czirják S., Vajda J., Halász P., Watanabe M., Palkovits M., Maglóczky Z., Freund T.F., Katona I. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J. Neurosci. 2008;28:2976–2990. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Goffin K., Van Paesschen W., Van Laere K. In Vivo activation of endocannabinoid system in temporal lobe epilepsy with hippocampal sclerosis. Brain. 2011;134:1033–1040. doi: 10.1093/brain/awq385. [DOI] [PubMed] [Google Scholar]
- 348.Falenski K.W., Carter D.S., Harrison A.J., Martin B.R., Blair R.E., DeLorenzo R.J. Temporal characterization of changes in hippocampal cannabinoid CB(1) receptor expression following pilocarpine-induced status epilepticus. Brain Res. 2009;1262:64–72. doi: 10.1016/j.brainres.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Karlócai M.R., Tóth K., Watanabe M., Ledent C., Juhász G., Freund T.F., Maglóczky Z. Redistribution of CB1 cannabinoid receptors in the acute and chronic phases of pilocarpine-induced epilepsy. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0027196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Sales-Carbonell C., Rueda-Orozco P.E., Soria-Gómez E., Buzsáki G., Marsicano G., Robbe D. Striatal GABAergic and cortical glutamatergic neurons mediate contrasting effects of cannabinoids on cortical network synchrony. Proc. Natl. Acad. Sci. USA. 2013;110:719–724. doi: 10.1073/pnas.1217144110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Vilela L.R., Medeiros D.C., Rezende G.H.S., de Oliveira A.C.P., Moraes M.F.D., Moreira F.A. Effects of cannabinoids and endocannabinoid hydrolysis inhibition on pentylenetetrazole-induced seizure and electroencephalographic activity in rats. Epilepsy Res. 2013;104:195–202. doi: 10.1016/j.eplepsyres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 352.Huizenga M.N., Wicker E., Beck V.C., Forcelli P.A. Anticonvulsant effect of cannabinoid receptor agonists in models of seizures in developing rats. Epilepsia. 2017;58:1593–1602. doi: 10.1111/epi.13842. [DOI] [PubMed] [Google Scholar]
- 353.Naderi N., Aziz Ahari F., Shafaghi B., Najarkolaei A.H., Motamedi F. Evaluation of interactions between cannabinoid compounds and diazepam in electroshock-induced seizure model in mice. J. Neural Transm. 2008;115:1501–1511. doi: 10.1007/s00702-008-0076-x. [DOI] [PubMed] [Google Scholar]
- 354.Turkanis S.A., Karler R. Central excitatory properties of delta 9-tetrahydrocannabinol and its metabolites in iron-induced epileptic rats. Neuropharmacology. 1982;21:7–13. doi: 10.1016/0028-3908(82)90204-0. [DOI] [PubMed] [Google Scholar]
- 355.Gordon E., Devinsky O. Alcohol and marijuana: Effects on epilepsy and use by patients with epilepsy. Epilepsia. 2001;42:1266–1272. doi: 10.1046/j.1528-1157.2001.19301.x. [DOI] [PubMed] [Google Scholar]
- 356.Clement A.B., Hawkins E.G., Lichtman A.H., Cravatt B.F. Increased seizure susceptibility and proconvulsant activity of anandamide in mice lacking fatty acid amide hydrolase. J. Neurosci. 2003;23:3916–3923. doi: 10.1523/JNEUROSCI.23-09-03916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Deshpande L.S., Sombati S., Blair R.E., Carter D.S., Martin B.R., DeLorenzo R.J. Cannabinoid CB1 receptor antagonists cause status epilepticus-like activity in the hippocampal neuronal culture model of acquired epilepsy. Neurosci. Lett. 2007;411:11–16. doi: 10.1016/j.neulet.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Braakman H.M.H., Van Oostenbrugge R.J., Van Kranen-Mastenbroek V.H.J.M., De Krom M.C.T.F.M. Rimonabant induces partial seizures in a patient with a history of generalized epilepsy: Letters/commentary. Epilepsia. 2009;50:2171–2172. doi: 10.1111/j.1528-1167.2009.02203.x. [DOI] [PubMed] [Google Scholar]
- 359.Di Marzo V. Targeting the endocannabinoid system: To enhance or reduce? Nat. Rev. Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 360.Rizzo V., Ferraro G., Carletti F., Lonobile G., Cannizzaro C., Sardo P. Evidences of cannabinoids-induced modulation of paroxysmal events in an experimental model of partial epilepsy in the rat. Neurosci. Lett. 2009;462:135–139. doi: 10.1016/j.neulet.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 361.Hill T.D.M., Cascio M.G., Romano B., Duncan M., Pertwee R.G., Williams C.M., Whalley B.J., Hill A.J. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br. J. Pharmacol. 2013;170:679–692. doi: 10.1111/bph.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Rizzo V., Carletti F., Gambino G., Schiera G., Cannizzaro C., Ferraro G., Sardo P. Role of CB2 receptors and cGMP pathway on the cannabinoid-dependent antiepileptic effects in an in vivo model of partial epilepsy. Epilepsy Res. 2014;108:1711–1718. doi: 10.1016/j.eplepsyres.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 363.Cao Q., Liu X., Yang F., Wang H. CB2R induces a protective response for epileptic seizure via the PI3K 110α-AKT signaling pathway. Exp. Ther. Med. 2018;16:4784–4790. doi: 10.3892/etm.2018.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Shapiro L., Wong J.C., Escayg A. Reduced cannabinoid 2 receptor activity increases susceptibility to induced seizures in mice. Epilepsia. 2019;60:2359–2369. doi: 10.1111/epi.16388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Wu Q., Wang H. The spatiotemporal expression changes of CB2R in the hippocampus of rats following pilocarpine-induced status epilepticus. Epilepsy Res. 2018;148:8–16. doi: 10.1016/j.eplepsyres.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 366.Romero J., Berrendero F., Pérez-Rosado A., Manzanares J., Rojo A., Fernández-Ruiz J.J., de Yebenes J.G., Ramos J.A. Unilateral 6-hydroxydopamine lesions of nigrostriatal dopaminergic neurons increased CB1 receptor mRNA levels in the caudate-putamen. Life Sci. 2000;66:485–494. doi: 10.1016/S0024-3205(99)00618-9. [DOI] [PubMed] [Google Scholar]
- 367.Di Marzo V., Hill M.P., Bisogno T., Crossman A.R., Brotchie J.M. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J. 2000;14:1432–1438. doi: 10.1096/fasebj.14.10.1432. [DOI] [PubMed] [Google Scholar]
- 368.Lastres-Becker I., Cebeira M., De Ceballos M.L., Zeng B.Y., Jenner P., Ramos J.A., Fernández-Ruiz J.J. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson’s syndrome and of MPTP-treated marmosets. Eur. J. Neurosci. 2001;14:1827–1832. doi: 10.1046/j.0953-816x.2001.01812.x. [DOI] [PubMed] [Google Scholar]
- 369.Pisani A., Fezza F., Galati S., Battista N., Napolitano S., Finazzi-Agrò A., Bernardi G., Brusa L., Pierantozzi M., Stanzione P., et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson’s disease patients. Ann. Neurol. 2005;57:777–779. doi: 10.1002/ana.20462. [DOI] [PubMed] [Google Scholar]
- 370.Pisani V., Moschella V., Bari M., Fezza F., Galati S., Bernardi G., Stanzione P., Pisani A., Maccarrone M. Dynamic changes of anandamide in the cerebrospinal fluid of Parkinson’s disease patients. Mov. Disord. 2010;25:920–924. doi: 10.1002/mds.23014. [DOI] [PubMed] [Google Scholar]
- 371.Mesnage V., Houeto J.L., Bonnet A.M., Clavier I., Arnulf I., Cattelin F., Le Fur G., Damier P., Welter M.L., Agid Y. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin. Neuropharmacol. 2004;27:108–110. doi: 10.1097/00002826-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 372.Sieradzan K.A., Fox S.H., Hill M., Dick J.P., Crossman A.R., Brotchie J.M. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: A pilot study. Neurology. 2001;57:2108–2111. doi: 10.1212/WNL.57.11.2108. [DOI] [PubMed] [Google Scholar]
- 373.Carroll C.B., Bain P.O., Teare L., Liu X., Joint C., Wroath C., Parkin S.G., Fox P., Wright D., Hobart J., et al. Cannabis for dyskinesia in Parkinson disease: A randomized double-blind crossover study. Neurology. 2004;63:1245–1250. doi: 10.1212/01.WNL.0000140288.48796.8E. [DOI] [PubMed] [Google Scholar]
- 374.Chaves-Kirsten G.P., Mazucanti C.H.Y., Real C.C., Souza B.M., Britto L.R.G., Torrão A.S. Temporal changes of CB1 cannabinoid receptor in the basal ganglia as a possible structure-specific plasticity process in 6-OHDA lesioned rats. PLoS ONE. 2013;8:e76874. doi: 10.1371/journal.pone.0076874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Lotan I., Treves T.A., Roditi Y., Djaldetti R. Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: An open-label observational study. Clin. Neuropharmacol. 2014;37:41–44. doi: 10.1097/WNF.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 376.García-Arencibia M., González S., de Lago E., Ramos J.A., Mechoulam R., Fernández-Ruiz J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: Importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007;1134:162–170. doi: 10.1016/j.brainres.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 377.Chung Y.C., Shin W.H., Baek J.Y., Cho E.J., Baik H.H., Kim S.R., Won S.Y., Jin B.K. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease. Exp. Mol. Med. 2016;48:e205. doi: 10.1038/emm.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Fernández-Ruiz J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br. J. Pharmacol. 2009;156:1029–1040. doi: 10.1111/j.1476-5381.2008.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Glass M., Faull R.L., Dragunow M. Loss of cannabinoid receptors in the substantia nigra in Huntington’s disease. Neuroscience. 1993;56:523–527. doi: 10.1016/0306-4522(93)90352-G. [DOI] [PubMed] [Google Scholar]
- 380.Richfield E.K., Herkenham M. Selective vulnerability in Huntington’s disease: Preferential loss of cannabinoid receptors in lateral globus pallidus. Ann. Neurol. 1994;36:577–584. doi: 10.1002/ana.410360406. [DOI] [PubMed] [Google Scholar]
- 381.Glass M., Dragunow M., Faull R.L. The pattern of neurodegeneration in Huntington’s disease: A comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience. 2000;97:505–519. doi: 10.1016/S0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 382.Denovan-Wright E.M., Robertson H.A. Cannabinoid receptor messenger RNA levels decrease in a subset of neurons of the lateral striatum, cortex and hippocampus of transgenic Huntington’s disease mice. Neuroscience. 2000;98:705–713. doi: 10.1016/S0306-4522(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 383.Lastres-Becker I., Fezza F., Cebeira M., Bisogno T., Ramos J.A., Milone A., Fernández-Ruiz J., Di Marzo V. Changes in endocannabinoid transmission in the basal ganglia in a rat model of Huntington’s disease. Neuroreport. 2001;12:2125–2129. doi: 10.1097/00001756-200107200-00017. [DOI] [PubMed] [Google Scholar]
- 384.Van Laere K., Casteels C., Dhollander I., Goffin K., Grachev I., Bormans G., Vandenberghe W. Widespread decrease of type 1 cannabinoid receptor availability in Huntington disease In Vivo. J. Nucl. Med. 2010;51:1413–1417. doi: 10.2967/jnumed.110.077156. [DOI] [PubMed] [Google Scholar]
- 385.Curtis A., Mitchell I., Patel S., Ives N., Rickards H. A pilot study using nabilone for symptomatic treatment in Huntington’s disease. Mov. Disord. 2009;24:2254–2259. doi: 10.1002/mds.22809. [DOI] [PubMed] [Google Scholar]
- 386.Sagredo O., González S., Aroyo I., Pazos M.R., Benito C., Lastres-Becker I., Romero J.P., Tolón R.M., Mechoulam R., Brouillet E., et al. Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: Relevance for Huntington’s disease. Glia. 2009;57:1154–1167. doi: 10.1002/glia.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Westlake T.M., Howlett A.C., Bonner T.I., Matsuda L.A., Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: An in vitro receptor autoradiography and In Situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neuroscience. 1994;63:637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- 388.Ramírez B.G., Blázquez C., Gómez Del Pulgar T., Guzmán M., De Ceballos M.L. Prevention of Alzheimer’s disease pathology by cannabinoids: Neuroprotection mediated by blockade of microglial activation. J. Neurosci. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Lee J.H., Agacinski G., Williams J.H., Wilcock G.K., Esiri M.M., Francis P.T., Wong P.T.H., Chen C.P., Lai M.K.P. Intact cannabinoid CB1 receptors in the Alzheimer’s disease cortex. Neurochem. Int. 2010;57:985–989. doi: 10.1016/j.neuint.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 390.Mulder J., Zilberter M., Pasquaré S.J., Alpár A., Schulte G., Ferreira S.G., Köfalvi A., Martín-Moreno A.M., Keimpema E., Tanila H., et al. Molecular reorganization of endocannabinoid signalling in Alzheimer’s disease. Brain. 2011;134:1041–1060. doi: 10.1093/brain/awr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Bedse G., Romano A., Cianci S., Lavecchia A.M., Lorenzo P., Elphick M.R., Laferla F.M., Vendemiale G., Grillo C., Altieri F., et al. Altered expression of the CB1 cannabinoid receptor in the triple transgenic mouse model of alzheimer’s disease. J. Alzheimer’s Dis. 2014;40:701–712. doi: 10.3233/JAD-131910. [DOI] [PubMed] [Google Scholar]
- 392.Ahmad R., Goffin K., Van den Stock J., De Winter F.L., Cleeren E., Bormans G., Tournoy J., Persoons P., Van Laere K., Vandenbulcke M. In vivo type 1 cannabinoid receptor availability in Alzheimer’s disease. Eur. Neuropsychopharmacol. 2014;24:242–250. doi: 10.1016/j.euroneuro.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 393.Solas M., Francis P.T., Franco R., Ramirez M.J. CB2 receptor and amyloid pathology in frontal cortex of Alzheimer’s disease patients. Neurobiol. Aging. 2013;34:805–808. doi: 10.1016/j.neurobiolaging.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 394.Eikelenboom P., Veerhuis R., Scheper W., Rozemuller A.J.M., Van Gool W.A., Hoozemans J.J.M. The significance of neuroinflammation in understanding Alzheimer’s disease. J. Neural Transm. 2006;113:1685–1695. doi: 10.1007/s00702-006-0575-6. [DOI] [PubMed] [Google Scholar]
- 395.Tolón R.M., Núñez E., Pazos M.R., Benito C., Castillo A.I., Martínez-Orgado J.A., Romero J. The activation of cannabinoid CB2 receptors stimulates in situ and in vitro beta-amyloid removal by human macrophages. Brain Res. 2009;1283:148–154. doi: 10.1016/j.brainres.2009.05.098. [DOI] [PubMed] [Google Scholar]
- 396.Wu J., Bie B., Yang H., Xu J.J., Brown D.L., Naguib M. Activation of the CB2 receptor system reverses amyloid-induced memory deficiency. Neurobiol. Aging. 2013;34:791–804. doi: 10.1016/j.neurobiolaging.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 397.Naguib M., Giordano T. DT-02-05: A novel therapeutic (NTRX-07) targeting neuroinflammation in alzheimer’s disease is undergoing phase I trials. Alzheimer’s Dement. 2019;15:1490–1491. doi: 10.1016/j.jalz.2019.08.014. [DOI] [Google Scholar]
- 398.Martin M., Ledent C., Parmentier M., Maldonado R., Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacol. Berl. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- 399.Viveros M.P., Marco E.M., File S.E. Endocannabinoid system and stress and anxiety responses. Pharmacol. Biochem. Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 400.Moreira F.A., Wotjak C.T. Cannabinoids and anxiety. Curr. Top. Behav. Neurosci. 2010;2:429–450. doi: 10.1007/7854_2009_16. [DOI] [PubMed] [Google Scholar]
- 401.Rubino T., Guidali C., Vigano D., Realini N., Valenti M., Massi P., Parolaro D. CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology. 2008;54:151–160. doi: 10.1016/j.neuropharm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 402.Rey A.A., Purrio M., Viveros M.P., Lutz B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA B receptors in the balance of gabaergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37:2624–2634. doi: 10.1038/npp.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Haller J., Bakos N., Szirmay M., Ledent C., Freund T.F. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur. J. Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- 404.Moreira F.A., Aguiar D.C., Terzian A.L.B., Guimarães F.S., Wotjak C.T. Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety-two sides of one coin? Neuroscience. 2012;204:186–192. doi: 10.1016/j.neuroscience.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 405.Rossi S., De Chiara V., Musella A., Kusayanagi H., Mataluni G., Bernardi G., Usiello A., Centonze D. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J. Neurosci. 2008;28:7284–7292. doi: 10.1523/JNEUROSCI.5346-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Häring M., Enk V., Rey A.A., Loch S., de Azua I.R., Weber T., Bartsch D., Monory K., Lutz B. Cannabinoid type-1 receptor signaling in central serotonergic neurons regulates anxiety-like behavior and sociability. Front. Behav. Neurosci. 2015;9:235. doi: 10.3389/fnbeh.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Su F., Yi H., Xu L., Zhang Z. Fluoxetine and S-citalopram inhibit M1 activation and promote M2 activation of microglia In Vitro. Neuroscience. 2015;294:60–68. doi: 10.1016/j.neuroscience.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 408.Stampanoni Bassi M., Gilio L., Maffei P., Dolcetti E., Bruno A., Buttari F., Centonze D., Iezzi E. Exploiting the multifaceted effects of cannabinoids on mood to boost their therapeutic use against anxiety and depression. Front. Mol. Neurosci. 2018;11:424. doi: 10.3389/fnmol.2018.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Onaivi E.S., Ishiguro H., Gong J.P., Patel S., Meozzi P.A., Myers L., Perchuk A., Mora Z., Tagliaferro P.A., Gardner E., et al. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann. N. Y. Acad. Sci. 2008;1139:434–449. doi: 10.1196/annals.1432.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.García-Gutiérrez M.S., Manzanares J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J. Psychopharmacol. 2011;25:111–120. doi: 10.1177/0269881110379507. [DOI] [PubMed] [Google Scholar]
- 411.García-Gutiérrez M.S., García-Bueno B., Zoppi S., Leza J.C., Manzanares J. Chronic blockade of cannabinoid CB 2 receptors induces anxiolytic-like actions associated with alterations in GABA A receptors. Br. J. Pharmacol. 2012;165:951–964. doi: 10.1111/j.1476-5381.2011.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Liu Q.R., Canseco-Alba A., Zhang H.Y., Tagliaferro P., Chung M., Dennis E., Sanabria B., Schanz N., Escosteguy-Neto J.C., Ishiguro H., et al. Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci. Rep. 2017;7:1–17. doi: 10.1038/s41598-017-17796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Nagayama T., Sinor A.D., Simon R.P., Chen J., Graham S.H., Jin K., Greenberg D.A. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J. Neurosci. 1999;19:2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Yu S.J., Reiner D., Shen H., Wu K.J., Liu Q.R., Wang Y. Time-dependent protection of CB2 receptor agonist in stroke. PLoS ONE. 2015;10:e0132487. doi: 10.1371/journal.pone.0132487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Caltana L., Saez T.M., Aronne M.P., Brusco A. Cannabinoid receptor type 1 agonist ACEA improves motor recovery and protects neurons in ischemic stroke in mice. J. Neurochem. 2015;135:616–629. doi: 10.1111/jnc.13288. [DOI] [PubMed] [Google Scholar]
- 416.Wang F., Han J., Higashimori H., Wang J., Liu J., Tong L., Yang Y., Dong H., Zhang X., Xiong L. Long-term depression induced by endogenous cannabinoids produces neuroprotection via astroglial CB1R after stroke in rodents. J. Cereb. Blood Flow Metab. 2019;39:1122–1137. doi: 10.1177/0271678X18755661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Kolb B., Saber H., Fadel H., Rajah G. The endocannabinoid system and stroke: A focused review. Brain Circ. 2019;5:1. doi: 10.4103/bc.bc_29_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Reichenbach Z.W., Li H., Ward S.J., Tuma R.F. The CB1 antagonist, SR141716A, is protective in permanent photothrombotic cerebral ischemia. Neurosci. Lett. 2016;630:9–15. doi: 10.1016/j.neulet.2016.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Knowles M.D., de la Tremblaye P.B., Azogu I., Plamondon H. Endocannabinoid CB1 receptor activation upon global ischemia adversely impact recovery of reward and stress signaling molecules, neuronal survival and behavioral impulsivity. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2016;66:8–21. doi: 10.1016/j.pnpbp.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 420.Bravo-Ferrer I., Cuartero M.I., Zarruk J.G., Pradillo J.M., Hurtado O., Romera V.G., Díaz-Alonso J., García-Segura J.M., Guzmán M., Lizasoain I., et al. Cannabinoid Type-2 Receptor Drives Neurogenesis and Improves Functional Outcome After Stroke. Stroke. 2017;48:204–212. doi: 10.1161/STROKEAHA.116.014793. [DOI] [PubMed] [Google Scholar]
- 421.Tang J., Miao H., Jiang B., Chen Q., Tan L., Tao Y., Zhang J., Gao F., Feng H., Zhu G., et al. A selective CB2R agonist (JWH133) restores neuronal circuit after Germinal Matrix Hemorrhage in the preterm via CX3CR1+ microglia. Neuropharmacology. 2017;119:157–169. doi: 10.1016/j.neuropharm.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 422.Zhang M., Martin B.R., Adler M.W., Razdan R.J., Kong W., Ganea D., Tuma R.F. Modulation of cannabinoid receptor activation as a neuroprotective strategy for EAE and stroke. J. Neuroimmune Pharmacol. 2009;4:249–259. doi: 10.1007/s11481-009-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Zhang M., Mahadevan A., Amere M., Li H., Ganea D., Tuma R.F. Unique Effects of Compounds Active at Both Cannabinoid and Serotonin Receptors During Stroke. Transl. Stroke Res. 2012;3:348–356. doi: 10.1007/s12975-012-0197-2. [DOI] [PubMed] [Google Scholar]
- 424.Lichtman A.H., Martin B.R. Spinal and supraspinal components of cannabinoid-induced antinociception. J. Pharmacol. Exp. Ther. 1991;258:517–523. [PubMed] [Google Scholar]
- 425.Mao J., Price D.D., Lu J., Keniston L., Mayer D.J. Two distinctive antinociceptive systems in rats with pathological pain. Neurosci. Lett. 2000;280:13–16. doi: 10.1016/S0304-3940(99)00998-2. [DOI] [PubMed] [Google Scholar]
- 426.Edsall S.A., Knapp R.J., Vanderah T.W., Roeske W.R., Consroe P., Yamamura H.I. Antisense oligodeoxynucleotide treatment to the brain cannabinoid receptor inhibits antinociception. Neuroreport. 1996;7:593–596. doi: 10.1097/00001756-199601310-00052. [DOI] [PubMed] [Google Scholar]
- 427.Lichtman A.H., Cook S.A., Martin B.R. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: Evidence supporting periaqueductal gray involvement. J. Pharmacol. Exp. Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- 428.Lichtman A.H., Martin B.R. The selective cannabinoid antagonist SR 141716A blocks cannabinoid- induced antinociception in rats. Pharmacol. Biochem. Behav. 1997;57:7–12. doi: 10.1016/S0091-3057(96)00121-9. [DOI] [PubMed] [Google Scholar]
- 429.Bridges D., Ahmad K., Rice A.S.C. The synthetic cannabinoid WIN55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br. J. Pharmacol. 2001;133:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Fox A., Kesingland A., Gentry C., McNair K., Patel S., Urban L., James I. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/S0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- 431.Lim G., Sung B., Ji R.-R., Mao J. Upregulation of spinal cannabinoid-1-receptors following nerve injury enhances the effects of Win 55,212-2 on neuropathic pain behaviors in rats. Pain. 2003;105:275–283. doi: 10.1016/S0304-3959(03)00242-2. [DOI] [PubMed] [Google Scholar]
- 432.Hama A.T., Urban M.O. Antihyperalgesic effect of the cannabinoid agonist WIN55,212-2 is mediated through an interaction with spinal metabotropic glutamate-5 receptors in rats. Neurosci. Lett. 2004;358:21–24. doi: 10.1016/j.neulet.2003.12.111. [DOI] [PubMed] [Google Scholar]
- 433.Naderi N., Shafaghi B., Khodayar M.J., Zarindast M.R. Interaction between gamma-aminobutyric acid GABAB and cannabinoid CB1 receptors in spinal pain pathways in rat. Eur. J. Pharmacol. 2005;514:159–164. doi: 10.1016/j.ejphar.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 434.Nackley A.G., Makriyannis A., Hohmann A.G. Selective activation of cannabinoid CB2 receptors suppresses spinal Fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003;119:747–757. doi: 10.1016/S0306-4522(03)00126-X. [DOI] [PubMed] [Google Scholar]
- 435.Elmes S.J.R., Winyard L.A., Medhurst S.J., Clayton N.M., Wilson A.W., Kendall D.A., Chapman V. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain. 2005;118:327–335. doi: 10.1016/j.pain.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 436.Racz I., Nadal X., Alferink J., Baños J.E., Rehnelt J., Martín M., Pintado B., Gutierrez-Adan A., Sanguino E., Bellora N., et al. Interferon-γ is a critical modulator of CB2 cannabinoid receptor signaling during neuropathic pain. J. Neurosci. 2008;28:12136–12145. doi: 10.1523/JNEUROSCI.3402-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Ibrahim M.M., Deng H., Zvonok A., Cockayne D.A., Kwan J., Mata H.P., Vanderah T.W., Lai J., Porreca F., Makriyannis A., et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: Pain inhibition by receptors not present in the CNS. Proc. Natl. Acad. Sci. USA. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Beltramo M., Bernardini N., Bertorelli R., Campanella M., Nicolussi E., Fredduzzi S., Reggiani A. CB2 receptor-mediated antihyperalgesia: Possible direct involvement of neural mechanisms. Eur. J. Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 439.Jhaveri M.D., Elmes S.J.R., Richardson D., Barrett D.A., Kendall D.A., Mason R., Chapman V. Evidence for a novel functional role of cannabinoid CB2 receptors in the thalamus of neuropathic rats. Eur. J. Neurosci. 2008;27:1722–1730. doi: 10.1111/j.1460-9568.2008.06162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Rahn E.J., Zvonok A.M., Thakur G.A., Khanolkar A.D., Makriyannis A., Hohmann A.G. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. J. Pharmacol. Exp. Ther. 2008;327:584–591. doi: 10.1124/jpet.108.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Wilkerson J.L., Gentry K.R., Dengler E.C., Wallace J.A., Kerwin A.A., Armijo L.M., Kuhn M.N., Thakur G.A., Makriyannis A., Milligan E.D. Intrathecal cannabilactone CB 2R agonist, AM1710, controls pathological pain and restores basal cytokine levels. Pain. 2012;153:1091–1106. doi: 10.1016/j.pain.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Quartilho A., Mata H.P., Ibrahim M.M., Vanderah T.W., Porreca F., Makriyannis A., Malan T.P. Inhibition of inflammatory hyperalgesia by activation of peripheral CB 2 cannabinoid receptors. Anesthesiology. 2003;99:955–960. doi: 10.1097/00000542-200310000-00031. [DOI] [PubMed] [Google Scholar]
- 443.Kinsey S.G., Mahadevan A., Zhao B., Sun H., Naidu P.S., Razdan R.K., Selley D.E., Imad Damaj M., Lichtman A.H. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharmacology. 2011;60:244–251. doi: 10.1016/j.neuropharm.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Wong S.S.C., Chan W.S., Cheung C.W. Analgesic Effects of Cannabinoids for Chronic Non-cancer Pain: A Systematic Review and Meta-Analysis with Meta-Regression. J. Neuroimmune Pharmacol. 2020:1–29. doi: 10.1007/s11481-020-09905-y. [DOI] [PubMed] [Google Scholar]
- 445.Colombo G., Agabio R., Diaz G., Lobina C., Reali R., Gessa G.L. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63:PL113–PL117. doi: 10.1016/S0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- 446.Di Marzo V., Goparaju S.K., Wang L., Liu J., Bátkai S., Járai Z., Fezza F., Miura G.I., Palmiter R.D., Sugiura T., et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 447.Di Marzo V., Ligresti A., Cristino L. The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. Int. J. Obes. 2009;33:S18–S24. doi: 10.1038/ijo.2009.67. [DOI] [PubMed] [Google Scholar]
- 448.Tucci S.A., Rogers E.K., Korbonits M., Kirkham T.C. The cannabinoid CB 1 receptor antagonist SR141716 blocks the orexigenic effects of intrahypothalamic ghrelin. Br. J. Pharmacol. 2004;143:520–523. doi: 10.1038/sj.bjp.0705968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Jo Y.H., Chen Y.J.J., Chua S.C., Talmage D.A., Role L.W. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron. 2005;48:1055–1066. doi: 10.1016/j.neuron.2005.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Kola B., Farkas I., Christ-Crain M., Wittmann G., Lolli F., Amin F., Harvey-White J., Liposits Z., Kunos G., Grossman A.B., et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Miller C.C., Murray T.F., Freeman K.G., Edwards G.L. Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol. Behav. 2004;80:611–616. doi: 10.1016/j.physbeh.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 452.Melis T., Succu S., Sanna F., Boi A., Argiolas A., Melis M.R. The cannabinoid antagonist SR 141716A (Rimonabant) reduces the increase of extra-cellular dopamine release in the rat nucleus accumbens induced by a novel high palatable food. Neurosci. Lett. 2007;419:231–235. doi: 10.1016/j.neulet.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 453.Koch M. Cannabinoid receptor signaling in central regulation of feeding behavior: A mini-review. Front. Neurosci. 2017;11:293. doi: 10.3389/fnins.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Ravinet Trillou C., Delgorge C., Menet C., Arnone M., Soubrié P. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int. J. Obes. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 455.Kim W., Doyle M.E., Liu Z., Lao Q., Shin Y.K., Carlson O.D., Kim H.S., Thomas S., Napora J.K., Lee E.K., et al. Cannabinoids inhibit insulin receptor signaling in pancreatic β-cells. Diabetes. 2011;60:1198–1209. doi: 10.2337/db10-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Shin H., Han J.H., Yoon J., Sim H.J., Park T.J., Yang S., Lee E.K., Kulkarni R.N., Egan J.M., Kim W. Blockade of cannabinoid 1 receptor improves glucose responsiveness in pancreatic beta cells. J. Cell. Mol. Med. 2018;22:2337–2345. doi: 10.1111/jcmm.13523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Rohrbach K., Thomas M.A., Glick S., Fung E.N., Wang V., Watson L., Gregory P., Antel J., Pelleymounter M.A. Ibipinabant attenuates β-cell loss in male Zucker diabetic fatty rats independently of its effects on body weight. Diabetesobes. Metab. 2012;14:555–564. doi: 10.1111/j.1463-1326.2012.01563.x. [DOI] [PubMed] [Google Scholar]
- 458.González-Mariscal I., Montoro R.A., Doyle M.E., Liu Q.R., Rouse M., O’Connell J.F., Santa-Cruz Calvo S., Krzysik-Walker S.M., Ghosh S., Carlson O.D., et al. Absence of cannabinoid 1 receptor in beta cells protects against high-fat/high-sugar diet-induced beta cell dysfunction and inflammation in murine islets. Diabetologia. 2018;61:1470–1483. doi: 10.1007/s00125-018-4576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Jourdan T., Godlewski G., Cinar R., Bertola A., Szanda G., Liu J., Tam J., Han T., Mukhopadhyay B., Skarulis M.C., et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat. Med. 2013;19:1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Jourdan T., Godlewski G., Kunos G. Endocannabinoid regulation of β -cell functions: Implications for glycaemic control and diabetes. Diabetesobes. Metab. 2016;18:549–557. doi: 10.1111/dom.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Cota D., Marsicano G., Tschöp M., Grübler Y., Flachskamm C., Schubert M., Auer D., Yassouridis A., Thöne-Reineke C., Ortmann S., et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J. Clin. Investig. 2003;112:423–431. doi: 10.1172/JCI17725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Ruby M.A., Nomura D.K., Hudak C.S.S., Mangravite L.M., Chiu S., Casida J.E., Krauss R.M. Overactive endocannabinoid signaling impairs apolipoprotein E-mediated clearance of triglyceride-rich lipoproteins. Proc. Natl. Acad. Sci. USA. 2008;105:14561–14566. doi: 10.1073/pnas.0807232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Matias I., Gonthier M.-P., Orlando P., Martiadis V., De Petrocellis L., Cervino C., Petrosino S., Hoareau L., Festy F., Pasquali R., et al. Regulation, Function, and Dysregulation of Endocannabinoids in Models of Adipose and β-Pancreatic Cells and in Obesity and Hyperglycemia. J. Clin. Endocrinol. Metab. 2006;91:3171–3180. doi: 10.1210/jc.2005-2679. [DOI] [PubMed] [Google Scholar]
- 464.Furuya D.T., Poletto A.C., Freitas H.S., Machado U.F. Inhibition of cannabinoid CB1 receptor upregulates Slc2a4 expression via nuclear factor-κB and sterol regulatory element-binding protein-1 in adipocytes. J. Mol. Endocrinol. 2012;49:97–106. doi: 10.1530/JME-12-0037. [DOI] [PubMed] [Google Scholar]
- 465.Han J.H., Shin H., Rho J.G., Kim J.-E., Son D.H., Yoon J., Lee Y.J., Park J.-H., Song B.J., Choi C.-S., et al. Peripheral cannabinoid 1 receptor blockade mitigates adipose tissue inflammation via NLRP3 inflammasome in mouse models of obesity. Diabetesobes. Metab. 2018;20:2179–2189. doi: 10.1111/dom.13350. [DOI] [PubMed] [Google Scholar]
- 466.Tedesco L., Valerio A., Cervino C., Cardile A., Pagano C., Vettor R., Pasquali R., Carruba M.O., Marsicano G., Lutz B., et al. Cannabinoid type 1 receptor blockade promotes mitochondrial biogenesis through endothelial nitric oxide synthase expression in white adipocytes. Diabetes. 2008;57:2028–2036. doi: 10.2337/db07-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Tedesco L., Valerio A., Dossena M., Cardile A., Ragni M., Pagano C., Pagotto U., Carruba M.O., Vettor R., Nisoli E. Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: The role of eNOS, p38 MAPK, and AMPK pathways. Diabetes. 2010;59:2826–2836. doi: 10.2337/db09-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Arrabal S., Lucena M.A., Canduela M.J., Ramos-Uriarte A., Rivera P., Serrano A., Pavón F.J., Decara J., Vargas A., Baixeras E., et al. Pharmacological blockade of cannabinoid CB1 receptors in diet-induced obesity regulates mitochondrial dihydrolipoamide dehydrogenase in muscle. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0145244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Wang X., Yu Q., Yue H., Zeng S., Cui F. Effect of Intermittent Hypoxia and Rimonabant on Glucose Metabolism in Rats: Involvement of Expression of GLUT4 in Skeletal Muscle. Med. Sci. Monit. 2015;21:3252–3260. doi: 10.12659/MSM.896039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Eckardt K., Sell H., Taube A., Koenen M., Platzbecker B., Cramer A., Horrighs A., Lehtonen M., Tennagels N., Eckel J. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetologia. 2009;52:664–674. doi: 10.1007/s00125-008-1240-4. [DOI] [PubMed] [Google Scholar]
- 471.Lipina C., Stretton C., Hastings S., Hundal J.S., Mackie K., Irving A.J., Hundal H.S. Regulation of MAP kinase-directed mitogenic and protein kinase B-mediated signaling by cannabinoid receptor type 1 in skeletal muscle cells. Diabetes. 2010;59:375–385. doi: 10.2337/db09-0979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 472.Cavuoto P., McAinch A.J., Hatzinikolas G., Cameron-Smith D., Wittert G.A. Effects of cannabinoid receptors on skeletal muscle oxidative pathways. Mol. Cell. Endocrinol. 2007;267:63–69. doi: 10.1016/j.mce.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 473.Pi-Sunyer F.X., Aronne L.J., Heshmati H.M., Devin J., Rosenstock J. RIO-North America Study Group, for the Effect of Rimonabant, a Cannabinoid-1 Receptor Blocker, on Weight and Cardiometabolic Risk Factors in Overweight or Obese Patients. JAMA. 2006;295:761. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 474.Mendizábal V.E., Adler-Graschinsky E. Cannabinoids as therapeutic agents in cardiovascular disease: A tale of passions and illusions. Br. J. Pharmacol. 2007;151:427–440. doi: 10.1038/sj.bjp.0707261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Moreira F.A., Crippa J.A.S. The psychiatric side-effects of rimonabant. Rev. Bras. Psiquiatr. 2009;31:145–153. doi: 10.1590/S1516-44462009000200012. [DOI] [PubMed] [Google Scholar]
- 476.Sam A.H., Salem V., Ghatei M.A. Rimonabant: From RIO to Ban. J. Obes. 2011;2011 doi: 10.1155/2011/432607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Boekholdt S.M., Peters R.J. Rimonabant: Obituary for a wonder drug. Lancet. 2010;376:489–490. doi: 10.1016/S0140-6736(10)61080-X. [DOI] [PubMed] [Google Scholar]
- 478.Klumpers L.E., Fridberg M., De Kam M.L., Little P.B., Jensen N.O., Kleinloog H.D., Elling C.E., Van Gerven J.M.A. Peripheral selectivity of the novel cannabinoid receptor antagonist TM38837 in healthy subjects. Br. J. Clin. Pharmacol. 2013;76:846–857. doi: 10.1111/bcp.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Cluny N.L., Vemuri V.K., Chambers A.P., Limebeer C.L., Bedard H., Wood J.T., Lutz B., Zimmer A., Parker L.A., Makriyannis A., et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br. J. Pharmacol. 2010;161:629–642. doi: 10.1111/j.1476-5381.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Tam J., Cinar R., Liu J., Godlewski G., Wesley D., Jourdan T., Szanda G., Mukhopadhyay B., Chedester L., Liow J.S., et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16:167–179. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Alonso M., Serrano A., Vida M., Crespillo A., Hernandez-Folgado L., Jagerovic N., Goya P., Reyes-Cabello C., Perez-Valero V., Decara J., et al. Anti-obesity efficacy of LH-21, a cannabinoid CB 1 receptor antagonist with poor brain penetration, in diet-induced obese rats. Br. J. Pharmacol. 2012;165:2274–2291. doi: 10.1111/j.1476-5381.2011.01698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Chen W., Shui F., Liu C., Zhou X., Li W., Zheng Z., Fu W., Wang L. Novel peripherally restricted cannabinoid 1 receptor selective antagonist TXX-522 with prominent weight-loss efficacy in diet induced obese mice. Front. Pharmacol. 2017;8 doi: 10.3389/fphar.2017.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Ma H., Zhang G., Mou C., Fu X., Chen Y. Peripheral CB1 receptor neutral antagonist, AM6545, ameliorates hypometabolic obesity and improves adipokine secretion in monosodium glutamate induced obese mice. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Nogueiras R., Veyrat-Durebex C., Suchanek P.M., Klein M., Tschöp J., Caldwell C., Woods S.C., Wittmann G., Watanabe M., Liposits Z., et al. Peripheral, but Not Central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes. 2008;57:2977–2991. doi: 10.2337/db08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485.Bowles N.P., Karatsoreos I.N., Li X., Vemuri V.K., Wood J.A., Li Z., Tamashiro K.L.K., Schwartz G.J., Makriyannis A.M., Kunos G., et al. A peripheral endocannabinoid mechanism contributes to glucocorticoid-mediated metabolic syndrome. Proc. Natl. Acad. Sci. USA. 2015;112:285–290. doi: 10.1073/pnas.1421420112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Tam J., Vemuri V.K., Liu J., Bátkai S., Mukhopadhyay B., Godlewski G., Osei-Hyiaman D., Ohnuma S., Ambudkar S.V., Pickel J., et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J. Clin. Investig. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Barutta F., Grimaldi S., Gambino R., Vemuri K., Makriyannis A., Annaratone L., di Marzo V., Bruno G., Gruden G. Dual therapy targeting the endocannabinoid system prevents experimental diabetic nephropathy. Nephrol. Dial. Transplant. 2017;32:1655–1665. doi: 10.1093/ndt/gfx010. [DOI] [PubMed] [Google Scholar]
- 488.Vidot D.C., Prado G., Hlaing W.M., Florez H.J., Arheart K.L., Messiah S.E. Metabolic Syndrome Among Marijuana Users in the United States: An Analysis of National Health and Nutrition Examination Survey Data. Am. J. Med. 2016;129:173–179. doi: 10.1016/j.amjmed.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Anker S.D., Chua T.P., Ponikowski P., Harrington D., Swan J.W., Kox W.J., Poole-Wilson P.A., Coats A.J. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–534. doi: 10.1161/01.CIR.96.2.526. [DOI] [PubMed] [Google Scholar]
- 490.Burckart K., Beca S., Urban R.J., Sheffield-Moore M. Pathogenesis of muscle wasting in cancer cachexia: Targeted anabolic and anticatabolic therapies. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:410–416. doi: 10.1097/MCO.0b013e328339fdd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Marco E.M., Romero-Zerbo S.Y., Viveros M.-P., Bermudez-Silva F.J., Bermú Dez-Silva F.J. The role of the endocannabinoid system in eating disorders: Pharmacological implications A brief update on the endocannabinoid system. Behav. Pharmacol. 2012;23:526–536. doi: 10.1097/FBP.0b013e328356c3c9. [DOI] [PubMed] [Google Scholar]
- 492.Osei-Hyiaman D. Endocannabinoid system in cancer cachexia. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:443–448. doi: 10.1097/MCO.0b013e3281900ecc. [DOI] [PubMed] [Google Scholar]
- 493.Strasser F., Luftner D., Possinger K., Ernst G., Ruhstaller T., Meissner W., Ko Y.D., Schnelle M., Reif M., Cerny T. Comparison of orally administered cannabis extract and delta-9- tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J. Clin. Oncol. 2006;24:3394–3400. doi: 10.1200/JCO.2005.05.1847. [DOI] [PubMed] [Google Scholar]
- 494.Cabeza C., Corsi O., Pérez-Cruz P. ¿Son los cannabinoides una opción para el síndrome anorexia-caquexia en pacientes con cáncer avanzado? Medwave. 2017;17:e7130. doi: 10.5867/medwave.2017.09.7130. [DOI] [PubMed] [Google Scholar]
- 495.Malinowska B., Baranowska-Kuczko M., Schlicker E. Triphasic blood pressure responses to cannabinoids: Do we understand the mechanism? Br. J. Pharmacol. 2012;165:2073–2088. doi: 10.1111/j.1476-5381.2011.01747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Siqueira S.W., Lapa A.J., Ribeiro Do Valle J. The triple effect ineuced by Δ9-tetrahydrocannabinol on the rat blood pressure. Eur. J. Pharmacol. 1979;58:351–357. doi: 10.1016/0014-2999(79)90305-4. [DOI] [PubMed] [Google Scholar]
- 497.Varga K., Lake K., Martin B.R., Kunos G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur. J. Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-J. [DOI] [PubMed] [Google Scholar]
- 498.Malinowska B., Kwolek G., Göthert M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 2001;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- 499.Niederhoffer N., Schmid K., Szabo B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn. Schmiedebergs. Arch. Pharmacol. 2003;367:434–443. doi: 10.1007/s00210-003-0755-y. [DOI] [PubMed] [Google Scholar]
- 500.Pacher P., Bátkai S., Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J. Physiol. 2004;558:647–657. doi: 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Pacher P., Bátkai S., Kunos G. Blood pressure regulation by endocannabinoids and their receptors. Neuropharmacology. 2005;48:1130–1138. doi: 10.1016/j.neuropharm.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Folkow B. Physiological aspects of primary hypertension. Physiol. Rev. 1982;62:347–504. doi: 10.1152/physrev.1982.62.2.347. [DOI] [PubMed] [Google Scholar]
- 503.Fisher J.P., Young C.N., Fadel P.J. Central sympathetic overactivity: Maladies and mechanisms. Auton. Neurosci. 2009;148:5–15. doi: 10.1016/j.autneu.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Fisher J.P., Paton J.F.R. The sympathetic nervous system and blood pressure in humans: Implications for hypertension. J. Human Hypertens. 2012;26:463–475. doi: 10.1038/jhh.2011.66. [DOI] [PubMed] [Google Scholar]
- 505.Haspula D., Clark M.A. Neuroinflammation and sympathetic overactivity: Mechanisms and implications in hypertension. Auton. Neurosci. 2018 doi: 10.1016/j.autneu.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 506.Seagard J.L., Dean C., Patel S., Rademacher D.J., Hopp F.A., Schmeling W.T., Hillard C.J. Anandamide content and interaction of endocannabinoid/GABA modulatory effects in the NTS on baroreflex-evoked sympathoinhibition. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H992–H1000. doi: 10.1152/ajpheart.00870.2003. [DOI] [PubMed] [Google Scholar]
- 507.Seagard J.L., Hopp F.A., Hillard C.J., Dean C. Effects of endocannabinoids on discharge of baroreceptive NTS neurons. Neurosci. Lett. 2005;381:334–339. doi: 10.1016/j.neulet.2005.02.044. [DOI] [PubMed] [Google Scholar]
- 508.Brozoski D.T., Dean C., Hopp F.A., Seagard J.L. Uptake blockade of endocannabinoids in the NTS modulates baroreflex-evoked sympathoinhibition. Brain Res. 2005;1059:197–202. doi: 10.1016/j.brainres.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 509.Durakoglugil M.S., Orer H.S. Cannabinoid receptor activation in the nucleus tractus solitaries produces baroreflex-like responses in the rat. Int. J. Biomed. Sci. 2008;4:229–237. [PMC free article] [PubMed] [Google Scholar]
- 510.Chen C.Y., Bonham A.C., Dean C., Hopp F.A., Hillard C.J., Seagard J.L. Retrograde release of endocannabinoids inhibits presynaptic GABA release to second-order baroreceptive neurons in NTS. Auton. Neurosci. Basic Clin. 2010;158:44–50. doi: 10.1016/j.autneu.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Padley J.R., Li Q., Pilowsky P.M., Goodchild A.K. Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anaesthetised rats. Br. J. Pharmacol. 2003;140:384–394. doi: 10.1038/sj.bjp.0705422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Dean C. Endocannabinoid modulation of sympathetic and cardiovascular responses to acute stress in the periaqueductal gray of the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300 doi: 10.1152/ajpregu.00391.2010. [DOI] [PubMed] [Google Scholar]
- 513.Ibrahim B.M., Abdel-Rahman A.A. Role of brainstem GABAergic signaling in central cannabinoid receptor evoked sympathoexcitation and pressor responses in conscious rats. Brain Res. 2011;1414:1–9. doi: 10.1016/j.brainres.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Ibrahim B.M., Abdel-Rahman A.A. Differential modulation of brainstem phosphatidylinositol 3-kinase/Akt and extracellular signal-regulated kinase 1/2 signaling underlies WIN55,212-2 centrally mediated pressor response in conscious rats. J. Pharmacol. Exp. Ther. 2012;340:11–18. doi: 10.1124/jpet.111.186858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Ibrahim B.M., Abdel-Rahman A.A. Enhancement of rostral ventrolateral medulla neuronal nitric-oxide synthase-Nitric-oxide signaling mediates the central cannabinoid receptor 1-evoked pressor response in conscious rats. J. Pharmacol. Exp. Ther. 2012;341:579–586. doi: 10.1124/jpet.112.192369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Ibrahim B.M., Abdel-Rahman A.A. A pivotal role for enhanced brainstem Orexin receptor 1 signaling in the central cannabinoid receptor 1-mediated pressor response in conscious rats. Brain Res. 2015;1622:51–63. doi: 10.1016/j.brainres.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Smiley K.A., Karler R., Turkanis S.A. Effects of cannabinoids on the perfused rat heart. Res. Commun. Chem. Pathol. Pharmacol. 1976;14:659–675. [PubMed] [Google Scholar]
- 518.Krylatov A.V., Maslov L.N., Ermakov S.Y., Lasukova O.V., Barzakh E.I., Crawford D., Pertwee R.G. Significance of cardiac cannabinoid receptors in regulation of cardiac rhythm, myocardial contractility, and electrophysiologic processes in heart. Biol. Bull. 2007;34:28–35. doi: 10.1134/S1062359007010049. [DOI] [PubMed] [Google Scholar]
- 519.Sterin-Borda L., Del Zar C.F., Borda E. Differential CB1 and CB2 cannabinoid receptor-inotropic response of rat isolated atria: Endogenous signal transduction pathways. Biochem. Pharmacol. 2005;69:1705–1713. doi: 10.1016/j.bcp.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 520.Liao Y., Bin J., Luo T., Zhao H., Ledent C., Asakura M., Xu D., Takashima S., Kitakaze M. CB1 cannabinoid receptor deficiency promotes cardiac remodeling induced by pressure overload in mice. Int. J. Cardiol. 2013;167:1936–1944. doi: 10.1016/j.ijcard.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 521.Pacher P., Mukhopadhyay P., Mohanraj R., Godlewski G., Bátkai S., Kunos G. Modulation of the endocannabinoid system in cardiovascular disease: Therapeutic potential and limitations. Hypertension. 2008;52:601–607. doi: 10.1161/HYPERTENSIONAHA.105.063651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Ledent C., Valverde O., Cossu G., Petitet F., Aubert J.F., Beslot F., Böhme G.A., Imperato A., Pedrazzini T., Roques B.P., et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 523.Liao Y., Bin J., Asakura M., Xuan W., Chen B., Huang Q., Xu D., Ledent C., Takashima S., Kitakaze M. Deficiency of type 1 cannabinoid receptors worsens acute heart failure induced by pressure overload in mice. Eur. Heart J. 2012;33:3124–3133. doi: 10.1093/eurheartj/ehr246. [DOI] [PubMed] [Google Scholar]
- 524.Li J., Kaminski N.E., Wang D.H. Anandamide-Induced Depressor Effect in Spontaneously Hypertensive Rats: Role of the Vanilloid Receptor. Hypertension. 2003;41:757–762. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]
- 525.White R., Vanessa Ho W.S.V., Bottrill F.E., Ford W.R., Hiley C.R. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br. J. Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.O’Sullivan S.E., Kendall D.A., Randall M.D. The effects of Δ9-tetrahydrocannabinol in rat mesenteric vasculature, and its interactions with the endocannabinoid anandamide. Br. J. Pharmacol. 2005;145:514–526. doi: 10.1038/sj.bjp.0706218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.O’Sullivan S.E., Kendall D.A., Randall M.D. Vascular effects of Δ9-tetrahydrocannabinol (THC), anandamide and N-arachidonoyldopamine (NADA) in the rat isolated aorta. Eur. J. Pharmacol. 2005;507:211–221. doi: 10.1016/j.ejphar.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 528.Wagner J.A., Abesser M., Karcher J., Laser M., Kunos G. Coronary vasodilator effects of endogenous cannabinoids in vasopressin-preconstricted unpaced rat isolated hearts. J. Cardiovasc. Pharmacol. 2005;46:348–355. doi: 10.1097/01.fjc.0000175437.87283.f2. [DOI] [PubMed] [Google Scholar]
- 529.Dannert M.T., Alsasua A., Herradon E., Martín M.I., López-Miranda V. Vasorelaxant effect of Win 55,212-2 in rat aorta: New mechanisms involved. Vascul. Pharmacol. 2007;46:16–23. doi: 10.1016/j.vph.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 530.Szekeres M., Nádasy G.L., Soltész-Katona E., Hunyady L. Control of myogenic tone and agonist induced contraction of intramural coronary resistance arterioles by cannabinoid type 1 receptors and endocannabinoids. Prostaglandins Other Lipid Mediat. 2018;134:77–83. doi: 10.1016/j.prostaglandins.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 531.Waldeck-Welermair M., Zoratti C., Osibow K., Balenga N., Goessnitzer E., Waldhoer M., Malli R., Graier W.F. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J. Cell Sci. 2008;121:1704–1717. doi: 10.1242/jcs.020958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Ronco A.M., Llanos M., Tamayo D., Hirsch S. Anandamide Inhibits Endothelin-1 Production by Human Cultured Endothelial Cells: A New Vascular Action of This Endocannabinoid. Pharmacology. 2007;79:12–16. doi: 10.1159/000097072. [DOI] [PubMed] [Google Scholar]
- 533.Bondarenko A.I. Endothelial atypical cannabinoid receptor: Do we have enough evidence? Br. J. Pharmacol. 2014;171:5573–5588. doi: 10.1111/bph.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Rosenkrantz H., Braude M. Acute, subacute and 23-day chronic marihuana inhalation toxicities in the rat. Toxicol. Appl. Pharmacol. 1974;28:428–441. doi: 10.1016/0041-008X(74)90228-2. [DOI] [PubMed] [Google Scholar]
- 535.Benowitz N.L., Jones R.T. Cardiovascular effects of prolonged delta-9-tetrahydrocannabinol ingestion. Clin. Pharmacol. Ther. 1975;18:287–297. doi: 10.1002/cpt1975183287. [DOI] [PubMed] [Google Scholar]
- 536.Crawford W.J., Merritt J.C. Effects of tetrahydrocannabinol on arterial and intraocular hypertension. Int. J. Clin. Pharmacol. Biopharm. 1979;17:191–196. [PubMed] [Google Scholar]
- 537.Vollmer R.R., Cavero I., Ertel R.J., Solomon T.A., Buckley J.P. Role of the central autonomic nervous system in the hypotension and bradycardia induced by (-)-delta 9-trans-tetrahydrocannabinol. J. Pharm. Pharmacol. 1974;26:186–192. doi: 10.1111/j.2042-7158.1974.tb09252.x. [DOI] [PubMed] [Google Scholar]
- 538.Brozoski D.T., Dean C., Hopp F.A., Hillard C.J., Seagard J.L. Differential endocannabinoid regulation of baroreflex-evoked sympathoinhibition in normotensive versus hypertensive rats. Auton. Neurosci. 2009;150:82–93. doi: 10.1016/j.autneu.2009.05.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Haspula D., Clark M.A. Heterologous regulation of the cannabinoid type 1 receptor by angiotensin II in astrocytes of spontaneously hypertensive rats. J. Neurochem. 2016;139:523–536. doi: 10.1111/jnc.13776. [DOI] [PubMed] [Google Scholar]
- 540.Anderson E.A., Sinkey C.A., Lawton W.J., Mark A.L. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertens. Dallas Tex. 1979. 1989;14:177–183. doi: 10.1161/01.HYP.14.2.177. [DOI] [PubMed] [Google Scholar]
- 541.Niederhoffer N., Szabo B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. J. Pharmacol. Exp. Ther. 2000;294:707–713. [PubMed] [Google Scholar]
- 542.Schaich C.L., Shaltout H.A., Brosnihan K.B., Howlett A.C., Diz D.I. Acute and chronic systemic CB1 cannabinoid receptor blockade improves blood pressure regulation and metabolic profile in hypertensive (mRen2)27 rats. Physiol. Rep. 2014;2 doi: 10.14814/phy2.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Schaich C.L., Grabenauer M., Thomas B.F., Shaltout H.A., Gallagher P.E., Howlett A.C., Diz D.I. Medullary Endocannabinoids Contribute to the Differential Resting Baroreflex Sensitivity in Rats with Altered Brain Renin-Angiotensin System Expression. Front. Physiol. 2016;7:207. doi: 10.3389/fphys.2016.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Slavic S., Lauer D., Sommerfeld M., Kemnitz U.R., Grzesiak A., Trappiel M., Thöne-Reineke C., Baulmann J., Paulis L., Kappert K., et al. Cannabinoid receptor 1 inhibition improves cardiac function and remodelling after myocardial infarction and in experimental metabolic syndrome. J. Mol. Med. 2013;91:811–823. doi: 10.1007/s00109-013-1034-0. [DOI] [PubMed] [Google Scholar]
- 545.Rajesh M., Bátkai S., Kechrid M., Mukhopadhyay P., Lee W.S., Horváth B., Holovac E., Cinar R., Liaudet L., Mackie K., et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes. 2012;61:716–727. doi: 10.2337/db11-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Tiyerili V., Zimmer S., Jung S., Wassmann K., Naehle C.P., Lütjohann D., Zimmer A., Nickenig G., Wassmann S. CB1 receptor inhibition leads to decreased vascular AT1 receptor expression, inhibition of oxidative stress and improved endothelial function. Basic Res. Cardiol. 2010;105:465–477. doi: 10.1007/s00395-010-0090-7. [DOI] [PubMed] [Google Scholar]
- 547.Szekeres M., Nádasy G.L., Turu G., Soltész-Katona E., Benyó Z., Offermanns S., Ruisanchez É., Szabó E., Takáts Z., Bátkai S., et al. Endocannabinoid-mediated modulation of Gq/11 protein-coupled receptor signaling-induced vasoconstriction and hypertension. Mol. Cell. Endocrinol. 2015;403:46–56. doi: 10.1016/j.mce.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 548.Mihrimah Ozturk H., Yetkin E., Ozturk S. Synthetic Cannabinoids and Cardiac Arrhythmia Risk: Review of the Literature. Cardiovasc. Toxicol. 2012;19:191–197. doi: 10.1007/s12012-019-09522-z. [DOI] [PubMed] [Google Scholar]
- 549.Rieder S.A., Chauhan A., Singh U., Nagarkatti M., Nagarkatti P. Cannabinoid-induced apoptosis in immune cells as a pathway to immunosuppression. Immunobiology. 2010;215:598–605. doi: 10.1016/j.imbio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.McKallip R.J., Lombard C., Martin B.R., Nagarkatti M., Nagarkatti P.S. Δ9-Tetrahydrocannabinol-induced apoptosis in the thymus and spleen as a mechanism of immunosuppression in vitro and in vivo. J. Pharmacol. Exp. Ther. 2002;302:451–465. doi: 10.1124/jpet.102.033506. [DOI] [PubMed] [Google Scholar]
- 551.Lombard C., Nagarkatti M., Nagarkatti P. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: Potential role for CB2-selective ligands as immunosuppressive agents. Clin. Immunol. 2007;122:259–270. doi: 10.1016/j.clim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Cencioni M.T., Chiurchiù V., Catanzaro G., Borsellino G., Bernardi G., Battistini L., Maccarrone M. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0008688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Kapellos T.S., Taylor L., Feuerborn A., Valaris S., Hussain M.T., Rainger G.E., Greaves D.R., Iqbal A.J. Cannabinoid receptor 2 deficiency exacerbates inflammation and neutrophil recruitment. FASEB J. 2019;33:6154–6167. doi: 10.1096/fj.201802524R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Ziring D., Wei B., Velazquez P., Schrage M., Buckley N.E., Braun J. Formation of B and T cell subsets require the cannabinoid receptor CB2. Immunogenetics. 2006;58:714–725. doi: 10.1007/s00251-006-0138-x. [DOI] [PubMed] [Google Scholar]
- 555.Muppidi J.R., Arnon T.I., Bronevetsky Y., Veerapen N., Tanaka M., Besra G.S., Cyster J.G. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J. Exp. Med. 2011;208:1941–1948. doi: 10.1084/jem.20111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Derocq J.M., Ségui M., Marchand J., Le Fur G., Casellas P. Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Lett. 1995;369:177–182. doi: 10.1016/0014-5793(95)00746-V. [DOI] [PubMed] [Google Scholar]
- 557.Miller A.M., Stella N. CB 2 receptor-mediated migration of immune cells: It can go either way. Br. J. Pharmacol. 2008;153:299–308. doi: 10.1038/sj.bjp.0707523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.BOUABOULA M., RINALDI M., CARAYON P., CARILLON C., DELPECH B., SHIRE D., LE FUR G., CASELLAS P. Cannabinoid-receptor expression in human leukocytes. Eur. J. Biochem. 1993;214:173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- 559.Lee S.F., Newton C., Widen R., Friedman H., Klein T.W. Differential expression of cannabinoid CB2 receptor mRNA in mouse immune cell subpopulations and following B cell stimulation. Eur. J. Pharmacol. 2001;423:235–241. doi: 10.1016/S0014-2999(01)01122-0. [DOI] [PubMed] [Google Scholar]
- 560.Correa F., Mestre L., Docagne F., Guaza C. Activation of cannabinoid CB 2 receptor negatively regulates IL-12p40 production in murine macrophages: Role of IL-10 and ERK1/2 kinase signaling. Br. J. Pharmacol. 2005;145:441–448. doi: 10.1038/sj.bjp.0706215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Richardson D., Pearson R.G., Kurian N., Latif M.L., Garle M.J., Barrett D.A., Kendall D.A., Scammell B.E., Reeve A.J., Chapman V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2008;10 doi: 10.1186/ar2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Ofek O., Karsak M., Leclerc N., Fogel M., Frenkel B., Wright K., Tam J., Attar-Namdar M., Kram V., Shohami E., et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA. 2006;103:696–701. doi: 10.1073/pnas.0504187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Mbvundula E.C., Bunning R.A.D., Rainsford K.D. Arthritis and cannabinoids: HU-210 and Win-55,212-2 prevent IL-1 α-induced matrix degradation in bovine articular chondrocytes in-vitro. J. Pharm. Pharmacol. 2006;58:351–358. doi: 10.1211/jpp.58.3.0009. [DOI] [PubMed] [Google Scholar]
- 564.Gui H., Liu X., Wang Z.-W., He D.-Y., Su D.-F., Dai S.-M. Expression of cannabinoid receptor 2 and its inhibitory effects on synovial fibroblasts in rheumatoid arthritis. Rheumatol. Oxf. 2014;53:802–809. doi: 10.1093/rheumatology/ket447. [DOI] [PubMed] [Google Scholar]
- 565.Fukuda S., Kohsaka H., Takayasu A., Yokoyama W., Miyabe C., Miyabe Y., Harigai M., Miyasaka N., Nanki T. Cannabinoid receptor 2 as a potential therapeutic target in rheumatoid arthritis. Bmc Musculoskelet. Disord. 2014;15:275. doi: 10.1186/1471-2474-15-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Bai J., Ge G., Wang Y., Zhang W., Wang Q., Wang W., Guo X., Yu B., Xu Y., Yang H., et al. A selective CB2 agonist protects against the inflammatory response and joint destruction in collagen-induced arthritis mice. Biomed. Pharmacother. 2019;116:109025. doi: 10.1016/j.biopha.2019.109025. [DOI] [PubMed] [Google Scholar]
- 567.Fechtner S., Singh A.K., Srivastava I., Szlenk C.T., Muench T.R., Natesan S., Ahmed S. Cannabinoid Receptor 2 Agonist JWH-015 Inhibits Interleukin-1β-Induced Inflammation in Rheumatoid Arthritis Synovial Fibroblasts and in Adjuvant Induced Arthritis Rat via Glucocorticoid Receptor. Front. Immunol. 2019;10:1027. doi: 10.3389/fimmu.2019.01027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Ashwood P., Wills S., Van de Water J. The immune response in autism: A new frontier for autism research. J. Leukoc. Biol. 2006;80:1–15. doi: 10.1189/jlb.1205707. [DOI] [PubMed] [Google Scholar]
- 569.Careaga M., Van de Water J., Ashwood P. Immune dysfunction in autism: A pathway to treatment. Neurotherapeutics. 2010;7:283–292. doi: 10.1016/j.nurt.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Siniscalco D., Sapone A., Giordano C., Cirillo A., De Magistris L., Rossi F., Fasano A., Bradstreet J.J., Maione S., Antonucci N. Cannabinoid receptor type 2, but not type 1, is up-regulated in peripheral blood mononuclear cells of children affected by autistic disorders. J. Autism Dev. Disord. 2013;43:2686–2695. doi: 10.1007/s10803-013-1824-9. [DOI] [PubMed] [Google Scholar]
- 571.Siniscalco D., Bradstreet J.J., Cirillo A., Antonucci N. The in vitro GcMAF effects on endocannabinoid system transcriptionomics, receptor formation, and cell activity of autism-derived macrophages. J. Neuroinflammation. 2014;11:78. doi: 10.1186/1742-2094-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Jean-Gilles L., Feng S., Tench C.R., Chapman V., Kendall D.A., Barrett D.A., Constantinescu C.S. Plasma endocannabinoid levels in multiple sclerosis. J. Neurol. Sci. 2009;287:212–215. doi: 10.1016/j.jns.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 573.Docagne F., Muñetón V., Clemente D., Ali C., Loría F., Correa F., Hernangómez M., Mestre L., Vivien D., Guaza C. Excitotoxicity in a chronic model of multiple sclerosis: Neuroprotective effects of cannabinoids through CB1 and CB2 receptor activation. Mol. Cell. Neurosci. 2007;34:551–561. doi: 10.1016/j.mcn.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 574.Benito C., Romero J.P., Tolón R.M., Clemente D., Docagne F., Hillard C.J., Guaza C., Romero J. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J. Neurosci. 2007;27:2396–2402. doi: 10.1523/JNEUROSCI.4814-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Docagne F., Mestre L., Loría F., Hernangómez M., Correa F., Guaza C. Therapeutic potential of CB2 targeting in multiple sclerosis. Expert Opin. Ther. Targets. 2008;12:185–195. doi: 10.1517/14728222.12.2.185. [DOI] [PubMed] [Google Scholar]
- 576.Ni X., Geller E.B., Eppihimer M.J., Eisenstein T.K., Adler M.W., Tuma R.F. Win 55212-2, a cannabinoid receptor agonist, attenuates leukocyte/endothelial interactions in an experimental autoimmune encephalomyelitis model. Mult. Scler. 2004;10:158–164. doi: 10.1191/1352458504ms1009oa. [DOI] [PubMed] [Google Scholar]
- 577.Malfitano A.M., Laezza C., D’Alessandro A., Procaccini C., Saccomanni G., Tuccinardi T., Manera C., Macchia M., Matarese G., Gazzerro P., et al. Effects on Immune Cells of a New 1,8-Naphthyridin-2-One Derivative and Its Analogues as Selective CB2 Agonists: Implications in Multiple Sclerosis. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0062511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Annunziata P., Cioni C., Mugnaini C., Corelli F. Potent immunomodulatory activity of a highly selective cannabinoid CB2 agonist on immune cells from healthy subjects and patients with multiple sclerosis. J. Neuroimmunol. 2017;303:66–74. doi: 10.1016/j.jneuroim.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 579.Sánchez A.J., González-Pérez P., Galve-Roperh I., García-Merino A. R-(+)-[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)-pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphtalenylmethanone (WIN-2) ameliorates experimental autoimmune encephalomyelitis and induces encephalitogenic T cell apoptosis: Partial involvement of the CB2 receptor. Biochem. Pharmacol. 2006;72:1697–1706. doi: 10.1016/j.bcp.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 580.Baker D., Pryce G., Ludovic Croxford J., Brown P., Pertwee R.G., Huffman J.W., Layward L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- 581.Pryce G., Baker D. Control of spasticity in a multiple sclerosis model is mediated by CB 1, not CB 2, cannabinoid receptors. Br. J. Pharmacol. 2007;150:519–525. doi: 10.1038/sj.bjp.0707003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Maresz K., Pryce G., Ponomarev E.D., Marsicano G., Croxford J.L., Shriver L.P., Ledent C., Cheng X., Carrier E.J., Mann M.K., et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat. Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- 583.Steffens S., Veillard N.R., Arnaud C., Pelli G., Burger F., Staub C., Zimmer A., Frossard J.L., Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. doi: 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- 584.Zhao Y., Liu Y., Zhang W., Xue J., Wu Y.Z., Xu W., Liang X., Chen T., Kishimoto C., Yuan Z. WIN55212-2 ameliorates atherosclerosis associated with suppression of pro-inflammatory responses in ApoE-knockout mice. Eur. J. Pharmacol. 2010;649:285–292. doi: 10.1016/j.ejphar.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 585.Netherland C.D., Pickle T.G., Bales A., Thewke D.P. Cannabinoid receptor type 2 (CB2) deficiency alters atherosclerotic lesion formation in hyperlipidemic Ldlr-null mice. Atherosclerosis. 2010;213:102–108. doi: 10.1016/j.atherosclerosis.2010.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Montecucco F., Burger F., Mach F., Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1145–H1155. doi: 10.1152/ajpheart.01328.2007. [DOI] [PubMed] [Google Scholar]
- 587.Zhang J.M., An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Ulmann L., Hirbec H., Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010;29:2290–2300. doi: 10.1038/emboj.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Hickernell T.R., Lakra A., Berg A., Cooper H.J., Geller J.A., Shah R.P. Should Cannabinoids Be Added to Multimodal Pain Regimens After Total Hip and Knee Arthroplasty? J. Arthroplast. 2018;33:3637–3641. doi: 10.1016/j.arth.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 590.Gazendam A., Nucci N., Gouveia K., Abdel Khalik H., Rubinger L., Johal H. Cannabinoids in the Management of Acute Pain: A Systematic Review and Meta-analysis. Cannabis Cannabinoid Res. 2020 doi: 10.1089/can.2019.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Richardson J.D., Kilo S., Hargreaves K.M. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- 592.Agarwal N., Pacher P., Tegeder I., Amaya F., Constantin C.E., Brenner G.J., Rubino T., Michalski C.W., Marsicano G., Monory K., et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Yu X.H., Cao C.Q., Martino G., Puma C., Morinville A., St-Onge S., Lessard É., Perkins M.N., Laird J.M.A. A peripherally restricted cannabinoid receptor agonist produces robust anti-nociceptive effects in rodent models of inflammatory and neuropathic pain. Pain. 2010;151:337–344. doi: 10.1016/j.pain.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 594.Amaya F., Shimosato G., Kawasaki Y., Hashimoto S., Tanaka Y., Ji R.R., Tanaka M. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. Pain. 2006;124:175–183. doi: 10.1016/j.pain.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 595.Burstein S., Levin E., Varanelli C. Prostaglandins and cannabis-II inhibition of biosynthesis by the naturally occurring cannabinoids. Biochem. Pharmacol. 1973;22:2905–2910. doi: 10.1016/0006-2952(73)90158-5. [DOI] [PubMed] [Google Scholar]
- 596.Fimiani C., Liberty T., Aquirre A.J., Amin I., Ali N., Stefano G.B. Opiate, cannabinoid, and eicosanoid signaling converges on common intracellular pathways nitric oxide coupling. Prostaglandins Other Lipid Mediat. 1999;57:23–34. doi: 10.1016/S0090-6980(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 597.Kunos G., Osei-Hyiaman D., Bátkai S., Sharkey K.A., Makriyannis A. Should peripheral CB1 cannabinoid receptors be selectively targeted for therapeutic gain? Trends Pharmacol. Sci. 2009;30:1–7. doi: 10.1016/j.tips.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Pertwee R.G. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009;156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 599.Di Marzo V. The endocannabinoid system: Its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol. Res. 2009;60:77–84. doi: 10.1016/j.phrs.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 600.McPartland J.M., Guy G.W., Di Marzo V. Care and Feeding of the Endocannabinoid System: A Systematic Review of Potential Clinical Interventions that Upregulate the Endocannabinoid System. PLoS ONE. 2014;9:e89566. doi: 10.1371/journal.pone.0089566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Bushlin I., Rozenfeld R., Devi L.A. Cannabinoid-opioid interactions during neuropathic pain and analgesia. Curr. Opin. Pharmacol. 2010;10:80–86. doi: 10.1016/j.coph.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Hojo M., Sudo Y., Ando Y., Minami K., Takada M., Matsubara T., Kanaide M., Taniyama K., Sumikawa K., Uezono Y. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: Electrophysiological and FRET assay analysis. J. Pharmacol. Sci. 2008;108:308–319. doi: 10.1254/jphs.08244FP. [DOI] [PubMed] [Google Scholar]
- 603.Zou S., Somvanshi R.K., Kumar U. Somatostatin receptor 5 is a prominent regulator of signaling pathways in cells with coexpression of Cannabinoid receptors 1. Neuroscience. 2017;340:218–231. doi: 10.1016/j.neuroscience.2016.10.056. [DOI] [PubMed] [Google Scholar]
- 604.Tóth A.D., Turu G., Hunyady L., Balla A. Novel mechanisms of G-protein-coupled receptors functions: AT1 angiotensin receptor acts as a signaling hub and focal point of receptor cross-talk. Best Pract. Res. Clin. Endocrinol. Metab. 2018;32:69–82. doi: 10.1016/j.beem.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 605.AbdAlla S., Lother H., El Massiery A., Quitterer U. Increased AT1 receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat. Med. 2001;7:1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- 606.Rozenfeld R., Devi L.A. Receptor heteromerization and drug discovery. Trends Pharmacol. Sci. 2010;31:124–130. doi: 10.1016/j.tips.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Moreno E., Cavic M., Krivokuca A., Casadó V., Canela E. The endocannabinoid system as a target in cancer diseases: Are we there yet? Front. Pharmacol. 2019;10:339. doi: 10.3389/fphar.2019.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Perrey D.A., Gilmour B.P., Thomas B.F., Zhang Y. Toward the development of bivalent ligand probes of cannabinoid CB1 and Orexin OX1 receptor heterodimers. Acs Med. Chem. Lett. 2014;5:634–638. doi: 10.1021/ml4004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Grant P.S., Kahlcke N., Govindpani K., Hunter M., MacDonald C., Brimble M.A., Glass M., Furkert D.P. Divalent cannabinoid-1 receptor ligands: A linker attachment point survey of SR141716A for development of high-affinity CB1R molecular probes. Bioorganic Med. Chem. Lett. 2019;29:126644. doi: 10.1016/j.bmcl.2019.126644. [DOI] [PubMed] [Google Scholar]
- 610.Cinar R., Iyer M.R., Kunos G. The therapeutic potential of second and third generation CB1R antagonists. Pharmacol. Ther. 2020;208:107477. doi: 10.1016/j.pharmthera.2020.107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 611.Morales P., Goya P., Jagerovic N., Hernandez-Folgado L. Allosteric Modulators of the CB1 Cannabinoid Receptor: A Structural Update Review. Cannabis Cannabinoid Res. 2016;1:22–30. doi: 10.1089/can.2015.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Kulkarni A.R., Garai S., Janero D.R., Thakur G.A. Methods in Enzymology. Volume 593. Academic Press Inc.; Cambridge, MA, USA: 2017. Design and Synthesis of Cannabinoid 1 Receptor (CB1R) Allosteric Modulators: Drug Discovery Applications; pp. 281–315. [DOI] [PubMed] [Google Scholar]
- 613.Horswill J.G., Bali U., Shaaban S., Keily J.F., Jeevaratnam P., Babbs A.J., Reynet C., Wong Kai In P. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB 1 receptors with hypophagic effects in rats. Br. J. Pharmacol. 2007;152:805–814. doi: 10.1038/sj.bjp.0707347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Qiao C.J., Ali H.I., Ahn K.H., Kolluru S., Kendall D.A., Lu D. Synthesis and biological evaluation of indole-2-carboxamides bearing photoactivatable functionalities as novel allosteric modulators for the cannabinoid CB1 receptor. Eur. J. Med. Chem. 2016;121:517–529. doi: 10.1016/j.ejmech.2016.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Wu J. Cannabis, cannabinoid receptors, and endocannabinoid system: Yesterday, today, and tomorrow. Acta Pharmacol. Sin. 2019;40:297–299. doi: 10.1038/s41401-019-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Dhopeshwarkar A., Mackie K. CB 2 Cannabinoid Receptors as a Therapeutic Target-What Does the Future Hold? Mol. Pharmacol. Mol Pharm. 2014;86:430–437. doi: 10.1124/mol.114.094649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Tai S., Vasiljevik T., Sherwood A.M., Eddington S., Wilson C.D., Prisinzano T.E., Fantegrossi W.E. Assessment of rimonabant-like adverse effects of purported CB1R neutral antagonist/CB2R agonist aminoalkylindole derivatives in mice. Drug Alcohol Depend. 2018;192:285–293. doi: 10.1016/j.drugalcdep.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618.Ostenfeld T., Price J., Albanese M., Bullman J., Guillard F., Meyer I., Leeson R., Costantin C., Ziviani L., Nocini P.F., et al. A randomized, controlled study to investigate the analgesic efficacy of single doses of the cannabinoid receptor-2 agonist GW842166, ibuprofen or placebo in patients with acute pain following third molar tooth extraction. Clin. J. Pain. 2011;27:668–676. doi: 10.1097/AJP.0b013e318219799a. [DOI] [PubMed] [Google Scholar]
- 619.Rogers N. Cannabinoid receptor with an “identity crisis” gets a second look. Nat. Med. 2015;21:966–967. doi: 10.1038/nm0915-966. [DOI] [PubMed] [Google Scholar]
- 620.Vogel C., Marcotte E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Clapper J.R., Henry C.L., Niphakis M.J., Knize A.M., Coppola A.R., Simon G.M., Ngo N., Herbst R.A., Herbst D.M., Reed A.W., et al. Monoacylglycerol lipase inhibition in human and rodent systems supports clinical evaluation of endocannabinoid modulatorss. J. Pharmacol. Exp. Ther. 2018;367:494–508. doi: 10.1124/jpet.118.252296. [DOI] [PubMed] [Google Scholar]
- 622.Huggins J.P., Smart T.S., Langman S., Taylor L., Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153:1837–1846. doi: 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 623.D’Souza D.C., Cortes-Briones J., Creatura G., Bluez G., Thurnauer H., Deaso E., Bielen K., Surti T., Radhakrishnan R., Gupta A., et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: A double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry. 2019;6:35–45. doi: 10.1016/S2215-0366(18)30427-9. [DOI] [PubMed] [Google Scholar]
- 624.Kerbrat A., Ferré J.-C., Fillatre P., Ronzière T., Vannier S., Carsin-Nicol B., Lavoué S., Vérin M., Gauvrit J.-Y., Le Tulzo Y., et al. Acute Neurologic Disorder from an Inhibitor of Fatty Acid Amide Hydrolase. N. Engl. J. Med. 2016;375:1717–1725. doi: 10.1056/NEJMoa1604221. [DOI] [PubMed] [Google Scholar]
- 625.Treister-Goltzman Y., Freud T., Press Y., Peleg R. Trends in Publications on Medical Cannabis from the Year 2000. Popul. Health Manag. 2019;22:362–368. doi: 10.1089/pop.2018.0113. [DOI] [PubMed] [Google Scholar]
- 626.Yeung A.W.K., Tzvetkov N.T., Arkells N., Milella L., Stankiewicz A.M., Huminiecki Ł., Horbanczuk O.K., Atanasov A.G. Molecular neuroscience at its “high”: Bibliometric analysis of the most cited papers on endocannabinoid system, cannabis and cannabinoids. J. Cannabis Res. 2019;1:4. doi: 10.1186/s42238-019-0004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Bisogno T., Ligresti A., Dimarzo V. The endocannabinoid signalling system: Biochemical aspects. Pharmacol. Biochem. Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 628.Mallipeddi S., Janero D.R., Zvonok N., Makriyannis A. Functional selectivity at G-protein coupled receptors: Advancing cannabinoid receptors as drug targets. Biochem. Pharmacol. 2017;128:1–11. doi: 10.1016/j.bcp.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Busquets-Garcia A., Bains J., Marsicano G. CB 1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity Signaling of CB 1 Receptors in the Brain: Intrinsic or Emerging Features? Neuropsychopharmacol. Rev. 2018;43:4–20. doi: 10.1038/npp.2017.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Hryhorowicz S., Kaczmarek-Ryś M., Andrzejewska A., Staszak K., Hryhorowicz M., Korcz A., Słomski R. Allosteric modulation of cannabinoid receptor 1—Current challenges and future opportunities. Int. J. Mol. Sci. 2019;20:5874. doi: 10.3390/ijms20235874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Urits I., Borchart M., Hasegawa M., Kochanski J., Orhurhu V., Viswanath O. An Update of Current Cannabis-Based Pharmaceuticals in Pain Medicine. Pain Ther. 2019;8:41–51. doi: 10.1007/s40122-019-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Onaivi E.S. Endocannabinoid system, pharmacogenomics and response to therapy. Pharmacogenomics. 2010;11:907–910. doi: 10.2217/pgs.10.91. [DOI] [PubMed] [Google Scholar]
- 633.Reddy V., Grogan D., Ahluwalia M., Salles É.L., Ahluwalia P., Khodadadi H., Alverson K., Nguyen A., Raju S.P., Gaur P., et al. Targeting the endocannabinoid system: A predictive, preventive, and personalized medicine-directed approach to the management of brain pathologies. EPMA J. 2020;11:217–250. doi: 10.1007/s13167-020-00203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]