Abstract
Objective
To evaluate the reliability and utility of the FIGO Nutrition Checklist to identify dietary and nutritional inadequacy in early pregnancy by comparing it against nutritional indicators and dietary quality indices (Dietary Approaches to Stop Hypertension [DASH] score, Mediterranean Diet Score [MDS], and Dietary Quality Index‐International [DQI‐I]), derived by a locally validated food frequency questionnaire (FFQ).
Methods
A prospective cohort study of healthy Chinese pregnant women randomly recruited between September 2017 and April 2018 at their first antenatal appointment. Women completed the FIGO Nutrition Checklist (translated into Chinese) and the FFQ. Spearman correlation was performed to examine association between the Checklist and dietary quality indices or food and nutrient intakes, calculated based on dietary data from the FFQ.
Results
Of 160 participants, 156 (97.5%) completed both the FIGO Nutrition Checklist and FFQ and were included. There were 148 (95%) women who reported at least one suboptimal dietary behavior using the Checklist. Checklist score was significantly associated with dietary quality indices (DASH ρ=0.344, P<0.001; DQI‐I ρ=0.304, P<0.001; MDS ρ=0.164, P=0.041). The Checklist question on fruit/vegetables was significantly associated with fiber, vitamin C, and fruit and vegetable intake as calculated from the FFQ (0.325 ≤ ρ ≤0.441, P<0.001). The question on dairy intake was significantly associated with intake of calcium, milk and dairy products captured via FFQ (0.576 ≤ ρ ≤0.655, P<0.001).
Conclusion
This study supports the use of the FIGO Nutrition Checklist to identify women with suboptimal dietary quality in early pregnancy.
Keywords: FIGO Nutrition Checklist, Maternal health, Nutrition, Obesity, Pregnancy
Short abstract
The FIGO Nutrition Checklist was significantly associated with dietary quality indices derived from a validated food frequency questionnaire. The Checklist can help identify nutritional issues in pregnancy.
1. Introduction
A healthy balanced diet providing good nutrition for women before and during pregnancy is not only important for women’s health, but also to support healthy fetal growth and development. 1 , 2 Furthermore, a large body of evidence supports the importance of maternal nutrition on long‐term noncommunicable disease risk for mothers as well as for future generations. 1 , 3 , 4 , 5
The nutritional status of women in Hong Kong has not been studied in detail, with only a few dietary and nutritional surveys conducted in the past. These studies highlighted inadequate intake of various nutrients, including iodine, 6 , 7 vitamin D, 8 fiber, calcium, and iron 9 among local women of childbearing age. However, no study focused specifically on maternal dietary intake and nutritional status.
The current gold standard for evaluating dietary and nutritional intake involves food‐based dietary assessments, such as a food diary, 24‐hour recall, and food frequency questionnaire (FFQ), which form the basis for investigating dietary and nutritional intake and dietary quality. The dietary assessment involves two important parts. First, collection of a dietary record using the selected dietary assessment tools, which is typically completed through direct interviews by a trained assistant. Details of food and beverage intakes and cooking and preparation methods are asked. The next step is conversion of the reported dietary record into nutrient data. The dietary records need to be coded into food analysis programs, which comprise country‐specific food composition tables. The final data are then converted into nutrient data or food groups as required. The whole process of traditional food‐based dietary assessment is complicated and requires a substantial amount of time and nutritional expertise. Therefore, it is unlikely to provide immediate feedback.
FIGO (the International Federation of Gynecology and Obstetrics) recognizes the importance of maternal nutrition in early pregnancy and has developed a set of recommendations regarding preconception and maternal nutrition. 1 To facilitate the identification of nutritional issues during pregnancy, the FIGO Nutrition Checklist (supporting information S1) was developed by a committee of experts to target prepregnant and early pregnant women. The FIGO Nutrition Checklist is simple, short, and easy to understand, with closed‐ended questions. It is practical and can be completed by women themselves, in conjunction with their healthcare professionals, before or during the antenatal check‐up, to identify nutritional risk and identify women who may benefit from more formal dietary assessment and counselling.
The aim of the present study was to evaluate the reliability and utility of the FIGO Nutrition Checklist to identify dietary and nutritional inadequacy in early pregnancy by comparing it against nutritional indicators (nutrients and food intake) and dietary quality indices (Dietary Approaches to Stop Hypertension [DASH] score, Mediterranean Diet Score [MDS], and Dietary Quality Index‐International [DQI‐I]), derived from a locally validated FFQ. 10 Furthermore, an exploratory analysis was performed to investigate whether there was any association between the FIGO Nutrition Checklist assessment and short‐term pregnancy outcomes in the study population.
2. Materials and methods
2.1. Study population and design
This was a prospective cohort study. A group of healthy Chinese pregnant women were recruited randomly at their first antenatal appointment at the antenatal clinic of the Prince of Wales Hospital, Shatin, Hong Kong, between September 28, 2017 and April 23, 2018. The Prince of Wales Hospital is one of eight public hospitals that provide maternity care in Hong Kong. Inclusion criteria were healthy Chinese women with a singleton pregnancy of less than 14 weeks at the time of recruitment, usually residing in Hong Kong, who were able to communicate in Cantonese Chinese and willing to follow study procedures. Women with pre‐existing diabetes, known psychiatric conditions including depression, any chronic medical condition requiring long‐term medications, previous surgical or medical interventions to treat obesity, participation in other intervention trials, or those who followed a restricted diet (e.g. vegetarian) were excluded.
Demographic information including pregnancy and medical history were collected using a standardized questionnaire. Anthropometric measurements such as body weight, body height, and blood pressure were obtained using standardized methods. The study involved two questionnaires: a Chinese translation of the FIGO Nutrition Checklist and a locally validated FFQ, 10 both collected at the antenatal clinic.
The study was conducted according to the Declaration of Helsinki and the research protocol was approved by the Clinical Research Ethics Committee of the Chinese University of Hong Kong. The study design was explained to each participant and written informed consent was obtained from women who expressed interest.
2.2. FIGO Nutrition Checklist
The FIGO Nutrition Checklist was developed by the FIGO Adolescent, Preconception, and Maternal Nutrition Working Group and its subgroup (supporting information S2). The Checklist was designed for women to complete, in conjunction with their healthcare professional, to assess nutritional intake before and during pregnancy. It consists of 11 questions, including weight and height, special dietary requirement, dietary quality, and other health behaviors. The present study focused on the six diet quality screening questions, including intake of meat or chicken, fruits or vegetables, fish, dairy products, wholegrains, carbohydrate foods, and packaged snacks. Each question was developed carefully and intended to focus on nutritional indicators such as nutrient or food intakes. However, some nutrient information was not available in the food composition databases, so a similar alternative nutrient was selected and assessed (supporting information S3).
Responses to the FIGO Nutrition Checklist are simple, and the respondent is only required to answer “Yes” or “No.” Each “Yes” response received a score of 1 and each “No” response received a score of 0, totaling a score of 6 if all answers were positive. An answer of “No” to any of the screening questions would potentially identify women with nutritional issues that would warrant further evaluation. To investigate the feasibility of the FIGO Nutrition Checklist to identify dietary and nutritional inadequacy, the study population was further divided into a dietary and nutritional risk groups according to responses (number of risk factors and score) for further analysis. The first classification categorized individuals into a “suboptimal diet” group if they had at least one “No” response, and a “good diet” group if they had six “Yes” responses. As a sensitivity analysis, we also evaluated an alternative classification of “suboptimal diet” group for individuals with at least two or more “No” responses and the rest categorized as “good diet” group if they had one or fewer “No” responses. All checklists were completed by the woman with the guidance of a well‐trained nutritionist.
The original FIGO Nutrition Checklist was designed and written in English, therefore translation into the local language was required prior to use in the study. The translation procedures involved the following steps: (1) forward translation; (2) committee evaluation; and (3) consolidation and content validation (supporting information S4). It was then pretested by a focus group and proposed modifications were incorporated before the final version was set (supporting information S5). 11
2.3. Food frequency questionnaire (FFQ)
The FFQ is often regarded as the gold standard for evaluating dietary intake, with local modifications that are necessary to reflect dietary practices in different cultures and regions. Dietary assessment was also performed using a locally validated FFQ. 9 Minor modifications were made to remove outdated items and include new food items that are commonly consumed in pregnancy. The FFQ contains over 200 food items, including 11 food categories: cereals, vegetables, fruits, meat and poultry, fish and seafood, eggs, milk and dairy products, beverages, dim sum and snacks, soups, and oil and condiments. Each participant was asked to fill in the amount and frequency of consumption of each food item over the past 3 months prior to the interview. A trained nutritionist was present throughout the interview and used food models, common household utensils and containers, and a catalogue of pictures of individual food portions to facilitate estimation of portion size. The amount of cooking oil was estimated based on the usual cooking methods, type of cooking oil, and the portion of different types consumed by participants. 12 Daily nutrient intake was estimated using nutrition analysis and fitness software Food Processor version 8.0 (ESHA Research, Salem, OR, USA) with additional local and Chinese food items. 13 , 14 , 15 Nutrient intake was energy adjusted by the nutrient density model residual method. 16
Individual food items from the FFQ were then aggregated into 32 food groups based on the similarity of food types and nutrient composition, as described in a previous study. 17 Some individual food items were combined into new groups to evaluate their association with individual questions on the FIGO Nutrition Checklist (e.g. red and processed meat, poultry). Both food and nutrients intake generated from the FFQ were used to calculate the dietary quality indices including DASH score, MDS, and DQI‐I.
2.4. Dietary score calculation
2.4.1. Dietary Approaches to Stop Hypertension (DASH) score
A score developed by Mellen et al. 18 was used to assess accordance with the DASH dietary pattern, which is a diet rich in fruits, vegetables, and low‐fat dairy foods, and reduced saturated and total fat. The score is entirely based on targeted intake of nine nutrients in the DASH diet, which includes total fat, saturated fat, protein, fiber, cholesterol, magnesium, calcium, potassium, and sodium. Individuals achieving the target of each nutrient receive 1 point; those achieving the intermediate target receive 0.5 points. The total DASH score is the sum of the score for each targeted nutrient, ranging from 0–9. A higher DASH score indicates better DASH diet accordance.
2.4.2. Mediterranean Diet Score (MDS)
Adherence to the Mediterranean diet was calculated based on the method proposed by Trichopoulou et al. 19 The MDS consists of nine components, which include a food group/nutrient index considered to be beneficial to health (vegetables, legumes, fruits and nuts, cereal, fish, and monosaturated to saturated lipids ratio), those presumed to be detrimental to health (meat and dairy products), and ethanol consumption. Individuals consuming beneficial food components at or above the sex‐specific median, or consuming detrimental food components below the median, receive 1 point. None of the pregnant women reported having consumed alcohol in the study. While changes in drinking habits due to pregnancy is expected, the ethanol consumption component might not be relevant and was excluded in the calculation. Therefore, the total MDS score ranged from 0 (minimal adherence) to 8 (maximal adherence) instead of 0–9.
2.4.3. Dietary Quality Index‐International (DQI‐I)
The DQI‐I was developed by Kim et al. 20 DQI‐I was calculated based on four major aspects: variety, adequacy, moderation, and overall balance of a healthy diet. Each aspect included its subcomponents. The calculation of DQI‐I in the present study adapted the modification method suggested by Chan et al. 21 This was due to inadequate information on empty calorie foods to calculate “moderation” score, therefore the range of score for “moderation” was 0–24 instead of 0–30, and the total DQI‐I score was 0–94 instead of 0–100 as in the original method.
2.5. Clinical investigations and pregnancy outcome
Hematological investigations including hemoglobin to detect anemia, white blood cell count, platelet count, and other hematological indices were taken at antenatal booking. Pregnancy complications such as gestational diabetes (GDM), results of the oral glucose tolerance test (OGTT 0/OGTT120), and pregnancy outcomes (e.g. gestational age, weight before delivery, birthweight) were obtained from the participants’ electronic health and medical records if available. GDM was diagnosed according to local adaptation of the World Health Organization 2013 criteria, using glucose values at 0 and 120 minutes. 22 , 23 , 24 Total gestational weight gain was calculated using [weight before delivery] − [weight at first antenatal appointment].
2.6. Statistical analysis
Statistical analyses were performed using SPSS version 24.0 (IBM, Armonk, NY, USA). Continuous variables (i.e. dietary quality indices score) are expressed as mean, median, and standard derivation. Categorical variables (e.g. education level, family income, GDM) are expressed as number and percentage. Mann–Whitney U test was used to compare dietary and nutritional risk groups with continuous variable, while χ2 test or Fisher exact test was used with categorical variables. Spearman correlation was used to examine: (1) association between the FIGO Nutrition Checklist and the dietary quality indices; and (2) association between the individual questions on the FIGO Nutrition Checklist and the respective nutritional indicators (e.g. nutrient and food intake).
Receiver operating characteristic (ROC) curve analysis was used to evaluate the performance of the FIGO Nutrition Checklist to identify less healthy dietary quality using DASH score, DQI‐I, and MDS. Since there is no cut‐off that can be used as a reference, the study population mean and median of each dietary quality indices were used to define healthy dietary quality, where above the mean and median was “healthy diet” and below was “unhealthy diet.” For all analyses, statistical significance was set at P<0.05 (2‐sided).
3. Results
A total of 160 women were recruited to the study but the analysis was restricted to 156 women who had completed both the FIGO Nutrition Checklist and FFQ. Mean gestational age of respondents was 12.0 ± 0.37 weeks at the time of dietary assessment. Mean age of the recruited women was 32.7 ± 3.9 years and mean body mass index (BMI, calculated as weight in kilograms divided by the square of height in meters) before pregnancy was 22.6 ± 3.83. Among the 156 women, 21 (13.5%) were diagnosed with GDM (Table 1).
Table 1.
Sociodemographic characteristics of the Hong Kong Chinese pregnant women included in the study (n=156). a
| Characteristics | |
|---|---|
| Age, y | 32.7 ± 3.9 |
| Gestational age at interview, wk | 12.0 ± 0.37 |
| BMI at antenatal visit | 22.6 ± 3.83 |
| Underweight (<18.5) | 14 (9.0) |
| Normal (18.5–22.9) | 85 (54.5) |
| Overweight and obese (≥23.0) | 57 (36.5) |
| Gravidity | |
| 1 | 75 (48.1) |
| ≥2 | 81 (51.9) |
| Education | |
| Secondary or below | 65 (41.7) |
| University and above | 91 (58.3) |
| Gestational diabetes | |
| Yes | 21 (13.5) |
| No | 135 (86.5) |
| Birth outcome | |
| Live birth | 148 (94.9) |
| Miscarriage/termination | 2 (1.3) |
| Unknown b | 6 (3.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters).
Values are given as mean ± SD or number (percentage).
Lost to follow‐up.
For analysis of birth outcome, data were not available for 18 births (1 miscarriage, 1 termination, 10 delivered in private hospitals or overseas, 6 lost to follow‐up and not contactable for follow‐up information), leaving 138 women included in the analysis. Two‐thirds of women (n=96) did not meet the recommended pregnancy weight gain according to their preconception BMI class. Two (1.4%) live births were macrosomic. Pregnancy outcomes are summarized in supporting information S6. In their responses to the FIGO Nutrition Checklist, 148 (95%) women had at least one answer of “No” to any of the questions on dietary or nutritional behaviors (supporting information S7).
FIGO Nutrition Checklist score and dietary quality indices scores (DASH, DQI‐I, and MDS) are summarized in Table 2. Results of the Spearman correlation showed that the FIGO Nutrition Checklist was significantly associated with the DASH score (ρ=0.344, P<0.001), DQI‐I (ρ=0.304, P<0.001), and MDS (ρ=0.164, P=0.041) (Fig. 1). FIGO Nutrition Checklist score was also significantly associated with the subcomponents of DQI‐I, variety (ρ=0.234, P=0.003) and adequacy (ρ=0.364, P<0.001), but not with moderation (ρ=−0.111, P=0.168) and overall balance (ρ=−0.014, P=0.862) (Fig. 1).
Table 2.
FIGO Nutrition Checklist score and dietary quality indices scores (DASH, DQI‐I, and MDS) among Hong Kong Chinese pregnant women (n=156)
| Dietary quality indices score | Mean | Median | ±SD |
|---|---|---|---|
| FIGO Nutrition Checklist (0–6) | 3.82 | 4.0 | 1.06 |
| DASH (0–9) | 3.79 | 4.0 | 1.34 |
| DQI (0–94) | 50.61 | 51.0 | 7.07 |
| Variety (0–10) | 4.69 | 5.0 | 0.82 |
| Adequacy (0–30) | 24.08 | 24.0 | 4.49 |
| Moderation (0–24) | 7.92 | 9.0 | 4.34 |
| Overall balance (0‐10) | 1.03 | 2.0 | 1.03 |
| MDS (0–8) | 4.10 | 4.0 | 1.23 |
Abbreviations: DASH, Dietary Approaches to Stop Hypertension; DQI‐I, Dietary Quality Index–International; MDS, Mediterranean Diet Score.
Figure 1.
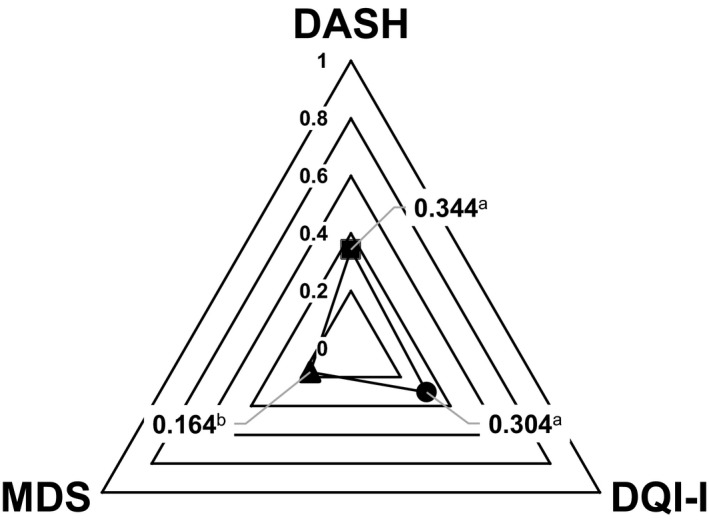
Spearman correlation between FIGO Nutrition Checklist score and dietary quality indices scores (DASH, DQI‐I, and MDS) among Hong Kong Chinese pregnant women (n=156). Abbreviations: DASH, Dietary Approaches to Stop Hypertension; DQI‐I, Dietary Quality Index–International; MDS, Mediterranean Diet Score. a P<0.01. b P<0.05.
The results of dietary quality indices scores on dietary and nutritional risk groups (i.e. “good diet” [FIGO Nutrition Checklist score of 6] and “suboptimal diet” [FIGO Nutrition Checklist score of 0–5]) are shown in Figure 2. The scores of DASH and DQI‐I were significantly higher in the “good diet” group compared to the “suboptimal diet” group (mean ± SD 5.19 ± 0.75 vs 3.72 ± 1.32, P=0.002; and 58.3 ± 5.70 vs 50.2 ± 6.92, P=0.003, respectively). The differences between the two groups remained significant when a different cut‐off on risk group definition was applied as a sensitivity analysis (supporting information S8). Furthermore, the subcomponent of the DQI‐I on variety and adequacy was significantly higher in the “good diet” group compared to the “suboptimal diet” group (4.95 ± 0.32 vs 4.6 ± 0.92, P=0.019; and 26.0 ± 4.71 vs 23.4 ± 4.24, P=0.002, respectively). The results for pregnancy outcomes in relation to dietary and nutritional risk groups defined by FIGO Nutrition Checklist scores are included in supporting information S9. There was a significant association between dietary and nutritional risk groups (“good diet” and “suboptimal diet”) and gestational weight gain (P=0.025) but not with GDM (P=0.684). In the ROC analysis, the FIGO Nutrition Checklist showed modest capacity to identify women with suboptimal dietary quality as defined by DASH (AUC 0.602 [0.514–0.691, P=0.027]); DQI‐I (AUC 0.675 [0.591–0.758, P<0.001]); and MDS (AUC 0.601 [0.510–0.692, P=0.041]).
Figure 2.
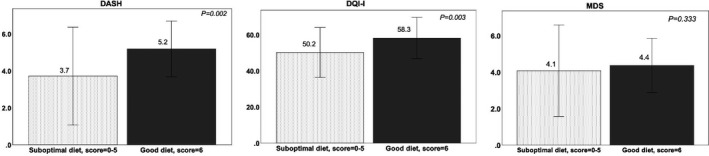
Summary of FIGO Nutrition Checklist score and mean ± SD of dietary quality indices scores (DASH, DQI‐I, and MDS) among 156 Hong Kong Chinese pregnant women (at ≤ 14 weeks of pregnancy) by dietary and nutritional risk groups (“suboptimal diet” and “good diet”) using Mann‐Whitney U test. Abbreviations: DASH, Dietary Approaches to Stop Hypertension; DQI‐I, Dietary Quality Index–International; MDS, Mediterranean Diet Score.
The association between the six individual FIGO Nutrition Checklist questions and their association with the respective nutrient and food group intakes assessed by FFQ are summarized in Table 3. The FIGO Nutrition Checklist questions on fruit and vegetables and dairy were associated with the respective nutrients and food intakes. The fruit and vegetables question was significantly associated with fiber (ρ=0.373, P<0.001), vitamin C (ρ=0.325, P<0.001), and fruit and vegetable intake (ρ=0.441, P<0.001). Moreover, the dairy question was associated with calcium (ρ=0.576, P<0.001) and milk and dairy products intake (ρ=0.655, P<0.001).
Table 3.
Spearman correlation between individual FIGO Nutrition Checklist questions and dietary nutritional indicators (nutrients and food group intake)
| FIGO Nutrition Checklist questions | Nutrients | Food group | |||||
|---|---|---|---|---|---|---|---|
| 1. Meat or chicken | Protein, g | Protein, en% | Iron, mg | Meat | |||
| Coefficient | 0.265 a | 0.275 a | 0.179 b | 0.290 a | |||
| P value | 0.001 | 0.001 | 0.025 | <0.001 | |||
| 2. Fruit or vegetables | Fiber, g | Vitamin C, mg | Fruit and vegetables | ||||
| Coefficient | 0.373 a | 0.325 a | 0.441 a | ||||
| P value | <0.001 | <0.001 | <0.001 | ||||
| 3. Fish | PUFA, g | Ratio: PUFA/SFA | Fish and seafood | ||||
| Coefficient | 0.14 | 0.034 | 0.472 a | ||||
| P value | 0.081 | 0.673 | <0.001 | ||||
| 4. Dairy | Calcium, mg | Milk and milk products | |||||
| Coefficient | 0.576 a | 0.655 a | |||||
| P value | <0.001 | <0.001 | |||||
| 5. Wholegrains | Fiber, g | Sugar, g | Refined grains | Wholegrain | |||
| Coefficient | 0.241 a | 0.062 | 0.005 | 0.359 a | |||
| P value | 0.002 | 0.444 | 0.954 | <0.001 | |||
| 6. Packaged snacks | Fiber, g | Sugar, g | Packaged snacks | Refined grain | Wholegrain | ||
| Coefficient | –0.149 | –0.389 a | 0.025 | 0.025 | −0.033 | ||
| P value | 0.064 | <0.001 | 0.756 | 0.753 | 0.682 | ||
Abbreviations: Protein en%, energy % from protein; PUFA, polyunsaturated fat; SFA, saturated fat.
P<0.01.
P<0.05.
In terms of food group intake, the question on meat or chicken consumption was significantly associated with energy percentage from protein (ρ=0.275, P=0.001), iron (ρ=0.179, P=0.025), and meat and meat products intake (ρ=0.290, P<0.001). The fish question was significantly associated with fish and seafood intake (ρ=0.472, P<0.001) but not with its respective nutrient intake: polyunsaturated fatty acids (PUFA) (ρ=0.14, P=0.081) and the ratio of PUFA/SFA (ρ=0.034, P=0.673). Furthermore, the wholegrain question was significantly associated with fiber (ρ=0.241, P=0.002) and wholegrain intake (ρ=0.359, P<0.001), but not with sugar or refined grains intake. Lastly, the packaged snacks question was only associated with sugar intake (ρ=−0.389, P<0.001), but not with any other parameters in food groups including wholegrains, refined grains, and packaged food.
4. Discussion
This is the first study to evaluate formally the usefulness of the FIGO Nutrition Checklist, translated into traditional Chinese, among pregnant women. Significant associations between the FIGO Nutrition Checklist and DASH, DQI‐I, and MDS provide evidence of the utility of using the FIGO Nutrition Checklist, as these dietary quality indices were developed to assess the healthiness of individual diet and have shown associations with disease preventions in various studies. 21 , 25 , 26 , 27 , 28 , 29
Since the aim of using the FIGO Nutrition Checklist is to provide a simple way of identifying women with dietary and nutritional inadequacy compared to long and detailed food diaries that are often difficult to administer and interpret, we also conducted an ROC analysis to investigate its performance using DASH, DQI‐I, and MDS scores as references. While there is no accepted reference cut‐off point from previous studies, we tested the study population mean and median to define healthy dietary quality, and found that both measures generated fair performance based on similar AUC results of DASH, DQI‐I, and MDS scores (AUC 0.60 to 0.67).
In addition, we used the FIGO Nutrition Checklist score (i.e. responses to each question) to classify women into dietary and nutritional risk groups, with arbitrarily defined “good diet” and “suboptimal diet,” using two different cut‐offs. Both cut‐offs demonstrated that dietary and nutritional risk groups were significantly associated with DASH and DQI‐I scores, where lower DASH and DQI‐I scores among women in the “suboptimal diet” group were observed. These results suggest that women who have lower scores on the FIGO Nutrition Checklist are more likely to have poor dietary and nutritional intake.
The “adequacy” subcomponent of the DQI‐I was significantly different when compared to “suboptimal diet” and “good diet” groups. This “adequacy” evaluates the dietary intake of fruits, vegetables, grains, and fibers, as well as nutrient intake of protein, iron, and vitamin C. Women in the “suboptimal diet” group attained a lower score, suggesting that their nutrient intakes were insufficient. The “variety” DQI‐I subcomponent was found to be significantly different when comparing “suboptimal diet” and “good diet” group. We observed a lower score for “variety” among the “suboptimal diet” group suggesting that intakes of nutrients were coming from a rather narrow variety of food sources. Therefore, these results suggest that the FIGO Nutrition Checklist can help to identify women with inadequate nutritional intake and dietary variety.
A further method used to study the utility of the FIGO Nutrition Checklist was to investigate the association between each individual Checklist question in terms of nutrient or food intakes. Our results demonstrated that the questions on meat and chicken, fruits and vegetables, dairy, and wholegrains were significantly associated with their respective nutrient and food intakes. The other two Checklist questions on fish and packaged food were also significantly associated with either nutrient or food intakes alone. These results provide evidence to support the application of the FIGO Nutrition Checklist to assess the sufficiency of nutrient or food intake in this population during pregnancy. Therefore, introduction of the FIGO Nutrition Checklist to identify dietary and nutritional risk in early pregnancy during the antenatal visit should be considered.
We also explored the association between the results of the FIGO Nutrition Checklist and pregnancy outcomes including inappropriate gestational weight gain, GDM, macrosomia, abnormal blood pressure, and hemoglobin level. We only observed a significant association between the FIGO Nutrition Checklist score‐defined dietary and nutritional risk groups (“suboptimal diet” and “good diet”) and gestational weight gain (P=0.025), where half of the women had less than the recommended gestational weight gain in the “suboptimal diet” group, but not the other parameters. This is likely due to the small sample size in our study, which was not powered to examine the relationship between diet and pregnancy outcome.
There are several strengths of our study, including enrolment of a representative population of local pregnant women. The interviews were conducted in parallel with a woman’s first antenatal visit in early pregnancy and the FFQ was focused on the women’s dietary habits over the 3 months prior to the interview. This timeframe should capture well the dietary habits and practices during the preconception and early pregnancy periods. In addition, our study used a locally validated FFQ and food composition table to calculate the intake of nutrients and food, as well as dietary quality scores, to assess their association with the FIGO Nutrition Checklist in order to strengthen the validity of the results. The FIGO Nutrition Checklist is quick, simple, and intelligible to the woman and her partner. It does not require input from a healthcare provider and can be completed in advance, for example in the waiting room. Coupled with more detailed information given to the healthcare provider, it acts as a stimulus for engaging with the woman and her partner in a discussion about the importance of good nutrition in early pregnancy, and also serves as a tool to identify women who need referral for more specialized nutritional advice.
We recognize several limitations of our study. We only included a relatively small sample size of 156 pregnant women for the analysis. In addition, the common limitations of self‐reporting assessment are expected, for example under‐reporting of energy intake or food generally considered unhealthy (e.g. sweet foods and desserts). Moreover, there is no nutrient biological marker available in the present study to confirm individual nutritional status and to provide biochemical validation of the FIGO Nutrition Checklist. Although our food composition table does not have data on vitamin B12, vitamin D, iodine, omega 3, and omega 6 PUFA, which were highlighted in relation to FIGO Checklist questions, the impact of this on our analysis should be limited as we selected appropriate alternative nutrients, based on the similarity of their properties and functions, for the analysis. For example, although we did not have separate information on omega 3 and omega 6 PUFA, we used total PUFA and ratio of PUFA/SFA instead. In addition, we included at least one specific nutrient and one food group to examine the association with each individual FIGO Nutrition Checklist question. Finally, in future it would be useful to tailor the Checklist to include specific nutrients that may be deficient locally. It is estimated that more than two‐thirds of women in Hong Kong had iodine deficiency and more than 80% of women have iodine intake below the daily recommendation. 7 Iodine is an important nutrient for fetal neurodevelopment in early pregnancy. 30 When using the FIGO Nutrition Checklist in pregnant women in Hong Kong it would therefore be beneficial to include an additional question that focuses on iodine‐containing foods (e.g. seaweed, kelp, and the practice of iodized salt use).
In conclusion, we observed significant associations between the FIGO Nutrition Checklist and dietary quality indices derived from a local FFQ, as well as between the individual FIGO Nutrition Checklist questions and the corresponding nutrient and food intakes. Women classified into the “suboptimal diet” group with a lower FIGO Nutrition Checklist score had significantly lower dietary quality scores on DASH and DQI‐I. Our results highlight the ability of the simple FIGO Nutrition Checklist to differentiate between women with better or less optimal diet when compared with more sophisticated dietary assessment such as the FFQ. The FIGO Nutrition Checklist therefore appears to be a suitable simple tool to help identify women with suboptimal dietary quality in early pregnancy.
Author contributions
RCWM, KYT, RSMC, and WHT conceived the study. Data collection was performed by KYT and RSMC. All authors contributed to the writing and revision of the article.
Conflicts of interest
The authors have no conflicts of interest.
Supporting information
Supporting information S1. FIGO nutrition checklist for pre‐pregnant/early pregnant women. Reproduced with permission from FIGO.
Supporting information S2. Members of the FIGO Adolescent, Pre‐conception, and Maternal Nutrition Working Group.
Supporting information S3. Summary of individual FIGO Nutrition Checklist questions, target nutritional indicators, and selected nutrients and food groups for analysis.
Supporting information S4. Process of translation of the FIGO Nutrition Checklist into the Chinese version used in the current study.
Supporting information S5. Translated FIGO Nutrition Checklist (traditional Chinese version).
Supporting information S6. Characteristics of pregnancy outcomes of the 138 live births with available follow‐up data.a
Supporting information S7. Frequency distribution of FIGO Nutrition Checklist score among Chinese pregnant women in Hong Kong (n=156).
Supporting information S8. Summary of FIGO Nutrition Checklist and dietary quality indices scores among 156 Hong Kong Chinese pregnant women (at ≤ 14 weeks of pregnancy) by dietary and nutritional risk groups (“suboptimal diet” and “good diet”).
Supporting information S9. Results for pregnancy outcomes in dietary and nutritional risk groups defined by FIGO Nutrition Checklist scores.
Acknowledgments
The FIGO Nutrition Checklist was developed by the FIGO Adolescent, Preconception, and Maternal Nutrition Working Group and Nutrition Checklist subgroup. RCMW acknowledges support from the Diabetes and Endocrine Research Fund of the Chinese University of Hong Kong in conducting this study.
References
- 1. Hanson MA, Bardsley A, De‐Regil LM, et al. The International Federation of Gynecology and Obstetrics (FIGO) recommendations on adolescent, preconception, and maternal nutrition: “Think Nutrition First”. Int J Gynecol Obstet. 2015;131(Suppl.4):S213–S253. [DOI] [PubMed] [Google Scholar]
- 2. O'Brien EC, Tsoi KY, Ma RC, Hanson MA, Hod M, McAuliffe FM. Nutrition through the life cycle: pregnancy In: Ferranti P, Berry E, and Jock A. (Eds). Encyclopedia of Food Security and Sustainability. Amsetrdam, Netherlands: Elsevier; 2019. [Google Scholar]
- 3. World Health Organization, Regional Office for Europe . Good Maternal Nutrition: The best start in life. Geneva: WHO; 2016. https://www.euro.who.int/__data/assets/pdf_file/0008/313667/Good-maternal-nutrition-The-best-start-in-life.pdf?ua=1. Accessed on July 2, 2020. [Google Scholar]
- 4. Ma RC, Chan JC. Pregnancy and diabetes scenario around the world: China. Int J Gynecol Obstet. 2009;104(Suppl.1):S42–S45. [DOI] [PubMed] [Google Scholar]
- 5. Ma RCW, Tsoi KY, Tam WH, Wong KCK. Developmental origins of type 2 diabetes: A perspective from China. Eur J Clin Nutr. 2017;71:870–880. [DOI] [PubMed] [Google Scholar]
- 6. Kung AW. Iodine nutrition of pregnant and lactating women in Hong Kong, where intake is of borderline sufficiency. Public Health Nutr. 2007;10:1600–1601. [DOI] [PubMed] [Google Scholar]
- 7. Tam WH, Chan RS, Chan MH, et al. Moderate iodine deficiency among pregnant women in Hong Kong: Revisit the problem after two decades. Hong Kong Med J. 2017;23:586–593. [DOI] [PubMed] [Google Scholar]
- 8. Woo J, Lam CW, Leung J, et al. Very high rates of vitamin D insufficiency in women of child‐bearing age living in Beijing and Hong Kong. Br J Nutr. 2008;99:1330–1334. [DOI] [PubMed] [Google Scholar]
- 9. Chan R, Li S, Lao TH, Woo J. Pilot cross‐sectional study examining nutrient intake fo Chinese Pregnant women in Hong Kong. Confernence paper presented at the 12th Asia Congress of Nutrition, Yokohama, Japan, May 14–18 2015. [Google Scholar]
- 10. Woo J, Leung SSF, Ho SC, Lam TH, Janus ED. A food frequency questionnaire for use in the Chinese population in Hong Kong: Description and examination of validity. Nutr Res. 1997;17:1633–1641. [Google Scholar]
- 11. Tsang S, Royse CF, Terkawi AS. Guidelines for developing, translating, and validating a questionnaire in perioperative and pain medicine. Saudi J Anaesth. 2017;11(Suppl.1):S80–S89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woo J, Leung SS, Ho SC, Lam TH, Janus ED. Dietary intake and practices in the Hong Kong Chinese population. J Epidemiol Community Health. 1998;52:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang Y, Wang G, Pan X. China Food Composition 2002. Peking: China University Medical Press; 2002. [Google Scholar]
- 14. Yang Y, Wang G, Pan X. China Food Composition 2004. Peking: China University Medical Press; 2004. [Google Scholar]
- 15. Centre for Food Safety . Nutrient Information Inquiry. Hong Kong SAR: Centre for Food Safety; 2006. [Google Scholar]
- 16. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1229S;65(4 Suppl):1220S–1228S; discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 17. Chan R, Chan D, Woo J. Associations between dietary patterns and demographics, lifestyle, anthropometry and blood pressure in Chinese community‐dwelling older men and women. J Nutr Sci. 2012;1:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mellen PB, Gao SK, Vitolins MZ, Goff DC Jr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168:308–314. [DOI] [PubMed] [Google Scholar]
- 19. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. [DOI] [PubMed] [Google Scholar]
- 20. Kim S, Haines PS, Siega‐Riz AM, Popkin BM. The Diet Quality Index‐International (DQI‐I) provides an effective tool for cross‐national comparison of diet quality as illustrated by China and the United States. J Nutr. 2003;133:3476–3484. [DOI] [PubMed] [Google Scholar]
- 21. Chan R, Wong VW, Chu WC, et al. Diet‐quality scores and prevalence of nonalcoholic fatty liver disease: A population study using proton‐magnetic resonance spectroscopy. PLoS One. 2015;10:e0139310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: A pragmatic guide for diagnosis, management, and care. Int J Gynecol Obstet. 2015;131(Supp.3):S173–S211. [DOI] [PubMed] [Google Scholar]
- 23. Hong Kong College of Obstetricians and Gynaecologists . Guideline for the management of gestational diabetes mellitus. A Foundation College of Hong Kong Academy of Medicine. The Hong Kong College of Obstetricians and Gynaecologists; 2016.
- 24. World Health Organization . Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 25. Tobias DK, Hu FB, Chavarro J, Rosner B, Mozaffarian D, Zhang C. Healthful dietary patterns and type 2 diabetes risk among women with a history of gestational diabetes. Arch Intern Med. 2012;172:1566–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, dietary approaches to Stop Hypertension Score, and Health Outcomes: An updated systematic review and meta‐analysis of cohort studies. J Acad Nutr Diet. 2018;118:74–100.e11. [DOI] [PubMed] [Google Scholar]
- 27. Sotos‐Prieto M, Bhupathiraju SN, Mattei J, et al. Changes in diet quality scores and risk of cardiovascular disease among US men and women. Circulation. 2015;132:2212–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vitale M, Masulli M, Calabrese I, et al. Impact of a mediterranean dietary pattern and its components on cardiovascular risk factors, glucose control, and body weight in people with type 2 diabetes: A real‐life study. Nutrients. 2018;10:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sotos‐Prieto M, Bhupathiraju SN, Mattei J, et al. Association of changes in diet quality with total and cause‐specific mortality. N Engl J Med. 2017;377:143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pehrsson PR, Patterson KY, Spungen JH, et al. Iodine in food‐ and dietary supplement‐composition databases. Am J Clin Nutr. 2016;104(Suppl.3):868S–S876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information S1. FIGO nutrition checklist for pre‐pregnant/early pregnant women. Reproduced with permission from FIGO.
Supporting information S2. Members of the FIGO Adolescent, Pre‐conception, and Maternal Nutrition Working Group.
Supporting information S3. Summary of individual FIGO Nutrition Checklist questions, target nutritional indicators, and selected nutrients and food groups for analysis.
Supporting information S4. Process of translation of the FIGO Nutrition Checklist into the Chinese version used in the current study.
Supporting information S5. Translated FIGO Nutrition Checklist (traditional Chinese version).
Supporting information S6. Characteristics of pregnancy outcomes of the 138 live births with available follow‐up data.a
Supporting information S7. Frequency distribution of FIGO Nutrition Checklist score among Chinese pregnant women in Hong Kong (n=156).
Supporting information S8. Summary of FIGO Nutrition Checklist and dietary quality indices scores among 156 Hong Kong Chinese pregnant women (at ≤ 14 weeks of pregnancy) by dietary and nutritional risk groups (“suboptimal diet” and “good diet”).
Supporting information S9. Results for pregnancy outcomes in dietary and nutritional risk groups defined by FIGO Nutrition Checklist scores.


