Abstract
Background
Calvarial bone grafts are successful in the reconstruction of the severely atrophied maxilla as a pre‐implant procedure. However, not much is known about graft incorporation at the microscopic level.
Purpose
This study aimed to assess calvarial bone conversion 4 months after being grafted in the edentulous maxillary bone.
Materials and methods
In 13 patients (age:65.3 ± 8.7 years) the atrophic maxilla was reconstructed with autologous calvarial bone. Biopsies were taken from fresh calvarial bone grafts and from the reconstructed maxillae after 4 months of healing. Micro‐CT, histomorphometric, and histological analysis were performed. From three patients biopsies were obtained after 9, 11, or 45 months.
Results
The micro‐CT analysis revealed that in the maxilla the calvarial bone was well preserved even after 45 months. Histology showed progressive incorporation of grafted bone within a maxillary bone. Osteoid and osteocytes were present in all biopsies indicating new bone formation and vital bone. Histomorphometrically, the percentage of grafted bone volume over total volume decreased from 79.8% (IQR78.7‐83.3) in fresh calvarial grafts to 59.3% (IQR44.8‐64.6) in healed grafts. The biopsies were taken after 9, 11, and 45 months showed similar values.
Conclusions
Calvarial bone grafts result in stable and viable bone, good incorporation into native maxillary bone, and a minor decrease in bone volume after healing. Consequently, they provide a solid base for implant placement in severely atrophied edentulous maxillary bone.
Keywords: alveolar ridge reconstruction, autogenous bone graft, bone augmentation, bone density, bone grafting, calvaria, edentulous atrophic maxilla, histological analysis, micro‐CT
1. INTRODUCTION
Autologous bone grafts are widely used to reconstruct bony defects in the craniofacial region. They are still the most favorable grafting material for reconstructions due to their unique osteogenic, osteoinductive, and osteoconductive properties. 1 , 2 , 3 The long‐term structural integrity and quality of the grafted bone depend on the donor site the bone graft is harvested from. Several sites are used to harvest autologous bone. These sites can be classified according to their embryological origin, that is, endochondral or intramembranous. The iliac crest, tibia, and ribs are endochondral in origin, while the maxilla, mandible, and skull (calvaria) are intramembranous in origin. 1 , 4 A major difference between the two is that the resorption rate of intramembranous bone is less than that of endochondral bone. 4 , 5 As a result, intramembranous bone is presumed to have better long‐term results with regard to three‐dimensional reconstructions of severely atrophied ridges.
Intra‐oral sites are frequently used as graft donor sites for bony reconstructive procedures prior to implant placement, but the amount of bone that can be harvested from the chin, mandibular ramus, and maxillary tuberosity is limited. When large volumes are needed, other intramembranous bone sites can be used as a donor, for example, the calvarium. 1 , 5 Although calvarial bone grafting bears the hazard of inducing severe complications, technique improvements have made its harvesting a safe and straightforward procedure. 6 , 7 , 8 , 9 , 10
The success of a bone graft is often assessed indirectly based on implant survival and macroscopic volumetric changes. 11 An important drawback of these approaches is that qualitative and quantitative factors, such as relative volumetric changes, mineral density, and maturation of the graft, remain out of scope, even though these parameters provide insight into the long‐term outcomes of the reconstruction. Current advancements in imaging technology have led to a significant improvement in the resolution of the skeletal structural architecture in vivo and ex vivo, enabling a more in‐depth analysis of bony reconstructions. Micro‐CT scanning provides a 3D image with a very high‐resolution, which can be used for quantitative analysis of the calcified tissue to assess graft site healing. The histomorphometric analysis provides insight into the cellular properties of the calcified tissue . 12 Utilizing a combination of these techniques facilitates the evaluation of the mineral and bioactive properties of bone. The aim of this study was to use micro‐CT and histomorphometric analyses to assess the material properties and incorporation of calvarial bone grafts into the reconstructed atrophied maxilla.
2. MATERIALS AND METHODS
2.1. Patient selection
Consecutive eligible patients who were referred to the Department of Oral and Maxillofacial Surgery of the Treant Scheper Hospital in Emmen, The Netherlands, and who suffered from problems with wearing an upper denture due to severe resorption of the edentulous maxilla, were asked to join this study. Inclusion criteria were an insufficient bone volume for reliable placement of dental implants as assessed on computed tomography (CT) scan, that is, <3 mm bone height in the maxillary sinus area, and <2 mm bone width in the anterior maxillary area. In order to harvest calvarial bone, the patients' parietal bone in the skull had to be at least 5 mm thick in the area between the articular tubercle and the end of the mastoid bone. Exclusion criteria were patients with an American Society of Anaesthesiologists (ASA) score of III or higher, 13 a history of radiotherapy in the head and neck region, former or current use of intravenous bisphosphonates, and previous cranial surgery.
2.2. Study approval
The study was approved by the Medical Ethical Committee (REF SH20141) of the Scheper Hospital, Emmen, The Netherlands.
2.3. Surgical procedure
The technique described by Schortinghuis et al. was used to harvest the calvarial bone grafts. 6 In short, after raising a full‐thickness flap from the parietal skull, the outer table graft was marked with a burr until the diploe was encountered. A bevel was created with a bone scraper around the calvarial outer table graft area to harvest cancellous bone and to facilitate piece‐by‐piece removal of the cortical bone grafts with a reciprocating saw. The remaining defect in the skull was reconstructed with bone cement (Palacos, Zimmer Biomet, Warsay, Indiana).
Maxillary sinus floor elevation surgery was performed with the cancellous calvarial bone on both sinuses. The cortical bone grafts were positioned at the exposed maxillary alveolar process as buccal onlay grafts. The cancellous portion of the graft was placed toward the recipient maxilla. The grafts were fixed with 1.3 mm osteosynthesis screws (Synthes, Wolhusen, Switzerland). The sharp bone edges were rounded to allow for smooth coverage of the grafted area with the overlying mucosa. Subsequently, dental implants (Straumann, Wolhusen, Switserland) were placed immediately in the reconstructed maxilla and the remaining gaps were covered with cancellous bone. Primary wound closure was accomplished using resorbable 4‐0 polyglactine sutures (Ethicon, Somerville, New Jersey).
2.4. Bone biopsies
Bone biopsies were obtained from the calvarial bone area immediately after harvesting and from the fresh grafts as well as 4 months later from the native and grafted bone in the reconstructed maxilla, whereby a small bone wedge of the reconstructed alveolar process was taken between two adjacent implants. A photograph and a schematic drawing of each biopsy were made to record the spatial orientation of the specimen (Figure 1). The biopsies were preserved in 4% phosphate‐buffered formaldehyde solution (Klinipath BV, Duiven, The Netherlands) for 24 hours and then stored in 70% ethanol until used for micro‐CT and histomorphometric analyses.
FIGURE 1.
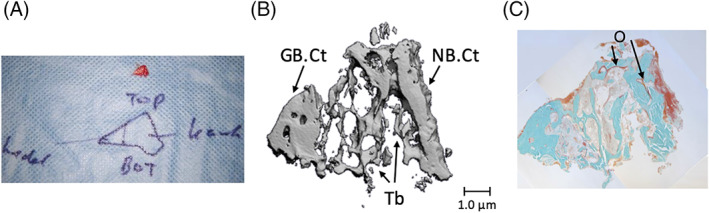
Method of micro‐CT and histomorphometrical analysis of biopsies taken from the edentulous maxillary alveolar process that was reconstructed using calvarial bone grafts, 4 months after reconstruction took place. A, Direct per‐operative notes. Biopsy obtained from the alveolar process, 4 months after it was reconstructed using calvarial bone grafts, with the orientation indicated by pencil marker. B, Micro‐CT image of the same biopsy. The thick cortical part (GB.Ct) (left) represents the grafted bone. In the middle, trabeculae (Tb) connect the grafted and native bone. C, Histological section of the same biopsy. New bone formation is observed, as red non‐mineralized osteoid aligning mature bone between native and grafted bone. Magnification: A, ×2; B, ×20; C, ×20. Top: top of the alveolar process; bot: maxillary jaw bone cranial from the alveolar process; Kaak: native maxillary bone; schedel: grafted calvarial bone used to reconstruct the alveolar process; GB.Ct: cortical part of grafted calvarial bone; NB.Ct: cortical part of native maxillary bone; O, osteoid; Tb: trabeculae
2.5. Micro‐CT evaluation
All the biopsies were scanned with a high‐resolution micro‐CT (μCT 40, Scanco Medical AG, Brüttisellen, Switzerland). This system was calibrated every 2 weeks using phantoms with densities of 0, 100, 200, 400, and 800 mg HA/cm3. Before scanning, the biopsies were fixed with synthetic foam in a polyetherimide tube (inner diameter, 28.5 mm; length, 75 mm). Then the tube was filled with 70% ethanol and covered with Parafilm M (SPI Supplies, West Chester, Pennsylvania) to prevent evaporation during scanning. The scanner settings were voltage, 70 kV; intensity, 113 A; integration time, 1000 ms; isometric resolution, 0.015 mm. 3D reconstructions were made with a cone‐beam reconstruction algorithm. All reconstructions were smoothed with a Gauss filter (0.8/1) and segmented with a visually determined threshold of 559.2 mg HA/cm3 (Figure 2). This threshold visualizes bone in the same way as it appears on histological sections. The orientation of the biopsy and transition zone were identified for each bone biopsy using the photographs and schematic drawings of the biopsies.
FIGURE 2.
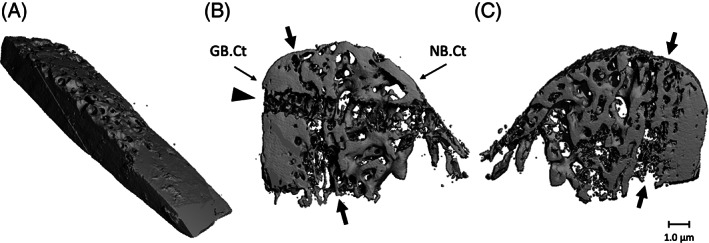
Micro‐CT scans of fresh calvarial bone biopsy and bone biopsy obtained from an edentulous maxillary alveolar process, 4 months after it was reconstructed using a calvarial bone graft. A, Fresh calvarial bone biopsy consisting mainly of cortical bone. The diploic bone is the more porous part of the piece. B, Biopsy after 4 months healing seen from a distal perspective. The left side exists of grafted bone. The compact cortex of the calvarium can be identified based on the morphology and density of the bone. Native maxillary bone is more porous, contains more trabeculae, and the cortical wall is thinner compared to the bone in the grafted area. C, Biopsy after 4 months healing seen from a mesial perspective. Arrows: the border between grafted and native bone. The horizontal path through the calvarial part and the native bone part of the removed fixation screw is clearly visible (arrowhead). Magnification: A, ×40; B, ×20; GB.Ct: cortical part of grafted calvarial bone; NB.Ct: cortical part of the native maxillary bone
To perform evaluations, volumes of interest (VOIs) were set by manually tracing the contours of the fragment of bone. For all biopsies, the VOI included the entire fragment of bone. Then for each of the biopsies taken after 4 months, one VOI was drawn, including only grafted bone and one including only native bone. For each VOI, the tissue mineral density (TMD, mg HA/cm3), defined as the mean mineral density of the whole volume of interest, was calculated. TMD can be used as a qualitative measure for the mineral density of compact bone. The bone mineral density (BMD, mg HA/cm3), defined as the mean mineral density of the segmented bone volume in the VOI, was also calculated. 14 Finally, the bone volume fraction (BVF), which is a quantitative measure defined as the ratio of the segmented bone volume to the total volume of the VOI (%), was assessed. In other words, BVF represents the percentage of biopsy volume or tissue volume that is occupied by bone volume.
2.6. Histology and histomorphometric analysis
After micro = CT scanning and dehydrating in ascending alcohol series, the bone biopsies were embedded without prior decalcification in low‐temperature polymerizing methyl methacrylate (MMA, Merck Schuchardt OHG, Hohenbrunn, Germany). 3D‐orientation of the biopsies was assessed using the clinical pictures. Longitudinal 5 μm thick sections were cut with a Jung K microtome (Reichert Jung, Heidelberg, Germany). Midsagittal histological sections of each biopsy were stained with Goldner's trichome to distinguish mineralized bone (green) and unmineralized osteoid tissue. 15 Digital images of the sections were acquired at ×100 magnification.
First, a qualitative histological analysis was performed. The 2D‐orientation, completeness, and outstanding features, such as signs of inflammation, were identified for each section. The orientation was determined from the notes and photographs taken during the surgery as well as the 3D‐reconstructions made by the micro‐CT‐software (Figure 3). The cortical bone percentage was determined for the fresh biopsies. Regarding the biopsies taken after 4 months, the native maxillary bone and grafted calvarial bone were identified visually. The presence and 2D distribution of bone, osteoid, and osteocytes were measured per section (presence and location). Bone is defined here as a mineralized bone matrix excluding osteoid. 16 Osteoid is a bone matrix that was not yet mineralized. 16 {Dempster, 2013 #11} The presence of osteoid indicates new bone formation. Osteocytes are mature osteoblasts located with their cell bodies in lacunae and with their cellular processes running through the canaliculi. They are encased by a mineral matrix and are normally supplied by vessels lying in the bone's canal system. Osteocytes are the mechanosensors of bone, and as such, they have an important regulatory function in bone resorption and bone formation. The presence of vital osteocytes in the grafted bone indicates that the canalicular system has been able to remain viable and functional. 17
FIGURE 3.
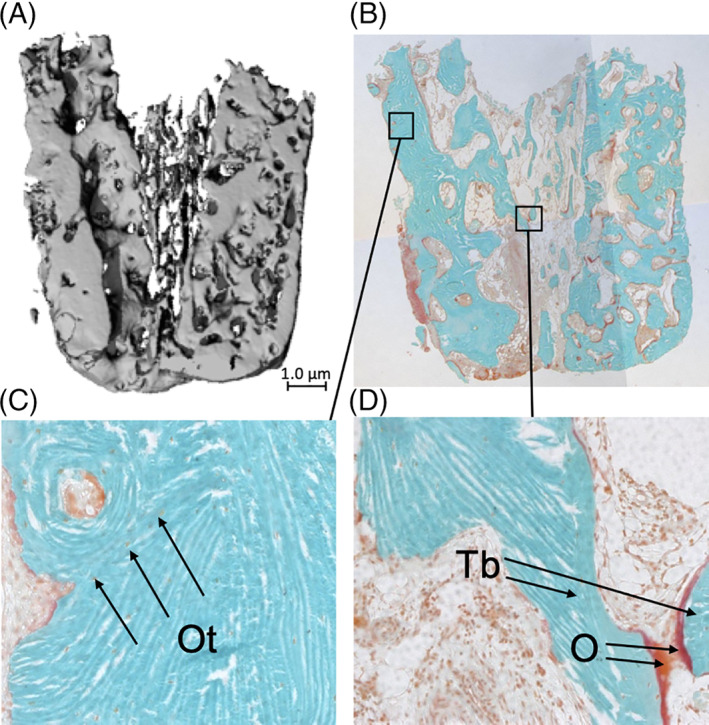
Histological and micro‐CT analysis of a biopsy taken from a double‐plated very thin knife‐edge alveolar ridge of an edentulous patient who received a reconstruction of the maxilla using calvarial bone grafts. A, Image of micro‐CT scan. Only bone with a mineral density of >559.2 mgHA/cm3 is visible. Magnification ×20. B, Histological section of the biopsy stained with Goldner's trichome to distinguish mineralized bone tissue (green) and unmineralized osteoid (red). Viable, mineralized mature bone(green) is visible. Osteoid is red. The morphology of the bone graft is still visible. Magnification ×20. C, Cortical region of interest (ROI), showing compact lamellar bone with several osteocytes visible as tiny black dots inside the green mineralized tissue, indicating vital bone. In the upper left corner, a haversian channel is visible. Magnification, ×100. Ot, osteocyte. D, The cancellous bone at the transition between grafted and native bone, the presence of osteoid (red; lower right corner) indicates the formation of new bone. Probably, the two trabeculae will be connected after maturation (mineralization) of the osteoid. Magnification, ×100. O, osteoid; Tb, trabecula
Histomorphometry was performed by dividing the sections into three pre‐defined zones, that is, (a) the cortical zone of the graft, which is the cortical outer side of the graft just underneath the periosteum; (b) the cancellous zone of the graft, which is the side of the graft toward the alveolar process; and (c) the transitional zone, which is the contact zone between the calvarial graft and the alveolar process, onto which the graft is fixed. For each zone, three regions of interest (ROIs) were determined for every biopsy zone following a pre‐defined pattern (Figure 4). The mean result of each zone was used to compare the biopsies.
FIGURE 4.
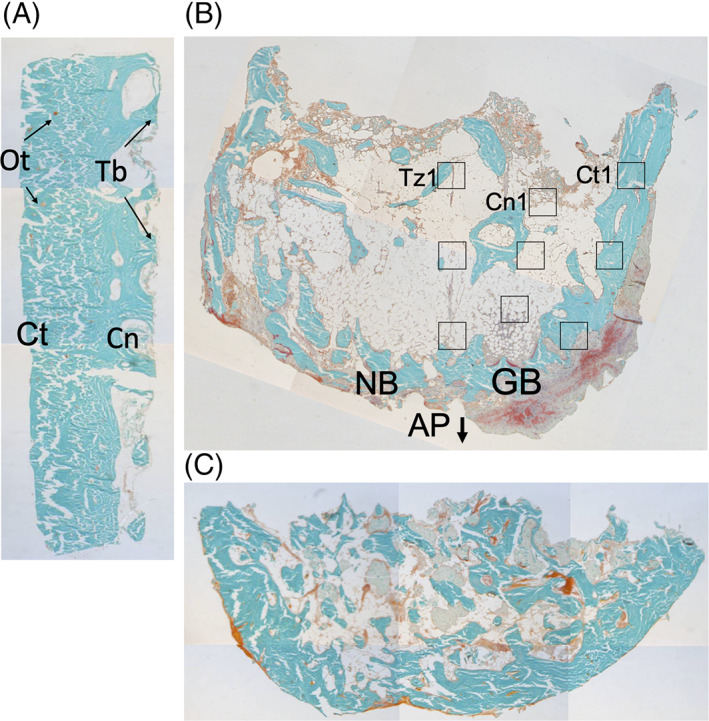
Histological sections of biopsies obtained from one edentulous patient who received reconstruction of the maxilla using calvarial bone grafts: fresh calvarial bone biopsy and biopsies from the maxillary alveolar ridge, four and 45 months post‐reconstruction surgery. A, Fresh calvarial bone showing dense cortical bone (Ct) and several thick trabeculae (Tb) on the right, where the more cancellous diploic bone (Cn) starts. B, Biopsy 4 months after grafting with a native maxillary bone (NB; left), and grafted calvarial bone (GB; right). The cortical part of the calvarial bone is denser compared to the native bone. Between the native bone and calvarial grafted bone, crossing trabeculae are scarce, and non‐mineralized connective tissue is present. C, Biopsy 45 months after grafting. The border between grafted and native bone has disappeared, and there is more homogenous mineralized, hard bone tissue visible throughout the section compared to the section obtained after 4 months of graft healing. Staining: Goldner's trichome to distinguish mineralized bone tissue (green) and unmineralized osteoid (red). All bone/biopsies are from one patient, showing a progression from fresh calvarial bone toward a healed, reconstructed alveolar process. Magnification, ×20. AP, alveolar process; Cn, cancellous bone; Cn1, first ROI of the cancellous zone of grafted bone; Ct, cortical bone; Ct1, first ROI of the cortical zone of grafted bone; GB, grafted (calvarial) bone; NB, native (maxillary) bone; Ot, osteocyte; Tb, trabecula; Tz1, first ROI of the transition zone between grafted and native bone
For each ROI, histomorphometrical measurements were manually performed using a computer with an electronic stage table and a Leica DC 200 digital camera. The computer software used was Leica QWin (Leica Microsystems Image Solutions, Rijswijk, The Netherlands). The primary variables bone area, osteoid area, and osteocyte number were determined. The percentage of bone area, percentage of osteoid, and osteocyte number per mm2 of tissue area were derived from these primary outcomes. 16 , 17 , 18
2.7. Statistical analysis
Data management and analysis were performed using SPSS 23.0 (IBM SPSS Statistics for Windows, Version 23.0. Armonk, New York: IBM Corp.) The data were tested for normal distribution with a Shiparo‐Wilk test and checked visually using a histogram with a distribution curve. Data are presented as mean ± SD, or in case of non‐normal distribution, as median and interquartile range. A dependent student T‐test, or the non‐parametric paired Wilcoxon Signed Rank test, was used to determine differences in TMD, BMD, and BVF between the fresh calvarial bone and the grafted calvarial bone in the biopsy obtained after 4 months healing from the same patient and to determine differences between the grafted and native bone in the biopsies obtained after 4 months healing, within the same biopsy. A Friedman Test, or in case of non‐parametric data, Kendall's W test, was used to determine differences in histomorphometric results between the three zones (cortical, cancellous, and transition zone) of the same section. A significance level of .05 was chosen for all tests.
3. RESULTS
3.1. Study population characteristics
The 13 participating patients were either male (7) or female (6) (Table 1). The mean age of all participating patients at the time of bone graft harvesting surgery was 65.3 ± 8.7 years. Three participating patients were smokers.
TABLE 1.
Micro‐CT analysis of tissue mineral density (TMD), bone mineral density (BMD) and bone volume fraction (BVF) in biopsies taken from fresh calvarial bone grafts and from reconstructed maxillary alveolar ridges with calvarial bone graft 4 months after reconstruction and prior to implant placement
| Healed reconstructed alveolar process biopsies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fresh calvarial bone biopsies (n = 13) | Native bone (n = 12) | Grafted bone (n = 12) | P value a | P value b | ||||||
| Median | IQR | Median | IQR | Median | IQR | Z | P | Z | P | |
| TMD (mg HA/cm3) | 617.9 | 510.3‐784.5 | 373.9 | 300.5‐484.9 | 596.3 | 444.4‐756.1 | −2.5 | .01* | −.87 | .43 |
| BMD (mg HA/cm3) | 983.4 | 962.9‐1016.2 | 866.7 | 823.7‐909.7 | 919.4 | 827.5‐985.1 | −2.1 | .04* | −1.9 | .06 |
| BVF (%) | 59.8 | 51.6‐72.7 | 30.5 | 24.4‐43.8 | 62.6 | 35.3‐74.1 | −2.5 | .01* | −.62 | .58 |
Abbreviation: IQR, interquartile range.
Equality of medians tested between native and grafted bone within the same biopsy using a Wilcoxon Signed Rank test.
Equality of medians tested between fresh calvarial bone and grafted calvarial bone after 4 months of healing.
Significant difference between native and grafted bone within the same biopsy obtained after 4 months healing, P < .05.
3.2. Clinical results
All 13 participating patients received reconstructive surgery of the maxilla. In three participating patients, the process involved a double plating technique, that is, both buccal and palatinal bone grafting was applied. There were no differences in clinical outcomes or micro‐CT, histological, and histomorphometric results between the patients that received buccal bone plating only and those that received both buccal and palatinal platings. It was possible to place all 66 implants immediately during the reconstruction. In total, six implants were lost in two patients (one patient lost five implants, and one patient lost one implant) after a 1‐year follow‐up. Both patients were smokers.
3.3. Biopsies
A total of 28 bone biopsies was obtained. Thirteen biopsies came from the freshly harvested calvarium, 15 were taken from the reconstructed maxillary alveolar wall after the grafted bone had healed. In 12 participants, biopsies were taken after 4 months of healing, and one participant's biopsy was taken after 9 months of healing due to a prolonged stay abroad. This biopsy's analysis was not added to the other results from the specimens that were taken after 4 months. From two participants, a biopsy could be obtained at a later point in time. One biopsy obtained after 11 months, was taken during surgical treatment of a peri‐implantitis, from a healthy area of the reconstructed maxilla. The other bone biopsy was taken 45 months after the calvarial bone grafting when this patient was surgically treated for a non‐dental related sinusitis condition.
3.4. Micro‐CT
In the CT scans the original orientation and the transition zone were identified on the 3D‐reconstruction by the same investigator (DW) and double‐checked (JS). Figure 2 shows a 3D‐reconstruction of a fresh block of calvarial bone graft and a specimen harvested during implant placement after a 4 month healing period.
In the fresh calvarial bone grafts, a smooth transition from dense cortical bone toward more cancellous diploic bone was seen. The trabecula of the diploic part was thick and short. In the biopsies obtained after 4 months, the grafted bone could be identified easily based on the compact cortical bone and morphology of the graft. The grafted bone looked as dense as the fresh biopsies. A more in‐depth observation of the biopsies revealed that the transition toward cancellous bone started similar to the fresh biopsies, but the trabeculae became thinner and longer toward the native maxillary bone. At the transition between both bone types, more space was seen between the trabeculae, while they were irregular in form and thickness indicating remodeling of the grafted bone. Further toward the native bone, the trabeculae remained thin and long but became more packed together. The cortical zone of the native bone was thinner compared to the calvarial bone.
All biopsies were included for micro‐CT analysis and the results are depicted in Table 1. The TMD measurements revealed that the density of the grafted bone after 4 months of healing was comparable to the fresh calvarial bone (Wilcoxon Signed Rank Test, Z = ‐.87, P = .43, Table 1). Comparison of BMD of fresh biopsies and BMD of the VOI of grafted bone in biopsies taken after 4 months showed no significant differences either (Wilcoxon Signed Rank Test, BMD: Z = ‐1.9, P = .06). In addition, the volume of the grafted bone (BVF) showed no significant decrease (Wilcoxon Signed Rank Test, Z = ‐.62, P = .58). Thus, calvarial bone grafts remain their volume and bone mass after being incorporated in the maxilla.
When grafted and native bone from the same biopsy was compared, the tissue mineral density of the grafted bone was significantly less compared to the maxillary bone (TMD: Wilcoxon Signed Rank Test, Z = −2.5, P = .01). The inorganic bone mass (BMD) and the volume fraction occupied by bone (BVF) were significantly lower in native bone as well (Wilcoxon Signed Rank Test, BMD: Z = −2.1, P = .02; BVF: Z = −2.5 P = .01, Table 1). In other words, after 4 months of healing, the grafted bone seemed to adapt to the maxillary bone with slight changes.
The TMD, BMD, and BVF values of the grafted bone biopsies taken at later moments (9, 11, and 45 months) lie within the confidence intervals of the biopsies taken after 4 months: the TMD was 768.6, 406.8, and 597.8 mg HA/cm3, the BMD was 990.3, 1009.1, and 867.5 mg HA/cm3, and the BVF was 49%, 32%, and 58% for the 9, 11, and 45 months biopsies, respectively. This suggests that calvarial bone grafts also remain stable over a longer time period than 4 months.
3.5. Histology
The fresh calvaria sections consisted of highly mineralized bony tissue, appearing dark green in the histological sections. The sections contained mostly dense cortical bone, but several sections showed both cortical and diploic bone, with a smooth transition from one type to the other bone type. The diploic bone consisted of short, thick trabeculae. Numerous osteocyte lacunae were visible throughout the sections (Figure 4A). In the sections obtained from biopsies taken at 4 months, the original alveolar process, transition zone, and grafted bone could still be identified in the bone morphology, irrespective of bone maturation. Several trabeculae were present at the transition zone between the grafted and native bone. These trabeculae connected the two bony parts, thus representing new bone formation. The trabeculae at the transition zone were irregular and thin and appeared more like maxillary bone. Next to these trabeculae, soft, mostly fat, tissue was seen between the graft and the native bone, and sometimes signs of inflammation. Moreover, low mineralized bony tissue was observed, possibly the result of the cancellous bone particles that were used to fill the gaps between the grafted and native bone. Apart from mineralized bone tissue, osteoid was present foremost in the transition zones (Figure 4B). Osteocytes were seen throughout the biopsies, but they were more concentrated in the transition zone and cancellous parts of the grafted bone than in the cortical part of the graft. In the biopsies taken at later time points, the osteocytes were more evenly distributed throughout the grafts (Figure 4C), indicating further maturation of the bone. The visual difference between the graft and the alveolar bone was less obvious in the later biopsies.
3.6. Histomorphometry
Histomorphometry was used to measure the cortical part and the cancellous zone of the graft, and the transition zone between the graft and the alveolar process. It was possible to set the nine ROIs following the pre‐defined pattern in 9 out of 11 biopsies taken after 4 months and all three late biopsies. In two biopsies, only two ROIs per zone were possible. Another two biopsies could not be measured due to inadequate orientation of the sectioning.
During the 4‐month healing period, the median bone percentage significantly decreased from 79.8% (IQR 78.7‐83.3%) in fresh biopsies and to 59.3% (IQR 51.5‐64.1%) in biopsies taken at 4 months (Wilcoxon Signed Rank Test, Z = ‐2.5, P = .01). Within the biopsies taken at 4 months, the median osteoid percentage was highest at the transition zone between the grafted and native bone, and lowest at the cortical zone (Kendall's W test, W = .412, P = .004, Table 2). As osteoid indicates new bone formation, it seems that bone had formed throughout the grafted bone. However, the highest activity took place at the border between the grafted and native bone. The median osteocyte count over the bone area was similar among the biopsies, that is, the highest number of osteocytes was found at the border between the grafted and native bone, and the lowest number of osteocytes was observed at the cortical part of the grafted bone (Kendall's W test, W = .05, P = .473, Table 2).
TABLE 2.
Histomorphometric analysis of bone percentage (Bp), osteoid percentage (Op), and osteocyte number per volume (OcN/Ba) in biopsies from grafted sites after 4 months in patients undergoing reconstruction of the edentulous maxilla prior to implant placement
| Cortical bone of calvarial graft | Cancellous bone of calvarial graft | Transition zone between calvarial graft and native maxillary bone | Significance a | |||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | W | P value | |
| Bp (%) | 59.3% | 44.8–64.6% | 36.2 | 29.5‐43.3 | 29.4% | 23.7‐34.6% | 0.387 | .001 |
| Op (%) | .7% | 0.3‐1.5% | 0.7% | 00‐1.3 | 1.6% | 0.9‐2.1% | 0.412 | .004 |
| OcN/Ba (1/mm2) | 159 | 87‐291 | 158 | 46‐506 | 316 | 83‐432 | 0.05 | .473 |
Significant difference between Bp, Op, or OcN/Ba measured in the cortical calvarial bone, cancellous calvarial bone, or transition zone between grafted calvarial bone and native maxillary bone, within the same section (Kendall's W test).
Abbreviation: IQR, interquartile range.
In the 9, 11, and 45 month‐biopsies, the cortical bone percentage was highest with 57.3%, 68.2%, and 66.9%, respectively, and the transition zone bone percentage was lowest, with 36.2%, 29.4%, and 20.3%, respectively. The osteoid percentage was highest at the transition zone (1.6%, 1.0%, and 0.2%, respectively), and lowest at the cortical part of the grafted bone (0.6%, 0.9%, and 0.1%, respectively). The osteocyte count over bone volume was highest at the transition zone (326.0, 1260.1, and 561.6 per mm2 bone), and lowest at the cortical part of the grafted bone (86.0, 135.9, and 395.3 per mm2 bone). These results lie within the confidence interval of the results from the biopsies obtained after 4 months.
4. DISCUSSION
The aim of this study was to provide insight into the incorporation of calvarial bone grafts into native maxillary bone following reconstruction of the severely resorbed edentulous maxilla. Histomorphometrical and micro‐CT analyses of bone biopsies revealed that (a) after 4 months of healing, calvarial bone grafts were viable and well incorporated as shown by the presence of living osteocytes throughout the histological sections, the presence of osteoid around the transition zone, and the formation of bony connections between grafted and native bone; (b) calvarial bone was well preserved even after 45 months; (c) compared to maxillary bone, calvarial grafts were less porous, contained a higher mineral density, and had a higher volumetric fraction that was occupied by bone, providing a more stable base for implant placement; (d) calvarial bone graft resorption was low as shown by the persistent high values for BVF and bone percentage.
Micro‐CT analyses demonstrated that the calvarial grafts consisted of bone with a large and strong mineral component. In other words, the grafts were a stable basis for implant placement. When compared to native maxillary bone, it seems as if the graft adapted to the native bone, but its superior features remained in terms of strength. Based on the similarities between the fresh biopsies, the biopsies taken at 4 months, and the biopsies obtained after more than 4 months, it can be surmised that these results are stable over time.
Histological analysis of the biopsies revealed bone graft viability and signs of the three important properties of autologous bone grafts namely, osteogenesis, osteoconduction, and osteoinduction, in the calvarial bone blocks. Osteogenic activity, the production of the osteoid by osteoblasts in the grafted bone, was proven by the presence of osteoid in the histological biopsy sections taken after 4 months. Bone trabeculae in the transition zone between grafted and native bone were a sign of osteoconduction, as this is the formation of new bone from adjacent bone or from the periosteum through a matrix that acts as a scaffold. Osteoinduction, the formation of bone by the biochemical transformation and stimulation of stem cells into bone‐producing cells, was not specifically assessed in this study. However, in the biopsies taken after more than 4 months, the border between the grafted and native bone had faded away, contained less voids, had no signs of inflammation and the bone volume had not changed much. This is a sign of the formation of new bone.
A decrease in BVF and BMD is considered normal as this results from resorption that comes along with bone graft healing. 17 Due to its dense microarchitecture, the cortical bone will start its healing in conjunction with osteoclastic activity, which is important to allow the revascularization of the haversian channels. This process will start 2 weeks after grafting and is at its highest about 6 months after transplantation. 19 , 20 , 21 Bone apposition starts around 12 weeks post‐surgery. 20 , 21 , 22 In the biopsies obtained in this study, bone apposition had only just started. This theory is in line with the results found in the biopsies obtained after 9, 11, and 45 months, which showed negligible (later) loss of native bone.
Several theories have arisen in the attempt to understand why calvarial bone demonstrates high volume maintenance after grafting to craniofacial bones. 21 , 23 Although some theories have focused on the embryologic origin, a specific mechanism to support this has not been identified, and the concept of innate embryological bone graft behavior continues to be a matter of controversy. 21 Others state that the microarchitecture of a bone graft is perhaps the most important determinant of graft volume maintenance. 21 , 23 In this theory, cortical bone serves as a space‐maintaining membrane, and cancellous bone facilitates a framework for rapid revascularization and contains marrow bone tissue with precursors of bone‐forming cells. Subsequently, graft incorporation and osteogenic and osteoconductive activity have been mainly addressed using cancellous bone. 20 Interestingly, the calvarial bone grafts in this study contained copious amounts of cortical and minor amounts of marrow bone and were well incorporated with signs of osteogenesis and osteoconduction. The explanation for high bone volume maintenance in calvarial bone is, therefore, not due to the microarchitecture.
It is also hypothesized that the low resorption of cranial bone grafts results from high mechanosensitivity. Due to specific mechanosensitive features of the local osteocytes, the parietal skull has efficient physiological load‐bearing and volume maintenance despite its relative mechanical disuse. 24 , 25 Possibly, calvarial osteocytes have the ability to successfully orchestrate bone apposition and resorption even after transplantation, 24 , 25 resulting in proper incorporation of a viable graft in combination with the preservation of its material properties. This theory seems to fit the findings in our study since the morphology of the grafted bone had only changed slightly during the healing period. The similarities are obvious between the biopsies taken at 4 months and at 45 months from the same patients.
Although in theory potential severe complications may occur, the bone graft harvesting technique used in the current study has been proven to be straightforward, safe, and effective in prospective studies with 1‐year follow‐up. 6 , 7 , 8 , 9 , 10 Previous studies have demonstrated a low donor site related morbidity and high patient appreciation of the procedure as well. 7 , 9 , 10
On attempting to clarify the incorporation of calvarial bone grafts into native maxillary bone following the reconstruction of the severely resorbed edentulous maxilla, this study revealed that (a) calvarial bone grafts were viable and well incorporated after 4 months of healing; (b) calvarial bone was well preserved even after 45 months; (c) calvarial grafts were less porous than native maxillary bone, contained a higher mineral density and had a higher volume fraction; and (d) areas grafted with calvarial bone showed less resorption.
CONFLICT OF INTEREST
All authors have no conflict of interest.
ACKNOWLEDGMENTS
The assistance in the processing and in the histomorphometrical analysis by Ing. Marion van Duin was greatly appreciated.
Wortmann DE, Klein‐Nulend J, van Ruijven LJ, Vissink A, Raghoebar GM, Schortinghuis J. Histomorphometric and micro‐CT analyses of calvarial bone grafts used to reconstruct the extremely atrophied maxilla. Clin Implant Dent Relat Res. 2020;22:593–601. 10.1111/cid.12936
REFERENCES
- 1. Zouhary KJ. Bone graft harvesting from distant sites: concepts and techniques. Oral Maxillofac Surg Clin North Am. 2010;22(3):301‐316. [DOI] [PubMed] [Google Scholar]
- 2. Sakkas A, Wilde F, Heufelder M, Winter K, Schramm A. Autogenous bone grafts in oral implantology‐is it still a "gold standard"? A consecutive review of 279 patients with 456 clinical procedures. Int J Implant Dent. 2017;3(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nkenke E, Weisbach V, Winckler E, et al. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: a prospective study. Int J Oral Maxillofac Surg. 2004;33(2):157‐163. [DOI] [PubMed] [Google Scholar]
- 4. Zins JE, Whitaker LA. Membranous versus endochondral bone: implications for craniofacial reconstruction. Plast Reconstr Surg. 1983;72(6):778‐785. [DOI] [PubMed] [Google Scholar]
- 5. Mertens C, Decker C, Seeberger R, Hoffmann J, Sander A, Freier K. Early bone resorption after vertical bone augmentation—a comparison of calvarial and iliac grafts. Clin Oral Implants Res. 2013;24(7):820‐825. [DOI] [PubMed] [Google Scholar]
- 6. Schortinghuis J, Putters TF, Raghoebar GM. Safe harvesting of outer table parietal bone grafts using an oscillating saw and a bone scraper: a refinement of technique for harvesting cortical and "cancellous"‐like calvarial bone. J Oral Maxillofac Surg. 2012;70(4):963‐965. [DOI] [PubMed] [Google Scholar]
- 7. Kuik K, Putters TF, Schortinghuis J, van Minnen B, Vissink A, Raghoebar GM. Donor site morbidity of anterior iliac crest and calvarium bone grafts: a comparative case‐control study. J Craniomaxillofac Surg. 2016;44(4):364‐368. [DOI] [PubMed] [Google Scholar]
- 8. Putters TF, Raghoebar GM, Klein‐Nulend J, Vissink A, Schortinghuis J. Immediate dental implant placement in calvarial bone grafts to rehabilitate the severely resorbed edentulous maxilla: a prospective pilot study. J Craniomaxillofac Surg. 2019;47(1):23‐28. [DOI] [PubMed] [Google Scholar]
- 9. Putters TF, Schortinghuis J, Vissink A, Raghoebar GM. A prospective study on the morbidity resulting from calvarial bone harvesting for intraoral reconstruction. Int J Oral Maxillofac Surg. 2015;44(4):513‐517. [DOI] [PubMed] [Google Scholar]
- 10. Putters TF, Wortmann DE, Schortinghuis J, et al. Morbidity of anterior iliac crest and calvarial bone donor graft sites: a 1‐year randomized controlled trial. Int J Oral Maxillofac Surg. 2018;47(11):1474‐1480. [DOI] [PubMed] [Google Scholar]
- 11. Chiapasco M, Zaniboni M, Boisco M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implants Res. 2006;17(Suppl 2):136‐159. [DOI] [PubMed] [Google Scholar]
- 12. Kamal M, Gremse F, Rosenhain S, et al. Comparison of bone grafts from various donor sites in human bone specimens. J Craniofac Surg. 2018;29(6):1661‐1665. [DOI] [PubMed] [Google Scholar]
- 13. Smeets EC, de Jong KJ, Abraham‐Inpijn L. Detecting the medically compromised patient in dentistry by means of the medical risk‐related history. A survey of 29,424 dental patients in The Netherlands. Prev Med. 1998;27(4):530‐535. [DOI] [PubMed] [Google Scholar]
- 14. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro‐computed tomography. J Bone Miner Res. 2010;25(7):1468‐1486. [DOI] [PubMed] [Google Scholar]
- 15.Plenk. Bone tissue and teeth. In: Böck, ed. Romeis Microscopic Technique. 17 ed. Munich, Germany: Urban & Schwarzenberg; 1989:527‐566.
- 16. Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry nomenclature committee. J Bone Miner Res. 2013;28(1):2‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983;(174):28‐42. [PubMed] [Google Scholar]
- 18. Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2(6):595‐610. [DOI] [PubMed] [Google Scholar]
- 19. Almaiman M, Al‐Bargi HH, Manson P. Complication of anterior iliac bone graft harvesting in 372 adult patients from may 2006 to may 2011 and a literature review. Craniomaxillofac Trauma Reconstr. 2013;6(4):257‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khan SN, Cammisa FP, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. J Am Acad Orthop Surg. 2005;13(1):77‐86. [PubMed] [Google Scholar]
- 21. Oppenheimer AJ, Tong L, Buchman SR. Craniofacial bone grafting: Wolff's law revisited. Craniomaxillofac Trauma Reconstr. 2008;1(1):49‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldberg VMAS. Biology of bone grafts In: Lieberman JRFGE, ed. Bone Regeneration and Repair. Totowa, NJ: Humana Press Inc; 2005:57‐65. [Google Scholar]
- 23. Marx RE. Bone and bone graft healing. Oral Maxillofac Surg Clin North Am. 2007;19(4):455‐466. [DOI] [PubMed] [Google Scholar]
- 24. Vatsa A, Breuls RG, Semeins CM, Salmon PL, Smit TH, Klein‐Nulend J. Osteocyte morphology in fibula and calvaria—is there a role for mechanosensing? Bone. 2008;43(3):452‐458. [DOI] [PubMed] [Google Scholar]
- 25. Santos A, Bakker AD, Klein‐Nulend J. The role of osteocytes in bone mechanotransduction. Osteoporos Int. 2009;20(6):1027‐1031. [DOI] [PubMed] [Google Scholar]


