Abstract
Sodium‐glucose cotransporter 2 (SGLT2) inhibitors reduce the occurrence of cardiovascular and renal complications in patients with type 2 diabetes mellitus (T2DM) and represent guideline‐recommended therapy in this indication. However, precise mechanisms underlying the beneficial cardiovascular effects of SGLT2 inhibitors are not fully understood. This study investigated the effects of the SGLT2 inhibitor, luseogliflozin, on arterial properties and home blood pressure (BP) in patients with T2DM. This multicenter, single‐arm study enrolled adults with T2DM, glycosylated hemoglobin (HbA1c) 6.5%‐8.9% in the previous 4 weeks, and an indication for new/additional antidiabetic therapy. Luseogliflozin 2.5 mg/d was given for 12 weeks. Primary outcome was change in cardio‐ankle vascular index (CAVI) from baseline to Week 4 and Week 12. Home and office BP, BP variability, and metabolic parameters were secondary endpoints. Forty‐seven patients (mean age 63.5 ± 10.7 years) treated with luseogliflozin were included in the full analysis set. CAVI did not change significantly from baseline (mean [95% confidence interval] 8.67 [8.37‐8.97]) to Week 12 (8.64 [8.38‐8.91]; P = .750), but there were significant reductions from baseline in morning home BP, HbA1c, body weight, body mass index, and serum uric acid levels during luseogliflozin therapy. The reduction in morning home systolic BP was ≥ 5 mm Hg and was independent of baseline BP and BP control status. In conclusion, there was no change in arterial stiffness (based on CAVI) during treatment with luseogliflozin, but changes in BP and metabolic parameters were consistent with the known beneficial effects of SGLT2 inhibitors in T2DM.
Keywords: arterial stiffness, cardio‐ankle vascular index, heart rate, home blood pressure, luseogliflozin, pulse wave velocity, SGLT2 inhibitors, type 2 diabetes mellitus
1. INTRODUCTION
A growing body of evidence from randomized clinical trials suggests that treatment with sodium‐glucose cotransporter 2 (SGLT2) inhibitors protects against the occurrence of cardiorenal complications in patients with type 2 diabetes mellitus. 1 , 2 , 3 , 4 Based on these data, recent guidelines recommend use of SGLT2 inhibitors as first‐line treatment for high cardiovascular risk patients with type 2 diabetes to reduce rates of major adverse cardiovascular events (MACE), hospitalization for heart failure, chronic kidney disease progression, or cardiovascular death, irrespective of baseline glycosylated hemoglobin (HbA1c). 5
Despite the positive results from clinical trials and evidence‐based guideline recommendations for the use of SGLT2 inhibitors, the precise mechanisms underlying the cardiorenal protective effects of these agents remain to be fully elucidated. A number of beneficial cardiovascular effects of SGLT2 inhibitors have been investigated, including reductions in blood pressure (BP), 6 , 7 attenuation of the salt sensitivity of BP, 8 improvements in arterial stiffness, 9 and sympatholytic effects. 8 We recently demonstrated that SGLT2 inhibitors significantly reduced out‐of‐office BP determined using ambulatory or home BP monitoring compared with placebo or control in patients with type 2 diabetes and uncontrolled nocturnal hypertension (a salt‐sensitive hypertension phenotype). 10 , 11
Arterial stiffness is gaining increased recognition as an important contributor to the development of hypertension, hypertensive end‐organ damage, cardiovascular disease, and all‐cause mortality. 12 , 13 , 14 , 15 , 16 The results of our recent prospective study in normotensive patients showed that increased arterial stiffness, evaluated using the cardio‐ankle vascular index (CAVI), was associated with the future development of hypertension. 17 In addition, arterial stiffness plays a central role in the vicious cycle of hemodynamic stress and vascular disease that triggers organ damage and increases the risk of cardiovascular events, known as the systemic hemodynamic atherosclerotic syndrome (SHATS). 18 , 19 , 20 , 21 Therefore, reducing arterial stiffness has the potential to attenuate this vicious cycle and reduce cardiovascular risk. This is especially the case for patients in the early stage of diabetes, who are at elevated risk of developing vascular complications. 22
The multicenter explorative study of beneficial effect of luseogliflozin on cardiovascular function in Japanese patients with type 2 diabetes mellitus (LUSCAR) investigated the effects of the SGLT2 inhibitor luseogliflozin on arterial properties and home BP in patients with type 2 diabetes.
2. METHODS
2.1. Study design
This multicenter (n = 7), open‐label, single‐arm study was conducted in Japan between March 2019 and October 2019. The trial was registered at the Japanese Registry of Clinical Trials (https://jrct.niph.go.jp/search; jRCTs031180434). The study protocol was approved by the Jichi Medical University Clinical Research Ethics Committee (Tochigi, Japan), and all patients provided written informed consent before study entry. Study procedures were performed in accordance with Clinical Trials Act (Japan) and the principles outlined in the Declaration of Helsinki.
2.2. Participants
Males or females aged 20‐74 years with type 2 diabetes mellitus (treated or untreated), HbA1c 6.5%‐8.9% within the 4 weeks prior to enrollment, and an indication for the initiation or addition of new antidiabetic therapy were eligible to participate in the study.
Exclusion criteria included type 1 diabetes mellitus; use of SGLT2 inhibitors, insulin or a glucagon‐like peptide‐1 (GLP‐1) receptor agonist; change in antihypertensive or antihyperglycemic therapy within the previous 12 weeks; history of serious adverse reactions to SGLT2 inhibitors; history of cerebral infarction; severe renal dysfunction (estimated glomerular filtration rate <30 mL/min/1.73 m2 or undergoing dialysis); severe liver dysfunction; pituitary dysfunction or adrenal insufficiency; pregnancy, possible pregnancy or breastfeeding; and participation in another clinical study.
2.3. Intervention
Patients were treated with luseogliflozin (Lusefi®) 2.5 mg/d, either as monotherapy (previously untreated patients) or added to existing antidiabetic therapy.
2.4. Outcomes
The primary outcome was the change in CAVI from baseline to Week 4 and Week 12. Secondary outcomes were changes in the following parameters from baseline to Week 4 and Week 12: ankle‐brachial pressure index (ABI); pulse wave velocity (PWV); ECG; BP (average systolic BP [SBP], diastolic BP [DBP], heart rate, and BP variability) based on home and office readings; and metabolic parameters (HbA1c, body weight, body mass index [BMI], lipid levels, and uric acid levels). Changes in the brachial and ankle pulse waveforms were evaluated as exploratory endpoints.
2.5. Assessments
There was an 8‐week observation period at baseline to allow for patient screening, consent, and enrollment. This was followed by a 12‐week treatment period; other treatments for diabetes plus nutritional and exercise therapy were continued unchanged throughout the trial. The following assessments were performed at baseline, Week 4, and Week 12: body weight, BMI, ECG, home (morning, evening) and office BP, HbA1c, CAVI, ABI, PWV, and blood and urinary laboratory tests.
BP measurements were performed in accordance with the 2014 Japanese Society of Hypertension guidelines. 23 Office BP was measured after ≥5 minutes of rest with the patient seated in a chair with the arm cuff level with the heart. Smoking was prohibited for 30 minutes before the measurement. Several consecutive measurements were taken at intervals of ≥1 minute, and the average of two measurements was used to define the office BP. Home BP measurement was performed using a cuff oscillometric device (HEM‐7080‐IC; Omron Healthcare Co., Ltd., Kyoto, Japan). Patients were instructed to measure their morning home BP (two readings within 1 hour after waking, taken after urination, before taking morning medications and after 1‐2 minutes of seated rest) and evening home BP (two readings before bedtime after 1‐2 minutes of seated rest) on 5 successive days immediately prior to their scheduled clinic visit at baseline, Week 4, and Week 12.
CAVI was measured using a CAVI device (Vasera VS1500 or VS3000; Fukuda Denshi, Tokyo, Japan). Examinations were performed after a 5‐minute rest period. The pressure of all cuffs was kept at 50 mm Hg to minimize the effect of cuff pressure on hemodynamics. BP was then measured. CAVI was determined using the following formula:
where a and b are constants, ρ is blood density, ΔP is Ps − Pd, Ps is systolic blood pressure, Pd is diastolic blood pressure, and PWV is pulse wave velocity.
PWV was determined by dividing vascular length by the time (T) taken for the pulse wave to travel from the aortic valve to the ankle. However, in practice, T was difficult to obtain because the time the blood left the aortic valve was difficult to identify from the sound of the valve opening. Therefore, because the time between the sound of the aortic valve closing and the notch of the brachial pulse wave is theoretically equal to the time between the sound of the aortic valve opening and the rise of the brachial pulse wave, T was determined by adding the time between the sound of the aortic valve closing and the notch of the brachial pulse wave, and the time between the rise of the brachial pulse wave and the rise of the ankle pulse wave.
The pulse waveforms of the right and left arms and ankles were measured, and digital data were stored in the CAVI device for arterial wave form analysis. The following arterial waveform indices were calculated: percent mean arterial pressure (%MAP) and upstroke time of ankle pressure waveforms 24 ; and augmentation index and reflection magnitude of the arm pressure waveforms (average of right and left arms). 25
2.6. Statistical analysis
To the best of our knowledge, there is no study to date using CAVI as an outcome measure for evaluating the effect of luseogliflozin on cardiovascular properties. Therefore, it was assumed that the effect of luseogliflozin on cardiovascular properties could be estimated by looking at the change in CAVI at the time during luseogliflozin treatment when a significant reduction in HbA1c was seen. Therefore, we set the HbA1c difference from baseline at 0.5% and the standard deviation of 1.0%. On this basis, it was calculated that inclusion of 40 patients would provide 80% power at P = .05 (2‐sided). The target sample size was set at 50 patients to allow for a 20% dropout rate.
Patients who were non‐compliant with the Ethical Guidelines for Clinical Research were excluded from all analyses (both efficacy and safety). The full analysis set (FAS) included all enrolled patients who received at least one dose of study medication after enrollment and had at least one set of data during treatment. The safety analysis set (SAS) included all patients who had received at least one dose of study medication after enrollment.
The efficacy analyses were conducted in the FAS. Pearson correlation analysis was used to calculate correlation coefficients for the relationship between changes in two different factors. Mixed‐effects model repeated measures (MMRM) analysis was used to evaluate changes in CAVI and other outcomes from baseline to Week 4 or Week 12. MMRM included the time point (baseline, 4, and 12 weeks) as fixed effects, and ages and sex as covariates. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC, USA) at The Institute of Japanese Union of Scientists & Engineers, Tokyo, Japan. A 2‐sided P‐value of < .05 was considered statistically significant.
3. RESULTS
3.1. Patients
A total of 51 patients were enrolled, three withdrew consent prior to treatment and 48 were treated with luseogliflozin. Of these, one additional patient withdrew consent, leaving 47 patients in the FAS (Figure S1). Nearly two‐thirds of patients were male, mean age showed an older population (63.5 ± 10.7 years), just over half the population were receiving antidiabetic therapy at baseline, and hypertension and dyslipidemia were the most common comorbidities (Table 1).
Table 1.
Patient demographic and clinical characteristics at baseline (full analysis set)
| Patients (n = 47) | |
|---|---|
| Age, y | 63.5 ± 10.7 |
| Male, n (%) | 30 (63.8) |
| Weight, kg | 72.4 ± 17.7 |
| BMI, kg/m2 | 26.8 ± 5.6 |
| Diabetes duration, y | 5.7 ± 6.0 |
| Current smoking, n (%) | 9 (19.1) |
| Current alcohol use, n (%) | 18 (38.3) |
| Comorbidities, n (%) | |
| Peripheral diabetic neuropathy | 5 (10.6) |
| Nephropathy | 5 (10.6) |
| Retinopathy | 3 (6.4) |
| Hypertension | 39 (83.0) |
| Dyslipidemia | 25 (53.2) |
| Cerebrovascular disease | 2 (4.3) |
| Cardiac disease | 2 (4.3) |
| Chronic kidney disease | 1 (2.1) |
| Liver disease | 2 (4.3) |
| Antidiabetic drugs, n (%) | 27 (57.4) |
| Sulfonylureas | 0 (0) |
| α‐glucosidase inhibitor | 0 (0) |
| DPP‐4 inhibitor | 12 (25.5) |
| Metformin | 16 (34.0) |
| Thiazolidinedione | 0 (0) |
| Glinide | 0 (0) |
| Antihypertensive drugs, n (%) | 36 (76.6) |
| ACE inhibitor | 0 (0) |
| ARB | 29 (61.7) |
| Alpha‐/beta‐blocker | 1 (2.1) |
| Alpha‐blocker | 0 (0) |
| CCB | 28 (59.6) |
| Diuretic | 10 (21.3) |
| CAVI | 8.8 ± 1.2 |
| ABI | 1.1 ± 0.1 |
| PWV, cm/s | 1789.4 ± 308.4 |
| Office SBP, mm Hg | 131.8 ± 14.8 |
| Office DBP, mm Hg | 75.9 ± 10.4 |
| Office HR, beats/min | 72.9 ± 11.3 |
| Morning home SBP, mm Hg | 129.6 ± 15.5 |
| Morning home DBP, mm Hg | 75.4 ± 10.6 |
| Morning home HR, beats/min | 68.8 ± 9.3 |
| Evening home SBP, mm Hg | 126.6 ± 15.1 |
| Evening home DBP, mm Hg | 71.7 ± 10.2 |
| Evening home HR, beats/min | 72.4 ± 9.1 |
| HbA1c, % | 7.0 ± 0.5 |
| LDL cholesterol, mg/dL | 110.1 ± 25.2 |
| HDL cholesterol, mg/dL | 52.6 ± 12.2 |
| Triglycerides, mg/dL | 178.3 ± 205.5 |
| Uric acid, mg/dL | 6.0 ± 1.2 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 74.4 ± 19.5 |
Data are presented as mean ± standard deviation or number of patients (%).
Abbreviations, ABI, ankle‐brachial pressure index; ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; CAVI, cardio‐ankle vascular index; CCB, calcium channel blocker; DBP, diastolic blood pressure; DPP‐4, dipeptidyl peptidase‐4; HbA1c, glycosylated hemoglobin; HDL, high‐density lipoprotein; HR, heart rate; LDL, low‐density lipoprotein; PWV, pulse wave velocity; SBP, systolic blood pressure.
3.2. CAVI
There was no significant change in CAVI during 12 weeks of treatment with luseogliflozin (P = .750 vs baseline at Week 12) (Figure 1A, Table 2).
Figure 1.
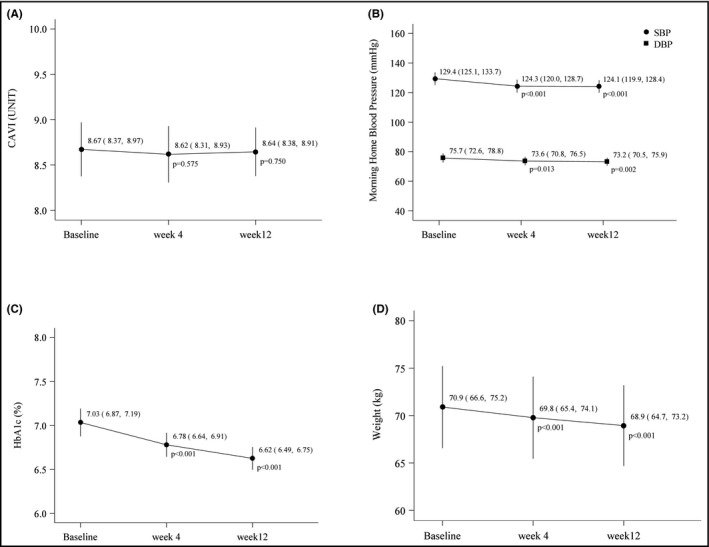
Changes in endpoint parameters during treatment with luseogliflozin. A, Cardio‐ankle vascular index (CAVI); B, Morning home blood pressure; C, Glycosylated hemoglobin (HbA1c); D, Body weight. DBP, diastolic blood pressure; SBP, systolic blood pressure. Data are adjusted for age and sex, and shown as mean values with 95% confidence intervals
Table 2.
Mixed‐effects model repeated measures analysis of changes in endpoint parameters
| Baseline | Week 4 | Week 12 | |||||
|---|---|---|---|---|---|---|---|
| Estimate | Estimate | Difference vs baseline | P‐value | Estimate | Difference vs baseline | P‐value | |
| CAVI | 8.67 (8.37, 8.97) | 8.62 (8.31, 8.93) | –0.05 (–0.25, 0.14) | .575 | 8.64 (8.38, 8.91) | –0.03 (–0.20, 0.14) | .750 |
| ABI | 1.12 (1.10, 1.14) | 1.13 (1.11, 1.15) | 0.01 (–0.01, 0.04) | .389 | 1.13 (1.11, 1.15) | 0.01 (–0.02, 0.04) | .448 |
| PWV, cm/s a | 1761.6 (1685.1, 1838.1) | 1742.3 (1662.8, 1821.8) | –19.4 (–62.9, 24.2) | .376 | 1738.4 (1663.4, 1813.5) | –23.2 (–66.0, 19.6) | .281 |
| Office SBP, mm Hg | 131.3 (126.7, 135.8) | 125.2 (120.3, 130.1) | –6.1 (–9.1, –3.0) | <.001 | 129.4 (124.7, 134.0) | –1.9 (–5.0, 1.3) | .234 |
| Office DBP, mm Hg | 75.9 (72.9, 78.9) | 72.5 (69.4, 75.7) | –3.4 (–6.3, –0.5) | .024 | 76.3 (73.3, 79.3) | 0.4 (–2.6, 3.3) | .799 |
| Office HR, beats/min | 73.5 (70.1, 76.9) | 71.7 (68.7, 74.8) | –1.7 (–3.9, 0.5) | .121 | 72.3 (69.2, 75.5) | –1.2 (–3.3, 1.0) | .290 |
| Morning home SBP, mm Hg | 129.4 (125.1, 133.7) | 124.3 (120.0, 128.7) | –5.0 (–7.4, –2.7) | <.001 | 124.1 (119.9, 128.4) | –5.2 (–7.5, –3.0) | <.001 |
| Morning home DBP, mm Hg | 75.7 (72.6, 78.8) | 73.6 (70.8, 76.5) | –2.1 (–3.7, –0.5) | .013 | 73.2 (70.5, 75.9) | –2.5 (–4.1, –0.9) | .002 |
| Morning home HR, beats/min | 68.4 (65.8, 70.9) | 68.0 (65.4, 70.6) | –0.4 (–1.6, 0.8) | .486 | 68.3 (65.8, 70.9) | 0.0 (–1.3, 1.2) | .970 |
| Evening home SBP, mm Hg | 126.5 (122.5, 130.6) | 122.4 (118.3, 126.6) | –4.1 (–6.7, –1.5) | .003 | 121.1 (117.5, 124.7) | –5.5 (–7.4, –3.5) | <.001 |
| Evening home DBP, mm Hg | 72.2 (69.7, 74.8) | 70.1 (67.7, 72.6) | –2.1 (–3.6, –0.7) | .006 | 69.4 (67.0, 71.7) | –2.9 (–4.1, –1.6) | <.001 |
| Evening home HR, beats/min | 72.4 (70.1, 74.7) | 72.6 (70.3, 74.9) | 0.2 (–1.4, 1.8) | .807 | 71.7 (69.5, 74.0) | –0.7 (–2.4, 1.0) | .436 |
| Weight, kg | 70.9 (66.6, 75.2) | 69.8 (65.4, 74.1) | –1.1 (–1.4, –0.9) | <.001 | 68.9 (64.7, 73.2) | –2.0 (–2.4, –1.5) | <.001 |
| BMI, kg/m2 | 26.8 (25.1, 28.5) | 26.4 (24.7, 28.1) | –0.4 (–0.5, –0.3) | <.001 | 26.1 (24.3, 27.8) | –0.7 (–0.9, –0.6) | <.001 |
| HbA1c, % | 7.0 (6.9, 7.2) | 6.8 (6.6, 6.9) | –0.3 (–0.3, –0.2) | <.001 | 6.6 (6.5, 6.8) | –0.4 (–0.5, –0.3) | <.001 |
| LDL cholesterol, mg/dL | 110.8 (101.1, 120.5) | 111.1 (101.6, 120.7) | 0.3 (–4.9, 5.6) | .903 | 112.2 (102.3, 122.0) | 1.4 (–2.9, 5.6) | .511 |
| HDL cholesterol, mg/dL | 53.1 (49.0, 57.2) | 53.3 (48.7, 57.8) | 0.2 (–1.6, 1.9) | .837 | 56.1 (51.2, 60.9) | 3.0 (0.4, 5.6) | .026 |
| Triglycerides, mg/dL | 178.6 (112.9, 244.3) | 163.0 (90.1, 235.8) | –15.6 (–95.6, 64.4) | .695 | 134.5 (108.1, 161.0) | –44.0 (–104.8, 16.7) | .151 |
| Uric acid, mg/dL | 5.8 (5.5, 6.1) | 4.9 (4.6, 5.2) | –0.9 (–1.1, –0.6) | <.001 | 5.0 (4.7, 5.2) | –0.8 (–1.0, –0.6) | <.001 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 75.1 (69.4, 80.8) | 70.8 (65.3, 76.3) | –4.2 (–7.0, –1.5) | .004 | 73.9 (68.6, 79.2) | –1.2 (–4.1, 1.7) | .399 |
Data are adjusted for age and sex, and shown as mean values with 95% confidence intervals.
Abbreviations: ABI, ankle‐brachial pressure index; BMI, body mass index; CAVI, cardio‐ankle vascular index; DBP, diastolic blood pressure; DPP‐4, dipeptidyl peptidase‐4; HbA1c, glycosylated hemoglobin; HDL, high‐density lipoprotein; HR, heart rate; LDL; low‐density lipoprotein; PWV, pulse wave velocity; SBP, systolic blood pressure.
Also adjusted for systolic blood pressure.
3.3. Secondary endpoints
Morning home SBP and DBP decreased significantly from baseline after 4 and 12 weeks of treatment with luseogliflozin (Figure 1B), but there was no change in morning heart rate or office BP over time (Table 2). There were also significant reductions in HbA1c (Figure 1C), body weight (Figure 1D), BMI (Table 2), and uric acid (Table 2) during luseogliflozin therapy. Statistically significant and comparable reductions in BP were seen when patients were divided into subgroups based on BP control status at baseline (baseline SBP ≥ 130 mm Hg [uncontrolled] or <130 mm Hg [controlled]) (Figure 2). There were marginally, but not statistically, significant correlations between the 12‐week change in morning home SBP and changes in BMI (r = 0.269, P = .089).
Figure 2.
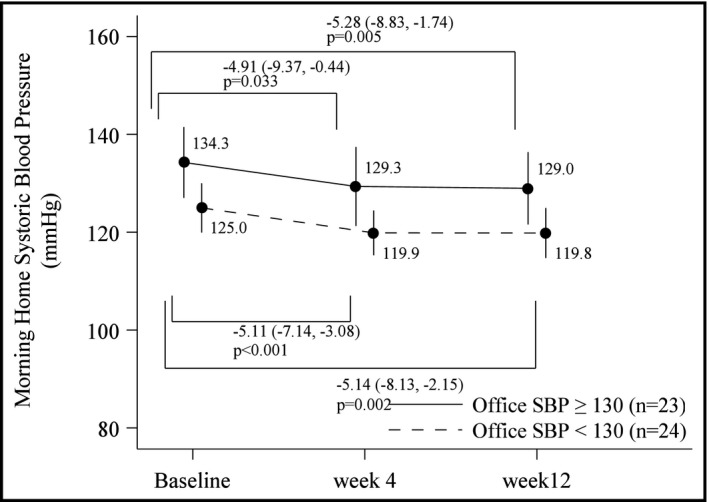
Changes in morning home systolic blood pressure (SBP) during treatment with luseogliflozin in patient subgroups based on office SBP control status at baseline. Data are adjusted for age and sex, and shown as mean values with 95% confidence intervals
Levels of high‐density lipoprotein (HDL) cholesterol were significantly higher than baseline after 12 weeks’ luseogliflozin therapy, but there were no significant changes in levels of low‐density lipoprotein cholesterol or triglycerides (Table 2).
3.4. Atrial waveform indices
After 12 weeks’ treatment with luseogliflozin, %MAP in the ankle was significantly lower than at baseline (Table 3); other waveform indices did not change significantly during treatment with luseogliflozin.
Table 3.
Mixed‐effects model repeated measures analysis of changes in arterial waveform index
| Baseline | Week 4 | Week 12 | |||||
|---|---|---|---|---|---|---|---|
| Estimate | Estimate | Difference vs baseline | P‐value | Estimate | Difference vs baseline | P‐value | |
| Ankle %MAP, % | 39.6 (38.2, 41.0) | 38.8 (37.4, 40.1) | –0.84 (–1.94, 0.27) | .134 | 37.8 (36.6, 39.0) | –1.79 (–3.02, –0.55) | .005 |
| Ankle upstroke time, msec | 148.9 (142.4, 155.5) | 150.1 (143.5, 156.6) | 1.13 (–5.51, 7.78) | .733 | 147.6 (140.8, 154.4) | –1.37 (–8.40, 5.67) | .698 |
| Brachial augmentation index, unit | 1.23 (1.14, 1.31) | 1.20 (1.12, 1.28) | –0.023 (–0.084, 0.037) | .443 | 1.16 (1.07, 1.25) | –0.069 (–0.143, 0.006) | .069 |
| Brachial reflection magnitude, unit | 0.48 (0.45, 0.51) | 0.49 (0.46, 0.51) | 0.007 (–0.012, 0.027) | .457 | 0.47 (0.44, 0.50) | –0.007 (–0.030, 0.016) | .548 |
Data are adjusted for age and sex, and shown as mean values with 95% confidence intervals.
Abbreviation: %MAP, percent mean arterial pressure.
3.5. Safety
Luseogliflozin was well tolerated, and no serious adverse events occurred during treatment (Table 4).
Table 4.
Adverse events
| Events (treatment‐related events) a , n | |
|---|---|
| Inflammation of the upper airway | 2 |
| Vulvitis | 1 (1) |
| Decreased estimated glomerular filtration rate | 1 (1) |
| Acute gastritis | 1 |
| Lower back pain | 1 |
All adverse events were of non‐serious severity.
4. DISCUSSION
Although there were significant improvements in BP and other metabolic parameters during treatment with luseogliflozin in patients with type 2 diabetes mellitus, no changes in arterial stiffness measured using CAVI were seen during this single‐center, open‐label study. However, %MAP, a measure of arterial properties, was significantly improved during luseogliflozin therapy.
In this study, we used CAVI as a measure of arterial stiffness. This approach is relatively independent of both BP and heart rate and is easy to use and reproducible. 26 , 27 CAVI has been shown to be associated with BP variability, 28 , 29 and elevated values have been reported in patients with hypertension and diabetes mellitus. 30 Preclinical data indicate a potential for SGLT2 inhibitors to improve arterial stiffness. 31 , 32 However, we did not see any change in arterial stiffness based on CAVI during treatment with luseogliflozin for 12 weeks in our study. These findings contrast with the results of previous clinical trials that included evaluation of the effects of SGLT2 inhibitors on measures of arterial stiffness. Significant reductions in PWV were seen after 8 weeks’ treatment with empagliflozin 25 mg/d in young patients with type 1 diabetes. 33 Post hoc analysis of data from the EMPA‐REG BP trial showed a trend toward a reduction in the ambulatory arterial stiffness index (P = .059) in patients with type 2 diabetes mellitus treated with empagliflozin for 24 weeks. 34 Looking specifically at CAVI, this was significantly reduced 6 months after switching from a dipeptidyl peptidase‐4 (DPP‐4) inhibitor to the SGLT2 inhibitor tofogliflozin in patients with type 2 diabetes. 35 The latter two trials had a longer duration of action than the current study, and it is possible that treatment with an SGLT2 inhibitor for longer than 12 weeks may be necessary to detect beneficial effects on arterial stiffness in patients with type 2 diabetes mellitus. However, the therapy duration in our study was selected based on data from previous studies, including a large randomized outcome study showing that the beneficial effects of SGLT2 inhibitor therapy on cardiovascular death and hospitalization for heart failure were seen within the first 3 months of therapy. 4
A number of factors influence both a single CAVI value and evaluation of this parameter over time, including age, sex, body mass index, frailty, serum uric acid levels, lipid levels, smoking status, and vascular tone. 36 , 37 , 38 , 39 , 40 , 41 The relative contribution of these factors to our study findings, and the extent to which they influenced the results of earlier studies, cannot be determined.
Although there was no significant change in CAVI and other arterial waveform indices during our study, there was a significant reduction in percent mean arterial pressure (%MAP) in the ankle from baseline to Week 12 (Table 3). The %MAP has been shown to be a significant predictor of peripheral arterial disease 24 , 42 and might therefore reflect the degree of stiffness in peripheral arteries. It is possible that significant improvements in MAP preceded significant improvements in CAVI, but additional research is needed to determine whether longer duration of therapy with luseogliflozin would be associated with a significant reduction in CAVI.
In this study, patients had relatively well‐controlled BP (mean 132/76 mm Hg for office BP and 130/75 mm Hg for morning home BP at baseline, Table 1), but morning home BP was significantly reduced after 12 weeks’ treatment with luseogliflozin, reaching 124/73 mm Hg. Although office BP was significantly reduced versus baseline at Week 4 (125/73 mm Hg), levels at Week 12 were not significantly different from baseline (129/76 mm Hg). The 5 mm Hg reduction seen in morning home SBP during treatment with luseogliflozin is expected to be clinically relevant because this corresponds to a 10 mm Hg reduction in office SBP, which has been shown to be associated with a 22‐25% reduction in the rate of coronary heart disease events and a 36%‐41% reduction in stroke rate. 43
Guideline‐recommended target office BP for patients with diabetes is <130/80 mm Hg 44 , 45 and for home BP is < 125/75 mm Hg. 44 There is a good rationale for this given that the results of the HONEST study showed that high‐risk patients with hypertension, including those with diabetes, obtained the greatest reduction in cardiovascular event risk when morning home SBP was < 125 mm Hg. 46 The morning home SBP achieved during treatment with luseogliflozin in the current study was below that 125 mm Hg threshold (124 mm Hg at Week 4 and Week 12). In addition, even in diabetic patients with well‐controlled BP at baseline (office SBP < 130 mm Hg), treatment with luseogliflozin was associated with a reduction in morning home SBP of approximately 5 mm Hg (from 125.0 to 119.8 mm Hg) (Figure 2), without any increase in morning heart rate (data not shown). Therefore, the significant home BP‐lowering effect of luseogliflozin appeared to be independent of BP controls status and did not activate sympathetic tonus.
During treatment with luseogliflozin, there were statistically significant improvements from baseline in a number of important secondary endpoint parameters. Glycemic control improved, as shown by a significant reduction in HbA1c, and body weight and BMI also decreased (Table 2). This reflects an overall improvement in the metabolic profile. Levels of low‐density lipoprotein cholesterol and triglycerides remained unchanged throughout the study, but there was a significant increase in HDL cholesterol levels. Levels of HDL have also been shown to increase during treatment with dapagliflozin. 47 A significant reduction in uric acid levels was seen during treatment with luseogliflozin, consistent with increased urinary excretion of uric acid secondary to luseogliflozin‐induced glycosuria. 48 Taken together, the findings of the current study are consistent with the documented clinical benefits of luseogliflozin in patients with diabetes. 49 , 50 , 51 , 52 , 53 , 54
The most important limitation of our study is the open‐label non‐comparative design. It is also possible that the lack of statistically significant changes from baseline in CAVI might, in part, be due to small sample size and/or inadequate treatment duration. Furthermore, the external validity of the findings is limited by the single ethnicity nature of the study population.
5. CONCLUSIONS
This study did not find any significant improvement in CAVI, a relatively BP‐independent measure of arterial stiffness parameter, during 12 weeks of treatment with luseogliflozin in patients with type 2 diabetes mellitus. Changes in BP, glucose control, and lipid levels during luseogliflozin therapy in this study were consistent with the documented beneficial effects of SGLT2 inhibitors. The ability to significantly reduce home BP without increasing heart rate in patients with diabetes and well‐controlled BP warrants further investigation in clinical trials.
Funding
This study was funded by Taisho Pharmaceutical Co. Ltd. The funding source had no role in the design of the study and did not participate in study execution, analysis or interpretation of the data, or the decision to submit the results for publication.
DISCLOSURES
Prof. K Kario has received research grants from Astellas Pharma Inc, Eisai Co., Otsuka Pharmaceutical Co., Sanofi KK, Shionogi & Co., Sanwa Kagaku Kenkyusho Co., Daiichi Sankyo Co., Sumitomo Dainippon Pharma Co., Takeda Pharmaceutical Co., Mitsubishi Tanabe Pharma Co., Boehringer Ingelheim Japan Inc, Pfizer Japan Inc, Bristol‐Myers Squibb KK and Mochida Pharmaceutical Co. and lecture fees from Idorsia Pharmaceuticals Japan, Daiichi Sankyo Co. and Takeda Pharmaceutical Co. All other authors have no conflicts of interest to disclose.
Supporting information
Fig S1
ACKNOWLEDGMENTS
We acknowledge all the patients, physicians, and medical staff who supported this study. We would like to thank Kyoichi Wada, Azusa Kaneko, Tomomi Okano and Kaori Iwatake, Satt Co., Ltd. (Tokyo, Japan), for their efforts on behalf of the contract research organization (CRO). We also thank Yuri Matsumoto, Kimiyo Saito, Tomoko Shiga, Chiharu Saito and Tomoko Morimoto, Jichi Medical University School of Medicine Center of Excellence Cardiovascular Research and Development (JCARD), for their study coordination and data management. Medical writing assistance was provided by Nicola Ryan, independent medical writer.
Kario K, Okada K, Murata M, et al. Effects of luseogliflozin on arterial properties in patients with type 2 diabetes mellitus: The multicenter, exploratory LUSCAR study. J Clin Hypertens. 2020;22:1585–1593. 10.1111/jch.13988
REFERENCES
- 1. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295‐2306. [DOI] [PubMed] [Google Scholar]
- 2. Solini A, Giannini L, Seghieri M, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 4. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 5. Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(2):221‐228. [DOI] [PubMed] [Google Scholar]
- 6. Sternlicht H, Bakris GL. Blood pressure lowering and sodium‐glucose Co‐transporter 2 inhibitors (SGLT2is): more than osmotic diuresis. Curr Hypertens Rep. 2019;21(2):12. [DOI] [PubMed] [Google Scholar]
- 7. Loutradis C, Papadopoulou E, Theodorakopoulou M, Karagiannis A, Sarafidis P. The effect of SGLT‐2 inhibitors on blood pressure: a pleiotropic action favoring cardio‐ and nephroprotection. Future Med Chem. 2019;11(11):1285‐1303. [DOI] [PubMed] [Google Scholar]
- 8. Wan N, Fujisawa Y, Kobara H, et al. Effects of an SGLT2 inhibitor on the salt sensitivity of blood pressure and sympathetic nerve activity in a nondiabetic rat model of chronic kidney disease. Hypertens Res. 2020;43(6):492‐499. [DOI] [PubMed] [Google Scholar]
- 9. Lunder M, Janic M, Japelj M, Juretic A, Janez A, Sabovic M. Empagliflozin on top of metformin treatment improves arterial function in patients with type 1 diabetes mellitus. Cardiovasc Diabetol. 2018;17(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kario K, Hoshide S, Okawara Y, et al. Effect of canagliflozin on nocturnal home blood pressure in Japanese patients with type 2 diabetes mellitus: the SHIFT‐J study. J Clin Hypertens (Greenwich). 2018;20(10):1527‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kario K, Okada K, Kato M, et al. 24‐hour blood pressure‐lowering effect of an SGLT‐2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the randomized, Placebo‐Controlled SACRA Study. Circulation. 2019;139:2089‐2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liao D, Arnett DK, Tyroler HA, et al. Arterial stiffness and the development of hypertension. The ARIC study. Hypertension. 1999;34(2):201‐206. [DOI] [PubMed] [Google Scholar]
- 13. Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51(14):1377‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55(13):1318‐1327. [DOI] [PubMed] [Google Scholar]
- 15. Park KH, Park WJ, Kim MK, et al. Noninvasive brachial‐ankle pulse wave velocity in hypertensive patients with left ventricular hypertrophy. Am J Hypertens. 2010;23(3):269‐274. [DOI] [PubMed] [Google Scholar]
- 16. Yamashina A, Tomiyama H, Arai T, et al. Brachial‐ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26(8):615‐622. [DOI] [PubMed] [Google Scholar]
- 17. Kario K, Kanegae H, Oikawa T, Suzuki K. Hypertension is predicted by both large and small artery disease. Hypertension. 2019;73(1):75‐83. [DOI] [PubMed] [Google Scholar]
- 18. Kario K, Chirinos JA, Townsend RR, et al. Systemic hemodynamic atherothrombotic syndrome (SHATS) ‐ Coupling vascular disease and blood pressure variability: Proposed concept from pulse of Asia. Prog Cardiovasc Dis. 2019;63(1):22–32. [DOI] [PubMed] [Google Scholar]
- 19. Kario K. Systemic hemodynamic atherothrombotic syndrome and resonance hypothesis of blood pressure variability: triggering cardiovascular events. Korean Circ J. 2016;46(4):456‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kario K. Systemic hemodynamic atherothrombotic syndrome (SHATS): diagnosis and severity assessment score. J Clin Hypertens (Greenwich). 2019;21(7):1011‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kario K. Essential Manual on Perfect 24‐Hour Blood Pressure Management from Morning to Nocturnal Hypertension: Up‐to‐Date for Anticipation Medicine. Japan: Wiley Publishing; 2018:1–309. [Google Scholar]
- 22. Beckman JA, Creager MA. Vascular complications of diabetes. Circ Res. 2016;118(11):1771‐1785. [DOI] [PubMed] [Google Scholar]
- 23. Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37(4):253‐390. [DOI] [PubMed] [Google Scholar]
- 24. Hashimoto T, Ichihashi S, Iwakoshi S, Kichikawa K. Combination of pulse volume recording (PVR) parameters and ankle‐brachial index (ABI) improves diagnostic accuracy for peripheral arterial disease compared with ABI alone. Hypertens Res. 2016;39(6):430‐434. [DOI] [PubMed] [Google Scholar]
- 25. Westerhof BE, Guelen I, Westerhof N, Karemaker JM, Avolio A. Quantification of wave reflection in the human aorta from pressure alone: a proof of principle. Hypertension. 2006;48(4):595‐601. [DOI] [PubMed] [Google Scholar]
- 26. Hayashi K, Yamamoto T, Takahara A, Shirai K. Clinical assessment of arterial stiffness with cardio‐ankle vascular index: theory and applications. J Hypertens. 2015;33(9):1742‐1757. discussion 1757. [DOI] [PubMed] [Google Scholar]
- 27. Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure‐independent arterial wall stiffness parameter; cardio‐ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13(2):101‐107. [DOI] [PubMed] [Google Scholar]
- 28. Boardman H, Lewandowski AJ, Lazdam M, et al. Aortic stiffness and blood pressure variability in young people: a multimodality investigation of central and peripheral vasculature. J Hypertens. 2017;35(3):513‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grillo A, Lonati LM, Guida V, Parati G. Cardio‐ankle vascular stiffness index (CAVI) and 24 h blood pressure profiles. Eur Heart J Suppl. 2017;19:B17‐B23. [Google Scholar]
- 30. Wang H, Liu J, Zhao H, et al. Arterial stiffness evaluation by cardio‐ankle vascular index in hypertension and diabetes mellitus subjects. J Am Soc Hypertens. 2013;7(6):426‐431. [DOI] [PubMed] [Google Scholar]
- 31. Aroor AR, Das NA, Carpenter AJ, et al. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc Diabetol. 2018;17(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee DM, Battson ML, Jarrell DK, et al. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc Diabetol. 2018;17(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bekki M, Tahara N, Tahara A, et al. Switching Dipeptidyl Peptidase‐4 inhibitors to tofogliflozin, a selective inhibitor of sodium‐glucose cotransporter 2 improve arterial stiffness evaluated by cardio‐ankle vascular index in patients with type 2 diabetes: a pilot study. Curr Vasc Pharmacol. 2019;17(4):411‐420. [DOI] [PubMed] [Google Scholar]
- 36. Nagayama D, Yamaguchi T, Saiki A, et al. High serum uric acid is associated with increased cardio‐ankle vascular index (CAVI) in healthy Japanese subjects: a cross‐sectional study. Atherosclerosis. 2015;239(1):163‐168. [DOI] [PubMed] [Google Scholar]
- 37. Tabara Y, Setoh K, Kawaguchi T, et al. Factors affecting longitudinal changes in cardio‐ankle vascular index in a large general population: the Nagahama study. J Hypertens. 2018;36(5):1147‐1153. [DOI] [PubMed] [Google Scholar]
- 38. Shirai K, Song M, Suzuki J, et al. Contradictory effects of β1‐ and α1‐ aderenergic receptor blockers on cardio‐ankle vascular stiffness index (CAVI)–CAVI independent of blood pressure. J Atheroscler Thromb. 2011;18(1):49‐55. [DOI] [PubMed] [Google Scholar]
- 39. Nagayama D, Watanabe Y, Saiki A, Shirai K, Tatsuno I. Lipid parameters are independently associated with cardio‐ankle vascular index (CAVI) in healthy Japanese subjects. J Atheroscler Thromb. 2018;25(7):621‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kamon T, Kaneko H, Itoh H, et al. Gender‐specific association between the blood pressure category according to the updated ACC/AHA guidelines for hypertension and cardio‐ankle vascular index: a community‐based cohort study. J Cardiol. 2020;75(5):578‐582. [DOI] [PubMed] [Google Scholar]
- 41. Xue Q, Qin MZ, Jia J, Liu JP, Wang Y. Association between frailty and the cardio‐ankle vascular index. Clin Interv Aging. 2019;14:735‐742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kiuchi S, Hisatake S, Watanabe I, et al. Pulse pressure and upstroke time are useful parameters for the diagnosis of peripheral artery disease in patients with normal ankle brachial index. Cardiol Res. 2016;7(5):161‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235‐1481. [DOI] [PubMed] [Google Scholar]
- 45. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269‐1324. [DOI] [PubMed] [Google Scholar]
- 46. Kushiro T, Kario K, Saito I, et al. Increased cardiovascular risk of treated white coat and masked hypertension in patients with diabetes and chronic kidney disease: the HONEST study. Hypertens Res. 2017;40(1):87‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hayashi T, Fukui T, Nakanishi N, et al. Dapagliflozin decreases small dense low‐density lipoprotein‐cholesterol and increases high‐density lipoprotein 2‐cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetol. 2017;16(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chino Y, Samukawa Y, Sakai S, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos. 2014;35(7):391‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seino Y, Sasaki T, Fukatsu A, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a 12‐week, randomized, placebo‐controlled, phase II study. Curr Med Res Opin. 2014;30(7):1219‐1230. [DOI] [PubMed] [Google Scholar]
- 50. Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double‐blind, placebo‐controlled, phase 3 study. Curr Med Res Opin. 2014;30(7):1245‐1255. [DOI] [PubMed] [Google Scholar]
- 51. Haneda M, Seino Y, Inagaki N, et al. Influence of renal function on the 52‐week efficacy and safety of the sodium glucose cotransporter 2 inhibitor luseogliflozin in Japanese patients with type 2 diabetes mellitus. Clin Ther. 2016;38(1):66‐88.e20. [DOI] [PubMed] [Google Scholar]
- 52. Sakai S, Kaku K, Seino Y, et al. Efficacy and safety of the SGLT2 inhibitor luseogliflozin in japanese patients with type 2 diabetes mellitus stratified according to baseline body mass index: pooled analysis of data from 52‐week phase III trials. Clin Ther. 2016;38(4):843‐862.e849. [DOI] [PubMed] [Google Scholar]
- 53. Sano M, Chen S, Imazeki H, Ochiai H, Seino Y. Changes in heart rate in patients with type 2 diabetes mellitus after treatment with luseogliflozin: Subanalysis of placebo‐controlled, double‐blind clinical trials. J Diabetes Investig. 2018;9(3):638‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sasaki T, Sugawara M, Fukuda M. Sodium‐glucose cotransporter 2 inhibitor‐induced changes in body composition and simultaneous changes in metabolic profile: 52‐week prospective LIGHT (Luseogliflozin: the Components of Weight Loss in Japanese Patients with Type 2 Diabetes Mellitus) Study. J Diabetes Investig. 2019;10(1):108‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


