Abstract
Breast cancer survivors with type 2 diabetes are at high risk for cancer recurrence, serious health complications, more severe symptoms, psychological distress, and premature death relative to breast cancer survivors without diabetes. Maintaining glycemic control is critical for decreasing symptoms and preventing serious health problems. Many breast cancer survivors with type 2 diabetes have difficulty maintaining diabetes self-management behaviors and achieving glycemic control. Both cancer and diabetes-related symptoms (e.g., physical symptoms and psychological distress) are often barriers to engaging in diabetes self-management strategies. This study evaluates a novel diabetes coping skills training (DCST) intervention for improving breast cancer survivors’ abilities to manage symptoms and adhere to recommended diabetes self-management behaviors. The telephone-based DCST protocol integrates three key theory-based strategies: coping skills training for managing symptoms, adherence skills training, and healthy lifestyle skills training. A randomized clinical trial will test the DCST intervention plus diabetes education by comparing it to diabetes education alone. Symptoms, distress, diabetes self-management behaviors, and self-efficacy will be assessed at baseline and 3, 6, and 12 months. Glycosylated hemoglobin (HbA1c) will be assessed at baseline, 6, and 12 months. This study addresses a critical gap in the care of breast cancer survivors by evaluating a novel behavioral intervention to improve the management of symptoms, adherence, and glycemic control in breast cancer survivors with type 2 diabetes. Special considerations for this medically underserved population are also provided. The findings of this study could lead to significant improvements in clinical care and beneficial outcomes for breast cancer survivors.
Keywords: Diabetes, Breast Cancer, Self-Management, Coping Skills
INTRODUCTION
Approximately 20% of the more than 268,000 women diagnosed with breast cancer in 2019 will have a diagnosis of type 2 diabetes.[1, 2] Type 2 diabetes is undiagnosed in almost one-third of individuals with the disease,[3] suggesting that an even greater number of breast cancer patients have type 2 diabetes.[4] Breast cancer survivors with comorbid type 2 diabetes are at high risk for breast cancer recurrence,[1, 4–22] have a 38% higher risk of breast cancer-specific mortality, and have a 49% higher risk of all-cause mortality[5] relative to women with breast cancer but without diabetes. For early-stage breast cancer survivors, type 2 diabetes is associated with significantly shorter median disease-free survival (36 vs. 81 months).[8] Together, breast cancer and type 2 diabetes represent a public health crisis.[23–25] A recent report by the American Cancer Society and the American Diabetes Association highlights the significance of this problem.[26] The prevalence of type 2 diabetes is rising rapidly in the U.S.,[27] and represents a growing health problem worldwide.[28, 29] Thus, the number of breast cancer survivors with type 2 diabetes will continue to grow.[24, 25, 28, 29]
Breast cancer survivors with type 2 diabetes experience more severe cancer and diabetes-related physical symptoms (e.g., pain, fatigue), poorer physical functioning, and greater psychological distress than those without diabetes.[30–37] Undergoing cancer treatment often exacerbates pre-existing symptoms (e.g., pain, fatigue, and diabetes-related neuropathy).[38] Women with breast cancer and type 2 diabetes are at greater risk for developing new issues including chemotherapy-induced neuropathy,[39–41] persistent post-surgical pain,[42] and severe cancer treatment-related toxicities, [1, 24, 40, 43–46] and cancer treatments may exacerbate diabetes-related complications.
Type 2 diabetes is a major cause of premature mortality and serious health complications (e.g., stroke, renal failure).[26, 47] Improving appropriate self-management of diabetes can dramatically reduce microvascular events, myocardial infarction, and diabetes-related mortality.[48] Data suggest that hyperglycemia and hyperinsulinemia, which are simultaneously present in most individuals with type 2 diabetes,[25] may play an important role in promoting breast cancer growth and progression.[17–20, 49–58] Thus, for breast cancer survivors with diabetes, maintaining glycemic control may be especially important.[25] However, many breast cancer survivors exhibit poor diabetes self-management behaviors, which negatively impact glycemic control and health outcomes.[38, 59, 60] Efforts to engage in diabetes self-management may be challenged by both cancer treatment-related symptoms (e.g., fatigue, arthralgia) and type 2 diabetes-related symptoms (e.g., neuropathy).[30, 38, 59] For women with type 2 diabetes, self-management includes physical activity, dietary modifications, medication (e.g., insulin), and blood glucose monitoring (when recommended).[47] Maintaining glycemic control and avoiding diabetes-related complications depends on daily engagement in these self-management behaviors.[61–66]
The present study evaluates a novel diabetes coping skills training (DCST) intervention for improving breast cancer survivors’ abilities to manage symptoms, reduce psychological distress, and adhere to recommended diabetes self-management. Although systematic training in skills for coping with symptoms and improving adherence could be beneficial for enhancing breast cancer survivors’ engagement in diabetes self-management behaviors, to our knowledge, no study has evaluated the effects of such training in patients with breast cancer and type 2 diabetes.
MATERIALS AND METHODS
A. Study Aims
Our first aim is to investigate the impact of the DCST protocol on both cancer- and diabetes-related physical symptoms and psychological distress, which are important factors that signficantly impact women’s abilities to manage their diabetes. Our second aim is to investigate the impact of the DCST protocol on diabetes self-management behaviors. Our third aim is to examine the impact of the DCST protocol on glycemic control. Finally, our fourth aim is to examine the impact of the DCST protocol on self-efficacy for managing symptoms and diabetes self-care. This study is being conducted in both a tertiary academic medical center and community cancer clinics to increase the generalizability of findings.
B. Patient Selection
a. Eligibility Criteria
Participants are recruited from a tertiary academic medical center and through community cancer clinics that are part of a cancer network affiliated with the tertiary academic medical center. The cancer network is a collaborative program that partners with community hospitals in medically underserved areas to provide access to oncology care. Eligible participants meet the following inclusion criteria: a) diagnosis of Stage I to III breast cancer, b) diagnosis of type 2 diabetes, c) completed primary treatment (surgery, chemotherapy, and radiation therapy), d) physician verification of ability to participate in the intervention, and e) English speaking. Women who meet any of the following criteria are excluded: a) <21 years of age, b) severe cognitive or hearing impairment as documented in the medical record, c) unable to provide meaningful consent (i.e., impairment such that descriptions of the research are not clearly understood), or d) presence of a health problem that precludes safe participation in the intervention (e.g., recent myocardial infarction, poorly controlled atrial fibrillation).
b. Subject Recruitment
This study was approved by the Institutional Review Board. Recruitment procedures comply with HIPAA guidelines. Patients meeting eligibility criteria are informed about the study in one of two ways. First, the study brochure and letter describing the study are given to women by a member of their treatment team at the time of an oncology follow-up visit. Second, the study brochure that briefly describes the study and a letter from their oncologist introducing the study are mailed to them. Prospective participants are contacted by telephone by study staff to determine if they are interested in hearing more about the study. For women who express interest in participating, study staff arrange an in-person meeting to further describe the study, confirm eligibility for the study, obtain informed consent, and complete the baseline assessment. The initial study visit ranges from 2–3 hours.
C. Procedures
See Figure 1 for the study design. Planned enrollment includes N=200 breast cancer survivors with comorbid type 2 diabetes (i.e., documented in the medical record), with an anticipated attrition rate of 20% for a final sample size of 160. Following the completion of informed consent, women complete the baseline assessment. All participants receive a single 60-minute, nurse-delivered diabetes education session after completion of the baseline assessment. Next, women are randomly assigned with equal allocation to either: 1) Diabetes Coping Skills Training (DCST), or 2) no additional intervention (i.e., patients only receive the single diabetes education session delivered during the baseline assessment). All patients receive usual health care. Randomization is stratified by clinic site to ensure equal allocation across sites, and is determined by a centralized randomization program. We selected clinic site as a key variable for stratification because the patient populations in these clinics vary demographically and reflect the diverse community settings in which these clinics are located. Following randomization, women in the DCST condition are given the intervention workbook materials and are contacted by the study interventionist to begin intervention phone sessions.
Figure 1.
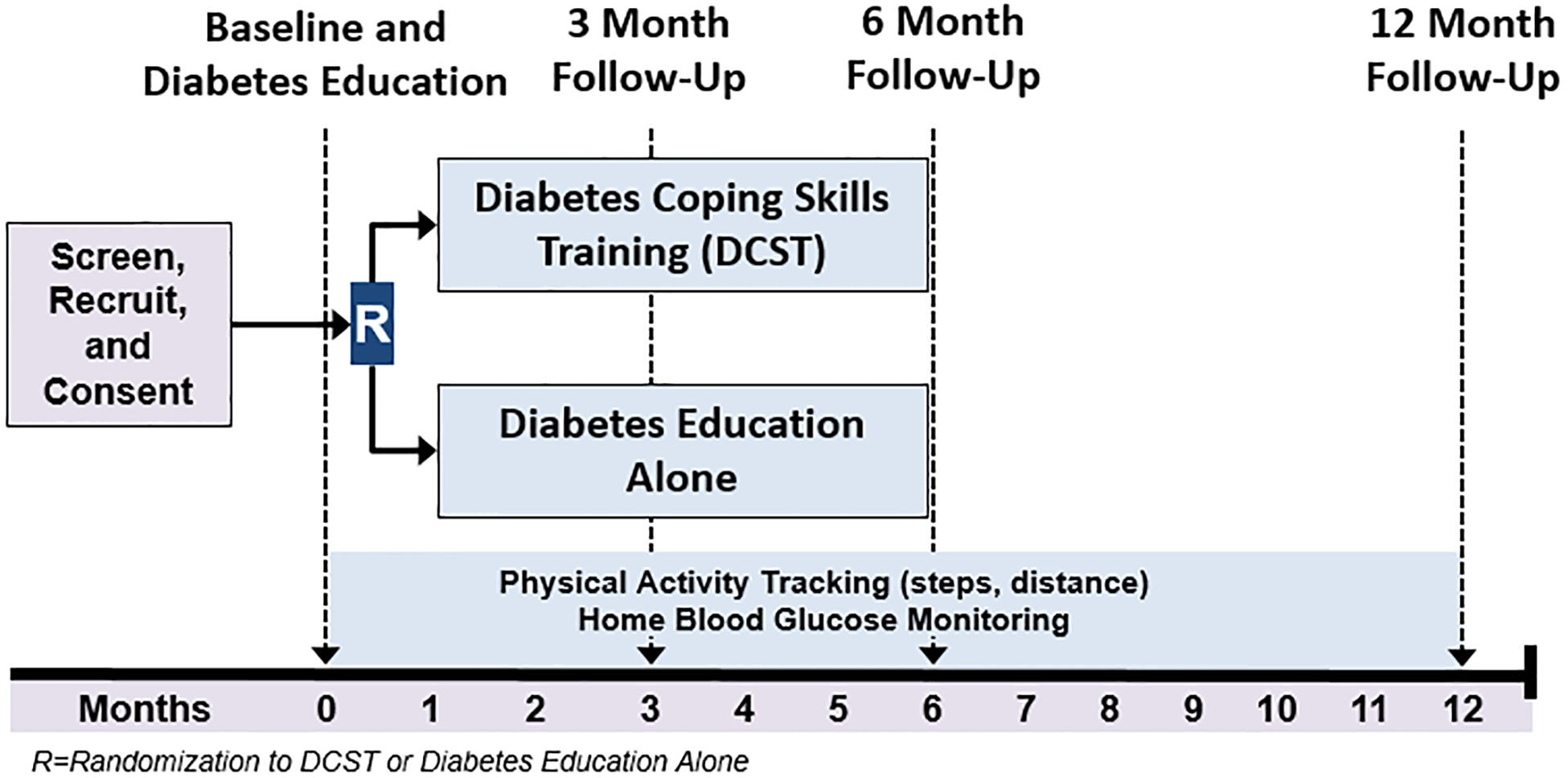
Study Design
In addition to the baseline assessment, all participants complete follow-up assessments at 3 months (mid-intervention), 6 months (post-intervention), and 12 months. During each of the four assessments, participants complete self-reported measures of physical symptoms, psychological distress, quality of life, self-efficacy, and diabetes self-management behaviors. Participants also complete the 6-minute walk test. To track daily physical activity (i.e., steps and distance), pedometers are set up by study staff during the baseline assessment and are given to study participants. Study staff obtain data from the pedometers during the follow-up assessments. When part of the participant’s recommended diabetes care, participants are asked to bring their home blood glucose monitors to each of the four assessments to collect data regarding the frequency of monitoring over the 12 months of study participation. Hemoglobin A1c (HbA1c) levels are assessed at baseline and again at 6 and 12 months to assess glycemic control. Given that HbA1c provides a measure of the cumulative glycemic history of the preceding two to three months, HbA1c levels are not assessed at 3 months as participants will have recently initiated the intervention. We do not anticipate seeing intervention-related changes in HbA1c until participants have completed the intervention.
Interventions
a. Diabetes Education (1 Session):
During the baseline study visit, all participants receive a single, individually-delivered, 60-minute diabetes education session with a study nurse using tele-video-conferencing (via Skype). This session mimics a traditional, in-person, face-to-face diabetes education session, and enables study nurses to deliver the diabetes education protocol to participants completing assessments at their community-based clinic or the tertiary medical center.
The content of the diabetes education session focuses on providing patients with information about diabetes care as recommended by the American Diabetes Association in 2016[67]: 1) healthy eating, 2) being active, 3) blood glucose monitoring (if applicable), 4) taking medication, 5) problem solving for barriers to care, 6) information about healthy coping, and 7) reducing risks of diabetes-related complications. The nurse works with the participant to establish tailored goals for diabetes self-care including the use of oral medication and insulin, glucose monitoring (if applicable), exercise, and foot care. Participants are provided bullet-pointed notes to assist with following along with the nurse-delivered content during the session, as well as an education workbook to use as a reference at home. The goal of the diabetes education session is to provide patients with the knowledge necessary to be active participants in their diabetes management.
b. Diabetes Coping Skills Training (DCST; 12 Sessions)
The DCST protocol integrates coping skills training for managing symptoms, adherence skills training, and healthy lifestyle skills training. The protocol is delivered via phone. Table 1 provides an overview of the approximate frequency and content of each of the DCST sessions. DCST is delivered by psychologists (PhD or Master’s level) over 12 sessions (45 to 60 minutes each) across approximately 6 months. The study interventionists schedule sessions using a faded contact model (i.e., 6 weekly sessions, 3 sessions delivered every two weeks, and 3 monthly sessions), but the session schedule may be altered to accommodate the participant’s circumstances (e.g., travel, illness).
Table 1.
Overview of DCST Telephone Session Frequency and Content
| Frequency | Session | Content |
|---|---|---|
| Weekly | 1 | Program introduction and rationale; Diabetes self-management adherence skills; Enjoying an active lifestyle and introduction to the home-based physical activity protocol |
| 2 | Healthy dietary patterns and eating well with diabetes; Staying motivated | |
| 3 | Portion control; Stress responses; Relaxation techniques | |
| 4 | Brief relaxation and breathing; Activity pacing skills | |
| 5 | Increasing lifestyle physical activity; Behavioral activation; Pleasant activity scheduling; Setting SMART goals | |
| 6 | Healthy shopping tips; Cognitive restructuring skills: identifying unhelpful thoughts; shifting to more neutral thoughts and using coping thoughts | |
| Every 2 weeks | 7 | Understanding eating triggers; Managing emotional and environmental eating triggers |
| 8 | Social influences on eating and physical activity; Communicating with significant others; Social eating and eating out | |
| 9 | Factors that maintain overeating; Appetite awareness training; Managing urges and cravings | |
| Monthly | 10 | Food awareness training for portion control and managing urges; Distraction techniques; Enhancing social support |
| 11 | Maintaining change; Problem solving; Plan for skills use and maintaining change | |
| 12 | Review of progress; Values; Review of maintenance plans and goal achievement strategies |
Each of the twelve sessions is delivered using the following three-section structure. 1) Home Practice Review: A review of the participant’s goals for diabetes self-management and adherence to these goals is conducted. Patients are provided with encouragement for using adherence, healthy lifestyle, and symptom management skills. If non-adherence is noted, brief problem solving is conducted that directs patients to the relevant adherence and coping skills (e.g., cuing strategies). 2) Skills Training: Each session includes psycho-education (e.g., information about healthy dietary patterns) and coping skills training for improving diabetes self-management. Coping, adherence, and lifestyle skills training addresses cognitive, behavioral, and emotional factors that influence engagement in self-management behaviors and heighten symptoms and disability. Instruction, modeling, and guided practice are used to teach patients skills for managing symptoms and improving engagement in self-management behaviors. 3) Skills Application and Goal Setting: Participants set goals for diabetes self-management and applying skills learned during the intervention. Potential barriers to working toward goals and applying skills are identified and participants develop plans for managing these barriers.
The DCST protocol also includes a home-based physical activity protocol developed by an exercise physiologist specifically for breast cancer survivors with type 2 diabetes. Participants are given information regarding the health benefits of exercise, tips for exercising safely, and suggestions and instructions for specific exercises. This program includes strategies specifically for breast cancer survivors managing long-term treatment side effects such as lymphedema and neuropathy. All participants in the study are provided a pedometer to track steps and distance. For women in the DCST condition, the pedometer is incorporated into activity goal setting and home practice review of physical activity. Strengthening exercises (15 minutes of resistance exercises, 3 or more days per week) are also encouraged, as strength training has benefits for glycemic control.[68] Individuals are given a set of resistance bands and instructions for using the resistance bands. A strength training program using the resistance bands was developed by an exercise physiologist for this study. Participants are provided with written, pictorial, and video instructions for the strength training exercises; an in-person demonstration is also provided by study staff during the baseline study visit.
A long-term goal of 150 minutes per week of moderate-intensity planned activity (e.g., walking 30 minutes, 5 or more days per week), and 15 minutes of exercises to increase strength (e.g., using resistance bands) on three non-consecutive days per week is encouraged. The interventionists work with participants to set safe and realistic physical activity goals during telephone sessions. Participants may be relatively inactive at the start of the intervention; interventionists assist participants with making gradual and small increases in physical activity working towards the goal of 150 minutes per week of walking (or moderate-intensity activity).[69] The interventionist also helps participants identify activities they enjoy that can easily be incorporated into their lifestyle, and to increase opportunities for physical activity in their daily lives (e.g., taking the stairs, parking further away from the store, completing housework). Intervention fidelity is increased by the interventionists participating in structured training, following a treatment manual, and provision of ongoing supervision with feedback regarding adherence to the intervention protocol.
In addition to the aforementioned diabetes education workbook provided during the diabetes education session, each participant in the DCST arm is provided with a DCST workbook, which includes written information, pictures, and diagrams for all content delivered during sessions as well as a home-based exercise manual providing instruction in physical activity recommendations and exercises. The workbooks provide guided exercises to help women apply the intervention content to their lives.
D. Measures
The measures included in the study assessments have extensive reliability and validity data. Table 2 presents the measures and the time points at which each measure is completed.
Table 2.
Timeline of Assessments, Self-report Measures, and Objective Measures
| Daily | Baseline | 3-months | 6-months | 12-months | |
|---|---|---|---|---|---|
| X | X | X | X | ||
| X | X | X | X | ||
| X | X | X | X | ||
| X | X | X | X | ||
| X | X | X | X | ||
| X | X | X | X | ||
| X | X | X | X | ||
| X | |||||
| X | X | X | X | ||
| X | X | X | X | ||
| X | X | X | X | ||
| Steps and Distance (from pedometers) | X | ||||
| X | X | X | X | ||
| X | X | X | |||
| X | X | X | X | ||
| X | X | X | X | ||
| X | |||||
| X | |||||
| X | X | X | X | ||
| X | |||||
| X | |||||
| X | X | X | X | ||
| X | X | X | X | ||
| X | X | X | X | ||
| X | |||||
| X | |||||
| X | X | X | X | ||
| X | X | X | X |
Aim 1: Investigate the impact of the DCST protocol on physical symptoms and psychological distress
Subjective, Self-report measures
a. Physical Symptoms
Pain Severity and Interference.
The Brief Pain Inventory – Short form (BPI-SF)[70] measures pain severity and interference in the last week over 9 areas (e.g., general activity, mood, sleep, enjoyment of life).
Fatigue.
The 8-item Patient-Reported Outcomes Measurement Information System Fatigue Scale (PROMIS Fatigue)[71] assesses fatigue over the past seven days.
Neuropathy Symptoms.
The 16-item taxane subscale of the Functional Assessment of Cancer Therapy (FACT)[72] assesses symptoms of neuropathy in cancer patients. Participants rate each item on a 0 (not at all) to 4 (very much) scale.
b. Psychological Distress
Depressive Symptoms.
The eight-item Patient-Reported Outcomes Measurement Information System Depression Scale (PROMIS Depression)[71] is used to assess depressive symptoms.
Symptoms of Anxiety.
The eight-item Patient-Reported Outcomes Measurement Information System Anxiety Scale (PROMIS Anxiety)[71] is used to assess symptoms of anxiety.
Aim 2: Investigate the impact of the DCST protocol on diabetes self-management behaviors
Medication Adherence.
A 16-item measure is used to assess self-reported adherence. This measure is based on the Medication Adherence Rating Scale[73] and our prior studies.[74] Items were revised to assess medication-taking behaviors related to adherence (e.g., forgetting) and capture intentional and unintentional non-adherence over the past month.
Adherence to Diabetes Self-Management Behaviors.
The 12-item Diabetes Self-Care Inventory-Revised[75] is used to assess diabetes self-management behaviors in the past month (e.g., exercising regularly, keeping food records, treating low blood sugar, taking the correct dose of pills/insulin).
Home Blood Glucose Monitoring.
When part of the participant’s recommended diabetes care, the frequency of use of home blood glucose monitoring is assessed. Data is obtained from the participant’s home blood glucose monitoring device using the manufacturer’s freely available software or study staff will manually write down the dates, times, and blood glucose values stored in the device from the last 30 days.
Diet.
The Five-A-Day Consumption and Evaluation Tool (FACET) is a dietary questionnaire that assesses changes in knowledge of, awareness of and access to fruits and vegetables.[76] The Dietary Instrument for Nutrition Education (DINE)[77] is used to assess the amount of fat and dietary fiber in an individual’s usual diet.
Physical Activity.
Patient-reported physical activity is assessed using the well-validated International Physical Activity Questionnaire (IPAQ).[78] This seven-item questionnaire assesses the amount of time participants have spent doing moderate and vigorous physical activities in the last seven days. Data is also obtained from participants’ pedometers to assess daily steps and distance. The 6-minute walk test is an objective assessment of womens’ abilities to exert effort in activity and the degree of pain experienced during activity.[79, 80] Women walk along an indoor hallway for 6-minutes with the goal of walking as far as possible within the allotted time.
Aim 3: Examine the impact of the DCST protocol on glycemic control
Glycosylated hemoglobin levels (HbA1c) will be obtained and analyzed by LabCorp in peripheral blood. HbA1c provides a measure of an individual’s average blood glucose levels during the previous two to three months, and it is the recommended standard of care for testing and monitoring type 2 diabetes.[81] HbA1c is an important indicator of glycemic control as it captures the cumulative glycemic history of the preceding two to three months.[82]
Aim 4: Examine the impactof the DCST protocol on self-efficacy
Self-Efficacy.
Self-efficacy for managing symptoms is assessed using an 8-item modified version of a standard self-efficacy scale.[83] Items are rated on a 10-point scale (1=not certain; 10=very certain) and averaged to create a total score (in our preliminary studies Cronbach’s α=.75).[84] Self-efficacy for diabetes self-management is assessed using the Confidence in Diabetes Self Care Scale,[85] which measures self-efficacy for performing 27 diabetes self-management behaviors (e.g., detect low levels of blood sugar in time to correct, ask a friend for help, etc.) and yields a total self-efficacy score. This measure has been used in prior studies of cancer survivors with diabetes (Cronbach’s α=.86).[38]
Other measures
Treatment Credibility and Satisfaction.
The Treatment Credibility Questionnaire [86–88] is a 5-item measure of the degree to which patients perceive a treatment as credible and expect positive outcomes (e.g., “How helpful does the therapist seem to you?”; “How confident are you that this treatment will help you manage your symptoms and health concerns?”). The Modified Satisfaction with Therapy and Therapist Scale[89] is a 13-item measure of satisfaction with and global improvement after intervention.
Participant sociodemographic and medical characteristics, health literacy, numeracy, health problems, and chronic life stressors are obtained. These variables will be considered as potential covariates in planned analyses. Participants are also asked about their participation in other programs that may impact the results of the present study.
Medical and Demographic Information.
Medical and demographic information are assessed by self-report (e.g., education, race, marital status, income, ability to pay for medication, health insurance coverage) and through medical record abstraction [e.g., age, height, cancer stage (TNM), ER/PR status, HER2 score, surgery, treatments]. Body weight is recorded at each study assessment. Type of pharmacological treatment for diabetes, duration of treatment, the indication of discontinuation, change in medications, and reasons for medication change or discontinuation are recorded.
Comorbidities and Complications.
The Adult Comorbidity Evaluation Scale (ACE-27)[90] is a 27-item comorbidity index for patients with cancer that assesses the severity of comorbidities. This assessment allows the study staff to identify and grade the severity of comorbidities via medical record. The Self-Report Disease Burden Scale [91] assesses the experience and interference of 25 different health problems (e.g., angina, depression, hypertension, osteoarthritis, stroke). If it is indicated that a problem has been experienced in the past, participants are asked to rate how much this problem has interfered with their daily activities in the past month. Response options range from 1 (not at all) to 5 (a lot). The Diabetes Complications Index[92] assesses diabetes-related complications. This assessment includes 17 items (e.g., ulcers, cramps, numbness in feet) and a checklist of potential comorbid conditions.
Barriers to Taking Medication.
Twelve items assess barriers to taking medication over the past month.[93, 94] Women rate how often certain situations (e.g., side effects, confusion, problems with injections) made it difficult for them to take their medications each day.
Literacy and Numeracy.
The Rapid Estimate of Adult Literacy in Medicine (REALM)[95] is used to assess health literacy. This assessment is a word recognition test during which participants are asked to de-code or pronounce health-related words. The test takes less than 2 minutes to administer and score. The Newest Vital Sign (NVS)[96] is a 6-item measure of numeracy and literacy in adults. Participants are given a nutrition label and must answer four questions involving understanding and using numbers and two questions involving reading and understanding text.
Chronic Life Stress.
The nine-item Chronic Life Stressors Scale[97] is used to assess stressors across nine domains: general/ambient problems, financial, work, relationship, parental concerns, family, social life, residence, and health issues. Participants also complete a questionnaire asking them to rate the economic pressures and concerns they have personally experienced in the past 12 months or since the last study assessment.[98–101]
E. Statistical Analysis
a. Sample Size and Power
Planned enrollment includes N=200 participants, with an anticipated attrition rate of 20% for a final sample size of 160 participants. The planned sample size was determined based on power calculations for comparisons between the DCST and control group in Aims 1–4, and the proposed mediation analyses for self-efficacy in Aim 4. Power analysis for group comparisons in Aims 1–4 were conducted based on the following assumptions: medium effect sizes (d=.5 or f=.25) based on prior preliminary studies, power of .80, two-sided tests, and a family wise error rate of α=.05 for each aim. A Bonferroni correction was used (i.e., α=.05/number of tests conducted) for each aim. For Aims 1 and 2, the total sample size required for adequate power ranges from N=80 to N=122, when correlations among repeated measures range from .2 to .5, respectively. For Aim 3, the total sample size required for adequate power ranges from N=62 to N=86 when correlations among repeated measures range from .2 to .5, respectively. For mediation analysis in Aim 4, sample size estimates were based on 80% power, α=.05, using a percentile bootstrap test of the indirect effect, and interpolating the standardized parameter estimates of Fritz and MacKinnon.[102] A total sample size of N=124 to N=160 will be required to detect an effect size effect size varying from 0.26 (reasonably small) to 0.39 (medium) for the treatment group to mediator path, and an effect size varying from 0.39 (medium) to 0.26 (reasonably small) for the mediator to outcomes path (i.e., an indirect effect size ranging from 0.07 to 0.10).
b. Analyses of Aims 1, 2, and 3
All analyses will be based on intention to treat (ITT) principles. We will examine incomplete data patterns. Data missing at random will be accommodated by the use of linear mixed models under the assumption of MAR or other approaches as necessary. For Aims with more than one outcome measure we will adopt a simple family-wise strategy for adjusting overall alpha for each aim by dividing alpha by the number of outcome variables examined, with alpha set to 0.05/k where k is the number of outcome variables used in each aim.
The primary analysis will examine treatment group differences in patient-reported outcomes (i.e., physical symptoms, psychological distress, self-management behaviors), daily physical activity (i.e., steps, distance), glucose monitoring (as appropriate), and glycemic control (i.e., HbA1c) using linear mixed-effects models. Patient effects will be entered as variance components to model within-patient correlations over time. We will also fit marginal models that account for within-patient correlations directly using an appropriate correlation matrix without introducing random-effects. As a model building strategy, we will first test a main-effects only model and then include the group × time interaction. We will follow up significant interactions using procedures described in Aiken and West[103] for cross-sectional models, and Preacher et al.[104] for longitudinal analyses. Best models will be selected using BIC information criterion to strike a balance between goodness of fit and model complexity. The outcome variables for Aim 1 will be physical symptoms, physical functioning, and psychological distress measured at baseline, 3, 6, and 12 months. For Aim 2, outcome variables will include patient-reported outcomes (medication adherence, self-management behaviors, physical activity, dietary patterns) measured at baseline, 3, 6, and 12 months and data from pedometers (steps and distance averages per month) and blood glucose monitors (% adherent per week) measured across 12 months of enrollment. The outcome variable for Aim 3 will be HbA1c measured at baseline, 6, and 12 months. Baseline values (baseline assessment value for patient-reported outcomes and measures of glycemic control; average steps and distance in week 1; and % days adherent to monitoring in week 1) of the outcome, demographic, and medical (e.g., comorbidities) variables will be used as covariates, and main effects of time, treatment arm, and time by treatment arm interaction will be included as predictors. Family-wise error will be controlled for within each aim as appropriate. By assessing demographic and medical variables, we may also be able to conduct exploratory post-hoc analyses to examine these variables as potential moderators of intervention effects in order to inform future studies.
c. Analysis of Aim 4
Analyses will examine whether changes in self-efficacy mediate the impact of DCST on study outcomes. We will use similar linear mixed-effects or marginal models as described for Aims 1, 2 and 3. The mediational hypothesis will be addressed using a set of three models: 1) the effect of treatment arm on study outcomes (e.g., patient-reported outcome will include 3, 6, and 12 month differences from baseline), 2) the effect of treatment arm on self-efficacy (outcome will include the changes in 3, 6 and 12 month measures of self-efficacy from the baseline), and 3) the effect of self-efficacy on study outcomes both measured as differences from their baseline. Following recent work on modeling of mediation and timing in mediational models[105–109] we will fit more parsimonious autoregressive cross-lag (ACL) models using SAS PROC GLM, MIXED or GENMOD/with GEE option, or Mplus.
DISCUSSION
The proposed study addresses a critical gap in the care of breast cancer survivors with type 2 diabetes by evaluating a novel behavioral intervention that aims to improve the management of symptoms, diabetes treatment adherence, and glycemic control. The demands of managing comorbid breast cancer and type 2 diabetes pose a formidable challenge for breast cancer survivors and their health care providers.[23–25] The significance of this challenge is highlighted in the 2010 joint consensus report of the American Cancer Society and the American Diabetes Association,[26] which called for research to develop treatments and improve outcomes for individuals with comorbid cancer and diabetes. Adherence to diabetes self-management has been shown to improve glycemic control and impact survival in patients with type 2 diabetes,[47, 48, 110] raising the possibility that the DCST has the potential to improve survival for women with breast cancer and diabetes. For breast cancer survivors with type 2 diabetes, the cost of non-adherence to diabetes self-management is high in terms of symptom-related disability, serious health complications (e.g., stroke, kidney failure), and premature mortality.[24, 25]
This clinical trial will be the first to evaluate a behavioral intervention for breast cancer survivors with type 2 diabetes, and its findings could lead to significant improvements in clinical care, beneficial outcomes for breast cancer survivors, and stimulate new research. If the DCST intervention is efficacious, results from this study could greatly heighten awareness of the role of behavioral interventions in promoting adherence and lead to the inclusion of these interventions in the routine medical management of cancer survivors. Finally, it could facilitate early referral to behavioral interventions before non-adherence leads to serious health problems.
Strengths of the Present Study and Methodological Considerations for the Study Population
The methodologies used in the present study were chosen to address the complexities of this patient population including a greater symptom burden that impacts daily functioning as well as diabetes self-management, competing demands of comorbid health conditions (e.g., increased doctor’s appointments, financial burden, interference with work), and greater life stressors (e.g., fear of cancer recurrence, changes in role functioning due to symptoms). For example, we chose to deliver the intervention sessions via telephone and provide a home-based physical activity program (e.g., home walking protocol, activity tracker, resistance bands with education around use) to reduce barriers to engaging in the intervention. Additionally, the diabetes education session was delivered using video conferencing technology by a trained study nurse. Both the education session and study assessments were conducted in the patient’s local, community-based clinic to reduce barriers associated with parking, travel, and cost (e.g., time and money).
The present study has several strengths. First, the developed intervention combines coping skills training for symptom management with adherence and lifestyle skills training, which is very likely to enhance breast cancer survivors’ diabetes self-management. Many breast cancer survivors with type 2 diabetes experience difficult, disabling symptoms (e.g., neuropathy, pain, fatigue) related to both their cancer treatments and their diabetes, and the symptom burden is greater than for those with breast cancer or type 2 diabetes alone;[39–42] these symptoms have a strong, negative impact on diabetes self-management.[37, 111–118] Past intervention work in patients with type 2 diabetes has focused on physical activity and diet, but has not explicitly addressed the physical symptoms that often interfere with engagement in these behaviors. A recent review of clinical trials to promote physical activity in these patients found that no trials targeted pain as part of the intervention or examined pain as a modifier of outcomes.[119]
Second, the DCST protocol is delivered via phone to increase the feasibility of participation among this unique population with difficult symptoms, competing health demands, and limited financial resources. A phone-based intervention confers several important benefits for dissemination, especially for patients who live in medically underserved areas, have lower income, and may be older and less comfortable with newer technologies.[42, 120] First, the financial demands of in-person sessions often prohibit cancer survivors from accessing this type of care, especially those with limited financial resources. Second, many cancer survivors travel a considerable distance to tertiary care centers, making in-person visits challenging. Third, phone-based interventions allow patients to complete sessions from home or a setting of their choosing. Participating by phone from one’s home may increase patients’ comfort level and ability to generalize skill use to the home setting and community setting.[41] Fourth, phone access is widely available and feasible for most patients. As other forms of communication (e.g., video-conferencing) become more widely available, the DCST protocol could be adapted for these methods of delivery.
Third, given the complexities of this patient population, the diabetes education session was delivered to all participants by a trained diabetes nurse educator, and the DCST intervention sessions were delivered by psychologists. It has been our experience that, even if a patient was diagnosed with diabetes prior to their cancer diagnosis, there are often changes in diabetes management (e.g., movement from oral medications to insulin) that occur following cancer treatment. Regardless of whether a patient was newly diagnosed with type 2 diabetes or had been diagnosed previously, patients may not have access to appropriate diabetes education as their cancer and treatments may have taken precedence during time. In this first trial, clinical psychologists (PhD and Master’s level) were chosen to deliver the DCST intervention because of the potential for greater psychological symptoms and the inclusion of skills based in cognitive-behavioral therapy. If found to be efficacious, other psychosocial providers (e.g., social workers, medical family therapists) and diabetes educators (e.g., nurses, nutritionists, dieticians) could be trained to deliver the intervention in the future. Non-behavioral health providers would require specialized training in cognitive behavioral theory-based strategies given the complexities and greater symptom burden often experienced by these patients.
Finally, if DCST is efficacious, future studies could adapt this intervention to address the challenges affecting cancer patients with comorbid diabetes during adjuvant treatment. Poor glycemic control during chemotherapy is associated with severe side effects and a high risk of severe complications,[24, 43–46, 121–123] which often result in the use of alternative chemotherapy agents or dose reductions.[1, 24, 40, 124–126] The DCST protocol could also be readily adapted to address specific challenges faced by individuals with other types of cancer and type 2 diabetes. For example, type 2 diabetes is prevalent among colorectal cancer survivors,[25] and these individuals often experience difficult symptoms (e.g., chronic bowel dysfunction) that interfere with diabetes self-management. The DCST protocol could be adapted for colorectal cancer survivors by focusing on the application of skills training on these symptoms. Finally, evidence suggests that adherence to healthy lifestyle behaviors is often better when a partner or caregiver is involved.[127–131] Future studies could examine the effects of training both the cancer survivor and their partner/caregiver in the intervention, which could enhance outcomes. [132–134]
Limitations of the Present Study
There are several limitations to be noted. First, this study is limited to breast cancer survivors with diabetes receiving treatment in North Carolina. However, we are recruiting participants from both a major academic medical center and community oncology clinics to capture the impact of the intervention on participants who live in diverse settings. Further, this study only includes breast cancer survivors with diabetes who have completed curative treatment (with the exception of adjuvant endocrine therapy); thus, we are unable to generalize these results to breast cancer patients on active treatment with diabetes, many of whom experience difficulties managing diabetes during surgery, radiation, and/or chemotherapy. Second, participants are followed for 12 months. While this allows us to examine the impact of the intervention over time, the longer-term impact of the intervention on diabetes self-management behaviors will be unknown. If the intervention is shown to be efficacious, future studies may benefit from a longer follow-up period. Finally, medication adherence is assessed using self-report measures. Future studies may benefit from collecting objective data related to medication adherence (e.g., patient medication refill records).
Clinical Implications
For breast cancer patients with type 2 diabetes, avoiding serious health complications depends on daily engagement in diabetes self-management behaviors (glucose monitoring, medication, dietary modifications, and physical activity).[61–66] Yet, breast cancer patients experience significant difficulty engaging in these behaviors during and after treatment, and need support for co-managing their diabetes and breast cancer.[38, 59, 60] Further, cancer treatments (e.g., estrogen suppression due to chemotherapy and/or adjuvant endocrine therapy)[135] may increase the risk of developing diabetes among women with pre-diabetes; thus, this intervention may prove to be beneficial for breast cancer patients with pre-diabetes as well. Future studies could adapt this intervention for use with cancer patients with diabetes during treatment. Studies suggest that chemotherapy and medications commonly given during chemotherapy, such as glucocorticoids, can dramatically impact blood glucose levels.[38, 45] Finally, results of this trial may provide insight into possible strategies for developing interventions that target individuals with comorbid diabetes and other chronic health conditions or who have symptoms that interfere with diabetes self-management.
Grant acknowledgement, Source of Support:
This work was supported by the American Cancer Society [RSG 341106].
Footnotes
Trials registration: ClinicalTrials.gov, NCT02970344, registered 11/22/2016
Conflicts of Interest: The authors have no actual or potential conflicts of interest to disclose.
References
- [1].Srokowski TP, Fang S, Hortobagyi GN, Giordano SH, Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 27(13) (2009) 2170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].American Cancer Society, Cancer Facts & Figures 2019, in: A.C. Society; (Ed.) Atlanta, 2019. [Google Scholar]
- [3].Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, Geiss LS, Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006, Diabetes care 32(2) (2009) 287–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Erickson K, Patterson RE, Flatt SW, Natarajan L, Parker BA, Heath DD, Laughlin GA, Saquib N, Rock CL, Pierce JP, Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29(1) (2011) 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, Brancati FL, Wolff AC, Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29(1) (2011) 40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Patterson RE, Flatt SW, Saquib N, Rock CL, Caan BJ, Parker BA, Laughlin GA, Erickson K, Thomson CA, Bardwell WA, Hajek RA, Pierce JP, Medical comorbidities predict mortality in women with a history of early stage breast cancer, Breast cancer research and treatment 122(3) (2010) 859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL, Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis, JAMA : the journal of the American Medical Association 300(23) (2008) 2754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kaplan MA, Pekkolay Z, Kucukoner M, Inal A, Urakci Z, Ertugrul H, Akdogan R, Firat U, Yildiz I, Isikdogan A, Type 2 diabetes mellitus and prognosis in early stage breast cancer women, Med Oncol 29(3) (2012) 1576–80. [DOI] [PubMed] [Google Scholar]
- [9].Schrauder MG, Fasching PA, Haberle L, Lux MP, Rauh C, Hein A, Bayer CM, Heusinger K, Hartmann A, Strehl JD, Wachter DL, Schulz-Wendtland R, Adamietz B, Beckmann MW, Loehberg CR, Diabetes and prognosis in a breast cancer cohort, Journal of cancer research and clinical oncology 137(6) (2011) 975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu X, Ji J, Sundquist K, Sundquist J, Hemminki K, The impact of type 2 diabetes mellitus on cancer-specific survival: a follow-up study in Sweden, Cancer 118(5) (2012) 1353–61. [DOI] [PubMed] [Google Scholar]
- [11].De Bruijn KM, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CH, Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer, The British journal of surgery 100(11) (2013) 1421–9. [DOI] [PubMed] [Google Scholar]
- [12].Du W, Simon MS, Racial disparities in treatment and survival of women with stage I-III breast cancer at a large academic medical center in metropolitan Detroit, Breast cancer research and treatment 91(3) (2005) 243–8. [DOI] [PubMed] [Google Scholar]
- [13].Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH, Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients, Annals of oncology : official journal of the European Society for Medical Oncology / ESMO 24(10) (2013) 2506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lipscombe LL, Goodwin PJ, Zinman B, McLaughlin JR, Hux JE, The impact of diabetes on survival following breast cancer, Breast cancer research and treatment 109(2) (2008) 389–95. [DOI] [PubMed] [Google Scholar]
- [15].van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR, Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis, International journal of cancer. Journal international du cancer 120(9) (2007) 1986–92. [DOI] [PubMed] [Google Scholar]
- [16].Chen WW, Shao YY, Shau WY, Lin ZZ, Lu YS, Chen HM, Kuo RN, Cheng AL, Lai MS, The impact of diabetes mellitus on prognosis of early breast cancer in Asia, The oncologist 17(4) (2012) 485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Duggan C, Irwin ML, Xiao L, Henderson KD, Smith AW, Baumgartner RN, Baumgartner KB, Bernstein L, Ballard-Barbash R, McTiernan A, Associations of insulin resistance and adiponectin with mortality in women with breast cancer, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29(1) (2011) 32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pasanisi P, Berrino F, De Petris M, Venturelli E, Mastroianni A, Panico S, Metabolic syndrome as a prognostic factor for breast cancer recurrences, International journal of cancer. Journal international du cancer 119(1) (2006) 236–8. [DOI] [PubMed] [Google Scholar]
- [19].Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, Hood N, Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 20(1) (2002) 42–51. [DOI] [PubMed] [Google Scholar]
- [20].Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Taylor SK, Hood N, Insulin- and obesity-related variables in early-stage breast cancer: correlations and time course of prognostic associations, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 30(2) (2012) 164–71. [DOI] [PubMed] [Google Scholar]
- [21].Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J, Diabetes mellitus, fasting glucose, and risk of cause-specific death, The New England journal of medicine 364(9) (2011) 829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].He DE, Bai JW, Liu J, Du CW, Huang WH, Zhang GJ, Clinicopathological characteristics and prognosis of breast cancer patients with type 2 diabetes mellitus, Molecular and clinical oncology 3(3) (2015) 607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ritchie CS, Kvale E, Fisch MJ, Multimorbidity: an issue of growing importance for oncologists, Journal of oncology practice / American Society of Clinical Oncology 7(6) (2011) 371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Richardson LC, Pollack LA, Therapy insight: Influence of type 2 diabetes on the development, treatment and outcomes of cancer, Nature clinical practice. Oncology 2(1) (2005) 48–53. [DOI] [PubMed] [Google Scholar]
- [25].Sciacca L, Vigneri R, Tumminia A, Frasca F, Squatrito S, Frittitta L, Vigneri P, Clinical and molecular mechanisms favoring cancer initiation and progression in diabetic patients, Nutrition, metabolism, and cardiovascular diseases : NMCD 23(9) (2013) 808–15. [DOI] [PubMed] [Google Scholar]
- [26].Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D, Diabetes and cancer: a consensus report, Diabetes care 33(7) (2010) 1674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fox CS, Pencina MJ, Meigs JB, Vasan RS, Levitzky YS, D’Agostino RB, Trends in the Incidence of Type 2 Diabetes Mellitus From the 1970s to the 1990s, The Framingham Heart Study 113(25) (2006) 2914–2918. [DOI] [PubMed] [Google Scholar]
- [28].Wild S, Roglic G, Green A, Sicree R, King H, Global prevalence of diabetes: estimates for the year 2000 and projections for 2030, Diabetes care 27(5) (2004) 1047–53. [DOI] [PubMed] [Google Scholar]
- [29].van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B, The global burden of diabetes and its complications: an emerging pandemic, European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology 17 Suppl 1 (2010) S3–8. [DOI] [PubMed] [Google Scholar]
- [30].Hershey DS, Given B, Given C, Von Eye A, You M, Diabetes and cancer: impact on health-related quality of life, Oncology nursing forum 39(5) (2012) 449–57. [DOI] [PubMed] [Google Scholar]
- [31].Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P, Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments, Diabetes care 33(10) (2010) 2285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA, The health care costs of diabetic peripheral neuropathy in the US, Diabetes care 26(6) (2003) 1790–5. [DOI] [PubMed] [Google Scholar]
- [33].Egede LE, Zheng D, Independent factors associated with major depressive disorder in a national sample of individuals with diabetes, Diabetes care 26(1) (2003) 104–11. [DOI] [PubMed] [Google Scholar]
- [34].Ali S, Stone MA, Peters JL, Davies MJ, Khunti K, The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis, Diabetic medicine : a journal of the British Diabetic Association 23(11) (2006) 1165–73. [DOI] [PubMed] [Google Scholar]
- [35].de Groot M, Kushnick M, Doyle T, Merrill J, McGlynn M, Shubrook J, Schwartz F, Depression Among Adults With Diabetes: Prevalence, Impact, and Treatment Options, Diabetes spectrum : a publication of the American Diabetes Association 23(1) (2010) 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Drivsholm T, de Fine Olivarius N, Nielsen AB, Siersma V, Symptoms, signs and complications in newly diagnosed type 2 diabetic patients, and their relationship to glycaemia, blood pressure and weight, Diabetologia 48(2) (2005) 210–4. [DOI] [PubMed] [Google Scholar]
- [37].Fritschi C, Quinn L, Fatigue in patients with diabetes: a review, Journal of psychosomatic research 69(1) (2010) 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hershey DS, Given B, Given C, Corser W, von Eye A, Predictors of diabetes self-management in older adults receiving chemotherapy, Cancer nursing 37(2) (2014) 97–105. [DOI] [PubMed] [Google Scholar]
- [39].Tanabe Y, Hashimoto K, Shimizu C, Hirakawa A, Harano K, Yunokawa M, Yonemori K, Katsumata N, Tamura K, Ando M, Kinoshita T, Fujiwara Y, Paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer, International journal of clinical oncology 18(1) (2013) 132–8. [DOI] [PubMed] [Google Scholar]
- [40].Bhatnagar B, Gilmore S, Goloubeva O, Pelser C, Medeiros M, Chumsri S, Tkaczuk K, Edelman M, Bao T, Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: a single-center experience, SpringerPlus 3 (2014) 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, Natale RB, Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer, Chemotherapy research and practice 2012 (2012) 913848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wilson GC, Quillin RC 3rd, Hanseman DJ, Lewis JD, Edwards MJ, Shaughnessy EA, Incidence and predictors of neuropathic pain following breast surgery, Annals of surgical oncology 20(10) (2013) 3330–4. [DOI] [PubMed] [Google Scholar]
- [43].Ellis ME, Weiss RB, Korzun AH, Rice MA, Norton L, Perloff M, Lesnick GJ, Wood WC, Hyperglycemic complications associated with adjuvant chemotherapy of breast cancer. A cancer and leukemia group B (CALGB) study, American journal of clinical oncology 9(6) (1986) 533–6. [DOI] [PubMed] [Google Scholar]
- [44].Ericson-Neilsen W, Kaye AD, Steroids: pharmacology, complications, and practice delivery issues, The Ochsner journal 14(2) (2014) 203–7. [PMC free article] [PubMed] [Google Scholar]
- [45].Leak A, Davis ED, Houchin LB, Mabrey M, Diabetes management and self-care education for hospitalized patients with cancer, Clinical journal of oncology nursing 13(2) (2009) 205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE, Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes, The Journal of clinical endocrinology and metabolism 87(3) (2002) 978–82. [DOI] [PubMed] [Google Scholar]
- [47].American Diabetes Association, Standards of medical care in diabetes--2014, Diabetes care 37 Suppl 1 (2014) S14–80. [DOI] [PubMed] [Google Scholar]
- [48].Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR, Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study, BMJ 321(7258) (2000) 405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Heuson JC, Legros N, Heimann R, Influence of insulin administration on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in intact, oophorectomized, and hypophysectomized rats, Cancer research 32(2) (1972) 233–8. [PubMed] [Google Scholar]
- [50].Milazzo G, Sciacca L, Papa V, Goldfine ID, Vigneri R, ASPB10 insulin induction of increased mitogenic responses and phenotypic changes in human breast epithelial cells: evidence for enhanced interactions with the insulin-like growth factor-I receptor, Molecular carcinogenesis 18(1) (1997) 19–25. [DOI] [PubMed] [Google Scholar]
- [51].Shukla A, Grisouard J, Ehemann V, Hermani A, Enzmann H, Mayer D, Analysis of signaling pathways related to cell proliferation stimulated by insulin analogs in human mammary epithelial cell lines, Endocrine-related cancer 16(2) (2009) 429–41. [DOI] [PubMed] [Google Scholar]
- [52].Papa V, Belfiore A, Insulin receptors in breast cancer: biological and clinical role, Journal of endocrinological investigation 19(5) (1996) 324–33. [DOI] [PubMed] [Google Scholar]
- [53].Belfiore A, Frittitta L, Costantino A, Frasca F, Pandini G, Sciacca L, Goldfine ID, Vigneri R, Insulin receptors in breast cancer, Annals of the New York Academy of Sciences 784 (1996) 173–88. [DOI] [PubMed] [Google Scholar]
- [54].Mathieu MC, Clark GM, Allred DC, Goldfine ID, Vigneri R, Insulin receptor expression and clinical outcome in node-negative breast cancer, Proceedings of the Association of American Physicians 109(6) (1997) 565–71. [PubMed] [Google Scholar]
- [55].Vona-Davis L, Howard-McNatt M, Rose DP, Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer, Obesity reviews : an official journal of the International Association for the Study of Obesity 8(5) (2007) 395–408. [DOI] [PubMed] [Google Scholar]
- [56].Irwin ML, Duggan C, Wang CY, Smith AW, McTiernan A, Baumgartner RN, Baumgartner KB, Bernstein L, Ballard-Barbash R, Fasting C-peptide levels and death resulting from all causes and breast cancer: the health, eating, activity, and lifestyle study, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 29(1) (2011) 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rose DP, Vona-Davis L, The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression, Endocrine-related cancer 19(6) (2012) R225–41. [DOI] [PubMed] [Google Scholar]
- [58].Ferroni P, Riondino S, Buonomo O, Palmirotta R, Guadagni F, Roselli M, Type 2 Diabetes and Breast Cancer: The Interplay between Impaired Glucose Metabolism and Oxidant Stress, Oxidative medicine and cellular longevity 2015 (2015) 183928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hershey DS, Tipton J, Given B, Davis E, Perceived impact of cancer treatment on diabetes self-management, The Diabetes educator 38(6) (2012) 779–90. [DOI] [PubMed] [Google Scholar]
- [60].Calip GS, Hubbard RA, Stergachis A, Malone KE, Gralow JR, Boudreau DM, Adherence to oral diabetes medications and glycemic control during and following breast cancer treatment, Pharmacoepidemiology and drug safety 24(1) (2015) 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Scain SF, Friedman R, Gross JL, A structured educational program improves metabolic control in patients with type 2 diabetes: a randomized controlled trial, The Diabetes educator 35(4) (2009) 603–11. [DOI] [PubMed] [Google Scholar]
- [62].Hartz A, Kent S, James P, Xu Y, Kelly M, Daly J, Factors that influence improvement for patients with poorly controlled type 2 diabetes, Diabetes research and clinical practice 74(3) (2006) 227–32. [DOI] [PubMed] [Google Scholar]
- [63].Gold R, Yu K, Liang LJ, Adler F, Balingit P, Luc P, Hernandez J, Toro Y, Modilevsky T, Synchronous provider visit and self-management education improves glycemic control in Hispanic patients with long-standing type 2 diabetes, The Diabetes educator 34(6) (2008) 990–5. [DOI] [PubMed] [Google Scholar]
- [64].Patel KL, Impact of tight glucose control on postoperative infection rates and wound healing in cardiac surgery patients, Journal of wound, ostomy, and continence nursing : official publication of The Wound, Ostomy and Continence Nurses Society / WOCN 35(4) (2008) 397–404; quiz 405–6. [DOI] [PubMed] [Google Scholar]
- [65].Krinsley JS, Glycemic variability: a strong independent predictor of mortality in critically ill patients, Critical care medicine 36(11) (2008) 3008–13. [DOI] [PubMed] [Google Scholar]
- [66].Honish A, Westerfield W, Ashby A, Momin S, Phillippi R, Health-related quality of life and treatment compliance with diabetes care, Disease management : DM 9(4) (2006) 195–200. [DOI] [PubMed] [Google Scholar]
- [67].American Diabetes A, Standards of Medical Care in Diabetes-2016 Abridged for Primary Care Providers, Clin Diabetes 34(1) (2016) 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dunstan DW, Vulikh E, Owen N, Jolley D, Shaw J, Zimmet P, Community center-based resistance training for the maintenance of glycemic control in adults with type 2 diabetes, Diabetes care 29(12) (2006) 2586–91. [DOI] [PubMed] [Google Scholar]
- [69].Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults, Medicine and science in sports and exercise 41(2) (2009) 459–71. [DOI] [PubMed] [Google Scholar]
- [70].Cleeland CS, Ryan KM, Pain assessment: global use of the Brief Pain Inventory, Ann Acad Med Singapore 23(2) (1994) 129–38. [PubMed] [Google Scholar]
- [71].Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai J-S, Pilkonis P, Revicki D, Rose M, Weinfurt K, Hays R, The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008, Journal of Clinical Epidemiology 63(11) (2010) 1179–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cella D, Peterman A, Hudgens S, Webster K, Socinski MA, Measuring the side effects of taxane therapy in oncology, Cancer 98(4) (2003) 822–831. [DOI] [PubMed] [Google Scholar]
- [73].Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P, New medication adherence scale versus pharmacy fill rates in seniors with hypertension, The American journal of managed care 15(1) (2009) 59–66. [PMC free article] [PubMed] [Google Scholar]
- [74].Kimmick G, Edmond SN, Bosworth HB, Peppercorn J, Marcom PK, Blackwell K, Keefe FJ, Shelby RA, Medication taking behaviors among breast cancer patients on adjuvant endocrine therapy, Breast 24(5) (2015) 630–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Weinger K, Butler HA, Welch GW, La Greca AM, Measuring diabetes self-care: a psychometric analysis of the Self-Care Inventory-Revised with adults, Diabetes care 28(6) (2005) 1346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ashfield-Watt PA, Welch AA, Godward S, Bingham SA, Effect of a pilot community intervention on fruit and vegetable intakes: use of FACET (Five-a-day Community Evaluation Tool), Public Health Nutr 10(7) (2007) 671–80. [DOI] [PubMed] [Google Scholar]
- [77].Roe L, Strong C, Whiteside C, Neil A, Mant D, Dietary Intervention in Primary Care: Validity of the DINE Method for Diet Assessment, Family Practice 11(4) (1994) 375–381. [DOI] [PubMed] [Google Scholar]
- [78].Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P, International physical activity questionnaire: 12-country reliability and validity, Medicine and science in sports and exercise 35(8) (2003) 1381–95. [DOI] [PubMed] [Google Scholar]
- [79].Du H, Newton PJ, Salamonson Y, Carrieri-Kohlman VL, Davidson PM, A review of the six-minute walk test: its implication as a self-administered assessment tool, European journal of cardiovascular nursing : journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology 8(1) (2009) 2–8. [DOI] [PubMed] [Google Scholar]
- [80].ATS statement: guidelines for the six-minute walk test, American journal of respiratory and critical care medicine 166(1) (2002) 111–7. [DOI] [PubMed] [Google Scholar]
- [81].World Health Organization, Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation, WHO, Geneva, 2011. [PubMed] [Google Scholar]
- [82].Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK, Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients, Biomark Insights 11 (2016) 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lorig K, Chastain RL, Ung E, Shoor S, Holman HR, Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis, Arthritis and rheumatism 32(1) (1989) 37–44. [DOI] [PubMed] [Google Scholar]
- [84].Shelby RA, Edmond SN, Wren AA, Keefe FJ, Peppercorn JM, Marcom PK, Blackwell KL, Kimmick GG, Self-efficacy for coping with symptoms moderates the relationship between physical symptoms and well-being in breast cancer survivors taking adjuvant endocrine therapy, Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer (2014). [DOI] [PubMed] [Google Scholar]
- [85].Van Der Ven NC, Weinger K, Yi J, Pouwer F, Ader H, Van Der Ploeg HM, Snoek FJ, The confidence in diabetes self-care scale: psychometric properties of a new measure of diabetes-specific self-efficacy in Dutch and US patients with type 1 diabetes, Diabetes care 26(3) (2003) 713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Borkoveck TD, Nau SD, Credibility of Analogue Therapy Rationales, J Behav Ther Exp Psy 3(4) (1972) 257–260. [Google Scholar]
- [87].Devilly GJ, Borkovec TD, Psychometric properties of the credibility/expectancy questionnaire, Journal of behavior therapy and experimental psychiatry 31(2) (2000) 73–86. [DOI] [PubMed] [Google Scholar]
- [88].Keefe FJ, Shelby RA, Somers TJ, Varia I, Blazing M, Waters SJ, McKee D, Silva S, She L, Blumenthal JA, O’Connor J, Knowles V, Johnson P, Bradley L, Effects of coping skills training and sertraline in patients with non-cardiac chest pain: a randomized controlled study, Pain 152(4) (2011) 730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Oei TPS, Green AL, The Satisfaction With Therapy and Therapist Scale--Revised (STTS-R) for Group Psychotherapy: Psychometric Properties and Confirmatory Factor Analysis, Prof Psychol-Res Pr 39(4) (2008) 435–442. [Google Scholar]
- [90].Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr., Prognostic importance of comorbidity in a hospital-based cancer registry, JAMA : the journal of the American Medical Association 291(20) (2004) 2441–7. [DOI] [PubMed] [Google Scholar]
- [91].Bayliss EA, Ellis JL, Steiner JF, Subjective assessments of comorbidity correlate with quality of life health outcomes: initial validation of a comorbidity assessment instrument, Health and quality of life outcomes 3 (2005) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Fincke BG, Clark JA, Linzer M, Spiro A 3rd, Miller DR, Lee A, Kazis LE, Assessment of long-term complications due to type 2 diabetes using patient self-report: the diabetes complications index, The Journal of ambulatory care management 28(3) (2005) 262–73. [DOI] [PubMed] [Google Scholar]
- [93].Ogedegbe G, Schoenthaler A, Richardson T, Lewis L, Belue R, Espinosa E, Spencer J, Allegrante JP, Charlson ME, An RCT of the effect of motivational interviewing on medication adherence in hypertensive African Americans: rationale and design, Contemporary clinical trials 28(2) (2007) 169–81. [DOI] [PubMed] [Google Scholar]
- [94].Ogedegbe G, Harrison M, Robbins L, Mancuso CA, Allegrante JP, Barriers and facilitators of medication adherence in hypertensive African Americans: a qualitative study, Ethnicity & disease 14(1) (2004) 3–12. [PubMed] [Google Scholar]
- [95].Parker RM, Baker DW, Williams MV, Nurss JR, The test of functional health literacy in adults: a new instrument for measuring patients’ literacy skills, Journal of general internal medicine 10(10) (1995) 537–41. [DOI] [PubMed] [Google Scholar]
- [96].Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, Mockbee J, Hale FA, Quick assessment of literacy in primary care: the newest vital sign, Ann Fam Med 3(6) (2005) 514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Turner RJ, Wheaton B, Lloyd DA, The epidemiology of social stress, American Sociological Review 60 (1995) 104–125. [Google Scholar]
- [98].Conger RD, Wallace LE, Sun Y, Simons RL, McLoyd VC, Brody GH, Economic pressure in African American families: A replication and extension of the family stress model, Developmental Psychology 38(2) (2002) 179–193. [PubMed] [Google Scholar]
- [99].D. CR, Xiaojia G, H. EG, O. LF, L. SR, Economic Stress, Coercive Family Process, and Developmental Problems of Adolescents, Child Development 65(2) (1994) 541–561. [PubMed] [Google Scholar]
- [100].Conger RD, Elder GH Jr.,, Families in troubled times: Adapting to change in rural America, Aldine, Hillsdale, NJ, 1994. [Google Scholar]
- [101].Spilman SK, Peng L, Critcial Transitions Project G3 Technical Reports, FY14 2007–2008, in: Project FT (Ed.) Iowa State University, Ames, IA, 2009. [Google Scholar]
- [102].Fritz MS, Mackinnon DP, Required sample size to detect the mediated effect, Psychol Sci 18(3) (2007) 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Aiken LS, West SG, Multiple regression: Testing and interpretting interactions, Sage Publications, Newbury Park, CA, 1991. [Google Scholar]
- [104].Preacher KJ, Curran PJ, Bauer DJ, Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis, J Educ Behav Stat 31 (2006) 437–448. [Google Scholar]
- [105].Yuan Y, MacKinnon DP, Bayesian mediation analysis, Psychol Methods 14(4) (2009) 301–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V, A comparison of methods to test mediation and other intervening variable effects, Psychol Methods 7(1) (2002) 83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Mackinnon DP, Integrating mediators and moderators in research design, Res Soc Work Pract 21(6) (2011) 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].MacKinnon DP, Fairchild AJ, Fritz MS, Mediation analysis, Annu Rev Psychol 58 (2007) 593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Maxwell SE, Cole DA, Bias in cross-sectional analyses of longitudinal mediation, Psychol Methods 12(1) (2007) 23–44. [DOI] [PubMed] [Google Scholar]
- [110].Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC, The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010, Diabetes care 36(8) (2013) 2271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Gonzalez JS, Delahanty LM, Safren SA, Meigs JB, Grant RW, Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes, Diabetologia 51(10) (2008) 1822–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, Safren SA, Depression and diabetes treatment nonadherence: a meta-analysis, Diabetes care 31(12) (2008) 2398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gonzalez JS, Safren SA, Delahanty LM, Cagliero E, Wexler DJ, Meigs JB, Grant RW, Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes, Diabetic medicine : a journal of the British Diabetic Association 25(9) (2008) 1102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE, Depression and poor glycemic control: a meta-analytic review of the literature, Diabetes care 23(7) (2000) 934–42. [DOI] [PubMed] [Google Scholar]
- [115].Krein SL, Heisler M, Piette JD, Makki F, Kerr EA, The effect of chronic pain on diabetes patients’ self-management, Diabetes care 28(1) (2005) 65–70. [DOI] [PubMed] [Google Scholar]
- [116].Bayliss EA, Ellis JL, Steiner JF, Barriers to self-management and quality-of-life outcomes in seniors with multimorbidities, Ann Fam Med 5(5) (2007) 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Choe HM, Mitrovich S, Dubay D, Hayward RA, Krein SL, Vijan S, Proactive case management of high-risk patients with type 2 diabetes mellitus by a clinical pharmacist: a randomized controlled trial, The American journal of managed care 11(4) (2005) 253–60. [PubMed] [Google Scholar]
- [118].Casey D, De Civita M, Dasgupta K, Understanding physical activity facilitators and barriers during and following a supervised exercise programme in Type 2 diabetes: a qualitative study, Diabetic medicine : a journal of the British Diabetic Association 27(1) (2010) 79–84. [DOI] [PubMed] [Google Scholar]
- [119].Riva JJ, Wong JJ, Brunarski DJ, Chan AH, Lobo RA, Aptekman M, Alabousi M, Imam M, Gupta A, Busse JW, Consideration of chronic pain in trials to promote physical activity for diabetes: a systematic review of randomized controlled trials, PloS one 8(8) (2013) e71021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Bosworth HB, Granger BB, Mendys P, Brindis R, Burkholder R, Czajkowski SM, Daniel JG, Ekman I, Ho M, Johnson M, Kimmel SE, Liu LZ, Musaus J, Shrank WH, Whalley Buono E, Weiss K, Granger CB, Medication adherence: a call for action, American heart journal 162(3) (2011) 412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Vokes EE, Ratain MJ, Mick R, McEvilly JM, Haraf D, Kozloff M, Hamasaki V, Weichselbaum RR, Panje WR, Wenig B, et al. , Cisplatin, fluorouracil, and leucovorin augmented by interferon alfa-2b in head and neck cancer: a clinical and pharmacologic analysis, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 11(2) (1993) 360–8. [DOI] [PubMed] [Google Scholar]
- [122].Weiser MA, Cabanillas ME, Konopleva M, Thomas DA, Pierce SA, Escalante CP, Kantarjian HM, O’Brien SM, Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen, Cancer 100(6) (2004) 1179–85. [DOI] [PubMed] [Google Scholar]
- [123].Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB 3rd, Fuchs CS, Impact of diabetes mellitus on outcomes in patients with colon cancer, Journal of clinical oncology : official journal of the American Society of Clinical Oncology 21(3) (2003) 433–40. [DOI] [PubMed] [Google Scholar]
- [124].Psarakis HM, Clinical Challenges in Caring for Patients with Diabetes and Cancer, Diabetes Spectrum 19(3) (2006) 157–162. [Google Scholar]
- [125].Wildiers H, Highley MS, de Bruijn EA, van Oosterom AT, Pharmacology of anticancer drugs in the elderly population, Clinical pharmacokinetics 42(14) (2003) 1213–42. [DOI] [PubMed] [Google Scholar]
- [126].Verstappen CC, Heimans JJ, Hoekman K, Postma TJ, Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management, Drugs 63(15) (2003) 1549–63. [DOI] [PubMed] [Google Scholar]
- [127].Trief P, Sandberg JG, Ploutz-Snyder R, Brittain R, Cibula D, Scales K, Weinstock RS, Promoting couples collaboration in type 2 diabetes: The diabetes support project pilot data, Families, Systems, & Health 29(3) (2011) 253–261. [DOI] [PubMed] [Google Scholar]
- [128].Trief PM, Ploutz-Snyder R, Britton KD, Weinstock RS, The relationship between marital quality and adherence to the diabetes care regimen, Annals of Behavioral Medicine 27(3) (2004) 148–154. [DOI] [PubMed] [Google Scholar]
- [129].Miller TA, Dimatteo MR, Importance of family/social support and impact on adherence to diabetic therapy, Diabetes, metabolic syndrome and obesity 6 (2013) 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Shao Y, Liang L, Shi L, Wan C, Yu S, The Effect of Social Support on Glycemic Control in Patients with Type 2 Diabetes Mellitus: The Mediating Roles of Self-Efficacy and Adherence, Journal of Diabetes Research 2017 (2017) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Mayberry LS, Osborn CY, Family support, medication adherence, and glycemic control among adults with type 2 diabetes, Diabetes care 35(6) (2012) 1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Porter LS, Keefe FJ, Garst J, Baucom DH, McBride CM, McKee DC, Sutton L, Carson K, Knowles V, Rumble M, Scipio C, Caregiver-Assisted Coping Skills Training for Lung Cancer: Results of a Randomized Clinical Trial, Journal of pain and symptom management 41 (2011) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Abbasi M, Dehghani M, Keefe FJ, Jafari H, Behtash H, Shams J, Spouse-assisted training in pain coping skills and the outcome of multidisciplinary pain management for chronic low back pain treatment: A 1-year randomized controlled trial, Eur J Pain 16(7) (2012) 1033–1043. [DOI] [PubMed] [Google Scholar]
- [134].Keefe FJ, Blumenthal J, Baucom D, Affleck G, Waugh R, Caldwell DS, Beaupre P, Kashikar-Zuck S, Wright K, Egert J, Lefebvre J, Effects of spouse-assisted coping skills training and exercise training in patients with osteoarthritic knee pain: a randomized controlled study, Pain 110(3) (2004) 539–49. [DOI] [PubMed] [Google Scholar]
- [135].Lipscombe LL, Fischer HD, Yun L, Gruneir A, Austin P, Paszat L, Anderson GM, Rochon PA, Association between tamoxifen treatment and diabetes, Cancer 118(10) (2012) 2615–2622. [DOI] [PubMed] [Google Scholar]


