Abstract
Introduction
Diagnosis, treatment monitoring and assessment of desmopressin effect in haemophilia A patients are performed by measurement of factor VIII activity (FVIII). The two assays commonly applied are the one‐stage assay and the chromogenic assay. Especially in non‐severe haemophilia A, discrepancies between these assays are common. It is still unestablished which assay corresponds best with bleeding phenotype and desmopressin effect.
Aim
To correlate FVIII levels measured by the one‐stage assay and by the chromogenic assay with bleeding phenotype and, additionally, to compare FVIII assay discrepancies before and after desmopressin administration.
Method
Factor VIII was measured in 130 non‐severe haemophilia A patients during routine visits to the outpatient clinic and/or during desmopressin testing. FVIII was measured by both the one‐stage assay and the chromogenic assay. Discrepancies between assays were defined as at least a twofold difference of FVIII or an absolute FVIII difference between measurements of ≥0.10 IU/mL. Bleeding phenotype was defined as annual number of treated bleedings (adjusted ABR).
Results
Hundred and thirty non‐severe haemophilia A patients were included. In 31/130 patients, assay results were discrepant. However, FVIII measurements with both assays correlated adequately with adjusted ABR. In addition, in 27/130 patients FVIII measurements at baseline and after desmopressin administration were analysed. In 13/27 patients, all measurements were either equivalent or discrepant when results were compared. In 14/27 patients, this was not the case as both equivalent measurements and discrepant measurements at different time points within one patient were observed.
Conclusion
Neither the one‐stage assay nor the chromogenic assay is superior in predicting bleeding phenotype. In addition, equivalent or discrepant FVIII results measured before desmopressin do not always predict FVIII assay results after desmopressin administration.
Keywords: bleeding phenotype, chromogenic assay, desmopressin, factor VIII, haemophilia A, one‐stage assay
1. INTRODUCTION
Haemophilia A is a rare X‐linked hereditary bleeding disorder characterized by a deficiency in coagulation factor VIII (FVIII). When haemophilia A is diagnosed, its severity is subsequently categorized according to FVIII activity: severe is defined as FVIII < 0.01 IU/mL, moderate as FVIII ≥ 0.01‐0.05 IU/mL and mild haemophiliac as FVIII > 0.05 IU/mL. Categorization according to severity plays a role in predicting bleeding risk and in determining treatment strategy. Measurement of FVIII activity is also of importance during treatment monitoring of either FVIII concentrate or desmopressin.
The most commonly used methods to measure FVIII are the one‐stage assay (OSA) and the chromogenic assay (CSA). 1 The OSA measures the ability of patient plasma to correct the activated partial thromboplastin time (APTT) of FVIII‐deficient plasma. The CSA consists of two stages. During the first stage, factor X is converted to activated factor X (FXa) by activated factor IX with FVIII as rate limiting factor. During the second stage, the generated FXa concentration is measured by a chromogenic substrate which is proportional to the FVIII concentration. 1 , 2
Factor VIII discrepancies between both assays, generally defined as at least a twofold difference in FVIII measurement, have been demonstrated in 15%‐50% of non‐severe haemophilia A patients. 1 , 3 Several studies have shown that discrepancies are associated with specific F8 mutations. In general, OSA measures higher FVIII levels than CSA when missense mutations are clustered in F8‐gene A1‐A2‐A3 domain interfaces. 1 , 2 , 4 , 5 Additionally, CSA is reported to measure higher FVIII levels when F8 mutations are clustered around the thrombin cleavage and factor IX binding sites. 1 Studies investigating correlations between both assays and bleeding phenotype report conflicting results. Some studies find CSA superior to OSA, whereas others show that regardless of assay type lowest measured FVIII level is most predictive of bleeding. 2 , 3 , 6
Factor VIII discrepancies between both assays after FVIII concentrate treatment have also been investigated. These reports conclude that some FVIII concentrates are best monitored by the CSA. 1 Only one study reports FVIII assay results after desmopressin administration. In this small case series, five patients with higher baseline FVIII results when measured by the OSA than when measured by the CSA were analysed at 1, 2 and 4 hours after desmopressin administration. FVIII increase was relatively lower when measured by the OSA 1 hour after desmopressin than measured by the CSA, leading to a decrease in relative differences between the two assays. However, this effect was not seen any longer at 2 and 4 hours after desmopressin infusion. 7 As desmopressin effect is tested in all haemophilia A patients, before it is used for treatment and usually measured by only one assay in clinical settings, these findings warrant further research as results may have major implications for therapeutic management.
Hence, we aimed to investigate the correlation between FVIII levels measured by OSA and CSA and bleeding phenotype. In addition, prevalence of FVIII assay discrepancies at baseline and after desmopressin administration was analysed.
2. METHODS
This study was a single‐centre retrospective cohort study. The study was not subject to the Medical Research Involving Human Subjects Act (WMO) and received a waiver from review by the Medical Ethics Committee of the Erasmus University Medical Centre Rotterdam, the Netherlands.
2.1. Patient population
All non‐severe haemophilia A patients with FVIII ≥ 0.01 as measured by the OSA, who routinely visited the outpatient clinic of the Erasmus University Medical Centre between 1 September 2011 and 31 March 2018 were screened for study inclusion. If residual plasma was stored, or both OSA and CSA FVIII measurements were available of the same blood sample, patients were included in this analysis. Only the first set of available FVIII measurements or blood samples was used to attain study data. Patients were excluded if they had received prophylactic treatment at the time of FVIII measurements.
In a subgroup of patients, FVIII was measured with both assays before and after intravenous desmopressin infusion. These patients were only included if FVIII measurements at two or more time points were available. In these patients, informed consent was obtained. 8 Blood sample sets from separate dates could be used for baseline measurement analyses and analyses during desmopressin testing when a baseline measurement was performed earlier than the desmopressin administration.
Data collection included patient characteristics, eg age, blood type and inhibitor presence (BU > 0.3).
2.2. FVIII assays
Factor VIII measurements were either collected from medical files retrospectively or tested using residual plasma samples stored in −80°C. For the OSA, the following settings were used, depending on the measurement date. From September 2011 until approximately May 2012, Sysmex CA1500 coagulation analyser with Triniclot reagent (TCoag, Kordia) (day‐to‐day variation of 3.8%) was used. Reference plasma was Cryocheck Reference plasma; FVIII‐deficient plasma was used from Kordia. From approximately May 2012 to March 2018 the Sysmex CS5100 coagulation analyser was used with Actin FS (Siemens) (day‐to‐day variation 3.7%). Reference plasma was Cryocheck Reference plasma until March 2015. On 13th of March 2015, reference plasma was altered to Standard Human Plasma (Siemens). FVIII‐deficient plasma was used from Siemens. For the CSA, the Sysmex CS5100 coagulation analyser was used in combination with the Biophen FVIII:C kit (Hyphen) (day‐to‐day variation 1.1% in abnormal control). The reference plasma was Standard Human Plasma (Siemens).
2.3. Assessment of bleeding phenotype
For bleeding, number of treated bleeding events occurring between 1 January 2011 and 1 January 2015 was calculated and divided by the number of follow‐up years. This ratio will be referred to as adjusted annual bleeding rate (a‐ABR). Treatment was defined as administration of factor VIII concentrate, bypassing agents, desmopressin and/or the need for (surgical) medical intervention. Treatment with tranexamic acid only was not included, as this intervention is often applied to prevent (further) bleeding.
2.4. Desmopressin administration and response classification
Desmopressin was administered intravenously in a standard dose of 0.3 μg/kg. FVIII measurements were measured before desmopressin administration and one, three or four, six and/or 24 hours after administration. Response 1 hour after desmopressin infusion was categorized according to FVIII levels: FVIII ≥ 0.50 IU/mL was a complete response, FVIII ≥ 0.30 IU/mL and <0.50 IU/mL a partial response and FVIII < 0.30 IU/mL no response, according to Stoof et al. 9
2.5. FVIII assay discrepancies
Factor VIII assay discrepancy was defined as at least a twofold difference in FVIII as measured by OSA and CSA, as used in previous studies. 1 , 2 However, as patients with higher FVIII levels (>0.10 IU/mL) are obliged to demonstrate a larger absolute difference to meet this criterion, an additional criterion was added to the definition. Assay results were also considered discrepant if absolute FVIII difference was ≥0.10 IU/mL, as this may lead to clinically relevant alterations in therapeutic management both with regard to diagnosis and classification of disease severity (baseline FVIII), as well as have implications for treatment strategy and/or classification in response categories (1 hour after desmopressin).
2.6. Statistical analyses
Demographic data are presented as medians with interquartile ranges. The difference between FVIII measured by the OSA and the CSA was tested with a paired Wilcoxon signed‐rank test. The association between FVIII level and a‐ABR was analysed by Spearman correlation testing with a significance level of P < .05. To see whether differences in FVIII results between assays were similar before and after the administration of desmopressin, relative differences (ratio OSA/CSA) and absolute differences (OSA minus CSA) were tested at all different time points with a Skillings‐Mack test. 10
3. RESULTS
One hundred thirty non‐severe haemophilia A patients were included. In 27/130 patients, FVIII assay results after desmopressin were available. Median age at first FVIII measurement was 45 years (range 17‐91 years; n = 130). All demographic data are depicted in Table 1.
TABLE 1.
Patient characteristics
| Age (y) | N = 130 | Median [range] | 45 | [17‐91] |
| Blood type O a | N = 123 | N [%] | 56 | [45.5] |
| FVIII at baseline – OSA | N = 130 | Median [IQR] | 0.10 | [0.05‐0.19]* |
| FVIII at baseline – CSA | N = 130 | Median [IQR] | 0.13 | [0.09‐0.22]* |
| Other bleeding disorders | N = 130 | |||
| VWD | N [%] | 4 | [3.1] | |
| Thrombocytopenia | N [%] | 1 | [0.8] | |
| Unknown | N [%] | 1 | [0.8] | |
| Oral anticoagulant medication | N = 130 | |||
| Vitamin K antagonist | N [%] | 1 | [0.8] | |
| Platelet aggregation inhibitor | N [%] | 2 | [1.5] | |
| Inhibitor measurements | N = 126 | |||
| Titre > 0.3 BU b | N [%] | 7 | [5.6] |
Blood type O refers to the ABO blood type system.
Abbreviations: FVIII‐CSA, FVIII measured by the chromogenic assay; FVIII‐OSA, FVIII measured by the one‐stage assay; VWD, von Willebrand disease.
Seven missing.
Positive inhibitor measurement at time of first FVIII measurements; only measured if patient ever had a positive inhibitor test; in four patients inhibitors were never tested.
Significant difference, P < .001.
3.1. Baseline FVIII measurements (N = 130)
At baseline, median FVIII with the OSA was 0.10 IU/mL [IQR 0.05‐0.19 IU/mL]. Median FVIII with the CSA was 0.13 IU/mL [IQR 0.09‐0.22 IU/mL]. The CSA showed significantly higher results than the OSA (P < .001, related samples Wilcoxon signed‐rank test).
In total, at baseline 31/130 patients (23.8%) showed discrepant results between assays according to at least one criterion (Figure 1). A discrepancy according to both criteria was seen in three patients. In 1/3 FVIII was higher according to the OSA (0.62 vs 0.15 IU/mL); in 2/3 FVIII was higher according to the CSA (0.45 vs 0.19 IU/mL; 0.30 vs 0.13 IU/mL). In 28/130 patients, only one criterion was met. A twofold discrepancy was seen in 22 patients; all had higher FVIII according to the CSA. Six patients presented with only an absolute FVIII assay difference ≥0.10 IU/mL; in 5/6 patients FVIII measured by the OSA was higher and in 1/6 FVIII measured with the CSA was higher. In the majority of patients (73.7%), severity of haemophiliac would have been categorized identically based on FVIII levels as measured by both assays (Figure 1 and Table 2).
FIGURE 1.
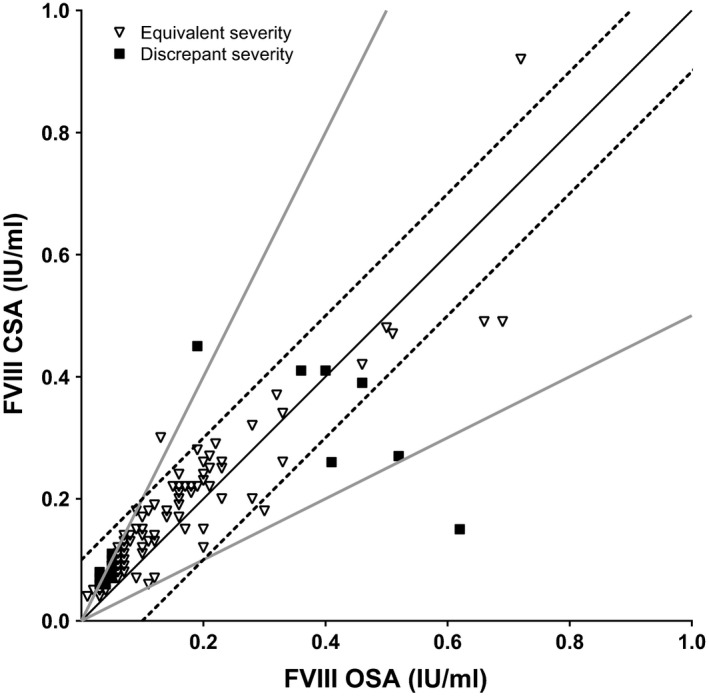
Baseline FVIII measurements. Baseline FVIII measured by the one‐stage assay (OSA) and by the chromogenic assay (CSA) (N = 130). Given are the line of unity (solid black line), the limits of twofold discrepancy (solid grey lines) and the limits of an absolute difference ≥0.10 IU/mL (dashed lines). In addition, agreement in categorical haemophilic severity is given: equivalent patients (open triangles; same severity category according to both assays) and discrepant patients (solid squares; different severity category according to different assays)
TABLE 2.
Differences in classification of haemophilia A severity
| Severity of haemophilia A according to the one‐stage assay | Severity of haemophilia A according to the chromogenic assay | |||
|---|---|---|---|---|
| Moderate | Mild | FVIII > 0.40 | Total | |
| Moderate | 6 | 28 | 0 | 34 |
| Mild | 0 | 83 | 3 | 86 |
| FVIII > 0.40 | 0 | 4 | 6 | 10 |
| Total | 6 | 115 | 9 | 130 |
Moderate haemophiliac: FVIII 0.01‐0.05 IU/mL; Mild haemophiliac: FVIII > 0.05‐0.40 IU/mL.
3.2. FVIII assays and bleeding phenotype (N = 126)
Adjusted ABR (a‐ABR) was calculated in 126 patients, as no bleeding event data were available in four patients. The number of years available for analyses differed between patients and varied from 1 to 5 years. Median a‐ABR was 0.25 bleedings per year (IQR 0‐0.80). An overview of bleeding types and FVIII levels is given in Table S1a,b. FVIII levels measured by the OSA, and by the CSA and lowest FVIII level measured by both assays showed a significant negative correlation with a‐ABR, meaning a‐ABR was significantly higher in patients with lower FVIII measurements (Figure 2).
FIGURE 2.
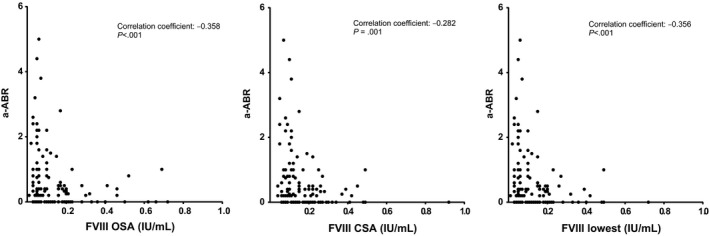
Correlations between FVIII measurements and adjusted annual bleeding rate for treated bleeding (a‐ABR). Spearman correlations between annual bleeding rate for treated bleedings (adjusted ABR; a‐ABR) and baseline FVIII measured by one‐stage assay (FVIII OSA), and by the chromogenic assay (FVIII CSA) or the lowest measurement as tested by both assays (FVIII lowest)
3.3. Assay discrepancies before and after desmopressin administration (N = 27)
Twenty‐seven patients had FVIII measured after desmopressin administration. Four of them had discrepant assay results before desmopressin infusion. One patient was discrepant according to both definitions and three only according to the absolute difference between assays. Number of measurements per time point are given in Table S2. As only one patient had FVIII measured at T4 instead of T3, these time points were taken together and depicted at T3 in all graphs. Differences between assays were different between time points (Figure 3), as absolute and relative differences between the OSA and the CSA varied significantly between time points (P = .002 and P = .005, respectively).
FIGURE 3.
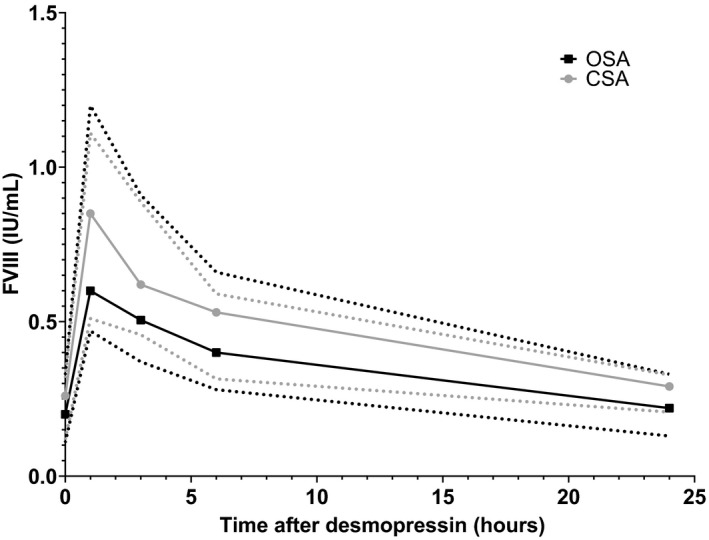
FVIII measurements before and after desmopressin administration. FVIII measurements measured by the one‐stage assay (black) and by the chromogenic assay (grey). T0 is before the administration of desmopressin, all other time points are after the end of desmopressin infusion. Depicted are the median (solid lines) and interquartile range (dotted lines)
More specifically, 13/27 (48.1%) patients showed only discrepant (3/13) or only equivalent measurements (10/13). The three patients with only discrepant results include the patient with a discrepancy at baseline according to both assays. However, in this patient a twofold difference was not observed at 1 and 3 hours after desmopressin infusion. In 14/27 patients, there was no consistency in differences and/or agreements between both assays during desmopressin tests. In 1/14 patients, FVIII was discrepant at baseline according to absolute difference, but one of two FVIII measurements after desmopressin showed equivalent results (T3). In the other 13/14 patients, baseline FVIII was equivalent, but at least one FVIII after desmopressin showed a discrepant result. In one patient, FVIII was discrepant according to both definitions 6 hours after desmopressin. In one patient, a twofold difference in FVIII between assays was present 24 hours after desmopressin. In the other 11 patients, absolute difference in FVIII between assays was ≥0.10 IU/mL at one to four measurements after desmopressin. In 13 of these 14 patients, FVIII measurements 1 hour after desmopressin were available. In 4/13 patients, differences in results may have led to a different use of desmopressin as results led to classification into different response categories of the desmopressin test when measured by different assays (Table S3). See Figure S1 for an overview of all 27 patients.
4. DISCUSSION
The aim of our study was to determine correlation of the one‐stage assay (OSA) and the chromogenic assay (CSA) with bleeding phenotype. Moreover, we aimed to investigate FVIII assay discrepancies before and after desmopressin testing as this has only been studied in one short report in a small patient sample. Most important findings were that FVIII measured by both the OSA and the CSA showed a clear correlation with bleeding phenotype, defined as number of treated bleedings per year (adjusted ABR; a‐ABR). In addition, we showed that discrepancy or equivalence between both FVIII assays before desmopressin administration is not predictive for discrepancy or equivalence of FVIII assays after desmopressin administration, as more than half of patients showed dissimilar results.
We believe these findings have clear implications for management of non‐severe haemophilia A patients. Firstly, differences in disease severity due to assay discrepancies may lead to different treatment strategies, such as initiation of prophylaxis, treatment with higher or lower FVIII concentrate doses or whether desmopressin is used. Therefore, choice of assay should be based on best correlation with clinical outcome. Our results demonstrate that fortunately both FVIII assays are associated with a‐ABR. Contrastingly, however, previous studies have reported better associations of the CSA with bleeding phenotype and that patients with higher FVIII levels according to the CSA, when compared to the OSA, do not experience spontaneous bleeding. 6 , 11 However, it is important to realize that different findings may be due to varying study approaches. In our analyses, patients with and without a FVIII assay discrepancy were included. In contrast, Cid et al only analysed patients with discrepant results while Bowyer et al only included patients with one specific F8 mutation. Although FVIII measurements by both the OSA and the CSA in each individual patient may be a solution, costs and assay availability will often necessitate a choice between assays. Additionally, an assay that is able to predict bleeding phenotype correctly in all patients may prevent patients from receiving unnecessary treatment. Until such a test is developed, in resource‐rich countries it is recommended to maintain testing with both assays to diagnose and monitor non‐severe haemophilia A patients due to reported discrepant results and therefore uncertainty of outcome.
The variation in assay discrepancies during desmopressin testing as we have reported is clearly relevant as applicability of desmopressin is determined by the achieved FVIII levels by an individual. In the study by Okoye et al, two of the five patients presented with discrepant FVIII measurements before desmopressin administration. However, these two patients showed no FVIII assay discrepancies 1 hour after desmopressin. 7 When classifying patients according to desmopressin response measured by the OSA or the CSA in our study, regularly patients were classified differently. Okoye et al also demonstrated that one patient would be classified otherwise according to assay results 1 hour after desmopressin. However, study inclusion criteria differed significantly from our study as patients all had discrepant FVIII assay results before desmopressin administration, with OSA measurements higher than CSA measurements. 7 In our study, we included patients with and without discrepancies before desmopressin administration and both patients with higher FVIII when measured by the OSA as well as the CSA. Moreover, not all FVIII assay discrepancies after desmopressin were reflected by discrepancies before desmopressin administration. Therefore, our findings suggest that desmopressin effect in patients is both underestimated and/or overestimated if only one FVIII assay is utilized. Underestimation of response may incorrectly dismiss desmopressin as a treatment option and may lead to overuse of FVIII concentrate. Inversely, overestimation of desmopressin effect may increase bleeding risk as desmopressin will be applied in patients without a sufficient FVIII response. As prevention and treatment of bleeding are the main priority in clinical settings, we underline the general opinion that lowest FVIII results independent of assay should be applied to determine treatment strategy. However, future research should focus on associations between FVIII assay results, and clinical outcome or development of alternative laboratory tests that measure haemostatic potential more than coagulation factor levels.
Multiple definitions for discrepancies are used, although all previous studies used a relative difference between assays. 1 , 3 Although this definition suffices in patients with low FVIII, clinically relevant differences may be missed in patients with higher FVIII levels. Therefore, we introduced a second definition of discrepancy, based on absolute difference. We chose a cut‐off of 0.10 IU/mL as we believe this is realistic and has clinical relevance. For example, when calculating a FVIII concentrate dose for an average man of 80 kg, this would mean a difference in dose of 400 IU (0.10/0.02*80) per infusion. In our study, this definition was also shown to be clinically relevant during desmopressin testing as one of three patients with only an absolute discrepancy at baseline fell in a different response category according to the different assays 1 hour after desmopressin infusion. Therefore, we believe, next to relative discrepancies, absolute discrepancies should be taken into account.
A few limitations were present in this study. Firstly, minor bleedings may have been missed due to underreporting of bleeding events due to data collection from medical files. However, as only treated bleedings, necessitating medical care, eg desmopressin, FVIII concentrate or medical interventions, were included, they were likely to have been reported directly or retrospectively. On the other hand, a‐ABR may have been overestimated in some patients as they may have skipped a regular visit to the outpatient clinic in cases of no bleeding, leading to a year of unanalysed missing data. The fact that we have applied treated bleedings and a‐ABR as an end point may also have biased results as our treatment centre is inclined to treat according to lowest FVIII assay result and therefore lowest result independent of assay may by definition correlate with bleeding phenotype as described by a‐ABR.
Furthermore, FVIII was significantly higher in our series when measured by the CSA than by the OSA. This may have been a coincidence, but could also be due to other reasons. Firstly, all included patients were diagnosed using the one‐stage assay. Therefore, we have potentially missed a possible group of patients with normal FVIII levels according to the one‐stage assay, but FVIII deficiency according to the chromogenic assay. Secondly, the difference could be due to calibration of the two assays. As no causes for this observation have been identified, this may be a potential bias, influencing study results. Especially in patients with low baseline FVIII, number of twofold discrepancies may have been overestimated by this observation. Finally, 13 patients had a FVIII of >0.40 IU/mL according to one or both assays. However, all patients in our study with FVIII > 0.40 IU/mL either had a previous FVIII ≤ 0.40 IU/mL or a proven F8‐gene mutation.
Overall, our study contributes to the improvement of treatment of non‐severe haemophilia A patients. We have demonstrated that both the OSA and the CSA correlate with bleeding phenotype. However, as previous studies report different results, additional studies are necessary to confirm our findings and to further evaluate clinical impact of discrepant assay results after desmopressin, such as prolonged or unexpected (surgical) bleeding.
5. CONCLUSION
No evidence was found in this study that either the one‐stage assay (OSA) or the chromogenic assay (CSA) was superior over the other in predicting bleeding phenotype. In addition, we demonstrate that equivalent or discrepant FVIII results measured by the OSA or the CSA before desmopressin administration are not able to predict results after desmopressin administration. Therefore, our data show there is no superiority and non‐inferiority of the OSA above the CSA or vice versa, in the care and management of non‐severe haemophilia A patients.
DISCLOSURES
L. M. Schütte, L.S Hodes and SCM Stoof have no conflicts of interest. FWG Leebeek received research funding from CSL Behring and Shire outside the submitted work. He is a consultant for UniQure and Shire, of which fees go to the institution. He is DSMB member for a study of Roche. M. H. Cnossen has received grants from governmental and societal research institutes such as NWO, ZonMW, Innovation fund, from private funds, institutional grants and unrestricted investigator research grants/educational and travel funding from the following companies over the years: Pfizer, Baxter/ Baxalta/ Shire, Bayer Schering Pharma, CSL Behring, Sobi Biogen, Novo Nordisk, Novartis and Nordic Pharma, and has served as a member on steering boards of Roche and Bayer. All grants, awards and fees go to the institution. MPM de Maat received research support or speaker fees from Siemens, Roche, Stago and Werfen. M. J. H. A. Kruip received grants from governmental and societal research institutes such as NWO, ZonMW, Innovation fund, institutional grants and unrestricted investigator research grants from Pfizer, Daiichi Sankyo, Boerhinger Ingelheim and research plus speakers fee from Bayer. All grants, awards and fees go to the institution.
AUTHOR CONTRIBUTIONS
LS designed and organized the study, collected and analysed data and wrote the manuscript; LH collected and analysed data and critically revised the manuscript; IM and SS critically revised the manuscript; MC, FL, MdM, and MK designed the study and critically revised the manuscript.
Supporting information
Figure S1
Table S1‐S3
Schütte LM, Hodes LS, van Moort I, et al. The one‐stage assay or chromogenic assay to monitor baseline factor VIII levels and desmopressin effect in non‐severe haemophilia A: Superiority or non‐inferiority? Haemophilia. 2020;26:916–922. 10.1111/hae.14106
REFERENCES
- 1. Potgieter JJ, Damgaard M, Hillarp A. One‐stage vs. chromogenic assays in haemophilia A. Eur J Haematol. 2015;94(Suppl 77):38‐44. [DOI] [PubMed] [Google Scholar]
- 2. Peyvandi F, Oldenburg J, Friedman KD. A critical appraisal of one‐stage and chromogenic assays of factor VIII activity. J Thromb Haemost. 2016;14:248‐261. [DOI] [PubMed] [Google Scholar]
- 3. Bowyer AE, Duncan EM, Antovic JP. Role of chromogenic assays in haemophilia A and B diagnosis. Haemophilia. 2018;24(4):578‐583. [DOI] [PubMed] [Google Scholar]
- 4. Provaznikova D, Houskova K, Radovska A, Salaj P, Hrachovinova I. Novel mutations associated with a discrepancy between one‐stage and chromogenic FVIII activity assays. Haemophilia. 2015;21:e330‐e332. [DOI] [PubMed] [Google Scholar]
- 5. Pavlova A, Delev D, Pezeshkpoor B, Muller J, Oldenburg J. Haemophilia A mutations in patients with non‐severe phenotype associated with a discrepancy between one‐stage and chromogenic factor VIII activity assays. Thromb Haemost. 2014;111:851‐861. [DOI] [PubMed] [Google Scholar]
- 6. Cid AR, Calabuig M, Cortina V, et al. One‐stage and chromogenic FVIII: C assay discrepancy in mild haemophilia A and the relationship with the mutation and bleeding phenotype. Haemophilia. 2008;14:1049‐1054. [DOI] [PubMed] [Google Scholar]
- 7. Okoye HC, Nielsen BI, Lee K, Abajas YL, Key NS, Rollins‐Raval MA. DDAVP trial in discrepant non‐severe haemophilia A patients. Haemophilia. 2018;24:e152‐e154. [DOI] [PubMed] [Google Scholar]
- 8. Stoof SC, Cnossen MH, de Maat MP, Leebeek FW, Kruip MJ. Side effects of desmopressin in patients with bleeding disorders. Haemophilia. 2016;22:39‐45. [DOI] [PubMed] [Google Scholar]
- 9. Stoof SCM, Schutte LM, Leebeek FWG, Cnossen MH, Kruip M. Desmopressin in haemophilia: the need for a standardised clinical response and individualised test regimen. Haemophilia. 2017;23:861‐867. [DOI] [PubMed] [Google Scholar]
- 10. Skillings JH, Mack GA. On the use of a friedman‐type statistic in balanced and unbalanced block designs. Technometrics. 1981;23:171‐177. [Google Scholar]
- 11. Bowyer AE, Goodeve A, Liesner R, Mumford AD, Kitchen S, Makris M. p.Tyr365Cys change in factor VIII: haemophilia A, but not as we know it. Br J Haematol. 2011;154:618‐625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1‐S3


