Abstract
Objective
Autologous stem cell transplantation (ASCT) has improved progression‐free survival (PFS) and overall survival in eligible patients with newly diagnosed multiple myeloma (NDMM); however, relapse occurs. Maintenance therapy with lenalidomide (Len‐Mt) extends survival and delays relapse and the subsequent initiation of costly second‐line regimens. Here, we report the cost‐effectiveness of Len‐Mt following ASCT from a Dutch healthcare service perspective.
Methods
A partitioned survival model was developed to assess the lifetime costs and benefits for patients with NDMM. Efficacy was taken from a pooled meta‐analysis of clinical trial data. Costs and subsequent therapy data were taken from sources appropriate for the Dutch market.
Results
Lenalidomide produced a quality‐adjusted life year gain of 2.46 and a life year gain of 2.79 vs no maintenance treatment. The cost of lenalidomide was partially offset by savings of EUR 77 462 in subsequent treatment costs. The incremental cost‐effectiveness ratio of Len‐Mt vs no maintenance treatment was EUR 30 143. Key model drivers included subsequent therapies, dosing schedule, and time horizon.
Conclusion
Lenalidomide is cost‐effective after ASCT vs no maintenance therapy in the Netherlands. By extending PFS, lenalidomide delays the cost burdens associated with relapse and subsequent treatment lines.
Keywords: cost‐effectiveness, lenalidomide, maintenance treatment, multiple myeloma
Novelty Statements.
1. What is the NEW aspect of your work?
This is the first examination of cost‐effectiveness of lenalidomide as maintenance therapy following ASCT in patients with newly diagnosed multiple myeloma from a Dutch healthcare perspective.
2. What is the CENTRAL finding of your work?
When compared with no maintenance therapy, lenalidomide is cost‐effective as a maintenance treatment for newly diagnosed multiple myeloma.
3. What is (or could be) the SPECIFIC clinical relevance of your work?
The cost burdens associated with relapse and subsequent treatment lines can be delayed by using lenalidomide maintenance to extend progression‐free survival.
1. INTRODUCTION
Maintenance treatment with the immunomodulatory drug lenalidomide has improved progression‐free survival (PFS) vs no maintenance therapy (no‐Mt) in four randomized trials in patients with newly diagnosed multiple myeloma (NDMM) who have undergone autologous stem cell transplantation (ASCT). 1 , 2 , 3 , 4 A meta‐analysis of pivotal trial data demonstrated that lenalidomide maintenance treatment (Len‐Mt) more than doubled median PFS vs no‐Mt (52.8 vs 23.5 months; hazard ratio [HR] 0.48; 95% confidence interval [CI] 0.41‐0.55; log‐rank P = .001). In addition to increased PFS, Len‐Mt extended the duration between initial and second‐line antimyeloma treatment (HR 0.57; 95% CI 0.49‐0.66). 5 This meta‐analysis also reported the median overall survival (OS), after a median follow‐up of 88.8 months, was 111.0 months for Len‐Mt, compared with 86.9 months for no‐Mt. 5 Lenalidomide is currently the only regulatory approved maintenance agent and is recognized as the standard of care in the post‐ASCT maintenance setting by national and international guidelines. 6 , 7
Despite the potential for prolonged response by following ASCT treatment with maintenance therapy, no current multiple myeloma (MM) treatment is curative. 8
From 1990 to 2016, incident cases of MM increased by 96% in Western Europe. 9 In the Netherlands, the 2017 projected incidence (crude rate) of MM for people aged 65‐70 years was 23.46 per 100 000. 10 Improvements in OS outcomes, and an aging population, are likely to increase the prevalence of myeloma. For this growing cohort of NDMM patients, sustaining response and extending the time until disease progression post‐ASCT are important clinical goals. There is also an increasing need to demonstrate novel treatment approaches are not only safe and effective but also cost‐effective. A healthcare cost‐impact analysis study in Europe has shown Len‐Mt reduces overall direct medical costs across the first 5 years post‐ASCT. 11 As no published study has considered the cost‐effectiveness of Len‐Mt for NDMM post‐ASCT from a Dutch perspective, our objective was to evaluate the cost‐effectiveness of Len‐Mt vs no‐Mt for patients with transplant‐eligible NDMM, from a Dutch healthcare perspective.
2. METHODS
2.1. Data sources
A systematic search was conducted to identify investigator‐initiated randomized controlled trials (RCTs) for inclusion in a meta‐analysis of outcomes for lenalidomide vs placebo. The inclusion criteria were: RCT in NDMM post‐ASCT; a Len‐Mt arm and a placebo/no‐Mt control arm; achievement of final database lock for the prespecified primary efficacy analysis; and availability of patient‐level data. Hence, the clinical effectiveness and safety data for lenalidomide and no‐Mt included in the economic model for the base case were derived from a pooled meta‐analysis of three RCTs (Table S1):
The Intergroupe Francophone du Myélome (IFM) study: IFM 2005‐02 (NCT00430365) 1
The Cancer and Leukemia Group B (CALGB) study: CALGB 100104 (NCT00114101) 2
The Gruppo Italiano Malattie EMatologiche dell'Adulto (GIMEMA) study: GIMEMA RV‐MM‐PI‐209 (NCT00551928) 3
These independently conducted trials varied in patient population, lenalidomide dosing, and follow‐up duration. The treatment effect of lenalidomide on OS varied between studies, whereas PFS results were relatively consistent (Table S2). 5
2.2. Patients
Simple pooling of the trials was applied; characteristics were broadly similar (72‐80% of patients in the HOVON study 12 had International Staging System (ISS) stage I/II at randomization, compared with 70.36% of patients included in our pooled analyses [Table S3]). This suggests that the pooled meta‐analysis patient characteristics were representative of a Dutch NDMM post‐ASCT population.
2.3. Model structure
A partitioned survival model structure, constructed in Microsoft Excel, was selected for this cost‐utility analysis.
The model structure (Figure 1) has six health states: preprogression (on maintenance treatment and off treatment); postprogression survival (off treatment and progressed, ie, presecond‐line treatment, second‐line treatment, and postsecond‐line treatment); and death.
FIGURE 1.
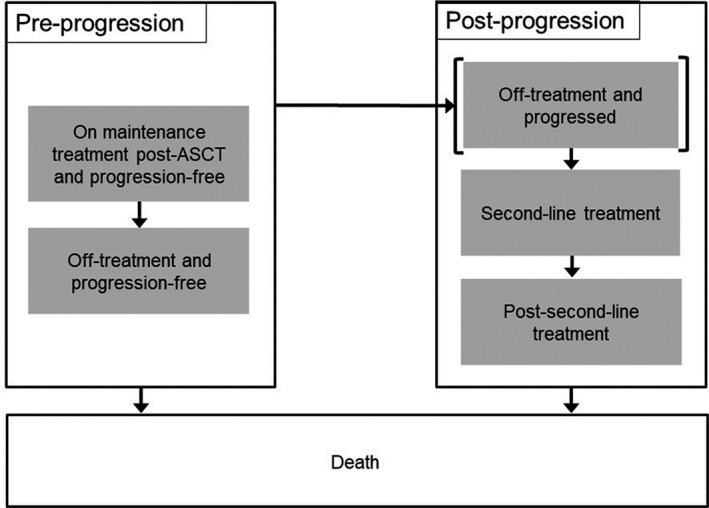
Model structure. †Death is an absorbing state; patients can transition to this state from anywhere in the model. Abbreviation: ASCT, autologous stem cell transplantation
A lifetime horizon allowed all key outcomes to be captured. Given the mean starting age of patients across the RCTs (56 years), this corresponded to a 40‐year time horizon in the base case. A cycle length of 28 days was considered sufficient to capture the speed of progression of NDMM and was in line with the most common pack size used for lenalidomide across the trials.
Model outcome measures included quality‐adjusted life years (QALYs), costs (in 2016 EUR), and life years (LYs). An incremental cost‐effectiveness ratio (ICER) was reported in terms of cost per QALY gained to allow evaluation against the willingness to pay (WTP) threshold of EUR 50 000 for MM. 13 The more conservative European‐wide threshold of EUR 50 000 was chosen over the EUR 80 000 Dutch figure, which takes the severe disease burden into account, in order to increase the generalizability of our analyses. 14 In line with Dutch guidelines, costs and effects (QALYs and LYs) were discounted at the annual rates of 4.0% and 1.5%, respectively. 15 A Dutch healthcare perspective was used as the data available were too limited to adequately capture a societal perspective for MM patients in the Netherlands.
2.4. Model parameters
Cost inputs and key model parameters are provided in Tables S5 and S6, respectively.
2.5. Clinical inputs
The key challenges presented by the trials, and modelling approaches applied to limit bias are described in Table 1. 8 (Data S1 “Covariate adjustment” and “Crossover analysis”).
TABLE 1.
Overview of trial comparability issues and model solutions
| Challenge | Assumptions applied in base case and sensitivity analyses |
|---|---|
| IFM Protocol amendment: Patients removed from treatment before the end of the study |
|
| Different dosing schedules used: 21/28 vs 28/28 |
|
| Prior consolidation therapy received in IFM |
|
| Imbalances in baseline patient characteristics across trials (including time between ASCT and maintenance) |
|
| Inconsistent dosing rules (titration) |
|
| Crossover in CALGB |
|
Abbreviations: ASCT, autologous stem cell transplantation; CALGB, Cancer and Leukemia Group B; GIMEMA, Gruppo Italiano Malattie EMatologiche dell'Adulto; IFM, Intergroupe Francophone du Myélome; RPSFTM, rank‐preserving structural failure time model.
The clinical trial data used to inform efficacy in the model were reasonably mature: maximum follow‐up durations of 66, 114, and 104 months were available from the GIMEMA, CALGB, and IFM datasets, respectively (unpublished data; data on file); however, some extrapolation was still required to predict what would happen over a patient's full lifetime.
Parametric survival models were fitted according to the UK National Institute for Health and Care Excellence (NICE) guidance to allow extrapolation of the OS and PFS outcomes beyond the observed duration of the RCTs. 16 Since extrapolation was not required for modelling time on treatment (ToT) as the proportion of patients remaining on treatment at the end of follow‐up was minimal on both arms across all trials (<2%), Kaplan‐Meier (KM) data were used directly in the model (Data S1 “Survival analysis”). Alternative curve selections presenting good statistical fit were explored in scenario analysis.
Based upon the extrapolated curves, the time spent alive was then calculated as the area under the OS curve, the area under the ToT curve gave the time spent in the PFS on‐treatment health state and the difference between the PFS and ToT curves gave the time spent in the PFS off‐treatment health state. Time spent postprogression yet prior to the receipt of second‐line treatment was calculated using the median treatment‐free interval (TFI) (Data S1 “Treatment‐free interval”). The duration of each subsequent treatment line was based on median ToT from the GIMEMA data set. Patients were assumed to begin postsecond‐line treatment immediately after completion of second‐line treatment (Data S1 “Subsequent therapy” and Table S4).
The frequencies of treatment‐related adverse events (AEs) were determined from grade 3‐4 events that occurred in ≥5% of patients receiving either Len‐Mt or no‐Mt during the studies. Due to data availability, this was limited to the CALGB and IFM trials. The probability of experiencing an AE or secondary primary malignancy per cycle was calculated based on ToT for application in the model.
2.6. Treatment cost and dosing
Dutch guidelines advise that Len‐Mt is prescribed for MM at the starting dose of 10 mg on days 1‐21 of each 28‐day cycle. 17 Doses may be either interrupted or reduced to 5 mg if the patient experiences treatment‐related toxicity. Treatment is given orally until either progression or unacceptable toxicity.
In the base case, the model uses Len‐Mt dosing from the trial data as this reflected the modelled efficacy outcomes. Although detailed dosing data were only available from the IFM trial, the individual study PFS results were considered similar enough that the data could be translated to the other studies. Therefore, IFM dosing was implemented on an individual cycle basis in the model.
List prices were used to cost lenalidomide, based on pack sizes of 21 tablets. This resulted in a weighted average cost of lenalidomide per cycle of EUR 4992 based upon the IFM dosing. As an oral therapy, it was assumed there was no cost associated with administration.
The type of subsequent treatment received at second line and postsecond line were based on those reported in the Zorginstituut Nederland (ZIN) submission for daratumumab in patients with MM previously treated with combination therapies. 18 These data, in combination with subsequent treatment market shares derived from a study investigating the cost impact of Len‐Mt post‐ASCT in Europe, 11 were used to calculate the expected Dutch market shares. The use of subsequent therapy market shares specific to the Dutch setting assumed that the resulting efficacy impact would be equivalent to subsequent therapies used in the trials.
Based on the ZIN submission data, daratumumab combination regimens have the potential to be the most used second‐line treatments, with daratumumab and lenalidomide monotherapies poised to account for most of the postsecond‐line treatments.
The assumed market shares for subsequent therapy in Dutch clinical practice and information on the clinical trial subsequent therapies utilized in the scenario analysis are presented in the Data S1 “Subsequent therapy”.
2.7. Health‐related Quality of Life (QoL)
Health‐related QoL data included in the economic model were based on a real‐world setting with EuroQol five dimensions (EQ‐5D®) data collected in the Connect® MM Registry (Data S1 “Connect® MM Registry data analysis”). 19 Utility values for the progression‐free health states were derived as 0.853 in both Len‐Mt and no‐Mt arms. Progressive disease, corresponding to the TFI and second‐line treatment health states, was determined to have a utility of 0.789.
The Connect® MM Registry data captured the impact of Len‐Mt on patient QoL 19 so disutility values for AEs were not applied in the base case to avoid double counting.
Utility data for subsequent lines of treatment in MM post‐ASCT are scarce. A postsecond‐line (and post‐ASCT) utility value of 0.640 was applied from a published analysis of the EMMOS registry (NCT01241396). 20
2.8. Healthcare resource use (HCRU)
HCRU was calculated separately for first‐line treatment, second‐line treatment, and postsecond‐line treatment. Except for granulocyte colony‐stimulating factor administration, which was based on IFM trial data, annual HCRU frequencies were obtained by analyzing patient‐level data derived from an EU5 retrospective chart review that assessed resource use in NDMM patients post‐ASCT in France, Germany, Italy, Spain, and the UK (Data S1 “Resource use analysis”). 21 As data were recorded separately for Len‐Mt and no‐Mt, differences in routine monitoring and care according to treatment regimen could be captured.
Costs attributable to HCRU and subsequent therapies were derived from standard sources appropriate for the Dutch market or published literature (Table S5). 22 , 23 , 24
AE costs were set to zero in the base case. This avoided double counting in addition to the hospital stays incorporated as part of the HCRU frequency data derived from the real‐world EU5 study.
Dutch guidelines advise that all healthcare system costs are captured in economic models, including those incurred from potential non‐related illness during gained LYs. Future unrelated healthcare costs were derived according to the Practical Application to Include Disease costs (PAID) data source. 25 The cost of terminal care was applied as a lump sum upon patients entering the death state, with the associated cost taken from a Dutch care product representing the value of all activities and actions required for palliative care associated with bone marrow cancers.
2.9. Sensitivity analysis
Sensitivity analysis was used to evaluate uncertainty and identify the key cost‐effectiveness drivers of the model. Deterministic analysis was conducted in the form of one‐way sensitivity analysis and scenario analysis. Probabilistic sensitivity analysis was used to evaluate uncertainty in the model parameters and the subsequent effects on the results.
2.10. Validation and clinical plausibility
The model was quality assured by external economists. They reviewed the model for coding errors and inconsistencies and evaluated the plausibility of inputs according to the Philips checklist. 26 A clinician provided external validation of the efficacy inputs (eg, long‐term survival projections), and their feedback was incorporated into the model assumptions where appropriate.
The model‐projected median OS and PFS were compared with the clinical outcomes published for each of the RCTs (Table S8) to evaluate their consistency. The modelled estimates largely lay within the 95% CIs reported for each trial.
3. RESULTS
3.1. Base case results
The model projected an increase in both OS and PFS for Len‐Mt vs no‐Mt. The modelled median OS was 77.3 months for no‐Mt and 107.6 months for Len‐Mt (Figure 2A). The modelled median PFS was 26.7 months for no‐Mt and 51.5 months for Len‐Mt (Figure 2B). The KM curve for ToT in the group receiving Len‐Mt is shown in Figure 2C.
FIGURE 2.
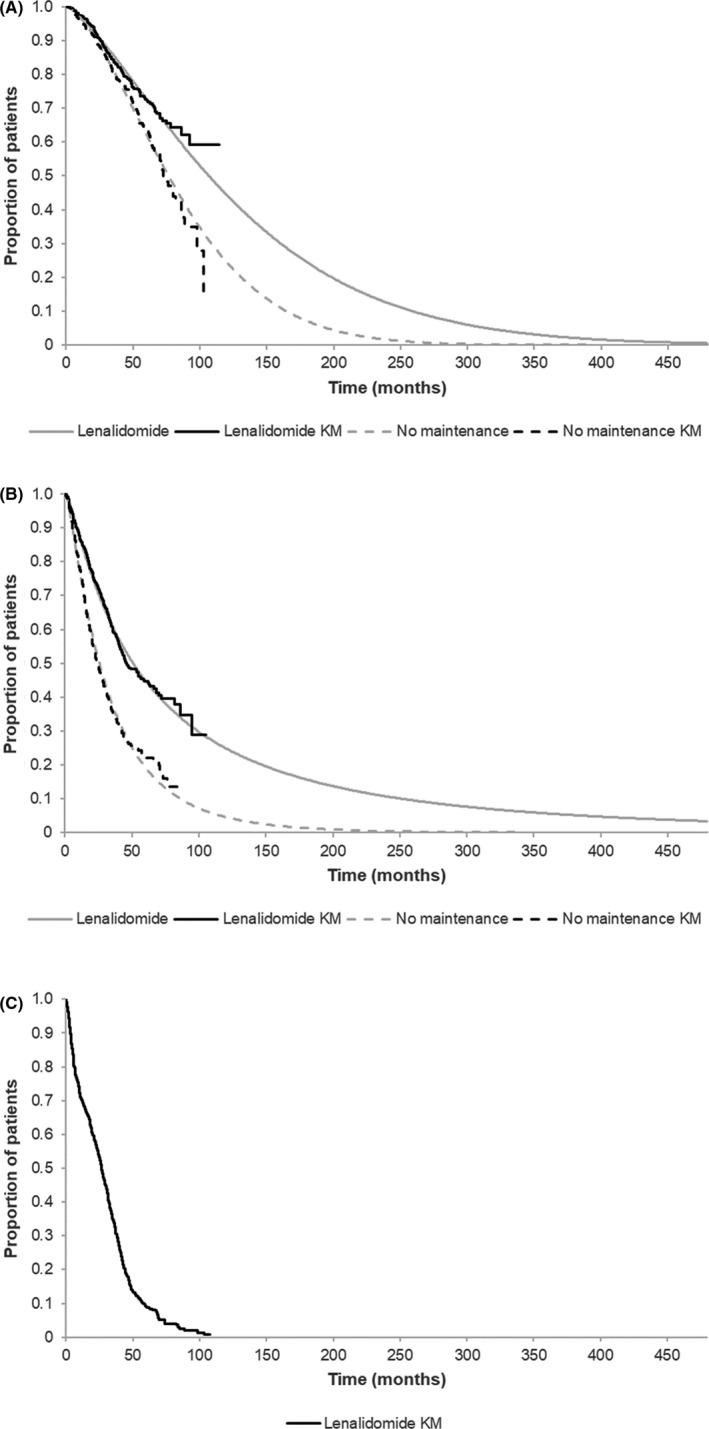
Model‐generated: (A) OS and (B) PFS for lenalidomide and no maintenance therapy vs KM; and (C) KM for lenalidomide ToT. All data shown for time horizon of model. Abbreviations: KM, Kaplan‐Meier; OS, overall survival; PFS, progression‐free survival; ToT, time on treatment
Len‐Mt gave 9.54 LYs and 7.49 QALYs with a total cost of EUR 360 480 (Table 2). No‐Mt gave 6.76 LYs and 5.04 QALYs at a total cost of EUR 286 444. Compared with no‐MT, Len‐Mt produced an incremental LY gain of 2.79 and an incremental QALY gain of 2.46. This resulted in an ICER of EUR 30 143 per QALY gained. The discounted results as determined by the base case model are shown in Table 2.
TABLE 2.
Discounted base case cost‐effectiveness results
| Cost item | Lenalidomide | No maintenance treatment | Incremental difference |
|---|---|---|---|
| Clinical outcomes a | |||
| LYs | 9.54 | 6.76 | 2.79 |
| QALYs | 7.49 | 5.04 | 2.46 |
| Cost outcomes, EUR b | |||
| Drug cost | |||
| First line | 147 707 | 0 | 147 707 |
| Second line | 67 242 | 114 958 | −47 716 |
| Postsecond line | 42 697 | 72 443 | −29 746 |
| HCRU cost | |||
| First line, on treatment | 3704 | 10 716 | −7012 |
| First line, off treatment | 5450 | 7118 | −1668 |
| Second line | 853 | 1483 | −629 |
| Postsecond line | 19 039 | 22 556 | −3516 |
| Terminal care | 16 846 | 18 800 | −1954 |
| SPM cost | 30 504 | 20 567 | 9938 |
| Future unrelated health costs | |||
| Other years | 24 116 | 15 215 | 8901 |
| Last year of life | 2321 | 2589 | −268 |
| Total | 360 480 | 286 444 | 74 036 |
| Deterministic ICER—cost/QALY, EUR | 30 143 | ||
| Probabilistic ICER—cost/QALY, EUR | 29 358 | ||
| Percentage chance cost‐effective | 99.9% at the threshold for MM of EUR 50 000/QALY | ||
Abbreviations: EUR, Euro; HCRU, healthcare resource use; ICER, incremental cost‐effectiveness ratio; LY, life years; MM, multiple myeloma; QALY, quality‐adjusted life year; SPM, secondary primary malignancy.
Based on a discount rate of 1.5% for QALYs and LYs.
Based on a 4.0% discount rate for costs.
Probabilistic results were consistent with those calculated from the deterministic analysis and indicated a 99.9% probability of Len‐Mt being cost‐effective at the WTP threshold for MM of EUR 50 000/QALY gained.
The model also predicted higher proportions of patients in the no‐Mt arm would receive subsequent therapy at both second and postsecond line within the modelled time horizon (40 years). No‐Mt was associated with 66% of patients progressing to second‐line treatment and 62% to postsecond‐line treatment. In comparison, Len‐Mt saw 38% of patients progress to second‐line treatment and 36% to postsecond‐line treatment. The total cost of second‐line and subsequent treatment for patients who received Len‐Mt or no‐Mt were EUR 109 939 and EUR 187 401, respectively, highlighting a savings of EUR 77 462 in treatment costs for patients who received first‐line Len‐Mt (Table 2).
3.2. Sensitivity analysis
One‐way sensitivity analysis assessed uncertainty in the model by setting parameters to their lower and upper bound values, usually aligning to the associated 95% CI range, and evaluating the impact on the results. The parameters with the greatest potential to influence the ICER were the subsequent therapy costs at both second‐ and postsecond‐line for no‐Mt, followed by the subsequent therapy costs for Len‐Mt. Of note, the resulting ICERs for all tested parameters remained below the WTP threshold of EUR 50 000/QALY, even at the most influential. The tornado diagram displaying the 10 most influential parameters on the ICER is shown in Figure 3.
FIGURE 3.

One‐way sensitivity analysis tornado diagram. Abbreviations: CI, confidence interval; HCRU, healthcare resource use; ICER, incremental cost‐effectiveness ratio; QALY, quality‐adjusted life year; SPM, secondary primary malignancy
Scenario analysis allowed evaluation of the impact of the key assumptions of the model on the cost‐effectiveness of Len‐Mt. Results demonstrate that most scenarios gave ICERs that remained cost‐effective at the WTP threshold of EUR 50 000 per QALY (Table 3).
TABLE 3.
Scenario analysis results
| Scenario | No maintenance | Lenalidomide | ICER, EUR | ||||
|---|---|---|---|---|---|---|---|
| Total cost, EUR | Total LYs | Total QALYs | Total cost, EUR | Total LYs | Total QALYs | ||
| Base case | 286 444 | 6.76 | 5.04 | 360 480 | 9.54 | 7.49 | 30 143 |
| Efficacy source: CALGB trial data | 145 783 | 6.09 | 4.68 | 302 405 | 10.52 | 8.32 | 43 045 |
| Time horizon | |||||||
| 30 y | 286 433 | 6.76 | 5.04 | 359 211 | 9.46 | 7.43 | 30 386 |
| 15 y | 311 912 | 6.58 | 4.91 | 348 670 | 8.29 | 6.54 | 39 727 |
| 10 y | 300 909 | 5.97 | 4.50 | 336 915 | 6.88 | 5.47 | 63 690 |
| Covariate adjustment | |||||||
| Four covariates a | 301 732 | 7.24 | 5.33 | 394 526 | 9.13 | 7.13 | 37 449 |
| Full list of covariates b | 302 988 | 7.28 | 5.36 | 397 475 | 9.22 | 7.20 | 37 276 |
| No crossover adjustment | 280 171 | 7.32 | 5.48 | 386 559 | 9.54 | 7.49 | 39 883 |
| OS extrapolation curves for both arms | |||||||
| Log‐logistic | 316 537 | 8.89 | 6.41 | 393 465 | 11.81 | 9.09 | 28 675 |
| Generalized gamma | 273 718 | 5.73 | 4.34 | 405 005 | 12.40 | 9.47 | 25 608 |
| PFS extrapolation curves: log‐logistic for both arms | 275 474 | 6.76 | 5.07 | 364 122 | 9.54 | 7.47 | 36 888 |
| Proportion of patients receiving 28 d out of 28‐day lenalidomide dosing: 88.9% as in pooled trial data | 286 444 | 6.76 | 5.04 | 404 255 | 9.54 | 7.49 | 47 966 |
| Lenalidomide dose: Dutch guidelines (10 mg) | 286 444 | 6.76 | 5.04 | 358 253 | 9.54 | 7.49 | 29 237 |
| Second‐line utility: 0.72 as in EMMOS registry | 286 444 | 6.76 | 5.01 | 360 480 | 9.54 | 7.48 | 29 999 |
| Subsequent treatment market shares: pooled trial data | 188 026 | 6.76 | 5.04 | 301 250 | 9.54 | 7.49 | 46 099 |
| HCRU frequencies: equal across both treatment arms | 278 364 | 6.76 | 5.04 | 370 036 | 9.54 | 7.49 | 37 324 |
Abbreviations: ASCT, autologous stem cell transplantation; CALGB, Cancer and Leukemia Group B; EUR, Euro; HCRU, healthcare resource use; ICER, incremental cost‐effectiveness ratio; LY, life year; OS, overall survival; PFS, progression‐free survival; QALY, quality‐adjusted life year.
Covariates adjusted: age (<60 vs ≥60 years), sex, International Staging System stage at diagnosis (I/II vs III vs missing), and response after ASCT by central review (complete response/very good partial response vs partial response/stable disease).
Additional covariates adjusted: adverse‐risk cytogenetics, creatinine clearance after ASCT, creatinine clearance at diagnosis, and Eastern Cooperative Oncology Group performance status.
The results remained relatively stable and cost‐effective for time horizon scenarios at 15 years and above. The resilience of the ICER to health‐related QoL assumptions was also demonstrated. Little change in the results was observed when applying an alternative second‐line utility value of 0.72, as was reported by the same source as the postsecond‐line utility. 20 Testing around the HCRU showed that Len‐Mt remained cost‐effective if equal frequencies of use were assumed in both treatment arms.
CALGB was deemed to have the better fit to the decision problem; as shown in Table 3, the CALGB scenario remained cost‐effective at the EUR 50 000 per QALY WTP threshold.
Application of log‐logistic and generalized gamma extrapolation curves in both arms for OS reduced the ICER due to the more optimistic predication of survival for Len‐Mt across the 40‐year time horizon. Similarly, extrapolation of PFS with a log‐logistic curve for both arms increased the ICER due to the optimistic predication of PFS for the no‐Mt arm.
Crossover unadjusted data increased LYs and QALYs for the no‐Mt arm. Using the rank‐preserving structural failure time method to adjust for crossover in the base case model reduces the potential for bias in the no‐Mt arm due to patients benefitting from receiving Len‐Mt, giving more plausible cost‐effective results. Covariate‐adjusted data with either four key prognostic variables or the full list had minimal impact on cost‐effectiveness.
A scenario where patients who received 15 mg lenalidomide in the trials were switched to 10 mg lenalidomide, to better reflect Dutch recommendations for clinical practice, reduced the ICER to EUR 29 237.
Scenarios that aligned the subsequent treatment market shares and proportion of patients receiving 28 days out of each 28‐day cycle lenalidomide dosing as per the trial data (88.9%) gave ICERs of EUR 46 099 and EUR 47 966, respectively (Table 3). However, as discussed previously, these treatment pathways were not considered to be generalizable to Dutch clinical practice. Incremental analysis revealed that the ICER remained below the WTP threshold of EUR 50 000 per QALY gained when up to 99.0% of patients received 28 out of 28‐day dosing.
4. DISCUSSION
This economic evaluation was performed to assess the cost‐effectiveness of Len‐Mt as a post‐ASCT treatment for patients with NDMM in the Netherlands. The base case deterministic ICER of EUR 30 143 shows that lenalidomide is a cost‐effective use of resources in the Netherlands when compared to the WTP threshold for MM of EUR 50 000/QALY.
Given that subsequent treatment lines are likely to include more expensive, newer combination treatments, delaying progression and time to subsequent therapy creates the potential for large budget savings; as illustrated by the finding that Len‐Mt post‐ASCT was associated with a 24% saving in overall direct medical costs, vs no‐Mt. 11 Although first‐line drug costs contributed EUR 147 707 to the increase in total costs vs no‐Mt, this was partially offset by savings of EUR 77 462 in subsequent treatment costs because lenalidomide delays time to progression and, hence, subsequent treatment. 18
Strengths of this analysis include the use of all relevant data to inform the base case analysis. Using the pooled meta‐analysis of three RCTs maximized the population size used to predict modelled outcomes, strengthening the applicability of the results. Using the CALGB study allowed the incorporation of more mature trial data and ToT required no extrapolation within the model. The incorporation of data from the EU5 retrospective chart review allowed HCRU to be representative of actual clinical practice. Dutch‐specific inputs for dosing and subsequent therapy captured clinical practice of the chosen perspective. Recently, results of the Myeloma XI trial were reported, indicating a significant improvement in both OS and PFS with Len‐Mt compared with observation in the subset of patients eligible for ASCT 4 ; while inclusion of data from this trial would have further strengthened our findings, the data were not available at the time of analysis.
Another strength is the utility value choice in this analysis. We calculated the utility values based on the real‐world EQ‐5D data collected in the Connect® MM Registry and further incorporated data from the Dutch tariff to derive Dutch‐specific utility scores. Therefore, results from the analysis are specific to the Dutch setting and reflect the preference of the Dutch population.
A limitation common to many cost‐effectiveness models is the uncertainty in long‐term outcomes, predicted from the extrapolation of trial data over a lifetime horizon. The ICER remained relatively stable for most time horizons tested during scenario analysis, with lenalidomide remaining cost‐effective for time horizons above 15 years. This demonstrated that cost‐effectiveness was not reliant on extrapolation beyond the availability of trial data. In addition, long‐term model survival projections (Figure S1) showed good clinical plausibility, being lower than Dutch general population mortality over the entire modelled time horizon. 27 The model was also robust to the selection of extrapolation curves for OS and PFS.
Furthermore, total treatment costs are likely to be overestimated as they are derived from their respective list prices, which do not account for confidential discounts. Inconsistent reporting of data across the trials required assumptions to be made in the derivation of some variables.
Another key limitation of this study was the deviation of some inputs from clinical trial data. Utility values were obtained from the best available literature as QoL data were lacking in the trials included. 19 The use of the days 1‐21 of each 28‐day cycle lenalidomide dosing schedule and Dutch subsequent therapy market shares were both based on the assumption that their efficacy outcomes were equivalent to those generated in the trials, without adjustment.
Sensitivity analysis revealed subsequent therapy use as a key driver of cost‐effectiveness. As the treatment pathway for MM is continually adapting, both in the Netherlands and globally, best practice methods for accurately assessing the impact on cost‐effectiveness when subsequent therapies vary from those used during clinical trials need to be determined. Further research is also required to establish the optimal dosing for Len‐Mt in MM post‐ASCT. However, the lack of difference in efficacy between 28‐ and 21‐day regimens suggests they are comparable. In addition to the data herein, the recently published Myeloma XI study also showed similar efficacy using a 21‐day regimen. 4 Although head‐to‐head data are lacking, RCT comparisons suggest a potential advantage for the 21‐day regimen in terms of tolerability, which may facilitate patients staying on treatment longer. Prevalence of key AEs was lower in the 21‐day Myeloma XI study 4 vs a previous RCT 1 using a 28‐day dosing schedule (grade 3‐4 neutropenia 33% vs 51%; grade 3‐4 thrombocytopenia 6% vs 14%; grade 3‐4 fatigue 1% vs 5%; respectively). The 21‐day regimen also has the added benefit of being more familiar to practitioners. Finally, as expected there are differences between the RCT populations used to generate our pooled analysis and those of newly diagnosed patients in Dutch registries. Inclusion/exclusion criteria‐driven differences between clinical trial populations and those seen day to day in the clinic are a well‐recognized issue throughout medical research. In a recent study on relapsed/refractory MM comparing RCT populations with those in an US electronic medical database, between half and three‐quarters of the patients were ineligible for at least one of six landmark MM trials. 28 Median age of patients receiving first‐line therapy in the Dutch PHAROS registry was 70 years old compared with 58 and 59 years old in our population. 1 , 2 , 3 , 29 Indeed, age‐based inclusion criteria would have excluded ≥50% of the PHAROS population in each of the three trials. 1 , 2 , 3 , 29 Although not directly comparable (mean vs median), demographic data suggest that the RCT patients also had better kidney function than real‐world patients in the Netherlands. 1 , 2 , 3 , 29 However, differences in staging were not particularly marked (ISS stage III: 26% vs 22‐24%, in real‐world and RCT populations, respectively). 1 , 2 , 3 , 29 Although these differences are likely to impact HCRU, the parameters of our sensitivity analyses suggest that Len‐Mt therapy would remain cost‐effective under most circumstances. However, real‐world cost analyses should be conducted when sufficient data are available.
5. CONCLUSIONS
The proportion of ASCT‐eligible patients with MM is expanding in the Netherlands due to improvements in supportive care, increasing the population requiring maintenance therapy. A cost‐utility analysis using a pooled meta‐analysis of the most relevant RCTs indicates the use of Len‐Mt increases both OS and PFS. By extending PFS, Len‐Mt delays the cost burdens associated with relapse and subsequent uncontrolled disease, including the cost of expensive later lines of treatment. Further research is needed to address the impact of changing subsequent treatment pathways, which are a key driver of cost‐effectiveness. The results of this analysis demonstrate that Len‐Mt post‐ASCT is a cost‐effective use of healthcare resources in the Netherlands vs no‐Mt, with a 99.9% probability of being cost‐effective at the WTP threshold for MM of EUR 50 000 per QALY gained. The present study provides a model for estimations in other EU countries; although the discount rates, HCRU rates, cost inputs, and utility values may vary across countries.
CONFLICT OF INTEREST
C. A. U‐dG. has received research funding from Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Genzyme, Gilead, Janssen‐Cilag, Merck, Roche, and Sanofi. RR is employed by BresMed and is a consultant for Bristol Myers Squibb. DL is employed by BresMed and is a consultant for Bristol Myers Squibb. JB is employed by Roche. SZ has received research funding and honoraria from Bristol Myers Squibb, Janssen, and Takeda. SD is employed by Celgene International, a Bristol Myers Squibb Company, and has equity interest.
AUTHOR CONTRIBUTIONS
CAUdeG wrote, reviewed, and edited the manuscript. RR designed the methodology and wrote the original draft. DL was involved in conceptualization, development of the methodology, supervision of the study, software, formal analysis, visualization, and review and editing of the manuscript. JB collected and interpreted the data, and wrote, reviewed, and edited the manuscript. SZ wrote, reviewed, and edited the manuscript. SD was involved in conceptualization, methodology, project administration, and writing, review, and editing of the manuscript. All authors contributed to the manuscript and approved the final version.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors received writing and editorial assistance in the preparation of this manuscript from Matt Bonnington (BresMed) and Rosie Morland, PhD (Excerpta Medica), funded by Bristol Myers Squibb. The authors are fully responsible for all content and editorial decisions for this manuscript.
Uyl‐de Groot CA, Ramsden R, Lee D, Boersma J, Zweegman S, Dhanasiri S. Lenalidomide as maintenance treatment for patients with multiple myeloma after autologous stem cell transplantation: A pharmaco‐economic assessment. Eur J Haematol. 2020;105:635–645. 10.1111/ejh.13497
Janneke Boersma: Employed by Celgene at time of data collection and initiation of manuscript.
REFERENCES
- 1. Attal M, Lauwers‐Cances V, Marit G, et al. Lenalidomide maintenance after stem‐cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782‐1791. [DOI] [PubMed] [Google Scholar]
- 2. McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem‐cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770‐1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371(10):895‐905. [DOI] [PubMed] [Google Scholar]
- 4. Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open‐label, randomised, phase 3 trial. Lancet Oncol. 2019;20(1):57‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem‐cell transplantation in newly diagnosed multiple myeloma: a meta‐analysis. J Clin Oncol. 2017;35(29):3279‐3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gay F, Jackson G, Rosiñol L, et al. Maintenance treatment and survival in patients with myeloma: a systematic review and network meta‐analysis. JAMA Oncol. 2018;4(10):1389‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sengsayadeth S, Malard F, Savani BN, Garderet L, Mohty M. Posttransplant maintenance therapy in multiple myeloma: the changing landscape. Blood Cancer J. 2017;7(3):e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rawstron AC, Gregory WM, de Tute RM, et al. Minimal residual disease in myeloma by flow cytometry: independent prediction of survival benefit per log reduction. Blood. 2015;125(12):1932‐1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cowan AJ, Allen C, Barac A, et al. Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol. 2018;4(9):1221‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Integraal Kankercentrum Nederland . The Netherlands Cancer Registry. Dutch cancer figures. (Updated: 2 February 2018). https://www.cijfersoverkanker.nl. Accessed September 2018.
- 11. Jackson G, Dutton R, Zamagni E, Hughes R, Dhanasiri S. Lenalidomide maintenance therapy post‐autologous stem cell transplant: a healthcare cost‐impact analysis in Europe. Blood. 2017;130:3405. Abstract. [Google Scholar]
- 12. Zweegman S, Stege CAM, Haukas E, et al. Ixazomib‐thalidomide‐low dose dexamethasone induction followed by maintenance therapy with ixazomib or placebo in newly diagnosed multiple myeloma patients not eligible for autologous stem cell transplantation; results from the randomized phase II HOVON‐126/NMSG21.13 trial. Haematologica. 2020;haematol.2019.240374. 10.3324/haematol.2019.240374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nederland Zorginstituut . Cost‐effectiveness in practice. 2015. https://english.zorginstituutnederland.nl/publications/reports/2015/06/16/cost‐effectiveness‐in‐practice. Accessed October 2019.
- 14. Reckers‐Droog VT, van Exel NJA, Brouwer WBF. Looking back and moving forward: on the application of proportional shortfall in healthcare priority setting in the Netherlands. Health Policy. 2018;122(6):621‐629. [DOI] [PubMed] [Google Scholar]
- 15. Nederland Zorginstituut . Guideline for economic evaluations in healthcare. 2016. https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline‐for‐economic‐evaluations‐in‐healthcare. Accessed October 2019.
- 16. Latimer N. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials ‐ extrapolation with patient‐level data. 2013. http://www.nicedsu.org.uk/NICE%20DSU%20TSD%20Survival%20analysis.updated%20March%202013.v2.pdf. Accessed August 2016. [PubMed]
- 17. Stichting Hemato‐Oncologie voor Volwassenen Nederland (HOVON) . Richtlijn Behandeling Multipel Myeloom 2019. 2019. (Updated 21 March 2019). https://www.hematologienederland.nl/hematline/node/816. Accessed October 2019.
- 18. Nederland Zorginstituut . Daratumumab (Darzalex®) voor de behandeling van volwassen patiënten met multipel myeloom die minstens één eerdere behandeling hebben gehad: in combinatie met bortezomib en dexamethason; in combinatie met lenalidomide en dexamethason. (Onze referentie ACP 70–4). https://www.zorginstituutnederland.nl/binaries/zinl/documenten/adviezen/2017/11/gvs‐advies‐oordruppels‐bij‐gehoorgangontsteking‐otitis‐externa/pakketadvies‐daratumumab‐darzalex‐bij‐multipel‐myeloom/combinatiebehandelingen+van+daratumumab+%28Darzalex%29+voor+de+behandeling+van+volwassen+pati%C3%ABnten+met.pdf. Accessed October 2017.
- 19. Abonour R, Wagner L, Durie BGM, et al. Impact of post‐transplantation maintenance therapy on health‐related quality of life in patients with multiple myeloma: data from the Connect® MM Registry. Ann Hematol. 2018;97(12):2425‐2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hatswell AJ, Couturier C, Ito T. Utility by treatment line in multiple myeloma: analysis of over 9,000 EQ‐5D‐3L questionnaires from the EMMOS registry. Value Health. 2016;19:A348. [Google Scholar]
- 21. Ashcroft J, Taylor‐Stokes G, Dhanasiri S, Judge D. Assessing real‐world treatment patterns, outcomes and resource use across Europe in newly diagnosed multiple myeloma (NDMM) patients post autologous stem cell transplant. Haematologica. 2017;102(Suppl 1):598. Abstract E1463. [Google Scholar]
- 22. Gaultney JG, Franken MG, Tan SS, et al. Real‐world health care costs of relapsed/refractory multiple myeloma during the era of novel cancer agents. J Clin Pharm Ther. 2013;38(1):41‐47. [DOI] [PubMed] [Google Scholar]
- 23. Nederland Zorginstituut . Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. 2016. https://www.zorginstituutnederland.nl/binaries/zinl/documenten/publicatie/2016/02/29/richtlijn‐voor‐het‐uitvoeren‐van‐economische‐evaluaties‐in‐de‐gezondheidszorg/Richtlijn+voor+het+uitvoeren+van+economische+evaluaties+in+de+gezondheidszorg+%28verdiepingsmodules%29.pdf. Accessed July 2017.
- 24. Nederland Zorginstituut . Medicijnkosten. 2017. https://www.medicijnkosten.nl/. Accessed January 2017.
- 25. van Baal PH, Wong A, Slobbe LJ, Polder JJ, Brouwer WB, de Wit GA. Standardizing the inclusion of indirect medical costs. Pharmacoeconomics. 2011;29(3):175‐187. [DOI] [PubMed] [Google Scholar]
- 26. Philips Z, Ginnelly L, Sculpher M, et al. Review of guidelines for good practice in decision‐analytic modelling in health technology assessment. Health Technol Assess. 2004;8(36):iii–iv, ix–xi, 1‐158. [DOI] [PubMed] [Google Scholar]
- 27. Statistics Netherlands . Deaths; underlying cause of death (shortlist), sex, age. 2015. http://statline.cbs.nl/Statweb/publication/?DM=SLEN&PA=7052eng&D1=0,92&D2=1‐2&D3=1‐21&D4=l&LA=EN&HDR=G1,G2,G3&STB=T&VW=T. Accessed November 2016.
- 28. Chari A, Romanus D, Palumbo A, et al. Randomized clinical trial representativeness and outcomes in real‐world patients: comparison of 6 hallmark randomized clinical trials of relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2020;20(1):8‐17.e16. [DOI] [PubMed] [Google Scholar]
- 29. Verelst SGR, Blommestein HM, De Groot S, et al. Long‐term outcomes in patients with multiple myeloma: a retrospective analysis of the Dutch Population‐based HAematological Registry for Observational Studies (PHAROS). Hemasphere. 2018;2(4):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


