Abstract
Objective
To assess the efficacy of erenumab at the ≥50%, ≥75%, and 100% reduction in monthly migraine days (MMD) response thresholds, using data from the 6‐month double‐blind treatment phase (DBTP) of the Study to Evaluate the Efficacy and Safety of Erenumab in Migraine Prevention (STRIVE) pivotal clinical trial.
Methods
Enrolled patients with episodic migraine (EM; ≥4 MMD and <15 monthly headache days) were randomized (1:1:1) to erenumab 70 mg (n = 312), erenumab 140 mg (n = 318), or placebo (n = 316) once monthly. We determined the proportions of patients with ≥50%, ≥75% and 100% reduction in MMD over the last 3 months of the STRIVE DBTP (months 4 through 6) and conducted post hoc analyses to contextualize the treatment benefit in patient subgroups achieving, and not achieving, these response thresholds. Outcome measures included changes in MMD, acute migraine‐specific medication days (MSMD), and patient‐reported outcomes.
Results
The proportions of patients with a reduction in MMD from baseline were greater for erenumab than for placebo at all response thresholds. As previously reported for the ≥50% response threshold, 135/312 (43.3%) of patients on erenumab 70 mg and 159/318 (50.0%) on erenumab 140 mg responded, vs 84/316 (26.6%) for placebo. At months 4 through 6, 65/312 (20.8%) and 70/318 (22.0%) of those on erenumab 70 mg and erenumab 140 mg, respectively, achieved ≥75% reductions vs 25/316 (7.9%) on placebo. A reduction of 100% response, which required no migraine days over 3 consecutive months based on observed data, was achieved by 10/312 (3.2%) of patients treated with erenumab 70 mg and 16/318 (5.0%) for erenumab 140 mg, vs 9/316 (2.8%) for placebo. At all response thresholds, responders achieved numerically greater reductions in mean MMD and MSMD, and greater improvements in disability than did the overall population; importantly, these remarkable responses were noted early. Meanwhile, 60/312 (19.2%) and 53/318 (16.7%) patients on erenumab 70 and 140 mg, respectively, had no reduction in MMD from baseline in months 4 through 6, compared with 104/316 (32.9%) patients on placebo.
Conclusions
The responses at the ≥50%, ≥75%, and 100% thresholds provide context for establishing realistic patient and physician expectations regarding the magnitude of treatment benefit that may be achieved by patients with EM responding to erenumab (STRIVE, NCT02456740).
Keywords: erenumab, episodic migraine, responder thresholds, STRIVE double‐blind treatment phase, efficacy
Abbreviations
- CGRP
calcitonin gene‐related peptide
- CI
confidence interval
- CM
chronic migraine
- CMH
Cochran‐Mantel‐Haenszel
- DBTP
double‐blind treatment phase
- eDiary
electronic diary
- EM
episodic migraine
- HIT‐6
6‐item Headache Impact Test
- MMD
monthly migraine days
- mMIDAS
modified Migraine Disability Assessment
- MPFID‐EA
Migraine Physical Function Impact Diary‐Everyday Activities
- MPFID‐PI
Migraine Physical Function Impact Diary‐Physical Impairment
- MSMD
migraine‐specific medication days
- NSAID
nonsteroidal anti‐inflammatory drug
- OR
odds ratio
- PRO
patient‐reported outcome
- Q3M
every 3 months
- SD
standard deviation
- STRIVE
Study to Evaluate the Efficacy and Safety of Erenumab in Migraine Prevention
Introduction
Migraine is a neurological disease 1 , 2 associated with a high personal and societal burden and is a leading cause of life years lost due to disability. 3 , 4 , 5 The calcitonin gene‐related peptide (CGRP) pathway, which involves signaling through the canonical CGRP receptor by binding of CGRP, plays a causal role in migraine. 6 , 7 Erenumab is a fully human monoclonal antibody that potently and selectively targets the canonical CGRP receptor, preventing binding of CGRP. 8
Clinical studies have demonstrated the efficacy and safety of erenumab in both episodic and chronic migraine. 9 , 10 , 11 , 12 , 13 , 14 Erenumab has been approved in several regions, including US and EU, for the preventative treatment of migraine in adults. 15 , 16
Clinical trials on migraine preventive therapy typically report the mean reduction in monthly migraine days (MMD) as primary endpoint, whereas the percentage of individuals achieving ≥50% reduction is a commonly used secondary endpoint and considered to be of high clinical relevance. 17 The most recent guidelines now allow responder thresholds (eg, ≥50%) to be used as a primary endpoint. 18 Other response thresholds (eg, ≥75% or 100% reduction in MMD) may also be used. 17 , 18 However, all response thresholds are by nature arbitrary and dichotomous and patients may still experience benefit from treatment even if they do not reach a certain level of response. 17 , 18 While efficacy results are summarized for the overall study population, patients may respond differently or may not respond at all. The mean reduction in MMD as a single point estimate does not capture the wide range of efficacy that patients may experience, from a remarkable response, for example, achieving 75‐100% reduction in MMD, to no improvement whatsoever, that is, nonresponse.
Here, we aim to contextualize the actual treatment benefits of erenumab in patients achieving ≥50% MMD response, as well as in patients with a remarkable (≥75% and 100%) MMD response, and those who showed no change in MMD or who worsened (ie, those without a reduction in MMD), using data from the Study to Evaluate the Efficacy and Safety of Erenumab in Migraine Prevention (STRIVE) pivotal clinical trial on episodic migraine (EM). 10 We also used waterfall plot analyses to examine the change in MMD for each individual patient. In addition, we present the results of exploratory analyses of the efficacy of erenumab in subgroups achieving or not achieving at least a 50%, 75%, and 100% reduction in MMD over months 4 through 6. As well as response at different thresholds, we also assessed outcome measures including change in MMD, in acute migraine‐specific medication days (MSMD), and in a number of patient‐reported outcomes (PROs) from baseline to the last 3 months of the 6‐month double‐blind treatment phase (DBTP). PROs included migraine impact on patients and their physical function, and migraine‐related disability (ClinicalTrials.gov: NCT02456740).
Methods
Summary of the STRIVE Study Design
Details of the study design are described in Goadsby et al. Enrolled patients had EM, defined as ≥4 and <15 migraine days per month and <15 headache days per month on average across the 3 months prior to screening and during the 4‐week baseline phase. 1 In brief, the trial consisted of a screening phase lasting up to 3 weeks, a 4‐week baseline phase, a 24‐week randomized, double‐blind, placebo‐controlled treatment phase, a 28‐week dose‐blinded active‐treatment phase, and a 12‐week safety follow‐up phase. The present analysis includes data collected throughout the 24‐week double‐blind placebo‐controlled phase, during which patients received either erenumab 70 mg, erenumab 140 mg, or placebo (randomization 1:1:1) in monthly subcutaneous injections.
From the beginning of the baseline phase onward, patients recorded on a daily basis in an electronic diary (eDiary) information about their migraine and non‐migraine headaches, use of migraine‐specific abortive therapies, and use of analgesic medications. The protocol was approved at each study site by an ethical review board, all patients gave written informed consent, and the study was conducted in accordance with principles specified by the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice.
Responder Threshold Analyses
The proportion of patients in the overall population with ≥50% reduction in mean MMD from baseline to months 4 through 6 (ie, the mean over the final, consecutive 3 months of the DBTP) was prespecified as a secondary endpoint (previously reported by Goadsby et al). A migraine day was any calendar day on which the patient experienced a qualified migraine headache (onset, continuation, or recurrence of the migraine headache) and/or used acute migraine‐specific medication as recorded in the eDiary. Mean MMD were calculated as the average number of migraine days per month during the last 3 months (months 4, 5, and 6) of the 24‐week DBTP (if at least 1 MMD was present). We present here the results of 2 additional responses that were prespecified as exploratory endpoints: the proportions of patients in whom the mean MMD was decreased from baseline to months 4 through 6 by ≥75% and by 100%. We also determined the proportions of patients in the erenumab and placebo groups achieving ≥50%, ≥75%, and 100% reduction in MMD in each of the last 3 months of the DBTP (ie, in month 4, in month 5 and in month 6) as a post hoc analysis. In addition, we present the post hoc analyses on the proportion of patients without reduction in MMD, that is, no change or worsening in mean MMD from baseline to months 4 through 6. Post hoc waterfall plot analyses were used to better assess the baseline MMD for each individual patient in comparison to his/her response to erenumab or placebo (as per change from baseline in MMD at months 4 through 6) and thus, allow the full spectrum of individual responses to be visualized across patients.
We present the results of the following outcome measures as post hoc analyses in the subgroups of patients who achieved and did not achieve ≥50%, ≥75%, and 100% reduction in MMD: change from baseline in MMD during each month of the 6‐month DBTP, change from baseline to the last 3 months (months 4 through 6) of the DBTP in MMD, MSMD, and in a number of PROs (the 6‐item Headache Impact Test [HIT‐6], 19 the Migraine Physical Function Impact Diary‐Everyday Activities [MPFID‐EA] and MPFID‐Physical Impairment [MPFID‐PI], 20 and the modified Migraine Disability Assessment [mMIDAS] questionnaire). The mMIDAS includes total days of lost productivity, absenteeism (complete disability), and presenteeism (reduced or impaired work) and has been modified for a recall period of 1 month, rather than a 3‐month recall period as in the traditional MIDAS to improve the accuracy of the results and reduce recall bias. 21 , 22 A post hoc analysis was performed in which the mMIDAS was converted into a 3‐monthly (Q3M) assessment of disability. The mMIDAS scores were summed for months 4, 5, and 6 by taking the average of these post‐baseline assessments and multiplying by 3 (if at least 1 mMIDAS was present).
Statistical Methods
The analysis population (efficacy analysis set) comprised all patients who received at least 1 dose of erenumab or placebo and had at least 1 measurement of change from baseline in MMD during the DBTP. When assessing continuous efficacy outcomes in subgroups that achieved or did not achieve response at the various response thresholds, descriptive statistics are provided based on observed data without imputation. A stratified Cochran‐Mantel‐Haenszel (CMH) test was used to estimate common odds ratios (ORs), 95% confidence intervals (95% CIs) for those ORs, and P values for achieving specific MMD reduction threshold in patients treated with erenumab, compared with placebo. Missing daily diary data were handled using proration method. MMDs were prorated to 28‐day equivalent if number of days with diary compliance was ≥14 days in the monthly interval or set to missing otherwise. Missing monthly response data (due to missing MMDs) were imputed to represent non‐response. No formal hypothesis testing was conducted for the endpoints included in this manuscript. Descriptive P values are reported without multiplicity adjustment. All descriptive statistics were generated using SAS System 9.3 or later (Institute Inc., Cary, NC, USA).
RESULTS
Patient Disposition and Baseline Characteristics
Baseline characteristics and patient disposition, as well as clinical responses and safety outcomes for erenumab 70 mg, erenumab 140 mg, and placebo groups were reported in the primary publication. 10 There were 317, 319, and 319 patients randomized to the erenumab 70 mg, erenumab 140 mg, and placebo treatment groups, respectively, and 312, 318, and 316 patients in the efficacy analysis set, respectively.
Spectrum of Response at the ≥50%, ≥75%, and 100% Response Thresholds With Respect to MMD Reduction
The proportions of patients in the overall population with ≥50% reduction in MMD in months 4 through 6 and in the individual months 4, 5, or 6 were reported previously. 10 In short, the percentage of patients with ≥50% reduction at months 4 through 6 was 43.3% for erenumab 70 mg and 50.0% for erenumab 140 mg, vs 26.6% for placebo (ORs: 2.13 and 2.80 for erenumab 70 and 140 mg, respectively, vs placebo, P < .001 for both comparisons) (Fig. 1). Greater proportions of patients in the erenumab groups also achieved ≥50% reduction in MMD in each of the last 3 months of the DBTP (ie, each person must have achieved ≥50% reduction in MMD in each of months 4, 5, and 6) in comparison with placebo: 27.6% (86/312) for erenumab 70 mg (OR vs placebo 2.49; 95 % CI, 1.65, 3.76; P < .001) and 28.3% (90/318) for erenumab 140 mg (OR vs placebo 2.64; 95% CI, 1.74, 3.99; P < .001) vs 13.3% (42/316) in the placebo group (Fig. S1A).
Fig. 1.
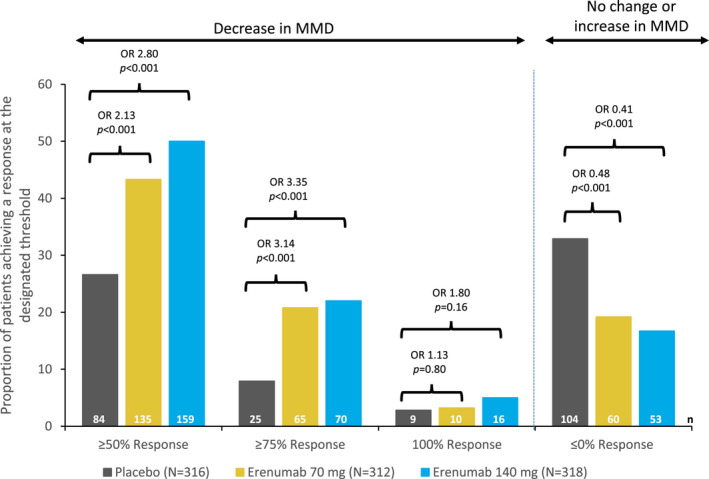
Proportions of patients achieving a ≥50%, ≥75%, or 100% decrease in monthly migraine days (MMD) and no change or increase in MMD during the last 3 months of the double‐blind treatment phase (DBTP) (months 4 through 6). The common odds ratios (ORs) and P values are obtained from a Cochran‐Mantel‐Haenszel (CMH) test, stratified by stratification factors region and prior/current treatment with migraine prophylactic medication. The P values for pairwise comparisons are nominal P values obtained from the CMH test using data including placebo and corresponding erenumab dose group only. Proportion (%) = n/N * 100. 95% confidence interval (CI) of the ORs for ≥50% response: 70 mg vs placebo (1.52, 2.99) and 140 mg (2.00, 3.92); for ≥75% response: 70 mg (1.91, 5.18) and 140 mg (2.05, 5.49); for 100%: 70 mg (0.45, 2.84) and 140 mg (0.78, 4.17). Breslow‐day test for homogeneity of the OR cross strata for the ≥75% responder over months 4, 5, and 6 is 0.52 for 70 mg and 0.20 for 140 mg. Breslow‐day test for homogeneity of the OR across strata for the 100% responder over months 4, 5, and 6 is 0.54 for 70 mg and 0.53 for 140 mg. Number in the efficacy analysis set (N); number in the subgroup (n).
The proportions of patients achieving ≥75% reductions in MMD in months 4 through 6 were 20.8% for erenumab 70 mg and 22.0% for erenumab 140 mg, compared with 7.9% in the placebo group (Fig. 1). The odds of having a ≥75% reduction in MMD were 3.14 times greater for erenumab 70 mg (95% CI, 1.91, 5.18) and 3.35 times greater for erenumab 140 mg (95% CI, 2.05, 5.49) vs placebo (P < .001 for both comparisons) (Fig. 1). The proportion of patients with ≥75% response in any of the individual months 4, 5, or 6 ranged from 21.5 to 25.6% for erenumab 70 mg, 24.5 to 28.6% for erenumab 140 mg, and 12.7 to 15.2% for placebo (Fig. 2A). In addition, greater proportions of patients in the erenumab groups achieved ≥75% reduction in MMD in each of the last 3 months of the DBTP in comparison with placebo: 8.3% (26/312) for erenumab 70 mg (OR vs placebo 2.11; 95 % CI, 1.06, 4.20; P = .029) and 11.6% (37/318) for erenumab 140 mg (OR vs placebo 3.13; 95% CI, 1.61, 6.06; P < .001) vs 4.1% (13/316) in the placebo group (Fig. S1B).
Fig. 2.
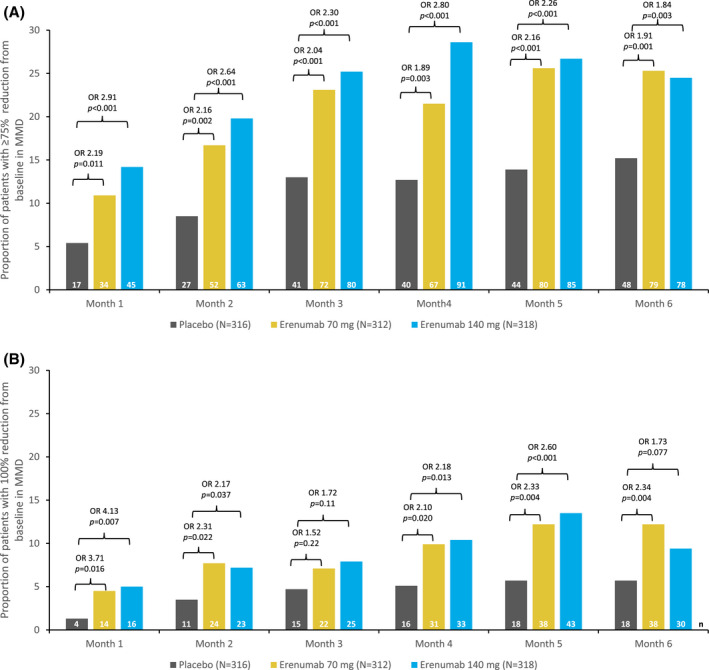
Proportion of patients with (A) ≥75% and (B) 100% reduction from baseline in monthly migraine days (MMD) during each month of the double‐blind treatment phase (DBTP). The common OR and P values are obtained from a Cochran‐Mantel‐Haenszel (CMH) test, stratified by stratification factors region and prior/current treatment with migraine prophylactic medication. The same analysis is repeated for each visit. The P values for pairwise comparisons are nominal P values obtained from the CMH test using data including placebo and corresponding erenumab dose group only. Proportion (%) = n/N * 100. (A) 95% confidence interval (CI) of the odds ratios (ORs) for month 1: 70 mg (1.19, 4.05) and 140 mg (1.62, 5.23); month 2: 70 mg (1.31, 3.55) and 140 mg (1.63, 4.27); month 3: 70 mg (1.33, 3.13) and 140 mg (1.51, 3.51); month 4: 70 mg (1.23, 2.92) and 140 mg (1.85, 4.25); month 5: 70 mg (1.43, 3.25) and 140 mg (1.51, 3.40); month 6: 70 mg (1.28, 2.85) and 140 mg (1.22, 2.76). (B) 95% CI of the ORs for month 1: 70 mg (1.20, 11.5) and 140 mg (1.36, 12.51); month 2: 70 mg (1.11, 4.81) and 140 mg (1.03, 4.53); month 3: 70 mg (0.77, 3.01) and 140 mg (0.88, 3.35); month 4: 70 mg (1.11, 3.95) and 140 mg (1.17, 4.07); month 5: 70 mg (1.29, 4.20) and 140 mg (1.46, 4.64); month 6: 70 mg (1.29, 4.23) and 140 mg (0.94, 3.21). Number in the efficacy analysis set (N); number in the subgroup (n).
Compared with placebo, numerically greater proportions of patients treated with erenumab achieved 100% response (complete elimination of migraine days) in months 4 through 6; the proportions that did so were small for this highly strict endpoint, which requires no migraine days over 3 consecutive months based on observed data (3.2% for erenumab 70 mg [95% CI, 0.45, 2.84] and 5.0% for erenumab 140 mg [95% CI, 0.78, 4.17], vs 2.8% for placebo, P = .80 and P = .16 for erenumab 70 and 140 mg, respectively) (Fig. 1). The proportion of patients with 100% response in any of the individual months 4, 5, or 6 ranged between 9.9 to 12.2% for erenumab 70 mg, 9.4 to 13.5% for erenumab 140 mg, and 5.1 to 5.7% for placebo (Fig. 2B).
Achievement of ≥75% and 100% response was substantially greater in either of the 2 erenumab groups than in the placebo group at the first prespecified time point for assessment of response, month 1 (P = .011 for erenumab 70 mg [OR vs placebo 2.19; 95% CI, 1.19, 4.05] and P < .001 for erenumab 140 mg [OR vs placebo 2.91, 95% CI, 1.62, 5.23] at the ≥75% threshold; P = .016 for erenumab 70 mg [OR vs placebo 3.71; 95% CI, 1.20, 11.5] and P = .007 for erenumab 140 mg [OR vs placebo 4.13; 95% CI, 1.36, 12.51] at the 100% threshold) (Fig. 2). Onset of efficacy by this first‐time point has also been reported at the ≥50% response threshold. 10
A total of 19.2%, 16.7%, and 32.9% patients had no reduction (ie, no change or increase) in MMD from baseline in months 4 through 6 on erenumab 70 mg, erenumab 140 mg and placebo, respectively (P < .001 vs placebo for both comparisons) (Fig. 1).
Waterfall Plot Analysis
To obtain insight into the variation in the response to erenumab or placebo across patients, we performed a waterfall plot analysis (Fig. 3), which shows the variation across individual responses in terms of change in MMD from baseline to the final 3 months (months 4‐6) of the DBTP against the individuals' baseline MMD. The bar chart shows individual patient baseline MMD, with the lowest to the left of the chart and the highest to the right; change in MMD is superimposed, with bars above the x‐axis indicating worsening, no bars indicating no change, and bars below the x‐axis indicating reduction in MMD, that is, improvement. The individuals' response levels did not appear to be related to their baseline MMD, as individuals ranged from being notably good responders to having no response, irrespective of baseline MMD. However, there was a clear trend for larger improvements correlated with higher MMD at baseline (Spearman correlation coefficient −0.349, −0.340, and −0.275 for erenumab 70 mg, erenumab 140 mg, and placebo, respectively).
Fig. 3.
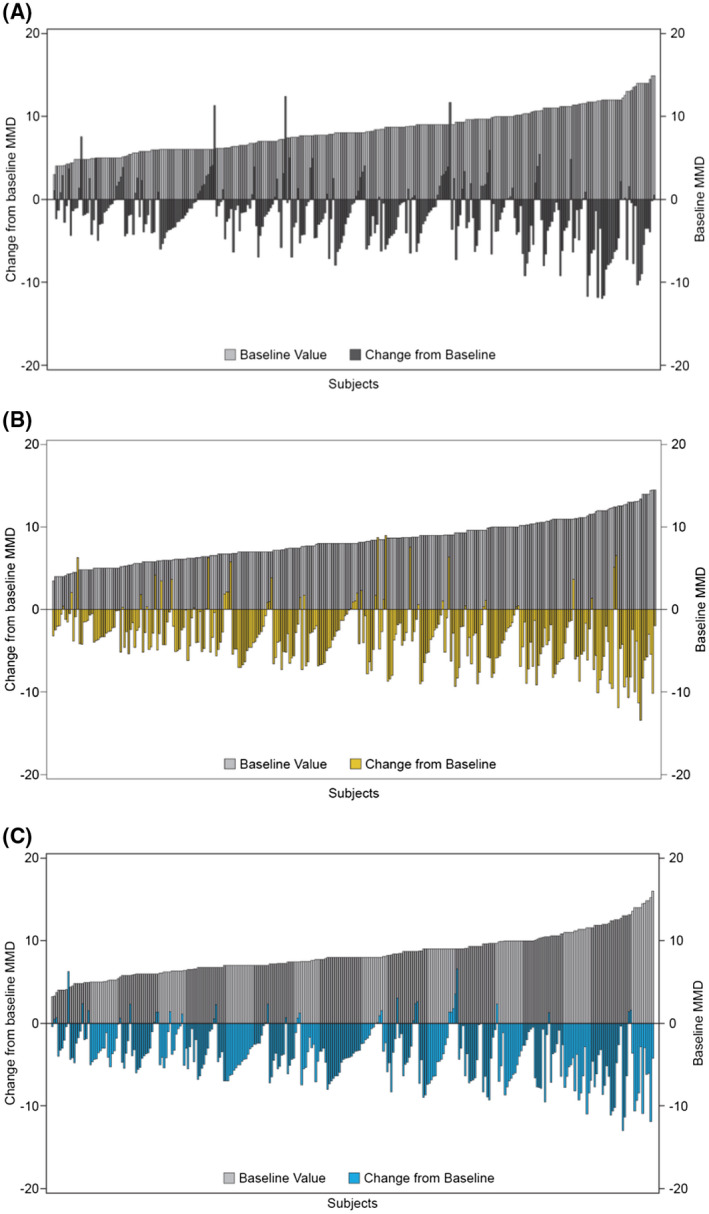
Waterfall plot of change in monthly migraine days (MMD) from baseline for placebo (A), erenumab 70 mg (B), and erenumab 140 mg (C). Waterfall plot showing change from baseline to primary endpoint against baseline MMD, Observed Efficacy Analysis Set. Baseline MMDs are shown in gray, with lowest baseline MMD to the left and highest to the right‐hand side. Change from baseline is shown in charcoal, yellow and blue, with reduction in MMD from baseline (improvement) below the x‐axis and worsening or no change above or at the x‐axis, respectively. The primary endpoint was the change in mean number of MMD from baseline to the final 3 months, months 4‐6, of the double‐blind treatment phase (DBPT).
Efficacy Based on Achievement of Response at the ≥50%, ≥75%, and 100% Response Thresholds
Baseline characteristics were similar for MMD across all response subgroups, whether the subgroup had achieved a response or not, and were similar to those for the overall population (Table 1). Baseline MSMD, which are given for the total population (ie, including patients not using acute migraine‐specific medications during baseline) were somewhat lower in those showing a response than those not achieving a response, across all response thresholds, irrespective of erenumab treatment group (Table 1). For those achieving a response, the range for baseline mean MSMD was 2.0‐2.4 days at the ≥75% response threshold and 1.4‐1.7 days at the 100% response threshold. By contrast, the range for mean baseline MSMD was 3.4‐3.8 days for the subgroups with no response across all response thresholds (Table 1). In terms of achievement of response at the ≥50% response threshold, lower proportions of patients achieving a response used acute migraine‐specific medications at baseline than did those who did not achieve a response (52.6% vs 64.0%, respectively, for the 70 mg erenumab group, and 59.1% vs 60.8%, respectively, for the 140 mg erenumab group). This proportion appeared to be lower again at the ≥75% and 100% response thresholds, although there were fewer patients at the higher response thresholds (Table 1).
Table 1.
Baseline Characteristics of Response Subgroups at the ≥50%, ≥75%, and 100% MMD Response Thresholds at Months 4 Through 6
| Threshold of MMD Response | Erenumab 70 mg | Erenumab 140 mg | ||||
|---|---|---|---|---|---|---|
| Achievement at Given Threshold | Overall Population | Achievement at Given Threshold | Overall Population | |||
| Yes† | No† | Yes† | No† | |||
| ≥50% response | ||||||
| N‡ | 135 | 161 | 296 | 159 | 143 | 302 |
| Baseline use of acute migraine‐specific medication, n (%) | 71 (52.6) | 103 (64.0) | 174 (58.8) | 94 (59.1) | 87 (60.8) | 181 (59.9) |
| MMD | 8.3 (2.5) | 8.3 (2.4) | 8.3 (2.5) | 8.2 (2.4) | 8.5 (2.5) | 8.3 (2.4) |
| MSMD | 2.9 (3.3) | 3.8 (3.5) | 3.4 (3.4) | 3.2 (3.4) | 3.6 (3.5) | 3.4 (3.4) |
| MPFID‐EA | 13.5 (8.5) | 14.1 (8.9) | 13.8 (8.7) | 12.6 (7.9) | 13.6 (8.6) | 13.1 (8.2) |
| MPFID‐PI | 11.8 (9.3) | 12.7 (9.6) | 12.3 (9.4) | 11.5 (8.3) | 12.8 (9.7) | 12.1 (9.0) |
| N‡ | 135 | 161 | 296 | 157 | 142 | 299 |
| HIT‐6 | 59.9 (6.3) | 60.4 (5.3) | 60.2 (5.7) | 59.3 (6.9) | 59.1 (5.8) | 59.2 (6.4) |
| mMIDAS | 13.7 (11.4) | 15.1 (11.6) | 14.5 (11.5) | 12.9 (10.6) | 13.0 (8.6) | 12.9 (9.7) |
| ≥75% response | ||||||
| N‡ | 65 | 231 | 296 | 70 | 232 | 302 |
| Baseline use of acute migraine‐specific medication, n (%) | 27 (41.5) | 147 (63.6) | 174 (58.8) | 35 (50.0) | 146 (62.9) | 181 (59.9) |
| MMD | 8.0 (2.4) | 8.4 (2.5) | 8.3 (2.5) | 8.1 (2.7) | 8.4 (2.4) | 8.3 (2.4) |
| MSMD | 2.0 (2.7) | 3.8 (3.5) | 3.4 (3.4) | 2.4 (3.1) | 3.7 (3.5) | 3.4 (3.4) |
| MPFID‐EA | 14.5 (9.0) | 13.6 (8.6) | 13.8 (8.7) | 13.5 (9.1) | 13.0 (8.0) | 13.1 (8.2) |
| MPFID‐PI | 13.2 (10.1) | 12.0 (9.3) | 12.3 (9.4) | 11.9 (8.8) | 12.2 (9.0) | 12.1 (9.0) |
| N‡ | 65 | 231 | 296 | 69 | 230 | 299 |
| HIT‐6 | 60.5 (6.5) | 60.1 (5.5) | 60.2 (5.7) | 60.9 (6.8) | 58.7 (6.2) | 59.2 (6.4) |
| mMIDAS | 13.4 (11.3) | 14.8 (11.5) | 14.5 (11.5) | 13.7 (11.3) | 12.7 (9.1) | 12.9 (9.7) |
| 100% response | ||||||
| N‡ | 10 | 286 | 296 | 16 | 286 | 302 |
| Baseline use of acute migraine‐specific medication, n (%) | 4 (40.0) | 170 (59.4) | 174 (58.8) | 8 (50.0) | 173 (60.5) | 181 (59.9) |
| MMD | 7.9 (2.4) | 8.3 (2.5) | 8.3 (2.5) | 6.9 (2.2) | 8.4 (2.4) | 8.3 (2.4) |
| MSMD | 1.4 (2.3) | 3.4 (3.4) | 3.4 (3.4) | 1.7 (2.3) | 3.5 (3.5) | 3.4 (3.4) |
| MPFID‐EA | 17.6 (9.7) | 13.7 (8.7) | 13.8 (8.7) | 11.3 (9.5) | 13.2 (8.2) | 13.1 (8.2) |
| MPFID‐PI | 15.7 (10.7) | 12.2 (9.4) | 12.3 (9.4) | 10.4 (10.1) | 12.2 (8.9) | 12.1 (9.0) |
| N‡ | 10 | 286 | 296 | 15 | 284 | 299 |
| HIT‐6 | 59.7 (6.6) | 60.2 (5.7) | 60.2 (5.7) | 61.6 (7.6) | 59.1 (6.3) | 59.2 (6.4) |
| mMIDAS | 20.2 (14.8) | 14.3 (11.3) | 14.5 (11.5) | 16.0 (18.5) | 12.8 (9.0) | 12.9 (9.7) |
Data are presented as mean (SD) unless otherwise specified.
Please refer to Goadsby PJ, et al for complete demographics, baseline disease characteristics.
Acute migraine‐specific medication: During the baseline phase, 557 (58.5%) patients used triptan‐based medications and 4 (0.4%) patients used ergotamine‐based medications (safety analysis set). 10
Yes or No indicates response/no response at given threshold.
Only patients with a value at both baseline and the given time point were included in the analyses.
HIT‐6 = Headache Impact Test (higher score indicates worse outcomes); MMD = monthly migraine days; mMIDAS = Migraine Disability Assessment (higher score indicates worse outcomes); MPFID‐EA = Migraine Physical Function Impact Diary‐Everyday Activities; MPFID‐PI = MPFID‐Physical Impairment; MSMD = migraine‐specific medication treatment days; N = total number of patients; n = number of patients with acute migraine‐specific medication use at baseline; SD = standard deviation; % = (n/N) × 100
HIT‐6 and mMIDAS baseline values were similar between responders and non‐responders across all thresholds, with one exception: at the 100% threshold, mMIDAS baseline values were consistently higher for responders compared with non‐responders (Table 1).
For those achieving a response at the ≥50% response threshold, mean (standard deviation, SD) reduction in MMD was 6.1 (2.1) for erenumab 70 mg and 6.0 (2.1) for 140 mg, from baselines of 8.3 (2.5) and 8.2 (2.4), respectively (Fig. 4A). For those not achieving the ≥50% response, the mean (SD) reduction in MMD was 1.1 (2.8) for erenumab 70 mg and 1.4 (2.3) for erenumab 140 mg, from baselines of 8.3 (2.4) and 8.5 (2.5), respectively (Fig. 4A).
Fig. 4.
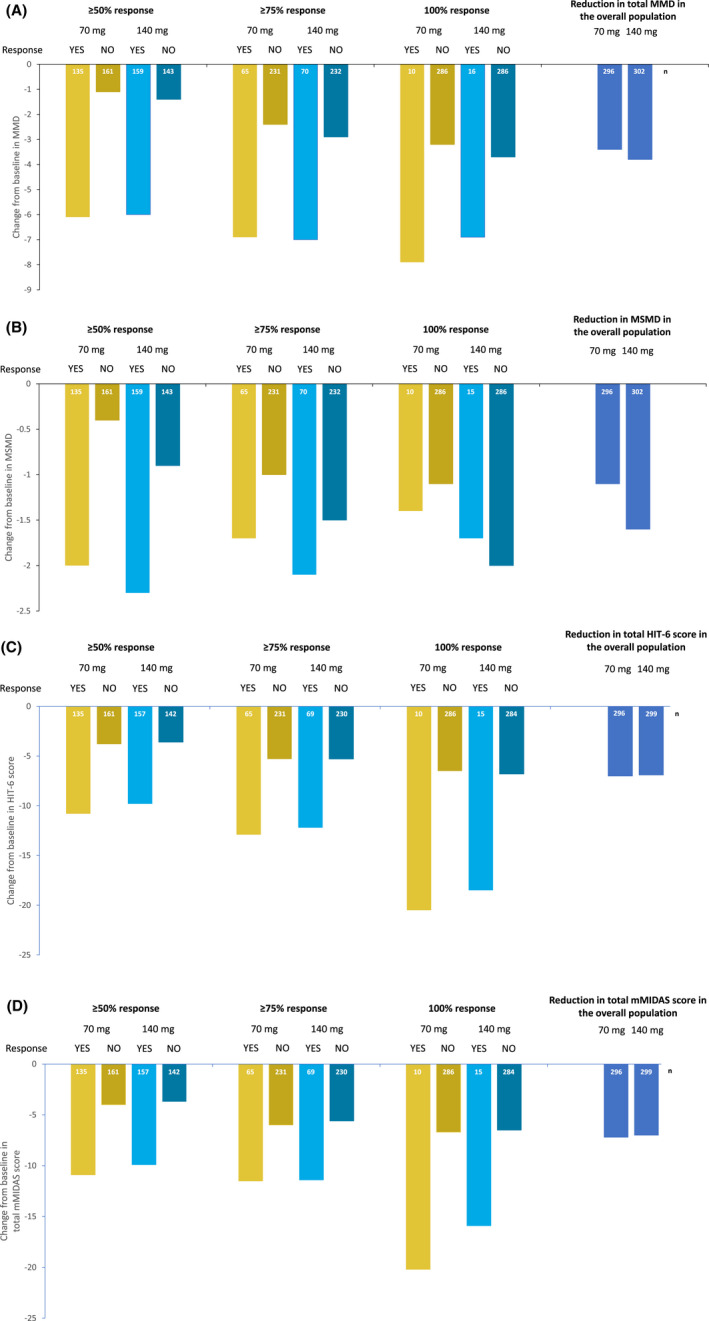
Exploratory measures of efficacy during the last 3 months of the double‐blind treatment phase (DBTP; months 4 through 6) in subgroups achieving ≥50%, ≥75%, and 100% response. (A) Mean change in monthly migraine days (MMD) from baseline. (B) Mean change in acute migraine‐specific medication treatment days (MSMD) from baseline. (C) Mean change in 6‐item Headache Impact Test (HIT‐6) from baseline. (D) Mean change in Modified Migraine Disability Assessment (mMIDAS) total score from baseline (every 3 months [Q3M]). The mMIDAS was converted into a Q3M assessment of disability. The mMIDAS scores were summed for months 4, 5, and 6 by taking the average of these post‐baseline assessments and multiplying by 3 (if at least 1 mMIDAS was present).
In comparison with the ≥50% response threshold, both doses of erenumab resulted in numerically greater reductions in MMD at the ≥75% and 100% response thresholds (Fig. 4A). For the subgroups that did not achieve a response at the various response thresholds (ie, those with <50%, <75% or <100% response), the reductions in MMD were slightly higher for those that did not achieve the ≥75% and 100% response thresholds than for those who did not achieve the ≥50% threshold (Fig. 4A). Similar trends were observed for MSMD and all PROs tested: HIT‐6, mMIDAS, MPFID‐EA, and MPFID‐PI (Figs. 4B‐D and S2), where a reduction in test score translates to improvement in disability. 9
For MSMD at the ≥50% threshold, a mean (SD) reduction of 2.0 (2.4) days was achieved for erenumab 70 mg and a mean (SD) of 2.3 (2.6) days was achieved for erenumab 140 mg, from baselines of 2.9 (3.3) and 3.2 (3.4), respectively (Fig. 4B). For those subgroups achieving the various response thresholds, reductions in MSMD were highest at the ≥50% threshold, and lowest at the 100% threshold. However, the baseline MSMDs were also lower at the ≥75% and 100% response thresholds (Table 1).
The reductions for those not achieving the ≥75% and 100% responses were numerically similar to those for the overall population (Fig. 2A,B); this is not unexpected given that the majority of the overall population did not achieve MMD response at the ≥75% and 100% thresholds.
Discussion
The exploratory and post hoc analyses of the STRIVE pivotal clinical trial data presented here corroborate previous analyses on the efficacy of erenumab as a preventive treatment in EM 9 , 10 , 11 , 12 , 13 , 14 , 23 and provide context for understanding the spectrum of response to erenumab. Erenumab has shown efficacy across a variety of response thresholds, from the standard ≥50% threshold 10 to the stricter ≥75% and 100% thresholds also presented here. It is worth noting that achievement of a 100% reduction in MMD over months 4 through 6 is an extremely strict endpoint, as it means that a patient must not have had a single migraine day during a 3‐month period.
STRIVE showed that 43.3% of patients on erenumab 70 mg and 50.0% of patients on erenumab 140 mg, as compared with 26.6% in the placebo group, achieved a ≥50% reduction in MMD at months 4 through 6 compared to the pre‐erenumab treatment baseline. 10 We show here that a clinically relevant number of patients achieved the more remarkable ≥75% or 100% response thresholds with either 70 or 140 mg erenumab. A most notable strength here is that the ORs allow anchoring of the response in the active group to that in the placebo group (which can vary considerably) and put the observed efficacy into context.
Erenumab treatment effect at the ≥75% and 100% response thresholds was already visible by month 1, as has been reported for the ≥50% response threshold, 10 and the proportions of patients responding increased over the first 3 months and were sustained thereafter, albeit with some month‐to‐month variation in response (Fig. 2). This month‐to‐month variation was not seen at the ≥50% response threshold, where a steady‐state in MMD reduction appeared to have been achieved by month 2, 10 and it is perhaps due, at least in part, to the fewer numbers of patients achieving ≥75% and 100% responses (Table 1). Lower proportions of patients achieved a response at the various response thresholds over months 4 through 6 than at months 4, 5, or 6 individually as the former is more difficult to achieve. This difference was more extreme for the 100% than for the ≥75% response threshold, since achieving a complete remission for a sustained period of time is a truly remarkable response and may not be achievable for a high proportion of patients with EM, especially over the time interval (4‐6 months) studied here. It may be helpful for future studies to divide the current 100% threshold into smaller ranges (75‐80%, 80‐89%, and 90‐99%) to have a clearer understanding of how high‐level responses are distributed among patients. A number of patients achieved a ≥75% response in MMD reduction in each of months 4, 5, and 6: 8.3% for erenumab 70 mg, and 11.2% for erenumab 140 mg vs 4.1% in the placebo group. About 20% of patients across treatment groups did not show any improvement, defined as no change or increase in MMD from baseline, during months 4 through 6 (Fig. 1). This is the first time that data on non‐responders has been described in this context, as previous erenumab trials did not report results for this group of patients. 9 , 10 , 11 , 12 , 13 , 14 The lack of response in a subgroup of patients raises the possibility that there may be as yet undefined migraine subtypes in which the CGRP pathway may not play a prominent role in the generation and pathogenesis of their migraine attack.
In migraine clinical trials, outcomes are reported for the overall population; however, response to treatment varies from patient to patient, as is shown by the waterfall plots presented here. This is relevant to treatment decisions in clinical practice, as even those patients who have not achieved response at a particular threshold may experience other benefits that reflect improvement in quality of life and functioning, which may have a considerable effect on patients' daily lives. Therefore, we analyzed change from baseline values during months 4 through 6 for a variety of efficacy and PRO endpoints according to whether a response at the ≥50%, ≥75%, and 100% response thresholds was achieved, with the aim of gaining insight into the actual treatment benefit for those patients who did or did not achieve an arbitrary threshold (eg, ≥50%) response.
Patients achieving MMD response at the various thresholds showed substantially greater reductions in mean MMD, HIT‐6, mMIDAS, MPFID‐EA, and MPIFID‐PI from baseline than did the overall population (Fig. 4); baseline values for each outcome measure were similar in subgroups achieving a given threshold of response compared with those that did not achieve it. Substantial reductions in all PRO scores were obtained in MMD responders across all response thresholds. Although improvements in PROs were also seen in the subgroups of patients not achieving a particular response threshold, the benefit was larger for responders. All patients treated with erenumab achieved a reduction in HIT‐6 over time, showing reduced impact of migraine even if they did not achieve a response at the ≥50%, ≥75% or 100% MMD thresholds; in the case of patients who achieved or did not achieve a ≥75% or 100% response, the reduction in HIT‐6 was ≥5 points, which is considered clinically relevant. 24 Those achieving responses at the ≥50%, ≥75%, and 100% response thresholds also had improved mMIDAS scores: the clinically significant reduction in total score of ≥5 points 25 was achieved at the ≥75% and 100% response thresholds (Fig. 4D). For both MPFID‐EA and MPFID‐PI, responders across all response thresholds achieved a clinically significant ≥5‐point change (Fig. S2). 25 , 26 Similar to HIT‐6 score results, mMIDAS, MPFID‐EA, and MPFID‐PI scores improved for patients showing any response, even for those who did not achieve response at the ≥50%, ≥75%, and 100% response thresholds. This shows that even subjects that do not achieve MMD responses at a given threshold may be deriving treatment benefit; in these cases, improvement in PROs can provide additional important outcome information even in the absence of large MMD responses.
These results highlight both the spectrum of treatment responses that exists and that outcomes obtained for the overall study population do not predict benefit at the individual level. Response thresholds are arbitrary and dichotomous, and as demonstrated here, patients who do not achieve a given level of response can still benefit from treatment as demonstrated by improvement in other clinically important and validated PRO measures. A number of factors likely influence an individual's ability to achieve a response to erenumab. However, the effects of these factors, which may include baseline characteristics, underlying pathophysiology, and genetics, among others, on response are not understood; therefore, there is no way at present to predict the level of response that a patient will experience, which represents an important gap in our knowledge. The various types of responses, coupled with the added placebo effect in all measures, most obvious in the waterfall plot, indicate the importance to act in all reasonable ways for this patient group.
In contrast to MMD and the various PROs, which were similar across all response thresholds, baseline acute migraine‐specific medication use and MSMD appeared to be lower in subgroups achieving MMD response at the higher thresholds (Table 1). These differences may be due, at least partly, to the smaller numbers of patients at the higher response thresholds. However, it is also possible that high level MMD responders are more responsive to acute migraine‐specific medication (triptans), and therefore, need to use less to achieve an improvement in their symptoms, or they may respond sufficiently well to other acute medications such as nonsteroidal anti‐inflammatory drugs (NSAIDs) so that they do not rely on triptans. This trend for baseline MSMD was also observed in chronic migraine (CM) for those achieving a response (baseline acute migraine‐specific medication was not included in this analysis); however, in contrast with EM, patients achieving a response also had lower baseline MMD than did those not achieving a response. 27 It is a plausible hypothesis that patients with more severe disease may not only use triptans more frequently, but they may also be somewhat less likely to achieve a high level response to preventive treatment.
While the efficacy results we present in this study are in line with the primary results of randomized, controlled erenumab clinical trials, 9 , 10 , 12 it should be noted that these are mostly post hoc analyses based on exploratory endpoints. It should also be noted that these analyses were based on descriptive statistics alone, meaning definitive conclusions cannot be drawn from our results. The response thresholds employed in MMD responder analyses are based on population data, and thus may underestimate individual responses, which is a limitation of this study. However, our waterfall plots and PRO results show that those who do not achieve a given level of response may still experience treatment benefit.
The results of our analysis – showing improvement in PROs regardless of response threshold reduction in MMD – suggest that measuring a single endpoint, even one as seemingly meaningful as MMD reduction, does not capture the totality of the benefit that patients derive from preventive treatment. In the future, we will need to continue to develop novel endpoints, including perhaps composite endpoints, that better reflect clinically meaningful benefit derived from a particular drug or intervention.
Conclusions
In conclusion, erenumab treatment groups achieved greater MMD responses than placebo, regardless of response threshold; individual response, however, was highly heterogeneous and showed that patients can still derive benefit from treatment even in the absence of a given response threshold. Patients who achieved responses at the ≥50%, ≥75%, or 100% thresholds displayed substantially greater reductions in migraine days and in migraine‐specific medication use as well as substantial improvement in migraine‐related disability and headache impact as determined by reductions in HIT‐6, mMIDAS, and MPFID test scores, as compared with the overall erenumab‐treated population. The findings presented here corroborate our findings in CM, 27 and together they provide context for establishing realistic patient and physician expectations regarding the magnitude of treatment benefit that may be achieved by patients treated with erenumab.
Statement of Authorship
Category 1
(a) Conception and Design
Daniel D. Mikol
(b) Acquisition of Data
Gregor Broessner, Jo H. Bonner, Yngve Hallström
(c) Analysis and Interpretation of Data
Gregor Broessner, Uwe Reuter, Jo H. Bonner, David W. Dodick, Yngve Hallström, Hernan Picard, Feng Zhang, Robert A. Lenz, Jan Klatt, Daniel D. Mikol
Category 2
(a) Drafting the Manuscript
Gregor Broessner
(b) Revising It for Intellectual Content
Gregor Broessner, Uwe Reuter, Jo H. Bonner, David W. Dodick, Yngve Hallström, Hernan Picard, Feng Zhang, Robert A. Lenz, Jan Klatt, Daniel D. Mikol
Category 3
(a) Final Approval of the Completed Manuscript
Gregor Broessner, Uwe Reuter, Jo H. Bonner, David W. Dodick, Yngve Hallström, Hernan Picard, Feng Zhang, Robert A. Lenz, Jan Klatt, Daniel D. Mikol
Supporting information
Fig S1A
Fig S1B
Fig S2A
Fig S2B
Legend
Acknowledgments
We thank the study investigators and patients for their participation and commitment to this work. The authors thank Joan Smyth (PhD) and Vanesa Martinez Lopez (PhD) of Novartis Ireland Limited, Dublin, Ireland for providing medical writing support.
Conflict of Interests: Gregor Broessner, MD, has received unrestricted grants, honoraria, personal fees, and travel grants from Allergan, Amgen, AstraZeneca, European Headache Foundation (EHF), Fresenius, Grünenthal, Janssen Cilag, Lilly, Linde AG, Menarini, Novartis, Österreichische Akademie der Wissenschaften (ÖAW), Österreichische Gesellschaft für Neurologie (ÖGN), Österreichische Kopfschmerzgesellschaft (ÖKSG), Pfizer, Reckitt Benkiser, St. Jude Medical, and Teva. Uwe Reuter, MD, has acted as a consultant for Allergan, Amgen, Eli Lilly, Novartis, Teva, has served on advisory boards for Allergan, Amgen, Autonomic Technologies, Eli Lilly, Medscape, Novartis, Teva, is a member of speakers' bureaus for Allergan, Amgen, Eli Lilly, Medscape, Novartis, StreamedUp, Teva, and has received research support from the German Federal Ministry of Education and Research (BMBF) and Novartis. Jo H. Bonner, MD, has nothing to disclose. David W. Dodick, MD, has received personal fees from AEON, Alder BioPharmaceuticals, Allergan, Amgen, Amzak Health, Association of Translational Medicine, Autonomic Technologies, Axsome, Biohaven, Charleston Laboratories, Clexio, Daniel Edelman Inc., Dr Reddy's Laboratories/Promius, Electrocore LLC, Eli Lilly, eNeura, Equinox, Foresite Capital, Impel, Ipsen, Neurolief, Nocira, Novartis, Oppenheimer, Pieris, PSL Group Services, Revance, Salvia, Satsuma, Sun Pharma (India), Supernus, Teva, Theranica, University Health Network, Upjohn (Division of Pfizer), Vedanta, WL Gore, XoC, Zosano, ZP Opco; has received speaking fees from Amgen, Eli Lilly, Lundbeck, Novartis Canada; has received CME fees or royalty payments from Academy for Continued Healthcare Learning, Cambridge University Press, Catamount, Chameleon, Global Access Meetings, Global Life Sciences, Global Scientific Communications, Haymarket, HealthLogix, Medicom Worldwide, MedLogix Communications, Mednet, Miller Medical, Oxford University Press, PeerView, Universal Meeting Management, UpToDate (Elsevier), WebMD Health/Medscape, Wolters Kluwer Health; owns stock options for Aural Analytics, Epien, Healint, King‐Devick Technologies, Matterhorn, Nocira, Ontologics, Precon Health, Second Opinion/Mobile Health, Theranica; has acted as consultant without fee for Aural Analytics, Epien, Healint, Second Opinion/Mobile Health; is a member of the board of Directors of Epien, King‐Devick Technologies, Matterhorn, Ontologics, Precon Health; owns the patent 17189376.1‐1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis without fee; has received research funding from American Migraine Foundation, Henry Jackson Foundation, PCORI, US Department of Defense; has received professional society fees or reimbursement for travel from American Academy of Neurology, American Brain Foundation, American Headache Society, American Migraine Foundation, Canadian Headache Society, International Headache Society. Yngve Hallström, MD, has served on advisory boards for Amgen, Novartis and Teva. Hernan Picard, MD, is an employee of and stockholder in Amgen. Feng Zhang, MS, is an employee of and stockholder in Amgen. Robert A. Lenz, MD, PhD, is an employee of and stockholder in Amgen. Jan Klatt, MD, is an employee of and stockholder in Novartis. Daniel D. Mikol, MD, PhD, is an employee of and stockholder in Amgen.
Funding: The study was funded by Amgen Inc. Erenumab is co‐developed by Amgen and Novartis.
REFERENCES
- 1. Headache Classification Committee of the International Headache Society . The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 2. Goadsby PJ, Holland PR, Martins‐Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev. 2017;97:553‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burch RC, Loder S, Loder E, Smitherman TA. The prevalence and burden of migraine and severe headache in the United States: Updated statistics from government health surveillance studies. Headache. 2015;55:21‐34. [DOI] [PubMed] [Google Scholar]
- 4. Global Burden of Disease Study 2016 . Disease and Injury Incidence and Prevalence Collaborators (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: A systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ford JH, Jackson J, Milligan G, Cotton S, Ahl J, Aurora SK. A real‐world analysis of migraine: A cross‐sectional study of disease burden and treatment patterns. Headache. 2017;57:1532‐1544. [DOI] [PubMed] [Google Scholar]
- 6. Schou WS, Ashina S, Amin FM, Goadsby PJ, Ashina M. Calcitonin gene‐related peptide and pain: A systematic review. J Headache Pain. 2017;18:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ong JJY, Wei DY, Goadsby PJ. Recent advances in pharmacotherapy for migraine prevention: From pathophysiology to new drugs. Drugs. 2018;78:411‐437. [DOI] [PubMed] [Google Scholar]
- 8. Shi L, Lehto SG, Zhu DX, et al. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene‐related peptide receptor. J Pharmacol Exp Ther. 2016;356:223‐231. [DOI] [PubMed] [Google Scholar]
- 9. Dodick DW, Ashina M, Brandes JL, et al. ARISE: A phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38:1026‐1037. [DOI] [PubMed] [Google Scholar]
- 10. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377:2123‐2132. [DOI] [PubMed] [Google Scholar]
- 11. Reuter U, Goadsby PJ, Lanteri‐Minet M, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two‐to‐four previous preventive treatments were unsuccessful: A randomised, double‐blind, placebo‐controlled, phase 3b study. Lancet. 2018;392:2280‐2287. [DOI] [PubMed] [Google Scholar]
- 12. Sun H, Dodick DW, Silberstein S, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: A randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Neurol. 2016;15:382‐390. [DOI] [PubMed] [Google Scholar]
- 13. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: A randomised, double‐blind, placebo‐controlled phase 2 trial. Lancet Neurol. 2017;16:425‐434. [DOI] [PubMed] [Google Scholar]
- 14. Sakai F, Takeshima T, Tatsuoka Y, et al. A randomized phase 2 study of erenumab for the prevention of episodic migraine in Japanese adults. Headache. 2019;59:1731‐1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. European Medicines Agency . Aimovig Label. Available at https://www.ema.europa.eu/en/documents/product‐information/aimovig‐epar‐product‐information_en.pdf. Accessed January 3, 2019. [Google Scholar]
- 16. Food and Drug Administration . Aimovig Label. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf. Accessed January 3, 2019. [Google Scholar]
- 17. Silberstein S, Tfelt‐Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28:484‐495. [DOI] [PubMed] [Google Scholar]
- 18. Tassorelli C, Diener H‐C, Dodick DW, et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia. 2018;38:815‐831. [DOI] [PubMed] [Google Scholar]
- 19. Kosinski M, Bayliss MS, Bjorner JB, et al. A six‐item short‐form survey for measuring headache impact: The HIT‐6. Qual Life Res. 2003;12:963‐974. [DOI] [PubMed] [Google Scholar]
- 20. Kawata AK, Hsieh R, Bender R, et al. Psychometric evaluation of a novel instrument assessing the impact of migraine on physical functioning: The migraine physical function impact diary. Headache. 2017;57:1385‐1398. [DOI] [PubMed] [Google Scholar]
- 21. Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) questionnaire to assess headache‐related disability. Neurology. 2001;56(Suppl. 1):S20‐S28. [DOI] [PubMed] [Google Scholar]
- 22. Buse DC, Lipton RB, Hallström Y, et al. Migraine‐related disability, impact, and health‐related quality of life among patients with episodic migraine receiving preventive treatment with erenumab. Cephalalgia. 2018;38:1622‐1631. [DOI] [PubMed] [Google Scholar]
- 23. Ashina M, Dodick D, Goadsby PJ, et al. Erenumab (AMG 334) in episodic migraine: Interim analysis of an ongoing open‐label study. Neurology. 2017;89:1237‐1243. [DOI] [PubMed] [Google Scholar]
- 24. Smelt AF, Assendelft WJ, Terwee CB, Ferrari MD, Blom JW. What is a clinically relevant change on the HIT‐6 questionnaire? An estimation in a primary‐care population of migraine patients. Cephalalgia. 2014;34:29‐36. [DOI] [PubMed] [Google Scholar]
- 25. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1‐18. [DOI] [PubMed] [Google Scholar]
- 26. Tepper SJ. History and review of anti‐calcitonin gene‐related peptide (CGRP) therapies: From translational research to treatment. Headache. 2018;58(Suppl. 3):238‐275. [DOI] [PubMed] [Google Scholar]
- 27. Brandes JL, Diener HC, Dolezil D, et al. The spectrum of response to erenumab in patients with chronic migraine and subgroup analysis of patients achieving ≥50%, ≥75%, and 100% response. Cephalalgia. 2020;40:28‐38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1A
Fig S1B
Fig S2A
Fig S2B
Legend


