Abstract
Introduction:
Pathologic complete response (pCR) after neoadjuvant chemotherapy has a demonstrated survival advantage; however, outcomes for non-pCR by receptor status are less understood. We sought to evaluate survival and distant recurrence by receptor status for patients with residual stage II/III breast cancer.
Methods:
A stage-stratified random sample of 11,366 patients with stage II-III breast cancer in 2006–2007 was selected from 1217 facilities in the National Cancer Database for a Commission on Cancer Special Study. We identified patients with residual pathologic stage II/III cancer who received standard of care therapy based on receptor status. Distant recurrence and 5-year survival were abstracted and Kaplan-Meier curves were generated by receptor status. Multivariable Cox regression was used to estimate hazard ratios for death and distant recurrence.
Results:
734 patients had residual disease; 58%, 28%, and 14% were ER or PR+/Her2neu−, ER and PR−/Her2neu−, and Her2neu+(any ER/PR) respectively. ER and PR−/Her2neu− cancers had the poorest 5-year overall (52% vs 82% for Her2neu+ and ER or PR+/Her2neu−, p<0.0001) and distant recurrence-free survival (57% vs 72% Her2neu+ and 77% ER or PR+/Her2neu, p<0.0001). Cox regression models demonstrated a higher likelihood of distant recurrence and death for patients with ER and PR−/Her2neu− disease (HR 2.25, 95% CI 1.56–3.24 and HR 3.19, 95% CI 2.20–4.64 respectively) compared to ER or PR+/Her2neu−.
Conclusion:
Patients with residual ER and PR−/Her2neu− cancer have a significant risk of distant recurrence and mortality compared to other breast cancer types, supporting the consideration for additional adjuvant therapy and novel clinical trials in this cohort.
Introduction:
Many studies have demonstrated variations by receptor status in the rates of pathologic complete response (pCR) following neoadjuvant chemotherapy.1–8 pCR has been associated with improved survival and decreased recurrence for most receptor subtypes.4–15 Even those with a small amount of residual cancer for many receptor subtypes have a worse prognosis than those who achieve a pCR.16,17 However, once a pCR is achieved, the majority of patients have a similar prognosis regardless of their receptor subtype.4,11,18,19
While pCR and its relationship to prognosis across receptor subtypes is well known, fewer studies have examined the difference in outcomes by receptor status for patients who do not achieve pCR.4,11,20 The studies that have evaluated differences in outcomes by receptor status in patients with residual breast cancer following neoadjuvant chemotherapy have been conducted at a single institution,4,11 had small numbers of patients,4,11,20 did not categorize receptor subtype beyond ER and PR−/Her2neu− and non-ER and PR−/Her2neu−,11 and/or were conducted prior to the routine use of Her2neu-targeted therapy.20 Understanding recurrence and survival outcomes for women with residual breast cancer following neoadjuvant chemotherapy may help inform further adjuvant treatment and follow-up recommendations in addition to allowing for more informed conversations with patients regarding prognosis.
As such, the objective of our study was to use a large, national database with five years of follow-up to evaluate the differences in overall and distant recurrence-free survival by receptor status in women who underwent neoadjuvant chemotherapy and had residual pathologic stage II/III breast cancer (yp II/III). We hypothesized that outcomes for patients with high volume residual disease after neoadjuvant chemotherapy would be better for those with options for ongoing targeted therapies (e.g. those with Her2neu and/or ER/PR positivity).
Methods:
Data Source
The National Cancer Data Base (NCDB), a collaborative effort between the American College of Surgeons Commission on Cancer (CoC) and the American Cancer Society, is a large national cancer registry capturing approximately 70% of all newly diagnosed cancers in the United States.21 This database includes patient and tumor characteristics, cancer staging, treatment received and mortality. Imaging and recurrence data and additional key fields were abstracted to supplement standard elements as part of a special study initiative. In the study, a stage-stratified sample of 10 patients with pathologic stage II-III breast cancer diagnosed between 2006–2007 were randomly selected from each of 1217 CoC facilities (n=11,366). This analysis of deidentified data was viewed as exempt by the University of Wisconsin Institutional Review Board.
Data Collection
Standard data elements from the NCDB included patient demographics (age, race, comorbidities, insurance status, zip code level median household income, percent with high school education), tumor characteristics (histology, grade, positive lymph nodes, tumor size, hormone receptor status, Her2neu receptor status), treatment characteristics (receipt of radiation, type of surgery, treatment facility type), and survival. Abstraction as part of the CoC special study confirmed recorded receptor status (estrogen, progesterone, and Her2neu receptors), treatment, comorbidities, and survival. Information regarding second breast events (new primary or local recurrence) and distant recurrence, recurrence detection, breast cancer imaging follow-up, and receipt of Her2neu-targeted therapy was abstracted. Patients were followed for a maximum of 5 years from the time of first surgery. Patients who were lost to follow-up were censored; only 3% of patients were censored prior to 5 years.
Study Inclusion/exclusion criteria
For the current analysis, females ≥18 years of age who had residual pathologic stage II/III breast cancer following neoadjuvant chemotherapy were identified (n=1,147). Patients with unknown tumor size, lymph node status, or receptor status were excluded (Figure 1). Patients who did not receive appropriate targeted therapy, i.e. Her2neu-targeted therapy or endocrine therapy, were also excluded. Finally, patients who had evidence of disease progression before surgery or had unknown distant recurrence and those with unknown histology or inflammatory breast cancer were excluded.
Figure 1.

Flowchart of study inclusion/exclusion
Data analysis
Patients were categorized into three groups based on receptor subtype: ER or PR+/Her2neu−, ER and PR−/Her2neu−, and Her2neu+ (including both ER/PR positive and negative patients). Her2neu+ patients were grouped into one category and not further subdivided based on ER and PR status due to low patient numbers. Descriptive statistics were generated for the cohort by receptor subtype. Chi-square testing was used to compare the proportion of events for patients experiencing distant recurrence and death by receptor subtype. Kaplan-Meier estimation was used to generate overall and distant recurrence-free survival curves for women with residual pathologic stage II/III breast cancer following neoadjuvant chemotherapy stratified by receptor status. Log-rank testing was used to assess differences in time-to-event data, distant recurrence and death, by receptor subtype. Multivariable Cox regression models were generated to assess the hazard ratios for death or distant recurrence by receptor subtype while controlling for patient, tumor, and treatment characteristics. For these analyses, residual tumor size, residual positive lymph nodes, race, age, grade, zip code level quartiles of median household income and population with less than a high school education, comorbidities, and facility type were included in the models as control variables. Stata software (version 15) was used for all statistical analyses with p <0.05 considered statistically significant.
Results:
A total of 734 patients met our inclusion/exclusion criteria for residual stage II/III breast cancer after neoadjuvant chemotherapy. Of these, 77% (n=567) had residual cancer within both the breast and lymph nodes. Patient and tumor characteristics for the overall cohort and by receptor subtype are summarized in Table 1. Overall, most women were between 50–70 years of age (48%) and the patient population was primarily white (78%). The majority (58%) of patients had ER or PR+/Her2neu− disease followed by ER and PR−/Her2neu− disease (28%) and Her2neu+ disease (14%). Significant differences among receptor subtypes existed for race (p<0.0005), residual lymph node status (p=0.002), and tumor grade (p<0.0005) (Table 1). Women with ER and PR−/Her2neu− disease were more likely to be black (27%) with negative residual lymph nodes (32%) and grade 3 tumors (84%) compared to ER or PR+/Her2neu− and Her2neu+ disease.
Table 1:
Patient Demographics of Patients with Residual Pathologic Stage II/III Cancer by Receptor Subtype
| Overall Cohort (734) | ER or PR+/Her2neu− (427) | ER and PR−/Her2neu− (207) | Her2neu+ (any ER or PR) (100) | P-value | |
|---|---|---|---|---|---|
| Age | 0.37 | ||||
| ≤50 years | 42.1% (309) | 43.3% (185) | 37.2% (77) | 47.0% (47) | |
| >50 – <70 years | 47.5% (349) | 46.1% (197) | 53.1% (110) | 42.0% (42) | |
| ≥70 years | 10.4% (76) | 10.5% (45) | 9.7% (20) | 11.0% (11) | |
| Race | <0.0005 | ||||
| White | 78.3% (575) | 82.2% (351) | 69.1% (143) | 81.0% (81) | |
| Black | 16.1% (118) | 11.9% (51) | 26.6% (55) | 12.0% (12) | |
| Other | 5.6% (41) | 5.9% (25) | 4.3% (9) | 7.0% (7) | |
| Comorbidities | 0.46 | ||||
| None | 73.3% (538) | 74.7% (319) | 69.1% (143) | 76.0% (76) | |
| 1 + | 21.1% (155) | 19.4% (83) | 25.6% (53) | 19.0% (19) | |
| Unknown | 5.6% (41) | 5.9% (25) | 5.3% (11) | 5.0% (5) | |
| Residual Tumor Size | 0.06 | ||||
| <2 cm | 17.7% (130) | 16.2% (69) | 17.4% (36) | 25.0% (25) | |
| 2–5 cm | 50.3% (369) | 53.6% (229) | 44.9% (93) | 47.0% (47) | |
| >5 cm or diffuse/inflam | 32.0% (235) | 30.2% (129) | 37.7% (78) | 28.0% (28) | |
| Residual Positive Nodes | 0.002 | ||||
| Negative | 22.7% (167) | 18.0% (77) | 32.4% (67) | 23.0% (23) | |
| 1–3 positive | 39.0% (286) | 40.1% (171) | 36.7% (76) | 39.0% (39) | |
| ≥4 positive | 38.3% (281) | 41.9% (179) | 30.9% (64) | 38.0% (38) | |
| Grade | <0.0005 | ||||
| 1 | 5.2% (38) | 8.4% (36) | 0.5% (1) | 1.0% (1) | |
| 2 | 37.5% (275) | 52.0% (222) | 12.1% (25) | 28.0% (28) | |
| 3 | 49.2% (361) | 30.0% (128) | 83.6% (173) | 60.0% (60) | |
| Unknown | 8.2% (60) | 9.6% (41) | 3.9% (8) | 11.0% (11) | |
| Median Household Income | 0.50 | ||||
| <$30,000 | 13.4% (98) | 12.4% (53) | 16.4% (34) | 11.0% (11) | |
| $30,000-$34,999 | 14.4% (106) | 15.2% (65) | 12.6% (26) | 15.0% (15) | |
| $35,000-$45,999 | 30.1% (221) | 30.7% (131) | 29.0% (60) | 30.0% (30) | |
| $46,000+ | 37.7% (277) | 38.6% (165) | 35.7% (74) | 38.0% (38) | |
| Unknown | 4.4% (32) | 3.0% (13) | 6.3% (13) | 6.0% (6) | |
| Zip Code Level Education | 0.19 | ||||
| >29% | 16.2% (119) | 15.7% (67) | 17.9% (37) | 15.0% (15) | |
| 20–28.9% | 21.4% (157) | 19.7% (84) | 26.1% (54) | 19.0% (19) | |
| 14–19.9% | 25.6% (188) | 26.7% (114) | 22.2% (46) | 28.0% (28) | |
| <14% | 32.4% (238) | 34.9% (149) | 27.5% (57) | 32.0% (32) | |
| Unknown | 4.4% (32) | 3.0% (13) | 6.3% (13) | 6.0% (6) | |
| Facility Type | 0.48 | ||||
| Community Cancer Program | 21.4% (157) | 21.8% (93) | 23.2% (48) | 16.0% (16) | |
| Comprehensive Community Cancer Program | 52.9% (388) | 54.1% (231) | 49.3% (102) | 55.0% (55) | |
| Academic | 25.7% (189) | 24.1% (103) | 27.5% (57) | 29.0% (29) |
Overall, the proportion of ER and PR−/Her2neu− patients that experienced distant recurrence (n=89, 43.0%) was higher than Her2neu+ (n=30, 30.0%) and ER or PR+/Her2neu− (n=113, 26.5%), (p<0.0001). Similarly, the proportion of ER and PR−/Her2neu− patients that experienced death (n=102, 49.3%) was higher than Her2neu+ (n=21, 21.0%) and ER or PR+/Her2neu (n=95, 22.3%), (p<0.0001). Kaplan-Meier curves for distant recurrence-free and overall survival stratified by receptor subtypes are depicted in Figure 2. Patients with ER and PR−/Her2neu− breast cancer had the poorest 5-year distant recurrence-free survival (57% vs 72% Her2neu+ and 77% ER or PR+/Her2neu−, p<0.0001). Overall survival was also lower for ER and PR−/Her2neu− cancers (52%) relative to Her2neu+ and ER or PR+/Her2neu− cancers (both at 82%, p<0.0001).
Figure 2.
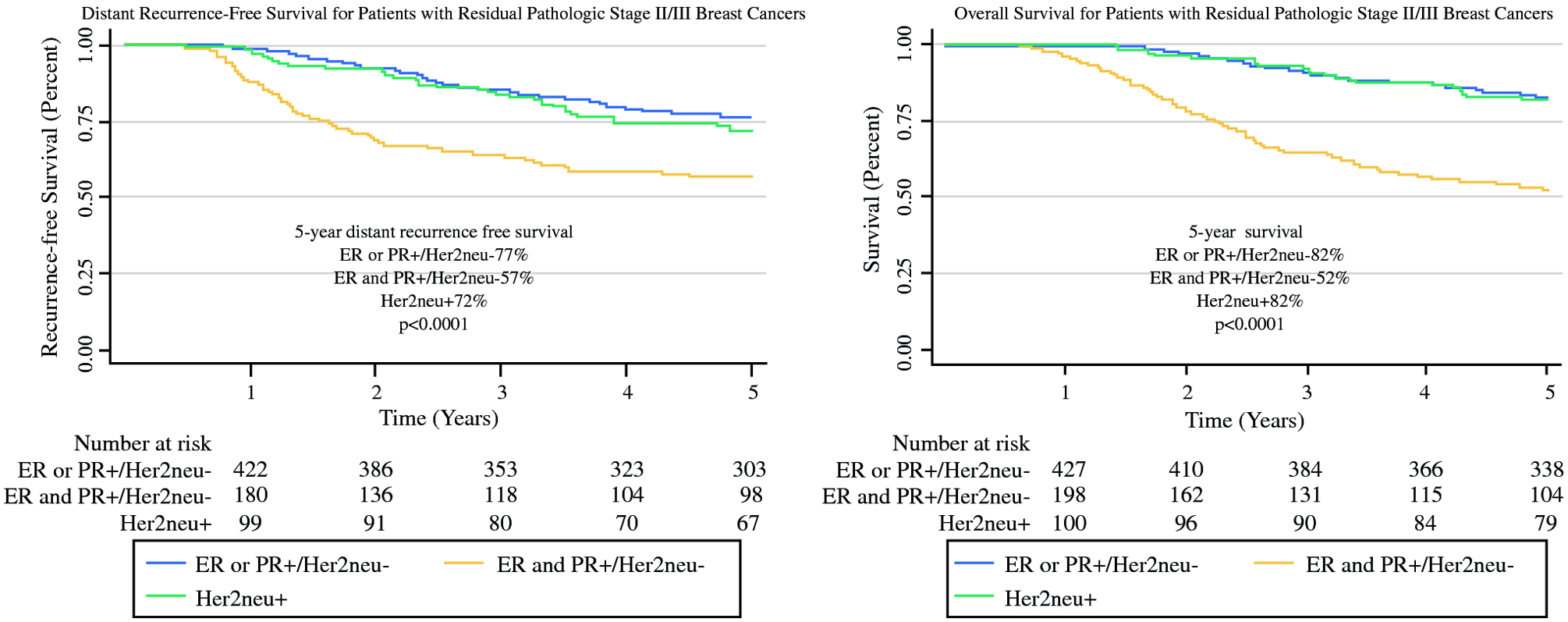
Kaplan–Meier curves for distant recurrent-free and overall survival for residual pathologic stage II/III breast cancers by receptor subtype
Multivariable Cox regression models also demonstrated a higher likelihood of distant recurrence for patients with ER and PR−/Her2neu− disease (HR 2.25, 95% CI 1.56–3.24). Larger residual tumor size (>5 cm, diffuse disease or inflammatory cancer; HR 2.19, 95% CI 1.39–3.45), more advanced residual nodal status (≥4 positive lymph nodes; HR 2.86, 95% CI 1.88–4.33), and identifying race as black (HR 1.78, 95% CI 1.25–2.53) also contributed to an increased likelihood of distant recurrence (Table 2). Similarly, multivariable Cox regression models demonstrated a higher likelihood of death for patients with ER and PR−/Her2neu− disease (HR 3.19, 95% CI 2.20–4.64). Again, larger residual tumor size (>5 cm, diffuse disease or inflammatory cancer; HR 1.99, 95% CI 1.25–3.15), more advanced residual nodal status (≥4 positive lymph nodes; HR 2.87, 95% CI 1.89–4.38), identifying race as black (HR 1.73, 95% CI 1.19–2.50), and older age (≥70 years of age; HR 2.23, 95% CI 1.38–3.60) have an increased likelihood of death (Table 2). There was no association between grade, zip code level median household income or education, comorbidities, or facility type and distant recurrence-free or overall survival.
Table 2:
Cox Regression Model for Distant Recurrence and Death
| Distant Recurrence | Death | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio (HR) | 95% Confidence Interval (CI) | P-value | Hazard Ratio (HR) | 95% Confidence Interval (CI) | P-value | |
| Receptor Status | 0.0001 | <0.0001 | ||||
| ERPR+Her2neu− | Reference | -- | Reference | -- | ||
| ER and PR−/Her2neu− | 2.25 | 1.56–3.24 | 3.19 | 2.20–4.64 | ||
| Any ER/PRHer2Neu+ | 1.25 | 0.80–1.95 | 1.08 | 0.63–1.85 | ||
| Age | 0.50 | 0.002 | ||||
| ≤50 years | Reference | -- | Reference | -- | ||
| >50 – <70 years | 0.83 | 0.61–1.13 | 1.10 | 0.78–1.54 | ||
| ≥70 years | 0.89 | 0.52–1.52 | 2.23 | 1.38–3.60 | ||
| Race | 0.006 | 0.01 | ||||
| White | Reference | -- | Reference | -- | ||
| Black | 1.78 | 1.25–2.53 | 1.73 | 1.19–2.50 | ||
| Other | 1.02 | 0.52–2.04 | 0.85 | 0.37–1.97 | ||
| Residual Tumor Size | 0.0001 | 0.0009 | ||||
| <2 cm | Reference | -- | Reference | -- | ||
| 2–5 cm | 1.21 | 0.77–1.91 | 1.16 | 0.73–1.84 | ||
| >5 cm or diffuse/inflam | 2.19 | 1.39–3.45 | 1.99 | 1.25–3.15 | ||
| Residual Positive Lymph Nodes | <0.0001 | <0.0001 | ||||
| Negative | Reference | -- | Reference | -- | ||
| 1–3 positive | 1.30 | 0.84–2.01 | 1.13 | 0.72–1.77 | ||
| ≥4 positive | 2.86 | 1.88–4.33 | 2.87 | 1.89–4.38 | ||
Also included in the model grade, median household income, zip code level education, comorbidities, and facility type
Discussion:
Achieving pCR is an important prognostic marker for women with breast cancer, as most women with a pCR experience improved survival and decreased recurrence.4–13,15,22 For patients with residual disease after neoadjuvant chemotherapy, prognosis is generally poorer.4–8,15–17,22 However, in this study using a large, national database with 5 years of follow-up, we demonstrated significant differences in outcomes for women with substantial residual disease after neoadjuvant chemotherapy based on receptor subtype. In our study, women with ER and PR−/Her2neu− residual pathologic stage II/III breast cancer had significantly worse outcomes when compared to women with ER or PR+ or Her2neu+ disease. Conversely, women with non-ER and PR−/Her2neu− residual breast cancer have a relatively favorable prognosis, especially in light of options for ongoing, targeted therapy.
Few studies have previously examined the difference in outcomes by receptor status for patients with residual disease after current standard of care neoadjuvant chemotherapy.4,11,20 In a single institution study by Liedtke et al, outcomes following neoadjuvant chemotherapy were compared for patients with ER and PR−/Her2neu− versus non-ER and PR−/Her2neu− disease. In a sub-analysis, the authors examined overall survival in patients with residual disease finding a worse overall survival for those that had ER and PR−/Her2neu− cancers.11 In a second study by Campbell, et al., the authors examined recurrence outcomes based on residual cancer burden (RCB, graded as 0, 1, 2, or 3) and pathologic American Joint Committee on Cancer staging (yAJCC). This study found that RCB and yAJCC identified patients at high risk of breast cancer recurrence. Using recursive partitioning, ER and PR−/Her2neu− cancers and Her2neu+ cancers were found to have the highest risk of recurrence among patients with RCB 3 or yAJCC III,20 similar to the findings in our study. A third study by Kim et al examined tumor response and outcomes by molecular subtype at a single institution finding similar results that those with ER and PR−/Her2neu− cancers have significant differences in overall and disease-free survival.4 However, these studies were limited by small sample size (n=162–329)4,20 and/or the treatment received.4,11,20 For example, the Liedtke study included patients treated over a 20 year period, with significant variations in the chemotherapeutic regimens used.11 Additionally, the Campbell study excluded patients with Her2neu+ cancers treated with trastuzumab.20 Our large, multi-institutional cohort of women with breast cancer diagnosed between 2006–2007 who received current standard of care chemotherapy and targeted therapies in a real-world setting supports and extends the findings of these earlier studies.
The results of our study may assist clinicians in discussing prognosis for women with residual disease. Studies have demonstrated that the majority of cancer patients want to have prognostic discussions with their provider.23 Ample data currently exists to support discussions regarding prognosis, treatment, and follow-up for women who achieve a pCR,9–13,16,18 but to date, less data is available for similar discussions for women with residual breast cancer following neoadjuvant chemotherapy.11,20 Our study helps add important prognostic information including 5-year overall and distant recurrence-free survival for women with residual pathologic stage II/III breast cancers based on their receptor subtype.
In addition to prognostic information, the observed 5-year overall survival and distant recurrence for women with residual disease after neoadjuvant therapy reinforces the potential benefit to adjuvant therapy, especially for women with residual ER and PR−/Her2neu− breast cancer. One such consideration is adjuvant capecitabine for women with residual ER and PR−/Her2neu− breast cancer. Masuda et al demonstrated a significantly improved 5-year disease-free survival and overall survival for women with residual ER and PR−/Her2neu− breast cancers when administered capecitabine.24 As ER and PR−/Her2neu− breast cancers also tend to have a high proportion of BRCA mutations associated with them, platinum compound chemotherapeutic agents have also shown promise in improving outcomes for those with BRCA-positive ER and PR−/Her2neu− breast cancer as well as poly ADP ribose polymerase (PARP) inhibitors.25 Several ongoing clinical trials are currently enrolling to assess the effectiveness of these agents in women with ER and PR−/Her2neu− breast cancers.25 Enrollment in these clinical trials should be considered for eligible women with residual ER and PR−/Her2neu− breast cancer due to their poor prognosis as demonstrated in our study cohort.
While women with residual breast cancers other than the ER and PR−/Her2neu− subtype have relatively favorable 5-year overall (~82%) and distant recurrence-free survival (>70%), there is still room for improvement and additional adjuvant therapy may still be considered. The KATHERINE trial assessed the impact of adjuvant trastuzumab emtansine in women with residual Her2neu positive early stage breast cancer following neoadjuvant chemotherapy finding significantly improved overall and distant recurrence-free survival compared to women who received trastuzumab alone.26 Additionally, extended schedule endocrine therapy, 10 years rather than 5 years, for hormone positive women may also be of benefit for those with residual disease following neoadjuvant chemotherapy.27–29
A few limitations of our study should be addressed. First, due to the collection parameters set forth by the CoC special study, patient selection was based on pathologic stage II/III, regardless of timing of chemotherapy; consequently, patients who achieved pCR after neoadjuvant chemotherapy were not eligible for the CoC special study and are not included in the data set. For this reason, we were unable to compare outcomes for those with a pCR versus residual cancer, especially minimal disease. However, this type of comparison has been well-documented in prior studies.9–13 We also did not have clinical stage information available in our dataset. Without clinical stage information, we were also unable to make direct comparisons to the Neo-Bioscore recently published by Mittendorf et al30 which uses clinical and pathologic staging, tumor grade, and hormone and Her2neu status to predict disease specific survival. Another limitation to our study was that we are unable to determine the specific chemotherapy regimens used for our patients. However, we were able to confirm receipt of Her2neu-targeted therapy. Additionally, the time frame of the study makes it likely that current standard of care chemotherapy would have been administered. Lastly, we are unable to determine the types of adjuvant treatments administered to these women with residual disease after neoadjuvant chemotherapy, as we were limited to the data fields routinely collected through the NCDB.
Conclusion:
Women with ER and PR−/Her2neu− residual pathologic stage II/III cancer after neoadjuvant chemotherapy have a higher risk of distant recurrence and mortality, when compared to patients with other breast cancer types. The relatively poor outcomes for patients with residual pathologic stage II/III ER and PR−/Her2neu− cancer supports the consideration of additional adjuvant therapy as well as novel clinical trials in this cohort. These data can also be used by clinicians to counsel their patients regarding prognosis, specifically the relatively favorable prognosis for women whose receptor status allows for targeted adjuvant therapy options in the face of residual disease.
Synopsis:
Women with ER and PR−/Her2neu− residual breast cancer following neoadjuvant chemotherapy have worse overall and distant recurrence-free survival compared to any other cancer subtype, supporting the consideration for additional adjuvant therapy and/or novel clinical trials for these patients.
Acknowledgements:
Funding/Support: This work was supported by the Patient Centered Outcomes Research Institute (PCORI) Award (Greenberg, Schumacher, Neuman, CE-1304-6543). This publication was further made possible by the National Institute of Health (NIH) funded University of Wisconsin Carbone Comprehensive Cancer Center Academic Oncologist Training Program (Neuman, NIH 5K12CA087718), Building Interdisciplinary Research Careers in Women’s Health Scholar Program (Neuman, NIH K12 HD055894), as well as the National Cancer Institute funded Surgical Oncology Research Training Program (Stankowski-Drengler, T32 CA090217) and Alliance Foundation Trials, LLC. The data used in the study are derived from a deidentified National Cancer Database file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator. Further, the contents of this publication, including its findings, are solely the responsibility of the authors and do not necessarily represent the official view of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee or the NIH.
Footnotes
ClinicalTrials.gov Identifier: NCT02171078
Financial Disclosures: LGW is a founder of Elucent Medical. CCG is a consultant for Johnson & Johnson.
References:
- 1.Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48(18):3342–3354. [DOI] [PubMed] [Google Scholar]
- 3.Ring AE, Smith IE, Ashley S, Fulford LG, Lakhani SR. Oestrogen receptor status, pathological complete response and prognosis in patients receiving neoadjuvant chemotherapy for early breast cancer. Br J Cancer. 2004;91(12):2012–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H, Park W, Huh SJ, et al. Clinical outcomes according to molecular subtypes in stage II-III breast cancer patients treated with neoadjuvant chemotherapy followed by surgery and radiotherapy. Asia Pac J Clin Oncol. 2017;13(4):329–336. [DOI] [PubMed] [Google Scholar]
- 5.Haque W, Verma V, Hatch S, Suzanne Klimberg V, Brian Butler E, Teh BS. Response rates and pathologic complete response by breast cancer molecular subtype following neoadjuvant chemotherapy. Breast Cancer Res Treat. 2018;170(3):559–567. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi N, Takahashi Y, Matsuda N, et al. The Prognostic Effect of Changes in Tumor Stage and Nodal Status After Neoadjuvant Chemotherapy in Each Primary Breast Cancer Subtype. Clin Breast Cancer. 2018;18(2):e219–e229. [DOI] [PubMed] [Google Scholar]
- 7.Spring L, Greenup R, Niemierko A, et al. Pathologic Complete Response After Neoadjuvant Chemotherapy and Long-Term Outcomes Among Young Women With Breast Cancer. J Natl Compr Canc Netw. 2017;15(10):12161223. [DOI] [PubMed] [Google Scholar]
- 8.Ohzawa H, Sakatani T, Niki T, Yasuda Y, Hozumi Y. Pathological responses and survival of patients with human epidermal growth factor receptor 2-positive breast cancer who received neoadjuvant chemotherapy including trastuzumab. Breast Cancer. 2014;21(5):563–570. [DOI] [PubMed] [Google Scholar]
- 9.Broglio KR, Quintana M, Foster M, et al. Association of Pathologic Complete Response to Neoadjuvant Therapy in HER2-Positive Breast Cancer With Long-Term Outcomes: A Meta-Analysis. JAMA Oncol. 2016;2(6):751–760. [DOI] [PubMed] [Google Scholar]
- 10.Chollet P, Amat S, Cure H, et al. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer. 2002;86(7):1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. [DOI] [PubMed] [Google Scholar]
- 12.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. [DOI] [PubMed] [Google Scholar]
- 13.Yee D, DeMichele A, Isaacs C, et al. Pathological complete response predicts event-free and distant disease-free survival in the I-SPY2 TRIAL [abstract]. In. Proceedings of the 2017 San Antonio Breast Cancer Symposium; 2017 Dec 5–9; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2018;78(4 Suppl):Abstract nr GS3–08. [Google Scholar]
- 14.Boughey JC, Ballman KV, McCall LM, et al. Tumor Biology and Response to Chemotherapy Impact Breast Cancer-specific Survival in Node-positive Breast Cancer Patients Treated With Neoadjuvant Chemotherapy: Long-term Follow-up From ACOSOG Z1071 (Alliance). Ann Surg. 2017;266(4):667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29(25):3351–3357. [DOI] [PubMed] [Google Scholar]
- 16.Symmans WF, Wei C, Gould R, et al. Long-Term Prognostic Risk After Neoadjuvant Chemotherapy Associated With Residual Cancer Burden and Breast Cancer Subtype. J Clin Oncol. 2017;35(10):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chollet P, Abrial C, Durando X, et al. A new prognostic classification after primary chemotherapy for breast cancer: residual disease in breast and nodes (RDBN). Cancer J. 2008;14(2):128–132. [DOI] [PubMed] [Google Scholar]
- 18.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. [DOI] [PubMed] [Google Scholar]
- 19.Bonnefoi H, Litiere S, Piccart M, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1–00 phase III trial. Ann Oncol. 2014;25(6):1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell JI, Yau C, Krass P, et al. Comparison of residual cancer burden, American Joint Committee on Cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat. 2017;165(1):181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mallin K, Browner A, Palis B, et al. Incident Cases Captured in the National Cancer Database Compared with Those in U.S. Population Based Central Cancer Registries in 2012–2014. Ann Surg Oncol. 2019. [DOI] [PubMed] [Google Scholar]
- 22.Kong X, Moran MS, Zhang N, Haffty B, Yang Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer. 2011;47(14):2084–2090. [DOI] [PubMed] [Google Scholar]
- 23.Kaplowitz SA, Campo S, Chiu WT. Cancer patients’ desires for communication of prognosis information. Health Commun. 2002;14(2):221–241. [DOI] [PubMed] [Google Scholar]
- 24.Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147–2159. [DOI] [PubMed] [Google Scholar]
- 25.Papadimitriou M, Mountzios G, Papadimitriou CA. The role of PARP inhibition in triple-negative breast cancer: Unraveling the wide spectrum of synthetic lethality. Cancer Treat Rev. 2018;67:34–44. [DOI] [PubMed] [Google Scholar]
- 26.von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380(7):617–628. [DOI] [PubMed] [Google Scholar]
- 27.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381(9869):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray R, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. In: J Clin Oncol; 2013:(18_suppl): 15–15. [Google Scholar]
- 29.Jinih M, Relihan N, Corrigan MA, O’Reilly S, Redmond HP. Extended Adjuvant Endocrine Therapy in Breast Cancer: Evidence and Update - A Review. Breast J. 2017;23(6):694–705. [DOI] [PubMed] [Google Scholar]
- 30.Mittendorf EA, Vila J, Tucker SL, et al. The Neo-Bioscore Update for Staging Breast Cancer Treated With Neoadjuvant Chemotherapy: Incorporation of Prognostic Biologic Factors Into Staging After Treatment. JAMA Oncol. 2016;2(7):929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]


