Abstract
Introduction
The pandemic of SARS CoV-2 has required urgent medical treatments for numerous patients. As no specific antiviral agents were available, different off-the-shelf alternatives have been explored.
Objective
Here, we review the rationale behind the use of Favipiravir, and report of the specific studies supporting this treatment being conducted.
Methods
Here we analyze the relevant literature to conclude about the present opportunities offered by this therapeutic agent.
Results
This antiviral drug approved influenza in Japan since 2014, has a demonstrated in vitro activity against SARS CoV-2 and is being investigated in several trials for SARS CoV-2. Signals of benefit were shown in a small trial for SARS CoV-2. However, in another small study, there was no advantage.
Conclusions
Further studies, statistically more significant, are urgently needed to understand the best opportunities offered by this treatment.
Keywords: SARS CoV-2, Favipiravir, Human trials, Animal studies, Laboratory experiments
Introduction
Initial estimations of the SARS CoV-2 mortality rate were extremely large, at about 1% of the infected in the simulations of [1]. Then [2], that first investigated the number of those with SARS CoV-2 antibodies in the supposed to be unaffected population, demonstrated the existence of a significant amount of people asymptomatic or mild. Thus, the revised SARS CoV-2 mortality rate is about 0.12–0.20%. The daily peak fatality rates for the United Kingdom, predicted by [1] were 210. The measured peak fatality rate for the United Kingdom (7-days rolling averages) has been less than 14. Countries that enforced less severe restrictions such as Sweden or the Netherlands did better at below 10 than countries such as the United Kingdom that had 14 or Belgium that had 30 [3]. For Saudi Arabia, the fatality rate (percentage of deaths in closed cases) is 0.83% (549 over 66,339, as per the data updated June 3) [4]. The above 0.83% is not the infection fatality rate, which is the number of deaths from the SARS CoV-2 disease divided by the total number of cases of SARS CoV-2, but only the fatality rate in medium-to-severe cases requiring medical attention. According to the World Health Organization (WHO), their data to early March were already suggesting that 80% of the infections were mild or asymptomatic, 15% were severe infection, requiring oxygen and 5% were critical infections, requiring ventilation [5]. By taking 20% of the 0.83% fatality rate in closed cases between the medium-to-severe SARS CoV-2 cases, the fatality rate of Saudi Arabia is, therefore, 0.166% [4], within the range indicated by [2]. As a reference, the death rate for influenza and pneumonia for Saudi Arabia [6] is 49.64 per 100,000 or 0.050%. Thus, the fatality rate of SARS CoV-2 is more than the flu. In addition, the fatality is mostly limited to the vulnerable [7–9]. In a healthy population, a strong immune system resulting from exercise, good nutrition, and regular supplements of vitamins and minerals is a guarantee of safety against SARS CoV-2. The Charles de Gaulle aircraft carrier case is a proof. Of almost 2000 people supposed to be healthy and with a strong immune system likely all uniformly challenged by the virus, only 1081 were infected, and of the 1081, only 24 ended up in the hospital, with only 1 of them in need of intensive care [9]. After less than 2 weeks, there were only two Marines still in the hospital, and one of them still in need of intensive care [9]. After 3 weeks, only the one previously in intensive care was still hospitalized but out of the intensive care [9]. Thus, the fatality rate in this sample of the total Charles de Gaulle aircraft carrier population was thus zero.
It is within this context, of therapies mostly needed in patients with an immune system compromised for age or comorbidities, where contraindications may exist for the use of the more toxic drugs, that SARS CoV-2 therapies must be applied. Within this context, it is necessary to carefully consider the safety-to-efficacy profile of the drugs used for SARS CoV-2 therapy.
In people with a weak immune system, for ages or comorbidities, drug toxicity may constitute a serious threat to the survival of the patients, producing in some cases more damage than benefit. The last controversy about chloroquine and hydroxychloroquine is focused on the safety-to-efficacy profile [10–12]. Thus, it is extremely relevant to evaluate therapies with minimal side effects and contraindications.
Favipiravir use for SARS CoV-2 infection
An emerging drug now becoming popular in Japan and Russia is Favipiravir.
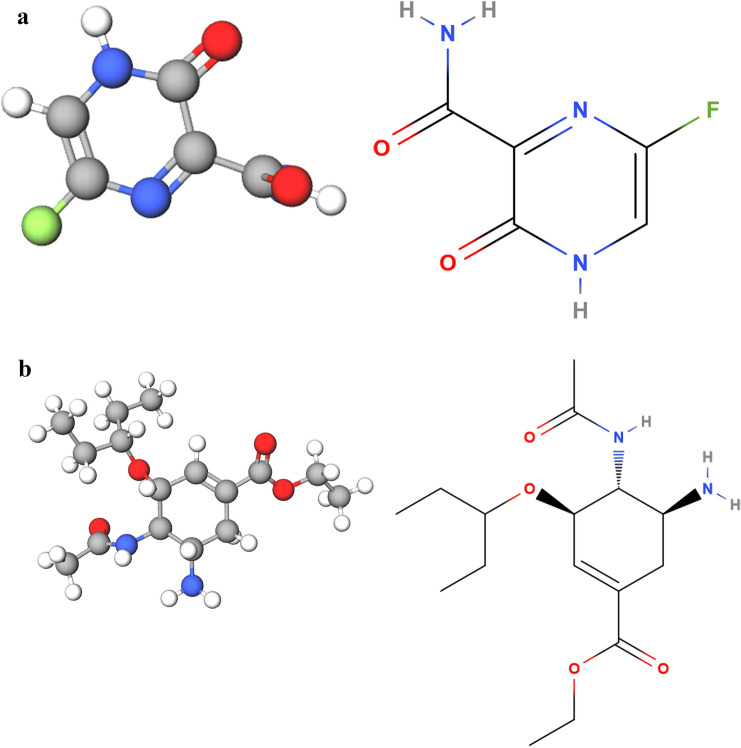
Figure 1a is the molecule of Favipiravir (from https://molview.org/?cid=492405).
Fig. 1.
a Favipiravir molecule from molview.org/?cid=492405. The formula is C5H4FN3O2. The molecular weight is 157.1 µg, hydrogen bond donors 2, hydrogen bond acceptors 4. Percent composition is C 12.0107 µg × 5 38.226%; H 1.00794 µg × 4, 2.5663%; F 18.998404 µg × 1, 12.093%; N 14.0067 µg × 3, 26.747%; and O 15.9994 µg × 2, 20.368%. Systematic name is 5-fluoro-2-oxo-1H-pyrazine-3-carboxamide. b Oseltamivir molecule from molview.org/?cid=65028. The formula is C16H28N2O4. The molecular weight is 312.4 µg, hydrogen bond donors 2, hydrogen bond acceptors 5. Percent composition is C 12.0107 µg × 16, 61.514%, H 1.00794 µg × 28, 9.0339%, N 14.0067 µg × 2, 8.9670%, and O 15.9994 µg × 4, 20.485%
Favipiravir (Avigan) is a known antiviral for influenza [13]. It is indicated for novel influenza strains that cause more severe disease rather than seasonal influenza [13]. Favipiravir triphosphate is a purine nucleoside analog. Favipiravir is a member of pyrazines and a primary carboxamide.
Figure 1b is the molecule of Oseltamivir, as Favipiravir and Oseltamivir are often combined. Oseltamivir is a cyclohexenecarboxylate ester. It is an antiviral prodrug being hydrolyzed to the active free carboxylic acid in the liver. Oseltamivir is used to slow the spread of influenza. It has a role as a prodrug, an EC 3.2.1.18 (exo-alpha-sialidase) inhibitor, an antiviral drug, an environmental contaminant, and a xenobiotic.
The mechanism of action is likely the selective inhibition of viral RNA-dependent RNA polymerase [14, 15]. In vitro studies on influenza A H1N1 viruses suggesting induced lethal RNA transversion mutations through the production of nonviable viral phenotype [16, 17] suggest that Favipiravir works as a chain terminator at the site of incorporation of the viral RNA thus reducing the viral load. Shiraki and Daikoku [17] advocate the use of Favipiravir against novel influenza strains. Favipiravir does not inhibit RNA or DNA synthesis in mammalian cells and it is not toxic to mammalian cells [18]. However, Favipiravir also seems not effective in primary human airway cells [19]. This may cast doubts about the efficacy in the use for SARS CoV-2 infection.
Favipiravir works against a broad range of influenza viruses [20]. These include A(H1N1) pdm09, A(H5N1) and A(H7N9) avian virus [20]. Favipiravir also inhibits influenza strains resistant to current antiviral drugs, and produce a synergistic effect in combination with Oseltamivir [20]. A small trial of 168 patients severely ill from influenza were treated with Favipiravir plus Oseltamivir (40) and Oseltamivir alone (128) [21]. A combination of Favipiravir and Oseltamivir accelerates clinical recovery [21].
For the specific use against SARS CoV-2, [22] evaluated the in vitro efficacy of different drugs, from Remdesivir to Chloroquine, also including Favipiravir. With Favipiravir, half‐maximal effective concentration EC50 = 61.88 μM, CC50 > 400 μM, SI > 6.46 were required to reduce the viral infection. Wang, Cao, Zhang, Yang, Liu, and Xu et al. [22] recommended further in vivo studies as efficacy against the Ebola virus challenge in mice was large despite an EC50 value in Vero E6 cells as high as 67 μM.
Li and De Clercq [23] include Favipiravir together with Remdesivir, Galidesivir, and Ribavirin in between the existing antiviral agents’ RNA‐dependent RNA polymerase inhibitors to repurpose to treat SARS CoV-2 infection. Dong et al. [24] also include Favipiravir, together with Chloroquine, Arbidol, and Remdesivir, all under clinical studies in China to test their efficacy and safety for SARS CoV-2 infection. Costanzo et al. [25] declare Favipiravir as one of the most promising drugs for SARS CoV-2, however, mentioning only the small study [26] discussed hereafter as supporting evidence. These are, however, experts’ opinions not supported by proper trials.
Favipiravir safety evidence in the treatment of other pathologies is reviewed in Ref. [27]. Study follow-up was between 5 and 21 days. The proportions of grade 1–4 adverse events (AE) on Favipiravir was 28.2% vs 28.4% in the comparison arms with Oseltamivir, Umifenovir, and Lopinavir/Ritonavir. The proportion of discontinuations due to AE on Favipiravir was 1.1% vs 1.2% in the comparison arms. Serious AEs were 0.4% in both arms. While Favipiravir demonstrates a favorable safety profile, safety concerns remain for hyperuricemia, teratogenicity, and QTc prolongation [28].
Regarding SARS CoV-2 application, according to [29], for analogies with the treatment with the same drug of the Ebola virus, while the drug EC50 against SARS CoV-2 is 9.4 µg/m they suggest a higher value of EC50, in the range of 40–80 µg/mL, about same of the Ebola dosage. Cardiac and hepatic monitoring during treatment is suggested, the same as monitoring of Favipiravir concentration [29]. In addition to the in vitro study [22] indicating that Favipiravir (T-705) inhibited SARS CoV-2 replication in Vero E6 cells with EC50 values of 61.88 μM (9.4 μg/mL), the independent study [30] indicated EC50 values > 100 μM (15. 7 μg/mL). As Favipiravir is a prodrug requiring metabolic activation in the host cells to form its triphosphate form, this may contribute to the differences between the two studies [28].
Up to date, there is not enough information from specific trials to infer any conclusion on the use of Favipiravir for SARS CoV-2 infection. An open-label non-randomized trial of only 80 patients performed in China [26] was reported in the literature. This work was published in one engineering rather than a medical journal. This small study found a reduced viral clearance time, improved CT scan, and fewer side effects in comparison to Lopinavir/Ritonavir [26].
Recently, in a small trial of 240 SARS CoV-2 patients, 120 were treated with Favipiravir and 120 with Arbidol [31]. Among patients with SARS CoV-2, in comparison, Favipiravir did not significantly improve the clinical recovery rate on Day 7 but improved the latency to relief for pyrexia and cough. Favipiravir had mild and manageable adverse effects.
There are a few ongoing trials. clinicaltrials.gov [32] reports as per June 6, 2020, 1416 SARS CoV-2 Studies from the World Health Organization Database. A search for Favipiravir returns 20 trials. The total number of Favipiravir trials for SARS CoV-2 and other applications is 73 (Table 1) (from [33]). Those with results are a small percentage of the total.
Table 1.
Current trials of Favipiravir (from [33])
| # | Title | Status | Study results | Conditions | Interventions |
|---|---|---|---|---|---|
| 1 | Bioequivalence study of Favipiravir 200 mg film tablet (ATABAY, Turkey) under fasting conditions | Completed | Has results | Bioequivalence | Drug: Favicovir 200-mg film tablet | Drug: Avigan 200-mg film tablets |
| 2 | Bioequivalence study of Favipiravir 200 mg film tablet (Novelfarma, Turkey) under fasting conditions | Completed | Has results | Bioequivalence | Drug: Favira 200-mg film tablet | Drug: Avigan 200-mg film tablets |
| 3 | Bioequivalence study of Favipiravir 200 mg film tablet (World Medicine, Turkey) under fasting conditions | Completed | No results available | Bioequivalence | Drug: test: Favipiravir 200 mg (LOQULAR) | Drug: reference: Favipiravir 200 mg (Avigan) |
| 4 | Tolerance and activity evaluation of high doses of Favipiravir against Ebola virus in the semen | Terminated | No results available | Ebola virus survivor | Drug: Favipiravir |
| 5 | A pharmacokinetics study of favipiravir in patients with severe influenza | Completed | No results available | Influenza, human | Critical illness | Influenza | Drug: Favipiravir | Drug: Oseltamivir 75-mg capsule |
| 6 | The effectivity and safety of favipiravir compared to Oseltamivir as adjuvant therapy for COVID-19 | Recruiting | No results available | Covid19 | Drug: Favipiravir | Drug: Oseltamivir 75 mg |
| 7 | Efficacy of Favipiravir against Ebola (JIKI) | Completed | No results available | Ebola Virus Disease | Drug: Favipiravir |
| 8 | Study on safety and efficacy of Favipiravir (Favipira) for COVID-19 patient in selected hospitals of Bangladesh | Recruiting | No results available | COVID-19 | Favipiravir (Favipira) | Drug: Favipiravir | Drug: only standard treatment |
| 9 | Efficacy and safety of Favipiravir in the treatment of COVID-19 patients over 15 years of age | Recruiting | No results available | COVID-19 | Drug: Favipiravir |
| 10 | Favipiravir and hydroxychloroquine combination therapy | Recruiting | No results available | COVID19 | Combination product: Favipiravir and Hydroxychloroquine |
| 11 | Study of the use of favipiravir in hospitalized subjects with COVID-19 | Active, not recruiting | No results available | COVID-19 | Drug: Favipiravir + standard of care | Drug: standard of care |
| 12 | Clinical trial evaluating the efficacy and safety of Favipiravir in moderate to severe COVID-19 patients | Recruiting | No results available | Covid19 | Drug: Avigan | Drug: placebo comparator |
| 13 | A multi-center, randomized, double-blind, placebo-controlled, phase 3 study evaluating Favipiravir in treatment of COVID19 | Not yet recruiting | No results available | COVID-19 | Drug: Favipiravir | Other: placebo |
| 14 | Bioequivalence study of Favir 200 mg film tablet Kocak under fasting conditions | Completed | No results available | Bioequivalence | Drug: Favir 200-mg FT|Drug: Avigan 200-mg FT |
| 15 | Favipiravir therapy in adults with mild COVID-19 | Recruiting | No results available | COVID-19 | Drug: Favipiravir | Drug: placebo |
| 16 | Early intervention in COVID-19: Favipiravir verses standard care | Recruiting | No results available | Coronavirus Infection | Drug: Favipiravir | Other: standard of care management |
| 17 | Favipiravir vs hydroxychloroquine in COVID-19 | Recruiting | No results available | SARS-CoV 2 | COVID-19 | Drug: Hydroxychloroquine | Drug: Favipiravir | Other: routine care for COVID-19 patients |
| 18 | Efficacy of Faviprevir in COVID-19 treatment | Recruiting | No results available | COVID | Drug: Favipiravir | Drug: placebos |
| 19 | Favipiravir combined with tocilizumab in the treatment of corona virus disease 2019 | Recruiting | No results available | COVID-19 | Drug: Favipiravir combined WITH Tocilizumab | Drug: Favipiravir | Drug: Tocilizumab |
| 20 | Bioequivalence study of Favipiravir from Flupirava 200 mg tablet (European Egyptian Pharmaceutical Industries, Egypt) versus Avigan 200 mg tablets (Man. by Toyama Chemical Co., Ltd Japan) | Completed | No results available | Healthy | Drug: Flupirava | Drug: Avigan |
| 21 | Efficacy and safety of Favipiravir in management of COVID-19 | Completed | No results available | Coronavirus Disease (COVID-19) | Drug: Favipiravir | Drug: standard of care therapy |
| 22 | Oral Favipiravir compared to placebo in subjects with mild COVID-19 | Enrolling by invitation | No results available | Sars-CoV2 | COVID-19 | Drug: Favipiravir | Drug: placebo | Other: standard of care treatment |
| 23 | Clinical study to evaluate the performance and safety of Favipiravir in COVID-19 | Active, not recruiting | No results available | COVID-19 | Drug: Favipiravir | Other: placebo |
| 24 | Clinical trial of Favipiravir tablets combine with chloroquine phosphate in the treatment of novel coronavirus pneumonia | Recruiting | No results available | Novel coronavirus pnuemonia | Drug: Favipiravir tablets + chloroquine phosphatetablets tablets | Drug: Favipiravir tablets | Drug: placebo |
| 25 | FLARE: Favipiravir ± Lopinavir: a RCT of early antivirals | Recruiting | No results available | COVID-19 | Drug: Favipiravir | Drug: Lopinavir/Ritonavir | Other: Favipiravir placebo | Other: Lopinavir/Ritonavir placebo |
| 26 | Favipiravir in hospitalized COVID-19 patients | Not yet recruiting | No results available | COVID-19 | Drug: Favipiravir | Drug: Hydroxychloroquine |
| 27 | Phase 3 efficacy and safety study of Favipiravir for treatment of uncomplicated influenza in adults—T705US316 | Completed | No results available | Influenza | Drug: favipiravir | Drug: placebo |
| 28 | Pharmacokinetics of Favipiravir in volunteers with hepatic impairment | Completed | No results available | Healthy | Hepatic impairment | Drug: Favipiravir |
| 29 | Dose-finding study of Favipiravir in the treatment of uncomplicated influenza | Completed | Has results | Influenza | Drug: Favipiravir | Drug: placebo comparator |
| 30 | Phase 3 efficacy and safety study of Favipiravir for treatment of uncomplicated influenza in adults | Completed | No results available | Influenza | Drug: favipiravir | Drug: placebo |
| 31 | Efficacy of Favipiravir against severe Ebola virus disease | Completed | No results available | Ebola virus disease | Other: WHO-recommended therapies | Drug: Favipiravir |
| 32 | Safety and efficacy of Maraviroc and/or Favipiravir vs currently used therapy in severe COVID-19 adults | Not yet recruiting | No results available | COVID-19 | Drug: Maraviroc + currently used therapy | Procedure: currently used therapy for COVID-19 non-critical patients | Drug: Favipiravir + currently used therapy | Drug: Maraviroc + Favipiravir + CT |
| 33 | Favipiravir plus hydroxychloroquine and Lopinavir/Ritonavir plus hydroxychloroquine in COVID-19 | Completed | No results available | COVID-19 | Favipiravir | Kaletra | Hydroxychloroquine | Lopinavir/Ritonavir | Drug: Favipiravir | Drug: Hydroxychloroquine | Drug: Lopinavir/Ritonavir |
| 34 | Control of COVID-19 outbreaks in long term care | Recruiting | No results available | COVID-19 | SARS-CoV-2 | Drug: Favipiravir | Drug: Favipiravir placebo |
| 35 | An adaptive study of Favipiravir compared to standard of care in hospitalized patients with COVID-19 | Active, not recruiting | No results available | COVID-19 | Drug: Favipiravir | Drug: standard of care |
| 36 | Study of Favipiravir compared to standard of care in hospitalized patients with COVID-19 | Completed | No results available | COVID-19 | Drug: Favipiravir | Drug: standard of care |
| 37 | Efficacy and safety of hydroxychloroquine and Favipiravir in the treatment of mild to moderate COVID-19 | Recruiting | No results available | Sars-CoV2 | COVID-19 | Drug: Favipiravir (3200 mg + 1200 mg) | Drug: Favipiravir (3600 mg + 1600 mg) | Drug: Favipiravir (3200 mg + 1200 mg) combined with Hydroxychloroquine | Drug: Favipiravir (3200 mg + 1200 mg) combined with Azithromycin|Drug: Hydroxychloroquine | Drug: Hydroxychloroquine combined with Azithromycin |
| 38 | An adaptive clinical trial of antivirals for COVID-19 infection | Recruiting | No results available | COVID | Drug: Favipiravir |
| 39 | Corona virus disease 2019 patients whose nucleic acids changed from negative to positive | Recruiting | No results available | COVID-19 | Drug: Favipiravir |
| 40 | T-705a multicenter study in adults subjects with uncomplicated influenza | Completed | No results available | Influenza | Drug: placebo | Drug: Favipiravir |
| 41 | Favipiravir, protease inhibitors, Oseltamivir -Gpo, hydroxychloroquine for treatment of COVID-19 | Recruiting | No results available | SARS-COV-2 infections | COVID-19 | Drug: oral |
| 42 | Study of efficacy and safety of TL-FVP-t vs. SOC in patients with mild to moderate COVID-19 | Active, not recruiting | No results available | COVID-19 | Drug: Favipiravir|Drug: standard of care (SOC) | Drug: standard concomitant therapy |
| 43 | An open non-comparative study of the efficacy and safety of Aprotinin in patients hospitalized with COVID-19 | Active, not recruiting | No results available | COVID-19 | Drug: Aprotinin |
| 44 | Convalescent plasma therapy in severe COVID-19 infection | Recruiting | No results available | Covid19 | Convalescence | Biological: convalescent plasma |
| 45 | COVID-19 treatment in South Africa | Recruiting | No results available | COVID-19 | Other: standard of care (Paracetamol)|Drug: Artesunate-amodiaquine | Drug: Pyronaridine-artesunate | Drug: Favipiravir plus Nitazoxanide|Drug: Sofosbuvir/daclatasvir |
| 46 | Assessment of safety and efficacy of CCP | Active, not recruiting | No results available | Covid19 | Biological: COVID convalescent plasma |
| 47 | Use of hydroxychloroquine alone or associated for inpatients with SARS-CoV2 virus (COVID-19) | Withdrawn | No results available | Coronavirus infections|SARS-CoV 2 | SARS (Severe Acute Respiratory Syndrome) | Pulmonary disease | Drug: Hydroxychloroquine sulfate | Drug: Hydroxychloroquine sulfate + Azythromycin |
| 48 | The use of dendritic cell/tumor hybridomas as a novel tumor vaccine in patients with advance melanoma | Completed | No results available | Metastatic melanoma | Biological: DC/tumor fusion vaccine |
| 49 | A phase I/II study to assess the safety and efficacy of vaccinations with allogeneic dendritic cells: autologous tumor-derived cells subjected to electrofusions in patients with AJCC stage IV renal cell carcinoma | Completed | No results available | Renal cell carcinoma | Biological: electrofusion DC vaccine |
| 50 | A phase I/II trial of the MUC1 inhibitor, GO-203-2C in patients with relapsed or refractory acute myeloid leukemia | Active, not recruiting | No results available | Acute myeloid leukemia, in relapse | Recurrent adult acute myeloid leukemia | Drug: GO-203-2c | Drug: GO-203-2c + Decitabine |
| 51 | Convalescent plasma of Covid-19 to treat SARS-COV-2 a randomized double blind 2 center trial | Recruiting | No results available | SARS pneumonia | Biological: Convalescent Plasma of patients with COVID-19 | Other: placebo (hartmann plus albumine) |
| 52 | PD-1 alone or with dendritic cell/renal cell carcinoma fusion cell vaccine | Terminated | Has results | Renal cell carcinoma | Drug: CT-011 | Biological: DC/RCC fusion vaccine |
| 53 | Blockade of PD-1 in conjunction with the dendritic cell/myeloma vaccines following stem cell transplantation | Active, not recruiting | No results available | Multiple myeloma | Drug: CT-011|Biological: dendritic cell fusion vaccine |
| 54 | Primary tumor harvest for the purpose of possible use in a future clinical trial in patients with ovarian, fallopian tube or primary peritoneal cancer | Completed | No results available | Ovarian cancer|Peritoneal cancer|Fallopian tube cancer | Procedure: tumor collection |
| 55 | Vaccination of patients with ovarian cancer with dendritic cell/tumor fusions with granulocyte macrophage colony-stimulating factor (GM-CSF) and imiquimod | Active, not recruiting | No results available | Ovarian cancer | Primary peritoneal cancer | Fallopian tube cancer | Drug: GM-CSF | Biological: dendritic cell/tumor fusion vaccine | Drug: imiquimod |
| 56 | Vaccination of patients with breast cancer with dendritic cell/tumor fusions and IL-12 | Terminated | Has results | Breast cancer | Biological: dendritic cell/tumor fusion vaccine | Drug: interleukin-12 |
| 57 | Vaccination with dendritic cell/tumor fusions with autologous stem cell transplants in patients with multiple myeloma | Completed | No results available | Multiple myeloma | Biological: dendritic cell tumor fusion |
| 58 | Vaccination of patients with renal cell cancer with dendritic cell tumor fusions and GM-CSF | Active, not recruiting | Has results | Renal cancer | Biological: dendritic cell tumor fusion vaccine | Drug: granulocyte macrophage colony-stimulating factor (GM-CSF) |
| 59 | Arsenic trioxide and tyrosine kinase inhibitors for chronic myelogenous leukemia (CML) | Terminated | No results available | Chronic myelogenous leukemia | Drug: arsenic trioxide |
| 60 | Chemotherapy and peripheral stem cell transplantation followed by Trastuzumab in treating women with metastatic breast cancer | Withdrawn | No results available | Breast cancer | Biological: trastuzumab | Drug: carboplatin|Drug: carmustine | Drug: cisplatin | Drug: cyclophosphamide | Drug: thiotepa | Procedure: peripheral blood stem cell transplantation |
| 61 | Reduced intensity conditioning with clofarabine, antithymocyte globulin (ATG), total lymphoid irradiation (TLI) followed by allogeneic stem cell transplant | Active, not recruiting | No results available | Acute myeloid leukemia | Myelodysplastic syndrome | Acute lymphocytic leukemia | Relapsed/refractory chronic lymphocytic leukemia | Relapsed/refractory non-Hodgkin’s lymphoma | Hodgkins disease | Relapsed refractory multiple myeloma | Drug: antithymocyte globulin | Drug: Clofarabine |
| 62 | The use of dendritic cell/tumor fusions as a novel tumor vaccine in patients with multiple myeloma | Completed | No results available | Multiple myeloma | Biological: dendritic cell tumor fusion vaccine |
| 63 | Interleukin-12 in treating women with metastatic breast cancer who have received high-dose chemotherapy and peripheral stem cell transplantation | Unknown status | No results available | Breast cancer | Biological: recombinant interleukin-12 |
| 64 | Vaccine therapy in treating patients with stage III or stage IV melanoma | Unknown status | No results available | Melanoma (skin) | Biological: autologous dendritic cell tumor fusion vaccine | Biological: gp100 antigen | Biological: therapeutic autologous dendritic cells |
| 65 | Nonmyeloablative allo SCT for the treatment of hematologic disorders | Completed | No results available | AML | ALL | CML chronic phase, accelerated phase, or blast crisis | CLL | MDS | Relapsed non-Hodgkin’s or Hodgkin’s lymphoma | Aplastic anemia | Multiple myeloma | Myeloproliferative disorder (P Vera, CMML, ET) | Drug: Cyclophosphamide | Drug: fludarabine | Drug: cyclosporine | Drug: methotrexate | Biological: G-CSF |
| 66 | Nonmyeloablative allogeneic stem cell transplantation from HLA-matched unrelated donor for the treatment of hematologic disorders | Completed | No results available | AML | ALL | CLL | Myelodysplastic syndrome | Non-Hodgkin’s lymphoma | Hodgkin’s lymphoma | Multiple myeloma | Aplastic anemia | Myeloproliferative disorder | Drug: cyclophosphamide; fludarabine; cyclosporine; CAMPATH-1H (Alemtuzumab); GM-CSF |
| 67 | Phase I study of sequential cord blood transplants | Completed | No results available | Lymphoma | Leukemia | Multiple myeloma | Myelodysplastic syndrome | Procedure: sequential cord blood transplantation |
| 68 | Study of parathyroid hormone following sequential cord blood transplantation from an unrelated donor | Terminated | Has results | Leukemia, myeloid, chronic | Anemia, aplastic | Myelofibrosis | Lymphoma | Hodgkin disease | Leukemia, lymphocytic, chronic | Leukemia, myelocytic, acute | Leukemia, lymphocytic, acute | Drug: Parathyroid Hormone (teriparatide) |
| 69 | A study of PVX-410, a cancer vaccine, and Citarinostat ± Lenalidomide for smoldering MM | Recruiting | No results available | Smoldering multiple myeloma | Drug: Hiltonol | Drug: Citarinostat | Drug: Lenalidomide | Biological: PVX-410 |
| 70 | Immuno-oncology drugs elotuzumab, anti-LAG-3 and anti-TIGIT | Recruiting | No results available | Multiple myeloma | Relapsed refractory multiple myeloma | Drug: Elotuzumab, pomalidomide, dexamethasone | Drug: Anti-LAG-3 | Drug: Anti-LAG-3 + Pomalidimide + Dexamethasone | Drug: Anti-TIGIT | Drug: Anti-TIGIT + Pomalidimide + Dexamethasone |
| 71 | Safety study of unlicensed, investigational cord blood units manufactured by the NCBP for unrelated transplantation | Recruiting | No results available | Infusion reactions | Biological: unlicensed CBU |
| 72 | A multicenter access and distribution protocol for unlicensed cryopreserved cord blood units (CBUs) | Recruiting | No results available | Hematologic malignancies | Inherited disorders of metabolism | Inherited abnormalities of platelets | Histiocytic disorders | Acute myelogenous leukemia (AML or ANLL) | Acute lymphoblastic leukemia (ALL) | Other acute leukemia | Chronic myelogenous leukemia (CML) | Myelodysplastic (MDS)/myeloproliferative (MPN) diseases | Other leukemia | Hodgkin lymphoma | Non-Hodgkin lymphoma | Multiple myeloma/Plasma cell disorder (PCD) | Inherited abnormalities of erythrocyte differentiation or function | Disorders of the immune system | Automimmune diseases | Severe aplastic anemia | Drug: A multicenter access and distribution protocol for unlicensed cryopreserved cord blood units (CBUs) |
| 73 | Expanded access protocol for GBM patients with already manufactured DCVax®-L who have screen-failed protocol 020221 | Available | No results available | GBM | Glioblastoma multiforme | Biological: DCVax-L |
Discussion and conclusion
Given the demonstrated in vitro of activity of Favipiravir against SARS CoV-2 and signals of benefit in early clinical experience for SARS CoV-2, but also the existence of contraindications that limit the window of cases where the safety-to-efficacy profile is promising, further studies are urgently needed.
The literature works that reviewed specific Favipiravir use for SARS CoV-2 are mostly comments, letters to the editors, or replies, and not research works reporting gold standard trials passed through a proper peer review. Results of trials have not yet been reported in the literature, with the only exception of one very small study and not all the parameters needed under control.
General for SARS CoV-2, the trials performed, under particularly challenging circumstances, without proper control of the relevant parameters, and based on a statistically irrelevant population, are not certainly the gold standard in medical research. These experiences have left more doubts than certainties, also because of the conflict of interest affecting the health sector [27], the rush for publishing in SARS CoV-2, and mostly the interference by the Mainstream Media.
Large, randomized, placebo-controlled studies of hospitalized SARS CoV-2 patients conducted without preconceived agenda and properly monitoring all the relevant parameters are urgently needed to understand if Favipiravir, as well as other products, are beneficial, and in which specific cases may be used to treat SARS CoV-2 infection with a positive safety-to-efficacy profile.
Author contributions
This is a single author paper.
Compliance with ethical standards
Conflict of interest
The author received no funding and has no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferguson N, Laydon D, Nedjati Gilani G, Imai N, Ainslie K, Baguelin M, Bhatia S, Boonyasiri A, Cucunuba Perez ZU, Cuomo-Dannenburg G, Dighe A. Report 9: impact of non-pharmaceutical interventions (NPIs) to reduce COVID19 mortality and healthcare demand. 10.25561/77482.
- 2.Bendavid E, Mulaney B, Sood N, Shah S, Ling E, Bromley-Dulfano R, Lai C, Weissberg Z, Saavedra R, Tedrow J, Tversky D. COVID-19 antibody seroprevalence in Santa Clara County, California. MedRxiv. 2020 doi: 10.1101/2020.04.14.20062463v1.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boretti A. After less than 2 months, the simulations that drove the world to strict lockdown appear to be wrong, the same of the policies they generated. Health Serv Res Manag Epidemiol. 2020;16(7):2333392820932324. doi: 10.1177/2333392820932324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boretti A. COVID-19 fatality rate for Saudi Arabia, updated 3 June 2020. J Glob Antimicrob Resist. 2020;1(22):845–846. doi: 10.1016/j.jgar.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200306-sitrep-46-covid-19.pdf?sfvrsn=96b04adf_4#:~:text=For%20COVID%2D19%2C,infections%2C%20requiring%20ventilation. Accessed 3 June 2020.
- 6.https://www.worldlifeexpectancy.com/cause-of-death/influenza-pneumonia/by-country/. Accessed 3 June 2020.
- 7.Boretti A. Sustainable post Covid19 lockdown strategy through evidence-based policy: analysis of Covid19 fatalities across Europe. Integr J Med Sci. 2020;7:172. doi: 10.15342/ijms.7.172. [DOI] [Google Scholar]
- 8.Boretti A. Analysis of the Charles De Gaulle aircraft carrier Covid19 epidemic: infectivity and fatality in the young, healthy, active population: lesson from the Charles de Gaulle aircraft carrier Covid19 experience. Integr J Med Sci. 2020;7:174. doi: 10.15342/ijms.7.174. [DOI] [Google Scholar]
- 9.Boretti A. Some doubt the Covid19 containment measures on the generally healthy population made any difference for Italy: Covid19 fatalities much larger in Europe, United States and Canada than elsewhere. Integr J Med Sci. 2020;7:179. doi: 10.15342/ijms.7.179. [DOI] [Google Scholar]
- 10.Mehra MR, Desai SS, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Mehra MR, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piller C, Servick K. Two elite medical journals retract coronavirus papers over data integrity questions. Science. 2020 (10.1126). https://www.sciencemag.org/news/2020/06/two-elite-medical-journals-retract-coronavirus-papers-over-data-integrity-questions. Accessed 5 June 2020
- 13.Du YX, Chen XP. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther. 2020 doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- 14.Jin Z, Smith LK, Rajwanshi VK, Kim B, Deval J. The ambiguous base-pairing and high substrate efficiency of T-705 (favipiravir) ribofuranosyl 5′-triphosphate towards influenza A virus polymerase. PLoS ONE. 2013;8(7):e68347. doi: 10.1371/journal.pone.0068347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coomes EA, Haghbayan H. Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother. 2020 doi: 10.1093/jac/dkaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baranovich T, Wong SS, Armstrong J, Marjuki H, Webby RJ, Webster RG, Govorkova EA. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol. 2013;87(7):3741–3751. doi: 10.1128/JVI.02346-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020;22:107512. doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, Gowen BB, Julander JG, Morrey JD. T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82(3):95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon JJ, Toots M, Lee S, Lee ME, Ludeke B, Luczo JM, Ganti K, Cox RM, Sticher ZM, Edpuganti V, Mitchell DG. Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob Agents Chemother. 2018;62(8):e00766–18. doi: 10.1128/AAC.00766-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antivir Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Fan G, Salam A, Horby P, Hayden FG, Chen C, Pan J, Zheng J, Lu B, Guo L, Wang C. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection. J Infect Dis. 2020;221(10):1688–1698. doi: 10.1093/infdis/jiz656. [DOI] [PubMed] [Google Scholar]
- 22.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Discov. 2020 doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 24.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 25.Costanzo M, De Giglio MA, Roviello GN. SARS-CoV-2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem. 2020 doi: 10.2174/0929867327666200416131117. [DOI] [PubMed] [Google Scholar]
- 26.Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y, Shen C. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pilkington V, Pepperrell T, Hill A. A review of the safety of favipiravir—a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020;6(2):45. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du YX, Chen XP. Response to "Dose rationale for favipiravir use in patients infected with SARS-CoV-2". Clin Pharmacol Ther. 2020 doi: 10.1002/cpt.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eloy P, Solas C, Touret F, Mentré F, Malvy D, de Lamballerie X, Guedj J. Dose rationale for favipiravir use in patients infected with SARS-CoV-2. Clin Pharmacol Ther. 2020 doi: 10.1002/cpt.1877. [DOI] [PubMed] [Google Scholar]
- 30.Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KP, Chu DK, Chan MC, Cheung PP, Huang X, Peiris M. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antivir Res. 2020;3:104786. doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C, Huang J, Cheng Z, Wu J, Chen S, Zhang Y, Chen B, Lu M, Luo Y, Zhang J, Yin P. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. MedRxiv. 2020 doi: 10.1101/2020.03.17.2003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.https://clinicaltrials.gov/ct2/who_table. Accessed 3 Oct 2020.
- 33.https://clinicaltrials.gov/ct2/results?cond=&term=Favipiravir&cntry=&state=&city=&dist=. Accessed 3 Oct 2020.