Abstract
Background
Venous thromboembolism (VTE) is a leading cause of morbidity and mortality in patients with cancer. Expert consensus recommends a risk‐based approach to guide prophylactic anticoagulation to prevent VTE in ambulatory patients with cancer receiving chemotherapy. However, oncology practice patterns for VTE prevention remain unclear.
Patients/Methods
We conducted (i) a retrospective, single‐center cohort study of patients with pancreatic and gastric cancers to examine rates of prophylactic anticoagulation prescription for eligible patients at high risk of VTE based on the validated Khorana score, and (ii) a 15‐question survey of oncology clinicians at the same institution to assess current practice patterns and knowledge regarding VTE risk assessment and primary thromboprophylaxis in February 2020.
Results
Of 437 patients who met study criteria, 181 (41%) had a score of ≥ 3 (high‐risk), and none had an anticoagulation prescription for prophylaxis without an alternate treatment indication. In a survey sent to 98 oncology clinicians, of which 34 participated, 67% were unfamiliar with the Khorana score or guideline recommendations regarding risk‐based VTE prophylaxis, and 90% “never” or “rarely” used VTE risk assessment.
Conclusions
Despite available evidence and existing guideline recommendations for VTE risk assessment for ambulatory patients with cancer, and primary prophylaxis for high‐risk patients, this study demonstrates that there is limited uptake in clinical practice.
Keywords: anticoagulation, neoplasm, primary prevention, risk assessment, venous thromboembolism
Essentials.
Existing guidelines outline strategies to prevent venous thromboembolism in outpatients with cancer.
We evaluated practice patterns of VTE prevention using retrospective cohort and survey data.
This study shows underuse of both assessing VTE risk and using primary prevention for VTE.
Uptake of recommended strategies to prevent VTE in outpatients with cancer is low in oncology practices..
1. INTRODUCTION
Venous thromboembolism (VTE) is a significant cause of morbidity and mortality in patients with cancer. Cancer‐associated thrombosis (CAT) is one of the leading causes of death in patients with cancer and is associated with a threefold increase in hospitalizations and health care costs. 1 , 2 , 3 Prophylactic anticoagulation can reduce VTE risk and is most beneficial in patients who are high risk based on a validated clinical scoring system, the Khorana score, which incorporates five clinical factors (cancer type; body mass index; and leukocyte, hemoglobin, and platelet count at time of chemotherapy). 4 In 2013, the American Society of Clinical Oncology published guidelines recommending periodic assessment of VTE risk and consideration of thromboprophylaxis for select high‐risk patients. 5 The next year, the ISTH recommended prophylactic anticoagulation for high‐risk patients (defined as Khorana score ≥ 3) in addition to VTE risk assessment for ambulatory patients starting chemotherapy. 6 Other hematology expert guidelines subsequently made similar recommendations. 7 More recently, two randomized controlled trials demonstrated both safety and efficacy of primary VTE prophylaxis in intermediate and high‐risk ambulatory cancer population. 8 , 9
However, clinician understanding of guideline recommendations are unknown; further, it is unclear if clinicians routinely assess VTE risk and prescribe primary thromboprophylaxis for high‐risk patients. We conducted a two‐part study to (i) describe the rate of anticoagulation prescriptions for primary prophylaxis in eligible, high‐risk oncology patients in a retrospective, single‐center, electronic health record (EHR)‐based study, and (2) evaluate knowledge and practice patterns regarding VTE‐risk assessment and primary prophylaxis of oncology clinicians affiliated with an academic medical center.
2. METHODS
2.1. Retrospective cohort analysis
To conduct the retrospective cohort analysis, we used data from the Northwestern Medicine (NM) Enterprise Data Warehouse (EDW), a comprehensive repository of clinical data, prescriptions, and administrative claims data from patients receiving treatment at NM. 10 We identified adult patients with a diagnosis of pancreatic or gastric cancer who received chemotherapy from January 1, 2015, to October 1, 2018, using International Classification of Diseases (ICD), Revision 9 and 10 codes and the Current Procedural Terminology (CPT) code for chemotherapy administration. We chose pancreatic and gastric cancers, as they are considered to be “very high‐risk” cancer types based on the Khorana score. We identified anticoagulant prescriptions (including apixaban, dabigatran, dalteparin, edoxaban, enoxaparin, fondaparinux, rivaroxaban, and warfarin, and trade names for each) through medication prescriptions and medication lists associated with outpatient encounters. We excluded patients with contraindications to anticoagulation (intracranial metastases or history of major bleeding) and alternate indications for anticoagulation (history of VTE before initiation of chemotherapy or atrial fibrillation) using ICD codes, and excluded patients with a prescription for anticoagulation >30 days before their first chemotherapy date. The primary study outcome was a prescription for anticoagulation within 30 days before or after the first chemotherapy date. A board‐certified hematologist (KM) reviewed charts to adjudicate indications for patients identified as having a prescription for anticoagulation as primary prophylaxis. The Northwestern University Institutional Review Board (IRB) waived the need for informed consent.
2.2. Survey of oncology clinicians
We surveyed oncology clinicians to assess current practice patterns surrounding VTE prevention. For broader generalizability among all cancer subtypes, we included all eligible hematology/oncology clinicians who practice within the NM system, which includes an academic‐based practice and four affiliated, formerly community‐based, practices in the greater Chicago area. Physicians, nurse practitioners, and physician assistants were eligible. We conducted the survey in February 2020, through REDCap, a secure, web‐based platform, through which we secured informed consent. We emailed oncology clinicians an initial survey invitation, followed by one email reminder a week later if the survey was not yet completed. The Northwestern IRB approved the study.
Topics of the 15‐question survey included familiarity with validated VTE risk‐assessment tools, current practice patterns of patient education for VTE, and use of risk‐assessment tools and primary prophylactic anticoagulation for high‐risk patients. Clinicians also were presented with two patient vignettes, both of which would be classified as high‐risk by Khorana score, and asked if they would routinely use anticoagulation prophylaxis. Next, participants were presented VTE risk reduction percentages derived from clinical trial data, 8 , 9 and asked if they would use anticoagulation prophylaxis given these reductions. (Survey is accessible at https://northwestern.box.com/s/jqtf7c9j0b9t1q85zvk0ncfua1vynsek.) We used Fisher’s exact test to assess associations between clinician and use of VTE education, familiarity with and use of validated risk prediction scores, and frequency of use of primary prophylaxis for high‐risk patients (P < .05). Analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC, USA).
3. RESULTS AND DISCUSSION
We identified 437 patients in the EDW meeting study criteria, of whom 316 (72%) had pancreatic cancer and 125 (29%) had gastric cancer (4 patients had both). Mean (SD) age was 64.2 (11.7) years; patients were predominately male (54%) and White (75%; Table 1). Using the sum of abstracted variables, 256 (58.9%) patients had a Khorana score of 2, 121 (27.7%) a score of 3, and 60 (13.7%) a score of ≥ 4. Of 16 patients identified with an active anticoagulation prescription within 30 days before or after the first chemotherapy date, manual chart review revealed that 7 were prescribed therapeutic anticoagulation to treat VTE, and 9 had other clinical indications for anticoagulation (eg, atrial fibrillation, peripheral arterial disease, acute stroke, mechanical heart valve, etc). None of the 16 patients received a prescription without a documented alternative indication for anticoagulation (Figure 1).
Table 1.
Retrospective cohort: characteristics of patients with pancreatic/gastric cancer
| Characteristic | Overall a (N = 437) | Gastric cancer (N = 125) | Pancreatic cancer (N = 316) |
|---|---|---|---|
| Age, mean y (SD) | 64.2 (11.7) | 59.9 (13.1) | 65.8 (10.8) |
| Female, N (%) | 202 (46.2) | 44 (35.2) | 162 (51.3) |
| Race | |||
| White, N (%) | 327 (74.8) | 87 (69.6) | 242 (76.6) |
| Black, N (%) | 48 (11.0) | 13 (10.4) | 35 (11.1) |
| Other/declined, N (%) | 62 (14.2) | 25 (20.0) | 39 (12.3) |
| Body mass index (kg/m2), mean (SD) | 26.2 (5.6) | 26.4 (5.7) | 26.2 (5.6) |
| Platelets (K/μL), mean (SD) | 277 (123.3) | 278.8 (115.8) | 276.9 (126.6) |
| Hemoglobin (g/dL), mean (SD) | 11.7 (1.8) | 11.5 (1.8) | 11.8 (1.8) |
| Leukocytes (K/μL), mean (SD) | 8.2 (5.9) | 7.6 (3.9) | 8.3 (6.4) |
| Khorana score | |||
| 2, N (%) | 49 (11.2) | 15 (12.0) | 34 (10.9) |
| ≥3, N (%) | 11 (2.5) | 4 (3.2) | 8 (2.5) |
Cancer type was not mutually exclusive—four patients had both types of cancer.
Figure 1.
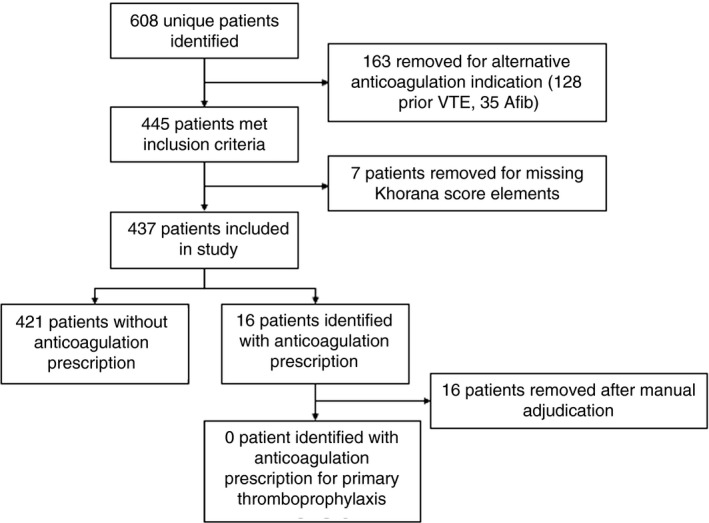
Retrospective cohort: flow diagram of patients. Afib, atrial fibrillation; VTE, venous thromboembolism
Of 98 surveys emailed to oncology clinicians, we received 36 responses (response rate, 37%). Two subjects declined informed consent. Of 34 participants, most (85%) described their practice as academic, and 33% had >10 years of clinical practice, 38% had 5‐10 years, and 29% had <5 years. Participants identified as physicians (50%), nurse practitioners (38%), physician assistants (6%); two participants (6%) identified as “other.” Twelve participants (35%) described their primary practice specialty as malignant hematology, eight (24%) gastrointestinal, and four (12%) breast oncology; five (15%) who selected three or more practice specialties were characterized as general oncology, and eight (24%) participants either reported “other” specialties or were categorized as “other” to maintain confidentiality (fewer than two participants per specialty [lung, genitourinary, gynecologic oncology]). Participants could identify more than one specialty.
Twenty‐four participants responded to questions about practice patterns. Of those, 38% reported “usually” discussing VTE risk with their patients, while 29% each reported “sometimes” and “rarely” discussing VTE risk (Table 2). Most (58%) reported “never” using validated VTE risk assessment scores in clinical practice, while 29% reported “rarely”; only one (4%, generalist) reported “usually” doing so. A majority (67%) of clinicians reported no familiarity with the Khorana score (“not at all”), 17% “a little bit,” 13% “somewhat,” and 4% “quite a bit.” Similarly, 67% reported no familiarity, and only 4% reported “quite a bit” of familiarity with ISTH recommendations. The frequency of reported use of primary prophylaxis, use of VTE education, and familiarity with risk prediction scores did not significantly differ by practice type, time in practice, or degree type.
Table 2.
Practice patterns and familiarity with primary VTE prevention (N = 24)
| 1. How often do you … | Never | Rarely | Sometimes | Usually | Always |
| Use risk scores to identify patients at high risk of VTE? | 58% | 29% | 8% | 4% | 0% |
| Talk to your patients with cancer about the risk of blood clots? | 0% | 29% | 29% | 38% | 4% |
| 2. How familiar are you with … | Not at all | A little bit | Somewhat | Quite a bit | |
| ISTH recommendations for VTE risk assessment and primary prophylaxis? | 67% | 21% | 8% | 4% | |
| The Khorana score? | 67% | 17% | 13% | 4% | |
Perceived VTE risk reduction with anticoagulation prophylaxis did not significantly affect reported decisions to use anticoagulation prophylaxis. Of respondents, 58% reported “never” or “rarely” using prophylactic anticoagulation if the risk declined from 6% to 3% over 6 months, whereas 55% reported “never” or “rarely” using prophylactic anticoagulation if the risk declined from 10% to 4%. Only three participants (13%) reported that they would “usually” prescribe in either situation; none indicated that they would “always” prescribe in either situation. In two patient vignettes, both high risk by Khorana score, most respondents in both scenarios would “never” recommend prophylaxis (60% in vignette 1 and 80% in vignette 2); no respondents would “always” prescribe, and only one would “usually” recommend prophylaxis.
Our study using complementary sources of EHR‐based patient data and survey‐based practice patterns of oncology clinicians demonstrates that the use of VTE risk assessment and primary anticoagulation prophylaxis for oncology patients at high‐risk of VTE is rare. For patients with cancer predicted to be at high risk of VTE based on the Khorana score, none were prescribed anticoagulation without another clinical indication, demonstrating that primary prophylactic anticoagulation is virtually never used. While this data preceded the publication of randomized clinical trials demonstrating safety and efficacy of direct oral anticoagulants for primary prophylaxis in CAT, international and national guidance publications recommending VTE risk assessment, and primary prophylaxis for high‐risk patients, have been available since 2013‐2014. 5 , 6 Additionally, despite the high incidence of VTE in this patient population, most oncology clinicians did not routinely discuss VTE risk, and most reported “never” using risk‐assessment scores in clinical practice. When presented with hypothetical high‐risk patients in vignettes, the majority would “never” recommend prophylaxis. Our data are consistent with a previously published study showing underuse of anticoagulation prophylaxis 11 and further demonstrate that factors related to underuse may be due to lack of perceived benefit of risk reduction with prophylaxis or lack of familiarity with ISTH recommendations and the validated Khorana score. Such lack of familiarity may result from hematology and oncology subspecialties having distinct practice foci and society organizations: the ISTH targets nonmalignant hematologists, who primarily manage thrombosis, rather than oncologists, who primarily manage cancer. Because of this division of care, a multidisciplinary approach to increase adherence is needed, and may benefit from the use of clinical decision support, recently shown to be successful in improving adherence to guidelines. 11
Strengths of our study include the complementary methods used to study oncology‐focused practice patterns surrounding risk‐based VTE prophylaxis and the use of oncology physicians and advanced practice providers in both academic and private practice settings. Limitations include a reliance on ICD, CPT, and medication coding for the EDW portion of the study, which may be inaccurate, though ICD‐9 codes for pulmonary embolism, deep vein thrombosis, and VTE have been found to appropriately identify VTE events. 12 Additionally, manual chart review was performed to confirm primary prophylaxis, strengthening the conclusions. Additional limitations include the retrospective nature of data collection, the use of only a single health system, which may affect generalizability, and the relatively low survey response rate, which may be a source of bias if those who did not respond to the survey have different VTE prophylaxis practice patterns than clinicians who responded to the survey.
Our study demonstrates limited uptake in clinical practice of a risk‐based approach to VTE prevention in ambulatory patients with cancer despite existing evidence and guidelines. Our data and emerging efficacy studies highlight the need to understand relevant barriers and facilitate expedited uptake of evidence‐based recommendations, with the ultimate goal to reduce the risk of CAT and improve outcomes of patients with cancer.
RELATIONSHIP DISCLOSURES
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
All authors contributed substantially to the study design, data analysis and interpretation, and writing of the article.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health NIH KL2TR001424 and American Heart Association #19TPA34890060 (SSK). Research reported in this publication was also supported, in part, by the National Institutes of Health's National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Martin KA, Molsberry R, Khan SS, Linder JA, Cameron KA, Benson A III. Preventing venous thromboembolism in oncology practice: Use of risk assessment and anticoagulation prophylaxis. Res Pract Thromb Haemost. 2020;4:1211–1215. 10.1002/rth2.12431
Handling Editor: Cihan Ay
Contributor Information
Karlyn A. Martin, Email: Karlyn.martin@northwestern.edu, MarKar24.
Sadiya S. Khan, @HeartDocSadiya.
REFERENCES
- 1. Khorana AA, Dalal MR, Lin J, Connolly GC. Health care costs associated with venous thromboembolism in selected high‐risk ambulatory patients with solid tumors undergoing chemotherapy in the United States. ClinicoEconomics Out Res. 2013;5:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007;5:632–4. [DOI] [PubMed] [Google Scholar]
- 3. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer‐associated venous thrombosis. Blood. 2013;122:1712–23. [DOI] [PubMed] [Google Scholar]
- 4. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood. 2008;111:4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus AI, Balaban EP, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(17):2189–204. [DOI] [PubMed] [Google Scholar]
- 6. Khorana AA, Otten HM, Zwicker JI, Connolly GC, Bancel DF, Pabinger I, et al. Prevention of venous thromboembolism in cancer outpatients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1928–31. [DOI] [PubMed] [Google Scholar]
- 7. Khorana AA, Carrier M, Garcia DA, Lee AY. Guidance for the prevention and treatment of cancer‐associated venous thromboembolism. J Thromb Thrombolysis. 2016;41:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carrier M, Abou‐Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711–9. [DOI] [PubMed] [Google Scholar]
- 9. Khorana AA, Soff GA, Kakkar AK, Vadhan‐Raj S, Riess H, Wun T, et al. Rivaroxaban for thromboprophylaxis in high‐risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–8. [DOI] [PubMed] [Google Scholar]
- 10. Starren JB, Winter AQ, Lloyd‐Jones DM. Enabling a learning health system through a unified enterprise data warehouse: the experience of the Northwestern University Clinical and Translational Sciences (NUCATS) Institute. Clin Transl Sci. 2015;8:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holmes CE, Ades S, Gilchrist S, Douce D, Libby K, Rogala B, et al. Successful model for guideline implementation to prevent cancer‐associated thrombosis: venous thromboembolism prevention in the ambulatory cancer clinic. JCO Oncol Pract. 2020;16(9):e868–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):154–62. [DOI] [PubMed] [Google Scholar]


