Abstract
Background
Anticoagulant treatment of splanchnic (SVT) and cerebral vein thrombosis (CVT) can be challenging due to the rarity of these conditions, the concomitantly high thrombotic and bleeding risks, and the available low‐quality evidence.
Objectives
To explore the current therapeutic approaches to SVT and CVT, and the rationale behind the anticoagulant treatment choice.
Methods
A cross‐sectional survey was conducted (October 2018‐April 2019) among members of three thrombosis and hemostasis societies. The survey consisted of four vignette cases: (i) SVT secondary to transient risk factor; (ii) cirrhotic SVT with esophageal varices; (iii) CVT secondary to transient risk factor; and (iv) unprovoked CVT with intracranial hemorrhage.
Results
A total of 397 physicians responded to the survey. There was wide variability in anticoagulant treatment options, starting time, and duration. Vitamin K antagonists were the commonest choice across the four vignette cases (44.2%‐63.0%). The direct oral anticoagulants (DOACs) were the second commonest choice in low‐bleeding‐risk scenarios (27.7% in case 1, 22.9% in case 3), while parenteral anticoagulation alone was the second commonest choice in high‐bleeding‐risk scenarios (39.9% in case 2, 39.8% in case 4). The most frequent reasons for selecting DOACs were oral route of administration (50.6%), lack of need for laboratory monitoring (48.1%), and favorable safety profile of these drugs (43.4%).
Conclusions
The results of our study showed that, despite being off‐label, the DOACs were considered for the treatment of unusual‐site venous thromboembolism. The wide variability among different physicians reflected the clinical difficulties and raised the need for more collaborative trials on these disorders.
Keywords: anticoagulants, cerebral veins, portal vein, splanchnic circulation, surveys and questionnaires, venous thromboembolism
Essentials.
Treatment of splanchnic (SVT) and cerebral vein thrombosis (CVT) can be challenging
We conducted an international physicians’ survey on the management of SVT and CVT.
Despite being off‐label, the direct oral anticoagulants were considered by several physicians.
Wide variability of treatment options and duration emerged, raising the need for more research.
1. INTRODUCTION
Splanchnic vein thrombosis (SVT) and cerebral vein thrombosis (CVT) are two uncommon manifestations of venous thromboembolism (VTE). 1 Compared to lower‐limb deep vein thrombosis and pulmonary embolism, the quality of the evidence on the management of SVT and CVT is lower, due to their relative rarity and difficulties in conducting large clinical studies. In addition, peculiar situations related to these conditions, such as cirrhosis with esophageal varices in SVT or concomitant intracerebral hemorrhage in CVT, contribute to the challenging risk‐benefit balance of anticoagulant treatment.
At the time of this study, the latest guidelines on the treatment of SVT were released by the European Association for the Study of the Liver in 2016 2 and the European Society of Vascular Surgery in 2017, 3 while those on CVT by the European Stroke Organization in 2017. 4 In these guidelines, the main therapeutic options were unfractionated heparin (UFH), low‐molecular‐weight heparin (LMWH), and vitamin K antagonists (VKAs). However, recent studies suggested that the direct oral anticoagulants (DOACs) are increasingly prescribed for unusual‐site VTE. 5 , 6 , 7 , 8 Furthermore, there are still some areas of uncertainty, such as the timing and duration of the anticoagulant treatment. Thus, the aim of this survey was to explore the current therapeutic approaches to patients with SVT and CVT and the rationale behind the anticoagulant treatment choice.
2. METHODS
2.1. Survey details
This survey was published online on a dedicated web page of https://www.surveygizmo.com and was accessible between October 2018 and April 2019. Members of the International Society on Thrombosis and Haemostasis (ISTH), Thrombosis Canada (TC), and the Italian Society for the Study of Haemostasis and Thrombosis (SISET) were invited to participate, to recruit a worldwide group of physicians with special interest in VTE. Invitations were sent twice by email to all members of TC (n = 130) and SISET (n = 675) and to the members of ISTH through the Subcommittees Control of Anticoagulation, Women’s Health Issues in Thrombosis and Hemostasis and Predictive/Diagnostic Variables (n = 1,691). A link to the survey was also included in the ISTH newsletter and advertised on social media.
The survey consisted of four vignette cases, based on real scenarios, on the management of unusual‐site VTE (Table 1): (i) a 40‐year‐old woman with portosplenomesenteric vein thrombosis, following laparoscopic cholecystectomy for acute calculous cholecystitis; (ii) a 55‐year‐old man with portal vein thrombosis and underlying liver cirrhosis with esophageal varices; (iii) a 25‐year‐old woman with thrombosis of the right transverse sinus secondary to oral contraceptive treatment; and (iv) a 45‐year‐old man with unprovoked thrombosis of the left sigmoid and transverse sinuses, complicated by acute intracerebral hemorrhage. The complete text of the survey, including details of each clinical vignette, questions, and possible answers, are reported in Appendix S1.
Table 1.
Survey design
|
Clinical vignette 1 A 40‐year‐old woman presented to the emergency department with acute abdominal pain, accompanied by nausea and vomiting. One month before, she underwent laparoscopic cholecystectomy for acute calculous cholecystitis. There were no other relevant medical conditions in the past medical history. Abdominal computed tomography (CT) scan showed thrombosis of the superior mesenteric vein, involving the confluence with the portal vein, and a minimal intraluminal perfusion defect in the splenic vein. Complete blood count, renal and liver function, and coagulation tests were normal. 1. Which anticoagulant treatment would you prescribe for the acute phase (initial 3 months)? 2. What is the rationale behind your choice regarding the anticoagulant treatment? 3. Which anticoagulant treatment duration would you recommend? |
|
Clinical vignette 2 A 55‐year‐old man (body weight, 72 kg) with a history of liver cirrhosis related to chronic hepatitis C virus infection (Child‐Pugh class B) was admitted to the hospital complaining of abdominal discomfort and increasing abdominal girth, which have developed gradually in the past month. Abdominal Doppler ultrasonography evidenced a thrombosis of the portal vein without any sign of portal cavernoma, and this finding was also confirmed by a CT scan. Blood test results were hemoglobin, 11.2 g/dL; platelet count, 165 000/mm3; and creatinine, 1.6 mg/dL (corresponding to creatinine clearance 53 mL/min, according to the Cockcroft‐Gault equation), international normalized ratio 1.3. Esophagogastroduodenoscopy showed grade 2 esophageal varices without evidence of recent hemorrhage. 1. When would you start the anticoagulant treatment? 2. Which anticoagulant treatment would you prescribe for the acute phase (initial 3 months)? 3. What is the rationale behind your choice regarding the anticoagulant treatment? 4. Which anticoagulant treatment duration would you recommend? |
|
Clinical vignette 3 A 25‐year‐old woman presented to the emergency department for severe, ongoing headache, which she described as “the worst headache of my life.” She had a past medical history of chronic migraine headaches, obesity, and anxiety. She had recently started the oral contraceptive pill for polycystic ovary syndrome. Neurological examination was unremarkable. CT venography showed thrombosis of the right transverse sinus. Angiography was negative for vascular malformations (aneurysm, arteriovenous malformation, or dural arteriovenous fistula). Complete blood count, renal and liver function, and coagulation tests were normal. She was advised to stop the oral contraceptive pill. 1. Which anticoagulant treatment would you prescribe for the acute phase (initial 3 months)? 2. What is the rationale behind your choice regarding the anticoagulant treatment? 3. Which anticoagulant treatment duration would you recommend? |
|
Clinical vignette 4 A 45‐year‐old man (body weight, 49 kg) was admitted to the hospital because of progressive headache, vomiting, and blurred vision. Previous medical history was unremarkable, and he was taking no medications. Physical examination showed mild dysarthria, weakness of the right side of the body, and bilateral papilledema on fundus examination. Cerebral magnetic resonance imaging revealed thrombosis of the left sigmoid sinus and the transverse sinus, with acute intracerebral hemorrhage in the left temporal lobe. The vital signs were stable. Blood test results were hemoglobin, 12.0 g/dL; platelet count, 130 000/mm3; and creatinine, 1.4 mg/dL (corresponding to creatinine clearance 46 mL/min, according to the Cockcroft‐Gault equation). 1. When would you start the anticoagulant treatment? 2. Which anticoagulant treatment would you prescribe for the acute phase (initial 3 months)? 3. What is the rationale behind your choice regarding the anticoagulant treatment? 4. Which anticoagulant treatment duration would you recommend? |
2.2. Statistical analysis
Data were expressed as count and proportions (based on the number of respondents to each question) and compared using the chi‐square or Fisher’s exact test. The rationale behind the choice of the anticoagulant treatment was analyzed by clinical case scenarios and by different anticoagulant options across the four cases. Subgroup analyses were performed by age (≤50 years vs >50 years), years of clinical experience in VTE (≤10 years vs >10 years), most represented geographic regions (Europe vs North America), and most common medical specialties (hematology vs internal medicine). Since four different subgroup analyses were performed, Bonferroni‐adjusted P values 9 were considered (significance level P < .0125). The statistical program STATA/SE v.12 (StataCorp LP, College Station, TX, USA) was used for the analysis.
3. RESULTS
3.1. Study population
Of the 2496 potential participants, 397 (15.9%) responded to the survey, and 263 of them (66.3%) answered all questions. Baseline characteristics of the respondent physicians are reported in Table 2. The majority were from Europe (53.2%) and North America (30.2%), particularly Italy (n = 82), Canada (n = 71), the United States (n = 47), and the United Kingdom (n = 28).
Table 2.
Characteristics of the respondent physicians
| Number of respondents, n = 397 (%) | |
|---|---|
| Sex | |
| Male | 217 (54.7) |
| Female | 180 (45.3) |
| Age categories | |
| Up to 30 y old | 19 (4.8) |
| 31‐40 y old | 107 (27.0) |
| 41‐50 y old | 104 (26.2) |
| 51‐60 y old | 102 (25.7) |
| 61‐70 y old | 56 (14.1) |
| >70 y old | 9 (2.3) |
| Continent of work | |
| Europe | 211 (53.2) |
| North America | 120 (30.2) |
| South America | 25 (6.3) |
| Asia | 23 (5.8) |
| Oceania | 11 (2.8) |
| Africa | 7 (1.8) |
| Specialty | |
| Hematology | 236 (59.5) |
| Internal medicine | 81 (20.4) |
| Vascular medicine | 30 (7.6) |
| Cardiology | 13 (3.3) |
| Others | 37 (9.3) |
| Years of clinical experience in venous thromboembolism | |
| Up to 5 y | 76 (19.1) |
| 6‐10 y | 84 (21.2) |
| 11‐20 y | 95 (23.9) |
| More than 20 y | 142 (35.8) |
Anticoagulant treatment choice and duration in each clinical case scenario are summarized in Figure 1.
Figure 1.
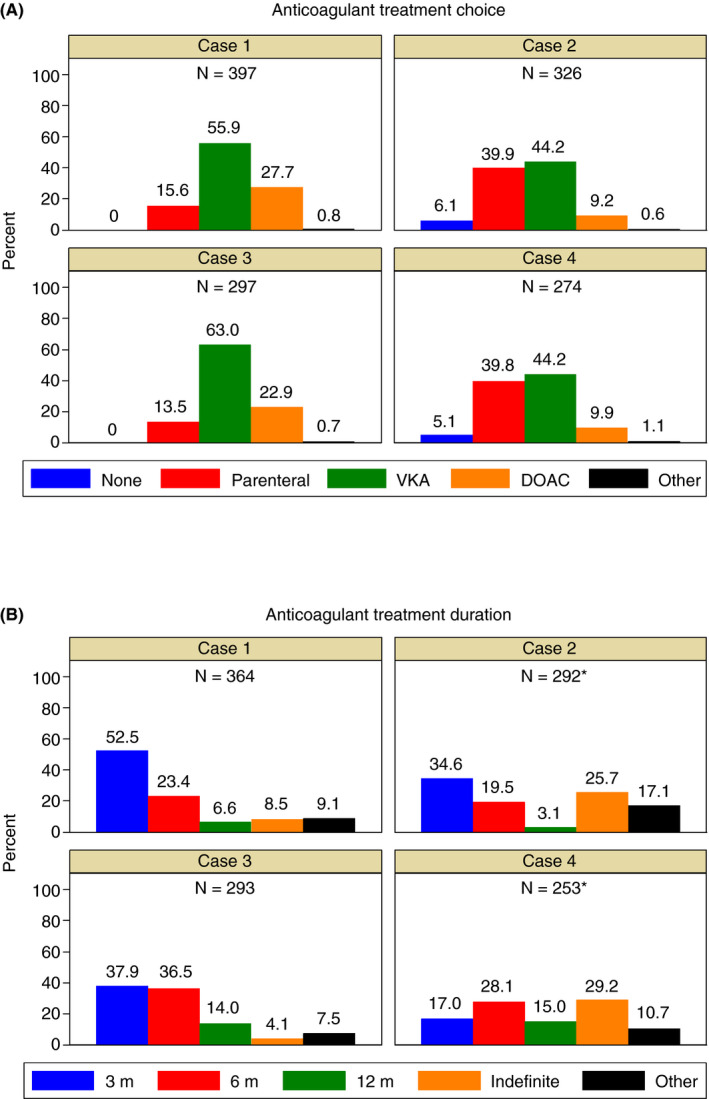
Anticoagulant treatment choice (A) and duration (B) in the four clinical case scenarios. DOAC = direct oral anticoagulant; VKA = vitamin K antagonist. * Excluding those physicians who chose no anticoagulant treatment
3.2. Case scenario 1: SVT secondary to transient risk factor
Among the 397 respondents to the first vignette, the majority would prescribe VKAs (55.9%), followed by the DOACs (27.7%) and parenteral anticoagulation alone (15.6%). When VKAs were chosen, in 97.3% of cases, the target international normalized ratio (INR) range was 2.0‐3.0. The most commonly suggested DOACs were rivaroxaban (53.4%) and apixaban (40.8%), followed by dabigatran (3.9%) and edoxaban (1.9%). In 87.0% of cases of rivaroxaban and in 83.3% of cases of apixaban, these drugs would have been prescribed at the standard initial dosages (rivaroxaban: 15 mg twice daily for 21 days, then 20 mg once daily; apixaban: 10 mg twice daily for 7 days, then 5 mg twice daily). Other physicians reported reduced dosages and/or an initial period of LMWH. The most common choice for parenteral anticoagulation alone was LMWH (91.9%), mainly at therapeutic (75.0%) or decreasing (eg, reduction after the first month of therapeutic dose, 19.6%) dosing. Overall, 52.5% of physicians would consider an anticoagulant treatment duration of 3 months, 23.4% 6 months, 6.6% 12 months, 8.5% indefinite, and 9.1% other options.
3.3. Case scenario 2: Cirrhotic SVT with esophageal varices
The second vignette was answered by 326 physicians: 6.1% of them would not prescribe any anticoagulant, 44.2% would prescribe VKAs, 39.9% parenteral anticoagulation alone, and 9.2% DOACs. Among those who chose the anticoagulant treatment, 80.0% would start immediately after diagnosis, together with beta‐blocker treatment for esophageal varices; 8.2% within 3‐7 days; and 11.8% after variceal band ligation. In 97.9% of cases in which VKAs were chosen, they were targeted to INR range 2.0‐3.0. The most commonly suggested DOACs were apixaban (50.0%) and rivaroxaban (42.9%), followed by dabigatran and edoxaban (3.6% each). Regarding parenteral anticoagulation alone, the most common choice was LMWH (92.3%) at therapeutic (44.7%), intermediate (25.4%), or decreasing (21.1%) dosing. Among those physicians who would prescribe anticoagulation, 34.6% considered an anticoagulant treatment duration of 3 months, 19.5% 6 months, 3.1% 12 months, 25.7% indefinite, and 17.1% other options.
3.4. Case scenario 3: CVT secondary to transient risk factor
Among the 297 respondents to the third vignette, the majority would prescribe VKAs (63.0%), followed by the DOACs (22.9%) and parenteral anticoagulation alone (13.5%). When VKAs were chosen, in 98.4% of cases the target INR range was 2.0‐3.0. The most commonly suggested DOACs were rivaroxaban (49.1%) and apixaban (38.6%), followed by dabigatran (7.0%) and edoxaban (5.3%). In 81.5% of cases of rivaroxaban and in 81.8% of cases of apixaban, these drugs would have been prescribed at the standard initial dosages. The most common choice for parenteral anticoagulation alone was LMWH (92.3%), mainly at therapeutic (83.3%) or decreasing (13.9%) dosing. Overall, 37.9% of physicians would consider an anticoagulant treatment duration of 3 months, 36.5% 6 months, 14.0% 12 months, 4.1% indefinite, and 7.5% other options.
3.5. Case scenario 4: Unprovoked CVT with concomitant intracranial hemorrhage
The fourth vignette was answered by 274 physicians: 5.1% of them would not prescribe any anticoagulant, 44.2% would prescribe VKAs, 39.8% parenteral anticoagulation alone, and 9.9% DOACs. Among those who chose the anticoagulant treatment, 52.3% would start immediately after diagnosis; 23.9% within 3‐7 days; and 23.9% after resolution of the intracerebral hemorrhage. In 98.2% of situations in which VKAs were chosen, they were targeted to INR range 2.0‐3.0. The most commonly suggested DOACs was apixaban (57.9%), followed by rivaroxaban (26.3%) and dabigatran (15.8%). In 63.6% of cases of apixaban and in 60% of cases of rivaroxaban, these drugs would have been prescribed at reduced dosages or following initial heparin treatment. Regarding parenteral anticoagulation alone, the most common choice was LMWH (77.6%), mainly at therapeutic (45.6%), increasing (eg, increment after the first month of prophylactic dose, 21.5%) or intermediate (20.3%) dosing, whereas UFH was preferred in 22.4% of cases. Among those physicians who would prescribe anticoagulation, 17.0% considered an anticoagulant treatment duration of 3 months, 28.1% 6 months, 15.0% 12 months, 29.2% indefinite, and 10.7% other options.
3.6. Subgroup analyses of anticoagulant treatment choice and duration
Some differences emerged in the subgroup analyses by geographic regions, showing a higher preference for the DOACs (cases 1‐3) and a shorter anticoagulant treatment duration (cases 1‐2) in North America compared to Europe (Figure 2).
Figure 2.
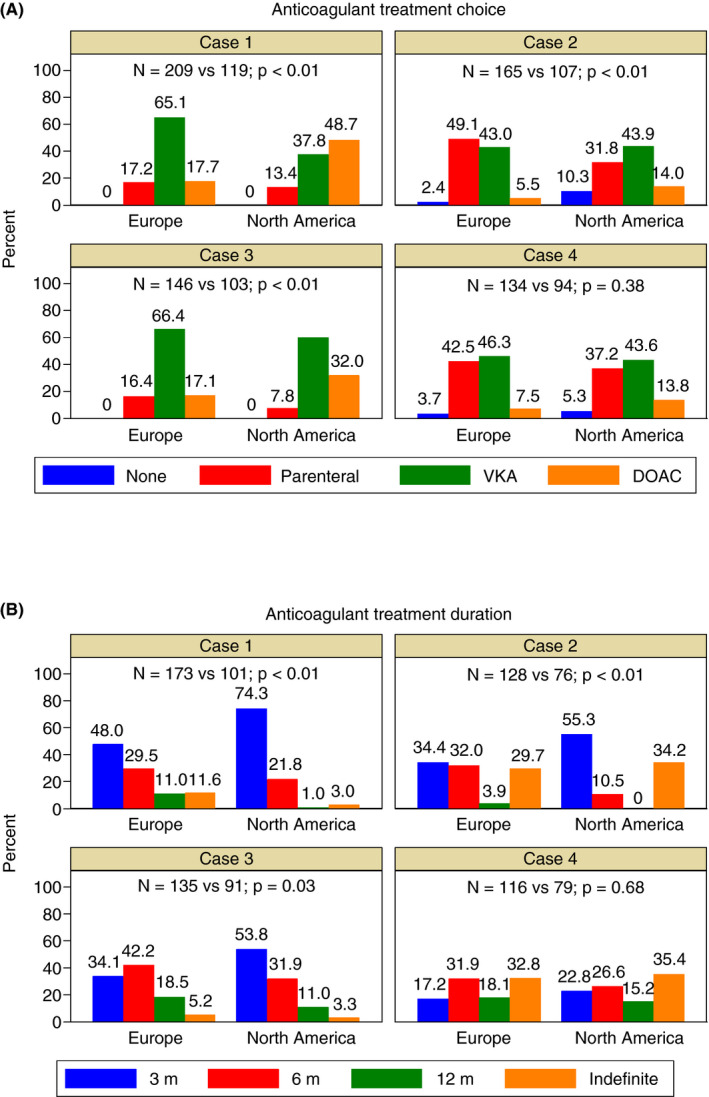
Anticoagulant treatment choice (A) and duration (B) by geographic regions. DOAC = direct oral anticoagulant; VKA = vitamin K antagonist
The analysis by medical specialties showed that internal medicine specialists, compared to hematologists, would choose VKA more frequently in case 3 and would prescribe a shorter anticoagulant treatment duration in case 1 (Figure S1). There were no statistically significant differences in anticoagulant treatment choice and duration according to clinical experience in VTE (Figure 3) or age categories (Figure S2).
Figure 3.
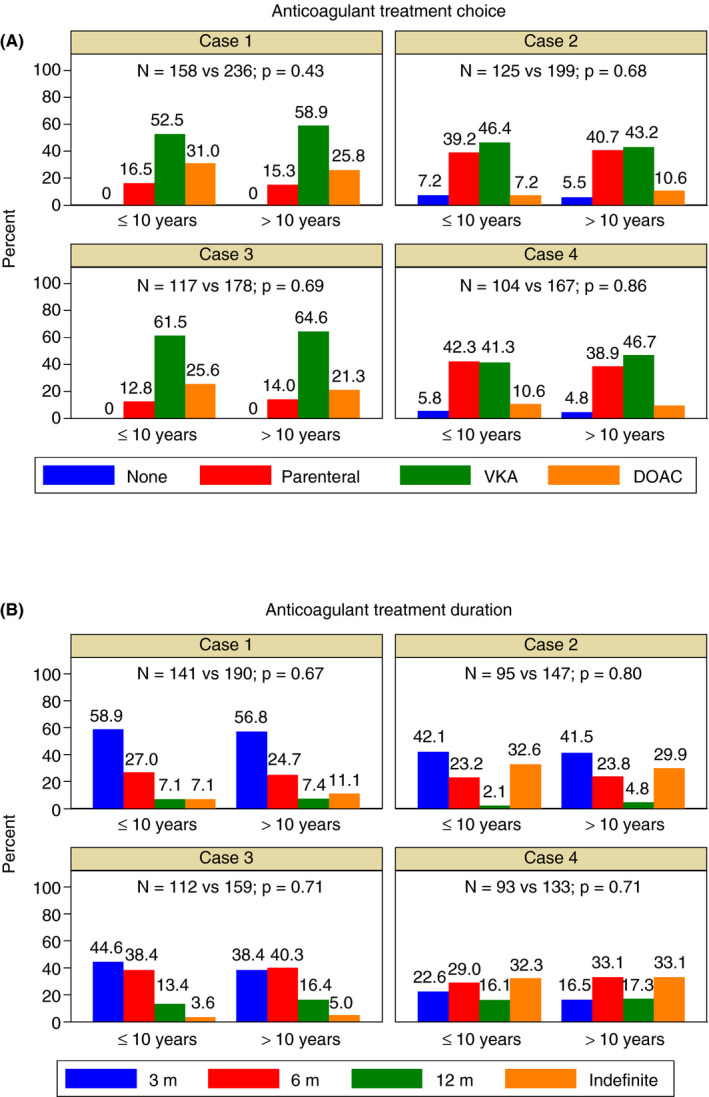
Anticoagulant treatment choice (A) and duration (B) by years of clinical experience in venous thromboembolism. DOAC = direct oral anticoagulant; VKA = vitamin K antagonist
3.7. Rationale behind the anticoagulant treatment choice
Table 3 summarizes the rationale behind the choice of the different anticoagulant treatment options. For instance, the most common reasons for choosing DOACs were the oral route of administration (50.6%), the lack of need for laboratory monitoring (48.1%), and the favorable safety profile of these drugs (43.4%); for VKAs, instead, the most common reasons were many years of clinical experience (38.0%), the proven efficacy in this setting (32.1%), and the available literature on these drugs (30.3%).
Table 3.
Rationale behind the choice of the anticoagulant treatment
| Reasons | Analyzed by clinical case scenarios | Analyzed by different anticoagulation options across the four clinical case scenarios | ||||||
|---|---|---|---|---|---|---|---|---|
|
Case 1 n = 397 (%) |
Case 2 n = 326 (%) |
Case 3 n = 297 (%) |
Case 4 n = 274 (%) |
No anticoagulation n = 34 (%) |
Parenteral drugs only n = 341 (%) |
VKAs n = 674 (%) |
DOACs n = 235 (%) |
|
| Route of administration of the drug |
86 (21.7) |
38 (11.7) |
67 (22.6) |
27 (9.9) |
0 (0) |
37 (10.9) |
60 (8.9) |
119 (50.6) |
| Pharmacological properties of the drug (eg, half‐life) |
37 (9.3) |
61 (18.7) |
22 (7.4) |
55 (20.1) |
0 (0) |
106 (31.1) |
20 (3.0) |
45 (19.2) |
| Availability of an antidote |
35 (8.8) |
64 (19.6) |
27 (9.1) |
62 (22.6) |
0 (0) |
40 (11.7) |
131 (19.4) |
15 (6.4) |
| No need for blood monitoring during follow‐up |
79 (19.9 |
23 (7.1) |
43 (14.5) |
9 (3.3) |
0 (0) |
38 (11.1) |
2 (0.3) |
113 (48.1) |
| Availability of laboratory tests to measure the anticoagulant effect |
62 (15.6) |
49 (15.0) |
44 (14.8) |
42 (15.3) |
0 (0) |
40 (11.7) |
150 (22.3) |
7 (3.0) |
| Many years of clinical experience with this drug |
136 (34.3) |
80 (24.5) |
98 (33.0) |
70 (25.6) |
0 (0) |
89 (26.1) |
256 (38.0) |
37 (15.7) |
| Favorable safety profile of the drug |
69 (17.4) |
58 (17.8) |
63 (21.2) |
50 (18.3) |
0 (0) |
99 (29.0) |
39 (5.8) |
102 (43.4) |
| Proven efficacy of the drug in this setting |
100 (25.2) |
51 (15.6) |
85 (28.6) |
48 (17.5) |
0 (0) |
60 (17.6) |
216 (32.1) |
8 (3.4) |
| Patient’s high risk of bleeding |
10 (2.5) |
107 (32.8) |
16 (5.4) |
96 (35.0) |
21 (61.8) |
128 (37.5) |
57 (8.5 |
20 (8.5) |
| Patient’s high risk of thrombosis extension |
44 (11.1) |
24 (7.4) |
19 (6.4) |
17 (6.2) |
0 (0) |
40 (11.7) |
48 (7.1) |
15 (6.4) |
| Patient’s demographic characteristics (age, sex) |
8 (2.0) |
1 (0.3) |
7 (2.4) |
0 (0) |
0 (0) |
2 (0.6) |
5 (0.7) |
9 (3.8) |
| Patient's comorbidities |
8 (2.0) |
79 (24.2) |
3 (1.0) |
23 (8.4) |
9 (26.5) |
56 (16.4) |
40 (5.9) |
8 (3.4) |
| Patient’s preference |
21 (5.3) |
2 (0.6) |
18 (6.1) |
2 (0.7) |
0 (0) |
2 (0.6) |
5 (0.7) |
34 (14.5) |
| Expected higher patient’s adherence |
15 (3.8) |
2 (0.6) |
12 (4.0) |
3 (1.1) |
0 (0) |
3 (0.9) |
5 (0.7) |
23 (9.8) |
| Drug licensed by regulatory authorities for this indication |
32 (8.1) |
9 (2.8) |
22 (7.4) |
10 (3.7) |
0 (0) |
3 (0.9) |
68 (10.1) |
2 (0.9) |
| Guidelinesrecommendations |
73 (18.4) |
47 (14.4) |
78 (26.3) |
54 (19.7) |
6 (17.7) |
42 (12.3) |
194 (28.8) |
10 (4.3) |
| Results of currently available literature |
88 (22.2) |
51 (15.6) |
77 (25.9) |
56 (20.4) |
8 (23.5) |
34 (10.0) |
204 (30.3) |
25 (10.6) |
| Personal experience when treating patients with this condition |
81 (20.4) |
50 (15.3) |
44 (14.8) |
39 (14.2) |
7 (20.6) |
45 (13.2) |
125 (18.6) |
36 (15.3) |
| Other reasons |
6 (1.5) |
6 (1.8) |
2 (0.7) |
2 (0.7) |
3 (8.8) |
3 (0.9) |
9 (1.3) |
1 (0.4) |
Up to three choices were possible in each case scenario. The three most common reasons in each column are highlighted.
Abbreviations: DOACs, direct oral anticoagulants; VKAs, vitamin K antagonists.
North American physicians, compared to European physicians, more frequently based their choice of anticoagulation on their personal experience when treating patients with these conditions (22.2% vs 14.0%; P < .01), on the perceived patient’s high‐bleeding‐risk (21.7% vs 14.0%; P < .01), on the route of administration of the drug (18.7% vs 13.1%; P = .01), and on the lack of need for laboratory monitoring (18.5% vs 7.1%; P < .01). Conversely, European physicians more frequently chose the following rationales, compared to North American physicians: proven efficacy of the drug in this setting (24.5% vs 17.8%; P < .01), guidelines recommendations (23.9% vs. 11.2%; P < .01), availability of laboratory tests to measure the anticoagulant effect (17.6% vs 10.3%; P < .01), and drug licensed by regulatory authorities for this indication (8.4% vs. 1.2%; P < .01). Results of the analysis of the rationale in different subgroups of physicians are presented in Figure 4.
Figure 4.
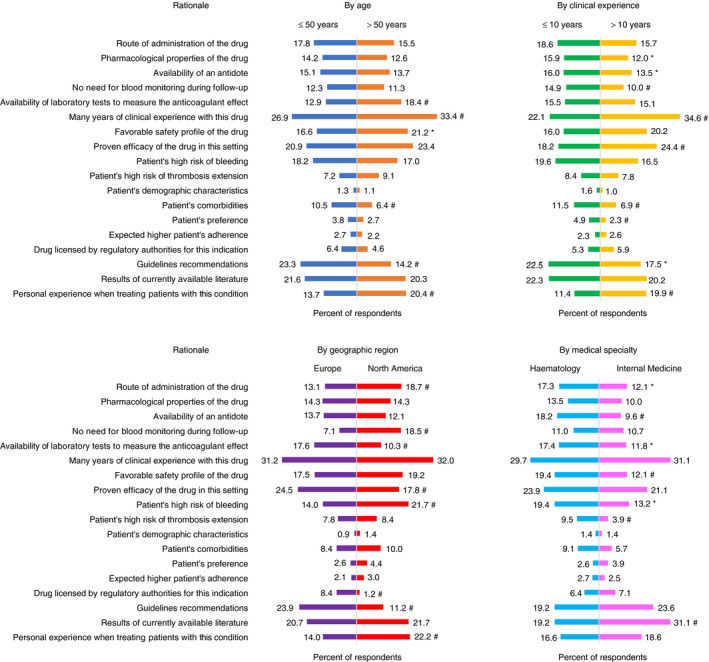
Rationale behind the choice of the anticoagulant treatment across the four clinical case scenarios (analyzed by age categories, years of clinical experience in venous thromboembolism, geographic regions, medical specialties). Level of statistical significance for the comparison between the two groups: * P value .0125 to <.05. # P value <.0125
4. DISCUSSION
Our study reported the results of an international survey on the management of unusual‐site VTE. The first two clinical vignettes evaluated SVT treatment and we found that VKAs were still the anticoagulant of choice. However, in case 1 (SVT secondary to transient risk factor and low‐bleeding‐risk), a significant proportion (27.7%) of physicians would consider the DOACs, most commonly the factor Xa inhibitors at the standard initial dosages (single‐drug approach). Conversely, in case 2 (cirrhotic SVT with signs of portal hypertension) the DOACs were considered only by a minority of physicians (9.2%). In this case of cirrhotic SVT, 80% of physicians would start anticoagulation and beta‐blockers immediately after diagnosis, in accordance with data suggesting that early anticoagulation is favorably associated with vessel recanalization 10 and with clinical practice guideline recommendations for the prevention of gastrointestinal bleeding. 2
The role of the DOACs in the management of SVT is still debated, and guidance documents and guidelines have produced conflicting recommendations. The 2020 guidelines of the American College of Gastroenterology highlighted the limited evidence available for the use of the DOACs in this context and suggested standard treatment with LMWH or VKA, 11 while in a recently published ISTH guidance the DOACs were suggested as first choice in nonmalignant, noncirrhotic patients with acute symptomatic SVT. 12
Lack of consensus emerged on anticoagulant treatment duration. In case 1, approximately 75% of physicians chose a definite anticoagulant duration of 3‐6 months, probably due to the transient risk factor (ie, surgery) associated with the SVT. 13 However, other physicians opted for indefinite duration, likely due to the involvement of the superior mesenteric vein, which warrants lifelong anticoagulation according to certain clinical practice guidelines. 2 In case 2, only a fourth of physicians considered indefinite treatment. Patients with SVT in the context of liver cirrhosis are indeed associated with the highest risk of both thrombotic and bleeding complications. 14 Despite liver cirrhosis being a permanent risk factor, guidelines for non–liver transplant candidates are vague, 2 and lack of consensus on treatment duration was also reported by a Spanish survey specifically focusing on patients with cirrhosis. 15
The last two clinical vignettes evaluated the treatment of CVT, and we found that VKAs were still regarded as the anticoagulant of choice, similarly to a recent Canadian survey on CVT management. 16 However, in case 3 (CVT secondary to transient risk factor and low‐bleeding‐risk), 22.9% of physicians would consider a DOAC, most commonly the factor Xa inhibitors at the standard initial dosages. It is important to note that this survey was conducted before the RE‐SPECT CVT (A Clinical Trial Comparing Efficacy and Safety of Dabigatran Etexilate With Warfarin in Patients With Cerebral Venous and Dural Sinus Thrombosis) trial, assessing dabigatran for the management of CVT, was published. 7 In case 4 (unprovoked CVT complicated by intracerebral hemorrhage), the DOACs were considered by only 9.9% of physicians. In addition, despite guideline recommendations to anticoagulate also patients with intracerebral hemorrhage at baseline, 4 only 52.3% of physicians in our survey would start anticoagulation immediately after diagnosis.
Lack of consensus on anticoagulant treatment duration emerged also for CVT. In case 3, approximately 75% of physicians chose a definite anticoagulant duration of 3‐6 months, considering that this episode of CVT was secondary to a transient risk factor. 17 , 18 In case 4, instead, 29% of physicians considered indefinite treatment. While the choice of indefinite treatment duration is likely derived from the treatment of unprovoked usual site VTE, 13 more specific clinical practice guidelines suggested 6‐12 months for unprovoked CVT and indefinite treatment duration for patients with history of recurrent VTE or strong prothrombotic risk factors (eg, severe thrombophilia). 17 , 18
Despite the fact that SVT and CVT involve different venous locations and are associated with different underlying risk factors, similarities in responses emerged between patients with provoked SVT or CVT and low‐bleeding‐risk (cases 1 and 3) and between patients with SVT or CVT and high‐bleeding‐risk (cases 2 and 4). Respondents seem to treat SVT and CVT in similar ways and to apply the basic principles of anticoagulation derived from the evidence on the anticoagulant treatment for deep vein thrombosis or pulmonary embolism. However, in patients at high risk of bleeding (eg, liver cirrhosis or concomitant intracerebral hemorrhage), a more cautious approach seems to be preferred.
Subgroup analyses showed some geographic differences with a preference for DOACs and shorter anticoagulant treatment duration in North America compared to Europe. Cost seems to be the major driving factor for the use of DOACs in North America, since LMWH is more expensive both in the United States and in Canada, and, consequently, European physicians were more likely to prescribe long‐term LMWH. Similarly, a study evaluating cancer‐associated thrombosis showed that, despite guideline recommendations, only a minority of patients in the United States were treated with LMWH. 19 In addition, the GARFIELD‐VTE registry (Global Anticoagulant Registry in the Field–Venous Thromboembolism) of patients with usual site VTE highlighted that the DOACs were most frequently used in North America and Australia. 20
We also noticed that the rationale behind the treatment choice and duration was different between North American and European physicians, the former giving more importance to pharmacological properties of the drugs and their personal experience in the management of unusual VTE, while the latter favoring guidelines recommendations and the established efficacy of certain drugs in the treatment of unusual VTE.
The main strengths of our study include the large number of respondents from different geographic locations and different clinical backgrounds, the evaluation of the rationale behind the anticoagulant treatment choice, and the vignette‐based survey design. Through a simulation of real scenarios, vignette studies allow to investigate the decision‐making process and were reported to have both good internal and external validity. 21 , 22
However, this study has also some limitations. First, we cannot exclude the presence of a self‐reporting bias, since participants’ responses could have been based on theoretical knowledge rather than actual experience, and thus might not exactly reproduce their actions in clinical practice. In addition, no information was collected on the number of patients with unusual VTE who were actually treated with DOAC by each respondent. Second, the low response rate (15.9%) could be partly due to a proportion of the membership of the involved societies being basic scientists or physicians more interested in bleeding disorders, and partly to the web‐based survey without incentives, as similar response rates were previously reported with this method. 23 , 24 Third, since our survey was sent to members of thrombosis and hemostasis societies, the respondents had a special interest for VTE; thus, their responses might not be generalizable to the most common treatment choices outside university hospitals or tertiary‐care hospitals.
In conclusion, our study provided an estimate of the current therapeutic approaches to SVT and CVT and evidenced that the DOACs were considered also in unusual‐site VTE. In addition, a wide variability of treatment options and duration emerged, reflecting the clinical difficulties of certain case scenarios but also the lack of high‐quality evidence in the literature, thus raising the need for more research and guidance on these topics.
AUTHOR CONTRIBUTIONS
N.R. contributed to study conception and design, analysis, and interpretation of data and drafted the article. W.A., M.C. and A.G. contributed to study conception and design, interpretation of data, and critical revision of the manuscript. All authors provided final approval of the manuscript.
RELATIONSHIP DISCLOSURE
N.R. and A.G. have no relevant conflict of interest to disclose. M.C. received research funding from LEO Pharma, BMS, and Pfizer; and honoraria for advisory boards from Bayer, Pfizer, Sanofi, Leo Pharma, Servier, and BMS. W.A. received research funding from Bayer Healthcare; and honoraria for advisory boards from Bayer, Boehringer Ingelheim, Daiichi Sankyo, Portola, Janssen, Sanofi, Aspen, and Leo Pharma.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This study was conducted under the auspices of the SSC Control of Anticoagulation Subcommittee of the ISTH. We thank the ISTH staff, in particular Shannon Brooks and Lacey Schmeidler, for their help in preparing the online survey and the support to this project; the ISTH, Thrombosis Canada, and the Italian Society for the Study of Haemostasis and Thrombosis for spreading this initiative among their members; and all the respondents who kindly completed the survey.
Riva N, Carrier M, Gatt A, Ageno W. Anticoagulation in splanchnic and cerebral vein thrombosis: An international vignette‐based survey. Res Pract Thromb Haemost. 2020;4:1192–1202. 10.1002/rth2.12424
Handling Editor: Dr Pantep Angchaisuksiri.
This study was supported by ISTH.
Contributor Information
Nicoletta Riva, Email: nico.riva@hotmail.it, Email: nicoletta.riva@um.edu.mt, @NicolettaRivaMD.
Marc Carrier, @MarcCarrier1.
REFERENCES
- 1. Riva N, Ageno W. Cerebral and splanchnic vein thrombosis: advances, challenges, and unanswered questions. J Clin Med. 2020;9:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: vascular diseases of the liver. J Hepatol. 2016;64:179–202. [DOI] [PubMed] [Google Scholar]
- 3. Björck M, Koelemay M, Acosta S, Bastos Goncalves F, Kölbel T, Kolkman JJ, et al. Editor's Choice – management of the diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460–510. [DOI] [PubMed] [Google Scholar]
- 4. Ferro JM, Bousser MG, Canhão P, Coutinho JM, Crassard I, Dentali F, et al. European Stroke Organization. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis ‐ endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24:1203–13. [DOI] [PubMed] [Google Scholar]
- 5. Hanafy AS, Abd‐Elsalam S, Dawoud MM. Randomized controlled trial of rivaroxaban versus warfarin in the management of acute non‐neoplastic portal vein thrombosis. Vascul Pharmacol. 2019;113:86–91. [DOI] [PubMed] [Google Scholar]
- 6. Naymagon L, Tremblay D, Zubizarreta N, Moshier E, Troy K, Schiano T, et al. The efficacy and safety of direct oral anticoagulants in noncirrhotic portal vein thrombosis. Blood Adv. 2020;4:655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferro JM, Coutinho JM, Dentali F, Kobayashi A, Alasheev A, Canhão P, et al. RE‐SPECT CVT Study Group. Safety and Efficacy of Dabigatran Etexilate vs Dose‐Adjusted Warfarin in Patients With Cerebral Venous Thrombosis: A Randomized Clinical Trial. JAMA Neurol. 2019;76:1457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wasay M, Khan M, Rajput HM, Farooq S, Memon MI, AlRukn SA, et al. New oral anticoagulants versus warfarin for cerebral venous thrombosis: a multi‐center, observational study. J Stroke. 2019;21:220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li G, Taljaard M, Van den Heuvel ER, Levine MA, Cook DJ, Wells GA, et al. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol. 2017;46:746–55. [DOI] [PubMed] [Google Scholar]
- 10. Delgado MG, Seijo S, Yepes I, Achécar L, Catalina MV, García‐Criado A, et al. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol. 2012;10:776–83. [DOI] [PubMed] [Google Scholar]
- 11. Simonetto DA, Singal AK, Garcia‐Tsao G, Caldwell SH, Ahn J, Kamath PS. ACG clinical guideline: disorders of the hepatic and mesenteric circulation. Am J Gastroenterol. 2020;115:18–40. [DOI] [PubMed] [Google Scholar]
- 12. Di Nisio M, Valeriani E, Riva N, Schulman S, Beyer‐Westendorf J, Ageno W. Anticoagulant therapy for splanchnic vein thrombosis: ISTH SSC Subcommittee Control of Anticoagulation. J Thromb Haemost. 2020;18:1562–8. [DOI] [PubMed] [Google Scholar]
- 13. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis. Chest. 2012;141(2):e419S–e496S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ageno W, Riva N, Schulman S, Beyer‐Westendorf J, Bang SM, Senzolo M, et al. Long‐term clinical outcomes of splanchnic vein thrombosis: results of an international registry. JAMA Intern Med. 2015;175:1474–80. [DOI] [PubMed] [Google Scholar]
- 15. Fortea JI, Puente Á, Ezcurra I, Cuadrado A, Arias‐Loste MT, Cabezas J, et al. Management of haemostatic alterations and associated disorders in cirrhosis in Spain: a national survey. Dig Liver Dis. 2019;51:95–103. [DOI] [PubMed] [Google Scholar]
- 16. Field TS, Camden MC, Al‐Shimemeri S, Lui G, Lee AY. Off‐label use of novel anticoagulants for treatment of cerebral venous thrombosis: a Canadian survey. Int J Stroke. 2017;12(9):NP16–NP18. [DOI] [PubMed] [Google Scholar]
- 17. Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the. Stroke. 2011;42:1158–92. [DOI] [PubMed] [Google Scholar]
- 18. Einhäupl K, Stam J, Bousser MG, De Bruijn SF, Ferro JM, Martinelli I, et al. European Federation of Neurological Societies. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17:1229–35. [DOI] [PubMed] [Google Scholar]
- 19. Khorana AA, Yannicelli D, McCrae KR, Milentijevic D, Crivera C, Nelson WW, et al. Evaluation of US prescription patterns: are treatment guidelines for cancer‐associated venous thromboembolism being followed? Thromb Res. 2016;145:51–3. [DOI] [PubMed] [Google Scholar]
- 20. Haas S, Ageno W, Weitz JI, Goldhaber SZ, Turpie AGG, Goto S, et al. Anticoagulation therapy patterns for acute treatment of venous thromboembolism in GARFIELD‐VTE patients. J Thromb Haemost. 2019;17:1694–706. [DOI] [PubMed] [Google Scholar]
- 21. Evans SC, Roberts MC, Keeley JW, Blossom JB, Amaro CM, Garcia AM, et al. Vignette methodologies for studying clinicians' decision‐making: validity, utility, and application in ICD‐11 field studies. Int J Clin Health Psychol. 2015;15:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Converse L, Barrett K, Rich E, Reschovsky J. Methods of observing variations in physicians’ decisions: the opportunities of clinical vignettes. J Gen Intern Med. 2015;30:S586–S594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. VanGeest JB, Johnson TP, Welch VL. Methodologies for improving response rates in surveys of physicians: a systematic review. Eval Health Prof. 2007;30:303–21. [DOI] [PubMed] [Google Scholar]
- 24. Mumoli N, Barco S, Cei M, Giorgi‐Pierfranceschi M, Campanini M, Fontanella A, et al. Prevention and treatment of venous thromboembolism in patients with solid brain neoplasms: results of a survey among Italian physicians. Intern Emerg Med. 2017;12:437–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


