Essentials.
Choice of anticoagulant therapy in thrombosis in unusual locations remains controversial.
Guideline lines on this topic are discordant.
Clinical practice is heterogeneous with regard to choice of drug class and duration of treatment.
Randomized controlled trials applying functional outcome and quality of life as important outcomes are urgently needed.
A large number of studies have focused on venous thromboembolism (VTE) over the past decades, accumulating to a staggering 23 595 articles indexed in Medline (August 3, 2020). Perhaps as a consequence of these efforts, mortality of VTE has decreased over time. 1 Despite this success, many aspects of the optimal care for patients at risk for or diagnosed with VTE remain uncertain. One of the topics for which current evidence falls short to support any strong‐level recommendation is the management of thromboses at unusual locations, such as splanchnic vein thrombosis and cerebral vein thrombosis.
Reasons for the lack of adequately sized randomized studies on this particular topic are its relative rarity, at least if compared with “usual” VTE, as well as logistical issues related to the complexity (and heterogeneity) of the clinical scenarios in which thrombosis at unusual locations occurs. For instance, splanchnic vein thrombosis is often associated with liver cirrhosis, bacterial infections, and cancer, conditions that are intrinsically associated with a substantial risk of anticoagulant treatment–associated bleeding. 2 , 3 Moreover, patients suffering from thromboses at unusual locations may not always be ideal candidates for participating in clinical trials in relation to usual eligibility criteria, including a long life expectancy, good adherence to the study procedures, and lack of contraindications to specific anticoagulant agents. In some cases, these patients are simply unable to consent to participation.
As a consequence, practice guidelines base their recommendations on available evidence from retrospective cohorts studies and extrapolation from interventional studies of deep vein thrombosis (DVT) and acute pulmonary embolism (PE). 4 , 5 , 6 The latter makes sense considering some shared pathophysiological mechanisms of thrombus formation but may diverge considering the ultimate goal of treatment. For DVT and PE, this is prevention of recurrent VTE, thrombus propagation, and progression to potentially fatal PE. This is less relevant for thromboses at unusual sites since splanchnic vein thrombosis and cerebral vein thrombosis do not progress to acute PE. Instead, the main goal of treating thromboses at unusual locations—in addition to prevention of extension or recurrence—is prevention of local infarction, venous hypertension with associated (bleeding) complications, and organ dysfunction.
Large randomized trials have undoubtedly demonstrated the better safety profile of oral direct anticoagulants (DOACs) over vitamin K antagonists (VKAs) when it comes to the treatment of DVT and acute PE. 7 The applicability of these findings for the choice of anticoagulant therapy for patients with thromboses at unusual locations is still debatable. Arguments used to promote or warn for the use of DOACs in this setting include the overall safety profile of anticoagulant drug classes, relevance of a first‐pass effect in the portal circulation of selected DOACs, the ability to reverse the anticoagulant in case of major bleeding, the potential presence (or risk) of relevant liver/renal dysfunction, the possibility to monitor anticoagulation intensity, the preference for oral over parenteral drugs, costs, availability, off‐label status, and the absence of high‐level evidence. As is often the case in areas of such uncertainty, recent guidelines provide conflicting recommendations, causing considerable heterogeneity in global treatment patterns. 4 , 5 , 6 The European Stroke Organization, for instance, recommends against the use of DOACs for the treatment of cerebral vein thrombosis. 4 The American College of Gastroenterology suggests using low‐molecular‐weight heparin (LMWH) and VKAs over DOACs for the initial and chronic treatment of splanchnic vein thrombosis. 5 In contrast, the ISTH Scientific and Standardization Committee (SSC) Control of Anticoagulation subcommittee suggests prescribing a DOAC at full dose over conventional anticoagulation, albeit only in noncirrhotic patients, with adequate renal function and without luminal gastrointestinal cancer and/or contraindications for the use of DOACs. 6 Notably, in our view, a preference for either of the available drug classes in patients with splanchnic vein thromboses and cerebral vein thromboses is surprising considering the minimal low‐quality data available.
In this issue of Research and Practice in Thrombosis and Haemostasis, Nicoletta Riva and colleagues illustrate the status quo of treatment of thrombosis at unusual locations. They performed a cross‐sectional survey among physicians, consisting of four vignette cases with either provoked or unprovoked splanchnic or cerebral vein thrombosis. Participants were asked to detail their preferred therapeutic approach in each of these vignettes: choice of drug, drug dose, and duration of treatment, including a rationale for the chosen treatment. The main findings of the study may be summarized as follows: (i) wide variability in all aspects of the treatment, (ii) DOACs were considered in all four vignettes, but (iii) the majority of responders preferred conventional anticoagulation over DOACs across all scenarios. In the vignettes with a clear provoked thrombosis, 23% and 28% of responders indicated to consider treatment with a DOAC. In the vignettes with the highest perceived risk of bleeding, this was only 9%–10%, respectively. Suggested duration of anticoagulant treatment also considerably differed in all four vignettes, without a clear common preference.
What does this important study tell us? First and foremost, it underlines the urgent need of performing properly designed randomized trials comparing the available therapeutic approaches, despite the foreseen efforts in the author’s conduction. The formal outcomes of such studies should ideally go beyond mortality, recurrent VTE, major bleeding, and ischemic complications, also accounting for functional outcomes, quality of life, and patient satisfaction (Figure 1). Given the substantial equivalence of DOACs and heparin/VKAs concerning the net clinical outcome of recurrence plus bleeding, it is more likely that such patient‐relevant functional outcomes may reflect differences in administration, monitoring, and drug‐specific complications of an anticoagulant strategy over the other. 8 This may also allow the conduction of pharmacoeconomic analyses. Second, the results of the study will definitely shape future discussions on this topic and contribute to designing the optimal trial to provide the relevant evidence required for strong and consistent guideline recommendations.
FIGURE 1.
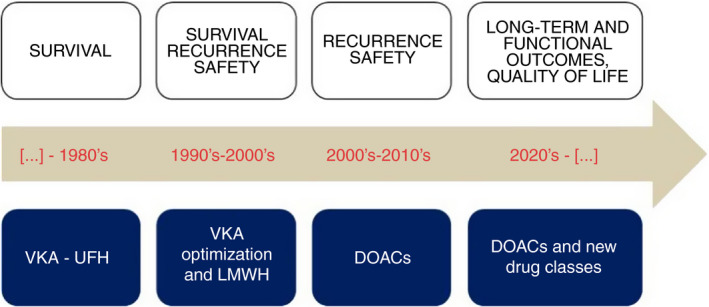
Evolution of outcomes in venous thromboembolism research over the past decades. DOAC, direct oral anticoagulant; LMWH, low‐molecular‐weight heparin; UFH, unfractionated heparin; VKA, vitamin K antagonist
What is to be done in our daily practice until the results of such studies are available? In this more or less evidence‐free zone, experience does count for something. Taking the paucity of comparative studies and the off‐label status of DOACs into account, as well as the long but relevant list of “ifs and buts” as stated in the SSC recommendation, 6 we feel strengthened by the results of the survey to be restrictive in the use of DOACs as primary treatment of splanchnic and cerebral vein thrombosis and consider DOACs only as an alternative to conventional treatment in selected cases without contraindications to the use of DOACs, rather than as preferred management.
AUTHOR CONTRIBUTIONS
FAK drafted the first version of the manuscript, and SB critically revised the draft for important intellectual content. Both authors approved the final version of the manuscript.
RELATIONSHIP DISCLOSURE
FK reports research grants from Bayer, Bristol‐Myers Squibb, Boehringer‐Ingelheim, Daiichi‐Sankyo, MSD, Actelion, the Dutch Heart Foundation, and the Dutch Thrombosis Association, all outside the submitted work. SB received congress and travel payments from Daiichi‐Sankyo and Bayer HealthCare, and personal fee from BTG/EKOS, outside the submitted work.
Klok FA, Barco S. Anticoagulation in splanchnic and cerebral vein thrombosis: Still groping in the dark. Res Pract Thromb Haemost. 2020;4:1080–1082. 10.1002/rth2.12427
Handling Editor: Mary Cushman
Contributor Information
Frederikus A. Klok, Email: f.a.klok@LUMC.nl, @Erik_Klok_MD.
Stefano Barco, @stebarco.
REFERENCES
- 1. Barco S, Mahmoudpour SH, Valerio L, Klok FA, Munzel T, Middeldorp S, et al. Trends in mortality related to pulmonary embolism in the European Region, 2000–15: analysis of vital registration data from the WHO Mortality Database. Lancet Respirat Med. 2020;8(3):277–87. [DOI] [PubMed] [Google Scholar]
- 2. Riva N, Ageno W. Approach to thrombosis at unusual sites: splanchnic and cerebral vein thrombosis. Vascular Med (London, England). 2017;22(6):529–40. [DOI] [PubMed] [Google Scholar]
- 3. Klok FA, Huisman MV. How I assess and manage the risk of bleeding in patients treated for venous thromboembolism. Blood. 2020;135(10):724–34. [DOI] [PubMed] [Google Scholar]
- 4. Ferro JM, Bousser MG, Canhao P, Coutinho JM, Crassard I, Dentali F, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis ‐ endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24(10):1203–13. [DOI] [PubMed] [Google Scholar]
- 5. Simonetto DA, Singal AK, Garcia‐Tsao G, Caldwell SH, Ahn J, Kamath PS. ACG Clinical Guideline: disorders of the hepatic and mesenteric circulation. Am J Gastroenterol. 2020;115(1):18–40. [DOI] [PubMed] [Google Scholar]
- 6. Di Nisio M, Valeriani E, Riva N, Schulman S, Beyer‐Westendorf J, Ageno W. Anticoagulant therapy for splanchnic vein thrombosis: ISTH SSC Subcommittee Control of Anticoagulation. J Thromb Haemost. 2020;18(7):1562–8. [DOI] [PubMed] [Google Scholar]
- 7. van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta‐analysis. J Thromb Haemost. 2014;12(3):320–8. [DOI] [PubMed] [Google Scholar]
- 8. Boon G, Barco S, Bertoletti L, Ghanima W, Huisman MV, Kahn SR, et al. Measuring functional limitations after venous thromboembolism: optimization of the Post‐VTE Functional Status (PVFS) Scale. Thromb Res. 2020;190:45–51. [DOI] [PubMed] [Google Scholar]


