Abstract
Background
Monitoring hemophilia treatment with extended half‐life products is challenging for coagulation laboratories since factor assays may show substantial differences between results obtained with the one‐stage assay (OSA) and the chromogenic substrate assay (CSA).
Objectives
The aim of this study was to evaluate and compare different factor assays and global coagulation methods.
Methods
Factor VIII (FVIII) and IX (FIX) activities and global assay parameters were analyzed in pre‐ and postinfusion samples (5 patients 2 samples/product/method).
Results
Samples containing FVIII products (NovoEight, Elocta, and Nuwiq) gave higher levels when measured with CSA compared to OSA. The correlation was excellent (r 2 ≥ .97) while biases of 42%‐72% of mean (CSA‐OSA) were obtained. With FVIII (OSA) as independent variable, the correlations to kaolin clot time (CT) and thrombin generation assay (TGA) peak were modest (r2 = .71‐.72 and .64‐.65, respectively), except for Nuwiq for which there was a poor correlation to TGA peak (r 2 = .08). Samples containing Alprolix, a FIX product, gave a smaller difference between activity levels (CSA‐OSA), and the correlation was excellent (r 2 = .96). With FIX (CSA) as independent variable for both Alprolix and Refixia, the correlations to Innovin CT and TGA peaks were weak (r 2 = .33‐.45 and .44‐.76, respectively).
Conclusions
Our data show that factor activity assays differ between methods used and agents. These discrepancies indicate the value of having more than one type of assay available in the coagulation laboratory when monitoring hemophilia treatment with extended half‐life products. Global assays gave complementary information indicated by the modest correlations to factor activities.
Keywords: blood coagulation tests, coagulants, drug monitoring, factor IX, factor VIII, hemophilia A, hemophilia B
Essentials.
Monitoring hemophilia treatment with extended half‐life products is a challenge.
Factor VIII and IX activities and global assay parameters were analyzed in postinfusion samples.
Assay discrepancy was shown for factor VIII activity, measured in chromogenic or one‐stage assays.
Global assays gave valuable information and could be used to complement activity measurements.
1. INTRODUCTION
Replacement therapy with factor VIII (FVIII) or IX (FIX) concentrates has been used in patients with hemophilia A and B, respectively, for decades. 1 New products have now been introduced including modifications by fusion or attachment of different molecules for an extended half‐life (EHL). 2 Factor activity assays are used for diagnostic purposes as well as for monitoring and to ensure optimal therapy, but have certain limitations. 3 , 4 , 5 , 6 , 7 In addition, monitoring treatment of modified EHL products has become a challenge for coagulation laboratories, since the factor assays used may show substantial differences with a potential impact on patient management. 8 , 9 Assay discrepancies have been reported for full‐length FVIII and B‐domain deleted FVIII with 20%‐30% higher chromogenic substrate assay (CSA) results compared to one‐stage assay (OSA) results. 10 , 11 In contrast, for recombinant FIX products, the activities measured by CSA are generally 30% lower than those for OSA. 10
The aim of this study was to evaluate and compare the results of different factor assays and global coagulation methods, for example, thrombin generation assay (TGA; Ceveron; Technoclone, Vienna, Austria) and viscoelastic whole blood measurement (rotational thrombelastometry [ROTEM]), measured in pre‐ and postinfusion samples of recently available FVIII and FIX products. Our data show that the results differ between methods used and agents. These discrepancies may have a significant impact on the clinical management of patients and indicate the value of having more than one type of assay available in the coagulation laboratory.
2. METHODS
2.1. Blood sampling and patient samples
This was a method comparison, in which coagulation assays in use in the clinical and laboratory routine follow‐up practice were analyzed in combination. Ethical approval for method comparisons was obtained from the local ethics board (Dnr2015/886). No additional blood sampling or information about the patients was obtained. Two blood samples (citrated, 0.129 M) were drawn at the routine follow‐up visit at the Clinical Coagulation Department, before infusion and 15 minutes after infusion upon change of treatment. Fifteen patients with hemophilia A and 10 patients with hemophilia B were included, changing to one of the following products: NovoEight (Novo Nordisk A/S, Bagsvaerd, Denmark), Elocta (Swedish Orphan Biovitrum AB, Solna, Sweden), Nuwiq (Octapharma AB, Stockholm, Sweden), Refixia (Novo Nordisk A/S, Bagsvaerd, Denmark), or Alprolix (Swedish Orphan Biovitrum AB, Solna, Sweden). The results were compared to the FVIII standard product Advate (Shire, Lexington, MA, USA) with postinfusion samples. None of the included patients had a current or history of inhibitor (<0.4 BU; half‐time as expected). Similar doses/kg per product (prophylaxis doses of 20‐50 IU/kg, 1‐3 times a week) were used. The tubes for plasma analyses were centrifuged for 20 minutes at 2000 g at room temperature and the plasma was frozen and stored at −70°C until analysis. ROTEM analyses were performed on citrated whole blood immediately after blood was drawn.
2.2. FVIII and FIX activity measurements
FVIII coagulant activity (FVIII:C) was measured with two methods (OSA and CSA) using the BCS‐XP instrument (Siemens Healthcare AB, Erlangen, Germany). The OSA was performed with activated partial thromboplastin time (aPTT) reagent PTT‐Automate (Stago, Parsippany, NJ, USA) and calibrator SHP (Siemens Healthcare AB). The CSA was with Coatest SP (ChromoGenix, Uppsala, Sweden) and calibrator NRP (Precision Biologics, Dallas, TX, USA).
FIX coagulant activity (FIX:C) was measured with two methods (OSA and CSA) on the BCS‐XP instrument. The OSA was performed with the aPTT reagent PTT‐Automate (Stago) and calibrator SHP. The CSA was with Rossix FIX (Rossix, Mölndal, Sweden) and calibrator NRP (Precision Biologics).
CSA for both FVIII and FIX was validated on BCS‐XP according to CLSI document EP10‐A3 AMD.
2.3. Global assays
ROTEM (ROTEM delta, TEM Innovations, Munich, Germany) was performed with kaolin (Haemonetics S.A, Vaud, Switzerland) and Innovin (diluted in 0.9% NaCl, final dilution 1:190 000) (Siemens) reagents. ROTEM was measured over time, and the results were presented as clot time (CT, seconds). TGA was performed using Ceveron alpha (Technoclone) with a fluorescent substrate and the TGA RB reagent (Technoclone). Coagulation was initiated through the addition of recombinant human tissue factor and low concentration of phospholipid micelles. Thrombin generation was measured over time, and the results were presented as lag time (minutes) and peak thrombin (nmol/L), which are recommended by the manufacturer.
2.4. Data analysis
The differences were observed using Bland‐Altman plots. Linear regression analysis and the correlation (Pearson r 2) were calculated. Bias plots were also evaluated, and generally an agreement interval of ±10%‐20% was considered as acceptable when comparing results by different methods.
3. RESULTS AND DISCUSSION
The data obtained from measurements in pre‐ and postinfusion samples are shown in Figures 1 and 3. An analysis of FVIII:C showed higher levels for CSA compared to OSA (Figure 1). The differences observed between methods were further compared in linear regression analysis, and the correlation (Pearson r 2) was excellent (r 2 ≥ .97) (Table 1). Bias plots for pre‐ and postinfusion samples from each patient on each FVIII product (n = 15 × 2 samples/method) showed biases between 42% and 72% of the mean (CSA‐OSA) (Table 1). The variation of the slope from 1.7 to 2.3 with a minimal intercept was the most consistent observation between patients (Table 1). For comparison, Advate in randomly collected postinfusion samples (n = 7) gave the regression line: y (CSA) = 1.4 × (OSA) + 0.006, r 2 = .87, bias = 0.14 (31%). Linear regression and bias plot for the difference between pre‐ and postinfusion (CSA‐OSA) for all three FVIII products (n = 15 × 2 results) was also calculated: y = 1.79 × +0.14, r 2 = .73 and bias 0.55 (67%) (Figure 2A,B). The range of mean differences was 0.3‐1.3 (Figure 2B). Our center has long experience with the CSA, especially for FVIII measurement, a method that performs very well in diagnostic samples, that is, to differentiate between severe and moderate hemophilia, and also based on external quality control results. However, the revealed large assay discrepancies (42%‐72%) between CSA and OSA and the better agreement with expected clinical recovery with the OSA, suggest in this comparison that OSA should be the preferred choice of assay for these FVIII products.
FIGURE 1.
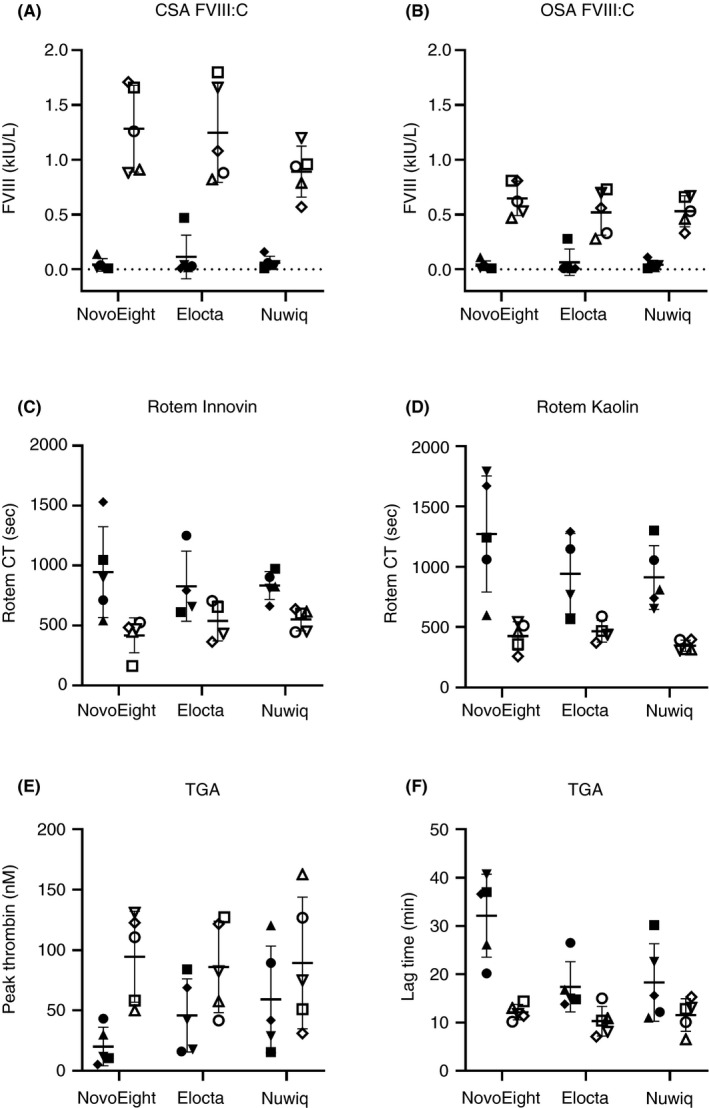
Measured parameters for preinfusion samples (filled symbols) and postinfusion samples (open symbols) for NovoEight, Elocta and Nuwiq (n = 5 samples/product shown as five different symbols). FVIII:C levels with CSA (A) or OSA (B). ROTEM CT levels using Innovin (C) or kaolin (D) as trigger. TGA parameter with peak thrombin (E) or lag time (F). CSA, chromogenic substrate assay; CT, clot time; FVIII, factor VIII; FVIII:C, FVIII coagulant activity; OSA, one‐stage assay; TGA, thrombin generation assay
FIGURE 3.
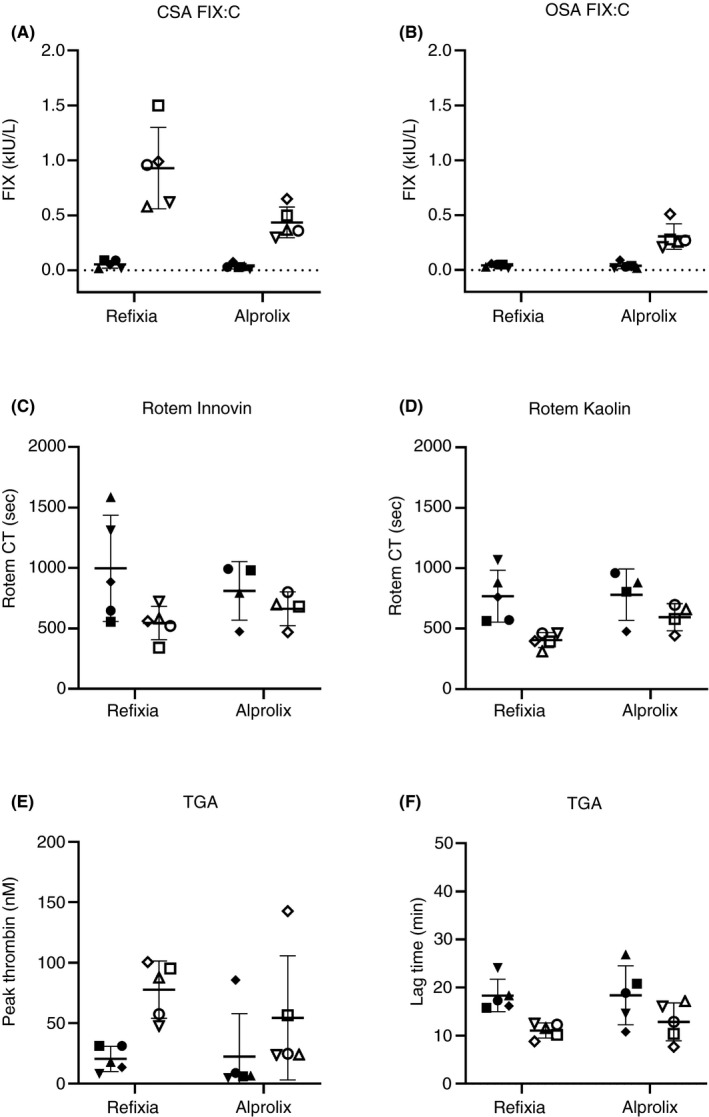
Measured parameters for preinfusion samples (filled symbols) and postinfusion samples (open symbols) for Refixia and Alprolix (n = 5 samples/product shown as five different symbols). FIX:C levels with CSA (A) or OSA (B). ROTEM CT levels using Innovin (C) or kaolin (D) as trigger. TGA parameter with peak thrombin (E) or lag time (F). Refixia was measured >2.0 kIE/L for OSA and not included in graph. CSA, chromogenic substrate assay; CT, clot time; FIX, factor IX; FIX:C, FIX coagulant activity; OSA, one‐stage assay; ROTEM, rotational thrombelastometry; TGA, thrombin generation assay
TABLE 1.
Comparisons of different methods (shown as Method 1 – Method 2) using Bland‐Altman plots for each FVIII‐ or FIX‐product (n = 5 * 2 samples/product)
| FVIII products | Comparison FVIII CSA – FVIII OSA | Comparison CT Kaolin – FVIII OSA | Comparison Peak – FVIII OSA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | r 2 | Bias | Slope | Inter‐cept | r 2 | Bias | Slope | Intercept | r 2 | Bias | |
| Novo‐Eight® | 2.1 | −0.050 | .99 | 42 | −5 × 10−4 | 0.78 | .71 | ND | 0.006 | 0.01 | .64 | ND |
| Elocta® | 2.3 | −0.002 | .97 | 72 | −8 × 10−4 | 0.86 | .71 | ND | 0.006 | −0.1 | .65 | ND |
| Nuwiq® | 1.7 | −0.006 | .98 | 41 | −7 × 10−4 | 0.71 | .72 | ND | 0.002 | 0.17 | .08 | ND |
| FIX products | Comparison FIX CSA – FIX OSA | Comparison CT Innovin – FIX CSA | Comparison Peak – FIX CSA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | r 2 | Bias | Slope | Intercept | r 2 | Bias | Slope | Intercept | r 2 | Bias | |
| Refixia® | ND | ND | ND | ND | 9 × 10−4 | 1.2 | .45 | ND | 0.01 | −0.16 | .76 | ND |
| Alprolix® | 1.4 | 0.010 | .96 | 14 | 7 × 10−4 | 0.78 | .33 | ND | 0.003 | 0.11 | .44 | ND |
Data shown are linear regression parameters (y = ax + b) such as slope (a) and intercept (b). Pearsons correlation coefficient (r 2) and bias shown as % (see Figure 2 as an example of parameters derived from Bland‐Altman plots) CSA (chromogenic assay using FVIII Coatest SP or FIX Rossix), OSA (one‐stage assay using PTT Automate), CT kaolin or Innovin (clot time in ROTEM using kaolin or Innovin as trigger), Peak (thrombin peak in thrombin generation assay). Comparisons that could not be calculated are indicated as not determined (ND).
Abbreviations: FIX, factor IX; FVIII, factor VIII.
[Correction added on Oct 7, 2020, after first online publication: “using Bland‐Altman” removed from the table headings.]
FIGURE 2.
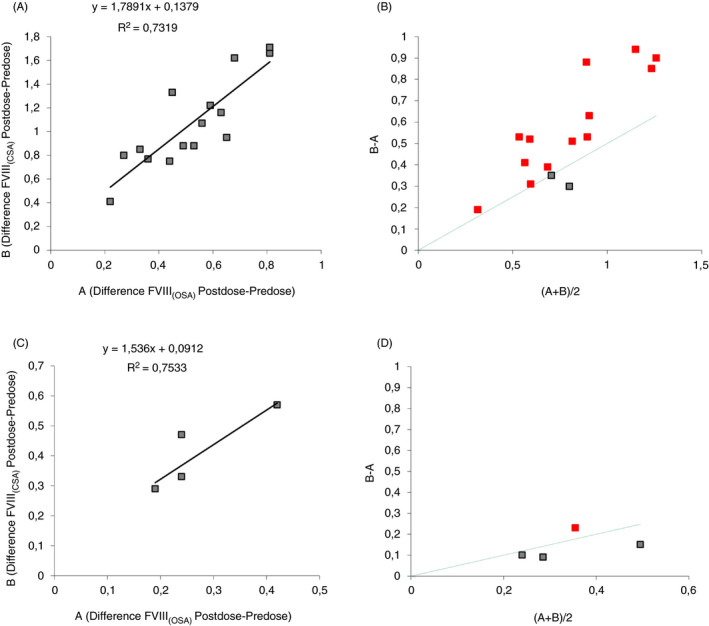
(A) Linear regression; and (B) bias plot. Difference between postinfusion and preinfusion results for FVIII (CSA) compared to FVIII (OSA) for all three tested products (green dotted line indicates 50% bias). (C) Linear regression. (D) Bias plot. Difference between postinfusion and preinfusion results for FIX (CSA) compared to FIX (OSA) for Alprolix (green dotted line indicates 50% bias). CSA, chromogenic substrate assay; FVIII, factor VIII; OSA, one‐stage assay [Correction added on Oct 9, 2020, after first online publication: "FVIII" corrected to "FIX" in Fig 2C axes.]
[Correction added on Oct 9, 2020, after first online publication: “FVIII” corrected to “FIX” in Fig 2C axes.]
An analysis of thrombin generation in FVIII samples indicated thrombin peak as the parameter with the largest observed difference between pre‐ and postinfusion results (Figure 1). Similar analysis using ROTEM showed kaolin CT as the parameter with the largest difference (Figure 1). Correlations were modest for comparison between the two ROTEM parameters CT Innovin and kaolin (r 2 = .61‐.80) and the two TGA parameters lag time and peak (r 2 = .58‐.73). In addition, with FVIII (OSA) as independent variable, the correlations to kaolin CT and TGA peak were modest (r 2 = .71‐.72 and .64‐.65, respectively, Table 1), with one exception, Nuwiq, which had a poor correlation to TGA peak (r 2 = .08). Comparison of biases between TGA or ROTEM parameters and FVIII activity were hampered by the large difference in absolute results.
There was a smaller difference in results between CSA and OSA for Alprolix, and both methods could be used for monitoring (Table 1 and Figure 3). The correlation was excellent (r 2 = .96), and the slope of the linear regression smaller (1.4) compared to that of the FVIII products. Bias plot for Alprolix indicated a small bias of 0.06 (14%) (CSA‐OSA). The difference between pre‐ and postinfusion (CSA‐OSA) for Alprolix (n = 5 × 2 results) was calculated: y = 1.15 × −0.09, r 2 = .75 with a bias of 0.13 (40%) (CSA‐OSA) (Figure 2C,D). The range of the mean differences was 0.2‐0.5, with the majority below 50% (Figure 2D). For Refixia, only the CSA was used as recommended by the manufacturer. 12
The ROTEM and TGA parameters with the largest observed difference between FIX results were the Innovin CT and thrombin peak, respectively (Figure 3). The correlations were modest for comparison between the two ROTEM parameters CT Innovin and CT kaolin (r 2 = .82‐.90) and the two TGA‐parameters lag time and peak (r 2 = .57‐.79). With FIX (CSA) as independent variable, the correlations to Innovin CT and TGA peak were modest (r 2 = .33‐.45 and .44‐.76, respectively, Table 1).
This study has the limitation that the samples were not drawn after a washout period but were “real‐life” pre‐ and postinfusion samples. In addition, FVIII or FIX activities were not <0.01 kIU/L in all preinfusion samples. The correlation between OSA and CSA was, however, very good both for FVIII and FIX in the preinfusion sample, which is in agreement with our experience in the diagnostic setting.
We chose to describe the agreement between results with a Bland‐Altman plot and generally an agreement interval of ±10%‐20% is considered acceptable if two methods measuring the same parameter are compared. For the FVIII:C measurements, however, the bias for the three products tested exceeded that with a mean bias of 41%‐72%, indicating a poor agreement despite an excellent correlation. The European Pharmacopoeia recommends the use of CSA for replacement factor potency labeling of FVIII and OSA for FIX, which has been followed for all products tested in this study, although information around choice of specific reagents is limited. Ideally, similar recovery results should be obtained in postinfusion samples with the same method as used for potency labeling. Generalized product‐by‐product–based guidance about systematic under‐ or overestimation of activity for many reagent combinations is needed. Field studies for many new products, and in Europe UK NEQAS and ECAT quality control programs have also been performed and provided valuable information for the coagulation laboratories. 13 , 14 , 15 , 16 For both Nuwiq and Elocta, field studies indicate that both OSA and CSA can be used for monitoring. However, all reagent‐product combinations have not been validated and, furthermore, the choice of calibrator has not been clarified. CSA and OSA differ in results depending on reagent composition (ie, ± thrombin) or activator composition (ellagic acid/phenol, silica/kaolin), respectively. One limitation of this study is the use of only one reagent for OSA and one for CSA. The best choice of assay would be the assay/reagent that most closely reflects expected values, for example, recovery of spiked samples tested for the reagent‐instrument combination used locally.
For the other parameters evaluated within global assays, the modest correlations to FVIII:C and FIX:C indicate that these might give complementary information, especially for ROTEM kaolin to FVIII (OSA), with r 2 = .71‐.72 for all tested products. For TGA parameters the results were more variable between products. This has also been described by Dargaud et al 17 for Nuwiq‐treated hemophilia patients in that a reduced thrombin generation was observed irrespective of trough levels. For FIX products the comparison to FIX (CSA) was also more variable for ROTEM Innovin and TGA parameters and no direct conclusion could be drawn but further studies are needed. If only the difference between pre‐ and postinfusion samples is considered the best discriminator, both ROTEM kaolin and TGA peak thrombin seem usable for both NovoEight and Refixia, whereas other products generally show more overlap in global than in factor analyses.
With many new products being introduced, it is not possible in each case to introduce the method used for potency labeling by the product manufacturer in all laboratories. More adequate factor levels are generally obtained with the chromogenic method in the low range, but for the three FVIII products in our comparison, the results in postinfusion samples differed much more than we expected.
To conclude, it is of major value for laboratories to have access to more than one method for the measurement of FVIII and FIX activities in patients with EHL products. Preferably, one CSA and one OSA should be available to cover the best method for each product. Our data revealed assay discrepancies between OSA and CSA for the FVIII products tested and with one set of reagents. In addition, global assays gave valuable information and could be used to complement activity measurements.
RELATIONSHIP DISCLOSURE
No external funding was received to perform the study. KS has received speaker fees from Octapharma, SOBI, Shire, and Novo Nordisk; and consultant fees from Novo Nordisk and SOBI. JA has received research grants from Shire, SOBI, and Octapharma; and consultant/speaker fees from Shire, SOBI, Novo Nordisk, and Octapharma. NGA has received speaker fees from SOBI, Bayer, Octapharma, Takeda, and CSL Behring. EZ has received research grants from Shire and Novo Nordisk and consultant fees from Shire, Novo Nordisk, CSL Behring, and SOBI.
AUTHOR CONTRIBUTIONS
KS and JA initiated and designed the study. KS and CA interpreted the data and wrote the manuscript. JA, NGA, EZ, and EN contributed with critical writing and revised the intellectual content. KS approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank My Chi Lam, Elena Mikheeva, Fikrije Rakip, Heidi Isacsson, Emma Engman Grahn, Malin Axelsson, and Eva Lindén for practical help and technical support.
Augustsson C, Norström E, Andersson NG, Zetterberg E, Astermark J, Strandberg K. Monitoring standard and extended half-life products in hemophilia: Assay discrepancies for factor VIII and IX in pre- and postinfusion samples. Res Pract Thromb Haemost. 2020;4:1114–1120. 10.1002/rth2.12421
Handling Editor: Pantep Angchaisuksiri
REFERENCES
- 1. Lee C, Berntorp E, Hoots W. Textbook of Haemophilia, 2nd ed Hoboken, NJ: Wiley‐Blackwells; 2011. [Google Scholar]
- 2. Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet (London, England). 2016;388:187. [DOI] [PubMed] [Google Scholar]
- 3. Kitchen S, McCraw A, Echenagucia M. Diagnosis of Hemophilia and Other Bleeding Disorders, 2nd ed Montreal, Canada: World Federation of Hemophilia; 2010. [Google Scholar]
- 4. Kitchen S, Olson J, Preston E. Quality in Laboratory Hemostasis and Thrombosis, 2nd ed Hoboken, NJ: Wiley‐Blackwell; 2013. [Google Scholar]
- 5. Bates SM, Weitz JI. Coagulation assays. Circulation. 2005;112:e53. [DOI] [PubMed] [Google Scholar]
- 6. Favaloro EJ, Meijer P, Jennings I, Sioufi J, Bonar RA, Kitchen DP, et al. Problems and solutions in laboratory testing for hemophilia. Semin Thromb Hemost. 2013;39:816. [DOI] [PubMed] [Google Scholar]
- 7. Potgieter JJ, Damgaard M, Hillarp A. One‐stage vs. chromogenic assays in haemophilia A. Eur J Haematol. 2015;94(Suppl 77):38. [DOI] [PubMed] [Google Scholar]
- 8. Adcock DM, Strandberg K, Shima M, Marlar RA. Advantages, disadvantages and optimization of one‐stage and chromogenic factor activity assays in haemophilia A and B. Int J Lab Hematol. 2018;40:621. [DOI] [PubMed] [Google Scholar]
- 9. Peyvandi F, Oldenburg J, Friedman KD. A critical appraisal of one‐stage and chromogenic assays of factor VIII activity. J Thromb Haemost. 2016;14:248. [DOI] [PubMed] [Google Scholar]
- 10. Kitchen S, Tiefenbacher S, Gosselin R. Factor activity assays for monitoring extended half‐life FVIII and factor IX replacement therapies. Semin Thromb Hemost. 2017;43:331. [DOI] [PubMed] [Google Scholar]
- 11. Makris M, Peyvandi F. Assaying FVIII activity: one method is not enough, and never was. Haemophilia. 2014;20:301. [DOI] [PubMed] [Google Scholar]
- 12. Rosen P, Rosen S, Ezban M, Persson E. Overestimation of N‐glycoPEGylated factor IX activity in a one‐stage factor IX clotting assay owing to silica‐mediated premature conversion to activated factor IX. J Thromb Haemost. 2016;14:1420. [DOI] [PubMed] [Google Scholar]
- 13. Sommer JM, Moore N, Mcguffie‐Valentine B, Bardan S, Buyue Y, Kamphaus GD, et al. Comparative field study evaluating the activity of recombinant factor VIII Fc fusion protein in plasma samples at clinical haemostasis laboratories. Haemophilia. 2014;20:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tiefenbacher S, Albisetti M, Baker P, Kappert G, Kitchen S, Kremer Hovinga JA, et al. Estimation of Nuwiq ® (simoctocog alfa) activity using one‐stage and chromogenic assays—results from an international comparative field study. Haemophilia. 2019;25:707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jimenez‐Yuste V, Lejniece S, Klamroth R, Suzuki T, Santagostino E, Karim FA, et al. The pharmacokinetics of a B‐domain truncated recombinant factor VIII, turoctocog alfa (NovoEight(R)), in patients with hemophilia A. J Thromb Haemost. 2015;13:370. [DOI] [PubMed] [Google Scholar]
- 16. Viuff D, Barrowcliffe T, Saugstrup T, Ezban M, Lillicrap D. International comparative field study of N8 evaluating factor VIII assay performance. Haemophilia. 2011;17:695. [DOI] [PubMed] [Google Scholar]
- 17. Dargaud Y, Negrier C, Rusen L, Windyga J, Georgiev P, Bichler J, et al. Individual thrombin generation and spontaneous bleeding rate during personalized prophylaxis with Nuwiq((R)) (human‐cl rhFVIII) in previously treated patients with severe haemophilia A. Haemophilia. 2018;24:619. [DOI] [PubMed] [Google Scholar]


