Abstract
Background
In multiple myeloma, venous thromboembolism (VTE) is common, and treatments for myeloma, such as lenalidomide, increase the risk of thrombosis while improving survival. The association between VTE and survival is not well known.
Objectives
To determine the association between VTE and survival in multiple myeloma (MM) while adjusting for known confounders that affect risk of thrombosis and survival, including patient characteristics and treatment in a retrospective cohort of US veterans.
Patients/Methods
A cohort of patients with newly diagnosed MM treated within Veterans Health Administration between September 1, 1999, and June 30, 2014, was created to assess the association between VTE and mortality using Cox proportional hazards regression modeling while accounting for known prognostic factors and treatments.
Results
The cohort comprised 4446 patients with myeloma, including 2837 patients diagnosed after lenalidomide approval in July 2006. VTE occurred in 327 (7.4%) patients within 1 year and occurred at a median of 77 days (interquartile range, 37‐153) after starting therapy for MM. In all patients, VTE was associated with increased mortality at 6 months (adjusted hazard ratio [aHR], 1.67; 95% confidence interval [CI], 1.18‐2.37). Patients in the post‐lenalidomide cohort with VTE had an increased mortality at both 6 months (aHR, 2.31; 95% CI, 1.52‐3.51) and 12 months (aHR, 1.66; 95% CI, 1.19‐2.33) after treatment initiation.
Discussion
This study shows that VTE during the first 6‐12 months of therapy is associated with increased mortality in patients with MM. Studies evaluating thromboprophylaxis in patients at high risk of thrombosis are needed.
Keywords: lenalidomide, mortality, multiple myeloma, thrombosis, venous thromboembolism, veterans
Essentials.
Deep vein thrombosis (DVT) in multiple myeloma is common and associated with therapy.
Retrospective analysis of patient from 1999‐2014 treated for myeloma within the Veterans Health Administration.
DVT associated with death at 6 and 12 months when adjusting for patient factors and therapy.
Risk of DVT was highest in the first 3 months of treatment.
1. INTRODUCTION
Patients with multiple myeloma (MM) have increased risk of venous thromboembolism (VTE) that is 7‐ to 9‐fold higher compared to patients without MM. 1 , 2 VTE is associated with increased morbidity 3 and is a leading cause of death in patients with cancer 4 however, evidence of the association of VTE with mortality in MM is limited and conflicting. Two single‐center studies in patients receiving aggressive MM‐directed therapy demonstrated no overall association of VTE on survival. 5 , 6 Conversely, a large population‐based study found inferior survival in patients with MM and VTE; however, that study had a limited ability to adjust for confounders, such as treatments or comorbid conditions. 7 Further understanding of the effect of VTE on survival in MM could inform strategies for VTE prevention.
MM treatment guidelines recommend use of thromboprophylaxis in patients with MM after risk stratification. 8 , 9 , 10 Two recent randomized controlled trials demonstrated a decrease in the risk of VTE with thromboprophylaxis in ambulatory patients with cancer identified as high risk for VTE. 11 , 12 However, a significant number of patients with MM and high risk of thrombosis do not receive thromboprophylaxis. 13 In clinical trials, rates of VTE in MM remain >10% during the first 6 months of treatment despite protocols for thromboprophylaxis. 14 The development of two clinical risk assessment scores for VTE in MM, SAVED 15 and IMPEDE VTE 16 , could improve identification of patients with MM at highest risk of VTE.
Considering prior conflicting evidence, an improved understanding of the impact of VTE on survival in MM could provide rationale for the benefit of thromboprophylaxis in patients with MM using risk assessment scores. Therefore, we used a large, nationwide cohort of US veterans to understand the impact of VTE on survival in patients with MM.
2. METHODS
2.1. Study population
We identified patients with newly diagnosed MM from September 1, 1999, to June 30, 2014, within the Veterans Health Administration (VHA) Central Cancer Registry using International Classification of Diseases (ICD)‐03 codes 9732/3 and received treatment within the VHA. To remove patients suspected of having monoclonal gammopathy of undetermined significance, solitary plasmacytoma, or smoldering myeloma, we excluded patients who did not receive MM‐directed therapy within 6 months of diagnosis. Additionally, we excluded patients who received a hematopoietic cell transplant (HCT) within 4 months of treatment initiation, as these patients likely received treatment outside of the VHA system (Figure 1). Before cohort creation, the Saint Louis VHA Medical Center and Washington University School of Medicine institutional review boards approved this study.
FIGURE 1.
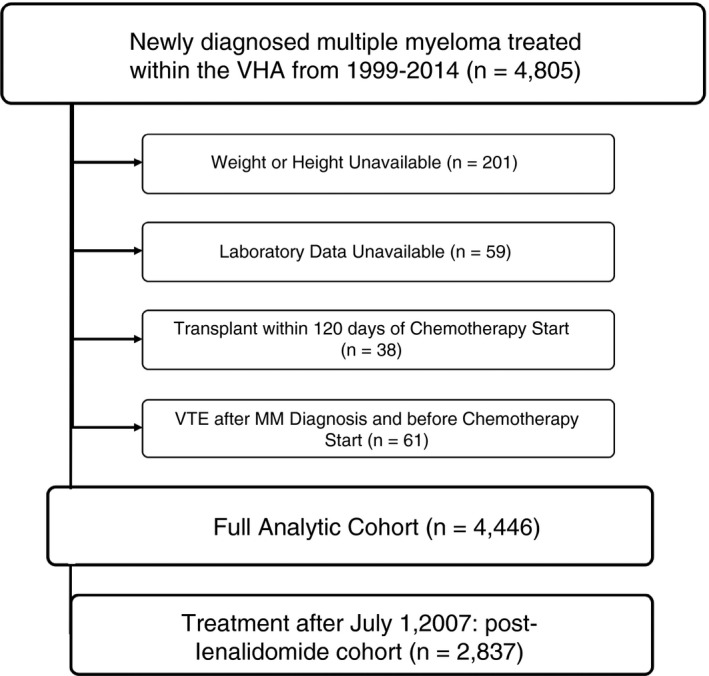
Flow diagram showing selection process of analytic cohorts. MM, multiple myeloma; VHA, Veterans Health Administration; VTE, venous thromboembolism
2.2. Measurements and covariates
We obtained ICD‐9‐Clinical Modification (CM) codes, Pharmacy Benefits Management (PBM) records, and laboratory data using the VA Informatics and Computing Infrastructure platform. Records from PBM included dates of administration of all medications, including MM‐directed therapy. We identified autologous HCT by the presence of at least two ICD‐9 codes or by the administration of high‐dose melphalan. We defined MM therapy as administration of melphalan, thalidomide, lenalidomide, bortezomib, doxorubicin, cyclophosphamide, vincristine, or etoposide and cisplatin within 6 months of diagnosis, which were recommended first‐line treatments in national guidelines during the study period. 17 The number of therapies in the initial treatment regimen was equal to the sum of all MM therapies administered within 1 month of first treatment initiation and included both dexamethasone or prednisone as therapies.
Baseline demographics collected included sex, race, and age at the time of MM diagnosis. We calculated body mass index (BMI) using height and weight within 1 month of diagnosis. Laboratory data included albumin, creatinine, and hemoglobin at the time closest to but within 2 months of MM diagnosis and before initiation of therapy. We calculated creatinine clearance (CrCL) using the Cockcroft‐Gault formula. Using at least two ICD‐9‐CM codes within 12 months before MM diagnosis, we calculated the Romano adaptation of the Charlson Comorbidity Index to account for comorbidities present at diagnosis. 18 We identified date of death using the VA Vital Status File, which combines the death dates from multiple sources including the VA Beneficiary Identification and Records Locator Subsystem Death File, the Social Security Administration Death Master File, the Medicare Vital Status File, and the Medical SAS Inpatient Dataset. Survival was measured as the time from date of MM diagnosis to date of death.
We identified VTE within 12 months of chemotherapy initiation using an algorithm that includes ICD‐9 codes for VTE (from both inpatient and outpatient encounters) as well as treatment for VTE, as previously validated for this cohort. 19 We confirmed all incident cases of VTE identified by the algorithm with manual chart abstraction. We defined a VTE event as present if there was imaging confirmation of an upper proximal extremity or lower‐extremity proximal or distal VTE and/or pulmonary embolism (PE). Imaging evaluated for diagnosis included ultrasonography, computed tomography, ventilation/perfusion imaging assessed as high probability for VTE, or clinician documentation of imaging‐proven VTE from an outside institution.
2.3. Statistical analyses
Demographic and clinical characteristics were compared between patients with and without VTE within 12 months of treatment initiation using chi‐square, Student’s t, or Cochran‐Mantel‐Haenszel tests as appropriate. The association between VTE and survival at 6 and 12 months was assessed using Cox proportional hazards regression modeling while adjusting for potential confounding variables. Confounders included age at diagnosis; Charlson Comorbidity Index; Black race 20 ; BMI 21 ; treatment with thalidomide, lenalidomide, or bortezomib; receipt of HCT; anemia (hemoglobin < 10 g/dL); impaired CrCL (Cockcroft‐Gault formula < 30 mL/min); albumin ≤ 3 g/dL; year of diagnosis; and number of therapies in the first regimen. VTE was included as a time‐varying covariate to account for immortal time bias.
To understand the impact of VTE on survival in a modern era of management, a subcohort of patients diagnosed with MM between July 1, 2006, and June 30, 2014, was created to account for the period after first US Food and Drug Administration (FDA) approval of lenalidomide for MM (post‐lenalidomide cohort). Using a Cox proportional hazards regression modeling with VTE as a time‐varying exposure, the association between VTE and survival in MM in the post‐lenalidomide cohort was assessed at 6 and 12 months, while adjusting for the same potential confounders as listed above. We used SAS 9.4 (SAS Inc, Cary, NC, USA) for all analyses. All P values were two‐tailed, with P < .05 determined as significant.
3. RESULTS
A total of 4446 patients were included. Mean age was 68.4 years, and the median year of diagnosis of myeloma was 2008 (Table 1). Consistent with the veteran population, 97.8% of patients were male and 29.8% were Black. Patients were predominantly treated with two agents (61.5%) while 20.0% were treated with a single agent, and 18.4% received three or more agents. Of the cohort, 2837 received a diagnosis of MM after July 1, 2006 (post‐lenalidomide cohort).
Table 1.
Demographics and clinical characteristics of US veterans treated for MM
| US veterans diagnosed with MM between 9/1/1999 and 6/30/2014 (full cohort, n = 4446) | US veterans diagnosed with MM between 7/1/2006 and 6/30/2014 (post‐lenalidomide cohort, n = 2837) | |||||
|---|---|---|---|---|---|---|
| VTE n = 327 (7.4%) | No VTE n = 4119 (92.6%) | P value | VTE n = 185 (6.5%) | No VTE n = 2652 (97.5%) | P value | |
| Mean age, y | 67.3 | 68.5 | .051 a | 67.6 | 68.5 | .28 a |
| Male, n (%) | 96.9 (317) | 98.0 (4035) | .22 b | 96.2 (178) | 97.8 (2594) | .19 b |
| Mean Charlson‐Romano Comorbidity Index | 2.9 | 3.4 | .005 a | 3.3 | 3.7 | .13 a |
| Race | .65 b | .59 b | ||||
| White/Other | 71.3 (233) | 70.1 (2886) | 71.4 (132) | 69.5 (1842) | ||
| Black | 28.7 (944) | 29.9 (1233) | 28.6 (53) | 30.5 (810) | ||
| BMI (%) | .009 c | .02 c | ||||
| <18.5 | 0.6 (2) | 2.5 (104) | 0.5 (1) | 2.3 (60) | ||
| 18.5‐<25 | 27.8 (91) | 31.1 (1281) | 25.4 (47) | 30.4 (805) | ||
| 25‐<30 | 38.5 (126) | 38.7 (1593) | 38.4 (71) | 37.8 (1003) | ||
| ≥30 | 33.0 (108) | 27.7 (1141) | 35.7 (66) | 30.0 (784) | ||
| VTE before MM (%, n) | 4.9 (16) | 2.3 (93) | .003 b | 5.4 (10) | 2.9 (77) | .06 b |
| Anemia (%) | 39.8 (130) | 40.6 (1671) | .77 b | 37.3 (69) | 40.2 (1065) | .44 b |
| CrCl < 30 mL/min (%) | 20.2 (66) | 22.7(933) | .33 b | 18.9 (35) | 22.1(586) | .31 b |
| Albumin (mean) | 3.3 | 3.3 | .66 | 3.3 | 3.3 | .81 |
| Transplant in 1 y after diagnosis (%, n) | 14.1 (46) | 8.9 (366) | .002 b | 12.4 (23) | 10.0 (266) | .30 b |
| Melphalan in 1 y after diagnosis (%, n) | 25.1 (82) | 25.7 (1181) | .17 b | 20.0 (37) | 19.2 (508) | .78 b |
| Lenalidomide use in 1 y after diagnosis (%, n) | 22.6(74) | 22.7 (933) | .99 b | 38.4(71) | 34.6 (918) | .30 b |
| Thalidomide use in 1 y after diagnosis (%, n) | 43.7 (143) | 30.1 (1239) | <.001 b | 30.8 (57) | 24.6 (651) | .06 b |
| Bortezomib use in 1 y after diagnosis (%, n) | 31.2 (102) | 33.5 (1379) | .40 b | 47.0 (87) | 48.6 (1289) | .68 b |
| Mean number of therapies in first regimen (mean) | 2.1 | 2.0 | .01 a | 2.2 | 2.0 | .04 a |
| Treatment with ≥3 therapies in first regimen (%, n) | 22.3 (73) | 18.1 (746) | .06 b | 25.9 (48) | 21.5 (571) | .16 b |
Abbreviations: BMI, body mass index; CrCl, creatinine clearance; MM, multiple myeloma; VTE, venous thromboembolism.
t test.
Chi‐square.
Cochran‐Mantel‐Haenszel (row mean score) test.
Within 1 year of initiation of therapy, 327 (7.4%) patients developed VTE, and 185 (6.5%) developed VTE within 6 months. Incidence of thrombosis was highest in the first 3 months after chemotherapy start date, with median time from chemotherapy start to VTE 77 days (interquartile range, 37‐153 days). Cumulative incidence of VTE at 12 months in the full cohort was 8.4%, and in the cohort treated after July 2006 it was 7.4% (Figure 2). Most of the events were lower‐extremity DVTs, but 92 patients had a PE (Table 2). Patients with VTE had a higher BMI (P < .001) and were more likely to receive multidrug treatment regimens (P = .01).
FIGURE 2.
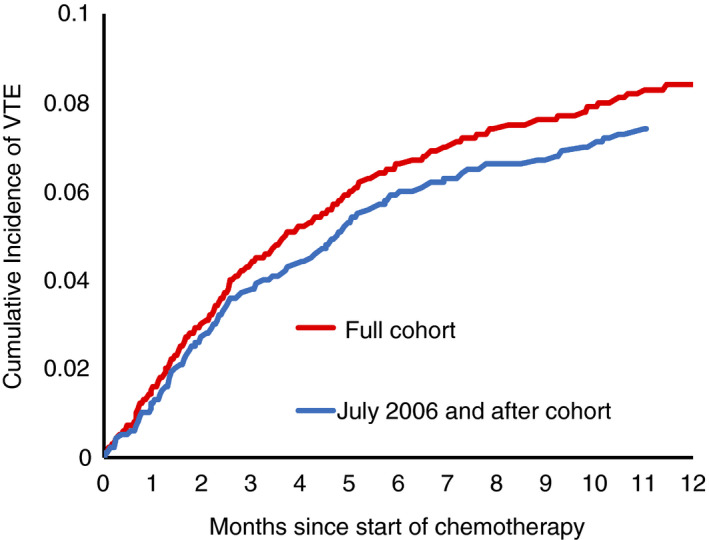
Cumulative incidence of VTE events over 1 y after initiation of therapy for multiple myeloma in the full cohort and July 2006 and after cohort. VTE, venous thromboembolism
Table 2.
Characteristics of VTE in 4446 veterans with multiple myeloma
| Characteristics of venous thromboembolism n = 327 | |
|---|---|
| Type and location of VTE | n |
| Pulmonary embolism | 50 |
| Pulmonary embolism and lower‐extremity deep vein thrombosis | 42 |
| Lower‐extremity deep vein thrombosis | |
| Distal | 17 |
| Proximal | 158 |
| Site not specified | 14 |
| Upper‐extremity deep vein thrombosis | |
| Line associated | 20 |
| Not line associated | 13 |
| Deep vein thrombosis, site not specified | 13 |
In our cohort, 821 (18.5%) patients died within 6 months after start of therapy, and 1208 of 4446 patients (27.2%) died within 1 year. Median survival of the overall cohort was 32.5 months (35.4 months in the post‐lenalidomide cohort). When restricting the survival analysis to patients treated with lenalidomide or thalidomide at the start of therapy, median survival was 41.7 months.
After adjusting for potential confounders, VTE was associated with an increased mortality at 6 months after initiation of therapy (adjusted hazard ratio [aHR], 1.67; 95% confidence interval [CI], 1.18‐2.37) in the full cohort, with a trend toward increased risk at 12 months (aHR, 1.22; 95% CI, 0.93‐1.60) (Table 3). Patient characteristics significantly associated with increased risk of mortality included BMI of <18.5, older age, non‐Black race, higher Charlson Comorbidity Index, CrCL < 30 mL/min, and albumin of ≤3 g/dL at diagnosis. Overweight and obesity, Black race, initial therapy with lenalidomide or thalidomide, and treatment with higher number of therapies in the first regimen were significantly associated with decreased mortality.
Table 3.
Association between VTE and mortality in US veterans diagnosed with MM between September 1, 1999 and June 30, 2014
| 6‐mo mortality | 12‐mo mortality | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| VTE (time varying) | 1.63 | 1.18‐2.37 | <.001 | 1.22 | 0.93‐1.60 | .16 |
| Year of diagnosis | 1.00 | 0.98‐1.03 | .84 | 1.00 | 0.98‐1.02 | .74 |
| BMI | ||||||
| < 18.5 | 1.79 | 1.28‐2.52 | <.001 | 1.53 | 1.14‐2.06 | .01 |
| 18.5‐25 | Referent | Referent | ||||
| 25‐30 | 0.81 | 0.69‐0.96 | .01 | 0.79 | 0.69‐0.90 | <.001 |
| ≥ 30 | 0.72 | 0.60‐0.87 | <.001 | 0.74 | 0.64‐0.86 | <.001 |
| Age (per y) | 1.01 | 1.00‐1.02 | .03 | 1.01 | 1.00‐1.01 | .02 |
| Race | ||||||
| Non‐Black | Referent | Referent | ||||
| Black | 0.78 | 0.67‐0.92 | <.001 | 0.74 | 0.65‐0.84 | <.001 |
| Charlson Comorbidity Index, per point | 1.08 | 1.06‐1.11 | <.001 | 1.07 | 1.05‐1.09 | <.001 |
| Baseline laboratory data | ||||||
| Hemoglobin < 10 g/dL | 1.15 | 0.99‐1.33 | .06 | 1.17 | 1.04‐1.32 | .01 |
| CrCl < 30 mL/min | 1.48 | 1.26‐1.73 | <.001 | 1.31 | 1.15‐1.50 | <.001 |
| Albumin ≤ 3 g/dL | 1.70 | 1.47‐1.98 | <.001 | 1.60 | 1.42‐1.81 | <.001 |
| Albumin unknown | 0.70 | 0.54‐0.91 | .01 | 0.70 | 0.57‐0.86 | <.001 |
| Myeloma‐specific therapy | ||||||
| Transplant | NE | <.001 | 0.03‐0.14 | <.001 | ||
| Thalidomide | 0.66 | 0.55‐0.79 | .11 | 0.66 | 0.58‐0.76 | <.001 |
| Bortezomib | 0.85 | 0.69‐1.04 | <.001 | 0.82 | 0.70‐0.96 | .01 |
| Lenalidomide | 0.42 | 0.32‐0.55 | .06 | 0.45 | 0.38‐0.55 | <.001 |
| Number of therapies in first regimen | 0.71 | 0.61‐0.82 | <.001 | 0.71 | 0.61‐0.82 | <.001 |
In the post‐lenalidomide cohort, VTE was associated with increased mortality at 6 months after start of chemotherapy (aHR, 2.31; 95% CI, 1.52‐3.51) when adjusting for potential confounders (Table 4). This risk of increased mortality remained significant at 12 months (aHR, 1.67; 95% CI, 1.19‐2.33). Patient characteristics significantly associated with increased mortality included a BMI < 18.5, older age, higher Charlson Comorbidity Index, CrCL < 30 mL/min, and albumin of ≤3 g/dL at diagnosis. Factors associated with decreased mortality included more recent year of diagnosis such that patients treated closer to 2014 had decreased mortality compared to those treated near 2006 as well as overweight (BMI > 25‐30) or obesity (BMI > 30). Treatment characteristics associated with decreased mortality included first‐line therapy with lenalidomide or thalidomide and higher number of initial treatment therapies at diagnosis.
Table 4.
Association between VTE and mortality in US veterans diagnosed with MM between July 1, 2006, and June 30, 2014
| 6‐mo mortality | 12‐mo mortality | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| VTE (time varying) | 2.37 | 1.56‐3.60 | <.001 | 1.69 | 1.21‐2.36 | <.001 |
| Year of diagnosis | 0.94 | 0.89‐0.98 | .01 | 0.95 | 0.92‐0.99 | .02 |
| BMI | ||||||
| < 18.5 | 1.70 | 1.10‐2.63 | .02 | 1.49 | 1.01‐2.21 | .05 |
| 18.5‐25 | Referent | Referent | ||||
| 25‐30 | 0.75 | 0.61‐0.93 | .01 | 0.74 | 0.62‐0.89 | .001 |
| ≥ 30 | 0.72 | 0.57‐0.92 | .01 | 0.78 | 0.64‐0.94 | .01 |
| Age (per y increase) | 1.01 | 1.00‐1.02 | .03 | 1.01 | 1.00‐1.02 | .07 |
| Race | ||||||
| Non‐Black | Referent | Referent | ||||
| Black | 0.84 | 0.69‐1.02 | .08 | 0.75 | 0.64‐0.89 | <.001 |
| Charlson Comorbidity Index, per point | 1.07 | 1.04‐1.10 | <.001 | 1.07 | 1.04‐1.09 | <.001 |
| Baseline laboratory data | ||||||
| Hemoglobin < 10 g/dL | 1.17 | 0.97‐1.41 | .11 | 1.15 | 0.99‐1.35 | .07 |
| CrCl < 30 mL/min | 1.50 | 1.23‐1.83 | <.001 | 1.30 | 1.09‐1.54 | <.001 |
| Albumin ≤ 3 g/dL | 1.70 | 1.41‐2.05 | <.001 | 1.60 | 1.36‐1.87 | <.001 |
| Albumin unknown | 0.59 | 0.39‐0.89 | .01 | 0.60 | 0.43‐0.82 | <.001 |
| Myeloma‐specific therapy | ||||||
| Transplant | NE | 0.05 | 0.02‐0.16 | <.001 | ||
| Thalidomide | 0.49 | 0.37‐0.63 | <.001 | 0.52 | 0.42‐0.64 | <.001 |
| Bortezomib | 0.91 | 0.73‐1.14 | .42 | 0.88 | 0.74‐1.05 | .16 |
| Lenalidomide | 0.43 | 0.32‐0.57 | <.001 | 0.46 | 0.38‐0.56 | <.001 |
| Number of therapies in first regimen | 0.71 | 0.59‐0.84 | <.001 | 0.84 | 0.73‐0.97 | .01 |
4. DISCUSSION
In this population‐based cohort of 4446 patients with newly diagnosed MM starting therapy, VTE was associated with a 67% increased relative risk of mortality at 6 months. In patients treated after FDA approval of lenalidomide in July 2006, development of VTE was associated with a twofold increased risk of mortality at 6 months, with risk persisting to 12 months after initiation of therapy.
Prior literature shows conflicting results regarding the association between VTE and mortality in MM. Studies assessing the impact of VTE on survival in clinical trials found no association between VTE in mortality. 6 , 22 However, modern therapies that have improved outcomes (such as lenalidomide and HCT) are also associated with risk of thrombosis. This creates confounding, as VTE in observational studies or retrospective analyses of clinical trials may be a marker of highly effective therapy. Therefore, adjustment for the presence of confounders that increase risk of VTE and also improve mortality is critical in any observational study. Additionally, the generalizability of outcomes from clinical trials is questionable, as selection criteria for trials result in enrollment of patients with better prognoses and reduced comorbidities compared to most patients with cancer in routine practice and only 5% of cancer patients participate in clinical trials. 23 , 24 Our study supports other population‐based studies that identify an association with between VTE and mortality. 7
This study has several limitations. We were unable to examine rate of VTE‐related death and cause‐specific mortality due to limitations in determination of death in nationwide VHA data. Additionally, our analysis likely underestimates the effect of VTE on mortality, as all patients had to survive long enough to receive therapy with adequate performance status for treatment. The generalizability of our findings to women may be limited because our population was 98% male. Also, because of the retrospective analysis of existing data, causality of adverse outcomes could not be determined and result may be affected by unmeasured confounders. Despite these limitations, the equal access to diagnostics and treatment, high rates of comorbidities, and integrated health data systems of the VHA allow for a robust analysis of patient characteristics, treatments, and VTE that is generally not possible in other retrospective studies.
This study has several strengths. We also included a measure of comorbidities and analyzed outcomes while adjusting for common treatments to help disentangle the thrombogenic effect of treatments, such as lenalidomide, or HCT. Patients in this study had a median age of 68 years, which is similar to the median age of patients in Surveillance, Epidemiology, and End Results data of 69 years. In comparison to other observational cohorts of MM and VTE, patients in our study were slightly younger (68 years) than those in the Swedish study (71 years), 7 and included more Blacks, who tend to develop MM at a younger age. 25 Our cohort was older than those in clinical trials of combination chemotherapy (57 years) 5 and immunomodulatory agents (63 years) 6 for MM.
Additionally, we used a previously validated method of thrombosis identification 19 that incorporates both diagnostic codes and treatment to identify VTE in conjunction with manual abstraction to verify events. This two‐factor identification allows for detection of VTE with high specificity across large populations and prevents overdiagnosis of adverse events that can occur based on ICD‐9 codes alone. In our cohort, 81.5% of patients were treated with two or fewer therapies within 6 months of diagnosis, which may have resulted in the decreased incidence of VTE.
This study found that VTE events during the first 6 months of therapy are associated with increased mortality. Recently, two large studies found that direct oral anticoagulants are safe and effective in prevention of VTE in patients with cancer when administered during the first 6 months of therapy. 11 , 12 In these trials, patients were identified as high risk for VTE based on a Khorana risk score ≥ 2, 26 which includes factors including site of cancer, platelet count, hemoglobin, leukocyte count, and elevated BMI. 26 While these studies contained < 3% of participants with myeloma, a similar practice may be effective in preventing VTE in MM. Recent work to define risk of VTE in patients with MM starting chemotherapy, such as the IMPEDE‐VTE or SAVED score, 15 , 16 could help to determine which patients are at highest risk for VTE to facilitate prospective study of the benefits of thromboprophylaxis in MM.
In conclusion, VTE is associated with increased mortality at 6 months after initiation of therapy in patients with MM and at both 6 and 12 months in a cohort of patients treated after July 2006, when lenalidomide was approved. With the availability of safe and effective anticoagulants, a strategy for thromboprophylaxis in patients with MM at high‐risk for VTE may improve survival.
RELATIONSHIP DISCLOSURE
KMS has received consulting fees for participation in advisory boards from Pfizer Inc and Bayer HealthCare Pharmaceuticals Inc, both outside of the work submitted; grant funding from the National Institutes of Health Loan Repayment Program and an American Cancer Society Institutional Research Grant outside of the work submitted; and travel support from AstraZeneca Pharmaceuticals Inc outside of the work submitted. KRC reports employment by Flatiron Health, a division of the Roche Group.
AUTHOR CONTRIBUTIONS
The study was conceptualized and designed by KMS and KRC. SL and MWS analyzed the data. The manuscript was drafted by MWS with critical revisions by KMS, KRC, BG, AL, and AA.
Supporting information
Additional supporting information may be found online in the supporting information tab for this article.
Appendix S1
ACKNOWLEDGMENTS
The research reported was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award K12 HL087107‐05 and Award 1K01HL136893‐01. The content is the responsibility of the authors and does not represent the views of the National Institutes of Health.
Schoen MW, Carson KR, Luo S, et al. Venous thromboembolism in multiple myeloma is associated with increased mortality. Res Pract Thromb Haemost. 2020;4:1203–1210. 10.1002/rth2.12411
Handling Editor: Dr Neil Zakai
Contributor Information
Martin W. Schoen, Email: martin.schoen@health.slu.edu, @mwschoen.
Ang Li, @AngLi_MD.
Kristen M. Sanfilippo, @KSanfilippoMD.
REFERENCES
- 1. Kristinsson SY, Fears TR, Gridley G, Turesson I, Mellqvist UH, Bjorkholm M, et al. Deep vein thrombosis after monoclonal gammopathy of undetermined significance and multiple myeloma. Blood. 2008;112:3582–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kristinsson SY, Pfeiffer RM, Bjorkholm M, Goldin LR, Schulman S, Blimark C, et al. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population‐based study. Blood. 2010;115:4991–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hogg K, Kimpton M, Carrier M, Coyle D, Forgie M, Wells P. Estimating quality of life in acute venous thrombosis. JAMA Intern Med. 2013;173:1067–72. [DOI] [PubMed] [Google Scholar]
- 4. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–34. [DOI] [PubMed] [Google Scholar]
- 5. Zangari M, Barlogie B, Cavallo F, Bolejack V, Fink L, Tricot G. Effect on survival of treatment‐associated venous thromboembolism in newly diagnosed multiple myeloma patients. Blood Coagul Fibrinolysis. 2007;18:595–98. [DOI] [PubMed] [Google Scholar]
- 6. Zangari M, Tricot G, Polavaram L, Zhan F, Finlayson A, Knight R, et al. Survival effect of venous thromboembolism in patients with multiple myeloma treated with lenalidomide and high‐dose dexamethasone. J Clin Oncol. 2010;28:132–35. [DOI] [PubMed] [Google Scholar]
- 7. Kristinsson SY, Pfeiffer RM, Bjorkholm M, Schulman S, Landgren O. Thrombosis is associated with inferior survival in multiple myeloma. Haematologica. 2012;97:1603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189–204. [DOI] [PubMed] [Google Scholar]
- 9. Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B, et al. Prevention of thalidomide‐ and lenalidomide‐associated thrombosis in myeloma. Leukemia. 2008;22:414–23. [DOI] [PubMed] [Google Scholar]
- 10. Swan D, Rocci A, Bradbury C, Thachil J. Venous thromboembolism in multiple myeloma ‐ choice of prophylaxis, role of direct oral anticoagulants and special considerations. Br J Haematol. 2018;183:538–56. [DOI] [PubMed] [Google Scholar]
- 11. Carrier M, Abou‐Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711–19. [DOI] [PubMed] [Google Scholar]
- 12. Khorana AA, Soff GA, Kakkar AK, Vadhan‐Raj S, Riess H, Wun T, et al. Rivaroxaban for thromboprophylaxis in high‐risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–28. [DOI] [PubMed] [Google Scholar]
- 13. Alexander M, Teoh KC, Lingaratnam S, Kirsa S, Mellor JD. Thromboprophylaxis prescribing and thrombotic event rates in multiple myeloma patients treated with lenalidomide or thalidomide at a specialist cancer hospital. Asia Pac J Clin Oncol. 2013;9:169–75. [DOI] [PubMed] [Google Scholar]
- 14. Bradbury CA, Jenner MW, Striha A, Cook G, Pawlyn C, Jones JR, et al. Thrombotic events in patients with myeloma treated with immunomodulatory drugs; results of the myeloma XI study. Blood. 2017;130:553. [Google Scholar]
- 15. Li A, Wu Q, Luo S, Warnick GS, Zakai NA, Libby EN, et al. Derivation and validation of a risk assessment model for immunomodulatory drug‐associated thrombosis among patients with multiple myeloma. J Natl Compr Canc Netw. 2019;17:840–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sanfilippo KM, Luo S, Wang TF, Fiala M, Schoen M, Wildes TM, et al. Predicting venous thromboembolism in multiple myeloma: development and validation of the IMPEDE VTE score. Am J Hematol. 2019;94:1176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderson KC, Alsina M, Bensinger W, Biermann JS, Chanan‐Khan A, Cohen AD, et al. NCCN clinical practice guidelines in oncology: multiple myeloma. J Natl Compr Canc Netw. 2009;7:908–42. [DOI] [PubMed] [Google Scholar]
- 18. Romano PS, Roos LL, Jollis JG. Presentation adapting a clinical comorbidity index for use with ICD‐9‐CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–79. [DOI] [PubMed] [Google Scholar]
- 19. Sanfilippo KM, Wang TF, Gage BF, Liu W, Carson KR. Improving accuracy of international classification of diseases codes for venous thromboembolism in administrative data. Thromb Res. 2015;135:616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waxman AJ, Mink PJ, Devesa SS, Anderson WF, Weiss BM, Kristinsson SY, et al. Racial disparities in incidence and outcome in multiple myeloma: a population‐based study. Blood. 2010;116:5501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beason TS, Chang S‐H, Sanfilippo KM, Luo S, Colditz GA, Vij R, et al. Influence of body mass index on survival in veterans with multiple myeloma. Oncologist. 2013;18:1074–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zangari M, Barlogie B, Thertulien R, Jacobson J, Eddleman P, Fink L, et al. Thalidomide and deep vein thrombosis in multiple myeloma: risk factors and effect on survival. Clinical lymphoma. 2003;4:32–5. [DOI] [PubMed] [Google Scholar]
- 23. Elting LS, Cooksley C, Bekele BN, Frumovitz M, Avritscher EB, Sun C, et al. Generalizability of cancer clinical trial results: prognostic differences between participants and nonparticipants. Cancer. 2006;106:2452–58. [DOI] [PubMed] [Google Scholar]
- 24. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race‐, sex‐, and age‐based disparities. JAMA. 2004;291:2720–26. [DOI] [PubMed] [Google Scholar]
- 25. Fillmore NR, Yellapragada SV, Ifeorah C, Mehta A, Cirstea D, White PS, et al. With equal access, African American patients have superior survival compared to white patients with multiple myeloma: a VA study. Blood. 2019;133:2615–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood. 2008;111:4902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found online in the supporting information tab for this article.
Appendix S1


