Abstract
Background/Objective
We report the first analysis of an extended half‐life recombinant factor IX, nonacog beta pegol (N9‐GP), in previously untreated patients (PUPs) and minimally treated patients with hemophilia B.
Methods
Paradigm 6 (Safety and Efficacy of Nonacog Beta Pegol [N9‐GP] in Previously Untreated Patients With Haemophilia B) is a multicenter, open‐label, single‐arm, phase 3 trial. Main inclusion criteria were males aged < 6 years, with hemophilia B with factor IX (FIX) activity ≤ 2%, who were previously untreated or with ≤ 3 exposure days (EDs) to FIX‐containing products. Patients received N9‐GP 40 IU/kg once weekly (prophylaxis) or individualized dosing (preprophylaxis). Bleeds were treated with N9‐GP 40 IU/kg (80 IU/kg if severe). The primary end point was incidence of anti‐FIX inhibitory antibodies (inhibitors). Secondary end points included safety outcomes and annualized bleeding rate (ABR).
Results
At data cutoff (August 31, 2018), 38 patients had been screened, and 37 had received N9‐GP (median age, 1.0 years [range, 0‐4]). Total in‐trial EDs amounted to 2833, representing ~ 65 patient‐years. Two (6.1%) of 33 “at‐risk” patients (patients with ≥ 10 EDs plus patients who developed inhibitors) developed high‐titer inhibitors and were withdrawn. No other safety concerns, including thromboembolic events, were identified. In the prophylaxis group (n = 28), 67.9% were bleed free; all bleeds (n = 15) were treated with one N9‐GP injection; and overall, spontaneous, and traumatic ABRs were low (median ABRs of 0.0, 0.0, and 0.0, respectively; modeled mean ABRs of 0.31, 0.08, and 0.23, respectively). Estimated mean FIX trough activity was 15.0%.
Conclusion
We report an inhibitor incidence of 6.1%, which is within the expected range for PUPs with hemophilia B. No other safety concerns were identified; moreover, N9‐GP provided effective hemostatic coverage.
Keywords: factor IX, hemophilia B, nonacog beta pegol, previously untreated patients, prophylaxis, recombinant proteins
Essentials.
Nonacog beta pegol (N9‐GP) was evaluated in previously untreated patients (PUPs) with severe hemophilia B (FIX ≤ 2%).
FIX inhibitor incidence (2/33 “at‐risk” patients) was within the expected range for PUPs.
~68% of PUPs on N9‐GP prophylaxis had no bleeds; all 15 bleeds resolved with one N9‐GP injection.
Median overall, spontaneous, and traumatic ABRs of PUPs on N9‐GP prophylaxis were 0.00.
1. INTRODUCTION
Therapeutic advances in hemophilia B, including development of recombinant factor IX (rFIX) products and implementation of routine prophylaxis, have improved patients’ lives. 1 , 2 Both the World Federation of Hemophilia (WFH) and the World Health Organization have stated that initiating prophylactic treatment with coagulation factor IX (FIX) at a young age, ideally ~ 1‐2 years, is the optimal treatment for children with severe hemophilia B (FIX activity < 1%). 3 , 4 However, frequent FIX injections may negatively impact quality of life and treatment adherence. 1 Frequent dosing is particularly challenging for younger children due to venous access difficulties, pain during factor administration, and complications associated with central venous access devices. 5 , 6 Extended half‐life (EHL) rFIX products aim to minimize this treatment burden by reducing dosing frequency while maintaining higher levels of FIX activity versus standard rFIX products. 7 , 8
Until now, clinical trial results have been published only for EHL rFIX products in previously treated patients (PTPs) with hemophilia B. 7 , 8 Previously untreated patients (PUPs) with severe disease represent a vulnerable patient population, as the greatest risk of developing anti‐FIX inhibitory (neutralizing) antibodies is during the first 10‐20 exposure days (EDs) of factor replacement therapy. 9 , 10 , 11 Development of inhibitory antibodies is a significant complication of hemophilia treatment, resulting in difficult‐to‐treat bleeds and an increased risk of morbidity. 12 , 13 Moreover, patients with anti‐FIX inhibitory antibodies may experience anaphylaxis or allergic reactions, and immune tolerance induction can be complicated by the risk of developing nephrotic syndrome. 14 , 15
Nonacog beta pegol (N9‐GP; REFIXIA/REBINYN; Novo Nordisk A/S, Bagsværd, Denmark) is a site‐specific glycoPEGylated EHL rFIX product that has demonstrated safety and efficacy in PTPs of all age groups in phase 3 clinical trials. 16 , 17 , 18 Here, we report interim safety, immunogenicity, and efficacy results of paradigm 6 (Safety and Efficacy of Nonacog Beta Pegol [N9‐GP] in Previously Untreated Patients With Haemophilia B), a phase 3 trial of N9‐GP for the prevention and treatment of bleeds in PUPs and minimally treated patients (MTPs) with severe and moderately severe hemophilia B (FIX activity ≤ 2%). The primary objective was to evaluate the incidence of anti‐FIX inhibitory antibodies; safety outcomes, efficacy, and FIX pharmacokinetics were also assessed.
2. METHODS
2.1. Trial design
Paradigm 6 (NCT02141074) is an ongoing, multinational, open‐label, single‐arm, phase 3 trial conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice Guideline, and the US Food and Drug Administration 21 Code of Federal Regulations Part 11. The protocol, consent form, and subject information sheet were approved according to local regulations by appropriate health authorities and an institutional review board/independent ethics committee.
The start date was July 2, 2014, and the estimated trial completion date is October 30, 2022. The trial has 4 phases. Phase 1 defines the screening visit. Phase 2 (main phase; 1‐3 years’ duration) comprises a patient’s first 50 N9‐GP EDs; patients receive either weekly prophylaxis with N9‐GP until 50 EDs are reached or preprophylaxis with N9‐GP (until maximum 24 months old or 20 EDs), after which they receive weekly prophylaxis until 50 EDs. Phase 3 comprises prophylactic N9‐GP for another 50 EDs until 100 EDs; thereafter, patients enter phase 4 and receive N9‐GP prophylaxis until trial end.
Following a protocol amendment (December 2017), a neurological examination was included as a baseline assessment, as was measurement of plasma polyethylene glycol (PEG) levels.
2.2. Patient eligibility
Key inclusion criteria were male sex, age < 6 years, hemophilia B with FIX activity ≤ 2%, and previously untreated or exposed to FIX‐containing products for ≤ 3 EDs (5 EDs to blood components were permitted). Informed consent was obtained from the primary caregiver(s) before any trial activities. A key exclusion criterion was history of anti‐FIX inhibitory antibodies.
2.3. N9‐GP regimen
Before starting treatment with N9‐GP, the family and investigator decided whether a patient initially underwent preprophylaxis or prophylaxis treatment.
2.3.1. Preprophylaxis treatment
To account for the difficulty of frequent infusions in very small children, caregivers and treating physicians could decide the frequency of infusions in patients up to 24 months of age or 20 EDs, whichever came first. Thus, patients in preprophylaxis received either N9‐GP only on demand for bleeds or N9‐GP 40 IU/kg at individually chosen intervals longer than a week, with additional doses given for treatment of bleeds if necessary. After 20 EDs or their second birthday, the patient was switched to the full prophylaxis dosing schedule. Patients could also switch before reaching either milestone. Any child aged ≥ 2 years at screening started directly on prophylaxis.
2.3.2. Prophylaxis treatment
N9‐GP was administered intravenously as a weekly (±1 day) 40 IU/kg dose. Patients could undergo major (after 20 EDs) or minor surgery. During surgery, the N9‐GP treatment pattern could be altered, with patients resuming the normal prophylaxis schedule upon surgery completion.
2.3.3. Treatment of bleeds
All bleeds were to be treated immediately. Mild/moderate bleeds were treated with N9‐GP 40 IU/kg, with an additional dose of 40 IU/kg permitted upon consultation with the investigator. Severe bleeds were treated with N9‐GP 80 IU/kg, which could be repeated (maximum dose, 200 IU/kg in 24 hours).
2.3.4. Treatment location requirements
The first 10, preferably 20, N9‐GP injections had to be administered at the trial site, per WFH recommendations. 11 N9‐GP administration outside the trial site before reaching 20 EDs was to be performed in the presence of an experienced health care professional.
2.4. Trial end points
2.4.1. Immunogenicity
The primary end point was incidence of anti‐FIX inhibitory (neutralizing) antibodies of ≥ 0.6 BU, calculated based on “at‐risk” patients, defined as those with ≥ 10 EDs or who had developed anti‐FIX inhibitory antibodies. The minimum exposure of 10 EDs was chosen because patients with fewer EDs might not have developed a measurable immune response. Samples were tested for N9‐GP–binding antibodies every 5 EDs until 20 EDs, every 10 EDs until 100 EDs, and every 24 EDs thereafter. A positive test for N9‐GP–binding antibodies and two consecutive positive anti‐FIX inhibitory antibody tests (Bethesda assay) performed at the central laboratory were required to confirm the presence of FIX inhibitors. The second test defined the anti‐FIX inhibitory antibody titer. 19 Patients with low‐titer (0.6 to < 5 BU) anti‐FIX inhibitory antibodies could continue N9‐GP treatment; patients with high‐titer (≥5 BU) anti‐FIX inhibitory antibodies were withdrawn from treatment, and follow‐up visits were planned.
Antibodies against anti‐Chinese hamster ovary (CHO) host cell proteins (HCPs) were assessed. Anti‐PEG antibodies were not directly measured but were considered possible in the event of N9‐GP–binding antibodies that did not cross‐react against rFIX.
2.4.2. Safety
Secondary safety end points included adverse events (AEs) thought possibly/probably related to treatment, serious AEs (SAEs), and medical events of special interest (MESIs). MESI categories were medication errors concerning the trial product, anti‐FIX inhibitory antibody formation, thromboembolic events, anaphylactic reaction, allergic reaction, and central nervous system–related AEs. Vital signs and laboratory parameters were also assessed. Following a protocol amendment (December 2017), PEG plasma levels were assessed at screening (or before dosing on the first day of dosing if the blood sampling limit had been reached at the screening visit), as well as at visits after 30, 50, 80, and 100 EDs, and every 24 EDs thereafter.
2.4.3. Efficacy
Secondary efficacy end points included hemostatic control, assessed on a 4‐point hemostatic response scale. An “excellent” response was defined as one N9‐GP dose within 8 hours and abrupt pain relief and/or clear improvement in objective signs of bleeding; “good” was one N9‐GP dose within 8 hours and noticeable pain relief and/or improvement in signs of bleeding; “moderate” was more than one N9‐GP dose within 8 hours and probable/slight beneficial effect after the first injection; and “poor” was more than one N9‐GP dose within 8 hours and no improvement/worsening of symptoms. Successful control of bleeding was defined as “excellent” or “good.” Number of breakthrough bleeds (annualized bleeding rate [ABR]), the amount of N9‐GP administered per bleed, the number of N9‐GP injections needed to treat a bleed, and N9‐GP consumption were assessed.
2.4.4. Pharmacokinetic parameters
Trough (predose) FIX activity was recorded every 5 EDs until 20 EDs, every 10 EDs until 100 EDs, and every 24 EDs thereafter. FIX activity at 30 minutes after dosing was recorded every 10 EDs until 20 EDs, as well as at 50 and 100 EDs. The resulting incremental FIX recovery at 30 minutes after dosing was calculated when both pre‐ and postdosing FIX activity were available.
2.5. Analytical assessments
Evaluation of anti‐FIX inhibitory antibodies was performed at a central laboratory using a heat‐modified Nijmegen FIX Bethesda assay. 20 Samples measuring above the assay cut point (≥0.6 BU) required a confirmation test.
Assays validated according to international guidelines were used for antibody screening. 21 , 22 Samples measuring above the predetermined assay cutoff point were characterized for specificity to N9‐GP and cross reactivity to FIX. If necessary, isotyping was performed.
FIX activity was measured with a FIX one‐stage clotting assay 23 using SynthAFax as activated partial thromboplastin time reagent. 23 FIX gene mutation status was determined by either in‐trial laboratory analysis or by post hoc classification of gene defects reported in patients’ medical records.
2.6. Statistical analysis
No formal sample size calculations were performed; the planned size of 40 trial completers was based on European Medicines Agency (EMA) guidelines. 24 With an estimated dropout rate of 20%, 50 patients needed to be enrolled. A protocol‐specified interim analysis was performed when ≥ 20 patients receiving N9‐GP had achieved ≥ 50 EDs. At time of trial design, EMA regulatory guidelines stated that approval of rFIX or human plasma‐derived FIX products for hemophilia B in PUPs should be based on a preauthorization safety/efficacy clinical trial in ≥ 20 patients with ≥ 50 EDs; in addition, a postapproval commitment to have follow‐up data for ≥ 20 additional PUPs for a minimum of 100 EDs was required. 24 Consequently, all patients in paradigm 6 were to continue in the ongoing extension phase to capture data for ≥ 100 EDs in ≥ 40 patients.
The incidence of anti‐FIX inhibitory antibodies was reported, and a one‐sided 97.5% upper confidence limit was determined based on an exact calculation for a binomial distribution: The numerator comprised patients with anti‐FIX inhibitory antibodies, and the denominator included all patients with ≥ 10 EDs plus any patient with ≤ 10 EDs with anti‐FIX inhibitory antibodies. The ABR was summarized, and a 95% two‐sided confidence interval (CI) was provided, based on a negative binomial regression model with the number of breakthrough bleeds as the outcome variable, and adjusting for exposure time. All other data were descriptive statistics. The full analysis and safety sets included all patients exposed to N9‐GP.
3. RESULTS
3.1. Patients
As of August 31, 2018 (data cutoff), 38 patients were screened and 37 dosed with N9‐GP; 13 patients started directly on prophylaxis. Of 24 patients who started preprophylaxis, 15 had moved to prophylaxis at the time of analysis (Figure 1).
Figure 1.
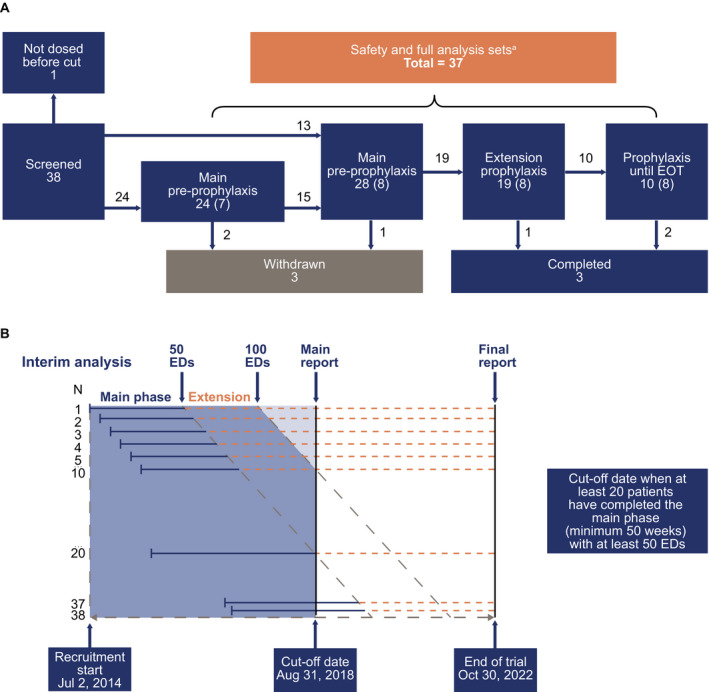
Patient flow chart showing patient attrition.A, the numbers of patients at various trial stages; B, schema to demonstrate the recruitment timeline. ED, exposure day; EOT, end of trial. aPatients who switched from preprophylaxis to prophylaxis are analyzed in all groups. Numbers in parentheses denote patients ongoing in the trial at time of analysis
Median (range) age of patients (N = 37) at baseline was 1.0 (0‐4) years; the most common FIX gene defect (determined in 36 patients) was missense mutations (n = 21; 58.3%), followed by nonsense mutations (n = 6; 16.7%; Table 1).
Table 1.
Patient demographics and characteristics
| Total (N = 37) | |
|---|---|
| Age, y | |
| Median | 1.0 |
| Min, max | 0, 4 |
| Race, n (%) a | |
| White | 18 (48.6) |
| Asian | 11 (29.7) |
| Black or African American | 5 (13.5) |
| Other | 3 (8.1) |
| Mutation, n b , c | |
| Missense | 21 |
| Nonsense | 6 |
| Splice site | 4 |
| Small deletion | 1 |
| Small duplication | 1 |
| Small insertion | 1 |
| No mutation identified b | 2 |
| Known family history | |
| Hemophilia, n (%) | 15 (40.5) |
| Inhibitor, n | 1 |
Patients who switched from preprophylaxis to prophylaxis are included in all columns.
Patients were enrolled from centers in Australia (n = 2), Austria (n = 2), Canada (n = 1), Israel (n = 2), Malaysia (n = 4), Spain (n = 3), Taiwan (n = 3), Thailand (n = 4), the United Kingdom (n = 3), and the United States (n = 13).
At the time of data cutoff.
Data analyzed in 36 patients so far.
Fourteen patients underwent minor surgery (preprophylaxis group, n = 5; prophylaxis group, n = 11): 17 surgeries for port placement or removal, 5 incidences of dental work, 2 circumcisions, and 2 bilateral myringotomy and tube surgeries. No major surgery was performed.
3.2. N9‐GP exposure
At data cutoff, 31 and 21 patients achieved ≥ 10 and ≥ 50 EDs, respectively, and total N9‐GP exposure amounted to 2833 EDs (preprophylaxis group, 222 EDs; prophylaxis group, 2611 EDs), representative of 65.0 patient years (preprophylaxis group, 15.4, prophylaxis group, 49.6 years). N9‐GP was administered for a median (range) duration of 0.64 (0.00‐1.63) years per patient (preprophylaxis group) and 1.52 (0.00‐3.77) years per patient (prophylaxis group). Median (range) total numbers of EDs per patient were 8.5 (1‐20; preprophylaxis) and 78.5 (1‐197; prophylaxis).
3.3. Safety
3.3.1. Immunogenicity
Two patients in the preprophylaxis group developed anti‐FIX inhibitory antibodies (≥0.6 BU) within 10 EDs, resulting in an estimated incidence of 6.1% (one‐sided 97.5% upper CI: 22.4%) among the 33 “at‐risk” patients exposed to N9‐GP (31 patients with ≥ 10 EDs plus the 2 patients who developed anti‐FIX inhibitory antibodies). Both patients were withdrawn (Table 2).
Table 2.
Immunogenicity outcomes
|
Preexisting (n = 37) |
Developed (n = 37) |
Total (N = 37) |
|
|---|---|---|---|
| Immunogenicity | |||
| N9‐GP–binding antibodies | 2 a | 3 b | 5 |
| Anti‐FIX inhibitory antibodies c | 0 | 2 | 2 |
| Anti–CHO‐HCP antibodies | 1 a | 4 d | 5 |
| Anti‐PEG antibodies e | 0 | 0 | 0 |
Abbreviations: CHO, Chinese hamster ovary; FIX, coagulation factor IX; HCP, host cell protein; n, number of patients; N9‐GP, nonacog beta pegol; PEG, plasma polyethylene glycol, rFIX, recombinant coagulation factor IX.
One of which was transient and reported no further positive tests thereafter.
Two of which were transient.
The two reported anti‐FIX inhibitory antibodies were from the preprophylaxis group.
All of which were transient.
Anti‐PEG antibodies were not directly measured but were considered possible in the event of N9‐GP–binding antibodies that did not cross‐react against rFIX.
The first patient to develop confirmed anti‐FIX inhibitory antibodies ≥ 0.6 BU was 17 months old with severe hemophilia B and a de novo nonsense mutation. He had an anaphylactic reaction < 5 minutes following the fourth dose of N9‐GP. Symptoms included vomiting, coughing, hypotonia, reduced level of consciousness, and swelling and cyanosis of the lips, but no respiratory insufficiency. The patient was treated with systemic steroids and fully recovered within approximately 1 hour. At the time of this reaction, FIX activity was < 1% and two local laboratory tests were positive for high‐titer (≥5 BU) anti‐FIX inhibitory antibodies. The patient was also positive for N9‐GP–binding antibodies and CHO‐HCP antibodies. At the end‐of‐treatment visit (2 weeks later), a central laboratory test confirmed that he was still positive for high‐titer (8 BU) anti‐FIX inhibitory antibodies. Two weeks later, the patient remained inhibitor positive (5 BU). One month and 4 days before the anaphylactic reaction, this patient had experienced vomiting after their second exposure to N9‐GP but showed no reaction at their third exposure. Before the trial, he had received two doses of another rFIX product (BeneFix; Pfizer Inc, New York, NY, USA).
The second patient who developed anti‐FIX inhibitory antibodies ≥ 0.6 BU was 15 months old with severe hemophilia B and a nonsense mutation who presented with persistent bleeding after the fourth and fifth N9‐GP exposures. Local laboratory measurements showed FIX activity < 1% and an anti‐FIX inhibitory antibody titer of 11 BU. However, when measured by the central laboratory, the anti‐FIX inhibitory antibody level was 4.5 BU (low titer). Thus, the patient stayed in the trial without further N9‐GP dosing and the anti‐FIX inhibitory antibody titer gradually decreased to a normal level (0.5 BU). When the patient approached 2 years of age and was about to enter the prophylaxis cohort, he received treatment with plasma‐derived activated prothrombin complex concentrate (containing FIX) during surgery for port insertion. He subsequently developed high‐titer anti‐FIX inhibitory antibodies (16 BU, as measured by the central laboratory) and was withdrawn. The patient had received two doses of a different rFIX product (BeneFix) during the trial—the first after screening but before the first N9‐GP dose, and another before the second N9‐GP dose.
Five patients tested positive for N9‐GP–binding antibodies. Two of these patients had preexisting N9‐GP binding antibodies before the first dose of N9‐GP (visit 1), one of whom had no positive tests thereafter, and one who developed concomitant high‐titer anti‐FIX inhibitory antibodies (the first inhibitor patient described above). The other three patients developed N9‐GP–binding antibodies during the trial, two of whom had transient N9‐GP–binding antibodies without clinical consequences, the third (the second inhibitor patient described above) developed persistent high‐titer N9‐GP–binding antibodies concomitant with anti‐FIX inhibitory antibody development. All N9‐GP–binding antibodies cross reacted to rFIX. No anti‐PEG antibodies were measured.
Five patients were positive for anti–CHO‐HCP antibodies: one had anti–CHO‐HCP antibodies at baseline, not measurable thereafter, and four developed transient anti–CHO‐HCP antibodies. These five patients included one of the patients who developed inhibitory anti‐FIX antibodies (the first inhibitor patient described above) and one of the patients with transient N9‐GP–binding antibodies (described above), though the latter was not positive for anti–CHO‐HCP antibodies at the same time as for N9‐GP–binding antibodies. The remaining three patients with transient anti–CHO‐HCP antibodies did not develop inhibitory anti‐FIX antibodies or anti–N9‐GP antibodies (Table 2).
3.3.2. Adverse events
Overall, 36 (97.3%) patients reported a total of 380 AEs (Table 3). Most were mild (n = 331 events) in severity. Most AEs (n = 367) were judged as unlikely to be related to N9‐GP.
Table 3.
Safety outcomes
|
Number of patients (N = 37) a |
Number of AEs (E/R) |
|
|---|---|---|
| Total years in trial b | 65.6 | |
| Exposure | ||
| Total number of exposure days per patient, median (range) | 59.0 (1‐197) | … |
| Number of prophylaxis doses per patient, median (range) | 78.5 (1‐196) | … |
| AEs, n (%) | 36 (97.3) | 380 (5.79) |
| AEs according to severity | ||
| Mild | 35 (94.6) | 331 (5.04) |
| Moderate | 19 (51.4) | 38 (0.58) |
| Severe | 9 (24.3) | 11 (0.17) |
| Most common AEs, n (%) | ||
| Pyrexia | 19 (51.4) | 51 (0.78) |
| Upper respiratory tract infection | 17 (45.9) | 24 (0.37) |
| Nasopharyngitis | 15 (40.5) | 31 (0.47) |
| Cough | 10 (27.0) | 15 (0.23) |
| Rhinorrhea | 8 (21.6) | 14 (0.21) |
| Diarrhea | 7 (18.9) | 8 (0.12) |
| SAEs, c n (%) | 14 (37.8) | 24 (0.37) |
| AEs judged by the investigator to be related to treatment, d n (%) | 7 (18.9) | 13 (0.20) |
| MESIs, n (%) | 9 (24.3) | 14 (0.21) |
| Drug hypersensitivity | 2 (5.4) | 3 (0.05) |
| Anaphylactic reaction e | 1 (2.7) | 1 (0.02) |
| Hypersensitivity | 1 (2.7) | 1 (0.02) |
| Rash | 1 (2.7) | 4 (0.06) |
| Papular rash | 1 (2.7) | 1 (0.02) |
| FIX inhibition e | 2 (5.4) | 2 (0.03) |
| Accidental underdose | 1 (2.7) | 1 (0.02) |
| ASD d | 1 (2.7) | 1 (0.02) |
| AEs leading to withdrawal, n (%) | 3 (8.1) | 5 (0.08) |
Abbreviations: AE, adverse event; ASD, autism spectrum disorder; ED, exposure day; E/R, number of AEs/patient‐years of exposure; FIX, coagulation factor IX; MESI, medical event of special interest; N9‐GP, nonacog beta pegol; SAE, serious adverse event; SOC, system organ class.
Includes all treated patients (preprophylaxis and prophylaxis).
Includes follow‐up period for patients with inhibitors who were off treatment and discontinued patients (from time of discontinuation until end of trial).
SAE SOCs included infections and infestations; gastrointestinal disorders; blood and lymphatic system disorders; investigations; immune system disorders; injury, poisoning, and procedural complications; skin and subcutaneous tissue disorders; and vascular disorders.
The patient with ASD was among the 17 who underwent a neurological exam. Communication with the patient’s family was complicated, as they communicated via a translator. This patient, aged 4 years, had abnormal findings in the neurological exam that were described by the investigator as possibly related to N9‐GP. He was enrolled in October 2015 at 15 months old with no abnormalities reported, and the first N9‐GP dose was administered 9 months later. The patient showed increasingly aberrant behavior over time and did not react to verbal or visual cues appropriately or exhibit normal social interaction. In January 2018, after ~ 27 months on the trial (101 EDs), the patient was diagnosed with hypoacusis (drainage of the ear was performed in May 2019). Nine months later, in October 2018, after > 130 EDs, the investigator reported that the patient (now aged ~ 4 years) was suspected to have ASD with the start dated to October 2017 (87 EDs). The patient received N9‐GP until June 2019 and remains in the trial for follow‐up. He has been referred to relevant medical specialists for further assessment and support.
SAE that was also considered a MESI.
Fourteen patients reported 24 SAEs (rate, 0.37 SAEs per patient‐year of exposure). The majority (13 SAEs in nine patients) were classified as “infections and infestations” (Table 3), consistent with the AE pattern expected at this age.
Seven patients had 13 AEs, including three SAEs, that were considered possibly/probably related to N9‐GP by the investigator (rate, 0.20 events per patient year; Table 3). Four of these, including the three SAEs, occurred in the two inhibitor patients who were withdrawn from the trial (FIX inhibition [x2], drug hypersensitivity [postdose vomiting], anaphylactic reaction). A third patient, aged 2 years, was withdrawn after developing a prolonged urticarial rash and generalized erythema after his tenth N9‐GP exposure. He received antihistamines and oral steroids; the rash resolved after 14 days. Laboratory assessments of immunogenicity were negative. The patient returned to prophylaxis with his previous FIX product without further complications. The remaining eight AEs, considered related to N9‐GP, occurred in four patients who remain on trial: accidental overdose and conjunctivitis in one patient each; hypoacusis and autism spectrum disorder (ASD) in another patient; and a rash reported as four distinct events in one patient, for which he received antihistamine treatment.
Overall, 14 AEs in nine patients were considered MESIs (rate, 0.21 events per patient‐year of exposure; details in Table 3). Of these, five were considered possibly related to N9‐GP, as previously described (FIX inhibitors, drug hypersensitivity, anaphylaxis, ASD).
Laboratory findings
There were no significant changes in laboratory assessments. No renal or hepatic safety concerns and no significant changes in creatinine profiles were seen. At the time of analysis, only two patients had PEG measurements performed at screening; both had PEG plasma levels below the lower limit of quantification of 0.75 µg/mL. Another 12 patients underwent PEG plasma measurements following N9‐GP exposure, with PEG levels falling in the expected steady‐state range of 3.5‐6.2 µg/mL.
3.4. Efficacy
Of the 37 patients exposed to N9‐GP, 18 (48.6%) were treated with N9‐GP for a total of 48 bleeds (33 and 15 bleeds in the preprophylaxis and prophylaxis groups, respectively). The majority (89.6%) were mild or moderate, and 5 (10.4%) were severe. Most bleeds (64.6%) were traumatic (Figure 2A). Two bleeds in one patient (who developed anti‐FIX inhibitory antibodies) were treated with another FIX product; the first occurred after screening but before visit 1, and the second occurred outside the trial site’s opening hours. These bleeds were included in ABR calculations.
Figure 2.
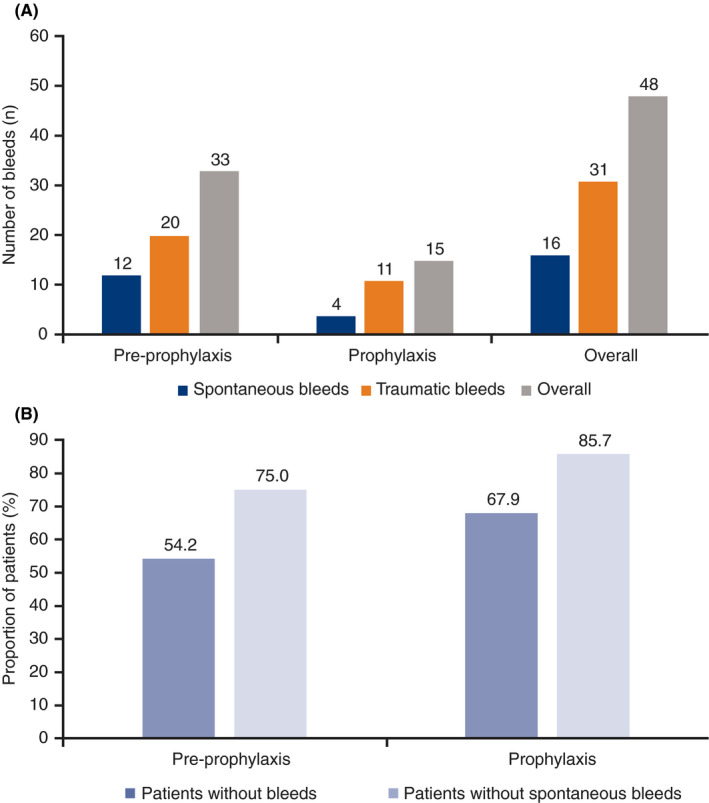
Number of bleedsa according to (A) type and (B) percentage of patients without bleeds and spontaneous bleeds. N9‐GP, nonacog beta pegol. aBleeds treated with N9‐GP. Note: Bleed type was unknown for one bleed in the preprophylaxis group
During weekly prophylaxis with N9‐GP, two‐thirds of patients (n = 19; 67.9%) were free from any bleed, and 85.7% (n = 24) were free from spontaneous bleeds (Figure 2B). Median overall, spontaneous, and traumatic ABRs were all 0.0 in the prophylaxis group (Table 4). Corresponding modeled mean ABRs were 0.31, 0.08, and 0.23, respectively.
Table 4.
Treatment outcomes
| Outcome |
Preprophylaxis a (n = 24) |
Prophylaxis (n = 28) |
|---|---|---|
| N9‐GP exposure period | ||
| N9‐GP median exposure duration, y (range) | 0.64 (0.00‐1.63) | 1.52 (0.00‐3.77) |
| N9‐GP median total number of EDs (range) | 8.5 (1‐20) | 78.5 (1‐197) |
| Efficacy outcomes | ||
| Number of bleeds/number of patients with bleeds | 33/11 | 15/9 |
| Patients without bleeds, n (%) | 13 (54.2) | 19 (67.9) |
| Patients without spontaneous bleeds, n (%) | 18 (75.0) | 24 (85.7) |
| Hemostatic control of bleeding b | ||
| Hemostatic success rate, c N (%) | ||
| Total | 31 (93.9) | 14 (93.3) |
| Spontaneous d | 12 (100.0) | 4 (100.0) |
| Traumatic d | 18 (90.0) | 10 (90.9) |
| ABR, median (IQR) | ||
| Total ABR | 0.95 (0.00‐3.12) | 0.00 (0.00‐0.32) |
| ABRspontaneous | 0.00 (0.00‐0.43) | 0.00 (0.00‐0.00) |
| ABRtraumatic | 0.00 (0.00‐1.88) | 0.00 (0.00‐0.27) |
| ABRjoint | 0.00 (0.00‐0.00) | 0.00 (0.00‐0.00) |
| Negative binomial estimate (modeled mean) of ABR (95% CI) e | ||
| Total ABR | 2.18 (1.32‐3.58) | 0.31 (0.16‐0.57) |
| ABRspontaneous | 0.69 (0.31‐1.56) | 0.08 (0.03‐0.21) |
| ABRtraumatic | 1.44 (0.78‐2.64) | 0.23 (0.11‐0.46) |
| ABRjoint | 0.33 (0.14‐0.78) | 0.02 (0.00‐0.14) |
| Injections required per bleed, N (%) | ||
| 1 | 27 (81.8) | 15 (100.0) |
| 2 | 3 (9.1) | … |
| 3 | 2 (6.1) | … |
| 4 | 1 (3.0) | … |
| Mean (SD) number of injections per bleed | 1.3 (0.7) | 1 (0.0) |
| N9‐GP consumption, mean (SD) | ||
| N9‐GP dose, IU/kg f | 41.3 (4.7) | 43.3 (3.3) |
| N9‐GP dose per bleed, IU/kg | 55.0 (28.7) | 48.8 (16.9) |
Abbreviations: ABR, annualized bleeding rate; ABRjoint, annualized joint bleeding rate; ABRspontaneous, annualized spontaneous bleeding rate; ABRtraumatic, annualized traumatic bleeding rate; CI, confidence interval; ED, exposure day; FIX, coagulation factor IX; IQR, interquartile range; n, number of patients; N, number of bleeds; N9‐GP, nonacog beta pegol; SD, standard deviation.
With respect to ABR calculations, bleeds treated with other FIX products were included; consequently, two additional traumatic bleeds were included in the preprophylaxis group.
Assessed on a 4‐point hemostatic response scale.
Determined as being either an “excellent” or a “good” response.
The percentages relate to the number of spontaneous and traumatic bleeds and not the overall number of bleeds.
Estimated from a negative binomial regression model with number of bleeding episodes as the outcome variable and adjusting for exposure time.
N9‐GP dose for preprophylaxis or prophylaxis only, not including the N9‐GP doses to treat bleeds.
Hemostatic control was rated “excellent” or “good” for 45 of 48 bleeds (93.8%) in the overall population and for 14 of 15 bleeds (93.3%) in the prophylaxis group (Figure 3). One traumatic bleed in a patient in the prophylaxis group was rated as a “moderate” response by the investigator, although not entirely consistent with the protocol definition. All 15 bleeds occurring in the prophylaxis group were treated with one N9‐GP injection. All spontaneous bleeds and 90.3% of traumatic bleeds were rated as successfully treated.
Figure 3.
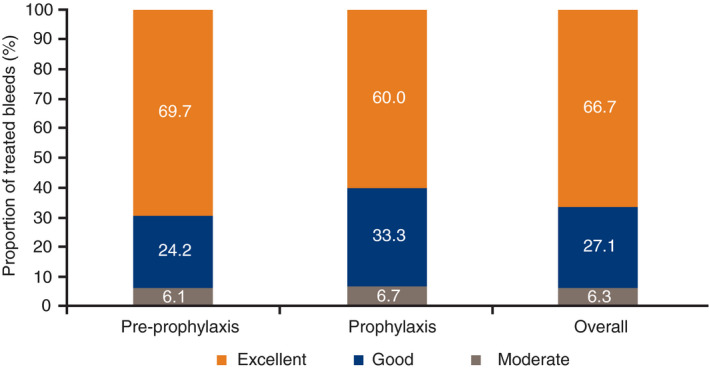
Hemostatic response of all 48 bleeds during the analysis period.a N9‐GP, nonacog beta pegol. aBleeds treated with N9‐GP. Note: Bleeds in the “Overall” column add up to 100.1% due to decimal point rounding
3.5. N9‐GP consumption
Median (range) N9‐GP consumption per patient was 701.8 (67.0‐1150.4) IU/kg/year in the preprophylaxis group and 2280.8 (2062.3‐2689.0) IU/kg/year in the prophylaxis group. Median (range) number of prophylactic doses per patient was 78.5 (1‐196), with a median (range) prophylactic dose of 43.7 (12.0‐52.1) IU/kg.
3.6. FIX activity
Geometric mean FIX trough activity (prophylaxis) was 0.6% at 1 ED, increasing to 13.4% at 5 EDs. This increased further over time, likely due to achieving steady state and the increasing age of patients (14.1% at 10 EDs, 15.5% at 15 EDs, 14.3% at 20 EDs, 14.7% at 70 EDs, and 17.1% at 148 EDs). Estimated mean FIX trough activity in the prophylaxis group was 15.0% (95% CI, 14.1%‐16.0%) over the entire trial duration (Figure 4). Mean incremental FIX recovery at 30 minutes was similar across EDs (0.012‐0.013 [IU/mL]/[IU/kg]) in both the preprophylaxis and prophylaxis groups.
Figure 4.
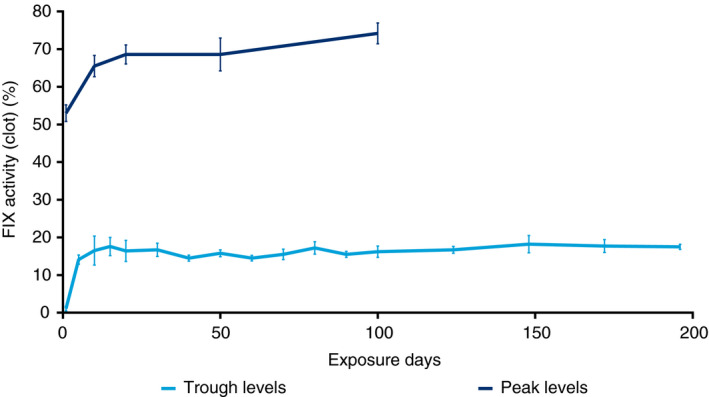
Mean profiles of FIX activity in prophylaxis. FIX, coagulation factor IX. Points are means; error bars represent standard error of means. The analysis is based on a mixed model on the log‐transformed plasma concentrations with patient as a random effect. The mean FIX activity level is presented back‐transformed to the natural scale. Only patients with a minimum of 4 weeks of prophylaxis were included. Measurements (n = 188) must have been taken ≥ 5 days and ≤ 10 days after the last dose. Measurements associated with doses within ≥ 14 days after a bleed were not included in the analysis. FIX activity up to 196 exposure days is reported
4. CONCLUSIONS
In this planned interim analysis of the paradigm 6 trial in PUPs and MTPs with hemophilia B and FIX activity ≤ 2%, we report an anti‐FIX inhibitory antibody incidence of 6.1% (2/33 “at‐risk” patients, both with nonsense mutations and in the preprophylaxis group). Both patients had previously received one or two doses of a different rFIX product before development of anti‐FIX inhibitory antibodies; as a result, it is not possible to definitively attribute the inhibitor development to either product. Historically, the risk of developing anti‐FIX inhibitory antibodies in hemophilia B was considered 1.5%‐5%; 13 , 25 however, these studies were not stratified by previous treatment status or disease severity. Recently, a systematic review analyzed seven studies (including data from European Haemophilia Safety Surveillance) comprising 176 PUPs with severe hemophilia B, reporting an overall anti‐FIX inhibitory antibody rate of 10.2% (interstudy range, 5%‐14%; one outlier study reported a rate of 37%). 26 , 27 Further studies in PUPs with severe hemophilia B found anti‐FIX inhibitory antibody incidences of 11.8%, 28 19%, 29 and 10.2; 30 the last of these also reported a 26.9% increased inhibitor risk associated with nonsense mutations. Therefore, the anti‐FIX inhibitory antibody incidence of 6.1% is within the expected range for this patient population.
N9‐GP was generally well tolerated, with 13 of 380 AEs judged possibly/probably related to treatment. Three were classified as SAEs, occurring in two patients: two of anti‐FIX inhibitory antibody development and one of anaphylactic reaction. Anaphylaxis is typically coincident with development of anti‐FIX inhibitory antibodies, occurring in up to 60% of patients with hemophilia B and anti‐FIX inhibitory antibodies. 31 , 32
One patient in this trial was diagnosed with ASD and further assessments are ongoing. In 2014, the prevalence of ASD in the United States was estimated at 16.8 cases per 1000 children aged 8 years. 33 Additionally, ASD may be more common in boys with hemophilia, with a recent UK study showing a prevalence of 50.6 cases per 1000 boys with hemophilia diagnosed aged < 8 years; this is higher than the general population (11.6 cases/1000) and unaffected boys aged 8 years (3.9 cases/1000). 34
Despite extensive clinical experience with marketed PEGylated products in adults, 35 , 36 some uncertainty remains regarding their safety in children with hemophilia due to potentially lifelong PEG exposure. 37 , 38 However, analysis of plasma samples from previous studies in the paradigm program showed that in adults and children treated with N9‐GP prophylaxis for up to 6.5 years, following the initial increase in plasma‐PEG levels to steady state, no further systemic PEG accumulation was observed. 39 PEG plasma measurements in paradigm 6 were within this previously reported range. Furthermore, in a study examining trends in AEs reported in pediatric populations treated with multiple different PEGylated therapies, no consistent pattern of AEs attributable to PEG was found. 40
FIX activity trough levels in the current paradigm 6 trial increased slightly over time, as expected with the increasing age of children.
During the prophylaxis phase, most patients (67.9%) were free from any bleed; notably, 85.7% experienced no spontaneous bleeds. In the overall patient population, 93.8% of bleeds were successfully controlled. Similar outcomes were found in the first interim analysis of paradigm 5 (performed after ≥ 52 weeks), with 92.9% of bleeds successfully controlled with 40 IU/kg N9‐GP. 18
Most bleeds (42/48 [87.5%]) required only one N9‐GP injection (27/33 [81.8%] in the pre‐prophylaxis group and all 15 bleeds [100.0%] in the prophylaxis group). This is in accordance with the hemostatic efficacy observed in other trials of prophylactic N9‐GP (40 IU/kg once weekly). 16 , 17 , 18
Also consistent with previous N9‐GP studies, median overall, spontaneous, and traumatic ABRs were very low in the prophylaxis group (0.0 for all). 16 These results show comparable or lower reported ABRs compared with the two other approved EHL FIX products, rFIX‐albumin fusion protein (rFIX‐FP) and rFIX‐Fc fusion protein (rFIXFc). In previously treated pediatric patients (aged < 6 years), the ABRs of patients receiving weekly rFIX‐FP (47 IU/kg) or rFIXFc (65 IU/kg) were 2.64 and 1.04, 41 , 42 respectively. However, these trials cannot be directly compared due to differences in study design and patient populations.
The main limitation of this analysis is the small sample size, which can impact the estimation of anti‐FIX inhibitory antibody incidence. However, this interim analysis was in line with regulatory guidelines. The final analysis will include a larger sample size.
In conclusion, the observed anti‐FIX inhibitory antibody incidence rate of 6.1% was within the expected range for PUPs with hemophilia B and FIX activity ≤ 2%, providing evidence for the safety of N9‐GP in this vulnerable population. N9‐GP was also generally well tolerated and provided effective hemostatic coverage.
RELATIONSHIP DISCLOSURE
AKC has received honoraria from Bayer, Novo Nordisk, Sanofi, and Shire (now Takeda). JA has received honoraria from Roche and SOBI; and nonfinancial support from Baxalta, CSL Behring, and Novo Nordisk. CB has received honoraria from Novo Nordisk and Pfizer; and was a consultant to Baxter Healthcare, Bayer, Biogen Idec, and Novo Nordisk. AC has received honoraria from Novo Nordisk and Shire (now Takeda). M‐LG and RMM are employees of Novo Nordisk A/S, Søborg, Denmark. GY has received honoraria and consultancy fees from Bayer, Bioverativ, CSL Behring, Freeline, Genentech/Roche, Grifols, Kedrion, Novo Nordisk, Shire (now Takeda), Spark, and UniQure; and research grants from Genentech, Grifols, and Shire (now Takeda). None of the authors received honoraria for activities related to the development of this manuscript.
AUTHOR CONTRIBUTIONS
AKC, JA, CB, AC, and GY were the clinical investigators during the trials. M‐LG was responsible for data collection, data interpretation, and for overseeing the trial in a medical capacity. AKC, JA, CB, AC, M‐LG, RMM, and GY provided critical revisions to the intellectual content of the drafts and a substantial contribution to interpretation of the data. RMM undertook the statistical analyses. All authors directed the data analysis and the development of the manuscript, and approved the final version.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The trial was sponsored by Novo Nordisk A/S (Bagsværd, Denmark). M Watson of Parexel and W Stainsby of Paragon, medical writers supported by funding from Novo Nordisk A/S, provided drafts and editorial assistance to the authors during preparation of this manuscript. Novo Nordisk's policy on data sharing may be found at https://www.novonordisk‐trials.com/how‐access‐clinical‐trial‐datasets.
Chan AK, Alamelu J, Barnes C, et al. Nonacog beta pegol (N9-GP) in hemophilia B: First report on safety and efficacy in previously untreated and minimally treated patients. Res Pract Thromb Haemost. 2020;4:1101–1113. 10.1002/rth2.12412
Handling Editor: Pantep Angchaisuksiri
Social media post: First report of EHL rFIX in haemophilia B PUPs: good safety/efficacy profile achieved with N9‐GP (median ABR 0; inhibitor incidence 6%) #extended‐half life, #PUPs, #hemophilia B, #Refixia
Contributor Information
Anthony K. Chan, Email: akchan@mcmaster.ca.
Guy Young, @GuyYoungMD.
REFERENCES
- 1. Franchini M, Frattini F, Crestani S, Bonfanti C. Haemophilia B: current pharmacotherapy and future directions. Expert Opin Pharmacother. 2012;13:2053–63. [DOI] [PubMed] [Google Scholar]
- 2. Poon MC, Lee A. Individualized prophylaxis for optimizing hemophilia care: can we apply this to both developed and developing nations? Thromb J. 2016;14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Federation of Hemophilia . Guidelines for the management of hemophilia. 2012. [Accessed July 22, 2019.] Available from http://www1.wfh.org/publications/files/pdf‐1472.pdf
- 4. Branchford BR, Monahan PE, Di Paola J. New developments in the treatment of pediatric hemophilia and bleeding disorders. Curr Opin Pediatr. 2013;25:23–30. [DOI] [PubMed] [Google Scholar]
- 5. Santagostino E, Mancuso ME. Barriers to primary prophylaxis in haemophilic children: the issue of the venous access. Blood Transfus. 2008;6(Suppl 2):s12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gringeri A, Lundin B, Von mackensen S, Mantovani L, Mannucci PM. von Mackensen S, Mantovani L, Mannucci PM, ESPIRIT Study Group. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study). J Thromb Haemost. 2011;9:700–10. [DOI] [PubMed] [Google Scholar]
- 7. Mancuso ME, Santagostino E. Outcome of clinical trials with new extended half‐life FVIII/IX concentrates. J Clin Med. 2017;6:E39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mahlangu JN. Updates in clinical trial data of extended half‐life recombinant factor IX products for the treatment of haemophilia B. Ther Adv Hematol. 2018;9:335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCarthy J, Mathew P. Treatment of hemophilia with inhibitors: an advance in options for pediatric patients. J Emerg Nurs. 2011;37:474–6. [DOI] [PubMed] [Google Scholar]
- 10. Puetz J, Soucie JM, Kempton CL, Monahan PE; Hemophilia Treatment Center Network Investigators. Prevalent inhibitors in haemophilia B subjects enrolled in the Universal Data Collection database. Haemophilia. 2014;20:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Federation of Hemophilia . Who is at risk of developing inhibitors? 2014. [Accessed July 22, 2019.] Available from https://www.wfh.org/en/page.aspx?pid=653
- 12. Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. 2014;124:3365–72. [DOI] [PubMed] [Google Scholar]
- 13. DiMichele D. Inhibitor development in haemophilia B: an orphan disease in need of attention. Br J Haematol. 2007;138:305–15. [DOI] [PubMed] [Google Scholar]
- 14. Jadhav M, Warrier I. Anaphylaxis in patients with hemophilia. Semin Thromb Hemost. 2000;26:205–8. [DOI] [PubMed] [Google Scholar]
- 15. Shibata M, Shima M, Misu H, Okimoto Y, Giddings JC, Yoshioka A. Management of haemophilia B inhibitor patients with anaphylactic reactions to FIX concentrates. Haemophilia. 2003;9:269–71. [DOI] [PubMed] [Google Scholar]
- 16. Collins PW, Young G, Knobe K, Karim FA, Angchaisuksiri P, Banner C, et al. Recombinant long‐acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trial. Blood. 2014;124:3880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young G, Collins PW, Colberg T, Chuansumrit A, Hanabusa H, Lentz SR, et al. Nonacog beta pegol (N9‐GP) in haemophilia B: A multinational phase III safety and efficacy extension trial (paradigm4). Thromb Res. 2016;141:69–76. [DOI] [PubMed] [Google Scholar]
- 18. Carcao M, Zak M, Abdul Karim F, Hanabusa H, Kearney S, Lu M‐Y, et al. Nonacog beta pegol in previously treated children with hemophilia B: results from an international open‐label phase 3 trial. J Thromb Haemost. 2016;14:1521–9. [DOI] [PubMed] [Google Scholar]
- 19. Carcao M, Goudemand J.World Federation of Hemophilia. Inhibitors in hemophilia: A primer. 2018. [Accessed October 10, 2019.] Available from http://www1.wfh.org/publications/files/pdf‐1122.pdf
- 20. Millner AH, Tiefenbacher S, Robinson M, Boesen HT. A variation of the Nijmegen‐Bethesda assay using heat or a novel heat/cold pretreatment for the detection of FIX inhibitors in the presence of residual FIX activity. Int J Lab Hematol. 2016;38:639–47. [DOI] [PubMed] [Google Scholar]
- 21. Mire‐Sluis AR, Barrett YC, Devanarayan V, Koren E, Liu H, Maia M, et al. Recommendations for the design and optimization of immunoassays used in the detection of host antibodies against biotechnology products. J Immunol Methods. 2004;289:1–16. [DOI] [PubMed] [Google Scholar]
- 22. Shankar G, Devanarayan V, Amaravadi L, Barrett YC, Bowsher R, Finco‐Kent D, et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J Pharm Biomed Anal. 2008;48:1267–81. [DOI] [PubMed] [Google Scholar]
- 23. Novo Nordisk . Coagulation assays: Haemophilia B. [Accessed August 27, 2019.] Available from https://www.coagulationassays.com/en_gb/home/diagnosis‐monitoring/algorithm‐of‐haemophilia‐b.html
- 24. European Medicines Agency . Guideline on clinical investigation of recombinant and human plasma‐derived factor IX products. 2011. [Accessed July 22, 2019.] Available from https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐clinical‐investigation‐recombinant‐human‐plasma‐derived‐factor‐viii‐products‐first‐version_en.pdf
- 25. Key NS. Inhibitors in congenital coagulation disorders. Br J Haematol. 2004;127:379–91. [DOI] [PubMed] [Google Scholar]
- 26. Franchini M, Santoro C, Coppola A. Inhibitor incidence in previously untreated patients with severe haemophilia B: a systematic literature review. Thromb Haemost. 2016;116:201–3. [DOI] [PubMed] [Google Scholar]
- 27. Ljung R, Petrini P, Tengborn L, Sjorin E. Haemophilia B mutations in Sweden: a population‐based study of mutational heterogeneity. Br J Haematol. 2001;113:81–6. [DOI] [PubMed] [Google Scholar]
- 28. Van den Berg M, Carcao M, Santagostino E, Chambost H, Fischer K, Williams M, et al. Inhibitor incidence in PUPs with severe haemophilia B is higher than usually reported; data from the PedNet registry. Haemophilia. 2018;24:23–31. [Google Scholar]
- 29. Martensson A, Letelier A, Hallden C, Ljung R. Mutation analysis of Swedish haemophilia B families ‐ high frequency of unique mutations. Haemophilia. 2016;22:440–5. [DOI] [PubMed] [Google Scholar]
- 30. Male C, Andersson NG, Rafowicz A, Liesner RI, Kurnik K, Fischer K, et al. Inhibitor incidence in an unselected cohort of previously untreated patients with severe haemophilia B: a PedNet study. Haematologica. 2020. 10.3324/haematol.2019.239160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mauro M, Bonetti E, Balter R, Poli G, Cesaro S. Recurrent episodes of anaphylaxis in a patient with haemophilia B: a case report. Blood Transfus. 2016;14:582–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chitlur M, Warrier I, Rajpurkar M, Lusher JM. Inhibitors in factor IX deficiency a report of the ISTH‐SSC international FIX inhibitor registry (1997–2006). Haemophilia. 2009;15:1027–31. [DOI] [PubMed] [Google Scholar]
- 33. Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years ‐ autism and developmental disabilities monitoring network, 11 Sites, United States, 2014. MMWR Surveill Summ. 2018;67:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bladen M, Thorpe N, Main E, Barrie A, Griffioen A, Hubert N, et al. What is the prevalence of autism spectrum disorder in boys with haemophilia? European Association for Haemophilia and Allied Disorders; Prague, Czech Republic 2019.
- 35. Ivens IA, Achanzar W, Baumann A, Brändli‐Baiocco A, Cavagnaro J, Dempster M, et al. PEGylated biopharmaceuticals: current experience and considerations for nonclinical development. Toxicol Pathol. 2015;43:959–83. [DOI] [PubMed] [Google Scholar]
- 36. Baumann A, Tuerck D, Prabhu S, Dickmann L, Sims J. Pharmacokinetics, metabolism and distribution of PEGs and PEGylated proteins: quo vadis? Drug Discov Today. 2014;19:1623–31. [DOI] [PubMed] [Google Scholar]
- 37. Zhang F, Liu MR, Wan HT. Discussion about several potential drawbacks of PEGylated therapeutic proteins. Biol Pharm Bull. 2014;37:335–9. [DOI] [PubMed] [Google Scholar]
- 38. European Medicines Agency . CHMP Safety Working Party’s response to the PDCO regarding the use of PEGylated drug products in the paediatric population. 2012. [Accessed March 26, 2020.] Available from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/11/WC500135123.pdf.
- 39. Sternebring O, Gabel‐Jensen C, Jacobsen H, Benie AJ, Bjørnsdottir I. Steady‐state plasma concentrations of polyethylene glycol (PEG) are reached in children and adults during once‐weekly prophylactic treatment with nonacog beta pegol (N9‐GP). BioDrugs. 2019;33:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stidl R, Denne M, Goldstine J, Kadish B, Korakas KI, Turecek PL. Polyethylene glycol exposure with antihemophilic factor (recombinant), PEGylated (rurioctocog alfa pegol) and other therapies indicated for the pediatric population: history and safety. Pharmaceuticals (Basel). 2018;11:E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kenet G, Chambost H, Male C, Lambert T, Halimeh S, Chernova T, et al. Long‐acting recombinant fusion protein linking coagulation factor IX with albumin (rIX‐FP) in children. Results of a phase 3 trial. Thromb Haemost. 2016;116:659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ragni M, Kulkarni R, Pasi KJ, Fischer K, Mahlangu J, Shapiro A, et al. B‐YOND final results confirm established safety, sustained efficacy, and extended dosing interval for up to 4 years of treatment With rFIXFc in previously treated subjects with severe hemophilia B. Blood. 2018;132:1214. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


