Abstract
Background
Tyrosyl‐tRNA synthetase (YRS) belongs to the family of enzymes that catalyzes the tRNA aminoacylation reaction for protein synthesis, and it has been recently shown to exert noncanonical functions. Although database results indicate extremely low levels of YRS mRNA in platelets, YRS protein is abundantly present. The source of YRS in platelets, as well as the physiological role of platelet‐stored YRS, remains largely unknown.
Objectives
To clarify how YRS accumulates in platelets and determine the potential role of platelet‐stored YRS.
Methods
Recombinant YRS proteins with epitope tags were prepared and tested in vitro for proteolytic cleavage in human plasma. Fluorescent‐labeled YRS was examined for uptake by platelets, as demonstrated by western blotting and confocal microscopy analysis. Using RAW‐Dual reporter cells, Toll‐like receptor and type I interferon activation pathways were analyzed after treatment with YRS.
Results
Full‐length YRS was cleaved by both elastase and matrix metalloproteinases in the plasma. The cleaved, N‐terminal YRS fragment corresponds to the endogenous YRS detected in platelet lysate by western blotting. Both full‐length and cleaved forms of YRS were taken up by platelets in vitro and stored in the α‐granules. The N‐terminal YRS fragment generated by proteolytic cleavage had monocyte activation comparable to that of the constitutive‐active mutant YRS (YRSY341A) previously reported.
Conclusion
Platelets take up both full‐length YRS and the active form of cleaved YRS fragment from the plasma. The cleaved, N‐terminal YRS fragment stored in α‐granules may have potential to activate monocytes.
Keywords: elastase, matrix metalloproteinases (MMPs), monocytes, platelets, tyrosyl‐tRNA synthetase (YRS), α‐granules
Essentials.
Tyrosyl‐tRNA synthetase (YRS) is an enzyme crucial for protein synthesis but also has nontranslational functions.
The potential functions of endogenous YRS stored in platelets were studied.
Secreted YRS is promptly cleaved by proteinases present in the plasma, generating active forms.
Cleaved YRS taken up by platelets has the potential to activate monocytes and regulate function.
1. INTRODUCTION
Aminoacyl‐tRNA synthetases (aaRSs) are a family of enzymes that catalyze the first step reaction, namely, the aminoacylation of tRNA, in protein synthesis. Over the course of evolution, aaRSs acquired additional functions beyond translation. 1 , 2 Tyrosyl‐tRNA synthetase (YRS) was first described to be secreted and split into two fragments with distinct cytokine/chemokine activities. 3 , 4 These nontranslational activities depend on two motifs: Glu‐Leu‐Arg (ELR) tripeptide in the catalytic domain and heptapeptide RVGKIIT in the C‐terminal EMAPII‐like domain. 5 These domains are buried in the native, full‐length YRS protein, and proteolytic cleavage or rationally designed mutagenesis (Y341A) exposes the key motifs and converts YRS to active forms. 4 , 6 We further showed in mouse models that a constitutive active‐form YRSY341A expands megakaryocytes (MK) and contributes to accelerated recovery from thrombocytopenia. 7 Interestingly, secreted YRS circulates in human plasma and, unlike other tRNA synthetases, is abundantly present in platelets. 8 , 9 However, database results indicate very low or undetectable levels of YRS mRNA in platelets. 10 These data suggest that platelet‐stored YRS may be derived from the circulating plasma pool, and that storage in platelets may have physiological roles associated with platelet biology. In this article, we clarify the mechanism of YRS accumulation in platelets and study the potential physiologic role of YRS stored in platelets.
2. MATERIALS AND METHODS
2.1. Blood sample preparation
Blood samples from healthy donors were obtained in accordance with the Declaration of Helsinki after obtaining written informed consent. The experimental protocol was approved by Institutional Review Board of the Scripps Research Institute (IRB‐15‐6643). Whole blood was collected from normal blood donors in 3.8% citrated tubes. The blood was centrifuged at 230 g for 7 minutes at room temperature to prepare platelet‐rich plasma (PRP). PRP was transferred into a new tube with an excess amount of Tyrode’s buffer (pH 7.4) with 2 mmol/L EDTA and 10 μmol/L prostaglandin E1 to prevent platelet activation, then was centrifuged again at 1460 g for 5 minutes. The platelet pellet was resuspended in Tyrode’s buffer, then added with equal volume of lysis buffer containing 2% SDS 50 mmol/L Tris pH 7.4 and boiled for 6 minutes. Protein concentration of platelet lysates was determined by spectrophotometer NanoDrop (Thermo Scientific, Waltham, MA, USA). A part of PRP was centrifuged at 1460 g for 5 minutes to obtain platelet‐poor plasma.
2.2. Animal procedures
Animal experiments were performed according to a protocol approved by the Institutional Animal Care and Usage Committee of The Scripps Research Institute.
2.3. Platelet analysis
Complete blood counts were obtained using Procyte Dx (IDEXX Laboratories, Westbrook, ME, USA).
2.4. Western blot analysis
Protein level of YRS in plasma and platelet lysates was assessed by western blotting. Samples were analyzed by SDS‐PAGE and electroblotted onto a polyvinylidene difluoride (PVDF) membrane using a transfer apparatus according to manufacturer’s protocols (Bio‐Rad Laboratories, Hercules, CA, USA). Equal amounts (20 µg) of platelet lysates were loaded for each sample. The membrane was incubated with anti‐YRS monoclonal antibody A‐10 (Santa Cruz) overnight, and the signal was detected with IRDye 680 goat anti‐mouse IgG. The image was obtained using LI‐COR Odyssey imaging system and the band intensity was quantified using Licor ImageStudio (LI‐COR Biosciences, Lincoln, NE, USA).
2.5. Inhibitor experiments using dual‐tagged recombinant YRS
Recombinant YRS with epitope tags on both ends (dual‐tagged YRS) (3.5 µmol/L) was incubated with platelet‐poor plasma in the absence or presence of elastase inhibitor Sivelestat (Bio Vision Group, Milpitas, CA, USA) (1000 µg/mL), matrix metalloproteinase (MMP) inhibitor GM6001 (Sigma‐Aldrich, St Louis, MO, USA) (500 µmol/L), or both inhibitors at 37°C for 1 hour. The samples were analyzed by SDS‐PAGE under reduced condition, transferred to PVDF membrane, and blotted with rat anti‐FLAG antibody L5 (BioLegend, San Diego, CA, USA) and mouse anti‐V5 antibody (Thermo Scientific) overnight.
2.6. Platelet uptake assay
Recombinant YRS proteins (dtYRS, YRSWT, YRSMini, or YRSMini+: 3.5 µmol/L) was incubated with PRP derived from healthy donors at 37°C for 17 hours. Platelets and plasma components were separated by centrifugation and loaded on SDS‐PAGE under reduced condition. YRS protein was detected by western blotting using rat anti‐FLAG antibody and mouse anti‐V5 antibody.
2.7. Flow cytometry
YRSWT, YRSMini, YRSMini+, and bovine serum albumin (BSA) were labeled with AlexaFluor647. Each protein was incubated with platelet rich plasma at a concentration of 500 nmol/L for 17 hours. Samples were analyzed using Novocyte flow cytometer (ACEA Biosciences, San Diego, CA, USA). Platelet population was gated by forward and side scatter, and the data were analyzed using FlowJo software (FlowJo LLC, Ashland, OR, USA).
2.8. Confocal analysis
Dual‐tagged YRS labeled with AlexaFluor647 (2 µmol/L) was incubated with PRP in the presence of AlexaFluor488‐labeled fibrinogen (Thermo Scientific) for 17 hours. Platelets were isolated by washing with Tyrode’s buffer with 2 mmol/L EDTA and 10 µmol/L prostaglandin E1 and centrifuged at 1460 g for 5 minutes. Platelets were resuspended in Tyrode’s buffer and then placed between the cover slip and glass slide. Images were taken using confocal microscope (LSM 880; Carl Zeiss AG, Jena, Germany).
2.9. Monocyte activation assay using RAW‐Dual reporter cells
The functional activities of YRSMini+ were tested by reporter macrophage cell line (RAW‐Dual reporter cells; InvivoGen, San Diego, CA, USA). These cells stably express two reporter genes, secreted embryonic alkaline phosphatase (SEAP) and Lucia luciferase (LUC). SEAP reports the activation of macrophage inflammatory protein 2 promoter, which reflects NF‐kB activation, and Luc is under interferon (IFN)‐stimulated response elements, reporting activation of interferon regulatory factors. After incubation with 500 nmol/L of YRSMini+ for 21 hours, the activation of the two reporter genes was simultaneously assessed using reagents QUANTI‐Blue and QUANTI‐Luc (InvivoGen) following manufacturer’s instructions.
2.10. Preparation of recombinant YRS proteins
The SUMO gene with an N‐terminal 6xHis tag was fused onto the N‐terminus of each YRS construct in the pET28a vector (EMD Millipore, Billerica, MA, USA). 11 Each construct was transformed into BL21‐CodonPlus (DE3)‐RIPL cells (Agilent Technologies, Santa Clara, CA, USA) or ClearColi BL21(DE3) (Lucigen Corporation, Middleton, WI, USA) for protein expression. After overnight culture and induction with 0.5 mmol/L IPTG for 5 hours, cells were collected by centrifugation, lysed using a cell homogenizer (Microfluidics, Westwood, MA, USA), and centrifuged at 30 000 g for 30 minutes. The lysate supernatant was loaded and purified using Ni‐NTA resin (Qiagen, Hilden, Germany). Following elution, the SUMO protease ubiquitin‐like specific protease 1 (ULP1) was added to the elution to cleave off the 6xHis‐SUMO tag. After desalting, proteins were concentrated and then separated using a Superdex 200 16/600 prep grade column (GE Healthcare, Princeton, NJ, USA) to fractionate out the SUMO tag, ULP1 protease, and aggregate proteins. Purified YRS protein was then poured over high capacity endotoxin removal resin (Pierce) and then passed through Acrodisc Mustang E membranes (Pall Corporation, Port Washington, NY, USA). Final endotoxin levels were tested using the Endosafe‐PTS system (Charles River Laboratories, Wilmington, MA, USA) and confirmed to be <4.0 EU/mg. The buffer of purified protein was exchanged against phosphate buffered saline using a desalting column.
3. RESULTS
3.1. Characterization of endogenous YRS present in circulating plasma and platelets
YRS, a cytoplasmic protein that is required and essential for protein synthesis, is known to be secreted extracellularly and exert nontranslational functions. 4 Thus, we first analyzed whether extracellular YRS was present in human plasma. Plasma samples derived from healthy donors were analyzed by western blotting using monoclonal antibody (A‐10), which recognizes the N‐terminal region of YRS. Plasma samples loaded under nonreduced conditions showed that most of the YRS was oligomerized in plasma and was detected as higher‐molecular‐weight ladders (Figure 1A, asterisk). Of note, a cleaved form of YRS with molecular weight of about 40 kDa was also detected (Figure 1A, arrowhead). When analyzed under reduced conditions, high‐molecular‐weight ladders disappeared as expected and YRS was detected as monomeric (Figure 1A, lower panel, arrow). We speculate that some of the six cysteines present in full‐length YRS formed disulfide bonds that cross‐linked molecules to each other. Western blotting of platelet lysates under nonreduced conditions detected YRS as a full‐length monomer (Figure 1B, arrow), with weak signals of high molecular weight oligomers, and partially present as a cleaved form (Figure 1B, arrowhead). 12 Platelet lysates derived from C57BL/6J mouse showed a similar pattern as human platelets. Interestingly, neither the ~40‐kDa cleaved YRS nor high‐molecular‐weight oligomers were detected with lysates of mouse melanoma cell line B16 and monocyte/macrophage cell line RAW.
FIGURE 1.
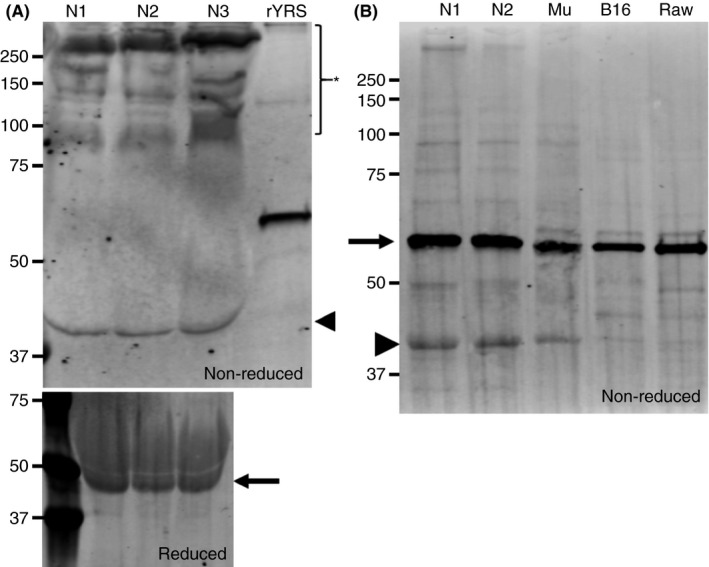
Western blot analysis of endogenous tyrosyl‐tRNA synthetase (YRS) in circulating plasma and platelets. (A) Plasma samples collected from healthy donors were analyzed by SDS‐PAGE under non reduced conditions and blotted with anti‐YRS monoclonal antibody (A‐10). Recombinant YRSWT was loaded as a positive control. The asterisk represents oligomerized YRS and the arrowhead points to the truncated form of YRS. In the lower panel, the same human plasma samples (N1‐N3) were analyzed by SDS‐PAGE under reduced conditions and blotted with the same antibody (A‐10). The arrow indicates full‐length YRS. (B) Platelet lysates obtained from healthy human donors and C57BL/6J mice were loaded onto SDS‐PAGE, together with whole cell lysates from B16 melanoma cell line and RAW cells. The arrow corresponds with full‐length YRS. The arrowhead represents the truncated form of YRS
3.2. YRS can be cleaved by elastase and matrix metalloproteinase in the plasma
YRS was previously shown to be cleaved by leukocyte elastase to generate a 41‐kDa N‐terminal fragment (residues 1‐366) similar to YRSMini (residues 1‐364) (Figure 2A). 4 , 6 In addition, MMPs have recently been shown to cleave YRS and generate a 43‐kDa N‐terminal fragment containing the chemotactic heptapeptide in the EMAPII domain (YRSMini+, residues 1‐386). 13 Based on these observations, we hypothesized that YRS secreted into plasma is cleaved by elastase and/or MMPs, and platelets incorporate both full‐length and cleaved forms of YRS from plasma and store them inside granules. To test possible cleavage by elastase and MMPs, a dual‐tagged YRSWT (dt‐YRS) was prepared with a FLAG‐tag at the N‐terminal end and a V5 tag at the C‐terminal end (Figure 2A, dt‐YRS). To determine the proteases responsible for the cleavage, dt‐YRS was incubated with plasma for 1 hour in the presence of specific inhibitors against MMP, elastase, or both. After incubation, samples were analyzed by Western blot using both N‐terminal (anti‐FLAG) and C‐terminal (anti‐V5) antibodies (Figure 2A, dt‐YRS). The intensity of remaining full‐length dt‐YRS was measured and shown as relative to the control sample in which dt‐YRS mixed with plasma was immediately mixed with sample buffer and boiled to prevent proteolysis. Both MMP inhibitor and elastase inhibitor prevented cleavage of dt‐YRS; the effect of the former was more robust, and the combination of the two inhibitors almost completely abolished cleavage (Figure 2B). This result suggests that full‐length YRS, when secreted into plasma, can be cleaved by MMP and elastase. 6 , 13
FIGURE 2.
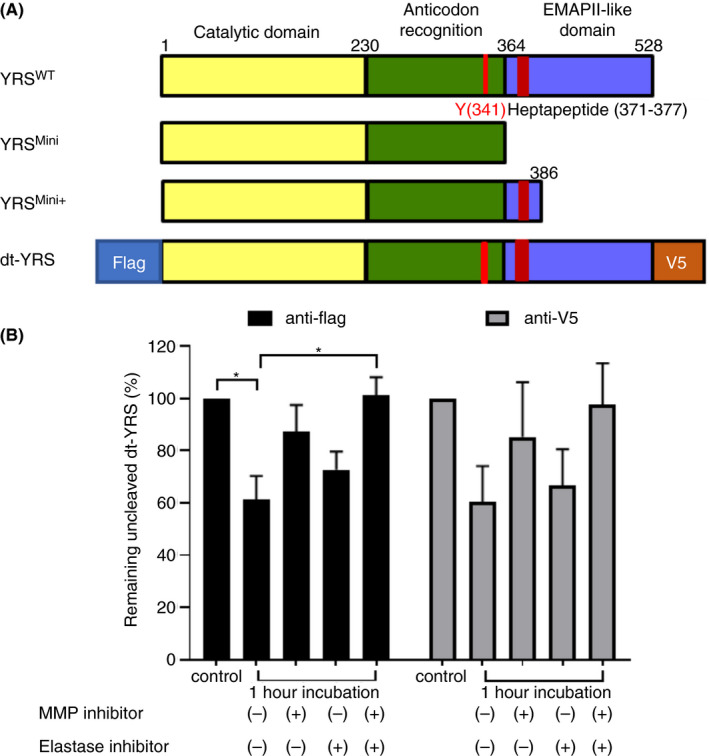
YRS can be cleaved by matrix metalloproteinase (MMP) and elastase present in plasma. (A) Schematic representation of YRS and its various forms. YRSMini and YRSMini+ are truncated, active forms. (B) Recombinant dual‐tagged YRS (dt‐YRS) was incubated in plasma with or without inhibitors against elastase, MMP, or both for 1 h. Samples were analyzed by Western blotting and the band intensity was quantified using LI‐COR ImageStudio. The intensity of recombinant dt‐YRS that was promptly denatured without incubation was used as a reference for normalization. The experiment was repeated 4 times using plasma derived from different donors, and the mean value with standard deviation is shown. *P < .05 by one‐way ANOVA with Dunn’s multiple comparison test
3.3. Recombinant YRS exogenously added to platelet rich plasma is taken up into platelets
Next, we analyzed whether exogenously added YRS can be taken up by platelets. Recombinant dt‐YRS was incubated with PRP overnight. After incubation, plasma and platelets were separated by centrifugation and analyzed by western blotting using anti‐FLAG antibody (Figure 3A). In the platelet fraction, both full‐length dtYRS (arrow) and an approximately 40‐kDa N‐terminal cleaved YRS fragment (arrowhead) were detected with anti‐FLAG tag antibody, confirming that platelet uptake of both full‐length and cleaved forms of YRS proteins occurs, and that YRS can be stored inside platelets. By comparing the signal intensity of full‐length dt‐YRS incubated in plasma in the presence of the inhibitors as a reference, we then calculated the percentage of exogenous full‐length dt‐YRS taken up by platelets and the percentage that remains in plasma. About half of the dt‐YRS added to PRP was cleaved and further degraded after overnight incubation. About 5% of exogenously added dt‐YRS was taken up into platelets and 50% of exogenous dt‐YRS remained in the plasma fraction (Figure 3B). Blotting with anti‐V5 antibody showed similar results.
FIGURE 3.
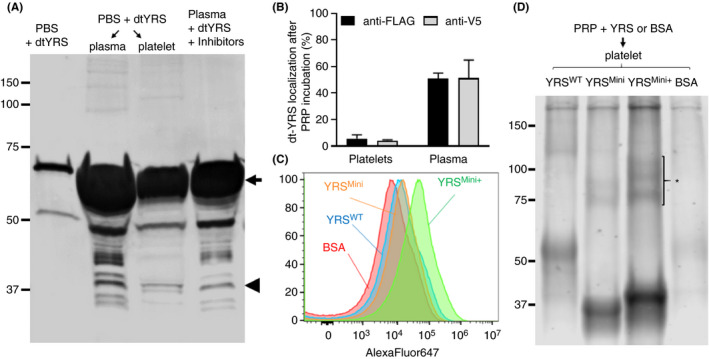
Recombinant tyrosyl‐tRNA synthetase (YRS) can be taken up by platelets when exogenously added to plasma. (A) Recombinant dual‐tagged YRS (dt‐YRS) was incubated in platelet rich plasma for 17 h. Platelets and plasma were separated by centrifugation as described in materials and methods. The samples were analyzed under reduced conditions, and the membrane was blotted with rat anti‐FLAG antibody. (B) The percentage of dt‐YRS taken up by platelets and dt‐YRS remaining in plasma was calculated by quantifying the signal using LI‐COR ImageStudio. The same amount of dt‐YRS incubated in plasma in the presence of inhibitors against elastase and matrix metalloproteinase (MMP) was used as reference. The results quantified from blotting with anti‐FLAG and anti‐V5 antibodies are shown. The experiment was repeated 3 times using plasma derived from different donors, and the mean value with standard deviation are shown. (C) Recombinant YRSWT, YRSMini, YRSMini+, and BSA labeled with AlexaFluor647 were incubated with platelet‐rich plasma (PRP) at a concentration of 500 nmol/L for 17 h. After the incubation, platelets were analyzed by flow cytometry. (D) After loading, platelets were lysed and loaded onto SDS‐PAGE under nonreduced conditions, transferred to polyvinylidene difluoride membrane, and AlexaFluor647 signals were detected with LI‐COR Odyssey imaging system. Oligomeric YRSMini+ is shown with an asterisk
To determine whether platelets can take up cleaved forms as well as full‐length forms of YRS from the plasma, recombinant YRSWT, YRSMini, YRSMini+ (see Figure 2A), and BSA labeled with AlexaFluor647 were incubated with PRP overnight. The uptake efficiency was evaluated by flow cytometry. All the recombinant YRS proteins were taken up into platelets more effectively than BSA (Figure 3C). Specifically, YRSMini+ was taken up by platelets 7.5 times higher than BSA as measured by geometric mean fluorescence intensity. After incubation with AlexaFluor647‐labeled recombinant proteins, platelets were lysed, loaded onto SDS‐PAGE, and transferred to PVDF membrane. Fluorescent signals were visualized using the Odyssey Infrared Fluorescent Image System. In agreement with flow cytometry results, YRSMini+ was more efficiently taken up by platelets, and a part of YRSMini+ was present as oligomerized form (Figure 3D, asterisk). These results support our hypothesis that the low‐molecular‐weight band of endogenous YRS identified in platelet lysates may be cleaved YRS fragments (eg YRSMini or YRSMini+) derived from plasma.
3.4. Platelets take up exogenously added dt‐YRS and store in α‐granules
Previous studies showed that platelets take up plasma proteins, such as fibrinogen and albumin, which are then stored in α‐granules to be subsequently released upon platelet activation. 14 , 15 Thus, we examined whether YRS taken up from circulating plasma is transported to and stored in platelet α‐granules. Fibrinogen and dt‐YRS labeled with different fluorochromes (AlexaFluor488 and AlexaFluor647, respectively) were incubated with PRP overnight. Confocal microscopy analysis showed partial colocalization of exogenously added dt‐YRS and fibrinogen in the α‐granules (Figure 4). To test the effect of exogenously added YRS on platelets, YRSMini+ was tested by platelet aggregation assay. Addition of ristocetin to platelet‐rich plasma induced expected aggregation, while YRSMini+ (500 nmol/L) did not induce any aggregation response (Figure S3A). The effect of YRSY341A on platelet activation was also tested by flow cytometry analysis of P‐selectin expression. As previously reported, treatment of platelets with Pam3CSK4 (a synthetic agonist of Toll‐like receptor [TLR] 2/TLR1) induced platelet P‐selectin expression. 16 Treatment with YRSY341A, on the other hand, did not promote P‐selectin expression (Figure S3B).
FIGURE 4.
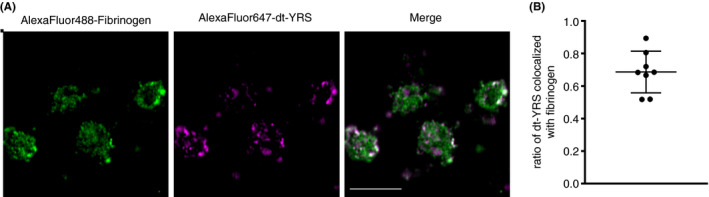
Tyrosyl‐tRNA synthetase (YRS) taken up by platelets is partially stored in the α‐granules. (A) AlexaFluor647‐labeled dt‐YRS and AlexaFluor488‐labeled fibrinogen were incubated with platelet‐rich plasma (PRP) for 17 h. After washing, platelets were resuspended and visualized using confocal microscopy (LSM 880; Carl Zeiss). Scale bar = 5 µm. (B) To evaluate the localization of dual‐tagged YRS (dt‐YRS) in the α‐granules, the colocalized area of dt‐YRS and fibrinogen was divided by the total area of dt‐YRS area using Fiji (n = 8)
Recent studies implicated interorganelle membrane contact and crosstalk between mitochondria and lysosomes. 17 Therefore, to examine localization of uptaken YRS in mitochondria, platelets preincubated with AlexaFluor488‐labeled YRSMini+ (500 nmol/L) were perfused over surfaces coated with immobilized recombinant human von Willebrand factor (VWF) A1 at a low shear rate of 100 s−1. Platelets adhered to VWF A1 were fixed, permeabilized, and immunostained with mitochondrial marker (Tom20). Confocal microscopy analysis showed that AlexaFluor488‐labeled YRSMini+ had little, if any colocalization with Tom20 (Figure S4). Taken together, these results indicate that active form of YRS proteins (YRSMini+ or YRSY341A) do not have direct stimulatory effect on platelets at the concentration that induce monocyte activation. YRS taken up by platelets are partially stored in α‐granules while maintaining functional integrity of platelets.
3.5. Functional implication of platelet‐stored YRS in monocyte activation
We have previously demonstrated that a constitutively active YRSY341A can stimulate megakaryopoiesis through activation of TLR2/4 of monocytic cells in the bone marrow. 7 Because recombinant YRSMini+ is effectively incorporated into platelets, we tested whether YRSMini+ induces monocyte activation. RAW‐Dual reporter cells are a mouse monocyte/macrophage cell line that stably expresses two reporter genes, SEAP and LUC, reflecting the activation of TLRs‐MyD88‐NF‐kB and type I interferon (IFN‐I) pathway, respectively. Treatment of RAW‐Dual cells with YRSMini+ significantly enhanced both signals (Figure 5A). To confirm that YRSMini+ taken up and released from platelets can induce signaling in monocytes, platelets pre‐loaded with YRSMini+ or BSA were washed and incubated with RAW‐Dual cells. Both SEAP and LUC signals were enhanced when platelets were preloaded with YRSMini+, compared to those incubated with BSA (Figure 5B). To further examine the thrombopoietic activity of YRSMini+, mouse bone marrow cells were cultured with YRSMini+ for 3 days, and MK number and ploidy were analyzed by flow cytometry. As expected, a cleaved, active form of YRS, YRSMini+ significantly enhanced MK expansion in a dose‐dependent manner (Figure S1A). Ploidy analysis showed enhanced MK maturation as evidenced by increase in the ploidies ≥ 16 N, which was similar to what we previously observed with a constitutively active YRSY341A (Figure S1B). 7 It is not clear yet whether the endogenous YRS protein contained in platelets is in the range of concentration to exert such biological activities when released. To estimate the amount of endogenous YRS contained in platelets, additional western blotting analysis was performed in a semiquantitative manner. Platelet lysates from healthy donors were loaded onto SDS‐PAGE with known concentration of purified recombinant YRS protein (Figure S2). The results of the signal intensities were back‐calculated, which estimated the concentration of platelet‐stored YRS in whole blood as 26.8 ± 10.5 nmol/L. Estimate made by western blotting may not accurately reflect the concentration of YRS in platelets. Nevertheless, this result indicates that a high level of YRS is stored in platelets and can be released upon stimulation.
FIGURE 5.
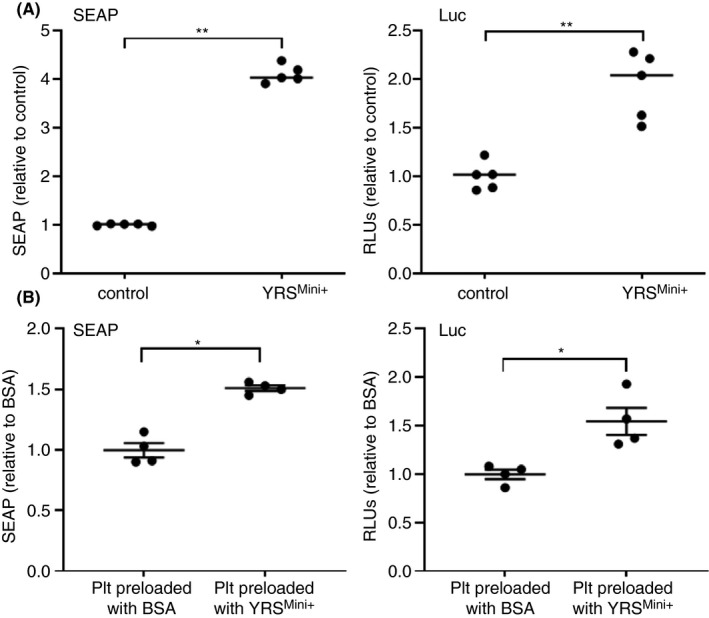
Tyrosyl‐tRNA synthetase (YRS)Mini+ activates monocyte via Toll‐like receptors (TLRs)–NF‐κB and type I interferon (IFN‐I) pathways. (A) RAW‐Dual reporter cells (InvivoGen) were incubated with 500 nmol/L of YRSMini+, and the activation of TLR‐NFκB pathway and IFN‐I pathway were measured by secreted embryonic alkaline phosphatase (SEAP) and Lucia luciferase (Luc), respectively. The mean and standard deviation of 5 experiments are shown. **P < .01 by unpaired t test. (B) Platelets were pre‐incubated with bovine serum albumin (BSA) or YRSMini+ (500 nmol/L) at 37˚C for 17 h. After washing, platelets (2 × 107 platelets) were added onto RAW‐Dual reporter cells seeded at 1 × 105 cells per well in a 96‐well plate, incubated for 21 h and assayed for the SEAP and Luc reporter activities. The experiment was repeated three times using platelets derived from different donors, and the representative result is shown. *P < .05 by unpaired t test
4. DISCUSSION
We report here, for the first time, a mechanism and potential function of YRS storage in platelets. We previously showed that YRSY341A targets TLR2/4 on monocytic cells and activates the TLR‐MyD88‐NFκB pathway, leading to cytokine secretion and MK expansion. 7 Our group and others showed that active forms of YRS can be naturally generated either by alternative splicing or proteolytic cleavage. 3 , 4 , 18 However, the physiological significance of endogenous, secreted YRS remains unclear. Another unresolved question is why the YRS protein is abundantly present in platelets while, in contrast, mRNA levels are extremely low. 8 In the current study, we showed that YRS, when secreted into plasma, is promptly cleaved and degraded by MMPs and elastase. Both full‐length and cleaved forms of YRS in the plasma can be taken up by platelets and transported to α‐granules, where they are protected from further degradation and stored for regulated release upon platelet activation. The exact cleavage site of the endogenous YRS fragment stored in platelets needs further study using mass spectrometry analysis.
Interestingly, a cleaved, activated form of YRS (YRSMini+) can be taken up by platelets more efficiently than the full‐length YRS (Figure 4A), consistent with a physiological function of active YRS stored in platelets. In support of this idea, YRSMini+ was shown to activate monocytes which play important roles in regulating inflammatory responses. Importantly, activated platelets form monocyte‐platelet aggregates (MPAs) and increases in the number of MPAs occur in patients with pathological conditions such as coronary artery diseases and microbial infections. 19 , 20 , 21 When activated, platelets form MPAs and undergo degranulation. We speculate that YRS stored in the α‐granules may be released and enhance activation of monocytes and release of cytokines. In addition, treatment of macrophages with YRS is reported to upregulate the expression of MMPs, which may enhance YRS cleavage and generate more activated YRS, and thus lead to a feed‐forward loop (Figure 6). 22 Regulatory mechanisms mediated by MMPs is a research focus in platelet biology, not only in the process of hemostasis but also in inflammation and immunity. 23 MMPs activity of monocytes may provide additional regulatory roles in platelet adheso‐signaling receptor density and function.
FIGURE 6.
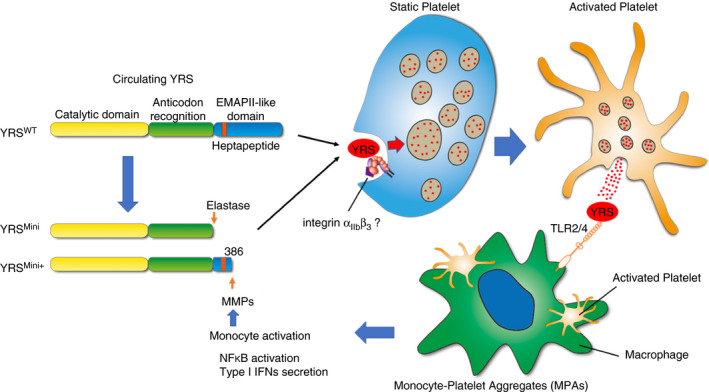
Schematic representation of tyrosyl‐tRNA synthetase (YRS) cleavage, storage in platelets, and potential function to activate monocytes. Circulating YRS can be cleaved by elastase or matrix metalloproteinases (MMPs) in plasma, producing a constitutively active form of YRS. YRSWT and its cleaved forms, YRSMini and YRSMini+, can be taken up by platelets, mediated by integrins or other receptors, and then partially stored in α‐granules where they are protected from further degradation. Upon activation, platelets may release the stored YRS, thereby activating monocytes/macrophages. Monocyte activation leads to MMP upregulation which may enhance YRS cleavage, producing more active YRS and feed‐forward loop
Monocytes produce both proinflammatory and anti‐inflammatory cytokines and, depending on the stimuli, can be differentiated into pro‐ or anti‐inflammatory macrophages. Thus, the overall effect of YRS released from platelets on the pathophysiology of inflammatory or thrombotic diseases remains to be elucidated. Platelets are reported to contribute to bacterial clearance and resolution of inflammation by regulating macrophage responses. 24 More recently, crosstalk between platelets and monocytes was shown to skew monocytes toward a proinflammatory phenotype, which is associated with increased survival in a septic model in mice. 25 It is intriguing to speculate that YRS released from platelets mediates the effect on monocyte/macrophage differentiation to strengthen host defense mechanisms. In addition, administration of YRSMini in a mouse model of myocardial infarction enhanced angiogenesis and tissue repair, resulting in improved cardiac function. 26 We previously showed that the active form of YRS can increase vascular endothelial growth factor A (VEGF‐A) expression, 7 raising the possibility that YRS released from platelets at sites of coronary artery occlusion and myocardial infarction may contribute to VEGF‐A secretion and enhanced angiogenesis. The amount of YRS stored in platelets of whole blood was estimated to be about 20 times lower than the doses we used in monocyte stimulation experiments (500 nmol/L). We speculate that regulated release of the granule content upon platelet activation enables high local concentration to exert biological activities. In an attempt to measure the level of YRS in the plasma, we set up an ELISA using anti‐YRS polyclonal antibodies generated in our laboratory. However, measurable amounts of YRS could not be detected with plasma samples derived from normal healthy donors, presumably due to low assay sensitivity (detection limit 0.16 ng/mL). Alternatively, recent studies have shown that aaRSs can be secreted via exosomes, and therefore we cannot exclude the possibility that YRS is present in such vesicles in plasma. 27 Interestingly, it has recently been reported that platelet‐derived extracellular vesicles (PEVs) infiltrate the bone marrow during inflammation and provide direct communication of plasma components with bone marrow cells. 28 It is possible that PEVs contain active form of endogenous YRS protein (eg, YRSMini+) and infiltrate the bone marrow, where it stimulates megakaryopoiesis under inflammatory stress conditions. Further studies using animal models will be needed to determine the functional consequence of YRS released from platelets in vivo.
We demonstrate here the capability of platelets to take in YRS from plasma. However, the exact mechanism by which YRS is taken up into platelets is not known. Platelets have been known to endocytose several plasma proteins, such as fibrinogen, IgG, and albumin, and subsequently translocate them into α‐granules. 14 , 15 , 29 Platelet‐trafficking of integrins (αIIbβ3, αVβ3) have also been characterized. 30 , 31 In our study, YRSMini+ is taken by platelets more efficiently than full‐length YRS and YRSMini (Figure 3C). YRSMini+ is a truncated form of YRS having the heptapeptide sequence (371RVGKIIT377) that is a critical component of the EMAPII domain and plays an important role in cytokine activities (Figure 6). 4 Structural analysis revealed that these residues are likely buried in full‐length YRS. 32 Interestingly, EMAPII is reported to be required for binding to integrin α5β1. 28 The heptapeptide sequence is not found as a common motif for Arg‐Gly‐Asp (RGD)‐independent integrin binding among proteins known to be endocytosed by platelets; however, having it exposed at the C‐terminus may facilitate YRSMini+ binding to the platelet membrane and allowing for endocytosis (Figure 6). This point also needs clarification through future studies.
YRS and tryptophanyl‐tRNA synthetase (WRS) are closely related, dual‐function enzymes that act in protein biosynthesis and angiogenesis. 33 Like YRS, WRS enhances macrophage activation by signaling through TLR2. 22 Interestingly, WRS can also be cleaved by MMPs. However, MMP‐cleaved WRS lacks the TLR signaling and pro‐inflammatory activities. 22 Thus, both YRS and WRS are cleaved by MMPs; the former is converted into an activated form while the latter loses its activity. Such opposite effects of YRS and WRS are also observed with vascular endothelial growth factor R2 (VEGFR2)‐mediated angiogenesis. YRSMini uses its ELR motif to bind to C‐X‐C motif chemokine receptor 1/2 and transactivate VEGFR2. 34 , 35 The active WRS, generated either by proteolytic cleavage or alternative splicing, binds to vascular endothelial cadherin and represses VEGFR2 signaling, leading to antiangiogenic activities. 18 , 36 , 37 , 38 During evolution, aaRSs have acquired a variety of additional domains, which are associated with new biological functions. 2 The EMAPII domain and the ELR motifs of YRS were added at the stage of insects and the WHEP domain of WRS appeared during vertebrate evolution. 2 , 33 It is intriguing to speculate that the YRS‐WRS system might regulate the hematovascular system by counterbalancing effects.
RELATIONSHIP DISCLOSURE
ZMR is founder, president, and CEO of MERU‐VasImmune, Inc. SK and TK have equity interest in MERU‐VasImmune, Inc, and JNO is a part‐time employee of the company. PS and XLY have a financial interest in aTyr Pharma. All other authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
EW performed experiments, analyzed data, and prepared the manuscript. YM, SK, RS, and MNV, performed experiments and analyzed data. JNO performed under shear platelet perfusion study. CDT contributed to the study design and revised the manuscript. XLY provided scientific advice and revised the manuscript. ZMR and PS supervised research and reviewed the manuscript. TK designed the study, performed experiments, analyzed data, and prepared the manuscript. The manuscript has been read and approved for submission by all authors.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants HL129011 (TK), HL135294 (ZM), GM125908 (PS), NS113583 (XLY), MERU Foundation (Italy), and by the National Foundation for Cancer Research.
Won E, Morodomi Y, Kanaji S, et al. Extracellular tyrosyl‐tRNA synthetase cleaved by plasma proteinases and stored in platelet α‐granules: Potential role in monocyte activation. Res Pract Thromb Haemost. 2020;4:1167–1177. 10.1002/rth2.12429
Handling Editor: Yotis Senis
REFERENCES
- 1. Guo M, Schimmel P. Essential nontranslational functions of tRNA synthetases. Nat Chem Biol. 2013;9:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo M, Yang XL, Schimmel P. New functions of aminoacyl‐tRNA synthetases beyond translation. Nat Rev Mol Cell Biol. 2010;11:668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wakasugi K, Schimmel P. Highly differentiated motifs responsible for two cytokine activities of a split human tRNA synthetase. J Biol Chem. 1999;274:23155–9. [DOI] [PubMed] [Google Scholar]
- 4. Wakasugi K, Schimmel P. Two distinct cytokines released from a human aminoacyl‐tRNA synthetase. Science. 1999;284:147–51. [DOI] [PubMed] [Google Scholar]
- 5. Vo MN, Yang XL, Schimmel P. Dissociating quaternary structure regulates cell‐signaling functions of a secreted human tRNA synthetase. J Biol Chem. 2011;286:11563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang XL, Kapoor M, Otero FJ, Slike BM, Tsuruta H, Frausto R, et al. Gain‐of‐function mutational activation of human tRNA synthetase procytokine. Chem Biol. 2007;14:1323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kanaji T, Vo MN, Kanaji S, Zarpellon A, Shapiro R, Morodomi Y, et al. Tyrosyl‐tRNA synthetase stimulates thrombopoietin‐independent hematopoiesis accelerating recovery from thrombocytopenia. Proc Natl Acad Sci U S A. 2018;115:E8228–E8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marcus K, Immler D, Sternberger J, Meyer HE. Identification of platelet proteins separated by two‐dimensional gel electrophoresis and analyzed by matrix assisted laser desorption/ionization‐time of flight‐mass spectrometry and detection of tyrosine‐phosphorylated proteins. Electrophoresis. 2000;21:2622–36. [DOI] [PubMed] [Google Scholar]
- 9. Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, et al. A draft map of the human proteome. Nature. 2014;509:575–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simon LM, Edelstein LC, Nagalla S, Woodley AB, Chen ES, Kong X, et al. Human platelet microRNA‐mRNA networks associated with age and gender revealed by integrated plateletomics. Blood. 2014;123:e37–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malakhov MP, Mattern MR, Malakhova OA, Drinker M, Weeks SD, Butt TR. SUMO fusions and SUMO‐specific protease for efficient expression and purification of proteins. J Struct Funct Genomics. 2004;5:75–86. [DOI] [PubMed] [Google Scholar]
- 12. Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochem Biophys Acta. 2008;1780:1273–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jobin PG, Solis N, Machado Y, Bell PA, Rai SK, Kwon NH, et al. Moonlighting matrix metalloproteinase substrates: enhancement of proinflammatory functions of extracellular tyrosyl‐tRNA synthetase upon cleavage. J Biol Chem. 2019;295(8):2186–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harrison P, Wilbourn B, Debili N, Vainchenker W, Breton‐Gorius J, Lawrie AS, et al. Uptake of plasma fibrinogen into the alpha granules of human megakaryocytes and platelets. J Clin Investig. 1989;84:1320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Handagama P, Scarborough RM, Shuman MA, Bainton DF. Endocytosis of fibrinogen into megakaryocyte and platelet alpha‐granules is mediated by alpha IIb beta 3 (glycoprotein IIb‐IIIa). Blood. 1993;82:135–8. [PubMed] [Google Scholar]
- 16. Blair P, Rex S, Vitseva O, Beaulieu L, Tanriverdi K, Chakrabarti S, et al. Stimulation of Toll‐like receptor 2 in human platelets induces a thromboinflammatory response through activation of phosphoinositide 3‐kinase. Circ Res. 2009;104:346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong YC, Kim S, Peng W, Krainc D. Regulation and function of mitochondria‐lysosome membrane contact sites in cellular homeostasis. Trends Cell Biol. 2019;29:500–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lo WS, Gardiner E, Xu Z, Lau CF, Wang F, Zhou JJ, et al. Human tRNA synthetase catalytic nulls with diverse functions. Science. 2014;345:328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shantsila E, Lip GY. The role of monocytes in thrombotic disorders. Insights from tissue factor, monocyte‐platelet aggregates and novel mechanisms. Thromb Haemost. 2009;102:916–24. [DOI] [PubMed] [Google Scholar]
- 20. Furman MI, Benoit SE, Barnard MR, Valeri CR, Borbone ML, Becker RC, et al. Increased platelet reactivity and circulating monocyte‐platelet aggregates in patients with stable coronary artery disease. J Am Coll Cardiol. 1998;31:352–8. [DOI] [PubMed] [Google Scholar]
- 21. Hottz ED, Medeiros‐de‐Moraes IM, Vieira‐de‐Abreu A, de Assis EF, Vals‐de‐Souza R, Castro‐Faria‐Neto HC, et al. Platelet activation and apoptosis modulate monocyte inflammatory responses in dengue. J Immunol. 2014;193:1864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jobin PG, Solis N, Machado Y, Bell PA, Kwon NH, Kim S, et al. Matrix metalloproteinases inactivate the proinflammatory functions of secreted moonlighting tryptophanyl‐tRNA synthetase. J Biol Chem. 2019;294:12866–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gardiner EE. Proteolytic processing of platelet receptors. Res Pract Thromb Haemost. 2018;2:240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ali RA, Wuescher LM, Dona KR, Worth RG. Platelets mediate host defense against Staphylococcus aureus through direct bactericidal activity and by enhancing macrophage activities. J Immunol. 2017;198:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carestia A, Mena HA, Olexen CM, Ortiz Wilczynski JM, Negrotto S, Errasti AE, et al. Platelets promote macrophage polarization toward pro‐inflammatory phenotype and increase survival of septic mice. Cell Rep. 2019;28(4):896–908.e5. [DOI] [PubMed] [Google Scholar]
- 26. McCormick ME, Rojas M, Moser‐Katz T, Tzima E, Reader JS. Natural aminoacyl tRNA synthetase fragment enhances cardiac function after myocardial infarction. PLoS One. 2014;9:e109325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim SB, Kim HR, Park MC, Cho S, Goughnour PC, Han D, et al. Caspase‐8 controls the secretion of inflammatory lysyl‐tRNA synthetase in exosomes from cancer cells. J Cell Biol. 2017;216:2201–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. French SL, Butov KR, Allaeys I, Canas J, Morad G, Davenport P, et al. Platelet‐derived extracellular vesicles infiltrate and modify the bone marrow during inflammation. Blood Adv. 2020;4:3011–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Handagama PJ, Shuman MA, Bainton DF. Incorporation of intravenously injected albumin, immunoglobulin G, and fibrinogen in guinea pig megakaryocyte granules. J Clin Investig. 1989;84:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Banerjee M, Joshi S, Zhang J, Moncman CL, Yadav S, Bouchard BA, et al. Cellubrevin/vesicle‐associated membrane protein‐3‐mediated endocytosis and trafficking regulate platelet functions. Blood. 2017;130:2872–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wencel‐Drake JD, Frelinger AL 3rd, Dieter MG, Lam SC. Arg‐Gly‐Asp‐dependent occupancy of GPIIb/IIIa by applaggin: evidence for internalization and cycling of a platelet integrin. Blood. 1993;81:62–9. [PubMed] [Google Scholar]
- 32. Yang X‐L, Liu J, Skene RJ, McRee DE, Schimmel P. Crystal structure of an EMAP‐II‐like cytokine released from a human tRNA synthetase. Helv Chim Acta. 2003;86:1246–57. [Google Scholar]
- 33. Yang XL, Schimmel P, Ewalt KL. Relationship of two human tRNA synthetases used in cell signaling. Trends Biochem Sci. 2004;29:250–6. [DOI] [PubMed] [Google Scholar]
- 34. Greenberg Y, King M, Kiosses WB, Ewalt K, Yang X, Schimmel P, et al. The novel fragment of tyrosyl tRNA synthetase, mini‐TyrRS, is secreted to induce an angiogenic response in endothelial cells. FASEB J. 2008;22:1597–605. [DOI] [PubMed] [Google Scholar]
- 35. Wakasugi K, Slike BM, Hood J, Ewalt KL, Cheresh DA, Schimmel P. Induction of angiogenesis by a fragment of human tyrosyl‐tRNA synthetase. J Biol Chem. 2002;277:20124–6. [DOI] [PubMed] [Google Scholar]
- 36. Wakasugi K, Slike BM, Hood J, Otani A, Ewalt KL, Friedlander M, et al. A human aminoacyl‐tRNA synthetase as a regulator of angiogenesis. Proc Natl Acad Sci U S A. 2002;99:173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tzima E, Reader JS, Irani‐Tehrani M, Ewalt KL, Schwartz MA, Schimmel P. VE‐cadherin links tRNA synthetase cytokine to anti‐angiogenic function. J Biol Chem. 2005;280:2405–8. [DOI] [PubMed] [Google Scholar]
- 38. Otani A, Slike BM, Dorrell MI, Hood J, Kinder K, Ewalt KL, et al. A fragment of human TrpRS as a potent antagonist of ocular angiogenesis. Proc Natl Acad Sci U S A. 2002;99:178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


