Abstract
Background
Second‐line treatment for immune thrombocytopenia (ITP) is not well reported for patients treated in real‐world clinical settings.
Objective
The purpose of this study was to compare outcomes of four second‐line treatments for ITP.
Patients/methods
Included adult patients had at least two medical records containing ITP diagnoses and second‐line eltrombopag, romiplostim, rituximab, or splenectomy. Date of treatment initiation or splenectomy was set as index date, between July 1, 2008, and March 31, 2017. Patients had first‐line corticosteroid or intravenous immune globulin treatment and continuous database activity from 6 months before to 12 months after index. Patient characteristics, treatment patterns, platelet counts, bleeding‐related episodes (BREs), and thrombotic events (TEs) were compared by second‐line treatment cohort.
Results
The sample included 3332 patients (mean age, 60.5 years; 52.3% female): eltrombopag (5.8%), romiplostim (9.9%), rituximab (73.3%), and splenectomy (11.0%). Patients having splenectomy were younger, more likely female and commercially insured, and less likely to require a third line of treatment than medical regimen cohorts. Proportions of patients having treatment‐free (≥180 days with no second‐line index or rescue agent) periods varied significantly (P = .01) by regimen: 33% for eltrombopag, 23% for romiplostim, 26% for rituximab, and 17% for splenectomy. All regimens significantly improved platelet counts, while TE and BRE rates differed significantly (P = .03 and P = .01, respectively) when all treatment groups were compared.
Conclusions
Over an average 7‐year follow‐up, all second‐line regimens improved platelet counts, but eltrombopag yielded the highest proportion of patients with completely treatment‐free periods of at least 180 days.
Keywords: eltrombopag, rituximab, romiplostim, splenectomy, thrombocytopenia
Essentials.
Comparative real‐world data on second‐line treatment of immune thrombocytopenia are scarce.
A total of 3332 adults had eltrombopag (6%), romiplostim (19%), rituximab (73%), or splenectomy (11%).
All regimens improved platelet count similarly, but bleeding and thrombotic event rates differed.
Patients discontinuing eltrombopag (33%) and romiplostim (23%) were treatment‐free for ≥6 months.
1. INTRODUCTION
Immune thrombocytopenia (ITP) involves the immune‐mediated destruction of platelets and suppression of platelet production, leading to an increased risk of bleeding events. Recent estimates from US health care claims databases (2010‐2016) found an annual incidence of ITP of 6.1 per 100 000 people. 1 New ITP diagnoses occur in nearly 20 000 children and adults each year in the United States, with collective medical care costs of more than $400 million within the first 12 months after diagnosis. 1
Corticosteroids and intravenous immune globulin (IVIG) are the most commonly used first‐line treatments; splenectomy, rituximab, and thrombopoietin receptor agonists (TPO‐RAs) are all acceptable second‐line options. 2 , 3 TPO‐RAs, including eltrombopag, romiplostim, and avatrombopag, now provide an alternative option among patients for whom second‐line therapy is indicated. 2 , 3 They have been shown to be effective and safe in randomized clinical trials, and to increase health‐related quality of life. 4 , 5
No direct comparison of second‐line treatment options has been conducted in the clinical trial setting. Comparative response rates, bleeding events, and treatment failure have been inferred only through single‐arm studies of individual treatments (mostly for splenectomy or rituximab) and randomized, placebo‐controlled trials of TPO‐RAs and rituximab. 6 Limited data are available comparing results with different TPO‐RAs, 7 although a cost‐consequence modeling comparison supported a preference for eltrombopag over romiplostim, driven by a reduction in severe bleeding events. 8 Grace et al 9 evaluated physician factors that determine choice of second‐line treatment. Their analysis demonstrated patient preference and physician perception of treatment attributes as most important in guiding treatment choice. However, real‐world second‐line treatment patterns and outcomes among adult patients treated in US clinical practice have not been published to date.
We undertook a retrospective study using electronic health records (EHR) among adult patients with ITP treated with second‐line therapies. Our primary objective was to examine treatment patterns with eltrombopag, romiplostim, rituximab, and splenectomy with a focus on platelet counts, bleeding‐related episodes, thrombotic events, and use of rescue medication.
2. METHODS
2.1. Study description and data source
This retrospective study used EHR data, including medical, pharmacy, and laboratory information obtained for the period July 1, 2008, to March 31, 2018. The data were obtained from the Optum Clinical Electronic Health Record Database, which aggregates extensive clinical treatment data from a network of >140 000 providers at more than 700 hospitals and 7000 clinics. The database currently has data for >90 million unique patients across the United States, with an average of 40 months of observed data per patient. Data are obtained without personal identifying information and used in compliance with the Health Insurance Portability and Accountability Act of 1996.
2.2. Patient sample: Inclusion and exclusion criteria
The first inclusion criterion was diagnosis of ITP: at least 2 medical records, dated ≥30 days apart, with diagnosis codes for ITP during the identification period (July 1, 2008 to March 31, 2017) were required. The diagnosis codes were International Classification of Diseases, Ninth Revision (ICD‐9), 287.3 primary thrombocytopenia, including 287.30 (primary thrombocytopenia, unspecified), 287.31 (immune thrombocytopenic purpura), 287.39 (other primary thrombocytopenia), 287.5 (thrombocytopenia, unspecified); or Tenth Revision (ICD‐10), D69 (primary thrombocytopenia), D69.3 (immune thrombocytopenic purpura), D69.49 (other primary thrombocytopenia), and D69.6 (thrombocytopenia, unspecified).
Additional inclusion criteria included a second line of treatment with eltrombopag, romiplostim, rituximab, or splenectomy (these define “second‐line index treatment or therapy”), between July 1, 2008, and March 31, 2017. The date of the first prescription order for a second‐line index medication or splenectomy was set as the “second‐line index date.” Medications were captured through Healthcare Common Procedure Coding System codes and National Drug Codes and splenectomy was captured through Current Procedural Terminology codes. Finally, patients were required to be at least 18 years of age during the year of the index date; to have continuous activity in the database for at least 6 months before the index date (fixed baseline period) and 12 months after the index date (Figure 1); and to have evidence of a first‐line therapy with corticosteroids, IVIG, or intravenous anti‐D immune globulin (anti‐D) before the second‐line index date. If there was no evidence of a first‐line agent before the initiation of the second‐line agent, the patient was excluded.
Figure 1.
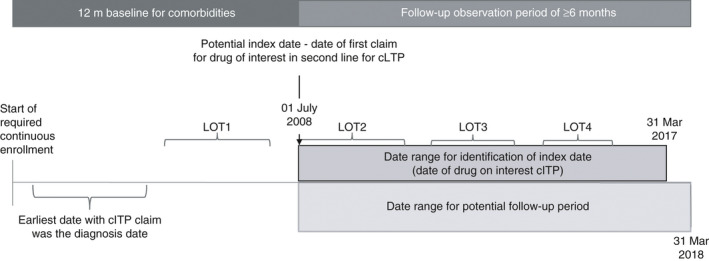
The second‐line of therapy (LOT2) index date was the date of the first prescription for medication (eltrombopag, rituximab, romiplostim) or splenectomy date, collectively referred to as LOT2 index therapy. Patient characteristics were described during the baseline period. Treatment patterns and outcomes were observed in the follow‐up period. ITP, immune thrombocytopenia; LOT, line of therapy
Patients were excluded for the following criteria: clinical trial enrollment, pregnancy, missing demographic data, or no available platelet counts during the baseline or follow‐up observation periods. Patients with records coded for secondary ITP (ICD‐9 287.41, posttransfusion purpura; 287.49, other secondary thrombocytopenia; or ICD‐10 D69.51, posttransfusion purpura; D69.59, other secondary thrombocytopenia) were also excluded.
2.3. Measures and outcomes
The following patient characteristics were obtained: age as of index date, sex, race, ethnicity, insurance type, and geographic region. 10 In addition, baseline clinical characteristics were obtained, including Quan‐Charlson comorbidity score. 11
Treatment patterns by regimen for each second‐line index therapy were obtained for the 12‐month postindex period by setting the regimen within the first 7 days of initiation of a second‐line treatment. This 7‐day period accounts for the minimum dosing interval for romiplostim. The treatment pattern measurements included duration of treatment with eltrombopag, romiplostim, or rituximab: treatment period ended by death, discontinuation of all agents in the regimen (90 days of no treatment after minimum 30‐day supply for oral agents; 1 day for injectable agent), start of a new regimen, or the end of the study period or EHR activity. In addition, the proportion of patients who started a third line of therapy with one of the index treatments of interest and the time between second and third lines of therapy were measured. Finally, the proportion of patients using a rescue medication (systemic corticosteroids, IVIG, and/or anti‐D) >30 days after the second‐line index regimen start date and time to initiation of those medications were measured.
The following clinical outcomes were measured for the 12 months after initiation of index therapy: (i) mean platelet count during each second‐line regimen, (ii) bleeding‐related episodes (BREs) requiring medical visits identified by ICD codes (see Appendix S1), and (iii) thrombotic events (TEs) including hemorrhagic stroke, transient ischemic attack, myocardial infarction, deep vein thrombosis, and pulmonary embolism identified by ICD codes (see Appendix S1).
2.4. Statistical analyses
Results were analyzed descriptively, stratified by treatment cohort (eltrombopag, romiplostim, rituximab, and splenectomy). Chi‐square and t tests were used to evaluate differences among the four treatment cohorts for most measures. A trend analysis was performed to identify significant changes observed in the proportions of patients receiving each treatment over the years during which the study was performed. For this, we tested for a linear trend with log odds using Wald chi‐square in a logistic regression with a linear cohort, comparing each year’s percentage within each treatment category.
3. RESULTS
3.1. Study sample and patient characteristics
After inclusion and exclusion criteria were applied, 3332 eligible patients were identified; these patients received the following index treatments: eltrombopag, n = 193 (5.8%); romiplostim, n = 329 (9.9%); rituximab, n = 2443 (73.3%); splenectomy, n = 367 (11.0%) (Figure 2). The analytic sample was focused upon the 3332 patients receiving eltrombopag, romiplostim, rituximab, or splenectomy.
Figure 2.
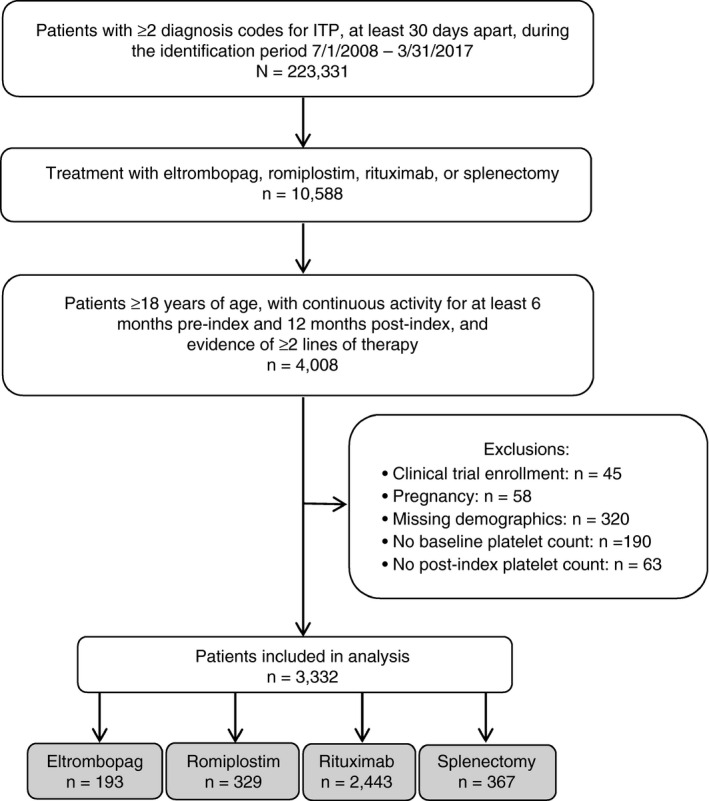
Patients were initially identified by diagnosis codes for immune thrombocytopenia (ITP), then by LOT2 regimens of interest (eltrombopag, rituximab, romiplostim) or splenectomy. Included patients also had requirements in age and continuous activity in the database, as well as evidence of at least a first line (LOT1) and second line (LOT2) of treatment. Patients were excluded by clinical trial enrollment, pregnancy, or missing demographic information or platelet count data
Demographic characteristics of eligible patients are summarized in Table 1. In the overall sample, the mean age was 60.5 years and 52.3% of patients were female. The majority of patients (83.0%) were White and insured either by a commercial plan (41.5%) or a Medicare plan (35.6%). Patients undergoing splenectomy were younger, more likely to be female, and more likely to have commercial insurance than those receiving medical therapy (P < .01). All patients received at least one prior therapy during the preindex period, with corticosteroids, IVIG, and anti‐D as monotherapy or combination therapy. Comorbidity scores differed across the cohorts. A greater percentage of patients in the eltrombopag and splenectomy cohorts had a Charlson Comorbidity Index score of 0 than in the romiplostim and rituximab cohorts.
Table 1.
Patient characteristics
| Variable | Total N = 3332 | Eltrombopag n = 193 | Romiplostim n = 329 | Rituximab n = 2443 | Splenectomy n = 367 | Overall P value | Eltrombopag vs Romiplostim P value | |
|---|---|---|---|---|---|---|---|---|
| Age at index, y, mean (SD) | 60.5 (17.3) | 63.4 (16.6) | 63.5 (16.8) | 60.7 (17.1) | 54.7 (18.1) | <.01 | .95 | |
| Female sex, n (%) | 1741 (52.3) | 106 (54.9) | 171 (52.0) | 1232 (50.4) | 232 (63.2) | <.01 | .52 | |
| Race, n (%) | White | 2764 (83.0) | 163 (84.5) | 271 (82.4) | 2020 (82.7) | 310 (84.5) | .58 | .76 |
| Black | 313 (9.4) | 13 (6.7) | 31 (9.4) | 244 (10.0) | 25 (6.8) | |||
| Asian | 47 (1.4) | 3 (1.6) | 5 (1.5) | 35 (1.4) | 4 (1.1) | |||
| Other/not available | 208 (6.2) | 14 (7.3) | 22 (6.7) | 144 (5.9) | 28 (7.6) | |||
| Ethnicity, n (%) | Non‐Hispanic | 3015 (90.5) | 173 (89.6) | 297 (90.3) | 2218 (90.8) | 327 (89.1) | .53 | .38 |
| Hispanic | 161 (4.8) | 8 (4.2) | 19 (5.8) | 110 (4.5) | 24 (6.5) | |||
| Other/unknown | 156 (4.7) | 12 (6.2) | 13 (4.0) | 115 (4.7) | 16 (4.4) | |||
| Insurance type, n (%) | Commercial | 1382 (41.5) | 75 (38.9) | 105 (31.9) | 1012 (41.4) | 190 (51.8) | <.01 | .07 |
| Medicare | 1185 (35.6) | 80 (41.5) | 126 (38.3) | 893 (36.6) | 86 (23.4) | |||
| Medicaid | 249 (7.5) | 14 (7.3) | 29 (8.8) | 178 (7.3) | 28 (7.6) | |||
| Uninsured | 77 (2.3) | 2 (1.0) | 14 (4.3) | 50 (201) | 11 (3.0) | |||
| Multiple types | 439 (13.2) | 22 (11.4) | 55 (16.7) | 310 (12.7) | 52 (14.2) | |||
| Geographic region, n (%) | Midwest | 1888 (56.7) | 108 (56.0) | 156 (47.4) | 1443 (59.1) | 181 (49.3) | <.01 | .11 |
| South | 825 (24.8) | 42 (21.8) | 103 (31.3) | 563 (23.1) | 117 (31.9) | |||
| Northeast | 248 (7.4) | 24 (12.4) | 41 (12.5) | 159 (6.5) | 24 (6.5) | |||
| West | 289 (8.7) | 12 (6.2) | 23 (7.0) | 218 (8.9) | 36 (9.8) | |||
| Other/unknown | 82 (2.5) | 7 (3.6) | 6 (1.8) | 60 (2.5) | 9 (2.5) | |||
| Baseline Charlson Comorbidity Index score, categorical, n (%) | 0 | 903 (27.1) | 87 (45.1) | 101 (30.7) | 562 (23.0) | 153 (41.7) | <.01 | <.01 |
| 1‐2 | 1277 (38.3) | 54 (28.0) | 111 (33.7) | 992 (40.6) | 120 (32.7) | <.01 | .17 | |
| 3‐4 | 653 (19.6) | 27 (14.0) | 54 (16.4) | 524 (21.5) | 48 (13.1) | <.01 | .46 | |
| 5+ | 499 (15.0) | 25 (13.0) | 63 (19.2) | 365 (14.9) | 46 (12.5) | .08 | .07 | |
3.2. Treatment patterns
As first‐line treatment observed in our study, 46.3% had a steroid monotherapy, 32.2% had multiple steroid types. The most common nonsteroid treatment was IVIG, at 20.3%; fewer than 1% of the patients had anti‐D or combinations of these three treatments.
Figure 3 shows the proportion of patients in the study who received each of the four second‐line index treatments over time. Rituximab remained the predominant index therapy during the entirety of the study. Over the 2009‐2017 study period, there was a statistically significant increase in the number of patients taking eltrombopag (trend P value <.01, Wald chi‐square using logistic regression to test for linear trend). No statistically significant trends were observed for the other types of treatment.
Figure 3.
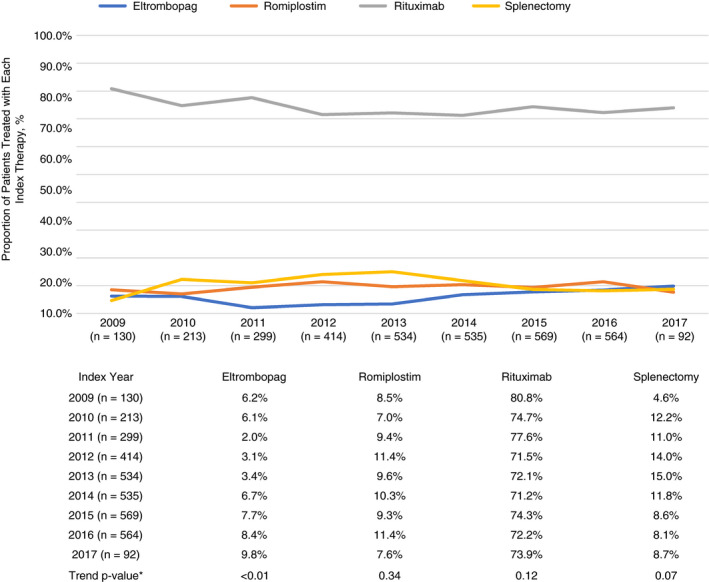
*Statistically significant trend within treatments, across years. Comparisons were not made between treatments. Wald chi‐square test in a logistic regression
Mean follow‐up time after initiation of the second‐line index therapy, during which patients remained active in the EHR data source, was >7 years. Mean treatment duration was similar for eltrombopag and romiplostim (227 days vs 198 days; P = .29; Table 2). Patients who underwent splenectomy were less likely to receive a third line of treatment, compared with those who received second‐line medical therapy (splenectomy, 20%; rituximab, 39%; eltrombopag, 47%; romiplostim, 44%; P < .01). The proportions who received a third line of treatment were similar between those treated with second‐line eltrombopag or romiplostim (P = .45).
Table 2.
Treatment patterns of LOT2 (index) treatments for immune thrombocytopenia
| Eltrombopag n = 193 | Romiplostim n = 329 | Rituximab n = 2,443 | Splenectomy n = 367 | Overall P value |
Eltrombopag vs Romiplostim P value |
|
|---|---|---|---|---|---|---|
| Follow‐up initiation of LOT2: Mean (SD) days | 2803 (791) | 2783 (751) | 2883 (729) | 2935 (677) | .02 | .78 |
| LOT2 index treatment duration: Mean (SD) days | 227 (252) | 198 (382) | 65 (131) | NA | <.01 | .29 |
| Proportion of patients who started a LOT3 after the LOT2 index treatment: n (%) | 91 (47.2) | 144 (43.8) | 941 (38.5) | 73 (20.0) | <.01 | .45 |
| Time from end of LOT2 index to LOT3: Mean (SD) days | 185 (254) | 161 (252) | 354 (417) | 311 (457) | <.01 | .48 |
| Patients with ≥ 1 period of 180 or more days without any LOT2 index regimen: n (%) | 92 (47.7) | 150 (45.6) | 1368 (56.0) | 263 (71.7) | <.01 | .65 |
| Proportion with ≥ 1 period of ≥ 180 days with no LOT2 or rescue agent (“completely treatment free”): n (%) a | 64 (33.2) | 75 (22.8) | 422 (17.3) | 96 (26.2) | <.01 | .01 |
| For rescue agent users, days to first use after LOT2: Mean (SD) | 90 (125) | 55 (96) | 41 (81) | 43 (88) | .01 | .14 |
Abbreviations: LOT2, second line of therapy (also referred to as LOT2 index therapy) among those studied (eltrombopag, romiplostim, rituximab, splenectomy); NA, not applicable.
Rescue agent, systemic corticosteroids, intravenous immune globulin, and/or anti‐D.
These patients discontinued LOT2 regimen and had no other treatment for remainder of follow‐up or moved on to LOT3 only after a period of at least 180 days' discontinuation. The rest of the original cohort stayed on their index regimen throughout their follow‐up period or switch to a new drug immediately or study period ended due to end of study, lack of evident medical care activity, or death.
Similar proportions (P = .65) of patients who received eltrombopag (48%) and romiplostim (46%) had at least one period of ≥180 days with no index therapy (eltrombopag, rituximab, romiplostim, splenectomy) after completing their second‐line treatment. A greater proportion of patients having second‐line eltrombopag (33%) also had no rescue agents (systemic corticosteroids, IVIG, and/or anti‐D) during this time, as compared with patients having second‐line romiplostim (23%; P = .01). Thus, patients who received eltrombopag were more likely to have a completely treatment‐free period of at least 180 days than those who received romiplostim.
3.3. Clinical outcomes
Mean platelet counts (×109/L) at the start of the index treatment were lower (P < .01) among patients who initiated eltrombopag (46) and romiplostim (42) compared with those who initiated rituximab (96) or underwent splenectomy (90) (Figure 4). All four index therapies led to a significant improvement in platelet count (P < .01 for all treatments). However, the mean platelet counts achieved during the 12 months following initiation of index treatment differed significantly across treatment cohorts, ranging from 101 × 109/L for eltrombopag to 251 × 109/L for splenectomy (P < .01) (Figure 4).
Figure 4.
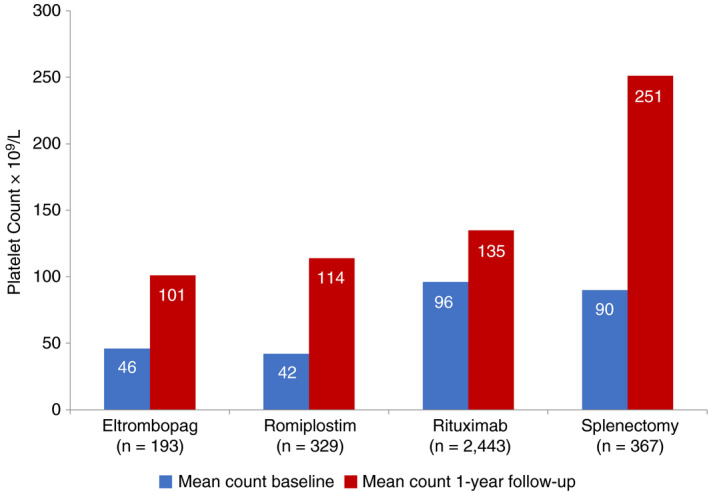
Platelet counts at baseline were collected within ± 14 days of initiation of the LOT2 index treatment; all available platelet counts were obtained for at least 1 year of follow‐up. Patients with no platelet count data in either period were excluded from the analytic sample. ITP, immune thrombocytopenia; LOT, line of therapy; SD, standard deviation. *Baseline and 1‐year mean counts differed significantly (P < .001) across all treatments. Platelet counts compared between eltrombopag and romiplostim were not significantly different, at baseline (P = .47) or follow‐up (P = .07).
The proportion of patients who experienced BREs in the 12‐month follow‐up period differed significantly across treatment cohorts, ranging from 27.5% for eltrombopag to 35.6% for romiplostim (P = .01) (Table 3). The most frequent BRE diagnostic codes included acute posthemorrhagic anemia, gastrointestinal hemorrhage, hematuria, hematemesis, and hemoptysis and epistaxis. Acute posthemorrhagic anemia was more common in the splenectomy cohort than in the other cohorts, possibly owing to surgical blood loss. Differing proportions of patients having TEs were observed across all treatment cohorts, ranging from 11.1% in the eltrombopag cohort to 18.1% in the splenectomy cohort (P = .03). Deep vein thrombosis (P = .03) and pulmonary embolism (P < .01) were more common in the splenectomy cohort, whereas rates of myocardial infarction, stroke, and transient ischemic attack were similar among the four treatment groups.
Table 3.
BREs and TEs among patients with a second‐line treatment for ITP
| Eltrombopag n = 193 | Romiplostim n = 329 | Rituximab n = 2,443 | Splenectomy n = 367 | Overall P value | Eltrombopag vs Romiplostim P value | |
|---|---|---|---|---|---|---|
| BREs identified by ICD code, n (%) | 53 (27.5) | 117 (35.6) | 678 (27.8) | 120 (32.7) | .01 | .06 |
| Most common BREs, n (%) | ||||||
| Acute post‐hemorrhagic anemia | 12 (6.2) | 26 (7.9) | 123 (5.0) | 54 (14.7) | <.01 | … |
| GI hemorrhage | 14 (7.3) | 30 (9.1) | 147 (6.0) | 29 (7.9) | .12 | … |
| Hematemesis | 10 (5.2) | 19 (5.8) | 105 (4.3) | 16 (4.3) | .64 | … |
| Hematuria | 11 (5.7) | 23 (7.0) | 148 (6.1) | 13 (3.5) | .21 | … |
| Hemoptysis and epistaxis | 7 (3.6) | 25 (7.6) | 102 (4.2) | 14 (3.8) | .03 | … |
| TEs identified by ICD code, n (%) | 19 (11.1) | 29 (10.7) | 274 (12.9) | 59 (18.1) | .03 | .88 |
| Most common TEs, n (%) | ||||||
| Deep vein thrombosis | 8 (4.4) | 24 (7.7) | 191 (8.3) | 40 (11.6) | .03 | … |
| Myocardial infarction | 6 (3.2) | 5 (1.6) | 66 (2.8) | 11 (3.0) | .60 | … |
| Pulmonary embolism | 9 (4.7) | 5 (1.6) | 78 (3.3) | 25 (7.0) | <.01 | … |
| Stroke | 6 (3.2) | 13 (4.2) | 56 (2.4) | 6 (1.7) | .17 | … |
| Transient ischemic attack | 2 (1.1) | 4 (1.2) | 29 (1.2) | 6 (1.7) | .90 | … |
Abbreviations: BREs, bleeding‐related episodes; ICD, International Classification of Diseases; ITP, immune thrombocytopenia; TEs, thrombotic events. International Classification of Diseases
4. DISCUSSION
Conventional first‐line treatments for ITP can provide a rapid platelet response, but the response is typically not lasting. Thus, second‐line treatments are needed to achieve more durable responses. TPO‐RAs have been developed as systemic treatments aimed at increasing production of platelets. Measures of successful treatment in the clinical trial setting include increase in platelet counts, reduced bleeding event rates, and reduced use of rescue medications, 6 as compared with placebo. TPO‐RAs have been demonstrated to be effective and safe, although no direct comparisons between eltrombopag and romiplostim or between TPO‐RAs and other second‐line treatment options have been performed to date. Systematic reviews and network meta‐analyses have suggested similar efficacy of eltrombopag and romiplostim and superior efficacy of both agents compared with rituximab. 12 , 13 In the current study, key outcomes were compared among patients receiving second‐line treatment with eltrombopag, romiplostim, rituximab, or splenectomy. In contrast to a clinical trial, a real‐world sample provides a more diverse population of patients treated in a routine practice setting.
Among this sample of 3332 patients, the most frequent (73.3%) second‐line treatment was rituximab, consistent with other small studies. 14 , 15 The observation period in the current study was nearly 9 years; thus, the proportions of patients receiving each treatment option could have varied across such a long study period. Such variance has been observed in an Italian retrospective study over 35 years. 16 However, a trend analysis showed significantly increased use only in eltrombopag over the years of the study; no other treatments showed statistically significant change in use. It is noteworthy that rituximab remained the predominant choice of index therapy throughout the study, even though studies suggest disappointing long‐term response rates with this agent. 17 , 18 , 19 This is an area for potential quality improvement in the care of patients with ITP.
In keeping with expectations, splenectomy offered the most durable response among the 4 treatments. Compared with rituximab, eltrombopag, and romiplostim, fewer patients undergoing splenectomy required third‐line treatment and more patients experienced a lengthy treatment‐free period. When comparing across all cohorts, treatment patterns differed significantly, yet in comparing the TPO‐RAs, the patterns were largely similar. Similar proportions of patients who received eltrombopag and romiplostim had at least one period of ≥180 days with no second‐line treatment. Among these patients, a significantly greater proportion of patients with eltrombopag also had no use of rescue agents (ie, corticosteroid, IVIG, anti‐D) during this time, as compared with patients with romiplostim (P = .01). These observations are interesting because TPO‐RAs are generally considered as indefinite therapy. However, the proportion of patients who were able to stop their TPO‐RA, and indeed be off all treatment for ≥6 months (33% for eltrombopag, 23% for romiplostim), suggests that for some patients, TPO‐RAs may not be needed indefinitely. These proportions are similar to what has been observed in smaller studies. 20 , 21 , 22 , 23
Significant differences were observed for platelet counts at the start of index treatment and mean values during the 12‐month follow‐up period for all cohorts. The lowest follow‐up platelet counts were observed for eltrombopag and romiplostim, likely because the prescribing information for these agents recommends use of the lowest dose sufficient to achieve and maintain a platelet count ≥50 × 109/L, whereas dose adjustment is not feasible for rituximab or splenectomy. 24 , 25 Lacking a head‐to‐head clinical trial, conclusive comparisons of treatment outcomes between second‐line therapies are not possible; however, small retrospective European studies 7 , 26 reported similar responses in terms of platelet counts.
Bleeding events and thrombotic events were identified by ICD code and compared across all treatment groups, and between TPO‐RAs. Significant differences in BREs were observed in statistical comparison across the four treatment groups (P = .01). Numerically higher rates of BREs occurred among patients receiving romiplostim as compared with eltrombopag during the follow‐up period, though the difference was not statistically significant (36% vs 28%; P = .06). This difference could reflect differences in protection from BREs offered by different treatments or it could be due to differences in baseline bleeding risk among different treatment cohorts. Specific types of BREs were observed in similar rates across groups, with the exception of acute posthemorrhagic anemia (P < .01 overall), which was most common in the splenectomy cohort, possibly due to the risk of surgical bleeding with splenectomy. The most common types of BREs observed (gastrointestinal hemorrhage, hematuria, and hematemesis) were similar to those reported in a retrospective study, as identified by claims. 27 TEs are also a complication of importance, which may be due to ITP therapy or to the thrombotic risk of ITP itself. TEs were also observed at different rates across groups overall (P = .03), with rates of deep vein thrombosis and pulmonary embolism greater in patients in the splenectomy cohort; yet between TPO‐RAs the differences were not significant. Similar findings were observed in two other studies, although the sample sizes were very small and no comparisons of individual types of TEs were available. 7 , 26
4.1. Limitations
In all analyses using EHR data, certain limitations should be considered when interpreting the findings including the possibility of coding errors. First, the ICD‐9 and ICD‐10 codes we used to identify patients with primary ITP have not been validated and ITP may be misdiagnosed in clinical practice. 28 , 29 Either misdiagnosis or inaccurate coding could lead to misclassification bias. Second, prescription orders are a proxy for, but do not confirm, accurate or complete administration or fills of medication. Third, we cannot determine whether differences in clinical outcomes among the treatment cohorts were due to the treatments themselves or to differences in baseline characteristics or baseline platelet values among the treatment cohorts. Further analyses with specific outcomes of interest could be conducted in future studies with larger sample sizes and adjustment for confounders to account for differences among the four groups.
5. CONCLUSIONS
Among a real‐world sample of patients with ITP treated with rituximab, splenectomy, eltrombopag, or romiplostim, rituximab was the predominant second‐line therapy. All four treatments were associated with significant increases in platelet count. Lengthy treatment‐free periods were observed in a substantial fraction of patients in the TPO‐RA cohorts and were more common after treatment with eltrombopag than romiplostim. Certain TEs and BREs including deep vein thrombosis, pulmonary embolism, and acute posthemorrhagic anemia were common in the splenectomy cohort.
RELATIONSHIP DISCLOSURE
LSL and KA are employees of Optum and report no other potential conflicts of interest. QS is an employee of Novartis Pharmaceuticals Corporation and reports stock ownership in NPC. AC has served as a consultant for Synergy. His institution receives research support on his behalf from Alexion, Bayer, Novo Nordisk, Pfizer, Sanofi, Spark, and Takeda. Support for this study was provided by Novartis Pharmaceuticals Corporation. Optum performed the work under contract; employment was not contingent upon this support. Portions of this work were presented at the 60th Annual ASH Meeting, San Diego, California, December 1‐4, 2018.
AUTHOR CONTRIBUTIONS
LSL contributed to concept and design, analysis/interpretation, and critical drafting and revising of intellectual content and final approval of the manuscript. QS contributed to design, interpretation, and critical revision and final approval of the manuscript. KA contributed to design; analysis, and interpretation and critical revision and final approval of the manuscript. AC contributed to concept and design, analysis/interpretation, and critical drafting and revising of intellectual content and final approval of the manuscript.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We gratefully acknowledge contributions of J. Anthony Graves posthumously. Caroline Jennermann, an employee of Optum, provided medical writing services, as contracted by Novartis Pharmaceuticals Corporation. We thank Brigette Nezami, Anuja Roy, and Isha Desai, as employees of Novartis Pharmaceuticals Corporation for their contributions to the design, analysis, and interpretation of the study.
Lal LS, Said Q, Andrade K, Cuker A. Second‐line treatments and outcomes for immune thrombocytopenia: A retrospective study with electronic health records. Res Pract Thromb Haemost. 2020;4:1131–1140. 10.1002/rth2.12423
Handling Editor: Dr Cihan Ay
REFERENCES
- 1. Weycker D, Hanau A, Hatfield M, Wu H, Sharma A, Bensink ME, et al. Primary immune thrombocytopenia in US clinical practice: incidence and healthcare burden in the first 12 months following diagnosis. J Med Econ. 2020;23(2):184–92. [DOI] [PubMed] [Google Scholar]
- 2. Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. American Society of Hematology. The American Society of Hematology 2011 evidence‐based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–207. [DOI] [PubMed] [Google Scholar]
- 3. Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raj AB. Immune thrombocytopenia: pathogenesis and treatment approaches. J Hematol Transfus. 2017;5(1):1056. [Google Scholar]
- 5. Khelif A, Saleh MN, Salama A, Portella MDSO, Duh MS, Ivanova J, et al. Changes in health‐related quality of life with long‐term eltrombopag treatment in adults with persistent/chronic immune thrombocytopenia: findings from the EXTEND study. Am J Hematol. 2019;94(2):200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bylsma LC, Fryzek JP, Cetin K, Callaghan F, Bezold C, Mehta B, et al. Systematic literature review of treatments used for adult immune thrombocytopenia in the second‐line setting. Am J Hematol. 2019;94(1):118–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mazza P, Minoia C, Melpignano A, Polimeno G, Cascavilla N, Di Renzo N, et al. The use of thrombopoietin‐receptor agonists (TPO‐RAs) in immune thrombocytopenia (ITP): a “real‐life” retrospective multicenter experience of the Rete Ematologica Pugliese (REP). Ann Hematol. 2016;95(2):239–44. [DOI] [PubMed] [Google Scholar]
- 8. Tremblay G, Dolph M, Bhor M, Said Q, Elliott B, Briggs A. Cost‐consequence model comparing eltrombopag versus romiplostim for adult patients with chronic immune thrombocytopenia. Clinico Econ Outcomes Res. 2018;1(10):705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grace RF, Despotovic JM, Bennett CM, Bussel JB, Neier M, Neunert C, et al. Physician decision making in selection of second‐line treatments in immune thrombocytopenia in children. Am J Hematol. 2018;93(7):882–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Department of Commerce . Census regions and divisions of the United States. At https://www2.census.gov/geo/pdfs/maps‐data/maps/reference/us_regdiv.pdf. Accessed 01 April 2020.
- 11. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82. [DOI] [PubMed] [Google Scholar]
- 12. Puavilai T, Thadanipon K, Rattanasiri S, Ingsathit A, McEvoy M, Attia J, et al. Treatment efficacy for adult persistent immune thrombocytopenia: a systematic review and network meta‐analysis. Br J Haematol. 2019;188(3):450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arai Y, Matsui H, Jo T, Kondo T, Takaori‐Kondo A. Comparison of treatments for persistent/chronic immune thromboycytopenia: a systematic review and network meta‐analysis. Platelets. 2019;30(8):946–56. [DOI] [PubMed] [Google Scholar]
- 14. Al Askar AS, Shaheen NA, Al Zahrani M, Al Otalibi MG, Al Qahtani BS, Amed F, et al. Splenectomy vs. rituximab as a second‐line therapy in immune thrombocytopenic purpura: a single center experience. Int J Hematol. 2018;107(1):69–74. [DOI] [PubMed] [Google Scholar]
- 15. Chater C, Terriou L, Duhamel A, Launay D, Chambon JP, Pruvot FR, et al. Reemergence of splenectomy for ITP second‐line treatment? Ann Surg. 2016;264(5):772–7. [DOI] [PubMed] [Google Scholar]
- 16. Palandri F, Polverelli N, Sollazzo D, Romano M, Catani L, Cavo M, et al. Have splenectomy rate and main outcomes of ITP changed after the introduction of new treatments? A monocentric study in the outpatient setting during 35 years. Am J Hematol. 2016;91:E267–E272. [DOI] [PubMed] [Google Scholar]
- 17. Ghanima W, Khelif A, Waage A, Michel M, Tjønnfjord GE, Romdhan NB, et al. Rituximab as second‐line treatment for adult immune thrombocytopenia (the RITP trial): a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385(9978):1653–61. [DOI] [PubMed] [Google Scholar]
- 18. Chugh S, Darvish‐Kazem S, Lim W, Crowther MA, Ghanima W, Wang G, et al. Rituximab plus standard of care for treatment of primary immune thrombocytopenia: a systematic review and meta‐analysis. Lancet Haematol. 2015;2(2):e75–e81. [DOI] [PubMed] [Google Scholar]
- 19. Patel VL, Mahévas M, Lee SY, Stasi R, Cunningham‐Rundles S, Godeau B, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood. 2012;119(25):5989–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Newland A, Godeau B, Priego V, Viallard JF, López Fernández MF, Orejudos A, et al. Remission and platelet responses with romiplostim in primary immune thrombocytopenia: final results from a phase 2 study. Br J Haematol. 2016;172(2):262–73. [DOI] [PubMed] [Google Scholar]
- 21. Leven E, Miller A, Boulad N, Haider A, Bussel JB. Successful discontinuation of eltrombopag treatment in patients with chronic ITP. Blood. 2012;120(21):1085. [Google Scholar]
- 22. Ghadaki B, Nazi I, Kelton JG, Arnold DM. Sustained remissions of immune thrombocytopenia associated with the use of thrombopoietin receptor agonists. Transfusion. 2013;53(11):2807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. González‐López TJ, Pascual C, Álvarez‐Román MT, Fernández‐Fuertes F, Sánchez‐González B, Caparrós I, et al. Successful discontinuation of eltrombopag after complete remission in patients with primary immune thrombocytopenia. Am J Hematol. 2015;90(3):E40–E43. [DOI] [PubMed] [Google Scholar]
- 24. Eltrombopag (Promacta®) prescribing information. Research Triangle Park, NC: GlaxoSmithKline (GSK group of companies). 2015. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207027s000lbl.pdf. Accessed 07 April 2020.
- 25. Romiplostim (Nplate™) prescribing information. Thousand Oaks, CA: Amgen Inc. 2008. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125268s0026lbl.pdf. Accessed 07 April 2020.
- 26. Mingot‐Castellano ME, Caparrós IS, Fernàndez F, Perera‐Alvarez MDM, Jiminez‐Barcenas R, Casaus Garcia A, et al. Treatment characteristics, efficacy and safety of thrombopoietin analogues in routine management of primary immune thrombocytopenia. Blood Coagul Fibrinolysis. 2018;29:374–80. [DOI] [PubMed] [Google Scholar]
- 27. Altomare I, Cetin K, Wetten S, Wasser JS. Rate of bleeding related episodes in adult patients with primary immune thrombocytopenia: a retrospective cohort study using a large administrative medical claims database in the US. Clin Epidemiol. 2016;20(8):231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Segal JB, Powe NR. Accuracy of identification of patients with immune thrombocytopenic purpura through administrative records: a data validation study. Am J Hematol. 2004;75(1):12–7. [DOI] [PubMed] [Google Scholar]
- 29. Arnold DM, Nazy I, Clare R, Jaffer AM, Aubie B, Li N, et al. Misdiagnosis of primary immune thrombocytopenia and frequency of bleeding: lessons from the McMaster ITP Registry. Blood Adv. 2017;1(25):2414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


