Abstract
Squid products are becoming more and more popular with consumers because of their high yields and nutrition, including novel textures with desirable sensory properties. However, it has not been determined whether the cooking method has effects on the flavor of the squid. In this study, the aroma and volatile substances of squid samples from different cooking methods (boiled, steamed, sous vide) were determined and analyzed by headspace–gas chromatography–ion mobility spectrometry and differentiated by using, as well, an electronic nose and sensory evaluation. A total of 43 characteristic flavor compounds were identified. Based on the signal intensity of the identified violate compounds, we established a fingerprint of heat‐treated squid from different cooking methods. Due to the long‐term low‐temperature heating conditions under vacuum, the flavor of sous vide squid is different from steamed and boiled squid, and it has unique special flavor compounds. Different cooking methods can affect the aroma of squid, providing support for the industrial production of squid.
Keywords: aroma, cooking method, GC‐IMS, sous vide, Squid, volatile components
Due to the long‐term low‐temperature heating conditions under vacuum, the flavor of sous vide squid is different from steamed and boiled squid, and it has unique special flavor compounds. Different cooking methods can affect the aroma of squid, providing support for the industrial production of squid.

1. INTRODUCTION
Illex Argentines, the Argentinian squid, is the most important cephalopod species and major component of commercial squid in the southwest Atlantic (Waluda, Rodhouse, Podestá, Trathan, & Pierce, 2001). The volume of cephalopods, including squid, cuttlefishes, and octopuses, harvested and consumed in the world during 2017 was 3,772,567 t (data from FISHSTAT, FAO). The edible portion of squid is as high as 80%, which is about 20% higher than that of fish in general. As a kind of invertebrate, the muscle protein of squid is slightly different from that of ordinary fish. The muscle protein of squid contains some paramyosin that has no ATPase activity to influence the protein stability, gel strength, and other properties of squid products (Mirzaei & Regnier, 2006), limiting the processing of squid. At present, research on the processing technology of squid can be divided into two categories. One is the optimization and improvement of traditional squid processing technology, such as squid rings (Tomac, Cova, Narvaiz, & Yeannes, 2017), dried squid (Dong, Zhu, Li, & Li, 2013), and frozen squid products (Gou, Lee, & Ahn, 2010). The other is the comprehensive processing and utilization of squid by‐products or minced meat leftovers to extract biologically active peptides (Alemán, Giménez, Pérez‐Santin, Gómez‐Guillén, & Montero, 2011; Alemán, Gómez‐Guillén, & Montero, 2013) or squid flavor condiments (Lyberg & Adlercreutz, 2008).
Sous vide cooking (SV) is a particular cooking method in which the raw materials are placed in a vacuum bag and placed in water with precise temperature (55–90°C) control heating for a long time (Schellekens, 1996). SV differs from traditional cooking methods, where vacuum packaging prevents food oxidation, reduces flavor and moisture loss during cooking, prevents the growth of aerobic bacteria, and improves shelf life (Baldwin, 2012). Additionally, precise temperature controlling can maintain the color of foods. Christensen et al. (Christensen et al., 2013) found that low‐temperature and long‐time (LTLT) heating can reduce the strength of connective tissue and increase the solubility of collagen, which makes beef as tender as veal. Roldán, Antequera, Martin, Mayoral, and Ruiz (2013) found the lamb loin tenderness positively correlated with cooking time (6, 12 hr, and 24 hr). However, as the cooking temperature increased, the weight loss of lamb loin increased, and the moisture content decreased. SV can improve lamb loin brightness and redness, as well as ensure biosafety at 60°C. Moreover, beef cooked by SV (59°C, 5 hr) did not exhibit an increase in thiobarbituric acid reactive substances (TBARS) and warmed‐over flavor (WOF) after 30 d of storage (Hansen, Knøchel, Juncher, & Bertelsen, 1995). Many scholars have also evaluated the safety of SV. After cooking at 50–62°C, the number of mesophiles and psychrotrophic bacteria in beef decreased significantly (Botinestean, Keenan, Kerry, & Hamill, 2016; Hansen et al., 1995; Vaudagna et al., 2002). Moreover, the number of Escherichia coli and mesophiles in pork cooked at 53°C for several hours also decreased significantly (Becker, Boulaaba, Pingen, Röhner, & Klein, 2015; Salaseviciene, Vaiciulyte‐Funk, & Koscelkovskienė, 2014). To reduce the nutrient loss while optimizing overall quality and shelf life of meat during cooking, lots of food processors use SV technique to replace traditional cooking methods, such as frying, microwaving, and grilling (del Pulgar, Gazquez, & Ruiz‐Carrascal, 2012; Roldan, Antequera, Perez‐Palacios, & Ruiz, 2014). Thus far, scholars have only focused on SV cooking technology for the protection of food nutrients, quality improvement, and food safety. In addition, they think that the lower heating temperature of SV produces less flavor (Calkins & Hodgen, 2007; Cross, Stanfield, & Koch, 1976). Therefore, there are fewer studies on the flavor of SV. The flavor characteristics of squid by different cooking methods have yet to be reported.
Ion mobility spectrometry (IMS) is a technology that analyzes trace chemical substances based on differences in the migration speed of different ions in the gas field in an electric field. IMS is a chemical substance analysis technology developed in the late 1960s and early 1970s (Eiceman, Karpas, & Hill, 2013). The most prominent advantages of IMS are that it can ionize analytes at atmospheric pressure, and the detection limit is as low as ng/L (Armenta, Alcala, & Blanco, 2011). IMS has been applied to the detection of drugs (Verkouteren & Staymates, 2011), chemical warfare agents (Rearden & Harrington, 2005), and explosives (Asbury, Klasmeier, & Hill, 2000). With the development and improvement of IMS technology, it has gradually been applied in food inspection, such as food adulteration (Garrido‐Delgado, Muñoz‐Pérez, & Arce, 2018), determination of food quality (Ivanov, Bilgucu, Ivanova, & Dimitrova, 2020; Snyder, Harden, Davis, Shoff, & Maswadeh, 1995), food process control (Karpas, Guamán, Calvo, Pardo, & Marco, 2012), and chemical food safety (Gloess, Yeretzian, Knochenmuss, & Groessl, 2018).
Therefore, the main objective of the present study was to analyze by GS‐IMS the volatile components of squid and differentiate the samples based on their volatile compound profile, using, as well, an electronic nose and sensory evaluation. Through principal component analysis, the establishment of fingerprints, and heat map analysis, the differences and correlations of volatile flavor substances in squid from different cooking methods were explored. The results provide a foundation for the replacement of traditional cooking methods by SV technology.
2. MATERIALS AND METHODS
2.1. Sample preparation
The Argentinian squid (approximate net weight 400 g) was purchased from an aquatic products market in Qingdao city, Shandong Province of China. The squid specimens were kept refrigerated with flake ice inside polystyrene boxes provided with a lid and holes for drainage. Samples were transported to the laboratory at −18°C. Prior to cooking, squid specimens were separated into the head, foot (wrist), and ketone body with scissors after thawing and washing. The average weight of the ketone body of squid was 20 ± 4 g (n = 16). Each squid specimen length, width, and thickness of the ketone body were 4 ± 1 cm, 4 ± 1 cm, and 1 ± 0.4 mm, respectively. All squid were randomly divided into four groups to prepare for processing and kept at 4°C until further analysis. From each specimen, one specimen remained raw (RAW) as a control, and the others were subjected to cooking methods.
2.2. Cooking methods
Different cooking methods were selected as the preparation procedures for squid samples in this study: boiling (BO), steaming (ST), and sous vide (SV).
2.2.1. BO method
The squid samples were placed in a stainless steel pot of boiling water (2 L) for 5 min. After cooking, the samples were removed and drained on absorbent papers.
2.2.2. ST method
The squid samples were placed in a steam oven (SCC WE 61, Germany Rational) at 100°C for 5 min. After cooking, the samples were removed and drained on absorbent papers.
2.2.3. SV method
The samples were put into a plastic vacuum bag (nylon/polyethylene pouches, operating temperature of −20°C/121°C) and sealed using a vacuum sealer (DZ‐260, Dajiang Holding Group Electric Co. LTD, China). The samples then were cooked in temperature‐controlled water bath (60°C) for 30 min (Cui, Dubova, & Mo, 2019).
All the samples were soaked in ice water after cooking for 30 min and stored at −20°C until analysis, including the control group.
2.3. Sensory evaluation
The sensory evaluation team consisted of 20 people, between 18 and 25 years old, all of whom had undergone sensory evaluation training. A hedonic scale method from 1 to 9 points was adopted, where 1 means extremely disliked and 9 means extremely liked.
2.4. Electronic nose analysis
The electronic nose (PEN 3, Germany AirSense) was used to preliminarily evaluate the aroma profile of the squid samples. It was conducted by the following procedure. Taking the center of the squid sample to be tested, each sample (1 g) was prepared in a 20 ml gas chromatographic analysis bottle at room temperature for 30 min. The injection rate was 600 ml/min; the carrier gas flow rate was 600 ml/min; and the measurement time was 60 s. Thereafter, the sensors were purged by clean dry air for 180 s. The parameters were optimized, and each analysis was repeated 3 times.
The types of sensitive substances corresponding to the ten sensors of the electronic nose are as follows: W1C: aromatic hydrocarbon compounds; W5S: nitrogen oxide compounds; W3C: ammonia, aromatic molecules; W6S: hydride; W5C: olefins, aromatic, polar molecules; W1S: alkanes; W1W: sulfur compounds; W2S: alcohols, partially aromatic compounds; W2W: aromatic compounds, sulfur organic compounds; and W3S: alkanes and fats.
2.5. Determination of volatile components by HS‐GC‐IMS
2.5.1. Isolation of volatile organic compounds in squid
Volatile compounds of squid from different cooking methods were identified by a GC‐IMS flavor analyzer (FlavourSpec®, Dortmund, Germany). Each squid sample (2 g) was placed in a 20 ml headspace vial and sealed. Samples were subsequently incubated at 60°C for 15 min. Finally, 500 μL headspace was injected automatically via a heated syringe (65°C) into the heated injector of the GC‐IMS equipment under conditions reported below. Three parallel samples were analyzed from the same processing method.
2.5.2. Chromatographic conditions
The gas chromatographic separation was performed at 60°C on a FS‐SE‐54‐CB‐1 capillary chromatographic column (15 m × 0.53 mm, 1 μm). High‐purity nitrogen (99.99%) was as a carrier gas with a flow rate of 150 ml/min and a programmed flow as follows: 2 ml/min for 2 min; linear increase to 100 ml/min over 18 min; and total run time of 20 min. To avoid cross‐contamination, the syringe automatically flushed for 30 s with nitrogen gas before each analysis and 5 min after each analysis.
The n‐ketones C4–C9 (Sinopharm Chemical Reagent Beijing Co., Ltd, China) were employed as external references to calculate the retention index (RI) of each volatile compound. By comparing the RI and drift time (DT) with the GC × IMS Library Search (FlavourSpec®, Dortmund, Germany), the volatile compounds in squid samples from different cooking methods were identified. The signal intensity represents the height or the peak area. Using laboratory analytical viewer, reporter, gallery plot, and GC × IMS Library Search database supported by HS‐GC‐IMS instrument, three‐dimensional (3D) and two‐dimensional (2D) fingerprint maps of the volatile organic components of squid samples were constructed.
2.6. Data analysis
The sensory data analysis was conducted using SPSS 13.0 software (IBM). Principal component analysis (PCA) of electronic nose was carried out with Win Muster software. Volatile organic components (VOCs) were analyzed by laboratory analytical viewer (LAV) and GC × IMS Library Search (FlavourSpec®).
3. RESULTS
3.1. Sensory evaluation
Sensory analysis of squid samples from different cooking methods was performed. Different cooking methods have different sensory evaluations of squid (Figure 1). The squid cooked by ST and BO contracted and curled to varying degrees, while the squid cooked by SV contracted less and did not curl. There was no difference in the color of the squid processed by the three cooking methods. In terms of texture, the SV sample was excellent and the softest. In terms of aroma and flavor, ST and BO were very outstanding, with a strong aroma of cooked squid. The SV samples had the best score in appearance, texture, and preference, while the ST samples performed best in terms of aroma and flavor. The comprehensive sensory score of SV samples was the best because of their tender texture and smoother appearance. However, the aroma and flavor of the SV samples were obviously insufficient and different from ST and BO. Further research is needed.
Figure 1.
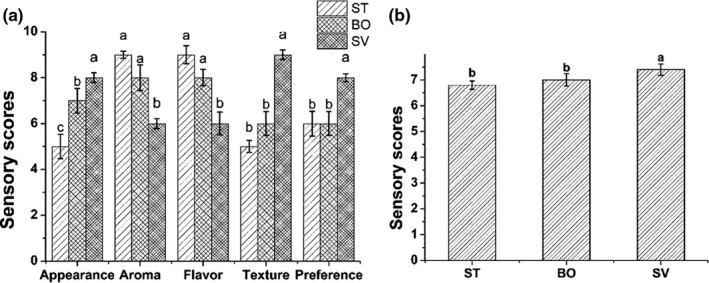
Sensory scores of squid in different cooking methods. a, Sensory scores of appearance, aroma, flavor, texture, and preference of squid prepared from different cooking methods. b, Comprehensive sensory score of squid from different cooking methods. BO: boiled squid; ST: steamed squid; SV: sous vide cooking squid
3.2. Analysis of flavor substances of squid via electronic nose
Compared with traditional sensory analysis, electronic nose is simple, fast, objective, and intuitive. According to the different response values of the electronic nose sensor to the aroma components of squid in different cooking methods, an intuitive radar chart was established, as shown in Figure 2a. The radar chart analysis method is mainly employed to study the sensors. Using this method, the contribution rate of each sensor to the squid sample can be distinguished, so as to investigate which type of volatile components play predominant roles in distinguishing the sample. From Figure 2a, the difference in volatile flavor compounds from different squid samples was mainly in the sensors W2W, W5S, W1W, and W1C, which are aromatic compounds, sulfur organic compounds, and nitrogen oxide compounds. It can be seen from the radar chart that the flavor of squid is greatly influenced by aromatic compounds. Conversely, the BO and ST squid samples had more obvious responses at W1W and W2W, which can be contributed to sulfide. Specific volatile components need further verification.
Figure 2.
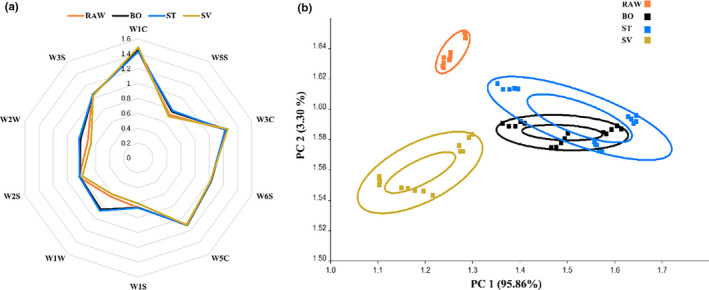
Electronic nose analysis. a, Radar charts of volatile compounds in squid from different cooking methods. b, Analysis of the predominant volatile compounds in squid samples. RAW: raw squid; BO: boiled squid; ST: steamed squid; SV: sous vide cooking squid
In order to highlight the aroma differences in squid from different cooking methods, a PCA scatter diagram (Figure 2b) was generated according to electronic nose data of the overall flavor substance composition. PCA is a multivariate statistical analysis technique that examines the correlation between multiple variables and reveals the internal structure between multiple variables through a few principal components (Jo et al., 2013). Generally, the PCA model is selected as the separation model when the cumulative contribution rate reaches 60% (Wu et al., 2015). PCA of aromatic substances from different heat‐treated squids (5 detection signals from 48s to 52s) was performed, and the result shows that the first principal component contribution rate was 95.86%; the second principal component contribution rate was 3.30%; and the cumulative contribution rate was 99.16%. This indicates that the two principal components can adequately represent predominant characteristics of the sample. Each sample in the group was relatively concentrated in a specific range and has a precise distance from the clustered areas of other groups. RAW samples and SV samples were clustered separately (the farthest distance in the PCA chart), while BO and ST were clustered together. This suggests that these methods have a high degree of similarity. Moreover, the results indicate that different cooking methods lead to differences in squid aroma.
3.3. Differences in VOCs in squid samples
A series of physical and chemical changes occur after meat is heated, which significantly affects the quality of the processing (Shahidi, Rubin, D'Souza, Teranishi, & Buttery, 1986). HS‐GC‐IMS analyzed the differences between volatile compounds in heat‐treated squid samples. Figure 3a shows the 3D topographic plot spectrum of volatile flavor compounds. From Figure 3a, the VOCs of squid from different cooking methods are very similar, but the signal intensities are slightly different. After heat treatment, the content of most flavor compounds changes to varying degrees.
Figure 3.
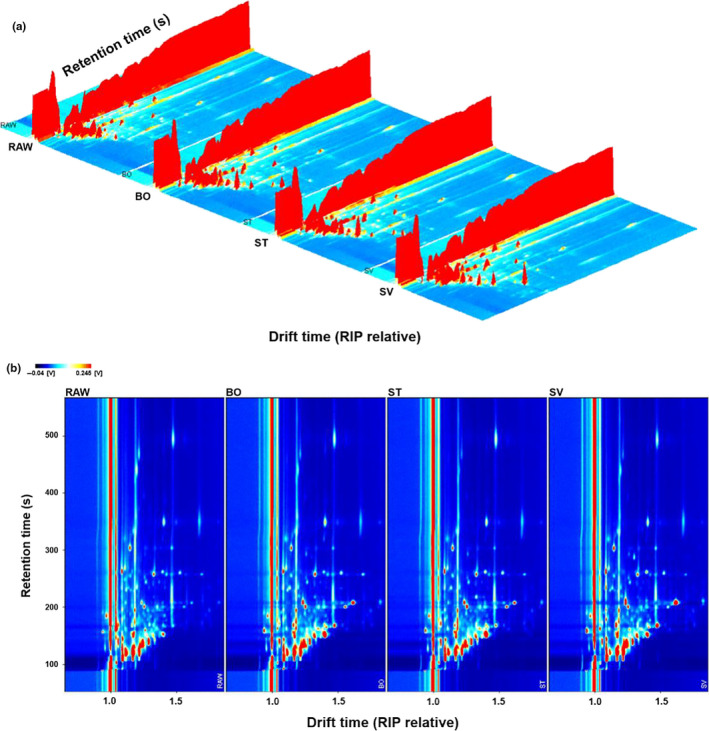
Gas chromatography–ion mobility spectrometry of squid from different cooking methods. a, Three‐dimensional topographic plots and chromatograms of squid samples. The y‐axis represents the retention time of the gas chromatograph; the x‐axis represents the ion migration time for identification, and the z‐axis represents the peak height for quantification. b, Two‐dimensional topographic plots and chromatograms of squid samples. RAW: raw squid; BO: boiled squid; ST: steamed squid; SV: sous vide cooking squid. The ordinate represents the retention time, and the abscissa represents the migration time. The red vertical line represents the reaction ion peak (RIP), and the migration time after normalization was 8.0 ms. Each point on the right of RIP represents a volatile substance. Color represents the signal intensity of the substance. White indicates low intensity, and red indicates high intensity. The darker the color, the greater the intensity
Although the differences in volatile flavor compounds can be visualized in the 3D spectrum, a 2D plot spectrum is more evident. Figure 3a shows a top view of the 3D GC‐IMS spectrum of Figure 3b projected onto a 2D plot, which can directly compare the differences in flavor substance for various heat‐treated squid. However, a volatile compound may produce one or more bright spots, representing monomers or dimers and trimers, depending on the concentration of volatile compounds. The entire spectrum represents all components found in the headspace sample. Most of the signals in the topographic plot of squid samples from different cooking methods appeared between 100 and 200 s, while in SV samples the signals were distributed between 100 s and 300 s. Moreover, the signal intensity was stronger than that the other heat treatments.
3.4. Identification of VOCs in squid from different cooking methods
Figure 3 shows the differences in VOCs in squid prepared from different cooking methods intuitively, but it is difficult to accurately determine the specific substance in the 2D and 3D spectrum maps. Through GC‐IMS separation, the differences in volatile substances in the squid samples are elucidated. According to the retention time and ion migration time of volatile substances in gas chromatography, 99 volatile substances were detected, and 43 specific volatile substances were determined (Table 1). Due to different concentrations of VOCs, certain compounds might produce multiple signals or spots (dimers or trimers).
Table 1.
Identified compounds in squid prepared from different cooking methods
| No. | Compound | CAS | Molecule Formula | MW | RI a | Rt b | Dt c | Comment |
|---|---|---|---|---|---|---|---|---|
| 1 | 1C | unidentified | * | 0 | 370.2 | 89.741 | 0.9376 | |
| 2 | Ethanol | C64175 | C2H6O | 46.1 | 433.3 | 103.409 | 1.0443 | |
| 3 | 2C | unidentified | * | 0 | 384.3 | 92.786 | 1.0901 | |
| 4 | Acetone | C67641 | C3H6O | 58.1 | 519.7 | 122.144 | 1.1168 | |
| 5 | 2‐butanone | C78933 | C4H8O | 72.1 | 604.1 | 140.442 | 1.0581 | mono |
| 6 | Isopropyl alcohol | C67630 | C3H8O | 60.1 | 514 | 120.912 | 1.1701 | mono |
| 7 | 3C | unidentified | * | 0 | 548.4 | 128.353 | 0.9589 | |
| 8 | 4C | unidentified | * | 0 | 682.4 | 159.787 | 0.9414 | |
| 9 | 5C | unidentified | * | 0 | 629.6 | 146.145 | 1.0412 | |
| 10 | Acetic acid ethyl ester | unidentified | * | 0 | 622.6 | 144.529 | 1.092 | mono |
| 11 | 6C | unidentified | * | 0 | 753.9 | 185.645 | 0.968 | |
| 12 | 1‐propene−3‐methylthio | C10152768 | C4H8S | 88.2 | 694.8 | 163.592 | 1.0421 | |
| 13 | 7C | unidentified | * | 0 | 739.1 | 179.623 | 1.0888 | |
| 14 | 3‐hydroxybutan−2‐one | C513860 | C4H8O2 | 88.1 | 720.4 | 172.421 | 1.0573 | mono |
| 15 | 8C | unidentified | * | 0 | 778.2 | 196.065 | 1.0896 | |
| 16 | 9C | unidentified | * | 0 | 766.6 | 191.051 | 0.94 | |
| 17 | 10C | unidentified | * | 0 | 775.8 | 195.031 | 1.1854 | |
| 18 | 11C | unidentified | * | 0 | 806.3 | 208.671 | 1.1153 | |
| 19 | Prop−1‐ene−3,3'‐thiobis | C592881 | C6H10S | 114.2 | 853.3 | 231.52 | 1.119 | |
| 20 | 12C | unidentified | * | 0 | 859.2 | 234.571 | 1.0879 | |
| 21 | 3‐methylthiopropanal | C3268493 | C4H8OS | 104.2 | 909.9 | 264.103 | 1.0867 | mono |
| 22 | Cyclohexen−2‐one | C930687 | C6H8O | 96.1 | 918 | 269.439 | 1.1098 | |
| 23 | N‐nitrosodiethylamine | C55185 | C4H10N2O | 102.1 | 897.9 | 256.562 | 1.1479 | |
| 24 | Benzaldehyde | C100527 | C7H6O | 106.1 | 963.7 | 304.911 | 1.1488 | mono |
| 25 | Octanal | C124130 | C8H16O | 128.2 | 1,007 | 349.69 | 1.4063 | mono |
| 26 | 13C | unidentified | * | 0 | 944.2 | 288.542 | 1.3026 | |
| 27 | Benzaldehyde | C100527 | C7H6O | 106.1 | 962.8 | 304.102 | 1.4666 | dimer |
| 28 | Heptanal | C111717 | C7H14O | 114.2 | 900.9 | 258.372 | 1.3292 | mono |
| 29 | 3‐methylthiopropanal | C3268493 | C4H8OS | 104.2 | 907.3 | 262.447 | 1.3961 | dimer |
| 30 | 14C | unidentified | * | 0 | 858.9 | 234.396 | 1.1369 | |
| 31 | 15C | unidentified | * | 0 | 877.5 | 244.54 | 1.2697 | |
| 32 | 16C | unidentified | * | 0 | 900.6 | 258.209 | 1.4269 | |
| 33 | 17C | unidentified | * | 0 | 788.2 | 200.514 | 1.3574 | |
| 34 | 18C | unidentified | * | 0 | 789.1 | 200.913 | 1.398 | |
| 35 | Furfural | C98011 | C5H4O2 | 96.1 | 827.7 | 218.686 | 1.3297 | |
| 36 | Pentan−1‐ol | C71410 | C5H12O | 88.1 | 763.3 | 189.603 | 1.2521 | |
| 37 | 3‐pentanone | C96220 | C5H10O | 86.1 | 694.4 | 163.468 | 1.1109 | mono |
| 38 | n‐propyl acetate | C109604 | C5H10O2 | 102.1 | 711.7 | 169.31 | 1.1648 | mono |
| 39 | 19C | unidentified | * | 0 | 672.1 | 156.833 | 1.1616 | |
| 40 | 1‐butanol | C71363 | C4H10O | 74.1 | 647.6 | 150.411 | 1.1741 | |
| 41 | 20C | unidentified | * | 0 | 729.4 | 175.826 | 1.186 | |
| 42 | Pentanal | C110623 | C5H10O | 86.1 | 697.4 | 164.416 | 1.1858 | |
| 43 | 2‐butanone | C78933 | C4H8O | 72.1 | 587.4 | 136.814 | 1.2451 | dimer |
| 44 | 21C | unidentified | * | 0 | 603.1 | 140.225 | 1.2916 | |
| 45 | 22C | unidentified | * | 0 | 647.5 | 150.404 | 1.3275 | |
| 46 | Acetic acid ethyl ester | C141786 | C4H8O2 | 88.1 | 618.6 | 143.642 | 1.3379 | dimer |
| 47 | 2‐methyl−1‐propanol | C78831 | C4H10O | 74.1 | 636.8 | 147.822 | 1.3716 | |
| 48 | 3‐pentanone | C96220 | C5H10O | 86.1 | 693.5 | 163.194 | 1.3562 | dimer |
| 49 | 23C | unidentified | * | 0 | 657.4 | 152.882 | 1.4039 | |
| 50 | 24C | unidentified | * | 0 | 686.2 | 160.899 | 1.2675 | |
| 51 | 25C | unidentified | * | 0 | 712.8 | 169.703 | 1.2969 | |
| 52 | 26C | unidentified | * | 0 | 686.2 | 160.902 | 1.3127 | |
| 53 | Hexanal | C66251 | C6H12O | 100.2 | 790.5 | 201.506 | 1.2571 | mono |
| 54 | Acetic acid butyl ester | C123864 | C6H12O2 | 116.2 | 806.2 | 208.622 | 1.2375 | mono |
| 55 | 27C | unidentified | * | 0 | 750.5 | 184.257 | 1.3049 | |
| 56 | 28C | unidentified | * | 0 | 712.8 | 169.703 | 1.4022 | |
| 57 | 29C | unidentified | * | 0 | 701.6 | 165.824 | 1.4157 | |
| 58 | 30C | unidentified | * | 0 | 702.6 | 166.16 | 1.4477 | |
| 59 | N,N‐diethylethanamine | C121448 | C6H15N | 101.2 | 689.6 | 161.95 | 1.2289 | |
| 60 | 31C | unidentified | * | 0 | 951.4 | 294.405 | 1.2635 | |
| 61 | 32C | unidentified | * | 0 | 831.7 | 220.638 | 1.2091 | |
| 62 | 33C | unidentified | * | 0 | 748.8 | 183.564 | 1.1066 | |
| 63 | 34C | unidentified | * | 0 | 755.3 | 186.255 | 1.4113 | |
| 64 | 35C | unidentified | * | 0 | 734.5 | 177.786 | 1.4245 | |
| 65 | 36C | unidentified | * | 0 | 676.5 | 158.048 | 1.078 | |
| 66 | 37C | unidentified | * | 0 | 788.2 | 200.488 | 1.469 | |
| 67 | Hexanal | C66251 | C6H12O | 100.2 | 792.5 | 202.431 | 1.561 | dimer |
| 68 | 38C | unidentified | * | 0 | 788.1 | 200.441 | 1.5285 | |
| 69 | Acetic acid butyl ester | C123864 | C6H12O2 | 116.2 | 805.9 | 208.485 | 1.6192 | dimer |
| 70 | n‐Propyl acetate | C109604 | C5H10O2 | 102.1 | 711.3 | 169.144 | 1.4752 | dimer |
| 71 | 39C | unidentified | * | 0 | 690 | 162.078 | 1.573 | |
| 72 | 40C | unidentified | * | 0 | 713.4 | 169.881 | 1.5378 | |
| 73 | Ethyl 2‐hydroxypropanoate | C97643 | C5H10O3 | 118.1 | 805.1 | 208.089 | 1.5362 | |
| 74 | Octamethylcyclotetrasiloxane | C556672 | C8H24O4Si4 | 296.6 | 1,009 | 352.022 | 1.6762 | |
| 75 | Octanal | C124130 | C8H16O | 128.2 | 1,007.2 | 349.831 | 1.8216 | dimer |
| 76 | 41C | unidentified | * | 0 | 1,006.7 | 349.269 | 1.4991 | |
| 77 | 42C | unidentified | * | 0 | 980.6 | 320.885 | 1.1066 | |
| 78 | Heptanal | C111717 | C7H14O | 114.2 | 901 | 258.418 | 1.6948 | dimer |
| 79 | 43C | unidentified | * | 0 | 811.2 | 210.943 | 1.8259 | |
| 80 | 44C | unidentified | * | 0 | 777.6 | 195.833 | 1.7521 | |
| 81 | Isopropyl alcohol | C67630 | C3H8O | 60.1 | 523 | 122.845 | 1.219 | dimer |
| 82 | 45C | unidentified | * | 0 | 737 | 178.771 | 1.285 | |
| 83 | 3‐hydroxybutan−2‐one | C513860 | C4H8O2 | 88.1 | 712.9 | 169.706 | 1.328 | dimer |
| 84 | 46C | unidentified | * | 0 | 904.1 | 260.356 | 1.528 | |
| 85 | Nonanal | C124196 | C9H18O | 142.2 | 1,109.5 | 494.176 | 1.4776 | |
| 86 | Compound from HS‐Vial Septum | GAS_00002 | * | 0 | 836 | 222.729 | 1.4664 | |
| 87 | 47C | unidentified | * | 0 | 860.4 | 235.177 | 1.2009 | |
| 88 | (E, E)−2,4‐heptadienal | C4313035 | C7H10O | 110.2 | 1,017.6 | 362.652 | 1.1919 | |
| 89 | 2‐heptanone | C110430 | C7H14O | 114.2 | 891 | 252.342 | 1.2602 | |
| 90 | Furaneol | C3658773 | C6H8O3 | 128.1 | 1,074.1 | 441.089 | 1.1985 | |
| 91 | Linalool | C78706 | C10H18O | 154.3 | 1,093.8 | 470.625 | 1.217 | |
| 92 | 48C | unidentified | * | 0 | 993.9 | 334.717 | 1.1761 | |
| 93 | 49C | unidentified | * | 0 | 577.3 | 134.63 | 1.2639 | |
| 94 | 50C | unidentified | * | 0 | 850 | 229.758 | 1.5154 | |
| 95 | 51C | unidentified | * | 0 | 469.3 | 111.221 | 1.0941 | |
| 96 | 52C | unidentified | * | 0 | 558.3 | 130.513 | 1.0884 | |
| 97 | 53C | unidentified | * | 0 | 1,286.3 | 759.457 | 1.3455 | |
| 98 | 54C | unidentified | * | 0 | 1,397.6 | 926.495 | 1.4833 | |
| 99 | Nonanoic acid | C112050 | C9H18O2 | 158.2 | 1,266.9 | 730.255 | 1.5409 | |
| 100 | 55C | unidentified | * | 0 | 1,205.2 | 637.789 | 1.8124 |
CAS is the registration number of chemical substances by Chemical Abstracts Service.
Represents the retention time in the capillary GC column.
Represents the retention index calculated on FS‐SE‐54‐CB column using n‐ketones C4‐C9 as external standard.
Represents the drift time in the drift tube
3.5. Fingerprints of squid samples
In order to compare the differences in VOCs more comprehensively, the gallery plot plug‐in of LAV software was used to generate the peak signal of the topographic plots of squid samples (Figure 4). In Figure 4, each row represents all selected signal peaks from a squid sample, and each column represents the signal peak of the same volatile organic compound. Brightness indicates the content of a substance. The higher the brightness, the higher the content of the substance.
Figure 4.
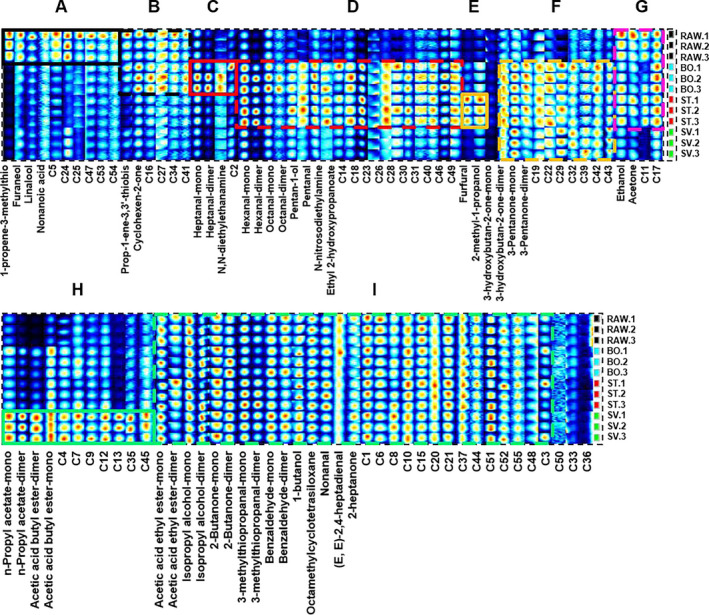
Fingerprint of squid in different cooking methods. RAW: raw squid; BO: boiled squid; ST: steamed squid; SV: sous vide cooked squid
The complete VOC information for each sample and the differences between the samples are depicted in Figure 4. The material marked by the solid black line in area A has the highest content in RAW samples, such as 1‐propene‐3‐methylthio, furaneol, linalool, and nonanoic acid. The substances marked by the solid red line in area C had the highest content in the BO squid, such as heptanal and N, N‐diethylethanamine. The substances marked by the solid yellow line in area E were found in ST squid. The compounds with the highest content include furfural and 2‐methyl‐1‐propanol. The solid green line in region H had the highest content in SV squid, including compounds such as n‐propyl acetate and acetic acid ethyl ester.
The substances marked by the black dotted line in area B were the highest in RAW and BO squid, such as prop‐1‐ene‐3, 3'‐thiobis, and cyclohexen‐2‐one. The substances marked by the red dotted line in the area D were the highest in BO and ST squid, including hexanal, octanal, pentan‐1‐ol, pentanal, N‐nitrosodiethylamine, and ethyl 2‐hydroxypropanoate. The substances marked by the yellow dotted line in area F were the highest in BO, ST, and SV squid, such as 3‐hydroxybutan‐2‐one and 3‐pentanone. The substances marked by the red dotted line in the area G were the highest in RAW, BO, and ST squid, including ethanol and acetone. The green dashed line in area I indicates the flavor substances that were common to the four kinds of the samples, such as acetic acid ethyl ester, isopropyl alcohol, 2‐butanone, 3‐methylthiopropanal, benzaldehyde, 1‐butanol, octamethylcyclotetrasiloxane, nonanal, (E,E)‐2,4‐heptadienal, and 2‐heptanone.
The heat map is an intuitive and visual method for analyzing the distribution of experimental data. It can cluster data and samples to determine the quality of the samples (Metsalu & Vilo, 2015). There were four treatment groups: RAW, BO, ST, and SV. The VOCs were classified into four clusters: group A, group B, group C, and group D in Figure 5. The volatile compounds in group A were mainly represented in RAW. Volatile compounds in group B were mainly represented in BO and ST. Volatile compounds in group C were represented in BO, and volatile compounds in group D were only represented in SV. From Figure 5, SV samples had particular volatile components that are different from ST samples, BO samples, and RAW samples, such as acetic acid butyl ester, n‐propyl acetate‐mono, and n‐propyl acetate‐dimer. Also, BO samples and ST samples had acetic acid ethyl ester‐mono, hexanal‐mono, hexanal‐dimer, N‐nitrosodiethylamine, 3‐pentanone‐dimer, and acetic acid butyl ester‐mono. This result was different from SV samples. Some unknown volatile compounds need to be further studied and determined.
Figure 5.
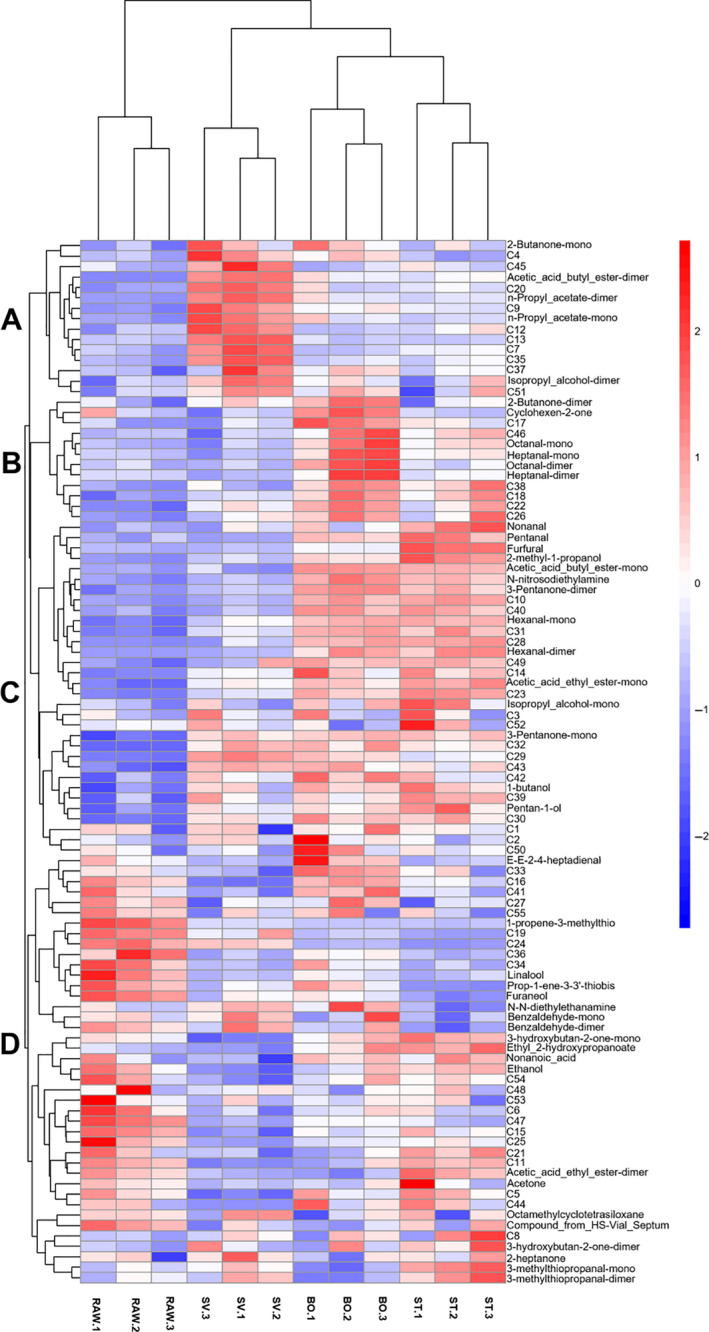
Heat map of squid from different cooking methods. RAW: raw squid; BO: boiled squid; ST: steamed squid; SV: sous vide cooked squid
4. DISCUSSION
Sensory evaluation is one of the critical indicators of food quality. In this study, the sensory evaluation could distinguish the squid samples with different cooking methods. The squid samples did not need other pretreatments, so only the professionalism and consistency of the evaluation were considered in the sensory assessment, ignoring the importance of consumers’ demand for the product. Consumers’ perceptions can be used for the assessment in the product development, which can significantly save time and labor costs (Vieira et al., 2020). It also suggested that evaluation based on preferred attribute elicitation methodology had the advantage of providing data on the importance of attributes for product acceptance (da Costa et al., 2020). Besides, in the sensory evaluation, the combination of the projective method (Judacewski et al., 2019), descriptive analysis method (H. Silva et al., 2018), and temporal methods (de Souza Paglarini et al., 2020) is more conducive to the development of new squid products.
The aroma is one of the most important evaluation indicators of meat products. Aroma contributes to the acceptability of the meat, and it is the result of a combination of various volatile compounds and chemical reactions that form flavor components, including Maillard reactions, lipid oxidation, interactions between Maillard reaction products, and lipid oxidation products (Shahidi, 1998). Different cooking methods affect the flavor of meat products (Grosch, 1982), especially the volatile components (Macleod, Seyyedain‐Ardebili, & Chang, 1981). The types of VOCs present in the BO and ST samples were similar. These techniques utilize the same temperature and pressure. SV heats the sample for a long time, and it utilizes low temperature under vacuum conditions. Thus, the VOCs are significantly different from BO and ST. Studies have shown that different heating methods affect the quality of food (F. A. Silva, Ferreira, Madruga, & Estevez, 2016). Even for SV, product quality is different under different vacuum degrees (Jeong, O, Shin, & Kim, 2018).
Volatile compounds of cooked meat include products of lipid and fatty acid oxidation, for example, aliphatic hydrocarbons, aldehydes, ketones, alcohols, carboxylic acids, and esters (Mottram, 1998). Aldehydes are the main products of the oxidative degradation of fatty acids and the characteristic aroma components of meat (Mottram, 1998). In this study, 43 VOCs were identified, including 14 aldehydes. The threshold value of aldehydes is relatively low, which contributes significantly to the flavor in cooked squid. In the experiment, 3‐methylthiopropanal was present in all four sample types. 3‐Methylthioprapanol was first identified as a volatile component in cooked squid by Kubota, Matsukage, Sekiwa, and Kobayashi (1996) It is a “baked potato” descriptor and considered an essential contributor to the scent of squid (Carrascon, Escudero, Ferreira, & Lopez, 2014) as well as cooked lobster (Lee, Suriyaphan, & Cadwallader, 2001). However, the difference in the contents of aldehydes in squid from different heating methods has an important impact on the sample odor. Ketones are also products of fatty acid oxidation, generally the products of automatic oxidation of unsaturated fatty acids (Thomas, 1971). There are ten ketones in the 43 VOCs detected in ST, BO, and SV samples, such as 3‐hydroxybutan‐2‐one, 3‐pentanone, acetone, 2‐butanone, and 2‐heptanone. In particular, furaneol (4‐hydroxy‐2,5‐dimethyl‐3(2H)furanone) in RAW samples is described as a “caramel‐like” flavor, and it is a flavor component of strawberry (Buechi, Demole, & Thomas, 1973) and pineapple (Rodin, Himel, Silverstein, Leeper, & Gortner, 1965). Kubota et al. (1996) considered furaneol a substance that contributes to the sweetness of squid after cooking. Ester compounds are produced by the esterification of alcohols and acids (Domínguez, Gómez, Fonseca, & Lorenzo, 2014) and esters are found in many flavors of crustacean fish products that are cooked and heated (Tanchotikul & Hsieh, 1989). SV squid has the highest content of n‐propyl acetate and acetic acid ethyl ester of all samples tested. The volatile substance n‐propyl acetate is a short‐chain aliphatic ester that produces a pleasant fruity aroma. It is a component of natural flavors (Mahapatra, Kumari, Garlapati, Banerjee, & Nag, 2009). This indicates the SV con ditions are conducive to the esterification reaction and can yield more desirable flavor in squid.
The type of volatile components was determined by the degree of oxidative degradation of fatty acids under different heating temperatures, heating pressures, and transfer medium. The results show that the aldehydes were more varied and in higher abundance when squid was heated at 100°C under normal pressure. Conversely, the aldehydes of SV were less abundant than ST and BO. The reason is high‐temperature and high‐pressure conditions are conducive to the thermal oxidative degradation of fatty acids (Cheah & Ledward, 1996), and more volatile components are generated when heated. The increase in heating temperature increased the degree of oxidative degradation of fatty acids, thus increasing the content of volatile components. Compared with ST and BO at normal pressure and 100°C, SV is heated under vacuum at 60°C, and the degree of fatty acid oxidation degradation is lower during heating (Oz & Zikirov, 2015). This reduces the content of volatile substances generated during heating. Falowo, Muchenje, and Hugo (2017) reported that there was no pronounced effect of SV cooking temperature on fatty acid of beef and liver compared to raw samples. Generally, low heating temperature is indeed a weakness of SV cooking, but long‐term heating sometimes compensates for this weakness. Long‐time heating increases the volatile substances derived from the degradation of amino acids and/or thiamine (Roldan, Antequera, Armenteros, & Ruiz, 2014; Roldan, Ruiz, del Pulgar, Perez‐Palacios, & Antequera, 2015), such as 2‐methyl‐thiophene, carbon disulfide, benzothiazole, and dimethyl disulfide (Calkins & Hodgen, 2007). Mortensen, Frøst, Skibsted, and Risbo (2012) indicated that the effect of SV heating time on flavor has a greater effect than the heating temperature. For beef and pork cooked in SV, the longer the cooking time, the better the flavor (Christensen et al., 2012). SV cooking still develops a pleasant flavor at a low temperature. 3‐Methylbutanal, a meaty‐nutty flavor of dry‐cured ham (Ruiz, Ventanas, Cava, Andrés, & García, 1999), was found in SV cooked meat after 24 hr of heating at 60°C (del Pulgar, Roldan, & Ruiz‐Carrascal, 2013; Roldan, Ruiz, et al., 2015). Of course, the prolonged heating time of SV reduces the lipid oxidation of carbonyl compounds in food, which indicates that they further react with other compounds (proteins, amino acids, etc.) to produce new, more desirable volatiles (del Pulgar et al., 2013; Roldan, Ruiz, et al., 2015). In this study, n‐propyl acetate and acetic acid ethyl ester (highlighted in group H in Figure 4) were produced after SV heating; however, they were not detected in BO and ST. Moreover, by adding reducing sugar or other flavor precursors, SV food flavor can also be enhanced (Roldan, Loebner, et al., 2015).
In addition to this, SV technology produces high‐quality sensory characteristics of meat products. Studies have shown that a slow heating rate is key to generating tender meat (Cover, 1943) and maintaining the meat core temperature closed to 60°C for a long time (Laakkonen, Sherbon, & Wellington, 1970). These observations have been further confirmed in the SV technique (Becker et al., 2015; Christensen et al., 2013; Roldan et al., 2013). SV means not only higher sensorial quality but LTLT heating can also minimize the nutrient loss (Rondanelli et al., 2017), improve bioaccessibility (da Silva et al., 2017), ensure the safety of food (El Kadri, Alaizoki, Celen, Smith, & Onyeaka, 2020; Nissen, Rosnes, Brendehaug, & Kleiberg, 2002), and extend the shelf life (Diaz, Nieto, Banon, & Garrido, 2009; Kim, Hong, Lim, Park, & Lee, 2015; Nissen et al., 2002). Given the many advantages of SV and the growing demand for nutritious and convenient foods, the application of SV technology in the industrial production of squid is feasible.
5. CONCLUSIONS
Through sensory evaluation and electronic analysis, it was found that different cooking methods have a great influence on the flavor of the squid. SV squid flavor is different from that of ST and BO. Additionally, a method was developed to evaluate the characteristic volatile compounds of squid samples from different cooking methods by establishing the fingerprint with HS‐GC‐IMS. A total of 43 volatile substances, including some dimers, were detected by GC‐IMS analysis from samples of squid from different cooking methods. The predominant compounds were mainly aldehydes, ketones, alcohols, and esters. Given the many advantages of SV technology, it could be used for the industrial production of squid.
6. INFORMED CONSENT
Written informed consent was obtained from all study participants.
CONFLICTS OF INTEREST
The authors declare that they do not have any conflict of interest.
AUTHORS CONTRIBUTION
Hao Zhang and Zhenkun Cui conceived the study; Zhenkun Cui, Hongbo Li, and Tatiana Manoli designed the methodology; Han Yan provided the software; Zhenkun Cui, Han Yan, and Hongbo Li involved in formal analysis; Zhenkun Cui and Han Yan wrote the original draft of the manuscript; Haizhen Mo and Tatiana Manoli wrote, reviewed, and edited the manuscript; Haizhen Mo and Hao Zhang involved in funding acquisition; and Hao Zhang investigated twehe study. All authors have read and agree to the published version of the manuscript.
ETHICAL APPROVAL
This study does not involve any human or animal testing.
ACKNOWLEDGMENTS
This work was supported by Henan Province Technology System for Conventional Freshwater Fish Industries (S2014‐10‐G02). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Cui Z, Yan H, Manoli T, Mo H, Li H, Zhang H. Changes in the volatile components of squid (illex argentinus) for different cooking methods via headspace–gas chromatography–ion mobility spectrometry. Food Sci Nutr. 2020;8:5748–5762. 10.1002/fsn3.1877
REFERENCES
- Alemán, A. , Giménez, B. , Pérez‐Santin, E. , Gómez‐Guillén, M. , & Montero, P. (2011). Contribution of Leu and Hyp residues to antioxidant and ACE‐inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chemistry, 125(2), 334–341. 10.1016/j.foodchem.2010.08.058 [DOI] [Google Scholar]
- Alemán, A. , Gómez‐Guillén, M. , & Montero, P. (2013). Identification of ace‐inhibitory peptides from squid skin collagen after in vitro gastrointestinal digestion. Food Research International, 54(1), 790–795. 10.1016/j.foodres.2013.08.027 [DOI] [Google Scholar]
- Armenta, S. , Alcala, M. , & Blanco, M. (2011). A review of recent, unconventional applications of ion mobility spectrometry (IMS). Analytica Chimica Acta, 703(2), 114–123. 10.1016/j.aca.2011.07.021 [DOI] [PubMed] [Google Scholar]
- Asbury, G. R. , Klasmeier, J. , & Hill, H. H. Jr (2000). Analysis of explosives using electrospray ionization/ion mobility spectrometry (ESI/IMS). Talanta, 50(6), 1291–1298. 10.1016/S0039-9140(99)00241-6 [DOI] [PubMed] [Google Scholar]
- Baldwin, D. E. (2012). Sous vide cooking: A review. International Journal of Gastronomy Food Science, 1(1), 15–30. 10.1016/j.ijgfs.2011.11.002 [DOI] [Google Scholar]
- Becker, A. , Boulaaba, A. , Pingen, S. , Röhner, A. , & Klein, G. (2015). Low temperature, long time treatment of porcine M. longissimus thoracis et lumborum in a combi steamer under commercial conditions. Meat Science, 110, 230–235. 10.1016/j.meatsci.2015.07.024 [DOI] [PubMed] [Google Scholar]
- Botinestean, C. , Keenan, D. F. , Kerry, J. P. , & Hamill, R. M. (2016). The effect of thermal treatments including sous‐vide, blast freezing and their combinations on beef tenderness of M. semitendinosus steaks targeted at elderly consumers. LWT‐Food Science and Technology, 74, 154–159. 10.1016/j.lwt.2016.07.026 [DOI] [Google Scholar]
- Buechi, G. , Demole, E. , & Thomas, A. F. (1973). Syntheses of 2, 5‐dimethyl‐4‐hydroxy‐2, 3‐dihydrofuran‐3‐one (furaneol), a flavor principle of pineapple and strawberry. The Journal of Organic Chemistry, 38(1), 123–125. 10.1021/jo00941a025 [DOI] [Google Scholar]
- Calkins, C. R. , & Hodgen, J. M. (2007). A fresh look at meat flavor. Meat Science, 77(1), 63–80. 10.1016/j.meatsci.2007.04.016 [DOI] [PubMed] [Google Scholar]
- Carrascon, V. , Escudero, A. , Ferreira, V. , & Lopez, R. (2014). Characterisation of the key odorants in a squid broth (Illex argentinus). LWT‐Food Science and Technology, 57(2), 656–662. 10.1016/j.lwt.2014.02.010 [DOI] [Google Scholar]
- Cheah, P. , & Ledward, D. (1996). High pressure effects on lipid oxidation in minced pork. Meat Science, 43(2), 123–134. 10.1016/0309-1740(96)84584-0 [DOI] [PubMed] [Google Scholar]
- Christensen, L. , Ertbjerg, P. , Løje, H. , Risbo, J. , van den Berg, F. W. , & Christensen, M. (2013). Relationship between meat toughness and properties of connective tissue from cows and young bulls heat treated at low temperatures for prolonged times. Meat Science, 93(4), 787–795. 10.1016/j.meatsci.2012.12.001 [DOI] [PubMed] [Google Scholar]
- Christensen, L. , Gunvig, A. , Tørngren, M. A. , Aaslyng, M. D. , Knøchel, S. , & Christensen, M. (2012). Sensory characteristics of meat cooked for prolonged times at low temperature. Meat Science, 90(2), 485–489. 10.1016/j.meatsci.2011.09.012 [DOI] [PubMed] [Google Scholar]
- Cover, S. (1943). Effect of extremely low rates of heat penetration on tendering of beef. Food Research, 8, 388–394. 10.1111/j.1365-2621.1943.tb16573.x [DOI] [Google Scholar]
- Cross, H. , Stanfield, M. S. , & Koch, E. J. (1976). Beef palatability as affected by cooking rate and final internal temperature. Journal of Animal Science, 43(1), 114–121. 10.2527/jas1976.431114x [DOI] [Google Scholar]
- Cui, Z. , Dubova, H. , & Mo, H. (2019). Effects of sous vide cooking on physicochemical properties of squid. Journal of Hygienic Engineering and Design, 29, 35–40. [Google Scholar]
- da Costa, G. M. , de Paula, M. M. , Costa, G. N. , Esmerino, E. A. , Silva, R. , de Freitas, M. Q. , … Pimentel, T. C. (2020). Preferred attribute elicitation methodology compared to conventional descriptive analysis: A study using probiotic yogurt sweetened with xylitol and added with prebiotic components. Journal of Sensory Studies, e12602, 10.1111/joss.12602 [DOI] [Google Scholar]
- da Silva, F. L. F. , de Lima, J. P. S. , Melo, L. S. , da Silva, Y. S. M. , Gouveia, S. T. , Lopes, G. S. , & Matos, W. O. (2017). Comparison between boiling and vacuum cooking (sous‐vide) in the bioaccessibility of minerals in bovine liver samples. Food Research International, 100, 566–571. 10.1016/j.foodres.2017.07.054 [DOI] [PubMed] [Google Scholar]
- de Souza Paglarini, C. , Vidal, V. A. S. , dos Santos, M. , Coimbra, L. O. , Esmerino, E. A. , Cruz, A. G. , & Pollonio, M. A. R. (2020). Using dynamic sensory techniques to determine drivers of liking in sodium and fat‐reduced Bologna sausage containing functional emulsion gels. Food Research International, 132, 109066 10.1016/j.foodres.2020.109066 [DOI] [PubMed] [Google Scholar]
- del Pulgar, J. S. , Gazquez, A. , & Ruiz‐Carrascal, J. (2012). Physico‐chemical, textural and structural characteristics of sous‐vide cooked pork cheeks as affected by vacuum, cooking temperature, and cooking time. Meat Science, 90(3), 828–835. 10.1016/j.meatsci.2011.11.024 [DOI] [PubMed] [Google Scholar]
- del Pulgar, J. S. , Roldan, M. , & Ruiz‐Carrascal, J. (2013). Volatile Compounds Profile of Sous‐Vide Cooked Pork Cheeks as Affected by Cooking Conditions (Vacuum Packaging, Temperature and Time). Molecules, 18(10), 12538–12547. 10.3390/molecules181012538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, P. , Nieto, G. , Banon, S. , & Garrido, M. D. (2009). Determination of Shelf Life of Sous Vide Salmon (Salmo Salard) Based on Sensory Attributes. Journal of Food Science, 74(8), S371–S376. 10.1111/j.1750-3841.2009.01317.x [DOI] [PubMed] [Google Scholar]
- Domínguez, R. , Gómez, M. , Fonseca, S. , & Lorenzo, J. M. (2014). Influence of thermal treatment on formation of volatile compounds, cooking loss and lipid oxidation in foal meat. LWT‐Food Science and Technology, 58(2), 439–445. 10.1016/j.lwt.2014.04.006 [DOI] [PubMed] [Google Scholar]
- Dong, L. , Zhu, J. , Li, X. , & Li, J. (2013). Effect of tea polyphenols on the physical and chemical characteristics of dried‐seasoned squid (Dosidicus gigas) during storage. Food Control, 31(2), 586–592. 10.1016/j.foodcont.2012.10.014 [DOI] [Google Scholar]
- Eiceman, G. A. , Karpas, Z. , & Hill, H. H. Jr (2013). Ion mobility spectrometry. Boca Raton, FL: CRC Press. [Google Scholar]
- El Kadri, H. , Alaizoki, A. , Celen, T. , Smith, M. , & Onyeaka, H. (2020). The effect of low‐temperature long‐time (LTLT) cooking on survival of potentially pathogenic Clostridium perfringens in beef. International Journal of Food Microbiology, 320, 108540 10.1016/j.ijfoodmicro.2020.108540 [DOI] [PubMed] [Google Scholar]
- Falowo, A. B. , Muchenje, V. , & Hugo, A. (2017). Effect of sous‐vide technique on fatty acid and mineral compositions of beef and liver from Bonsmara and non‐descript cattle. Annals of Animal Science, 17(2), 565–580. 10.1515/aoas-2016-0078 [DOI] [Google Scholar]
- Garrido‐Delgado, R. , Muñoz‐Pérez, M. E. , & Arce, L. (2018). Detection of adulteration in extra virgin olive oils by using UV‐IMS and chemometric analysis. Food Control, 85, 292–299. 10.1016/j.foodcont.2017.10.012 [DOI] [Google Scholar]
- Gloess, A. , Yeretzian, C. , Knochenmuss, R. , & Groessl, M. (2018). On‐line analysis of coffee roasting with ion mobility spectrometry–mass spectrometry (IMS–MS). International Journal of Mass Spectrometry, 424, 49–57. 10.1016/j.ijms.2017.11.017 [DOI] [Google Scholar]
- Gou, J. , Lee, H.‐Y. , & Ahn, J. (2010). Effect of high pressure processing on the quality of squid (Todarodes pacificus) during refrigerated storage. Food Chemistry, 119(2), 471–476. 10.1016/j.foodchem.2009.06.042 [DOI] [Google Scholar]
- Grosch, W. (1982). Lipid degradation products and flavour In Morton I. D., & MacLeod A. J. (Eds.), Food Flavours (pp. 325–398). Amsterdam: Elsevier Scientific Publication. [Google Scholar]
- Hansen, T. B. , Knøchel, S. , Juncher, D. , & Bertelsen, G. (1995). Storage characteristics of sous vide cooked roast beef. International Journal of Food Science & Technology, 30(3), 365–378. 10.1111/j.1365-2621.1995.tb01384.x [DOI] [Google Scholar]
- Ivanov, G. , Bilgucu, E. , Ivanova, I. , & Dimitrova, M. (2020). Volatile organic compound profiles of yoghurt produced from cow's milk with different somatic cell counts. International Journal of Dairy Technology, 10.1111/1471-0307.12702 [DOI] [Google Scholar]
- Jeong, K. , O, H. , Shin, S. Y. , & Kim, Y.‐S. (2018). Effects of sous‐vide method at different temperatures, times and vacuum degrees on the quality, structural, and microbiological properties of pork ham. Meat Science, 143, 1–7. 10.1016/j.meatsci.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Jo, D. , Kim, G.‐R. , Yeo, S.‐H. , Jeong, Y.‐J. , Noh, B. S. , & Kwon, J.‐H. (2013). Analysis of aroma compounds of commercial cider vinegars with different acidities using SPME/GC‐MS, electronic nose, and sensory evaluation. Food Science and Biotechnology, 22(6), 1559–1565. 10.1007/s10068-013-0251-1 [DOI] [Google Scholar]
- Judacewski, P. , Los, P. R. , Lima, L. S. , Alberti, A. , Zielinski, A. A. F. , & Nogueira, A. (2019). Perceptions of Brazilian consumers regarding white mould surface‐ripened cheese using free word association. International Journal of Dairy Technology, 72(4), 585–590. 10.1111/1471-0307.12649 [DOI] [Google Scholar]
- Karpas, Z. , Guamán, A. V. , Calvo, D. , Pardo, A. , & Marco, S. (2012). The potential of ion mobility spectrometry (IMS) for detection of 2, 4, 6‐trichloroanisole (2, 4, 6‐TCA) in wine. Talanta, 93, 200–205. 10.1016/j.talanta.2012.02.012 [DOI] [PubMed] [Google Scholar]
- Kim, J. H. , Hong, G. E. , Lim, K. W. , Park, W. , & Lee, C. H. (2015). Influence of citric acid on the pink color and characteristics of sous vide processed chicken breasts during chill storage. Korean Journal for Food Science of Animal Resources, 35(5), 585–596. 10.5851/kosfa.2015.35.5.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota, K. , Matsukage, Y. , Sekiwa, Y. , & Kobayashi, A. (1996). Identification of the characteristic volatile flavor compounds formed by cooking squid (Todarodes pacificus Steenstrup). Food Science and Technology International, Tokyo, 2(3), 163–166. 10.3136/fsti9596t9798.2.163 [DOI] [Google Scholar]
- Laakkonen, E. , Sherbon, J. W. , & Wellington, G. H. (1970). Low‐temperature, long‐time heating of bovine muscle 3. Collagenolytic activity. Journal of Food Science, 35(2), 181–184. 10.1111/j.1365-2621.1970.tb12133.x [DOI] [Google Scholar]
- Lee, G.‐H. , Suriyaphan, O. , & Cadwallader, K. R. (2001). Aroma components of cooked tail meat of American lobster (Homarus americanus). Journal of Agricultural and Food Chemistry, 49(9), 4324–4332. 10.1021/jf001523t [DOI] [PubMed] [Google Scholar]
- Lyberg, A. M. , & Adlercreutz, P. (2008). Lipase‐catalysed enrichment of DHA and EPA in acylglycerols resulting from squid oil ethanolysis. European Journal of Lipid Science and Technology, 110(4), 317–324. 10.1002/ejlt.200700217 [DOI] [Google Scholar]
- Macleod, G. , Seyyedain‐Ardebili, M. , & Chang, S. S. (1981). Natural and simulated meat flavors (with particular reference to beef). Critical Reviews in Food Science & Nutrition, 14(4), 309–437. 10.1080/10408398109527309 [DOI] [PubMed] [Google Scholar]
- Mahapatra, P. , Kumari, A. , Garlapati, V. K. , Banerjee, R. , & Nag, A. (2009). Enzymatic synthesis of fruit flavor esters by immobilized lipase from Rhizopus oligosporus optimized with response surface methodology. Journal of Molecular Catalysis B: Enzymatic, 60(1–2), 57–63. 10.1016/j.molcatb.2009.03.010 [DOI] [Google Scholar]
- Metsalu, T. , & Vilo, J. (2015). ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Research, 43(W1), W566–W570. 10.1093/nar/gkv468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei, H. , & Regnier, F. (2006). Identification and quantification of protein carbonylation using light and heavy isotope labeled Girard's P reagent. Journal of Chromatography A, 1134(1–2), 122–133. 10.1016/j.chroma.2006.08.096 [DOI] [PubMed] [Google Scholar]
- Mortensen, L. M. , Frøst, M. B. , Skibsted, L. H. , & Risbo, J. (2012). Effect of time and temperature on sensory properties in low‐temperature long‐time sous‐vide cooking of beef. Journal of Culinary Science & Technology, 10(1), 75–90. 10.1080/15428052.2012.651024 [DOI] [Google Scholar]
- Mottram, D. S. (1998). Flavour formation in meat and meat products: A review. Food Chemistry, 62(4), 415–424. 10.1016/S0308-8146(98)00076-4 [DOI] [Google Scholar]
- Nissen, H. , Rosnes, J. T. , Brendehaug, J. , & Kleiberg, G. H. (2002). Safety evaluation of sous vide‐processed ready meals. Letters in Applied Microbiology, 35(5), 433–438. 10.1046/j.1472-765x.2002.01218.x [DOI] [PubMed] [Google Scholar]
- Oz, F. , & Zikirov, E. (2015). The effects of sous‐vide cooking method on the formation of heterocyclic aromatic amines in beef chops. LWT‐Food Science and Technology, 64(1), 120–125. 10.1016/j.lwt.2015.05.050 [DOI] [Google Scholar]
- Rearden, P. , & Harrington, P. B. (2005). Rapid screening of precursor and degradation products of chemical warfare agents in soil by solid‐phase microextraction ion mobility spectrometry (SPME–IMS). Analytica Chimica Acta, 545(1), 13–20. 10.1016/j.aca.2005.04.035 [DOI] [Google Scholar]
- Rodin, J. , Himel, C. M. , Silverstein, R. M. , Leeper, R. W. , & Gortner, W. A. (1965). Volatile Flavor and Aroma Components of Pineapple. l. Isolation and Tentative Identification of 2,5‐Dimethyl‐4‐Hydroxy‐3(2H)‐Furanone. Journal of Food Science, 30(2), 280–285. 10.1111/j.1365-2621.1965.tb00302.x [DOI] [Google Scholar]
- Roldan, M. , Antequera, T. , Armenteros, M. , & Ruiz, J. (2014). Effect of different temperature‐time combinations on lipid and protein oxidation of sous‐vide cooked lamb loins. Food Chemistry, 149, 129–136. 10.1016/j.foodchem.2013.10.079 [DOI] [PubMed] [Google Scholar]
- Roldan, M. , Antequera, T. , Martin, A. , Mayoral, A. I. , & Ruiz, J. (2013). Effect of different temperature‐time combinations on physicochemical, microbiological, textural and structural features of sous‐vide cooked lamb loins. Meat Science, 93(3), 572–578. 10.1016/j.meatsci.2012.11.014 [DOI] [PubMed] [Google Scholar]
- Roldan, M. , Antequera, T. , Perez‐Palacios, T. , & Ruiz, J. (2014). Effect of added phosphate and type of cooking method on physico‐chemical and sensory features of cooked lamb loins. Meat Science, 97(1), 69–75. 10.1016/j.meatsci.2014.01.012 [DOI] [PubMed] [Google Scholar]
- Roldan, M. , Loebner, J. , Degen, J. , Henle, T. , Antequera, T. , & Ruiz‐Carrascal, J. (2015). Advanced glycation end products, physico‐chemical and sensory characteristics of cooked lamb loins affected by cooking method and addition of flavour precursors. Food Chemistry, 168, 487–495. 10.1016/j.foodchem.2014.07.100 [DOI] [PubMed] [Google Scholar]
- Roldan, M. , Ruiz, J. , del Pulgar, J. S. , Perez‐Palacios, T. , & Antequera, T. (2015). Volatile compound profile of sous‐vide cooked lamb loins at different temperature‐time combinations. Meat Science, 100, 52–57. 10.1016/j.meatsci.2014.09.010 [DOI] [PubMed] [Google Scholar]
- Rondanelli, M. , Daglia, M. , Meneghini, S. , Di Lorenzo, A. , Peroni, G. , Faliva, M. A. , & Perna, S. (2017). Nutritional advantages of sous‐vide cooking compared to boiling on cereals and legumes: Determination of ashes and metals content in ready‐to‐eat products. Food Science & Nutrition, 5(3), 827–833. 10.1002/fsn3.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz, J. , Ventanas, J. , Cava, R. , Andrés, A. , & García, C., (1999). Volatile compounds of dry‐cured Iberian ham as affected by the length of the curing process. Meat Science, 52(1), 19–27. 10.1016/S0309-1740(98)00144-2 [DOI] [PubMed] [Google Scholar]
- Salaseviciene, A. , Vaiciulyte‐Funk, L. , & Koscelkovskienė, I. (2014). Impact of low temperature, prolonged time treatment and vacuum depth on the porcine muscle quality and safety. Paper presented at the 9th Baltic Conference on Food Science and Technology “Food for Consumer Well‐Being” [Google Scholar]
- Schellekens, M. (1996). New research issues in sous‐vide cooking. Trends in Food Science and Technology, 7(8), 256–262. 10.1016/0924-2244(96)10027-3 [DOI] [Google Scholar]
- Shahidi, F. (1998). Flavor of meat, meat products and seafoods. London: Blackie Academic and Professional. [Google Scholar]
- Shahidi, F. , Rubin, L. J. , D'Souza, L. A. , Teranishi, R. , & Buttery, R. G. (1986). Meat flavor volatiles: A review of the composition, techniques of analysis, and sensory evaluation. Critical Reviews in Food Science & Nutrition, 24(2), 141–243. 10.1080/10408398609527435 [DOI] [PubMed] [Google Scholar]
- Silva, F. A. , Ferreira, V. C. , Madruga, M. S. , & Estevez, M. (2016). Effect of the cooking method (grilling, roasting, frying and sous‐vide) on the oxidation of thiols, tryptophan, alkaline amino acids and protein cross‐linking in jerky chicken. Journal of Food Science and Technology, 53(8), 3137–3146. 10.1007/s13197-016-2287-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, H. , Balthazar, C. , Silva, R. , Vieira, A. , Costa, R. , Esmerino, E. , … Cruz, A. (2018). Sodium reduction and flavor enhancer addition in probiotic prato cheese: Contributions of quantitative descriptive analysis and temporal dominance of sensations for sensory profiling. Journal of Dairy Science, 101(10), 8837–8846. 10.3168/jds.2018-14819 [DOI] [PubMed] [Google Scholar]
- Snyder, A. P. , Harden, C. S. , Davis, D. M. , Shoff, D. B. , & Maswadeh, W. M. (1995). Hand‐portable Gas Chromatography‐Ion Mobility spectrometer for the determination of the freshness of fish. Paper presented at the Third International Workshop on Ion Mobility Spectrometry, Galveston, Texas, USA. [Google Scholar]
- Tanchotikul, U. , & Hsieh, T. C. Y. (1989). Volatile flavor components in crayfish waste. Journal of Food Science, 54(6), 1515–1520. 10.1111/j.1365-2621.1989.tb05149.x [DOI] [Google Scholar]
- Thomas, C. (1971). Sources of flavor in poultry skin. Food Technol, 25, 109–115. [Google Scholar]
- Tomac, A. , Cova, M. C. , Narvaiz, P. , & Yeannes, M. I. (2017). Sensory acceptability of squid rings gamma irradiated for shelf‐life extension. Radiation Physics and Chemistry, 130, 359–361. 10.1016/j.radphyschem.2016.09.016 [DOI] [Google Scholar]
- Vaudagna, S. R. , Sánchez, G. , Neira, M. S. , Insani, E. M. , Picallo, A. B. , Gallinger, M. M. , & Lasta, J. A. (2002). Sous vide cooked beef muscles: Effects of low temperature–long time (LT–LT) treatments on their quality characteristics and storage stability. International Journal of Food Science and Technology, 37(4), 425–441. 10.1046/j.1365-2621.2002.00581.x [DOI] [Google Scholar]
- Verkouteren, J. R. , & Staymates, J. L. (2011). Reliability of ion mobility spectrometry for qualitative analysis of complex, multicomponent illicit drug samples. Forensic Science International, 206(1–3), 190–196. 10.1016/j.forsciint.2010.08.005 [DOI] [PubMed] [Google Scholar]
- Vieira, A. H. , Balthazar, C. F. , Rocha, R. S. , Silva, R. , Guimaraes, J. T. , Pagani, M. M. , … Tonon, R. V. (2020). The free listing task for describing the sensory profiling of dairy foods: A case study with microfiltered goat whey orange juice beverage. Journal of Sensory Studies, e12594, 10.1111/joss.12594 [DOI] [Google Scholar]
- Waluda, C. , Rodhouse, P. , Podestá, G. , Trathan, P. , & Pierce, G. (2001). Surface oceanography of the inferred hatching grounds of Illex argentinus (Cephalopoda: Ommastrephidae) and influences on recruitment variability. Marine Biology, 139(4), 671–679. 10.1007/s002270100615 [DOI] [Google Scholar]
- Wu, Z. , Chen, L. , Wu, L. , Xue, X. , Zhao, J. , Li, Y. , … Lin, G. (2015). Classification of Chinese honeys according to their floral origins using elemental and stable isotopic compositions. Journal of Agricultural and Food Chemistry, 63(22), 5388–5394. 10.1021/acs.jafc.5b01576 [DOI] [PubMed] [Google Scholar]


