Abstract
During the study, we determined the antimicrobial activity of different selected essential oils (thyme, lemongrass, juniper, oregano, sage, fennel, rosemary, mint, rosehips, dill) on some pathogenic and spoilage bacteria isolated from the surface of various fresh vegetables. At the same time, in the case of some volatile oil combinations we followed the phenomena of synergism and antagonism. The identification of the isolated bacterial strains was made using 16S rDNA gene sequence analysis. The most resistant isolates appeared to be Curtobacterium herbarum, Achromobacter xylosoxidans, and Enterobacter ludwigii, while Pseudomonas hibiscicola was the most sensitive. Of the chosen plant essential oils, the most pronounced antimicrobial effect was detected in the case of oregano. The essential oils of thyme and mint also showed elevated antimicrobial activity. A synergistic effect was observed in case of five combinations of essential oil. Based on the results, we find that some individual essential oils and mixture compositions (due to synergic effect) could be good candidates for the preservation of fresh vegetables. These preliminary findings suggest that essential oils from locally grown spices could contribute to decreasing the health risk and also to the suppression of emergence of antibiotic resistance.
Keywords: antimicrobial effects, essential oils, foodborne pathogenic bacteria, fresh vegetables, spoilage bacteria
Is very useful to know the pathogenic strains occurrence in different geographic regions, to prevent disease occurrence in population and among the tourists, how are more susceptible to foodborne pathogens. The main goal was the detection of active antimicrobial essential oil compositions that will be used as ingredients in active packaging materials for fresh vegetables and fruits, for decreasing the health risk and increasing the product shelf‐life.
1. INTRODUCTION
Different parts of vegetables are edible, such as roots, tubers, leaves, flowers, and bulbs. Generally, these plant components are rich in carbohydrates, with pH values ranging from 5.0 to 7.0. In favorable conditions, different microorganisms such as bacteria, yeasts, and molds can colonize them. The main source of microbes in vegetables includes air, soil, water, domestic or wild animals, insects, or different equipments. Microorganisms can differ with the types of vegetables (Ray & Bhunia, 2014). The microbiota associated with most vegetables is dominated by the Gram‐negative bacteria. In the case of many vegetables, Pseudomonas spp. dominate the microflora and normally represent 50%–80% of the microbial population (Ramos, Miller, Brandão, Teixeira, & Silva, 2013). In the development of the food spoilage microbial community on the vegetable surface (beside external environmental factors), the prevailing internal properties of the vegetables (chemical composition, water activity, pH‐value, redox potential) play an important role. The physiological state of the plant is an important factor in resistance to the microbial spoilage. The main cause of spoilage of fresh vegetables is bacteria, where their proliferation rate exceeds the growth rate of the yeasts and molds, particularly in a cooled environment. Among the Gram‐negative bacterial strains Acinetobacter, Chromobacterium, Alcaligenes, Enterobacter, Flavobacterium, Klebsiella, Pseudomonas, Serratia, and Xanthomonas species can often be isolated. The most common Gram‐positive bacteria that occur on vegetables are Micrococcus, Lactobacillus, Corynebacterium, and Bacillus species (Deák, 2006). At refrigeration temperature storage, the dominant bacterial populations on the surface of minimally processed vegetables belong to different families such as Pseudomonadaceae, Enterobacteriacae, and also different species of lactic acid bacteria. The most representative species are P. fluorescens, Rahnella aquatilis, Erwinia herbicola, and Leuconostoc mesenteroides (Prakash, Baskaran, Paramasivam, & Vadivel, 2018). Enteric pathogens from vegetables are a public health concern. In general, transmission of these pathogens is possible from manure contaminated soil, feces of animals and birds, irrigation water (Adams & Moss, 2008). Major pathogen bacteria from vegetables associated with outbreaks are as follows: Clostridium botulinum, E. coli O157:H7, Listeria monocytogenes, Salmonella spp. Shigella spp., Staphylococcus spp., Vibrio cholerae, and Yersinia enterocolitica (Cayalvizhi & Balachandhar, 2019; Ramos et al., 2013).
Minimally processed vegetables are one of the largest rising sectors in food industry. Ongoing advancement in natural food preservatives has led to the use of essential oils or their components in food packaging, and they represent an alternative to the synthetic additives (Patrignani, Siroli, Serrazanetti, Gardini, & Lanciotti, 2015). Essential oils have exhibited antagonistic properties against different pathogenic or spoilage microorganisms. In vitro studies have shown antibacterial activity of essential oils against Bacillus cereus, Escherichia coli, Listeria monocytogenes, Salmonella typhimurium, and Staphylococcus aureus with concentration ranging from 0.2 to 10 μl/ml (Scollard, Francis, & O'Beirne, 2013). Some essential oils such as Citrus spp., cinnamon, oregano, and thyme have been utilized as natural antimicrobials in different food preparations, while uncommon, plant‐derived essential oils have received minimal attention. For example, Thymbra capitata essential oil, containing mainly carvacrol (93.31%) as oxygenated monoterpenes, exhibited strong antimicrobial activity. It was assayed as a potential sanitizing solution in food industry for food contact surfaces (Falcó, Verdeguer, Aznar, Sánchez, & Randazzo, 2019). Essential oils of Azadirachta indica and Litsea cubeba were recognized as promising and successful new antimicrobials with significant antimicrobial activity against the Staphylococcus aureus and Escherichia coli, concerning possible applicability in food (Thielmann, Muranyi, & Kazman, 2019). Subinhibitory concentrations of Origanum vulgare and Rosmarinus officinale combined essential oils effectively inhibited the growth and survival of different pathogenic (Listeria monocytogenes, Yersinia enterocolitica, Aeromonas hydrophila) and spoilage (Pseudomonas fluorescens) bacteria related to minimally processed vegetables. Sensory evaluation showed that consumers find it acceptable for the different essential oils to be used together at subinhibitory concentrations as vegetable sanitizer. These forms of essential oils may contribute to the extension of the storage and shelf‐life of vegetables (Azeredo et al., 2011). The effectiveness of essential oil combination is associated with the increased membrane damages showing a stronger antimicrobial effect (Yuan, Teo, & Yuk, 2018). A recent study proposes the use of essential oils as natural antibacterial inhibitors in nanoemulsion formulations against foodborne pathogens isolated from fresh fruits and vegetables (Amrutha, Sundar, & Shetty, 2017). The microbial quality and safety of minimally processed vegetables have been remarkably improved with the application of essential oil‐based nanoemulsions. These formulations were used as washing disinfectant or incorporated into edible coatings on packaging (Dvir et al., 2019) or product surface (Prakash et al., 2018). A new application is small concentrations of cinnamon essential oil, which are capable of reducing the attachment of Salmonella strains to the lettuce surface during refrigerated storage, with a remarkably positive effect on food safety (Rossi et al., 2019).
For the prevention of foodborne disease occurrence, it could be useful to know the specific pattern of pathogenic strains in different geographic regions. Susceptibility of these bacteria to essential oils could be a good candidate for combating them using antimicrobial packaging, without risk of the emergence of antibiotic resistance. In this research, we determined the antimicrobial effect of different essential oils and certain combinations of essential oils, with the agar diffusion method on pathogenic as well as spoilage bacteria isolated on selective culture media and identified, from the surface of various fresh vegetables.
2. MATERIALS AND METHODS
The first step of our research was to examine the microbial contamination of the surface of various fresh vegetables (cucumber, lettuce, tomatoes, peppers, hot peppers, cabbage, radishes, broccoli, and onions) with cultivation methods on different selective media. Pseudomonas isolation agar base was used to detect the presence of Pseudomonas species. TBX Chromo Agar was used for the detection and enumeration of Escherichia coli. XLD Agar and BPLS agar were used for the detection of Salmonella species. Campylobacter‐Blood Free Selective Agar was used for the detection of Campylobacter species. TCBS Agar was applied for the detection of Vibrio species, for the detection of Staphylococcus sp. Mannitol Salt Agar was used. Cereus Selective Agar was applied for the detection of Bacillus cereus, and Listeria mono Differential Agar was used for the detection of Listeria species.
The vegetables were purchased from the local market. The bacterial strains occurring at highest amount on selective media were identified by molecular biological method. Subsequently, the antibacterial activity of a variety of individual herbal essential oils and certain combinations of essential oils were studied by agar diffusion method in the case of nine isolated and identified bacterial species: Pseudomonas hibiscicola, Brevibacillus agri, Enterobacter ludwigii, Curtobacterium herbarum, Acinetobacter beijerinckii, Acinetobacter calcoaceticus, Achromobacter xylosoxidans, Staphylococcus succinus, and Staphylococcus sciuri. Ten commercially available essential oils were used in this study: thyme, lemongrass, juniper, oregano, sage, fennel, rosemary, mint, rosehips, and dill. These oils were selected based on their herbal use in traditional culinary practices. These oils were produced by different companies (Solaris Plant, Adams Vision, Herbavit, Aromax, Fares Bio Vital, and Hofigal Export‐Import) and extracted from different parts of the herbs.
The identification of the isolated bacterial strains was obtained using 16S rDNA sequence analysis. AccuPrep® Genomic DNA Extraction Kit from Bioneer was used for Genomic DNA isolation. Isolations were performed according to the manufacturer's protocol. For the amplification of one part of the bacterial 16S rDNA gene, universal oligonucleotides were used 27f and 1492r (5’ AGAGTTTGATCMTGGCTCAG 3’, 5’ TACGGYTACCTTGTTACGACTT 3’). The PCR program for amplification was as follows: an initial denaturation at 94°C for 5 min, which was followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min, and a final extension at 72°C for 7 min. Sequencing was performed by Biomi KFT (Hungary). The sequences were edited and aligned using Chromas (Technelysium Pty. Ltd., South Brisbane, Australia); Molecular Evolutionary Genetics Analysis 4 system was used for the phylogenetic analyses. The isolates were identified through comparison of the sequences using the EzTaxon server on the basis of 16SrDNA sequence data (Laslo, György, Ábrahám, & Mara, 2017).
In the agar diffusion method, 20 ml nutrient agar medium was poured in a sterilized Petri dish. After solidification, the medium was inoculated on the surface with a 0.1 ml suspension of bacteria (108 CFU/ml) taken in study. In the center of all of the inoculated media, an 8 mm diameter hole was cut with the help of a sterile test‐tube. In this hole 0.05 ml of essential oil was dropped. The incubation was carried out at the temperature of 30ºC, 48 hr. In the case of the essential oil combinations, in the holes made in the inoculated media, 0.025 ml essential oils were added. After incubation, the results were read and expressed in accordance with the size of the inhibition zone (György, Laslo, & András, 2015).
The statistical analysis was performed with Statistica 8.0 (StatSoft, Inc., Oklahoma, USA).
3. RESULTS AND DISCUSSION
Among the studied vegetables, in the case of the lettuce, cucumber, radishes, and onions, the contamination was higher compared to the other vegetables. The results show that the microbiota occurring on the surface of fresh vegetables is highly variable and the molecular biological studies have identified a variety of pathogenic, saprophytic, and food spoilage bacteria (Table 1). It is important to highlight that the majority of the isolated and identificated bacteria during our research are not commonly found on the vegetable surfaces. It has been shown that different factors contribute to the diversity and composition of bacterial communities associated with the surfaces of fresh vegetables (Leff & Fierer, 2013).
TABLE 1.
Results of the identification of the bacterial strains isolated from the surface of different fresh vegetables
| Most closely related organism | Source of isolation | % Gene identity |
|---|---|---|
| Achromobacter xylosoxidans | Radish | 99.99 |
| Acinetobacter calcoaceticus | Radish | 100 |
| Acinetobacter beijerinckii | Lettuce | 99.71 |
| Bacillus thuringiensis berliner | Onion | 98.32 |
| Bacillus subtilis subsp. inaquosorum | Cabbage | 98.24 |
| Brevibacterium frigoritolerans | Broccoli | 67 |
| Brevibacillus agri | Radish | 98.77 |
| Cellulomonas pakistan e nsis | Onion | 90.07 |
| Curtobacterium herbarum | Cabbage | 98.95 |
| Curtobacterium herbarum | Onion | 87.42 |
| Enterococcus casseliflavus | Broccoli | 99,69 |
| Enterococcus casseliflavus | Cucumber | 98.35 |
| Enterobacter ludwigii | Radish | 99.8 |
| Enterobacter ludwigii | Radish | 99.48 |
| Pseudomonas geniculate | Cucumber | 98.55 |
| Pseudomonas hibiscicola | Cucumber | 99.9 |
| Pseudomonas hibiscicola | Tomato | 98.98 |
| Pseudomonas hibiscicola | Cucumber | 99.19 |
| Pseudomonas hibiscicola | Cucumber | 99.17 |
| Pseudomonas hibiscicola | Cucumber | 99.15 |
| Pseudomonas monteilii. | Lettuce | 99.61 |
| Pseudomonas putida | Cucumber | 98.49 |
| Pseudomonas oryzihabitans | Cucumber | 99.45 |
| Pseudomonas oryzihabitans | Cucumber | 98.94 |
| Staphylococcus succinus subsp. succinus | Red pepper | 99.85 |
| Staphylococcus sciuri subsp. sciuri | Tomato | 99.9 |
| Staphylococcus sciuri subsp. sciuri | Tomato | 99.7 |
| Staphylococcus sciuri subsp. sciuri | Tomato | 100 |
| Staphylococcus sciuri subsp. sciuri | Red pepper | 98.87 |
| Stenotrophomonas maltophilia | Lettuce | 98.78 |
The identified bacterial strains were isolated from the used selective media as follows: a Pseudomonas hibiscicola from Mannitol Salt Agar; another Pseudomonas hibiscicola, P. putida, and P. monteilii from Pseudomonas isolation agar base; Brevibacillus agri and P. geniculata from BPLS agar (for the isolation of Salmonella); Enterobacter ludwigii and Achromobacter xylosoxidans from XLD Agar; Curtobacterium herbarum, Enterococcus casseliflavus, and Cellulomonas pakistanensis from Listeria mono Differential Agar; Acinetobacter beijerinckii and Acinetobacter calcoaceticus from Campylobacter‐Blood Free Selective Agar; Staphylococcus succinus and Staphylococcus sciuri from Mannitol Salt Agar, Bacillus thuringiensis berliner, Bacillus subtilis subsp. inaquosorum, and Brevibacterium frigoritolerans from Cereus Selective Agar; and Pseudomonas oryzihabitans from TBX Chromo Agar.
According to the result of the 16S rDNA sequence analysis, the bacterial isolates belong to 11 genera, showing 98%–100% similarity, with the exception of three bacterial isolates. In these cases, the gene identity is below 98%.
The most commonly occurring species was Pseudomonas hibiscicola. Pseudomonas genus includes large numbers of species. The majority of spoilage bacteria can transform due to the metabolization wide variety of food components as carbohydrates, proteins, and lipids. The Pseudomonas hibiscicola bacterium was firstly isolated by Monis in 1963, but further research shows that this strain can be found in the microbial nomenclature also known as Xanthomonas maltophilia and Stenotrophomonas maltophilia. On the basis of the phylogenetic tree of the γ and the γ‐β subclasses of the Proteobacteria derived from the similarities of the 16S rDNA sequence, Pseudomonas hibiscicola belong to the Xanthomonas group, next to Stenotrophomonas maltophilia (Anzai, Kim, Park, Wakabayashi, & Oyaizu, 2000). Bacterial strain designated SD8 was isolated from sea muds of the Qinhuangdao sea area in China. This marine bacterial strain was able to produce alkaline protease approximatively at a low yield. The bacterial strain was identified as Pseudomonas hibiscicola based on 16S rDNA sequence analysis and morphological, physiological, and biochemical characterization (Cui, Yang, Wang, & Xian, 2015). Pseudomonas hibiscicola was isolated from ticks (Murrell et al., 2003). This bacterium is naturally occurring on the surface of roots, in the soil, and is an opportunistic plant pathogen. Other isolated Pseudomonas species were as follows: Pseudomonas oryzihabitans, P. geniculata, P. putida, and P. monteilii. Other isolated bacteria with highest occurrence on the surface of fresh vegetables were as follows: Acinetobacter beijerinckii, A. calcoaceticus, Achromobacter xylosoxidans, Bacillus thuringiensis berliner, Bacillus subtilis subsp. inaquosorum, Staphylococcus sciuri subsp. sciuri, Staphylococcus succinus subsp. succinus, Enterococcus casseliflavus, Enterobacter ludwigii, Brevibacillus agri, Brevibacterium frigoritolerans, Curtobacterium herbarum, Cellulomonas pakistanensis, and Stenotrophomonas maltophilia.
Based on the results, the antimicrobial activity of essential oils shows differences in the function of bacterial strains. The higher efficiency was observed for oregano essential oil, when total inactivation was observed for some strains (Pseudomonas hibiscicola, Brevibacillus agri), and for the other studied strains, the formation of large inhibition zone was observed. The highest number of Brevibacillus strains have been isolated from different natural environments. The origin of these strains is soils, where they occur as saprophytes. Some strains have also been isolated from human clinical samples and from human diseases. Brevibacillus agri is an aerobic, motile, oxidase negative, catalase positive, Gram‐positive bacteria with rod shaped cell morphology, isolated from soil, water, sterilized milk, clinical specimens, pharmaceutical manufacturing plants, and a public water supply where it was implicated in an outbreak of waterborne illness (Vos et al., 2009).
The essential oils of thyme and mint also show elevated antimicrobial activity (Table 2). The mint essential oil determined total inhibition to Pseudomonas hibiscicola, Enterobacter ludwigii, and Achromobacter xylosoxidans. Enterobacter ludwigii strains are motile rods, Gram‐negative bacteria. These strains are characterized by the possession of catalase, oxidase, and the lack of DNAase. They are fermentative bacteria and nonpigmented. They exhibit the same general characteristic as the family Enterobacteriaceae, the genus Enterobacter, and the E. cloacae complex. The capacity to grow on myo‐inositol and 3–0‐methyl‐D‐glucopyranose can differentiate the E. ludwigii from the other species of the genus (Hoffmann et al., 2005). Enterobacter species, such Enterobacter ludwigii, can cause different infections connected to abdominal, urinary tract, meningeal, and surgical sites (Khajuria, Praharaj, Grover, & Kumar, 2013). Achromobacter xylosoxidans belong to the Alcaligenaceae family. This strain is an aerobic characterized by motility, nonfermenting Gram‐negative rod. This bacterium is oxidase—and catalase positive, and is ubiquitous in aqueous environments. A. xylosoxidans is generally considered as an opportunistic microorganism with low virulence. It is most often isolated from humans. It was detected in adults and neonates suffering from simultaneous chronic diseases comorbidities and from indwelling medical devices (Claassen, Reese, Mysliwiec, & Mahlen, 2011). Primarily, it has also been detected in different infectious etiologies as in immunocompromised individuals. A case was also reported where A. xylosoxidans caused osteomyelitis in a patient with diabetes mellitus. Generally, this bacterium is characterized by multidrug resistance (Shinha & Oguagha, 2015). In patients with skin and soft tissue infections or with vascular diseases, A. xylosoxidans should be treated as potential pathogen. In humans, after surgery or trauma, pathogenic bacteria should also be considered (Tena, Martínez, Losa, & Solís, 2014).
TABLE 2.
The effect of the essential oils on growth of the studied bacteria I. (Inhibition zone in mm, average ± S.D., n = 10)
| Studied bacteria | Thyme | Oregano | Mint | Lemongrass | Sage |
|---|---|---|---|---|---|
| Pseudomonas hibiscicola | Total inhibition | Total inhibition | Total inhibition | 10.2 ± 0.20 | 2.8 ± 0.20 |
| Brevibacillus agri | Total inhibition | Total inhibition | 3.4 ± 0.24 | 10.6 ± 0.24 | 3.8 ± 0.37 |
| Enterobacter ludwigii | 9.0 ± 0.0 | 18.2 ± 1.07 | Total inhibition | 2.0 ± 0,0 | No inhibition |
| Curtobacterium herbarum | 13.2 ± 1.39 | 19.2 ± 0.80 | 1.0 ± 0.0 | 1.0 ± 0.0 | No inhibition |
| Acinetobacter beijerinckii | 22.8 ± 0.20 | 17.6 ± 0.81 | 4.8 ± 0.20 | 7.0 ± 0.0 | 1.0 ± 0.0 |
| Acinetobacter calcoaceticus | 41.2 ± 1.11 | 27.4 ± 1.25 | 3.6 ± 0.24 | 3.2 ± 0.20 | 3.0 ± 0.0 |
| Achromobacter xylosoxidans | 4.8 ± 0.37 | 11.4 ± 0.51 | Total inhibition | 2.0 ± 0.0 | No inhibition |
| Staphylococcus succinus | 23.6 ± 1.81 | 31.2 ± 4.37 | 1.4 ± 0.24 | 5.6 ± 0.24 | 1.0 ± 0.0 |
| Staphylococcus sciuri | 11.2 ± 0.20 | 20.0 ± 2.12 | 2.6 ± 0.24 | 8.0 ± 0.0 | 2.4 ± 0.24 |
The sensitivity of bacterial strains isolated from the surface of fresh‐cut vegetables relative to other selected essential oils was low or even shows lack of effects. The weakest activity on bacteria was shown by rosehips and sage essential oils (Tables 2 and 3).
TABLE 3.
The effect of the essential oils on growth of the studied bacteria II. (Inhibition zone in mm, average ± S.D., n = 10)
| Studied bacteria | Rosemary | Fennel | Juniper | Dill | Rose hips |
|---|---|---|---|---|---|
| Pseudomonas hibiscicola | 6.5 ± 0.39 | 4.0 ± 0.45 | 5.4 ± 0.24 | 5.6 ± 0.40 | 4.1 ± 0.40 |
| Brevibacillus agri | 5.7 ± 0.30 | 1.4 ± 0.24 | 5.0 ± 0.45 | 12.2 ± 1.74 | No inhibition |
| Enterobacter ludwigii | 9.4 ± 0.40 | 1.0 ± 0.0 | 6.6 ± 1.05 | No inhibition | No inhibition |
| Curtobacterium herbarum | 13.0 ± 0.0 | 1.0 ± 0.0 | No inhibition | 2.0 ± 0.0 | No inhibition |
| Acinetobacter beijerinckii | 8.8 ± 1.96 | 7.8 ± 0.37 | 6.2 ± 0.37 | Total inhibition | No inhibition |
| Acinetobacter calcoaceticus | 7.0 ± 0.0 | 3.6 ± 0.24 | 9.2 ± 0.86 | 18.0 ± 0.45 | No inhibition |
| Achromobacter xylosoxidans | 5.6 ± 0.24 | No inhibition | No inhibition | No inhibition | 1.0 ± 0.0 |
| Staphylococcus succinus | 14.8 ± 0.37 | 1.0 ± 0.0 | 3.6 ± 0.4 | 1.0 ± 0.0 | No inhibition |
| Staphylococcus sciuri | 11.6 ± 0.24 | 2.4 ± 0.24 | 5.6 ± 0.40 | 2.0 ± 0.0 | No inhibition |
In case of the dill essential oil, total inactivation was observed for Acinetobacter beijerinckii. Acinetobacters are present naturally in soil and water and occur in sewage. They have been detected in different foods such as raw, washed, and frozen vegetables, and they have also occurred in fresh, frozen, and stored fish products, as well as in spoiled animal‐origin foods such as meat, milk, and cheese (Brenner, Krieg, & Staley, 2005). Different strains of Acinetobacter beijerinckii were isolated from human clinical samples (Nemec et al., 2009), from ready to eat fruit and lettuce (Carvalheira, Silva, & Teixeira, 2017).
The most sensitive bacterium against essential oils was Pseudomonas hibiscicola, and the most resistant was Curtobacterium herbarum. Strains belonging to Curtobacterium have been isolated from different plants and rice, and C. herbarum was isolated from grass. Curtobacterium strains are rarely isolated from clinical specimens. It is recommended for clinical microbiologists to be aware of the possible occurrence of these bacteria in human samples. Because of the everyday exposure of people to Curtobacterium their pathogenicity is considered rather low (Funke, Aravena‐Roman, & Frodl, 2005).
Based on the results, the most resistant strain against the essential oil combinations was the Curtobacterium herbarum. The essential oils exert only a small antimicrobial effect on them (the most effective was the oregano essential oil), and in 7 cases, a lack of inhibition was observed (Tables 4, 5, 6 and 7). Similar results were obtained for the strain Achromobacter xylosoxidans, 7 essential oil combinations did not inhibit them, and generally a slight antibacterial effect was observed. The Enterobacter ludwigii strain shows resistance to essential oils, since in most cases, minimal inhibition was detectable (likewise, the oregano was the most effective). However, in this last two cases, the combination of thyme and dill essential oils showed synergism. The most susceptible to single essential oil inhibition was the bacteria Pseudomonas hibiscicola, alongside the essential oil combinations. In case of five combinations of essential oil, synergistic effect was observed (i.e., a larger inhibition zone was observed for combinations in comparison with the effect of the individual essential oils). The synergistic combinations were as follows: lemongrass‐rosemary, sage‐rosemary, dill‐rosemary, juniper‐cumin, and cumin‐dill. For the bacterial strain Acinetobacter calcoaceticus juniper‐cumin essential oil combination show an enhanced antibacterial effect. Species belonging to the Acinetobacter calcoaceticus–Acinetobacter baumannii complex group are believed to be nosocomial pathogens. This group of bacteria is nonfermenting, aerobic, and Gram‐negative coccobacilli (Lai et al., 2012).
TABLE 4.
The effect of the combination of essential oils on the studied bacteria I. (Inhibition zone in mm, average ± S.D., n = 10)
| Studied bacteria | Lemongrass + Juniper | Lemongrass + Fennel | Lemongrass + Sage | Lemongrass + Rosemary | Lemongrass + Rose hips |
|---|---|---|---|---|---|
| Pseudomonas hibiscicola | 11.2 ± 0.58 | 8.8 ± 0.25 | 5.4 ± 0.24 | 29.2 ± 5.45 | 5.2 ± 0.37 |
| Brevibacillus agri | 4.0 ± 0.45 | 5.8 ± 0.66 | 1.4 ± 0.24 | 4.6 ± 0.24 | 4.0 ± 0.32 |
| Enterobacter ludwigii | 3.4 ± 0.87 | 1.6 ± 0.24 | 3.2 ± 0.0 | 5.8 ± 0.58 | 1.0 ± 0.0 |
| Curtobacterium herbarum | No inhibition | 1.0 ± 0.0 | No inhibition | 6.8 ± 0.2 | 1.0 ± 0.0 |
| Acinetobacter beijerinckii | 6.6 ± 0.24 | 9.6 ± 0.51 | 9.6 ± 0.24 | 11.2 ± 0.2 | 8.8 ± 0.37 |
| Acinetobacter calcoaceticus | 10.2 ± 0.58 | 3.8 ± 0.2 | No inhibition | 12.8 ± 0.37 | 8.0 ± 0.55 |
| Achromobacter xylosoxidans | 4.8 ± 0.20 | 1.0 ± 0.0 | No inhibition | 10.0 ± 0.32 | 1.0 ± 0.0 |
| Staphylococcus succinus | 8.0 ± 0.45 | 4.8 ± 0.37 | 3.6 ± 0.4 | 10.0 ± 0.0 | 6.4 ± 0.4 |
| Staphylococcus sciuri | 5.8 ± 0.2 | 6.4 ± 0.24 | 5.0 ± 0.0 | 8.0 ± 0.32 | 5.4 ± 0.24 |
TABLE 5.
The effect of the combination of essential oils on the studied bacteria II. (Inhibition zone in mm, average ± S.D., n = 10)
| Studied bacteria | Juniper + Fennel | Juniper + Sage | Juniper + Rosemary | Dill + Fennel | Dill + Sage |
|---|---|---|---|---|---|
| Pseudomonas hibiscicola | 19.8 ± 0.37 | 3.6 ± 0.40 | 11.4 ± 0.51 | 14.2 ± 1.02 | 1.4 ± 0.24 |
| Brevibacillus agri | 6.6 ± 0.51 | 10.8 ± 1.07 | 2.8 ± 0.20 | 10.8 ± 1.39 | 9.6 ± 0.24 |
| Enterobacter ludwigii | No inhibition | No inhibition | 2.8 ± 0.37 | 2.4 ± 0.24 | 2.4 ± 0.24 |
| Curtobacterium herbarum | 2.8 ± 0.37 | 2.6 ± 0.24 | 9.6 ± 0.24 | 2.6 ± 0.4 | 1.8 ± 0.2 |
| Acinetobacter beijerinckii | 10.2 ± 0.49 | No inhibition | 13.0 ± 0.32 | 16.2 ± 0.97 | 26.0 ± 1.55 |
| Acinetobacter calcoaceticus | Total inhibition | No inhibition | 14.6 ± 0.24 | 5.6 ± 0.6 | 15.0 ± 1.41 |
| Achromobacter xylosoxidans | 1.4 ± 0.24 | No inhibition | No inhibition | 3.4 ± 0.51 | No inhibition |
| Staphylococcus succinus | 2.0 ± 0.0 | 1.0 ± 0.0 | 5.0 ± 0.0 | 1.8 ± 0.2 | 2.8 ± 0.37 |
| Staphylococcus sciuri | 2.0 ± 0.0 | 6.8 ± 0.2 | 9.4 ± 0.24 | 1.0 ± 0.0 | 3.2 ± 0.37 |
TABLE 6.
The effect of the combination of essential oils on the studied bacteria III. (Inhibition zone in mm, average ± S.D., n = 10)
| Studied bacteria | Dill + Rosemary | Sage + Rosemary | Sage + Fennel | Thyme + Mint | Thyme + Dill |
|---|---|---|---|---|---|
| Pseudomonas hibiscicola | 29.2 ± 1.74 | 22.4 ± 0.93 | 10.0 ± 0.71 | Total inhibition | 29.2 ± 1.74 |
| Brevibacillus agri | 13.0 ± 0.89 | 4.8 ± 0.58 | 2.9 ± 0.10 | 4.0 ± 0.45 | 21.6 ± 1.29 |
| Enterobacter ludwigii | 3.8 ± 0.20 | 3.6 ± 0.68 | No inhibition | 3.4 ± 0.24 | 17.2 ± 1.66 |
| Curtobacterium herbarum | 9.2 ± 0.2 | 7.4 ± 0.24 | 3.2 ± 0.49 | 4.6 ± 0.24 | 5.0 ± 0.0 |
| Acinetobacter beijerinckii | 11.0 ± 0.32 | 10.0 ± 0.32 | 2.6 ± 0.24 | 10.6 ± 0.51 | 14.4 ± 0.51 |
| Acinetobacter calcoaceticus | 10.6 ± 0.51 | 10.8 ± 0.37 | 1.6 ± 0.24 | 14.4 ± 0.75 | 23.0 ± 0.71 |
| Achromobacter xylosoxidans | 9.2 ± 0.2 | 9.0 ± 0.32 | No inhibition | 5.6 ± 0.93 | 10.0 ± 1.14 |
| Staphylococcus succinus | 9.4 ± 0.24 | 8.8 ± 0.2 | 1.0 ± 0.0 | 10.0 ± 0.0 | 7.0 ± 0.95 |
| Staphylococcus sciuri | 8.2 ± 0.2 | 9.4 ± 0.24 | 5.6 ± 0.51 | 3.0 ± 0.0 | 2.8 ± 0.2 |
TABLE 7.
The effect of the combination of essential oils on the studied bacteria IV. (Inhibition zone in mm, average ± S.D., n = 10)
| Studied bacteria | Thyme + Juniper | Mint + Juniper | Mint + Sage | Oregano + Sage | Rosehips + sage |
|---|---|---|---|---|---|
| Pseudomonas hibiscicola | 26.4 ± 2.46 | 6.4 ± 1.03 | 2.4 ± 0.24 | 10.4 ± 0.75 | 1.0 ± 0.0 |
| Brevibacillus agri | 28.8 ± 3.31 | 4.2 ± 0.73 | 4.0 ± 0.55 | 11.0 ± 0.32 | No inhibition |
| Enterobacter ludwigii | 6.8 ± 0.8 | 4.2 ± 0.37 | 2.0 ± 0.0 | 3.2 ± 0.0 | No inhibition |
| Curtobacterium herbarum | No inhibition | No inhibition | No inhibition | No inhibition | No inhibition |
| Acinetobacter beijerinckii | 7.6 ± 0.24 | 12.6 ± 0.68 | 6.6 ± 0.81 | 9.6 ± 0.24 | No inhibition |
| Acinetobacter calcoaceticus | 18.4 ± 0.68 | 10.6 ± 0.51 | 10.6 ± 0.4 | No inhibition | 4.2 ± 0.37 |
| Achromobacter xylosoxidans | 6.8 ± 0.66 | 2.0 ± 0.0 | No inhibition | 1.0 ± 0.0 | No inhibition |
| Staphylococcus succinus | 10.6 ± 0.68 | 4.0 ± 0.0 | 2.4 ± 0.24 | 1.0 ± 0.0 | No inhibition |
| Staphylococcus sciuri | 2.0 ± 0.0 | 3.0 ± 0.0 | 1.0 ± 0.0 | 6.6 ± 0.24 | 1.0 ± 0.0 |
For evaluation of antibacterial effect of isolated strains of pure and combined essential oils, a multivariate statistical method, principal component analysis (PCA) was used. The PCA results showed that the first principal component accounted for 54.44%, the second component 30.50% and 12.59% the third component of the total variance. The first three principal components (PC) together accounted for 97.53% of the total variance.
According to eigenvalues, which represent the total amount of variance explained by a given principal component, the components (factor) with an eigenvalue >1.00 are retained and interpreted. The essential oil antibacterial effects on different strains are represented on the biplot analysis on the PC1–PC2, PC1–PC3, and PC2–PC3 coordinate systems (Figure 1). The PCA shows that the essential oils had a selective antibacterial effect. Oregano and thyme showed closer association and exerted the greatest antimicrobial effect on the majority of bacterial strains. It can be observed that the majority of essential oils (grouped in the left quadrant of the biplot PC1‐PC2, PCA figure (b)) exhibited low or lack of activity. The mint with three total inhibition cases is also represented on PCA figure (b). It was also shown that mint possesses good antibacterial activity against Enterobacter aerogenes, E. cloacae, resulting damage to membrane (Bouyahya et al., 2020; Raut & Karuppayil, 2014). The rosemary is situated in the same quadrant with oregano, possessing a high selectivity against Staphylococcus species and Curtobacterium herbarum, as well as moderate antimicrobial effect against the other isolated bacterial strains.
FIGURE 1.
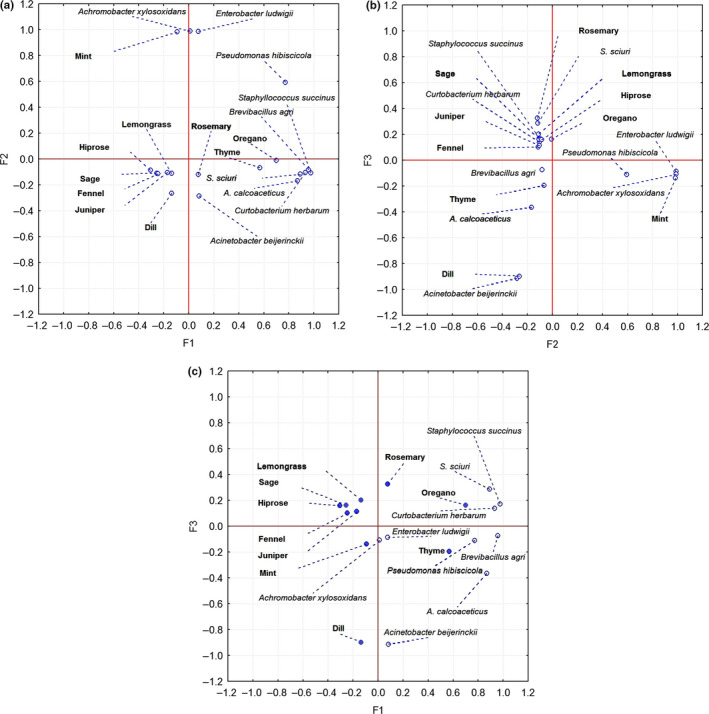
PCA biplot analysis of PCs—(a) PC1‐PC2, (b) PC1‐PC3, (c) PC2‐PC3—to elucidate the efficiency of antibacterial effects of ten essential oils on different isolated bacterial strains
To evidentiate the inhibition synergy between the components of EO combinations, the inhibition zone diameter measurement results were subjected to PCA. The results showed that the first principal component (PC) accounted for 46.78%, the second 20.03%, and the third 11.43% of the total variation.
The statistical evaluation of the antimicrobial effect of the EO combinations (expressed by inhibition zone mean diameter) resulted in the greatest sensitivity effect for T + J (thyme + juniper) and T + D (thyme + dill) combination. Based on Figure 2 (PC1‐PC2 scatterplot), other adjacent associations that present synergism are J + R (juniper + rosemary), L + R (lemongrass + rosemary), S + R (sage + rosemary), and D + R (dill + rosemary). These combinations possess synergistic antibacterial effect. The synergistic effect of rosemary, thyme with other spice essential oils was reported by other researcher too. It was observed that less‐active compounds, as in the case of rosemary, are enhanced by compounds from other EOs. The complementary chemical constituents contribute to the damage of the outer membrane or metabolic activities (García‐Díez et al., 2017; Ambrosio et al., 2019; Nikkhah, Hashemi, Habibi Najafi, & Farhoosh, 2017).
FIGURE 2.
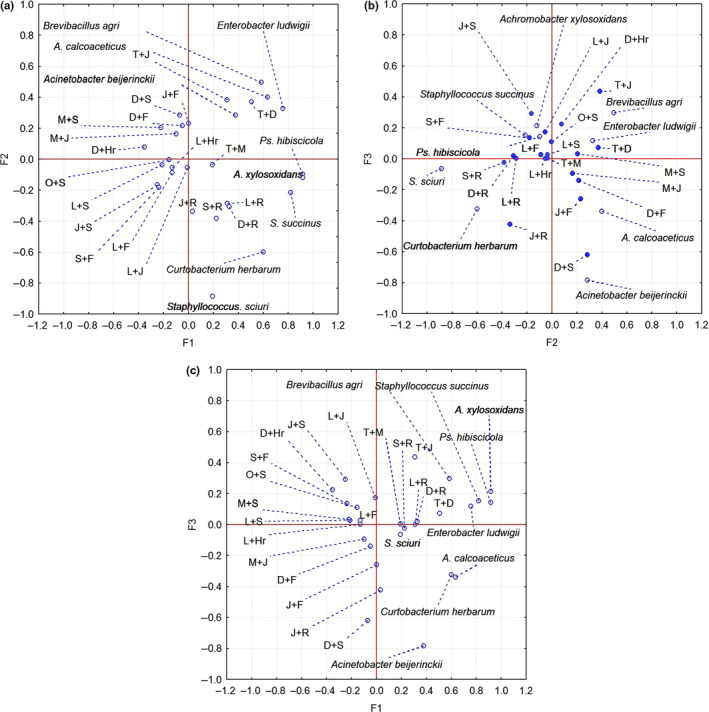
PCA biplot analysis of PCs—(a) PC1‐PC2, (b) PC1‐PC3, (c) PC2‐PC3 of the antibacterial activity of the studied essential oil combinations (L‐Lemongrass, J‐ Juniper,F‐ Fennel, S‐Sage, R‐ Rosemary, Hr‐ Rosehips, D‐Dill, T‐Thyme, M‐Mint, O‐Oregano) on isolated strains
In the PC1‐PC3 scatterplot in the two right quadrants are placed the combination of EOs with only weak antibacterial effect. PC1 contains the strains Pseudomonas hibiscicola, Acinetobacter calcoaceticus, Achromobacter xylosoxidans, Staphylococcus succinus, and PC3 containing Brevibacillus agri. It was observed that the aforementioned strains were the most sensitive to EOs combinations. According to recent researches (Raut & Karuppayil, 2014; Reddy, 2019), various essential oils exhibit antibacterial activity against human pathogenic as well as food spoilage bacteria and against the isolated strains from fresh vegetable surface.
Using essential oil as antimicrobial agent (e.g., in food preservation and packaging) may decrease the risk of foodborne infections and could decrease the overuse of antibiotics, especially the emergence of antimicrobial resistance (AR). Essential oil compounds affect the bacterial cells by different mechanisms in comparison with traditional antibiotics. A synergic effect between these two compound classes may even occur. Another important strategy in the antibacterial fight is the reversing the antibiotic resistance (Kristiansen, Thomsen, Martins, Viveiros, & Amaral, 2010). The general principle of this strategy is given by the concept of collateral sensitivity, namely that microbial populations adapted to one class of antibiotics will have low fitness for one other class of antimicrobial compounds (Pál, Papp, & Lázár, 2015). A main AR mechanism is the increased activity of efflux pumps. The decreased activity of these pumps make bacteria vulnerable (Lázár et al., 2013), and active blocking of the efflux pump function or cell wall disruption is an effective way to counteract the AR (Langeveld, Veldhuizen, & Burt, 2014; Mouwakeh, Telbisz, Spengler, Mohácsi‐Farkas, & Kiskó, 2018). As these two mechanisms are very common for essential oil compounds, this could be the key for AR reversing activity of the EOs (Yap, Yiap, Ping, & Lim, 2014). The efficiency of this strategy was confirmed experimentally for Gram‐negative multidrug‐resistant strains (Lorenzi et al., 2009; Yap, Lim, Hu, & Yiap, 2013). In case of multidrug resistance, blocking the efflux pumps with essential oil compounds may prevent the ejecting of multiple drugs from microbial cells, which have lost their efficiency due by AR. Previously, the strategy of regaining antibiotic efficiency by incorporation of nonantibiotic compounds (e.g., clavulanic acid, a β‑lactamase inhibitor) was effective in the fight with AR in the case of penicillin‐resistant strains (Cheesman, Ilanko, Blonk, & Cock, 2017). Until now, only several EOs possess a demonstrated strong individual antimicrobial effectiveness and more research is needed. Even though the antimicrobial effect of the majority of EO compounds is significantly weaker compared to antibiotics, their low toxicity level, as well as their natural origin, makes them attractive in both the food and cosmetic industries (Wińska et al., 2019).
4. CONCLUSION
The present study has shown that different pathogenic and food spoilage bacteria occur on the surface of fresh vegetables. The antimicrobial activity of studied essential oils of locally grown spices on the growth of identified bacteria shows differences in function of bacterial strains. The highest efficiency was observed for oregano essential oil. Some of the essential oil used in our experiments contains thymol and carvacrol (oregano and thyme) as main active component, with demonstrated efflux pump inhibitor effects, that are good candidate in prevention or even reversal of antibiotic resistance. Based on the results, it can be concluded that some individual essential oils and mixture compositions have the potential for practical antimicrobial application. These could be good candidates for the preservation of fresh vegetables, decreasing the health risk of foodborne infections, and also could contribute to the suppression of antibiotic resistance.
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest.
ACKNOWLEDGMENTS
We would like to thank the Sapientia Foundation—Institute for Scientific Research for financial support.
György É, Laslo É, Hajnalka Kuzman I, Dezső András C. The effect of essential oils and their combinations on bacteria from the surface of fresh vegetables. Food Sci Nutr. 2020;8:5601–5611. 10.1002/fsn3.1864
REFERENCES
- Adams, M. R. , & Moss, M. O. (2008). Food Microbiology (pp. 153–158). Cambridge, IL: The Royal Society of Chemistry. [Google Scholar]
- Ambrosio, C. M. S. , Ikeda, N. Y. , Miano, A. C. , Saldaña, E. , Moreno, A. M. , Stashenko, E. , … Da Gloria, E. M. (2019). Unraveling the selective antibacterial activity and chemical composition of citrus essential oils. Scientific Reports, 9, 17719 10.1038/s41598-019-54084-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrutha, B. , Sundar, K. , & Shetty, P. H. (2017). Spice oil nanoemulsions: Potential natural inhibitors against pathogenic E. coli and Salmonella spp. from fresh fruits and vegetables. LWT –. Food Science and Technology, 79, 152–159. 10.1016/j.lwt.2017.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzai, Y. , Kim, H. , Park, J.‐Y. , Wakabayashi, H. , & Oyaizu, H. (2000). Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. International Journal of Systematic and Evolutionary Microbiology, 50, 1563–1589. 10.1099/00207713-50-4-1563 [DOI] [PubMed] [Google Scholar]
- Bouyahya, A. , Lagrouh, F. , Omari, N. E. , Bourais, I. , Jemli, M. E. , Marmouzi, I. , … Bakri, Y. (2020). Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatalysis and Agricultural Biotechnology, 23, 101471, 10.1016/j.bcab.2019.101471 [DOI] [Google Scholar]
- Brenner, D. J. , Krieg, N. R. , & Staley, J. T. (2005). Bergey's Manual of Systematic Bacteriology: Volume 2: The Proteobacteria (p. 425). New York, IL: Springer, US. [Google Scholar]
- Carvalheira, A. , Silva, J. , & Teixeira, P. (2017). Lettuce and fruits as a source of multidrug resistant Acinetobacter spp. Food Microbiology, 64, 119–125. 10.1016/j.fm.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Cayalvizhi, B. S. , & Balachandhar, D. (2019). Prevalence of Shiga‐like toxin producing Escherichia coli strain (E. coli O157) in freshly consumed vegetables and its characterization. Journal of Food Safety, 39, 1–8. 10.1111/jfs.12577 [DOI] [Google Scholar]
- Cheesman, M. J. , Ilanko, A. , Blonk, B. , & Cock, I. E. (2017). Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacognosy Reviews, 11(22), 57–72. 10.4103/phrev.phrev_21_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen, S. L. , Reese, J. M. , Mysliwiec, V. , & Mahlen, S. D. (2011). Achromobacter xylosoxidans infection presenting as a pulmonary nodule mimicking cancer. Journal of Clinical Microbiology, 49(7), 2751–2754. 10.1128/JCM.02571-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, H. , Yang, M. , Wang, L. , & Xian, C. J. (2015). Identification of a new marine bacterial strain SD8 and optimization of its culture conditions for producing alkaline protease. PLoS One, 10(12), 1–13. 10.1371/journal.pone.0146067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Azeredo, G. A. , Stamford, T. L. M. , Nunes, P. C. , Neto, N. J. G. , de Oliveira, M. E. G. , & de Souza, E. L. (2011). Combined application of essential oils from Origanum vulgare L. and Rosmarinus officinalis L. to inhibit bacteria and autochthonous microflora associated with minimally processed vegetables. Food Research International, 44, 1541–1548. 10.1016/j.foodres.2011.04.012 [DOI] [Google Scholar]
- Deák, T. (2006). Élelmiszer mikrobiológia (pp. 269–270). Budapest, IL: Mezőgazda Kiadó. [Google Scholar]
- Dvir, I. M. , Weizman, O. , Lewitus, D. , Weintraub, S. , Ophir, A. , & Dotan, A. (2019). Antimicrobial active packaging combining essential oils mixture: Migration and odor control study. Polymers for Advanced Technologies, 30(10), 2558–2566. 10.1002/pat.4642 [DOI] [Google Scholar]
- Falcó, I. , Verdeguer, M. , Aznar, R. , Sánchez, G. , & Randazzo, W. (2019). Sanitizing food contact surfaces by the use of essential oils. Innovative Food Science and Emerging Technologies, 51, 220–228. 10.1016/j.ifset.2018.02.013 [DOI] [Google Scholar]
- Funke, G. , Aravena‐Roman, M. , & Frodl, R. (2005). First description of Curtobacterium spp. isolated from human clinical specimens. Journal of Clinical Microbiology, 43(3), 1032–1036. 10.1128/JCM.43.3.1032-1036.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Díez, J. , Alheiro, J. , Pinto, A. L. , Falco, V. , Fraqueza, M. J. , & Patarata, L. (2017). Synergistic activity of essential oils from herbs and spices used on meat products against food borne pathogens. Natural Product Communications, 12(2), 281–286. 10.1177/1934578X1701200236 [DOI] [PubMed] [Google Scholar]
- György, É. , Laslo, É. , András, C. S. D. (2015). The effect of essential oils on foodborne pathogenic and spoilage bacteria occurring on the surface of fresh vegetables In Engelhardt T., Dalmadi I., Baranyai L., & Mohácsi‐Farkas C. S. (Eds.), Book of proceedings Food Science Conference 2015, Integration of science in food chain. (pp. 86–89)Corvinus University of Budapest. [Google Scholar]
- Hoffmann, H. , Stindl, S. , Stumpf, A. , Mehlen, A. , Monget, D. , Heesemann, J. , … Roggenkamp, A. (2005). Description of Enterobacter ludwigii sp. nov., a novel Enterobacter species of clinical relevance. Systematic and Applied Microbiology, 28, 206–212. 10.1016/j.syapm.2004.12.009 [DOI] [PubMed] [Google Scholar]
- Khajuria, A. , Praharaj, A. K. , Grover, N. , & Kumar, M. (2013). First report of an Enterobacter ludwigi isolate coharboring NDM‐1 and OXA‐48 carbapenemases. Antimicrobial Agents and Chemotherapy, 57(10), 5189–5190. 10.1128/AAC.00789-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen, J. E. , Thomsen, V. F. , Martins, A. , Viveiros, M. , & Amaral, L. (2010). Non‐antibiotics reverse resistance of bacteria to antibiotics. Vivo, 24(5), 751–754. [PubMed] [Google Scholar]
- Lai, C.‐C. , Hsu, H.‐L. , Tan, C.‐K. , Tsai, H.‐Y. , Cheng, A. , Liu, C.‐Y. , … Hsuehe, P.‐R. (2012). Recurrent bacteremia caused by the Acinetobacter calcoaceticus‐Acinetobacter baumannii complex. Journal of Clinical Microbiology, 50(9), 2982–2986. 10.1128/JCM.01194-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langeveld, W. T. , Veldhuizen, E. J. , & Burt, S. A. (2014). Synergy between essential oil components and antibiotics: A review. Critical Reviews in Microbiology, 40(1), 76–94. 10.3109/1040841X.2013.763219 [DOI] [PubMed] [Google Scholar]
- Laslo, É. , György, É. , Ábrahám, B. , & Mara, G. Y. (2017). Bacterial strains with nutrient mobilisation ability from Ciuc Mountains (Transylvania Region, Romania) In Singh D., Singh H., & Prabha R. (Eds.), Plant‐Microbe Interactions in Agro‐Ecological Perspectives. Singapore: Springer. [Google Scholar]
- Lázár, V. , Pal Singh, G. , Spohn, R. , Nagy, I. , Horváth, B. , Hrtyan, M. , … Pál, C. (2013). Bacterial evolution of antibiotic hypersensitivity. Molecular Systems Biology, 9, 700 10.1038/msb.2013.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff, J. W. , & Fierer, N. (2013). Bacterial communities associated with the surfaces of fresh fruits and vegetables. PLoS One, 8(3), e59310 10.1371/journal.pone.0059310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi, V. , Muselli, A. , Bernardini, A. F. , Berti, L. , Pagès, J. M. , Amaral, L. , & Bolla, J. M. (2009). Geraniol restores antibiotic activities against multidrug‐resistant isolates from Gram‐negative species. Antimicrobial Agents and Chemotherapy, 53(5), 2209–2211. 10.1128/AAC.00919-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouwakeh, A. , Telbisz, Á. , Spengler, G. , Mohácsi‐Farkas, C. S. , & Kiskó, G. (2018). Antibacterial and resistance modifying activities of Nigella sativa essential oil and its active compounds against Listeria monocytogenes . Vivo, 32(4), 737–743. 10.21873/invivo.11302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell, A. , Dobson, S. J. , Yang, X. , Lacey, E. , & Barkey, S. C. (2003). A survey of bacterial diversity in ticks, lice and fleas from Australia. Parasitology Research, 89, 326–334. 10.1007/s00436-002-0722-4 [DOI] [PubMed] [Google Scholar]
- Nemec, A. , Musílek, M. , Maixnerová, M. , Baere, T. D. , Reijden, T. J. K. , Vaneechoutte, M. , & Dijkshoorn, L. (2009). Acinetobacter beijerinckii sp. nov. and Acinetobacter gyllenbergii sp. nov., haemolytic organisms isolated from humans. International Journal of Systematic and Evolutionary Microbiology, 59, 118–124. 10.1099/ijs.0.001230-0 [DOI] [PubMed] [Google Scholar]
- Nikkhah, M. , Hashemi, M. , Habibi Najafi, M. B. , & Farhoosh, R. (2017). Synergistic effects of some essential oils against fungal spoilage on pear fruit. International Journal of Food Microbiology, 18(257), 285–294. 10.1016/j.ijfoodmicro.2017.06.021 [DOI] [PubMed] [Google Scholar]
- Pál, C. S. , Papp, B. , & Lázár, V. (2015). Collateral sensitivity of antibiotic‐resistant microbes. Trends in Microbiology, 23(7), 401–407. 10.1016/j.tim.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrignani, F. , Siroli, L. , Serrazanetti, D. I. , Gardini, F. R. , & Lanciotti, R. (2015). Innovative strategies based on the use of the essential oils and their components to improve safety, shelf‐life and quality of minimally processed fruits and vegetables. Trends in Food Science & Technology, 46, 311–319. 10.1016/j.tifs.2015.03.009 [DOI] [Google Scholar]
- Prakash, A. , Baskaran, R. , Paramasivam, N. , & Vadivel, V. (2018). Essential oil‐based nanoemulsions to improve the microbial quality of minimally processed fruits and vegetables: A review. Food Research International, 111, 509–523. 10.1016/j.foodres.2018.05.066 [DOI] [PubMed] [Google Scholar]
- Ramos, B. , Miller, F. A. , Brandão, T. R. S. , Teixeira, P. , & Silva, C. L. M. (2013). Fresh fruits and vegetables ‐ An overview on applied methodologies to improve its quality and safety. Innovative Food Science and Emerging Technologies, 20, 1–15. 10.1016/j.ifset.2013.07.002 [DOI] [Google Scholar]
- Raut, J. S. , & Karuppayil, S. M. (2014). Bioprospecting of plant essential oils for medicinal uses In Fulekar M., Pathak B., & Kale R. (Eds.), Environment and Sustainable Development (pp. 59–77). New Delhi, IL: Springer. [Google Scholar]
- Ray, B. , & Bhunia, A. (2014). Fundamental Food Microbiology (pp. 44–45). Boca Raton, IL: CRC Press. [Google Scholar]
- Reddy, D. M. (2019). Essential oils extracted from medicinal plants and their applications In Akhtar M. S., Swamy M. K., & Sinniah U. R. (Eds.), Natural bio‐active compounds Volume 1: Production and applications (pp. 238–267). Singapore, IL: Springer. [Google Scholar]
- Rossi, C. , Chaves‐López, C. , Možina, S. S. , Mattiaa, C. D. , Scuota, S. , Luzzi, I. , … Serio, A. (2019). Salmonella enterica adhesion: Effect of Cinnamomum zeylanicum essential oil on lettuce. LWT ‐ Food Science and Technology, 111, 16–22. 10.1016/j.lwt.2019.05.026 [DOI] [Google Scholar]
- Scollard, J. , Francis, G. A. , & O'Beirne, D. (2013). Some conventional and latent anti‐listerial effects of essential oils, herbs, carrot and cabbage in fresh‐cut vegetable systems. Postharvest Biology and Technology, 77, 87–93. 10.1016/j.postharvbio.2012.11.011 [DOI] [Google Scholar]
- Shinha, T. , & Oguagha, I. C. (2015). Case report osteomyelitis caused by Achromobacter xylosoxidans . IDCases, 2, 11–12. 10.1016/j.idcr.2015.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena, D. , Martínez, N. M. , Losa, C. , & Solís, S. (2014). Skin and soft tissue infection caused by Achromobacter xylosoxidans: Report of 14 cases. Scandinavian Journal of Infectious Diseases, 46, 130–135. 10.3109/00365548.2013.857043 [DOI] [PubMed] [Google Scholar]
- Thielmann, J. , Muranyi, P. , & Kazman, P. (2019). Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus . Heliyon, 5(6), e01860 10.1016/j.heliyon.2019.e01860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos, P. D. , Garrity, G. M. , Jonnes, D. , Krieg, N. R. , Ludwig, W. , Rainey, F. A. , … Whitman, W. B. (2009). Bergey's Manual of Systematic Bacteriology: Volume 3: The Firmicutes (p. 306–313). New York, IL: Springer‐Verlag. [Google Scholar]
- Wińska, K. , Mączka, W. , Łyczko, J. , Grabarczyk, M. , Czubaszek, A. , & Szumny, A. (2019). Essential oils as antimicrobial agents‐myth or real alternative? Molecules, 24(11), 2130 10.3390/molecules24112130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, P. S. , Lim, S. H. , Hu, C. P. , & Yiap, B. C. (2013). Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid‐conferred multidrug resistant bacteria. Phytomedicine, 20(8–9), 710–713. 10.1016/j.phymed.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Yap, P. S. , Yiap, B. C. , Ping, H. C. , & Lim, S. H. (2014). Essential oils, a new horizon in combating bacterial antibiotic resistance. The Open Microbiology Journal, 8, 6–14. 10.2174/1874285801408010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, W. , Teo, C. H. M. , & Yuk, H.‐G. (2018). Combined antibacterial activities of essential oil compounds against Escherichia coli O157:H7 and their application potential on fresh‐cut lettuce coli. Food Control, 96, 112–118. 10.1016/j.foodcont.2018.09.005 [DOI] [Google Scholar]


