Abstract
Background
The role of high-dose chemotherapy with autologous stem cell transplantation (ASCT) in the treatment of soft-tissue sarcoma (STS) remains an unsettled issue. Prospective clinical trials failed to prove a benefit of the procedure but were limited by small and heterogeneous patient cohorts. Thus, it is unknown if ASCT may be a valuable treatment option in specific patient subgroups.
Methods
The purpose of this study was to investigate the value of ASCT according to histological subtype in STS patients who were registered in the European Society for Blood and Marrow Transplantation database between 1996 and 2016.
Results
Median progression-free (PFS) and overall survival (OS) in the entire cohort of 338 patients were 8.3 and 19.8 months, respectively, and PFS and OS at 5 years were 13% and 25%, respectively. Analysis of outcomes in different subgroups showed that younger age, better remission status before transplantation and melphalan-based preparative regimen were predictive of benefit from ASCT, whereas histology and grading had no statistically significant impact.
Conclusions
Outcomes after ASCT compared favorably to those of recent trials on conventional chemotherapies and targeted therapies in STS, including histology-tailored approaches. ASCT, thus, should be reinvestigated in clinical trials focusing on defined patient subgroups.
Keywords: high-dose chemotherapy, stem cell transplantation, soft-tissue sarcoma
Key questions.
What is already known about this subject?
Soft-tissue sarcomas (STSs) are a heterogeneous group of mesenchymal tumours with variable biology and clinical course. Clinical trials including all histological subtypes may, therefore, miss potential benefits of a specific treatment in a particular histological subtype.
High-dose chemotherapy and autologous stem cell transplantation (ASCT) have proven value in the treatment of only a few solid tumours and have also been investigated in STS with negative results. However, due to the heterogeneity of STS, it remains unclear if certain histological subgroups may derive benefit from ASCT.
To date, no study thoroughly investigated predictors of benefit from ASCT in a sufficiently large STS patient cohort.
What does this study add?
This, to our knowledge, is the most extensive retrospective study of ASCT in STS patients and the first to thoroughly investigate potential predictors of benefit from this treatment.
Median progression-free and overall survival in this pretreated patient cohort were 8.3 and 19.8 months, respectively, which compares favourably to recent non-transplant treatments for STS, although most patients included in the analysis were transplanted before 2006 when treatment options for STS patients were quite limited.
Predictors of benefit from ASCT were younger age, better remission status before transplantation and melphalan-based preparative regimens.
How might this impact on clinical practice?
Based on this retrospective analysis, ASCT cannot be recommended as routine treatment for the STS subgroups investigated.
The findings of favourable outcomes associated with ASCT and potential predictors of benefit in a heterogenous population of STS patients support the reinvestigation of ASCT in randomised trials with histological stratification.
Introduction
Soft-tissue sarcomas (STSs) are a group of rare, mesenchymal malignancies, which account for about 1% of adult malignancies.1 2 The current WHO classification differentiates more than 70 histological subtypes of STS, with leiomyosarcoma, liposarcoma, synovial sarcoma and undifferentiated pleomorphic sarcoma being most common.3 Although a substantial proportion of patients with localised disease can be cured with surgery and adjuvant radiotherapy and/or chemotherapy, the prognosis of patients with metastatic disease remains dismal with a median survival of less than 2 years in recent studies.4–7 Several drugs have shown activity in STS with doxorubicin and ifosfamide being the most active in terms of objective response. The notion of a dose–response relationship for ifosfamide, for example,8–10 fueled interest in high-dose chemotherapy (HDCT) as a treatment option for STS, but none of the few trials performed to date could prove a benefit of intensified treatment with autologous stem cell transplantation (ASCT). However, most studies were performed as single-arm phase II trials and included all STS histological subgroups.11–14 The only published randomised phase III trial reporting on 87 patients did not show a benefit for ASCT, but also was done in a highly heterogenous population with 18 different histologies included.15 Likewise, a meta-analysis of 294 patients included 19 different histologies, and no attempt was made to decipher a possible benefit restricted to some histological subtype.16 As there is growing evidence that clinical course and response to specific treatments differs significantly between histological subgroups of STS,2 17–20 we aimed to investigate the efficacy of HDCT and ASCT in distinct histological subtypes of STS.
Methods
Patient population
The European Society for Blood and Marrow Transplantation (EBMT) is a non-profit organisation established in 1974 to allow scientists and physicians involved in clinical SCT to share their experience and develop cooperative studies. The EBMT is divided into working parties, whose mission is the implementation of EBMT scientific and educational policy, the development and management of scientific proposals with the support of the Data and Executive Offices and assisting the definition of guidelines and policies. The Cellular Therapy and Immunobiology Working Party that includes the solid tumour subcommittee is dedicated to preclinical, translational and clinical (including retrospective) studies, including ASCT and allogeneic SCT, active and adoptive immunotherapy. EBMT centres, which are distributed in over 60 countries, are required to send patient data, including demographic and clinical, to the central EBMT database on a yearly basis. Informed consent for transplantation and data collection was obtained locally according to regulations applicable at the time of transplantation. Since 1 January 2003, all transplant centres have been required to obtain written informed consent prior to data registration with the EBMT following the Helsinki Declaration 1975. Policies were recently updated to comply with EU General Data Protection Regulation.
The present retrospective study analysed the EBMT registry data regarding adult patients with STS who underwent a first HDCT and ASCT between 1996 and 2016. All centres with eligible patients were requested to provide additional data including details on pretreatment, post-ASCT treatments and histology. Analyses were each carried out including all patients with the relevant information available for the respective analyses.
Primary outcomes were overall survival (OS; time to death from any cause) and progression-free survival (PFS; defined as survival with no evidence of relapse or progression). PFS and OS were measured from the date of first ASCT.
Statistical analysis
Probabilities of OS and PFS were calculated using the Kaplan-Meier method. Univariate analyses were done using the log-rank test. Factors studied were histological subtype of STS, grading, status prior transplant, age and gender, year of ASCT and preparative regimen. A Cox proportional hazards model was used for multivariate regression. All variables associated with one outcome in univariate analysis were included in the Cox model. In order to test for a centre effect, we introduced a random effect or frailty for each centre into the model.21 22 Results were expressed as the HR with the 95% CI. Statistical analyses were performed with SPSS V.24.0 (SPSS) and R 3.6.2 (R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
Results
Patient and treatment characteristics
A total of 338 patients met the eligibility criteria of this study. Median age at first ASCT was 37.3 years (range 18–69), 201 (59%) of patients were male. Most common histologies were leiomyosarcoma (n=66), synovial sarcoma (n=52), angiosarcoma (n=40) and liposarcoma (n=34). In 120 patients, no further information was available regarding histological subclassification. These patients, together with diagnoses occurring in 10 or less cases were grouped together as ‘other sarcomas’ in further analyses.
Regarding patients with respective information available, 45.7% had metastatic disease at diagnosis (n=92 with available information); 66.4%, 30.6% and 89.4% had prior surgery, radiotherapy and/or chemotherapy, respectively (n=140, n=134, and n=142 with available information, respectively). The median number of chemotherapy regimens before ASCT was 1 (range 1–7), with 24.6% being treated with two or more lines. Remission status prior ASCT was complete remission/no evidence of disease (CR) in 20.1%, partial remission in 39.1%, stable disease in 10.2% and progressive disease in 30.7% of patients. Patients transplanted in CR were younger but otherwise showed similar characteristics compared with patients not in CR prior ASCT (online supplemental table 1).
esmoopen-2020-000860supp001.pdf (36.4KB, pdf)
Preparative regimens were various, with platinum/etoposide/ifosfamide being used most frequently (42.5%). Stem cells were mobilised mostly with anthracycline-based or platinum-based chemotherapy (45.5%) in combination with G-CSF (Granulocyte-Colony Stimulating Factor; 98%); >95% of ASCTs were performed using mobilised peripheral blood stem cells. Relevant patient and treatment characteristics are summarised in table 1.
Table 1.
Patientand treatment characteristics
| Characteristic | n=338 | |
| Age at first ASCT, years | ||
| median (range) (IQR) | 37.3 (18.1–69.6) (27.6–49.7) | |
| Histology | n | % |
| Leiomyosarcoma | 66 | 19.5 |
| Synovial sarcoma | 52 | 15.4 |
| Angiosarcoma* | 40 | 11.8 |
| Liposarcoma | 34 | 10.1 |
| Desmoplastic small round cell tumour | 10 | 3 |
| other STS† | 136 | 40.2 |
| Tumour grading | n | % |
| Grade 1 | 4 | 3.8 |
| Grade 2 | 18 | 17.3 |
| Grade 3 | 82 | 78.8 |
| missing | 234 | – |
| Remission status before ASCT | n | % |
| Complete response/no evidence of disease | 55 | 20.1 |
| Partial response | 107 | 39.1 |
| Stable disease | 28 | 10.2 |
| Progressive disease | 84 | 30.7 |
| Missing | 64 | – |
| Year of first ASCT | n | % |
| 1996–2000 | 152 | 45.0 |
| 2001–2005 | 111 | 32.8 |
| 2006–2016 | 75 | 22.2 |
| Preparative regimen | n | % |
| PEI/CEI | 71 | 42.5 |
| Other platinum based | 31 | 18.6 |
| Melphalan based | 42 | 25.1 |
| Other | 23 | 13.8 |
| missing | 171 | – |
| Remission status after last ASCT | n | % |
| Complete response/no evidence of disease | 94 | 58.4 |
| Partial response | 19 | 11.8 |
| Stable disease | 22 | 13.7 |
| Progressive disease | 26 | 16.1 |
| missing | 177 | – |
*Including haemangiosarcoma and lymphangiosarcoma.
†Including: fibrosarcoma: n=8, malignant fibrous histiocytoma: n=3, sarcoma NOS: n=2, malignant peripheral nerve sheath tumour: n=2, fibromyxoid sarcoma: n=1, sarcoma not further subclassified: n=120.
ASCT, autologous stem cell transplantation; CEI, Carboplatinum, Etoposide, Ifosfamide; NOS, not otherwise specified; PEI, Cisplatinum, Etoposide, Ifosfamide; STS, soft-tissue sarcoma.
Outcomes after ASCT
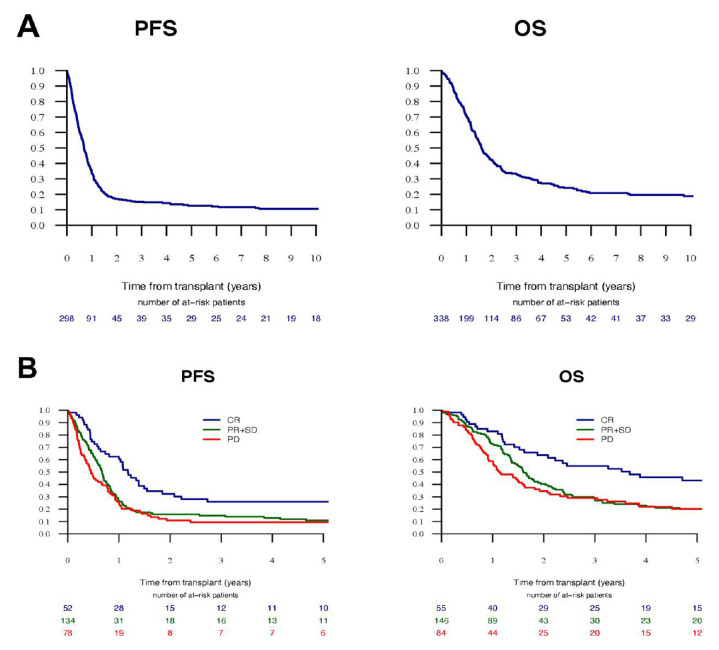
The median follow-up of survivors was 8.2 years. PFS and OS at 5 years were 12.6% and 25.2%, respectively, and median PFS and OS were 8.3 and 19.8 months, respectively. In univariate analyses, remission status prior ASCT was a significant predictor for better outcomes (figure 1). Patients in CR before ASCT had PFS and OS of 14.1 (95% CI 10.7 to 17.5) and 44.1 months (95% CI 15.3 to 72.9), respectively, whereas patients with documented non-CR status prior ASCT had PFS and OS of 7.2 (95% CI 5.8 to 8.5) and 17.8 months (95% CI 15.8 to 19.9), respectively (online supplemental table S1). Grading had no significant impact on outcomes while younger age was associated with improved survival (online supplemental figure S1). Patients treated with platinum-based preparative regimens had inferior PFS at 2 years than patients treated with melphalan based and other regimens (12% vs 25% vs 24%, respectively), but without significant impact on OS. Leiomyosarcoma patients had inferior PFS compared with patients with synovial sarcoma and angiosarcoma (7.4%, 15% and 21.3%, respectively, table 2, online supplemental figure S1).
Figure 1.
Kaplan-Meier estimates of PFS and OS in (A) the whole-study population and (B) stratified according to remission status prior ASCT. ASCT, autologous stem cell transplantation; OS, overall survival; PFS, progression-free survival.
Table 2.
Univariate analyses
| PFS | OS | |||
| 2 years | 5 years | 2 years | 5 years | |
| Age at first ASCT, years | ||||
| ≤37.3 (median) | 21.6% (15.1–28.8) | 18.6% (12.5–25.5) | 50.6% (42.1–58.6) | 31.1% (23.4–39) |
| >37.3 | 12.4% (7.5–18.6) | 6.2% (2.8–11.5) | 37.6% (29.5–45.6) | 18.9% (12.4–26.4) |
| P value | 0.006 | 0.03 | ||
| Patient sex | ||||
| Male | 15% (10–21) | 12.4%(7.8–18) | 42.2%(34.5–49.7) | 22.3%(15.9–29.3) |
| Female | 20%(13.1–28) | 13% (7.4–20.2) | 47.3% (37.9–56.1) | 29.4% (21.1–38.2) |
| p value | 0.38 | 0.26 | ||
| Histology | ||||
| Leiomyosarcoma | 9.2% (3.4–18.7) | 7.4% (2.4–16.3) | 34.2% (21.7–47.1) | 18.4% 8.9–30.6) |
| Liposarcoma | 18% (6.6–33.8) | 13.5% (3.9–29.1) | 52.5% (34–68.2) | 20.6% (8.2–36.9) |
| Synovial sarcoma | 22.5% (11.2–36.2) | 15% (6.1–27.6) | 45.3% (29.8–59.6) | 24.9% (12.9–39) |
| Angiosarcoma | 21.3% (9.4–36.4) | 21.3% (9.4–36.4) | 42.9% (25.9–58.9) | 31.8% (16.4–48.4) |
| Other sarcoma | 17.1% (10.9–24.6) | 11.3% (6.3–18.1) | 46.3% (37.3–54.8) | 27.8% (19.8–36.4) |
| P value | 0.49 | 0.63 | ||
| Remission status prior ASCT | ||||
| CR/NED | 32.4% (19.7–45.7) | 25.9% (14.5–38.9) | 63.7% (48.6–75.4) | 43.2% (28.7–56.9) |
| PR+SD | 15.8% (9.9–22.9) | 10.9% (6–17.5) | 40.3% (31.4–49.1) | 20.1% (13.1–28.1) |
| PD | 10.8% (5.1–19.1) | 9.5% (4.2–17.4) | 34.6% (24.1–45.3) | 20.1% (11.8–30.1) |
| P value | 0.001 | 0.002 | ||
| Tumour grading | ||||
| Grade 2 | 16.7% (4.1–36.5) | 5.6% (0.4–22.4) | 71.4% (44.3–87) | 33.3% (11.2–57.6) |
| Grade 3 | 18.5% (10.5–28.3) | 17% (9.4–26.6) | 50.4% (38.1–61.5) | 25.9% (15.9–37) |
| P value | 0.62 | 0.7 | ||
| Preparative regimen | ||||
| Platinum based | 12% (6.2–19.7) | 9.6% (4.5–16.9) | 49.2% (38.2–59.4) | 23% (14.4–32.8) |
| Melphalan based | 24.6% (12.2–39.1) | 21.8% (10.3–36.1) | 47.9% (31.4–62.6) | 36.6% (21.6–51.8) |
| Other | 24.5% (9–43.9) | 12.2% (2.3–31.2) | 39.1% (19–58.8) | 16.8% (4.3–36.2) |
| P value | 0.059 | 0.25 | ||
| Year of ASCT | ||||
| 1996–2000 | 18.3% (12.1–25.5) | 11.8% (6.8–18.4) | 40.8% (32.4–48.9) | 20.6% (14–28) |
| 2001–2005 | 15.1% (8.6–23.3) | 11.5% (5.9–19.2) | 42.8% (32.5–52.7) | 26.1% (16.9–36.2) |
| 2006–2016 | 17.2% (8.9–27.8) | 15.5% (7.6–25.8) | 55% (40.8–67.2) | 35.8% (22.8–49) |
| P value | 0.51 | 0.07 | ||
Bold numbers denote statistical significance (p < 0.05).
ASCT, autologous stem cell transplantation; CR, complete remission; NED, no evidence of disease; OS, overall survival; PFS, progression-free survival; PR, partial remission; SD, stable disease.
esmoopen-2020-000860supp002.pdf (125.4KB, pdf)
Cox regression analysis regarding the factors histology, age, remission status prior ASCT and preparative regimen were performed and showed better remission status prior ASCT to independently predict better PFS and OS, whereas histology had no impact on outcomes. Younger patients had better OS, whereas patients treated with melphalan-based preparative regimens experienced a significant better PFS, but not OS than the other patients (table 3).
Table 3.
Cox regression analyses
| 145 patients | 157 patients | |||
| PFS | OS | |||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age at first ASCT | 1.1 (0.93 to 1.31) | 0.24 | 1.21 (1.02 to 1.43) | 0.029 |
| Histology | ||||
| Leiomyosarcoma (reference) | ||||
| Liposarcoma | 0.86 (0.43 to 1.72) | 0.67 | 0.75 (0.37 to 1.55) | 0.44 |
| Synovial sarcoma | 1.2 (0.63 to 2.26) | 0.58 | 1.4 (0.72 to 2.72) | 0.32 |
| Angiosarcoma | 1.24 (0.66 to 2.32) | 0.50 | 1.25 (0.64 to 2.47) | 0.51 |
| Other sarcoma | 1.24 (0.76 to 2.01) | 0.40 | 1.11 (0.65 to 1.89) | 0.70 |
| Remission status prior ASCT | ||||
| CR/NED (reference) | ||||
| PR+SD | 1.49 (0.92 to 2.41) | 0.10 | 1.48 (0.87 to 2.53) | 0.15 |
| PD | 2.78 (1.62 to 4.77) | 0.0002 | 3 (1.69 to 5.32) | 0.0002 |
| Preparative regimen | ||||
| Platinum based (reference) | ||||
| Melphalan based | 0.61 (0.38 to 0.97) | 0.036 | 0.85 (0.52 to 1.4) | 0.53 |
| Other | 0.7 (0.41 to 1.22) | 0.21 | 1.2 (0.68 to 2.13) | 0.52 |
Bold numbers denote statistical significance (p < 0.05).
ASCT, autologous stem cell transplantation; CR, complete remission; NED, no evidence of disease; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial remission; SD, stable disease.
Treatment-related mortality (TRM), secondary malignancies and clinical course post-ASCT
Death without relapse occurred in seven patients, with all cases occurring in patients being transplanted before 2003. Six patients died of infectious complications after a median of 10 days after ASCT (range 4–121) and one after a myelodysplastic syndrome at 4.6 years post-ASCT. Out of 301, 244 (81.1%) patients had experienced relapse or progression at a median of 7 months after last ASCT. Data on treatments after ASCT were available in 93 patients. 36 patients had surgical resections and 27 had radiotherapy. Seventy-two per cent of the patients were treated with a median number of 1 (range 1–3) chemotherapy regimens.
Discussion
Despite the advent of new drugs and the implementation of a multidisciplinary approach for the treatment of STS in the past decades, nearly all patients with metastatic STS and a substantial proportion of patients with localised STS die of the disease. Whereas a dose–response correlation has been shown for chemotherapy in STS, the effect of further dose escalation with HDCT and ASCT is unclear, since the studies performed in the past included relatively small and heterogeneous patient populations. Our study, reporting on a retrospective data analysis of ASCT in STS, is one of the largest series in the field and, to our knowledge, the first one to attempt a thorough investigation of predictors for benefit of ASCT. Another large study, a metaanalysis of 62 trials on ASCT including 294 patients with 19 different STS histologies,16 23 also included 109 patients with desmoplastic small round cell tumour, a disease with a unique biology and clinical course,24–26 and thus is not representative for the more common STS histologies. Regarding OS, only a rough estimate was given with 20%–51% and 32%–40% of patients being alive at 2 and 3 years, respectively, which is in accordance with the OS probabilities of 44% and 35% at 2 and 3 years, respectively, in our study. The only randomised trial of ASCT in STS patients performed so far included 87 patients with various histologies and showed no benefit of ASCT vs standard dose treatment (SDT), with a median OS of 26.1 vs 28.2 months, respectively,15 which is superior to the median OS of 19.8 months observed in our study. However, in the aforementioned trial, only patients with an objective response to first-line chemotherapy were randomised between SDT and ASCT, and only half of those randomised to ASCT were actually treated per protocol. In addition, one-third of these patients had surgery prior to randomisation and were randomised in CR; thus, the data on inferior outcomes associated with ASCT in this trial are difficult to interpret, and the possibility that some subgroups might benefit from ASCT cannot be excluded. In contrast, the purpose of our study was to investigate factors that might predict benefit from ASCT to generate hypotheses for future prospective clinical trials. We, therefore, aimed to analyse a large population and included all STS patients reported to the EBMT from multiple centres in various countries, without excluding specific age groups, preparative regimens, or patients with chemorefractory disease.
Most patients in the aforementioned trials as well as our study were transplanted before 2006. Our data show a substantial higher OS in patients transplanted after 2005, which did not reach statistical significance, but is supported by the notion that experience in ASCT influences outcomes, and thus is relevant when comparing transplant results over decades and, importantly, when putting our study in the context of more recent trials on non-transplant treatments in STS.27 28
Median PFS and OS of the total population of our study were 8.3 and 19.8 months, respectively. Yet, patients transplanted in CR clearly experienced better outcomes and are not comparable to patients with macroscopic residual disease regarding outcomes. However, in patients with remission status other than CR prior ASCT, PFS and OS still were 7.2 and 17.8 months, respectively, and thus compare very well with recent data regarding conventional chemotherapies or targeted therapies: in latest phase 3 trials in metastatic STS, median PFS and OS in first line ranged about 5–7 and 13–20 months, respectively4–7 and around 2–5 and 11–13 months, respectively, in second-line trials.29–31 Likewise, the outcomes of our cohort compare favourably to the reported PFS and OS of about 4 and 12 months, respectively, of over 2500 STS patients treated with first-line anthracycline-based chemotherapy in trials of the EORTC.32 33 When taking into account that most of the patients in our study had high grade sarcomas, were transplanted at relapse, and, most importantly, were treated at times when the therapeutic options for STS were much more limited, and TRM of ASCT was higher than today,34 these results are remarkable.
Due to lacking data, we cannot exclude a potential impact of local and/or systemic treatments after ASCT on outcomes. However, post-ASCT treatments unlikely affect PFS, and the problem of an unknown impact of poststudy treatments is inherent to every trial.
Our data show differences in PFS in some histologies in univariate analyses: Compared with leiomyosarcoma, more patients with synovial sarcoma and even more with angiosarcoma were free from progression at 2 and 5 years, without reaching statistical significance. Although this fits well to the notion that synovial sarcomas and maybe also angiosarcomas are more chemosensitive than other histological subtypes,33 35 we were not able to prove a significant impact of histology in multivariate analyses. However, in view of data supporting histotype tailored treatment of STS,2 20 30 36–39 we assume the still small patient numbers of our study, rather than an irrelevance of histology to be the cause of these results.
Our finding of remission status prior ASCT being predictive for better outcome after ASCT is a recurrent observation across many groups of malignant diseases, but as most studies on ASCT in STS excluded patients refractory to standard-dose chemotherapy, this has not yet been shown in a sufficient patient number to our knowledge.
Finally, our data show superior PFS in patients treated with melphalan-based vs platinum-based preparative chemotherapy. Notably, the aforementioned randomised trial which found no benefit of ASCT in STS, did employ a platinum-based preparative regimen.15 This may be a finding with clinical implications, as platinum-based salvage regimens are still in use today, whereas melphalan in fact has no role in STS aside from its use in isolated limb perfusion.40 41
In summary, our study provides evidence that age and remission status prior to transplantation are predictors of favourable outcome after ASCT in STS and suggests melphalan-based preparative regimens to be superior to platinum-based therapies. However, our data do not allow for conclusions as to whether specific histological subgroups benefit more from ASCT than others. Thus, ASCT should not be performed in routine clinical practice. However, as metastatic STS remains an incurable disease with few treatment options, we believe that a well-designed clinical trial of HDCT and ASCT in STS is worthwhile. Based on our data, we suggest investigating melphalan-based conditioning and ASCT versus SDT in patients with chemosensitive disease. Importantly, only a histologically stratified trial may answer the question if and which STS patients derive benefit from ASCT.
Footnotes
Twitter: @ChrisHeiligMD, @TMO_Heidelberg, @CChabannon
Contributors: Conception and design: CEH, CC and PP. Collection and assembly of data: CEH, MB, ML, SS, JH, EN-V, DB, CK, AS, MV, WK, SS, JRP, MDN, JR, PD and PP. Data analysis and interpretation: CEH, MB, ML, SF, UK, CC, PP. Important intellectual contribution: all authors. Manuscript writing: CEH, MB, ML, SF, PP. Final approval of manuscript: all authors. Accountable for all aspects of the work: all authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplemental information.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Gatta G, van der Zwan JM, Casali PG, et al. . Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer 2011;47:2493–511. 10.1016/j.ejca.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 2.Casali PG, Abecassis N, Aro HT, et al. . Soft tissue and visceral sarcomas: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv51–67. 10.1093/annonc/mdy096 [DOI] [PubMed] [Google Scholar]
- 3.Fletcher CDM. Who classification of tumours of soft tissue and bone 2013.
- 4.Tap WD, Wagner AJ, Papai Z, et al. . ANNOUNCE: A randomized, placebo (PBO)-controlled, double-blind, phase (Ph) III trial of doxorubicin (dox) + olaratumab versus dox + PBO in patients (pts) with advanced soft tissue sarcomas (STS). JCO 2019;37:LBA3 10.1200/JCO.2019.37.18_suppl.LBA3 [DOI] [Google Scholar]
- 5.Ryan CW, Merimsky O, Agulnik M, et al. . PICASSO III: a phase III, placebo-controlled study of doxorubicin with or without Palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol 2016;34:3898–905. 10.1200/JCO.2016.67.6684 [DOI] [PubMed] [Google Scholar]
- 6.Seddon B, Strauss SJ, Whelan J, et al. . Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol 2017;18:1397–410. 10.1016/S1470-2045(17)30622-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judson I, Verweij J, Gelderblom H, et al. . Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014;15:415–23. 10.1016/S1470-2045(14)70063-4 [DOI] [PubMed] [Google Scholar]
- 8.Patel SR, Vadhan-Raj S, Papadopolous N, et al. . High-dose ifosfamide in bone and soft tissue sarcomas: results of phase II and pilot studies--dose-response and schedule dependence. J Clin Oncol 1997;15:2378–84. 10.1200/JCO.1997.15.6.2378 [DOI] [PubMed] [Google Scholar]
- 9.Le Cesne A, Antoine E, Spielmann M, et al. . High-Dose ifosfamide: circumvention of resistance to standard-dose ifosfamide in advanced soft tissue sarcomas. J Clin Oncol 1995;13:1600–8. 10.1200/JCO.1995.13.7.1600 [DOI] [PubMed] [Google Scholar]
- 10.Rosen G, Forscher C, Lowenbraun S, et al. . Synovial sarcoma. uniform response of metastases to high dose ifosfamide. Cancer 1994;73:2506–11. [DOI] [PubMed] [Google Scholar]
- 11.Blay JY, Bouhour D, Ray-Coquard I, et al. . High-Dose chemotherapy with autologous hematopoietic stem-cell transplantation for advanced soft tissue sarcoma in adults. J Clin Oncol 2000;18:3643–50. 10.1200/JCO.2000.18.21.3643 [DOI] [PubMed] [Google Scholar]
- 12.Bokemeyer C, Franzke A, Hartmann JT, et al. . A phase I/II study of sequential, dose-escalated, high dose ifosfamide plus doxorubicin with peripheral blood stem cell support for the treatment of patients with advanced soft tissue sarcomas. Cancer 1997;80:1221–7. [DOI] [PubMed] [Google Scholar]
- 13.Kasper B, Scharrenbroich I, Schmitt T, et al. . Consolidation with high-dose chemotherapy and stem cell support for responding patients with metastatic soft tissue sarcomas: prospective, single-institutional phase II study. Bone Marrow Transplant 2010;45:1234–8. 10.1038/bmt.2009.333 [DOI] [PubMed] [Google Scholar]
- 14.Kasper B, Lehnert T, Bernd L, et al. . High-Dose chemotherapy with autologous peripheral blood stem cell transplantation for bone and soft-tissue sarcomas. Bone Marrow Transplant 2004;34:37–41. 10.1038/sj.bmt.1704520 [DOI] [PubMed] [Google Scholar]
- 15.Bui-Nguyen B, Ray-Coquard I, Chevreau C. High-Dose chemotherapy consolidation for chemosensitive advanced soft tissue sarcoma patients: an open-label, randomized controlled trial. Annals of … 2012. [DOI] [PubMed] [Google Scholar]
- 16.Peinemann F, Labeit AM. Autologous haematopoietic stem cell transplantation following high-dose chemotherapy for non-rhabdomyosarcoma soft tissue sarcomas: a Cochrane systematic review*. BMJ Open 2014;4:e005033. 10.1136/bmjopen-2014-005033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sleijfer S, Ouali M, van GM, et al. . Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European organization for research and treatment of Cancer-Soft tissue and bone sarcoma group (EORTC-STBSG). Eur J Cancer 2010;46:72–83. 10.1016/j.ejca.2009.09.022 [DOI] [PubMed] [Google Scholar]
- 18.Gronchi A, Ferrari S, Quagliuolo V, et al. . Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol 2017;18:812–22. 10.1016/S1470-2045(17)30334-0 [DOI] [PubMed] [Google Scholar]
- 19.Grosso F, Jones RL, Demetri GD, et al. . Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007;8:595–602. 10.1016/S1470-2045(07)70175-4 [DOI] [PubMed] [Google Scholar]
- 20.Higham CS, Steinberg SM, Dombi E, et al. . SARC006: phase II trial of chemotherapy in sporadic and neurofibromatosis type 1 associated Chemotherapy-Naive malignant peripheral nerve sheath tumors. Sarcoma 2017;2017:8685638 10.1155/2017/8685638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hougaard P. Frailty models for survival data. Lifetime Data Anal 1995;1:255–73. 10.1007/BF00985760 [DOI] [PubMed] [Google Scholar]
- 22.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med 1999;18:1489–500. [DOI] [PubMed] [Google Scholar]
- 23.Peinemann F, Enk H, Smith LA, et al. . Autologous hematopoietic stem cell transplantation following high-dose chemotherapy for nonrhabdomyosarcoma soft tissue sarcomas. Cochrane Db Syst Rev 2017;27 10.1002/14651858.CD008216.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gani F, Goel U, Canner JK, et al. . A national analysis of patterns of care and outcomes for adults diagnosed with desmoplastic small round cell tumors in the United States. J Surg Oncol 2019;119:880–6. 10.1002/jso.25426 [DOI] [PubMed] [Google Scholar]
- 25.Bulbul A, Fahy BN, Xiu J, et al. . Desmoplastic small round blue cell tumor: a review of treatment and potential therapeutic genomic alterations. Sarcoma 2017;2017:1–12. 10.1155/2017/1278268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes-Jordan AA, Coakley BA, Green HL, et al. . Desmoplastic small round cell tumor treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: results of a phase 2 trial. Ann Surg Oncol 2018;25:872–7. 10.1245/s10434-018-6333-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Passweg JR, Baldomero H, Bader P, et al. . Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant 2016;51:786–92. 10.1038/bmt.2016.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gratwohl A, Pasquini MC, Aljurf M, et al. . One million haemopoietic stem-cell transplants: a retrospective observational study. Lancet Haematol 2015;2:e91–100. 10.1016/S2352-3026(15)00028-9 [DOI] [PubMed] [Google Scholar]
- 29.Demetri GD, von Mehren M, Jones RL, et al. . Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016;34:786–93. 10.1200/JCO.2015.62.4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schöffski P, Chawla S, Maki RG, et al. . Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 2016;387:1629–37. 10.1016/S0140-6736(15)01283-0 [DOI] [PubMed] [Google Scholar]
- 31.van der Graaf WTA, Blay J-Y, Chawla SP, et al. . Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86. 10.1016/S0140-6736(12)60651-5 [DOI] [PubMed] [Google Scholar]
- 32.Kroep JR, Ouali M, Gelderblom H, et al. . First-Line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: an EORTC soft tissue and bone sarcoma group study. Ann Oncol 2011;22:207–14. 10.1093/annonc/mdq338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young RJ, Natukunda A, Litière S, et al. . First-Line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European organisation for research and treatment of cancer soft tissue and bone sarcoma group trials. Eur J Cancer 2014;50:3178–86. 10.1016/j.ejca.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 34.Styczyński J, Tridello G, Koster L, et al. . Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant 2020;55:126–36. 10.1038/s41409-019-0624-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlenterie M, Litière S, Rizzo E, et al. . Outcome of chemotherapy in advanced synovial sarcoma patients: review of 15 clinical trials from the European organisation for research and treatment of cancer soft tissue and bone sarcoma group; setting a new landmark for studies in this entity. Eur J Cancer 2016;58:62–72. 10.1016/j.ejca.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 36.Canter RJ, Qin L-X, Maki RG, et al. . A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res 2008;14:8191–7. 10.1158/1078-0432.CCR-08-0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.del MXG, Lopez-Pousa A, Martinez-Trufero J, et al. . Phase II study of gemcitabine (GEM) plus sirolimus (Sir) in previously treated patients with advanced soft tissue sarcoma (STS): a Spanish group for research on sarcomas (GEIS) study. J Clin Oncol 2014;32:10594. [DOI] [PubMed] [Google Scholar]
- 38.Maki RG, Wathen JK, Patel SR, et al. . Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007;25:2755–63. 10.1200/JCO.2006.10.4117 [DOI] [PubMed] [Google Scholar]
- 39.Gronchi A, Bui BN, Bonvalot S, et al. . Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol 2012;23:771–6. 10.1093/annonc/mdr265 [DOI] [PubMed] [Google Scholar]
- 40.Hayes AJ, Neuhaus SJ, Clark MA, et al. . Isolated limb perfusion with melphalan and tumor necrosis factor alpha for advanced melanoma and soft-tissue sarcoma. Ann Surg Oncol 2007;14:230–8. 10.1245/s10434-006-9040-x [DOI] [PubMed] [Google Scholar]
- 41.Jakob J, Smith HG, Wilkinson MJ, et al. . Regional chemotherapy by isolated limb perfusion prior to surgery compared with surgery and post-operative radiotherapy for primary, locally advanced extremity sarcoma: a comparison of matched cohorts. Clin Sarcoma Res 2018;8:12. 10.1186/s13569-018-0098-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000860supp001.pdf (36.4KB, pdf)
esmoopen-2020-000860supp002.pdf (125.4KB, pdf)