Abstract
Introduction:
Growing evidence indicates the prognostic importance of the crosstalk between cancer cells and stroma through the induction of epithelial-mesenchymal transition (EMT). This study aimed to clarify the prognostic value of evaluating primary tumor histology with the anatomical extent of disease in patients with colorectal liver metastasis (CRLM).
Methods:
Prognostic analyses were performed in 411 CRLM patients who underwent hepatectomy at two institutions. Tumors were graded into one of three histological categories based on integrated assessment of EMT-associated histology (HistologyEMT) in primary tumors, i.e., poorly differentiated clusters (PDCs) and desmoplastic reaction (DR).
Results:
A prognostic grouping system for the anatomical extent of disease (N stage, liver metastasis number and size, and extrahepatic disease; Gradeanatomical) stratified patients into three groups with different five-year relapse-free survival (RFS) rates after hepatectomy: A, 31% (191 patients); B, 15% (124 patients); and C, 6% (96 patients; P < 0.0001). HistologyEMT (A, G1 PDC and mature-type DR; C, G3 PDC and immature-type DR; and B, others) identified 49, 120, and 242 patients with 46%, 5%, and 22% five-year RFS, respectively (P < 0.0001). Among prognostic factors, the Akaike information criterion was most favorable in Gradeanatomical, followed by HistologyEMT. Multivariate analysis demonstrated that these two factors independently impacted RFS; two-year RFS after hepatectomy in different patient groups had a wide range (10%-76%).
Conclusions:
Histological assessment of dedifferentiation and the stromal environment of primary tumors contributed to effective risk stratification of early relapse after hepatectomy, which could be useful to determine clinical management for CRLM patients.
Keywords: colorectal liver metastasis, desmoplastic reaction, epithelial-mesenchymal transition (EMT), hepatectomy, poorly differentiated cluster
Introduction
CRC patients with resectable liver metastases comprise a prognostically heterogeneous population that requires treatment on a case-to-case basis, especially in accordance with the accurate evaluation of recurrence risk within a short period after hepatectomy. Since the 1990s, several prognostic prediction systems have been proposed for CRC liver metastasis patients undergoing hepatectomy (1), (2), (3), (4). Most of these systems are intended to determine the characteristics of patients suitable for hepatectomy. These comprise conventional prognostic markers of the anatomical extent of disease, such as liver metastasis number (1), (2), (3), (4) and size (1), (2), (4), resection margin (1), extrahepatic disease (4), disease-free interval (1), (2), (3), primary tumor nodal status (1), (2), (4), and serum carcinoembryonic antigen (CEA) levels (1), (2). However, their broad application currently has limited value in patient stratification for clinical management (5), principally owing to the inability to accurately stratify patients according to the prognostic outcome.
Recent accumulated knowledge in cancer biology research highlights the importance of the molecular mechanism of epithelial-mesenchymal transition (EMT), a means by which transformed epithelial cells can acquire the ability to invade and disseminate (6). Under the EMT program, loss of differentiation is induced at the tumor-host interface in CRC, which enables cellular detachment, dissemination, and eventually, metastasis (7), (8). In the cancer microenvironment, cancer-associated fibroblasts (CAFs) play a central role in mediating the EMT program (8), (9), thereby facilitating the progression of cancer cells toward a dedifferentiated state. One of the most promising methods for characterizing dedifferentiation to assess the migratory phenotype of a tumor is to quantify poorly differentiated clusters (PDCs) (10), (11). Additionally, desmoplastic reaction (DR), histological consequences of extracellular matrix remodeling generated by CAFs, can be morphologically categorized on the basis of the presence of specific types of fibrous cancer stroma (12).
Recently, we proposed a histological categorization of EMT (HistologyEMT) by the integrated assessment of PDC and DR on the premise that HistologyEMT represents the potential of induction of EMT in CRC (Figure 1) (13). Our previous study indicated that this categorization greatly influences oncological results not only in patients with curatively resected CRC but also in stage IV patients with unresectable distant metastasis (13). The aims of the present study were to examine the prognostic value of HistologyEMT in a multi-institutional patient dataset that underwent curative hepatectomy for CRC metastases and to clarify whether comprehensive assessment of the histological expression of CRC metastatic potential in primary tumors and the anatomical extent of disease were useful for evaluating the survival outcome, particularly the risk of early relapse after hepatectomy.
Figure 1.
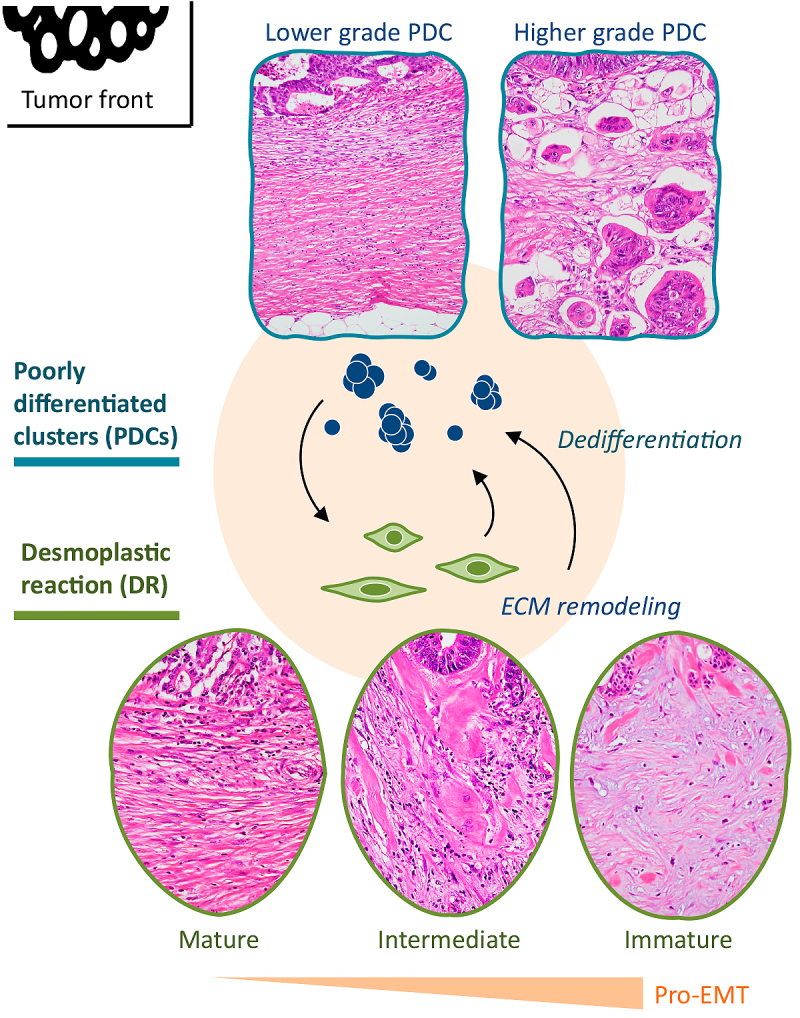
Scheme of histological heterogeneity at the primary tumor front in terms of the association between desmoplastic patterns and dedifferentiation.
CAFs are being increasingly recognized as central players in modulating the metastatic capacity of most cancers. Directly and indirectly, the oncological outcome of tumors is determined during the process of DR through the formation of the extracellular matrix, which causes EMT signals to induce tumor cells into a dedifferentiated phenotype. In the present study, comprehensive assessment of PDCs and the DR pattern (HistologyEMT) was used as an index to assess the individual tumor microenvironments associated with the EMT status. PDCs are cancer cell clusters composed of ≥ 5 cancer cells that lack gland formation and are located in the stroma. PDCs were graded as 1, 2, or 3 using a hot-spot method. According to the existence of hyalinized collagens and myxoid stroma, which are distinctive fibrotic stromal components that exclusively appear at the desmoplastic front and are thought to be morphological results of activated CAF function, DR was classified into three patterns: mature (neither hyalinized collagens nor myxoid stroma), intermediate (fibrotic stroma with hyalinized broad bundles), or immature (fibrotic stroma with myxoid changes).
PDCs, poorly differentiated clusters; CAFs, cancer-associated fibroblasts; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition.
Materials and Methods
Patients
The study included CRC patients (n = 411) who had undergone potentially curative surgery for their primary tumors and synchronous/metachronous liver metastases at two independent institutions: the National Defense Medical College Hospital (1984-2010) and the Cancer Institute Hospital (1995-2007). The average age of the patients was 61.4 years (range, 28-91 years), with 271 males and 140 females. Of these, 245 patients had colon cancer and 166 had rectal cancer. The average and median follow-up period after hepatectomy for survivors was 61 and 48 months, respectively (range, 11-188 months). This study protocol was approved by the Ethics Committee of the National Defense Medical College (No.2954) and the Cancer Institute Hospital (No. 2010-1056).
Primary tumor histology
One of the authors (HU) pathologically reviewed primary tumors to evaluate PDCs and DR with no prior knowledge of the patients' clinical outcomes. Hematoxylin-eosin (HE)-stained glass slides prepared from a single longitudinal section of the whole tumor, including its deepest part, were used to determine the grade of PDCs and pattern of DR.
PDCs
PDCs were defined as cancer cell clusters comprising ≥ 5 cancer cells infiltrating the stroma and lacking gland formation (10), (11). After selecting one field where PDCs were the most intensive, the number of clusters was counted under a 20× objective lens, and the grade was determined on the basis of the number of clusters present. Tumors with < 5, 5-9 and ≥10 clusters were classified as G1, G2, and G3, respectively.
DR
The DR pattern was histologically classified into one of three categories (mature, intermediate, and immature) on the basis of the existence of keloid-like collagen and myxoid stroma in the reactive fibrous zone at the advancing edge of the tumor (12). Keloid-like collagen consists of broad bundles of hypocellular collagen with brightly eosinophilic hyalinization, typically observed in a keloid. Myxoid stroma can be defined as an amorphous stromal substance composed of an amphophilic or slightly basophilic material that is usually intermingled with randomly oriented keloid-like collagen.
DR was regarded as a mature pattern when fibrotic stroma did not contain keloid-like collagen or myxoid stroma and consisted of fine mature collagen fibers stratified into multiple layers. When keloid-like collagen was intermingled with mature stroma, the fibrotic stroma was designated as undergoing intermediate maturation. Stroma with myxoid changes was regarded as immature stroma. In each case, stroma was classified according to the most immature stromal area.
Categorization criteria for HistologyEMT
Using PDC and DR as components, a three-tiered categorization system (HistologyEMT) was established as the model to estimate the potential of EMT of a tumor (Figure 2) (13). Category A included tumors with both G1 PDC and mature-type stroma; category C included tumors with both G3 PDC and immature-type stroma; and category B indicated tumors with other histological types.
Figure 2.
Categorization systems of HistologyEMT, H stage, and Gradeanatomical.
JSCCR, Japanese Society for Cancer of the Colon and Rectum.
Anatomical extent of disease
The T and N stages in the primary tumor, liver metastasis number and size, index for the extent of liver metastases defined by the Japanese Society for Cancer of the Colon and Rectum (JSCCR; H stage) (14), and extrahepatic disease were analyzed as parameters for the anatomical extent of disease. We evaluated prognostic value of the number and size of liver metastasis with cutoff values used to define H stage, i.e., five for the number and 5 cm for the size of metastasis, respectively. The prognostic grouping system defined by JSCCR (Gradeanatomical) described below was used as a prognostic prediction model to comprehensively assess the anatomical extent of disease (Figure 2) (14).
H stage
The extent of liver metastasis was stratified as follows: H1, 1-4 metastatic tumors, all of which are ≤ 5 cm in maximum diameter; H3, ≥ 5 metastatic tumors, at least one of which is ≥5 cm in maximum diameter; and H2, other than H1 or H3 (14).
Gradeanatomical
The three-tiered grading system of the anatomical extent of disease was determined on the basis of the primary tumor N stage, H stage, and extrahepatic metastases (14). In summary, the modified TNM staging system was used to determine the primary tumor N stage: N0, no lymph node (LN) metastasis; N1, 1-3 LN metastasis; N2, ≥4 LN metastasis; N3, positive findings in the main LNs (LNs located at the origin of each colic artery and inferior mesenteric trunk LNs) or iliac LNs (14). Patients without any extrahepatic metastases were classified as Gradeanatomical A or B: grade A denoted patients with N0/1 and H1 and grade B denoted patients with N2 and H1 or N0/1 and H2. Grade C denoted patients with extrahepatic metastasis or the following combinations of N and H stages: N0/1 and H3, N2 and H2/3, or N3 and any H stage.
Statistical analyses
Survival rates were calculated using the Kaplan-Meier method, and comparisons were made using the log-rank test. After categorization, each clinical and pathological variable was entered into Cox’s proportional hazard regression analysis to determine the hazard ratio and 95% confidence interval for survival after hepatectomy and to compare the prognostic power of each factor using the Akaike information criterion (AIC) (15); the simplest, most effective model with the least information loss while predicting the outcome provides the lowest AIC value. The associations among prognostic factors and their associations with recurrence were analyzed using the chi-square test. The chi-square test was used to assess the differences in recurrence rates according to HistologyEMT. Statistical analyses were conducted using SPSS Statistics 17.0 (SPSS, Inc., Chicago, IL, USA), Stata/SE 10 (StataCorp LP, College Station, TX, USA), and StatView ver.5.0 (SAS Institute, Inc., Cary, NC, USA).
Results
Correlation between PDCs and DR and distribution of HistologyEMT categories
There was a significant correlation between PDCs and DR (P < 0.0001), but multivariate analysis indicated an independent impact of PDCs and DR on both relapse-free survival (RFS; P = 0.0016 and 0.0066, respectively) and overall survival (OS; P = 0.0135 and 0.0022, respectively).
According to HistologyEMT, primary tumors were classified as categories A, B, and C (49, 242, and 120 tumors, respectively). HistologyEMT was significantly associated with the T stage, N stage, tumor grade, venous invasion, metastasis timing, extrahepatic disease, and Gradeanatomical (P ≤ 0.0001-0.001) and not with liver-associated factors (number and size as well as H stage).
Prognostic impact of HistologyEMT
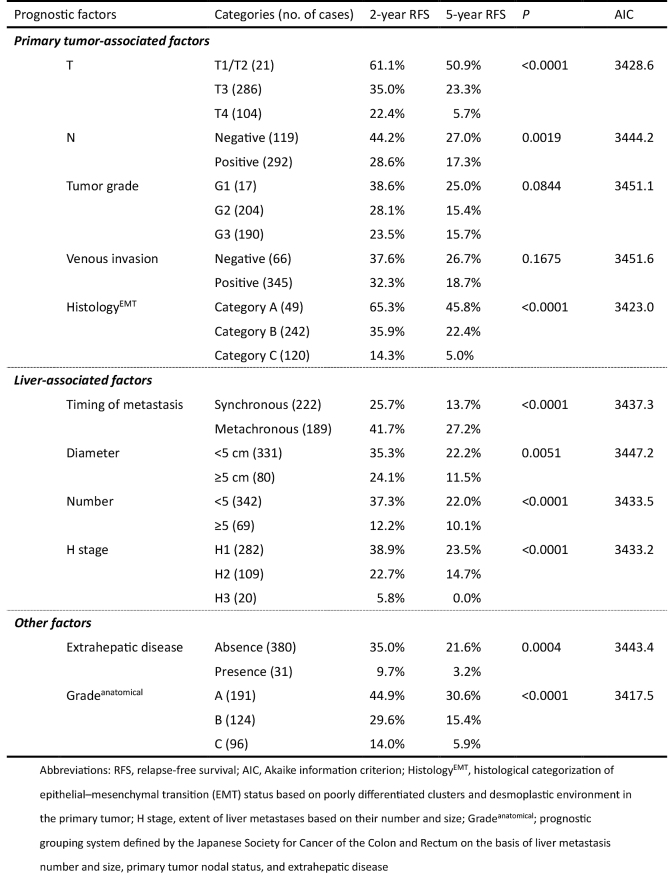
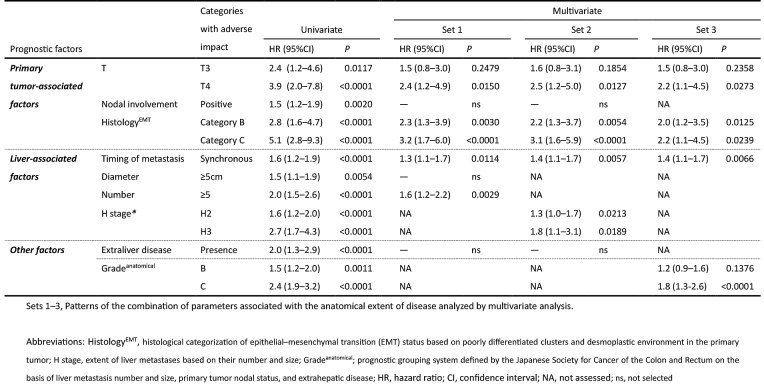
Prognostic factors associated with RFS after hepatectomy are shown in Table 1. According to HistologyEMT, five-year RFS after hepatectomy for categories A, B, and C were 45.8%, 22.4%, and 5.0%, respectively (P < 0.0001). Among the prognostic factors examined, HistologyEMT had the second most favorable AIC (3423.0) following Gradeanatomical (3417.5) which stratified patients with different five-year RFS as 30.6% for grade A, 15.4% for grade B, and 5.9% for grade C (P < 0.0001). Figures 3 and 4 show the Kaplan-Meier estimates for RFS after hepatectomy in patients stratified into the three groups according to HistologyEMT and Gradeanatomical, respectively.
Table 1.
Prognostic Factors Associated with Relapse-Free Survival after Hepatectomy for Colorectal Liver Metastasis.
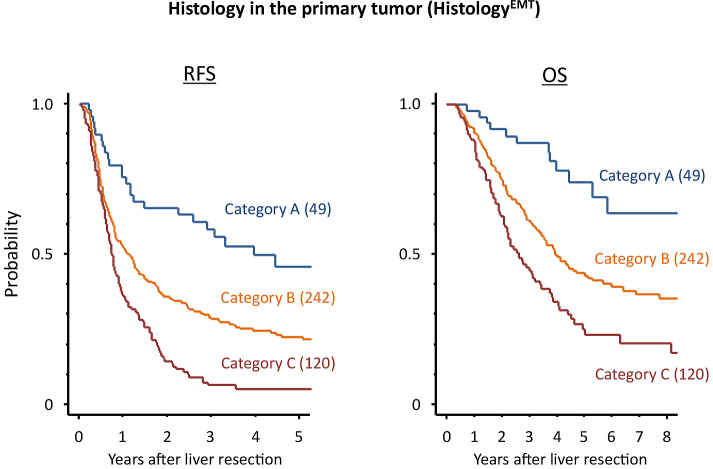
Figure 3.
Survival estimates of patients undergoing hepatectomy for colorectal liver metastases according to primary tumor histology (HistologyEMT) using the Kaplan-Meier method.
Five-year RFS by HistologyEMT: category A, 45.8%; category B, 22.4%; category C, 5.0% (P < 0.0001); Five-year OS by HistologyEMT: category A, 74.1%; category B, 43.8%; category C, 25.1% (P < 0.0001).
RFS, relapse-free survival; OS, overall survival. The numbers in parentheses denote the number of patients.
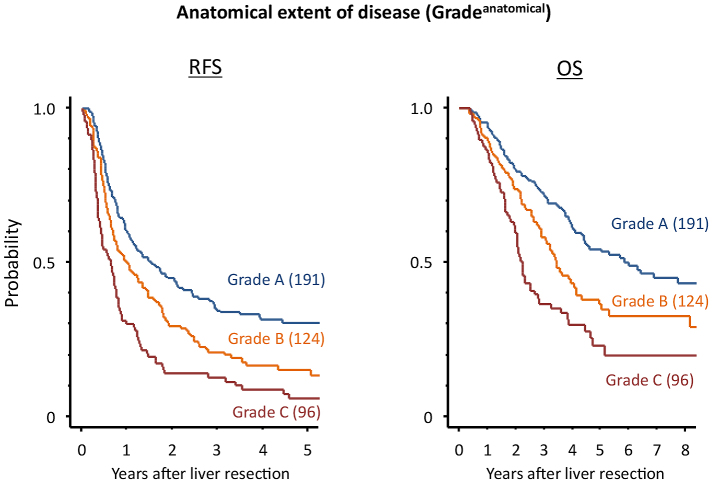
Figure 4.
Survival estimates of patients undergoing hepatectomy for colorectal liver metastases according to the anatomical extent of disease (Gradeanatomical) using the Kaplan-Meier method.
Five-year RFS by Gradeanatomical: grade A, 30.6%; grade B, 15.4%; grade C, 5.9% (P < 0.0001); Five-year OS by Gradeanatomical: grade A, 54.4%; grade B, 36.5%; grade C, 23.1% (P < 0.0001).
RFS, relapse-free survival; OS, overall survival. The numbers in parentheses denote the numbers of patients.
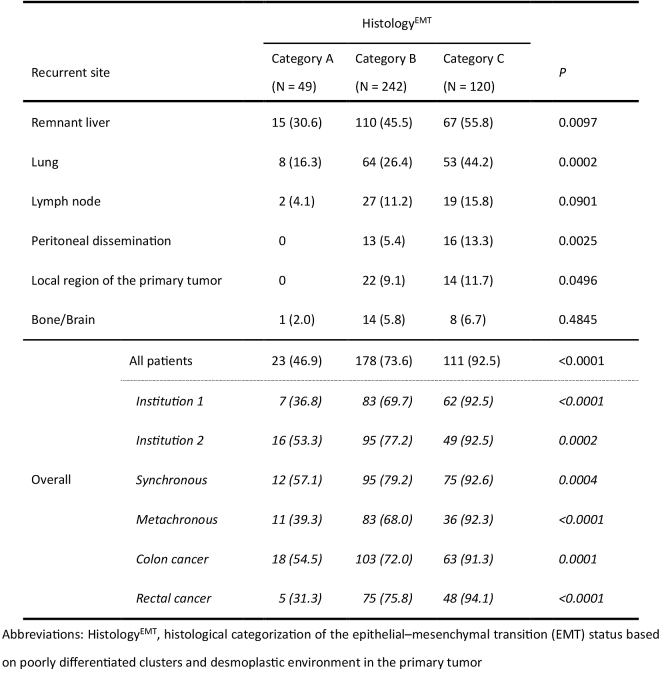
With regard to the recurrence pattern, HistologyEMT was significantly associated with hepatic, lung, peritoneal, and local recurrence (Table 2). The incidence of overall recurrence was the highest in category C (92.5%), followed by categories B (73.6%) and A (46.9%; P < 0.0001). A significant impact of HistologyEMT on the overall recurrence rate was observed in the subset analyses of institution cohort, metastasis timing, and primary tumor location.
Table 2.
Impact of Histological Categorization of the EMT Status in the Primary Tumor (HistologyEMT) on Recurrence Pattern after Hepatectomy.
Multivariate analysis
In multivariate analyses for RFS, parameters associated with the anatomical extent of disease were analyzed under three different conditions (Table 3): set 1, all factors entered the multivariate analysis individually; set 2, H stage entered the multivariate analysis instead of liver metastasis number and size; and set 3, Gradeanatomical entered the multivariate analysis instead of liver metastasis number and size, primary CRC nodal status, and extrahepatic disease.
Table 3.
Univariate and Multivariate Analyses of Relapse-Free Survival after Hepatectomy in Patients with Colorectal Liver Metastasis.
Consequently, HistologyEMT was shown to significantly impact RFS independent of the anatomical extent of disease in any condition.
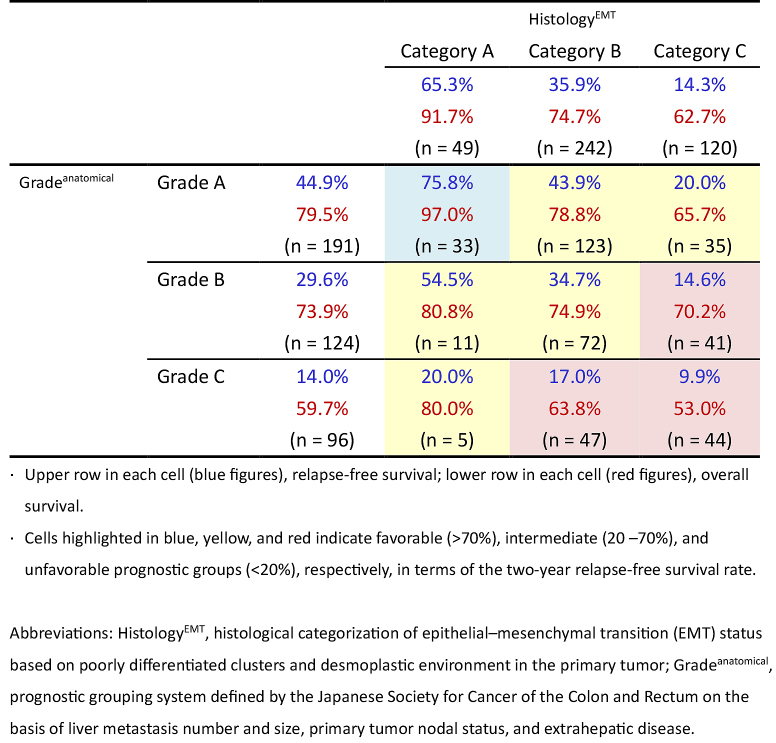
Comprehensive assessment of HistologyEMT and Gradeanatomical
The two-year RFS after hepatectomy was calculated for nine populations grouped by the two independent three-tiered grading systems (HistologyEMT and Gradeanatomical). The survival results ranged widely: 9.9%-75.8% (Table 4).
Table 4.
Relapse-Free and Overall Survival Rates at Two Years after Hepatectomy According to Comprehensive Assessments of HistologyEMT and Gradeanatomical.
Discussion
In the last two decades, indications for hepatectomy for CRC liver metastasis have changed considerably. Improvements in the preoperative management of hepatectomy preparations and in surgical techniques, including portal vein embolization, aggressive approaches combined with resection of adjacent major vessels, and two-stage hepatectomy, have allowed hepatobiliary surgeons to extend surgical indications for multiple or large-sized liver metastases. Additionally, even extrahepatic diseases such as lung metastases, hilar LN involvement, or peritoneal seeding are not currently regarded as contraindications of hepatectomy (16). In this recent trend of widening the indication of hepatectomy, conventional prognostic prediction systems have limited value for patient stratification for determining surgical indications (5).
Nevertheless, prognostic prediction systems with high accuracy are still valuable because they could affect clinical management in controversial areas such as delaying resection or preoperative chemotherapy (5). Japan has a prognostic grouping system for colorectal liver metastasis defined by the Japanese Society for Cancer of the Colon and Rectum (JSCCR; Gradeanatomical), which is composed of the number and size of liver metastasis, N stage, and extrahepatic metastasis (14). Although Gradeanatomical has been used nationwide since 2006 when the Japanese Classification of Colorectal Carcinoma (6th edition) was issued, its clinical value remains unclear; for example, its exact role in routine clinical practice has still not been defined in the Japanese guidelines (17). Similarly, international guidelines, including the National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) guidelines, do not use any proposed prognostic scoring systems (1), (2), (3), (4) as treatment indicators for CRC liver metastasis. This situation may indicate that there is insufficient accuracy in prognostic prediction systems using only parameters associated with the extent of disease, and novel methodologies to predict survival outcome are required.
In the present study, we focused on HistologyEMT comprising two EMT-associated histological factors in primary tumors: PDC and desmoplastic environment. PDC is a cancer morphology representative of dedifferentiation and mostly appears at the invasive front of the tumor. Tumor grading by quantifying PDCs has been shown to be a robust parameter for prognosis after resecting primary CRC (10), (18). In non-metastatic CRC, it was demonstrated that PDCs affect outcome independent of T and N stages and more effectively stratify the recurrence risk than the TNM tumor stage (10).
Fibrotic stroma heterogeneity at the desmoplastic front represents the variety of tumor utilization of stromal components for its development. The mechanism underlying tumor cell invasion involves complex interactions between neoplastic cells and the surrounding matrix, and recent findings have indicated that cancer develops by utilizing fibrotic stroma to modify CAF function (19). DR histological categorization based on the appearance of keloid-like collagen and myxoid stroma, which represent deviant CAF function under the influence of cancer cells, has been shown to be associated with tumor lymphocyte infiltration (12), microvascular density (20), pro-tumor extracellular matrix components (including fibronectin and tenascin-C) (20), mismatch repair status (21), and prognostic outcome in CRC patients (12), (20).
We previously found that both PDCs and DR have a prognostic value in patients with CRC liver metastasis (22), (23), and the present study clarified that these two factors are independently associated with survival results. This result is consistent with that of another previous study which showed that PDCs and DR are independent in terms of their survival impact in stage II-III and stage IV CRC settings (13) and are associated with the contribution of HistologyEMT to the effective identification of patient groups with very favorable as well as unfavorable survival results in case of CRC liver metastasis. Notably, multivariate prognostic analyses showed that HistologyEMT exerts a significant impact on postoperative survival after hepatectomy independent of the liver metastasis status, and AIC is the second most favorable following Gradeanatomical, a comprehensive assessment of liver metastasis number and size, primary tumor nodal status, and extrahepatic disease. It should also be noted that HistologyEMT impacts postoperative survival results not only in patients with synchronous liver metastases but also in those with metachronous liver metastasis. To the best of our knowledge, this is the first study to find that CRC histology is comparable to the anatomical extent of disease in its power to stratify the prognostic outcome.
Patients with resectable liver metastases belong to a heterogeneous population in terms of oncological outcome because of the greatly diverse potential of aggressiveness that is histologically expressed in primary tumors and in the anatomical extent of disease in this group. We could resolve this issue of heterogeneity using Gradeanatomical and HistologyEMT. In particular, one of the most important findings in this study was that it is possible to stratify the heterogeneous population into subgroups with wide-ranging RFS two years after hepatectomy (10%-76%) on the basis of comprehensive assessment of HistologyEMT and Gradeanatomical, both of which can be determined prior to liver metastasis treatment. We believe that this approach will help resolve clinical issues originating from population heterogeneity, including effective patient selection for delaying resection and perioperative chemotherapy in patients with resectable liver metastasis who had undergone resection of the primary tumor.
The reason why the histopathological characteristics of metastatic origin have been undervalued compared to the anatomical extent of disease is possibly owing to a lack of promising findings of conventional primary site factors. In some reports analyzing patient prognostic outcome after hepatectomy for CRC liver metastasis, poor primary tumor differentiation has had an adverse prognostic impact (24), albeit inconsistently (4). Similarly, reports of the prognostic significance of primary tumor vascular invasion are conflicting, with some reports being positive (25) and others being negative (4). We believe that our study changes this because it provides statistical evidence, including AIC and c-index, indicating that HistologyEMT is more powerful than these conventional primary site factors. Another reason for the underestimation of primary tumor histology in metastatic CRC is that most studies investigating prognostic outcomes after hepatectomy have been conducted by hepatobiliary surgeons who face limitations in obtaining detailed histopathological information regarding the primary tumor. We believe it would be valuable to raise awareness of the importance of prognostic stratification by adopting the latest findings in the field of gastrointestinal pathology.
In conclusion, primary tumor histology, particularly EMT-associated new prognostic parameters, should be fully considered as prognostic determinants for patients with resectable liver metastases. The information obtained by comprehensively evaluating the biological aggressiveness expressed in the primary tumor and the anatomical extent of disease could allow us to identify patients at a high risk of early relapse after hepatectomy.
Article Information
Conflicts of Interest
None
Sources of Funding
This work was supported by a Grant-in-Aid for Scientific Research (C) by the Japan Society for the Promotion of Science (JSPS) KAKENHI grant number 25462075.
Approval by Institutional Review Board (IRB)
The study protocol was approved by the Ethics Committee of the National Defense Medical College (No.2954) and the Cancer Institute Hospital (No. 2010-1056).
Addresses of the Institutions at which this Work Was Carried Out
National Defense Medical College (3-2 Namiki, Tokorozawa, Saitama 359-8513, Japan)
Cancer Institute Hospital of the Japanese Foundation for Cancer Research (3-8-31, Ariake, Koto, Tokyo 135-8550, Japan)
References
- 1.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371(9617):1007-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer. analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ueno H, Mochizuki H, Hatsuse K, et al. Indicators for treatment strategies of colorectal liver metastases. Ann Surg. 2000;231(1):59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beppu T, Sakamoto Y, Hasegawa K, et al. A nomogram predicting disease-free survival in patients with colorectal liver metastases treated with hepatic resection: multicenter data collection as a Project Study for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2012;19(1):72-84. [DOI] [PubMed] [Google Scholar]
- 5.Gomez D, Cameron IC. Prognostic scores for colorectal liver metastasis: clinically important or an academic exercise? HPB. 2010;12(4):227-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-74. [DOI] [PubMed] [Google Scholar]
- 7.Brabletz T, Jung A, Spaderna S, et al. Migrating cancer stem cells-an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5(9):744-9. [DOI] [PubMed] [Google Scholar]
- 8.Karagiannis GS, Poutahidis T, Erdman SE, et al. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol Cancer Res. 2012;10(11):1403-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392-401. [DOI] [PubMed] [Google Scholar]
- 10.Ueno H, Shimazaki H, Shinto E, et al. New criteria for histologic grading of colorectal cancer. Am J Surg Pathol. 2012;36(2):193-201. [DOI] [PubMed] [Google Scholar]
- 11.Ueno H, Hase K, Hashiguchi Y, et al. Site-specific tumor grading system in colorectal cancer: multicenter pathological review of the value of quantifying poorly differentiated clusters. Am J Surg Pathol. 2014;38(2):197-204. [DOI] [PubMed] [Google Scholar]
- 12.Ueno H, Jones AM, Wilkinson KH, et al. Histological categorisation of fibrotic cancer stroma in advanced rectal cancer. Gut. 2004;53(4):581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueno H, Shinto E, Kajiwara Y, et al. Prognostic impact of histological categorisation of epithelial-mesenchymal transition in colorectal cancer. Br J Cancer. 2014;111(11):2082-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal carcinoma (second English edition). Tokyo: Kanehara & Co., Ltd; 2009. [Google Scholar]
- 15.Akaike H. Information theory and an extension of the maximum likelihood principle. Budapest: Akademia Kiado; 1973. [Google Scholar]
- 16.Pulitanò C, Bodingbauer M, Aldrighetti L, et al. Liver resection for colorectal metastases in presence of extrahepatic disease: results from international multi-institutional analysis. Ann Surg Oncol. 2011;18(5):1380-8. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23(1):1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barresi V, Bonetti LR, Branca G, et al. Colorectal carcinoma grading by quantifying poorly differentiated cell clusters is more reproducible and provides more robust prognostic information than conventional grading. Virchows Arch. 2012;461(6):621-8. [DOI] [PubMed] [Google Scholar]
- 19.Tsujino T, Seshimo I, Yamamoto H, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13(7):2082-90. [DOI] [PubMed] [Google Scholar]
- 20.Ueno H, Shinto E, Shimazaki H, et al. Histological categorization of desmoplastic reaction: its relevance to the colorectal cancer microenvironment and prognosis. Ann Surg Oncol. 2015;22(5):1504-12. [DOI] [PubMed] [Google Scholar]
- 21.Wright CL, Stewart ID. Histopathology and mismatch repair status of 458 consecutive colorectal carcinomas. Am J Surg Pathol. 2003;27(11):1393-406. [DOI] [PubMed] [Google Scholar]
- 22.Ueno H, Konishi T, Ishikawa Y, et al. Histological categorization of fibrotic cancer stroma in the primary tumor is an independent prognostic index in resectable colorectal liver metastasis. Am J Surg Pathol. 2014;38(10):1380-6. [DOI] [PubMed] [Google Scholar]
- 23.Ueno H, Konishi T, Ishikawa Y, et al. Prognostic value of poorly differentiated clusters in the primary tumor in patients undergoing hepatectomy for colorectal liver metastasis. Surgery. 2015;157(5):899-908. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka K, Shimada H, Ueda M, et al. Long-term characteristics of 5-year survivors after liver resection for colorectal metastases. Ann Surg Oncol. 2007;14(4):1336-46. [DOI] [PubMed] [Google Scholar]
- 25.Ueno H, Mochizuki H, Hashiguchi Y, et al. Predictors of extrahepatic recurrence after resection of colorectal liver metastases. Br J Surg. 2004;91(3):581-6. [DOI] [PubMed] [Google Scholar]