Abstract
Aims
To estimate the association of smoking status with rates of (i) infection, (ii) hospitalization, (iii) disease severity and (iv) mortality from SARS‐CoV‐2/COVID‐19 disease.
Design
Living rapid review of observational and experimental studies with random‐effects hierarchical Bayesian meta‐analyses. Published articles and pre‐prints were identified via MEDLINE and medRxiv.
Setting
Community or hospital, no restrictions on location.
Participants
Adults who received a SARS‐CoV‐2 test or a COVID‐19 diagnosis.
Measurements
Outcomes were SARS‐CoV‐2 infection, hospitalization, disease severity and mortality stratified by smoking status. Study quality was assessed (i.e. ‘good’, ‘fair’ and ‘poor’).
Findings
Version 7 (searches up to 25 August 2020) included 233 studies with 32 ‘good’ and ‘fair’ quality studies included in meta‐analyses. Fifty‐seven studies (24.5%) reported current, former and never smoking status. Recorded smoking prevalence among people with COVID‐19 was generally lower than national prevalence. Current compared with never smokers were at reduced risk of SARS‐CoV‐2 infection [relative risk (RR) = 0.74, 95% credible interval (CrI) = 0.58–0.93, τ = 0.41]. Data for former smokers were inconclusive (RR = 1.05, 95% CrI = 0.95–1.17, τ = 0.17), but favoured there being no important association (21% probability of RR ≥ 1.1). Former compared with never smokers were at somewhat increased risk of hospitalization (RR = 1.20, CrI = 1.03–1.44, τ = 0.17), greater disease severity (RR = 1.52, CrI = 1.13–2.07, τ = 0.29) and mortality (RR = 1.39, 95% CrI = 1.09–1.87, τ = 0.27). Data for current smokers were inconclusive (RR = 1.06, CrI = 0.82–1.35, τ = 0.27; RR = 1.25, CrI = 0.85–1.93, τ = 0.34; RR = 1.22, 95% CrI = 0.78–1.94, τ = 0.49, respectively), but favoured there being no important associations with hospitalization and mortality (35% and 70% probability of RR ≥ 1.1, respectively) and a small but important association with disease severity (79% probability of RR ≥ 1.1).
Conclusions
Compared with never smokers, current smokers appear to be at reduced risk of SARS‐CoV‐2 infection, while former smokers appear to be at increased risk of hospitalization, increased disease severity and mortality from COVID‐19. However, it is uncertain whether these associations are causal.
Keywords: COVID‐19, e‐cigarettes, hospitalization, infection, living review, mortality, nicotine replacement therapy, SARS‐CoV‐2, smoking, tobacco
Introduction
COVID‐19 is a respiratory disease caused by the SARS‐CoV‐2 virus. Large age and gender differences in case severity and mortality have been observed in the ongoing COVID‐19 pandemic [1]; however, these differences are currently unexplained. SARS‐CoV‐2 enters epithelial cells through the angiotensin‐converting enzyme 2 (ACE‐2) receptor [2]. Some evidence suggests that gene expression and subsequent receptor levels are elevated in the airway and oral epithelium of current smokers [3, 4], thus putting smokers at higher risk of contracting SARS‐CoV‐2. Other studies, however, suggest that nicotine down‐regulates the ACE‐2 receptor [5]. These uncertainties notwithstanding, both former and current smoking is known to increase the risk of respiratory viral [6, 7] and bacterial [8, 9] infections and is associated with worse outcomes once infected. Cigarette smoke reduces the respiratory immune defence through peri‐bronchiolar inflammation and fibrosis, impaired mucociliary clearance and disruption of the respiratory epithelium [10]. There is also reason to believe that behavioural factors (e.g. regular hand‐to‐mouth movements) involved in smoking may increase SARS‐CoV‐2 infection and transmission in current smokers. However, early data from the COVID‐19 pandemic have not provided clear evidence for a negative impact of current or former smoking on SARS‐CoV‐2 infection or COVID‐19 disease outcomes, such as hospitalization or mortality [11]. It has also been hypothesized that nicotine might protect against a hyperinflammatory response to SARS‐CoV‐2 infection, which may lead to adverse outcomes in patients with COVID‐19 disease [12].
There are several reviews that fall within the scope of smoking and COVID‐19 [11, 13, 14, 15, 16, 17, 18]. We aimed to produce a rapid synthesis of available evidence pertaining to the rates of infection, hospitalization, disease severity and mortality from SARS‐CoV‐2/COVID‐19 stratified by smoking status. Given the increasing availability of data on this topic, this is a living review with regular updates. As evidence accumulates, the review will be expanded to include studies reporting COVID‐19 outcomes by alternative nicotine use (e.g. nicotine replacement therapy or e‐cigarettes).
Methods
Study design
This is a living evidence review, which is updated as new evidence becomes available [19]. We adopted recommended best practice for rapid evidence reviews, which involved limiting the search to main databases and having one reviewer extract the data and another verify [20]. This study was not pre‐registered, but evolved from a report written for a UK medical society [21]. The most recent (and all future) version(s) of this living review is https://www.qeios.com/read/latest‐UJR2AW. A completed Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) checklist is included in Supporting information, Fig. S1.
Eligibility criteria
Studies were included if they:
Were primary research studies using experimental (e.g. randomized controlled trial), quasi‐experimental (e.g. pre‐ and post‐test;) or observational (e.g. case–control, retrospective cohort, prospective cohort) study designs;
Included adults aged 16 + years;
Recorded as outcome (i) results of a SARS‐CoV‐2 diagnostic test (including antibody assays), (ii) clinical diagnosis of COVID‐19, (iii) hospitalization with COVID‐19, (iv) severity of COVID‐19 disease in those hospitalized or (v) mortality from COVID‐19;
Reported any of the outcomes of interest by self‐reported or biochemically verified smoking status (e.g. current smoker, former smoker, never smoker) or current vaping or nicotine replacement therapy (NRT) use;
Were available in English; and
Were published in a peer‐reviewed journal, as a pre‐print or a public health report by reputable agents (e.g. governments, scientific societies).
Search strategy
The following terms were searched for in Ovid MEDLINE (2019‐search date) as free text or Medical Subject Headings:
Tobacco Smoking/ or Smoking Cessation/ or Water Pipe Smoking/ or Smoking/ or Smoking Pipes/ or Cigar Smoking/ or Smoking Prevention/or Cigarette Smoking/ or smoking.mp. or Pipe Smoking/or Smoking, Non‐Tobacco Products/or Smoking Water Pipes/
Nicotine/or nicotine.mp. or Electronic Nicotine Delivery Systems/ or Nicotine Chewing Gum/
vaping.mp. or Vaping/
1 or 2 or 3
Coronavirus/ or Severe Acute Respiratory Syndrome/or Coronavirus Infections/ or covid.mp.
4 and 5
The following terms were searched for in titles, abstracts and full texts in medRxiv no time limitations):
covid (this term captures both covid and SARS‐CoV‐2) AND smoking
covid AND nicotine
covid AND vaping
Additional articles/reports of interest were identified through mailing lists, Twitter, the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) and the US Centers for Disease Control and Prevention (CDC). Where updated versions of pre‐prints or public health reports were available, old versions were superseded.
Selection of studies
One reviewer screened titles, abstracts and full texts against the inclusion criteria.
Data extraction
Data were extracted by one reviewer and verified (i.e. independently checked against pre‐prints and published reports) by another on (i) author (year); (ii) date published; (iii) country; (iv) study design; (v) study setting; (vi) sample size; (vii) sex; (viii) age; (ix) smoking status (e.g. current, former, never, not stated, missing); (x) use of alternative nicotine products; (xi) SARS‐CoV‐2 testing; (xii) SARS‐CoV‐2 infection; (xiii) diagnosis of COVID‐19; (xiv) hospitalization with COVID‐19; (xv) disease severity in those hospitalized with COVID‐19; and (xvi) mortality.
Quality appraisal
The quality of included studies was assessed to determine suitability for inclusion in meta‐analyses. Studies were judged as ‘good’ quality if they: (i) had < 20% missing data on smoking status and used a reliable self‐report measure that distinguished between current, former and never smoking status; AND (ii) used biochemical verification of smoking status and reported results from adjusted analyses; OR reported data from a representative/random sample. Studies were rated as ‘fair’ if they fulfilled only criterion (i) and were otherwise rated as ‘poor’. The quality appraisal was conducted by one reviewer and verified by a second.
Evidence synthesis
A narrative synthesis was conducted. Data from ‘good’ and ‘fair’ quality studies were pooled in R version 3.6.3 [22]. In a living review where new data are regularly added to the analyses, it may be more appropriate to use a Bayesian (as opposed to frequentist) approach where prior knowledge is used in combination with new data to estimate a posterior risk distribution. A Bayesian approach mitigates against the issue of performing multiple statistical tests, which can inflate family‐wise error. A series of random‐effects hierarchical Bayesian meta‐analyses were performed with the brms [23] package to estimate the relative risk for each comparison with accompanying 95% credible intervals (CrIs). We first defined prior distributions for the true pooled effect size (μ) and the between‐study heterogeneity (τ), with μ specified as a normal distribution with a mean equal to the derived point estimate from each comparison of interest in the immediately preceding version of this living review [24], and τ specified as a half‐Cauchy distribution with a mean of 0 and standard deviation of 1. The half‐Cauchy distribution was selected to reflect prior knowledge that high levels of between‐study heterogeneity are more likely than lower levels. Markov chain Monte Carlo methods (20 000 burn‐ins followed by 80 000 iterations) were then used to generate a risk distribution for each study, in addition to a pooled effect for the posterior risk distribution. We report forest plots with the pooled effect for the posterior risk distribution displayed as the median relative risk (RR) with an accompanying 95% CrIs. We used the empirical cumulative distribution function (ECDF) to estimate the probability of there being a 10% reduction or 10% increase in the RR (i.e. RR ≥ 1.1 or RR ≤ 0.9). Due to a lack of indication as to what constitutes a clinically or epidemiologically meaningful effect (e.g. with regard to onward disease transmission or requirements for intensive care beds), we deemed a 10% change in risk as small, but important. Where data were inconclusive (as indicated by CrIs crossing RR = 1.0), to disambiguate whether data favoured no effect or there being a small but important association, we estimated whether there was ≥ 75% probability of RR ≥ 1.1 or RR ≤ 0.9.
Two sensitivity analyses were performed. First, a minimally informative prior for μ was specified as a normal distribution with a mean of 0 and standard deviation of 1 and τ as described above. Second, an informative prior as described above for μ was used with τ specified as a half‐Cauchy distribution with a mean of 0.3 and standard deviation of 1 to reflect greater between‐study heterogeneity.
To aid in the visualization of smoking prevalence in the included studies, 95% bootstrap percentile confidence intervals (CIs) were calculated for each study. We performed 1000 bootstrap replications, with the 2.5th and 97.5th percentiles of the empirical distribution forming the 95% bootstrap percentile CIs [25]. It should be noted that prevalence estimates in the included studies were not adjusted for age, sex, socio‐economic position or region within countries.
Data availability
All data contributing to the current and future review versions are https://doi.org/10.6084/m9.figshare.12756020. All code required to reproduce the current and future analyses are https://doi.org/10.5281/zenodo.4002046.
Results
In the current review (version 7) with searches up to 25 August 2020, a total of 347 new records were identified, with 233 studies included in a narrative synthesis and 32 studies included in meta‐analyses (see Fig. 1).
FIGURE 1.

Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram of included studies.
Study characteristics
Characteristics of included studies are presented in Table 1. Studies were conducted across 33 countries. Sixty‐two studies were conducted in the United States, 53 in China, 26 in the United Kingdom, 13 in Spain, 12 in Mexico, 11 in France, seven in Italy, six across multiple international sites, four in Brazil and Iran, three in Israel and Turkey, two in Bangladesh, Chile, Denmark, Finland, India, Japan and Qatar and one from 15 further countries (see Supporting information, Fig. S1). The majority of studies used observational designs (see Supporting information, Table S1). One hundred and fifty‐five studies were conducted in hospital settings, 62 studies included a community component in addition to hospitalized patients, 14 studies were conducted exclusively in the community, one study was conducted in a quarantine centre and one did not state the study setting. Studies had a median of 404 (interquartile range = 115–1631) participants. The majority of studies (93.5%) used reverse transcriptase–polymerase chain reaction (RT–PCR) for confirmation of SARS‐CoV‐2 infection, 2.6% used an antibody test to confirm prior infection and 3.9% further studies relied on a combination of RT–PCR and clinical diagnosis (see Supporting information, Table S1).
TABLE 1.
Characteristics of included studies.
| Ref. | Lead author | Date published | Country | Sample size | Study setting | Median (IQR) | Female % | Current smoker % | Former smokers % | Current/former smokers % | Never smokers % | Never/unknown smokers % | Missing % | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [1] | Guan, Ni | 2020–02–28 | China | 1099 | Hospital | 47 (35–58) | 41.9 | 12.5 | 1.9 | – | 84.3 | – | 1.27 | Fair |
| [50] | Guan, Liang | 2020–03–26 | China | 1590 | Hospital | 49 (33–64) | 42.7 | – | – | 7.0 | 93.0 | – | 0.00 | Poor |
| [51] | Lian | 2020–03–25 | China | 788 | Hospital | NA | 38.5 | 6.9 | – | – | – | – | 93.15 | Poor |
| [52] | Jin | 2020–03–24 | China | 651 | Hospital | 46 (32–60) | 49.2 | 6.3 | – | – | – | – | 93.70 | Poor |
| [53] | Chen | 2020–03–26 | China | 548 | Hospital | 62 (44–70) | 37.6 | 4.4 | 2.6 | – | – | – | 93.07 | Poor |
| [54] | Zhou, Yu | 2020–03–11 | China | 191 | Hospital | 56 (46–67) | 38.0 | 5.8 | – | – | – | – | 94.24 | Poor |
| [55] | Mo | 2020–03–16 | China | 155 | Hospital | 54 (53–66) | 44.5 | 3.9 | – | – | – | – | 96.13 | Poor |
| [56] | Zhang, Dong | 2020–02–19 | China | 140 | Hospital | 57 † (25–87) | 46.3 | 1.4 | 5.0 | – | – | – | 93.57 | Poor |
| [57] | Wan | 2020–03–21 | China | 135 | Hospital | 47 (36–55) | 46.7 | 6.7 | – | – | – | – | 93.33 | Poor |
| [58] | Liu, Tao | 2020–02–28 | China | 78 | Hospital | 38 (33–57) | 50.0 | – | – | 6.4 | – | – | 93.59 | Poor |
| [59] | Huang, Wang | 2020–01–24 | China | 41 | Hospital | 49 (41–58) | 27.0 | 7.3 | – | – | – | – | 92.68 | Poor |
| [60] | Zhang, Cai | 2020–03–20 | China | 645 | Hospital | NA | 49.1 | 6.4 | – | – | – | – | 93.64 | Poor |
| [61] | Guo | 2020–03–27 | China | 187 | Hospital | 59 (45–73) | 51.3 | 9.6 | – | – | – | – | 90.37 | Poor |
| [62] | Liu, Ming | 2020–03–12 | China | 41 | Hospital | 39 (30–48) | 58.5 | 9.8 | – | – | – | – | 90.24 | Poor |
| [63] | Huang, Yang | 2020–03–05 | China | 36 | Hospital | 69 (60–78) | 30.6 | – | – | 11.1 | – | – | 88.89 | Poor |
| [64] | Xu | 2020–03–08 | China | 53 | Hospital | NA | 47.2 | 11.3 | – | – | – | – | 88.68 | Poor |
| [65] | Li | 2020–02–12 | China | 17 | Hospital | 45 (33–57) | 47.1 | 17.6 | – | – | – | – | 82.35 | Poor |
| [31] | Rentsch | 2020–04–14 | USA | 3528 | Community and Hospital | 66 (60–70) | 4.6 | 27.2 | 30.6 | – | 36.9 | – | 5.30 | Fair |
| [66] | Hu | 2020–03–25 | China | 323 | Hospital | 61 † (23–91) | 48.6 | – | – | 11.8 | – | – | 88.24 | Poor |
| [67] | Wang, Pan | 2020–03–24 | China | 125 | Hospital | 41 (26–66) | 43.2 | – | – | 12.8 | – | – | 87.20 | Poor |
| [68] | Chow (US CDC) | 2020–03–31 | USA | 7162 | Community and Hospital | NA | – | 1.3 | 2.3 | – | – | – | 96.36 | Poor |
| [69] | Dong, Cao | 2020–03–20 | China | 9 | Hospital | 44 (30–46) | 66.7 | 11.1 | – | – | – | – | 88.89 | Poor |
| [70] | Kim | 2020–04–01 | South Korea | 28 | Hospital | 43 (30–56) | 46.4 | 17.9 | – | – | – | – | 82.14 | Poor |
| [71] | Shi, Yu | 2020–03–18 | China | 487 | Hospital | 46 (27–65) | 46.8 | – | – | 8.2 | – | – | 91.79 | Poor |
| [72] | Yang, Yu | 2020–02–24 | China | 52 | Hospital | 60 (47–73) | 37.0 | 3.8 | – | – | – | – | 96.15 | Poor |
| [73] | Argenziano | 2020–05–29 | USA | 1000 | Hospital | 63 (50–75) | 40.4 | 4.9 | 17.9 | – | 77.2 | – | 0.00 | Fair |
| [74] | Solis | 2020–04–25 | Mexico | 650 | Hospital | 46 (NA) | 42.1 | 9.4 | – | – | – | – | 90.62 | Poor |
| [75] | Richardson | 2020–04–22 | USA | 5700 | Hospital | 63 (52–75) | 39.7 | – | – | 9.8 | 52.8 | – | 37.42 | Poor |
| [76] | Fontanet | 2020–04–23 | France | 661 | Community and Hospital | 37 (16–47) | 62.0 | 10.4 | – | – | – | 89.6 | 0.00 | Poor |
| [77] | Zheng, Gao | 2020–04–19 | China | 66 | Hospital | 47 † (NA) | 25.8 | 12.1 | – | – | – | – | 87.88 | Poor |
| [78] | Liao, Feng | 2020–04–24 | China | 1848 | Hospital | 55 (48–61) | 54.7 | – | – | 0.4 | – | – | 99.57 | Poor |
| [79] | Gil–Agudo | 2020–04–24 | Spain | 7 | Hospital | 68 (34–75) | 28.6 | – | – | 42.9 | 57.1 | – | 0.00 | Poor |
| [80] | Shi, Ren | 2020–04–23 | China | 134 | Hospital | 46 (34–58) | 51.5 | – | – | 10.4 | – | – | 89.55 | Poor |
| [81] | Hadjadj | 2020–04–23 | France | 50 | Hospital | 55 (50–63) | 22.0 | 2.0 | 18.0 | – | 80.0 | – | 0.00 | Fair |
| [82] | Gold (US CDC) | 2020–04–20 | USA | 305 | Hospital | NA | 50.5 | 5.2 | – | – | – | – | 94.75 | Poor |
| [83] | Yu, Cai | 2020–04–27 | China | 95 | Hospital | NA | 44.2 | 8.4 | – | – | – | – | 91.58 | Poor |
| [84] | Zheng, Xiong | 2020–04–30 | China | 73 | Hospital | 43 † (NA) | 45.2 | – | – | 11.0 | 89.0 | – | 0.00 | Poor |
| [85] | de la Rica | 2020–05–11 | Spain | 48 | Hospital | 66 † (33–88) | 33.0 | – | – | 20.8 | – | – | 79.17 | Poor |
| [86] | Yin, Yang | 2020–05–10 | China | 106 | Hospital | 73 (61–85) | 39.6 | – | – | 17.0 | – | – | 83.02 | Poor |
| [87] | Shi, Zuo | 2020–05–17 | USA | 172 | Hospital | 63 † (44–82) | 44.0 | – | – | 26.2 | – | – | 73.84 | Poor |
| [88] | Cho | 2020–05–11 | UK | 322 341 | Community and Hospital | NA | 49.2 | 14.2 | 21.4 | – | 64.4 | – | 0.00 | Fair |
| [89] | Allenbach | 2020–05–08 | France | 152 | Hospital | 77 (60–83) | 31.1 | – | – | 6.6 | – | – | 93.42 | Poor |
| [90] | Robilotti | 2020–05–08 | USA | 423 | Hospital | NA | 50.0 | 2.1 | 37.6 | – | 58.6 | – | 1.65 | Fair |
| [91] | The OpenSAFELY Collaborative | 2020–07–01 | UK | 17 278 392 | Community and Hospital | NA | 50.1 | 17.0 | 32.9 | – | 45.9 | – | 4.17 | Fair |
| [92] | Borobia | 2020–05–06 | Spain | 2226 | Hospital | 61 (46–78) | 52.0 | 7.1 | – | – | – | – | 92.95 | Poor |
| [93] | Giacomelli | 2020–05–06 | Italy | 233 | Hospital | 61 (50–72) | 31.9 | – | – | 30.0 | 70.0 | – | 0.00 | Poor |
| [94] | Shah | 2020–05–06 | USA | 316 | Hospital | 63 (43–72) | 48.1 | 16.5 | 17.7 | – | 42.1 | – | 23.73 | Poor |
| [95] | Kolin | 2020–05–05 | UK | 502 536 | Community and Hospital | 56.5 (48–64) | 54.4 | 10.5 | 34.4 | – | 54.4 | – | 0.59 | Fair |
| [96] | Lubetzky | 2020–05–08 | USA | 54 | Hospital | 57 (29–83) | 62.0 | – | – | 22.2 | – | – | 77.78 | Poor |
| [97] | Goyal | 2020–04–17 | USA | 393 | Hospital | 62.2 (49–74) | 39.3 | 5.1 | – | – | – | – | 94.91 | Poor |
| [98] | Feng | 2020–04–10 | China | 476 | Hospital | 53 (40–64) | 43.1 | 9.2 | – | – | – | – | 90.76 | Poor |
| [99] | Yao | 2020–04–24 | China | 108 | Hospital | 52 (37–58) | 60.2 | 3.7 | – | – | – | – | 96.30 | Poor |
| [100] | Sami | 2020–05–19 | Iran | 490 | Hospital | 56.6 (41–71) | 39.0 | 14.1 | – | – | – | 85.9 | 0.00 | Poor |
| [101] | Almazeedi | 2020–05–15 | Kuwait | 1096 | Hospital | 41 (25–57) | 19.0 | 4.0 | – | – | – | 96.0 | 0.00 | Poor |
| [102] | Carillo‐Vega | 2020–05–14 | Mexico | 10 544 | Community and Hospital | 46.5 † (30–62) | 42.3 | 8.9 | – | – | – | – | 91.12 | Poor |
| [103] | Yanover | 2020–05–13 | Israel | 4353 | Community and Hospital | 35 (22–54) | 44.5 | 11.8 | 3.0 | – | 85.2 | – | 0.00 | Fair |
| [104] | Hamer | 2020–05–13 | UK | 387 109 | Hospital | 56.2 (48–64) | 55.1 | 9.7 | 34.8 | – | 55.5 | – | 0.00 | Fair |
| [105] | Regina | 2020–05–14 | Switzerland | 200 | Hospital | 70 (55–81) | 40.0 | 4.5 | – | – | – | – | 95.50 | Poor |
| [39] | de Lusignan | 2020–05–15 | UK | 3802 | Community and Hospital | 58 (34–73) | 57.6 | 10.9 | 46.1 | – | 29.6 | – | 13.44 | Fair |
| [106] | Targher | 2020–05–13 | China | 339 | Hospital | 48.4 † (NA) | 52.8 | 8.3 | – | – | – | – | 91.74 | Poor |
| [107] | Valenti | 2020–05–18 | Italy | 789 | Community | 40.7 † (NA) | 35.0 | 25.9 | – | – | – | – | 74.14 | Poor |
| [108] | Feuth | 2020–05–18 | Finland | 28 | Hospital | 56 (47–72) | 46.0 | 10.7 | 28.6 | – | 60.7 | – | 0.00 | Fair |
| [109] | Ge | 2020–05–18 | China | 51 | Hospital | 70 (58–79) | 27.5 | 13.7 | – | – | – | – | 86.27 | Poor |
| [110] | Parrotta | 2020–05–18 | USA | 76 | Community and Hospital | 44.9 (13–71) | 61.8 | 2.6 | 26.3 | – | 68.4 | – | 2.63 | Fair |
| [111] | Shekhar | 2020–05–18 | USA | 50 | Hospital | 55.5 (20–85) | 54.0 | 48.0 | – | – | – | – | 52.00 | Poor |
| [112] | Mejia‐Vilet | 2020–05–16 | Mexico | 329 | Hospital | 49 (41–60) | 36.0 | – | – | 7.0 | – | – | 93.01 | Poor |
| [113] | Chen, Jiang | 2020–05–16 | China | 135 | Hospital | NA | 42.2 | – | – | 9.6 | – | – | 90.37 | Poor |
| [114] | Li, Chen | 2020–05–16 | China | 1008 | Hospital | 55 (44–65) | 43.6 | 5.7 | – | – | – | – | 94.35 | Poor |
| [27] | Rimland | 2020–05–19 | USA | 11 | Hospital | 59 (48–65) | 18.2 | 9.1 | – | – | – | – | 81.82 | Poor |
| [115] | Palaiodimos | 2020–05–15 | USA | 200 | Hospital | 64 (50–73.5) | 51.0 | – | – | 32.5 | 67.5 | – | 0.00 | Poor |
| [116] | Ip | 2020–05–25 | USA | 2512 | Hospital | 64 (52–76) | 37.6 | 3.1 | 17.8 | – | 64.5 | – | 14.61 | Fair |
| [117] | Heili‐Frades | 2020–05–25 | Spain | 4712 | Hospital | 62 (47–77) | 50.5 | 4.9 | 17.4 | – | – | 66.5 | 11.16 | Poor |
| [118] | Vaquero‐Roncero | 2020–05–24 | Spain | 146 | Hospital | 66 † (59–72) | 32.2 | – | – | 6.8 | – | – | 93.15 | Poor |
| [119] | Kim, Garg | 2020–05–22 | USA | 2491 | Hospital | 62 (50–75) | 46.8 | 6.0 | 25.8 | – | – | 68.1 | 0.08 | Poor |
| [120] | Wu | 2020–05–21 | Italy | 174 | Hospital | 61.2 † (50–71) | 30.5 | – | – | 33.3 | – | – | 66.67 | Poor |
| [121] | Shi, Zhao | 2020–05–20 | China | 101 | Hospital | 71 (59–80) | 40.6 | – | – | 5.0 | – | – | 95.05 | Poor |
| [122] | Al‐Hindawi | 2020–05–20 | UK | 31 | Hospital | 61 (NA) | 12.9 | 3.2 | 71.0 | – | 25.8 | – | 0.00 | Fair |
| [123] | Basse | 2020–05–19 | France | 141 | Hospital | 62 (52–72) | 72.0 | 17.7 | – | – | – | – | 82.27 | Poor |
| [124] | Freites | 2020–05–19 | Spain | 123 | Hospital | 59.88 † (44–74) | 69.9 | 3.3 | – | – | – | – | 96.75 | Poor |
| [125] | Alshami | 2020–05–19 | Saudi Arabia | 128 | Quarantine Centre | 39.6 † (24–55) | 53.9 | 15.6 | 2.3 | – | – | – | 82.03 | Poor |
| [126] | Berumen | 2020–05–26 | Mexico | 102 875 | Hospital | NA | 49.1 | – | – | 9.6 | – | 90.4 | 0.00 | Poor |
| [127] | Gianfrancesco | 2020–05–29 | Multiple | 600 | Community and Hospital | 56 (45–67) | 71.0 | – | – | 21.5 | 64.8 | – | 13.67 | Poor |
| [128] | Li, Long | 2020–05–28 | China | 145 | Not Stated | 49 † (13–80) | 61.0 | – | – | 5.5 | – | – | 94.48 | Poor |
| [129] | Batty | 2020–06–17 | UK | 908 | Hospital | 57.27 † (48–66) | 44.3 | 11.2 | – | – | – | – | 88.77 | Poor |
| [130] | Israel | 2020–06–01 | Israel | 24 906 | Community and Hospital | 40 (27–59) | 48.7 | 16.8 | 12.7 | – | 70.5 | – | 0.00 | Fair |
| [131] | del Valle | 2020–05–30 | USA | 1484 | Hospital | 62 (52–72) | 40.6 | 5.5 | 23.3 | – | – | – | 71.16 | Poor |
| [132] | Chaudhry | 2020–05–29 | USA | 40 | Community and Hospital | 52 (45.5–61) | 60.0 | – | – | 15.0 | – | – | 85.00 | Poor |
| [133] | Louis | 2020–05–28 | USA | 22 | Hospital | 66.5 † (55–77) | 36.4 | – | – | 45.5 | – | – | 54.55 | Poor |
| [134] | Soto‐Mota | 2020–06–05 | Mexico | 400 | Hospital | NA | 30.0 | – | – | 12.0 | – | – | 88.00 | Poor |
| [135] | Garibaldi | 2020–05–26 | USA | 832 | Hospital | 63 (49–75) | 47.0 | 5.5 | 22.6 | – | – | – | 71.88 | Poor |
| [136] | Docherty | 2020–05–22 | Multiple | 20 133 | Hospital | 72.9 (58–82) | 40.0 | 4.2 | 21.7 | – | 44.5 | – | 29.55 | Poor |
| [137] | Boulware | 2020–06–03 | Multiple | 821 | Community | 40 (33–50) | 51.6 | 3.3 | – | – | – | – | 96.71 | Poor |
| [138] | Kuderer | 2020–05–28 | Multiple | 928 | Community and Hospital | 66 (57–76) | 50.0 | 4.6 | 35.1 | – | 50.5 | – | 9.70 | Fair |
| [139] | Romao | 2020–06–08 | Portugal | 34 | Community | 41 † (26–66) | 67.7 | – | – | 26.5 | – | – | 73.53 | Poor |
| [140] | Giannouchos | 2020–06–07 | Mexico | 236 439 | Community and Hospital | 42.5 † (25–59) | 49.1 | 9.1 | – | – | – | 90.9 | 0.00 | Poor |
| [141] | Ramlall | 2020–06–06 | USA | 11 116 | Community and Hospital | 52 (34.7–69.5) | 55.2 | – | – | 26.8 | 73.2 | – | 0.00 | Poor |
| [142] | Wang, Oekelen | 2020–06–05 | USA | 58 | Community and Hospital | 67 (NA) | 48.0 | – | – | 36.2 | – | – | 63.79 | Poor |
| [143] | Perrone | 2020–06–05 | Italy | 1189 | Hospital | NA | 21.2 | – | – | 21.9 | – | – | 78.13 | Poor |
| [144] | Sharma | 2020–06–05 | India | 501 | Hospital | 35.1 † (18–51) | 36.0 | – | – | 4.2 | – | – | 95.81 | Poor |
| [145] | Eugen‐Olsen | 2020–06–02 | Denmark | 407 | Hospital | 64 (47–77) | 57.7 | 20.6 | 36.9 | – | 39.6 | – | 2.95 | Fair |
| [146] | Martinez‐Portilla | 2020–06–02 | Mexico | 224 | Community and Hospital | 29 (26–33) | 100.0 | – | – | 3.1 | – | – | 96.88 | Poor |
| [147] | Raisi‐Estabragh | 2020–06–02 | UK | 4510 | Hospital | NA | 48.8 | – | – | 51.8 | – | – | 48.20 | Poor |
| [148] | Luo | 2020–06–02 | China | 625 | Hospital | 46 (NA) | 47.7 | 3.0 | – | – | – | – | 96.96 | Poor |
| [149] | Houlihan | 2020–06–09 | UK | 200 | Community | 34 (29–44) | 61.0 | 11.0 | 16.5 | – | 66.5 | – | 6.00 | Fair |
| [150] | Cen | 2020–06–08 | China | 1007 | Hospital | 61 (49–68) | 51.0 | – | – | 8.7 | – | – | 91.26 | Poor |
| [151] | Klang | 2020–05–23 | USA | 3406 | Hospital | NA | 61.8 | – | – | 23.3 | – | – | 76.72 | Poor |
| [152] | Maraschini | 2020–06–12 | Italy | 146 | Hospital | 32.5 † (27–38) | 100.0 | – | 9.6 | – | 80.8 | – | 9.59 | Poor |
| [153] | Wang, Zhong | 2020–06–12 | USA | 7592 | Community and Hospital | NA | 45.1 | 3.6 | 17.1 | – | 51.9 | – | 27.42 | Poor |
| [154] | McQueenie | 2020–06–12 | UK | 428 199 | Community and Hospital | NA | 54.9 | – | – | 44.4 | 55.0 | – | 0.59 | Poor |
| [26] | Miyara | 2020–06–12 | France | 479 | Community and Hospital | NA | 44.7 | 6.7 | 31.6 | – | 59.5 | – | 1.87 | Fair |
| [155] | Apea | 2020–06–12 | UK | 1737 | Hospital | 63.4 † (NA) | 30.4 | – | – | 10.0 | – | – | 90.04 | Poor |
| [156] | Woolford | 2020–06–11 | UK | 4510 | Community and Hospital | 70.5 (NA) | 51.2 | 13.0 | 38.1 | – | 48.1 | – | 0.80 | Fair |
| [157] | Hultcrantz | 2020–06–11 | USA | 127 | Community and Hospital | 68 (41–91) | 46.0 | – | – | 26.8 | 72.4 | – | 0.79 | Poor |
| [158] | Rajter | 2020–06–10 | USA | 280 | Hospital | 59.6 † (41–77) | 45.5 | 5.7 | 10.7 | – | 74.6 | – | 8.93 | Fair |
| [159] | Lan | 2020–06–09 | USA | 104 | Community | 49 † (34–63) | 47.1 | – | – | 24.0 | – | – | 75.96 | Poor |
| [160] | Zeng | 2020–06–16 | China | 1031 | Hospital | 60.3 † (46–74) | 47.8 | – | – | 10.2 | – | – | 89.82 | Poor |
| [161] | Suleyman | 2020–06–16 | USA | 463 | Hospital | 57.5 † (40–74) | 55.9 | – | – | 34.6 | – | – | 65.44 | Poor |
| [162] | Chen, Yu | 2020–06–16 | China | 1859 | Hospital | 59 (45–68) | 50.0 | 2.4 | 3.6 | – | 94.0 | – | 0.00 | Fair |
| [163] | Garassino | 2020–06–12 | Multiple | 200 | Community and Hospital | 68 (61.8–75) | 30.0 | 24.0 | 55.5 | – | 18.5 | – | 2.00 | Fair |
| [164] | Hernandez‐Garduno | 2020–06–11 | Mexico | 32 583 | Community and Hospital | 45 (34–56) | 48.7 | – | – | 11.0 | – | 88.8 | 0.15 | Poor |
| [165] | Govind | 2020–06–20 | UK | 6309 | Community and Hospital | 46.5 † (31–61) | 38.3 | 66.3 | 26.8 | – | 5.5 | – | 1.49 | Fair |
| [166] | Siso‐Almirall | 2020–06–20 | Spain | 322 | Community and Hospital | 56.7 † (38–74) | 50.0 | – | – | 25.2 | – | – | 74.84 | Poor |
| [167] | Gu | 2020–06–18 | USA | 5698 | Community and Hospital | 47 † (26–67) | 62.0 | 7.0 | 24.7 | – | 50.8 | – | 17.53 | Fair |
| [168] | Kibler | 2020–06–16 | France | 702 | Community and Hospital | 82 † (75–88) | 56.0 | 3.7 | – | – | – | – | 96.30 | Poor |
| [169] | Ikitimur | 2020–06–03 | Turkey | 81 | Hospital | 55 † (38–72) | 44.0 | – | – | 28.4 | – | – | 71.60 | Poor |
| [170] | Sierpinski | 2020–06–03 | Poland | 1942 | Community | 50 (NA) | 60.0 | 6.3 | – | – | – | 49.7 | 44.03 | Poor |
| [171] | Zhou, He | 2020–06–10 | China | 238 | Hospital | 55.5 (35–67) | 57.0 | 2.9 | – | – | – | – | 97.06 | Poor |
| [172] | Crovetto | 2020–06–19 | Spain | 874 | Community and Hospital | 33.7 † (28–38) | 100.0 | 1.1 | – | – | – | 13.2 | 85.70 | Poor |
| [173] | Veras | 2020–06–09 | Brazil | 32 | Hospital | 58.9 † (40–77) | 47.0 | – | – | 25.0 | – | – | 75.00 | Poor |
| [174] | Sterlin | 2020–06–11 | France | 135 | Hospital | 61 (50–72) | 41.0 | 3.7 | 38.5 | – | 57.8 | – | 0.00 | Fair |
| [175] | Rossi | 2020–06–09 | France | 246 | Hospital | 68 † (53–83) | 39.0 | – | – | 25.2 | – | – | 74.80 | Poor |
| [176] | Duan | 2020–06–22 | China | 616 | Hospital | 64 (53–70) | 57.5 | 3.7 | – | – | – | – | 96.27 | Poor |
| [177] | Martin‐Jimenez | 2020–06–09 | Spain | 339 | Hospital | 81.6 (72–87) | 39.5 | – | – | 30.7 | – | – | 69.32 | Poor |
| [178] | Elezkurtaj | 2020–06–17 | Germany | 26 | Hospital | 70 (61.8–78.3) | 34.6 | – | – | 19.2 | – | – | 80.77 | Poor |
| [179] | Lenka | 2020–06–22 | USA | 32 | Hospital | 62.2 † (51–73) | 37.5 | – | – | 50.0 | – | – | 50.00 | Poor |
| [180] | Olivares | 2020–06–16 | Chile | 21 | Hospital | 61 † (26–85) | 76.2 | – | – | 9.5 | – | – | 90.48 | Poor |
| [181] | Salton | 2020–06–20 | Italy | 173 | Hospital | 64.4 † (NA) | 34.9 | – | – | 29.5 | – | – | 70.52 | Poor |
| [182] | Wei | 2020–06–18 | USA | 147 | Hospital | 52 † (34–70) | 41.0 | 14.3 | – | – | – | – | 85.71 | Poor |
| [183] | Zuo, Estes | 2020–06–17 | China | 172 | Hospital | 61 † (25–95) | 44.0 | – | – | 26.2 | – | – | 73.84 | Poor |
| [184] | Killerby | 2020–06–17 | USA | 531 | Community and Hospital | 51.6 (38–62) | 57.1 | – | – | 17.1 | 71.4 | – | 11.49 | Poor |
| [185] | Petrilli | 2020–05–22 | USA | 5279 | Community and Hospital | 54 (38–66) | 51.5 | 5.5 | 17.1 | – | 61.9 | – | 15.55 | Fair |
| [186] | Magagnoli | 2020–06–05 | USA | 807 | Hospital | 70 (60–75) | 4.3 | – | – | 15.9 | – | – | 84.14 | Poor |
| [33] | Niedzwiedz | 2020–05–29 | UK | 392 116 | Community and Hospital | NA | 54.9 | 9.8 | 34.8 | – | 55.4 | – | 0.00 | Fair |
| [187] | Bello‐Chavolla | 2020–05–31 | Mexico | 177 133 | Community and Hospital | 42.6 (26–59) | 48.9 | – | – | 9.3 | – | – | 90.72 | Poor |
| [188] | Zuo, Yalavarthi | 2020–04–24 | USA | 50 | Hospital | 61 (46–76) | 34.0 | – | – | 36.0 | – | – | 64.00 | Poor |
| [189] | Sigel | 2020–06–28 | USA | 493 | Hospital | 60 (55–67) | 24.1 | – | – | 28.6 | – | – | 71.40 | Poor |
| [190] | Nguyen | 2020–06–29 | USA | 689 | Community and Hospital | 55 (40–68) | 57.0 | – | – | 24.8 | – | – | 75.18 | Poor |
| [191] | de Melo | 2020–06–29 | Brazil | 181 | Hospital | 55.3 † (34–76) | 60.8 | 9.9 | 12.2 | – | 38.1 | – | 39.78 | Poor |
| [192] | Auvinen | 2020–06–29 | Finland | 61 | Hospital | 53 (41–67) | 36.0 | 18.0 | 27.9 | – | 54.1 | – | 0.00 | Fair |
| [193] | Souza | 2020–06–28 | Brazil | 8443 | Hospital | NA | 53.0 | – | – | 1.7 | – | 96.3 | 2.01 | Poor |
| [194] | Mendy | 2020–06–27 | USA | 689 | Community and Hospital | 49.5 (35.2–67.5) | 47.0 | – | – | 24.7 | – | – | 75.33 | Poor |
| [195] | Pongpirul | 2020–06–26 | Thailand | 193 | Hospital | 37 (29–53) | 41.5 | – | – | 15.0 | 66.3 | – | 18.65 | Poor |
| [196] | Jin, Gu | 2020–06–25 | China | 6 | Hospital | 60.5 † (51–75) | 33.3 | 33.3 | – | – | – | – | 66.67 | Poor |
| [197] | Favara | 2020–05–23 | UK | 70 | Community and Hospital | 41 (23–64) | 87.1 | 10.0 | – | – | – | – | 90.00 | Poor |
| [198] | Fisman | 2020–06–23 | Canada | 21 922 | Community and Hospital | NA | 57.0 | – | – | 2.3 | – | – | 97.65 | Poor |
| [199] | Madariaga | 2020–06–23 | USA | 103 | Community and Hospital | 41.8 † (27–55) | 48.5 | – | – | 25.2 | 74.8 | – | 0.00 | Poor |
| [200] | Senkal | 2020–07–07 | Turkey | 611 | Hospital | 57 † (18–98) | 40.6 | 11.3 | – | – | – | – | 88.71 | Poor |
| [201] | Mohamud | 2020–07–02 | USA | 6 | Hospital | 65.8 † (55–78) | 16.7 | – | – | 16.7 | – | – | 83.33 | Poor |
| [202] | Magleby | 2020–06–30 | USA | 678 | Hospital | 68 (50–81) | 38.9 | – | – | 28.6 | – | – | 71.39 | Poor |
| [203] | Kimmig | 2020–07–06 | USA | 111 | Hospital | 63 † (48–78) | 44.1 | 7.2 | 36.0 | – | 56.8 | – | 0.00 | Fair |
| [204] | Bello‐Chavolla, Antonio‐Villa | 2020–07–04 | Mexico | 60 121 | Community and Hospital | 45.5 † (29–61) | 47.0 | – | – | 10.5 | – | – | 89.52 | Poor |
| [205] | Zacharioudakis | 2020–07–04 | USA | 314 | Hospital | 64 (54–72) | 34.7 | – | – | 22.8 | – | – | 77.22 | Poor |
| [206] | Antonio‐Villa | 2020–07–04 | Mexico | 34 263 | Community and Hospital | 40 † (29–50) | 62.9 | 9.7 | – | – | – | – | 90.32 | Poor |
| [207] | Patel | 2020–07–03 | USA | 129 | Hospital | 60.8 † (47–74) | 45.0 | 37.2 | – | – | – | 55.8 | 6.98 | Poor |
| [208] | Merzon | 2020–07–03 | Israel | 7807 | Community and Hospital | 46.2 † (NA) | 58.6 | – | – | 16.2 | – | – | 83.82 | Poor |
| [34] | Trubiano | 2020–07–02 | Australia | 2935 | Community and Hospital | 39 (29–53) | 63.5 | – | – | 8.8 | – | – | 91.18 | Poor |
| [209] | Fan | 2020–07–11 | UK | 1425 | Community and Hospital | NA | 46.7 | 12.2 | 40.1 | – | 46.9 | – | 0.84 | Fair |
| [210] | Shi, Resurreccion | 2020–07–11 | UK | 1521 | Community and Hospital | 61.5 † (57–66.8) | 45.9 | – | – | 54.9 | – | – | 45.10 | Poor |
| [211] | Maucourant | 2020–07–10 | Sweden | 27 | Hospital | 57 (18–78) | 22.2 | 11.1 | 25.9 | – | 40.7 | – | 22.22 | Poor |
| [212] | Elmunzer | 2020–07–09 | Multiple | 1992 | Hospital | 60 † (43–76) | 43.0 | 6.3 | 28.6 | – | 59.0 | – | 6.12 | Fair |
| [213] | Alizadehsani | 2020–07–09 | Iran | 319 | Hospital | 45.48 † (26–63) | 55.5 | – | – | 0.3 | – | – | 99.69 | Poor |
| [214] | Xie | 2020–07–07 | China | 619 | Hospital | NA | 52.0 | – | – | 8.2 | – | – | 91.76 | Poor |
| [36] | Merkely | 2020–07–17 | Hungary | 10 474 | Community | 48.7 † (30–66) | 53.6 | 28.0 | 20.5 | – | 51.4 | – | 0.16 | good |
| [215] | Fox | 2020–07–17 | UK | 55 | Community and Hospital | 63 (23–88) | 31.0 | 1.8 | 10.9 | – | 56.4 | – | 30.91 | Poor |
| [56] | Zhang, Cao | 2020–07–14 | China | 289 | Hospital | 57 (22–88) | 46.6 | 3.5 | 6.2 | – | – | – | 90.31 | Poor |
| [216] | Martinez‐Resendez | 2020–07–20 | Mexico | 8 | Hospital | 57 (48–69) | 25.0 | – | – | 12.5 | – | – | 87.50 | Poor |
| [217] | Hoertel | 2020–07–20 | France | 12 612 | Hospital | 58.7 † (39–77) | 49.6 | – | – | 9.3 | – | – | 90.72 | Poor |
| [218] | McGrail | 2020–07–19 | USA | 209 | Hospital | 62.5 (NA) | 38.8 | – | – | 18.7 | – | – | 81.34 | Poor |
| [219] | Pandolfi | 2020–07–17 | Italy | 33 | Hospital | 62 (52–65) | 21.1 | 3.0 | 24.2 | – | 72.7 | – | 0.00 | Fair |
| [28] | Girardeau | 2020–07–17 | France | 10 | Community | 30 (29–33) | 50.0 | 40.0 | 10.0 | – | – | – | 40.00 | Poor |
| [220] | Kurashima | 2020–07–17 | Japan | 53 | Hospital | 62.9 † (49–76) | 35.8 | – | – | 50.9 | – | – | 49.06 | Poor |
| [221] | Zhan | 2020–07–16 | China | 75 | Hospital | 57 (25–75) | 48.0 | – | – | 12.0 | – | – | 88.00 | Poor |
| [222] | Omrani | 2020–07–16 | Qatar | 1409 | Community and Hospital | 39 (30–50) | 17.2 | – | – | 9.2 | – | – | 90.77 | Poor |
| [223] | Gupta | 2020–07–16 | USA | 496 | Hospital | 70 (60–78) | 46.0 | – | – | 7.3 | – | 31.7 | 61.09 | Poor |
| [87] | Shi, Zuo | 2020–07–15 | USA | 172 | Hospital | 61.48 † (25–96) | 44.0 | – | – | 26.2 | – | – | 73.84 | Poor |
| [224] | Hussein | 2020–07–15 | USA | 502 | Hospital | 60.9 † (45–76) | 52.0 | 9.0 | 22.1 | – | – | 68.9 | 0.00 | Poor |
| [225] | Bian | 2020–07–15 | China | 28 | Hospital | 56 † (42–67) | 42.9 | 7.1 | – | – | – | – | 92.86 | Poor |
| [226] | Eiros | 2020–07–14 | Spain | 139 | Community and Hospital | 52 (41–57) | 72.0 | 4.3 | 50.4 | – | – | – | 45.32 | Poor |
| [227] | Marcos | 2020–07–14 | Spain | 918 | Hospital | 72.8 † (58–87) | 42.2 | 6.1 | – | 15.3 | – | – | 78.65 | Poor |
| [228] | Hoertel, Sanchez‐Rico | 2020–07–14 | France | 7345 | Hospital | NA | 49.3 | 8.5 | – | – | – | – | 91.52 | Poor |
| [229] | Soares | 2020–07–16 | Brazil | 10 713 | Community and Hospital | NA | 55.0 | 2.0 | – | – | – | 98.0 | 0.00 | Poor |
| [230] | Zobairy | 2020–07–28 | Iran | 203 | Community and Hospital | 49.2 † (32–65) | 44.8 | 5.9 | – | – | – | 94.1 | 0.00 | Poor |
| [231] | Altamimi | 2020–07–27 | Qatar | 68 | Hospital | 49 † (40–58) | 2.0 | 16.4 | – | – | – | 83.6 | 0.00 | Poor |
| [232] | Thompson | 2020–07–27 | UK | 470 | Hospital | 71 (57–82) | 46.0 | 14.0 | 27.2 | – | 58.7 | – | 0.00 | Fair |
| [233] | Reiter | 2020–07–26 | Austria | 235 | Community | 44.2 † (32–55) | 70.0 | 22.6 | 22.6 | – | 54.7 | – | 0.00 | Fair |
| [234] | Motta | 2020–07–26 | USA | 374 | Hospital | 64.7 † (46–82) | 41.4 | – | – | 33.2 | 66.8 | – | 0.00 | Poor |
| [235] | Santos | 2020–07–25 | USA | 43 | Community and Hospital | 50 (34–73) | 63.0 | – | – | 4.7 | – | – | 95.35 | Poor |
| [236] | Schneeweiss | 2020–07–22 | USA | 24 313 | Community and Hospital | 67 † (53–80) | 53.0 | – | – | 2.9 | – | – | 97.12 | Poor |
| [237] | Concha‐Mejia | 2020–07–24 | Colombia | 72 | Community and Hospital | 46 (28–64) | 47.0 | 8.3 | 11.1 | – | – | – | 80.56 | Poor |
| [238] | Izquierdo | 2020–07–24 | Spain | 71 192 | Community and Hospital | 42 † (18–66) | 59.0 | 10.0 | – | – | – | 90.0 | 0.00 | Poor |
| [239] | Bernaola | 2020–07–21 | Spain | 1645 | Hospital | NA | 38.5 | 2.5 | 10.9 | – | 86.6 | – | 0.00 | Fair |
| [30] | Islam | 2020–08–18 | Bangladesh | 1016 | Community and Hospital | 37 (28–49) | 35.9 | 18.2 | – | – | – | – | 77.85 | Poor |
| [240] | Qi | 2020–03–03 | China | 267 | Hospital | 48 (35–65) | 45.2 | 19.9 | – | – | – | 80.1 | 0.00 | Poor |
| [241] | Peters | 2020–08–15 | Netherlands | 1893 | Hospital | 66.8 † (52–81) | 39.4 | 4.9 | – | – | – | – | 95.14 | Poor |
| [242] | Ouyang | 2020–08–14 | China | 217 | Hospital | 46.5 † (30–62) | 53.5 | 16.6 | – | – | – | – | 83.41 | Poor |
| [47] | Ward | 2020–08–21 | UK | 99 908 | Community | NA | 56.1 | 10.6 | – | – | – | 88.4 | 0.98 | Poor * |
| [243] | Valenzuela | 2020–08–14 | Chile | 29 | Hospital | 56.9 † (43–70) | 6.9 | 17.2 | – | – | – | 82.8 | 0.00 | Poor |
| [244] | Monteiro | 2020–08–14 | USA | 112 | Hospital | 61 (45–74) | 34.0 | 6.2 | 17.9 | – | 68.8 | – | 7.14 | Fair |
| [245] | Philipose | 2020–08–14 | UK | 466 | Hospital | 67 (6–97) | 41.8 | 6.0 | 73.2 | – | 16.5 | – | 4.29 | Fair |
| [246] | Weerahandi | 2020–08–14 | USA | 394 | Community | 63 (55–70) | 37.0 | 5.3 | 25.9 | – | 55.8 | – | 12.94 | Fair |
| [29] | Ebinger | 2020–08–04 | USA | 6062 | Community | 41.5 † (29–53) | 67.8 | 1.7 | – | – | – | – | 96.88 | Poor |
| [247] | Altibi | 2020–08–11 | USA | 706 | Hospital | 66.7 † (51–81) | 43.0 | 4.0 | 37.3 | – | 58.8 | – | 0.00 | Fair |
| [248] | Izzi‐Engbeaya | 2020–08–11 | UK | 889 | Hospital | 65.8 † (48–83) | 40.0 | – | – | 21.3 | 33.2 | – | 45.6 | Poor |
| [249] | Rizzo | 2020–08–11 | USA | 76 819 | Hospital | 54 (38–67) | 55.2 | 6.7 | 20.8 | – | 50.4 | – | 22.05 | Poor |
| [250] | Dashti | 2020–08–04 | USA | 4140 | Community and Hospital | 52 (36–65) | 55.0 | – | – | 28.4 | 51.6 | – | 19.95 | Poor |
| [251] | Morshed | 2020–08–02 | Bangladesh | 103 | Community | 37 (31–53) | 28.2 | 31.1 | – | – | – | 68.9 | 0.00 | Poor |
| [252] | Jun | 2020–08–01 | USA | 3086 | Hospital | 66 (56–77) | 40.9 | 3.7 | 21.3 | – | 52.8 | – | 22.23 | Poor |
| [253] | Higuchi | 2020–07–30 | Japan | 57 | Hospital | 52 (35–70) | 43.9 | 12.3 | 29.8 | – | 57.9 | – | 0.00 | Fair |
| [254] | Zhou, Sun | 2020–07–29 | China | 144 | Hospital | 47 (38–56) | 46.5 | 9.0 | – | – | – | 91.0 | 0.00 | Poor |
| [255] | Salerno | 2020–08–22 | USA | 15 920 | Hospital | 49 (30–65) | 57.0 | – | – | 36.8 | 55.9 | – | 7.29 | Poor |
| [256] | Kumar | 2020–07–29 | India | 91 | Hospital | 47 † (41–52) | 21.0 | 44.0 | – | – | – | – | 56.04 | Poor |
| [257] | Hao | 2020–06–01 | China | 788 | Hospital | 46 (35–56) | 48.4 | 6.9 | – | – | – | – | 93.15 | Poor |
| [258] | Iversen | 2020–08–03 | Denmark | 28 792 | Community and Hospital | 44.4 † (31–57) | 78.9 | 16.0 | 6.5 | – | 76.8 | – | 0.67 | Fair |
| [259] | Hippisley‐Cox | 2020–07–13 | UK | 8 275 949 | Community and Hospital | 48.5 † (30–66) | 50.3 | 17.2 | 21.4 | – | 57.3 | – | 4.04 | Fair |
| [260] | Fillmore | 2020–08–24 | USA | 22 914 | Community and Hospital | NA | – | 37.5 | 40.7 | – | 15.5 | – | 6.38 | Fair |
| [261] | Rashid | 2020–08–22 | UK | 517 | Hospital | 72.8 † (59–86) | 31.9 | 9.9 | 29.0 | – | 29.4 | – | 31.72 | Poor |
| [262] | Pan | 2020–08–22 | USA | 12 084 | Community and Hospital | 45.5 † (27–63) | 54.3 | – | – | 17.5 | – | – | 82.49 | Poor |
| [263] | Alkurt | 2020–08–20 | Turkey | 932 | Community and Hospital | 34.8 † (25–44) | 64.4 | 24.5 | – | – | – | – | 75.54 | Poor |
| [264] | Zhao, Chen | 2020–07–30 | USA | 641 | Hospital | 60 (NA) | 40.1 | 21.7 | – | – | – | – | 78.32 | Poor |
| [265] | Holman | 2020–08–13 | UK | 10 989 | Community and Hospital | NA | 38.8 | 5.5 | 42.6 | – | 49.0 | – | 2.82 | Fair |
| [266] | Qu | 2020–07–29 | China | 246 | Hospital | 53.6 † (38–68) | 53.3 | 42.3 | – | – | – | – | 57.72 | Poor |
| [267] | Chand | 2020–08–19 | USA | 300 | Hospital | 58.2 † (45–70) | 39.3 | 22.3 | – | – | – | – | 77.67 | Poor |
NA Age not provided for total sample.
‐ Not reported for total sample.
Denotes mean ± standard deviation.
This study was rated as ‘poor’ quality as the manuscript only presents data for current (but not former) smokers despite having obtained complete smoking status, thus resulting in > 20% missing data on smoking status.
Smoking status
Categorization of smoking status was heterogeneous (see Table 1). One hundred and forty‐five studies collected data on smoking status through routine electronic health records (EHRs), 59 studies used a bespoke case report form for COVID‐19 and 29 studies did not state the source for information on smoking status. None of the studies verified smoking status biochemically. Notably, only 57 (24.4%) studies reported current, former and never smoking status (see Supporting information, Table S2a), with a further 17 studies reporting ever and never smoking status (see Supporting information, Table S2b). The remaining 159 studies reported current, current/former or current and former smoking status but did not explicitly state whether remaining participants were never smokers or if data were missing on smoking status (see Supporting information, Table S2c). Seventy‐eight studies explicitly reported the proportion with missing data on smoking status, which ranged from 0.08 to 96.4%.
Use of alternative nicotine products
Five studies recorded the use of alternative nicotine products in current and/or former smokers but did not report COVID‐19 outcomes stratified by nicotine use [26, 27, 28, 29, 30].
Quality appraisal
One study was performed in a random, representative population sample and was rated as ‘good’ quality. Forty‐six studies were rated as ‘fair’ quality. The remaining 186 studies were rated as ‘poor’ quality (see Table 1).
Smoking prevalence by country
Unadjusted smoking prevalence compared with overall estimates for national adult smoking prevalence split by country and study setting is presented in Fig. 2a,b. Lower than expected current smoking prevalence was generally observed. Former smoking prevalence was more similar to expected prevalence when reported. National smoking prevalence estimates used for comparison are presented in Supporting information, Table S3.
FIGURE 2.

(a) Weighted mean prevalence of current smoking in included studies with 95% bootstrap confidence intervals (CIs) compared with national current smoking prevalence (solid red lines), split by country. Shape corresponds to study setting (community, community and hospital, hospital) and shape size corresponds to relative study sample size. (b) Weighted mean prevalence of former smoking in included studies (where this was reported) with 95% bootstrap CIs compared with national former smoking prevalence (solid red lines), split by country. Shape corresponds to study setting (community, community and hospital, hospital) and shape size corresponds to relative study sample size. [Colour figure can be viewed at wileyonlinelibrary.com]
SARS‐CoV‐2 testing by smoking status
Three studies provided data on access to SARS‐CoV‐2 diagnostic testing for those meeting local testing criteria by smoking status. In a cohort study of US military veterans aged 54–75 years [31], current smokers were more likely to receive a test: 42.3% (1603 of 3789) of the sample were current smokers compared with 23.8% of all veterans aged 50+ years using any tobacco product between 2010 and 2015 [32]. In the UK Biobank cohort [33], former (RR = 1.29, 95% CI = 1.14–1.45, P < 0.001) and current (RR = 1.44, 95% CI = 1.20–1.71, P < 0.001) compared with never smokers were more likely to receive a test in a multivariable analysis. In an Australian rapid assessment screening clinic for COVID‐19 [34], 9.4% (397 of 4226) of the self‐referred sample (subsequently assessed by a health‐care professional to decide on testing) were current smokers. Current compared with former or never smokers were less likely to require a test (RR = 0.93, 95% CI = 0.86–1.0, P = 0.045).
SARS‐CoV‐2 infection by smoking status
Forty‐five studies provided data on SARS‐CoV‐2 infection for people meeting local testing criteria by smoking status (see Table 2). Meta‐analyses were performed for one ‘good’ and 16 ‘fair’ quality studies (see Figs 3 and 4). Current smokers were at reduced risk of testing positive for SARS‐CoV‐2 compared with never smokers (RR = 0.74, 95% CrI = 0.58–0.93, τ = 0.41, 95% CI = 0.24–0.64). The probability of current smokers being at reduced risk of infection compared with never smokers (RR ≤ 0.9) was 95%. Former compared with never smokers were at increased risk of testing positive, but data were inconclusive (RR = 1.05, 95% CrI = 0.95–1.17, τ = 0.17, 95% CI = 0.10–0.26) and favoured there being no important association. The probability of former smokers being at increased risk of infection (RR ≥ 1.1) compared with never smokers was 21%. Results were materially unchanged in the two sensitivity analyses (see Supporting information, Fig. S2).
TABLE 2.
SARS‐CoV‐2 infection by smoking status.
| SARS‐CoV‐2‐negative | SARS‐CoV‐2‐positive | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Total population tested | n (%) | Current smoker (%) | Former smoker (%) | Current/former smoker (%) | Never smoker (%) | Not stated (%) | n (%) | Current smoker (%) | Former smoker (%) | Current/former smoker (%) | Never smoker (%) | Not stated (%) |
| Rentsch | 3528 | 2974 (84.30%) | 1444 (48.55%) | 704 (23.67%) | – | 826 (27.77%) | – | 554 (15.70%) | 159 (28.70%) | 179 (32.31%) | – | 216 (38.99%) | – |
| Fontanet | 661 | 490 (74.13%) | 64 (13.06%) | – | – | 426 (86.94%) | – | 171 (25.87%) | 5 (2.92%) | – | – | 166 (97.08%) | – |
| Cho | 1331 | 793 (59.58%) | 142 (17.91%) | 214 (26.99%) | – | 437 (55.11%) | – | 538 (40.42%) | 111 (20.63%) | 145 (26.95%) | – | 282 (52.42%) | – |
| Shah | 243 | 212 (87.24%) | 52 (24.53%) | 47 (22.17%) | – | 113 (53.30%) | – | 29 (11.93%) | 0 (0.00%) | 9 (31.03%) | – | 20 (68.97%) | – |
| Kolin | 1474 | 805 (54.61%) | 141 (17.52%) | 307 (38.14%) | – | 354 (43.98%) | 3 (0.37%) | 669 (45.39%) | 72 (10.76%) | 285 (42.60%) | – | 303 (45.29%) | 9 (1.35%) |
| de Lusignan | 3291 | 2740 (83.26%) | 366 (13.36%) | 1450 (52.92%) | – | 924 (33.72%) | – | 551 (16.74%) | 47 (8.53%) | 303 (54.99%) | – | 201 (36.48%) | – |
| Valenti | 789 | 689 (87.33%) | 197 (28.59%) | – | – | – | 492 (71.41%) | 40 (5.07%) | 7 (17.50%) | – | – | – | 33 (82.50%) |
| Parrotta | 76 | 39 (51.32%) | 1 (2.56%) | 10 (25.64%) | – | 27 (69.23%) | 1 (2.56%) | 37 (48.68%) | 1 (2.70%) | 10 (27.03%) | – | 25 (67.57%) | 1 (2.70%) |
| Berumen | 102 875 | 71 353 (69.36%) | – | – | 7173 (10.05%) | 64 180 (89.95%) | – | 31 522 (30.64%) | – | – | 2748 (8.72%) | 28 774 (91.28%) | – |
| Israel | 24 906 | 20 755 (83.33%) | 3783 (18.23%) | 2671 (12.87%) | – | 14 301 (68.90%) | – | 41 151 (165.23%) | 406 (0.99%) | 483 (1.17%) | – | 3262 (7.93%) | – |
| del Valle | 1108 | 143 (12.91%) | 27 (18.88%) | 53 (37.06%) | – | – | 63 (44.06%) | 965 (87.09%) | 55 (5.70%) | 293 (30.36%) | – | – | 617 (63.94%) |
| Romao | 34 | 20 (58.82%) | – | – | 5 (25.00%) | – | 15 (75.00%) | 14 (41.18%) | – | – | 4 (28.57%) | – | 10 (71.43%) |
| Ramlall | 11 116 | 4723 (42.49%) | – | – | – | – | – | 6393 (57.51%) | – | – | 1643.001 (25.70%) | 4749.999 (74.30%) | – |
| Sharma | 501 | 267 (53.29%) | – | – | 1 (0.37%) | – | 266 (99.63%) | 234 (46.71%) | – | – | 20 (8.55%) | – | 214 (91.45%) |
| Eugen‐Olsen | 407 | 290 (71.25%) | 76 (26.21%) | 104 (35.86%) | – | 102 (35.17%) | – | 117 (28.75%) | 8 (6.84%) | 46 (39.32%) | – | 59 (50.43%) | – |
| Raisi‐Estabragh | 4510 | 3184 (70.60%) | – | – | 1653 (51.92%) | – | 1531 (48.08%) | 1326 (29.40%) | – | – | 683 (51.51%) | – | 643 (48.49%) |
| Houlihan | 177 | 97 (54.80%) | 14 (14.43%) | 14 (14.43%) | – | 69 (71.13%) | – | 80 (45.20%) | 7 (8.75%) | 19 (23.75%) | – | 54 (67.50%) | – |
| McQueenie | 428 199 | 424 355 (99.10%) | – | – | 189 299 (44.61%) | 235 056 (55.39%) | – | 1311 (0.31%) | – | – | 669 (51.03%) | 642 (48.97%) | – |
| Woolford | 4474 | 3161 (70.65%) | 441 (13.95%) | 1194 (37.77%) | – | 1526 (48.28%) | – | 1313 (29.35%) | 145 (11.04%) | 525 (39.98%) | – | 643 (48.97%) | – |
| Lan | 104 | 83 (79.81%) | – | – | 24 (28.92%) | – | 59 (71.08%) | 21 (20.19%) | – | – | 1 (4.76%) | – | 20 (95.24%) |
| Hernandez‐Garduno | 32 583 | 20 279 (62.24%) | – | – | 2399 (11.83%) | 17 861 (88.08%) | – | 12 304 (37.76%) | – | – | 1191 (9.68%) | 11 083 (90.08%) | – |
| Govind | 6215 | 6207 (99.87%) | 4104 (66.12%) | 1669 (26.89%) | – | 342 (5.51%) | – | 102 (1.64%) | 78 (76.47%) | 20 (19.61%) | – | 2 (1.96%) | – |
| Gu | 4699 | 3815 (81.19%) | 360 (9.44%) | 1142 (29.93%) | – | 2313 (60.63%) | – | 884 (18.81%) | 40 (4.52%) | 264 (29.86%) | – | 580 (65.61%) | – |
| Kibler | 702 | 680 (96.87%) | 25 (3.68%) | – | – | – | 655 (96.32%) | 22 (3.13%) | 1 (4.55%) | – | – | – | 21 (95.45%) |
| Petrilli | 10 620 | 5341 (50.29%) | 3454 (64.67%) | 816 (15.28%) | – | 541 (10.13%) | 530 (9.92%) | 5279 (49.71%) | 3268 (61.91%) | 902 (17.09%) | – | 288 (5.46%) | 821 (15.55%) |
| Bello‐Chavolla | 150 200 | 98 567 (65.62%) | – | – | 9624 (9.76%) | – | 88 943 (90.24%) | 51 633 (34.38%) | – | – | 4366 (8.46%) | – | 47 267 (91.54%) |
| Auvinen | 61 | 33 (54.10%) | 10 (30.30%) | 8 (24.24%) | – | 15 (45.45%) | – | 28 (45.90%) | 1 (3.57%) | 9 (32.14%) | – | 18 (64.29%) | – |
| Favara | 70 | 55 (78.57%) | 5 (9.09%) | – | – | – | 50 (90.91%) | 15 (21.43%) | 2 (13.33%) | – | – | – | 13 (86.67%) |
| Antonio‐Villa | 34 263 | 23 338 (68.11%) | 2293 (9.83%) | – | – | – | 21 045 (90.17%) | 10 925 (31.89%) | 1023 (9.36%) | – | – | – | 9902 (90.64%) |
| Merzon | 7807 | 7025 (89.98%) | – | – | 1136 (16.17%) | – | 5889 (83.83%) | 782 (10.02%) | – | – | 127 (16.24%) | – | 655 (83.76%) |
| Trubiano | 2935 | 2827 (96.66%) | – | – | 256 (9.06%) | – | 2586 (91.48%) | 108 (3.68%) | – | – | 3 (2.78%) | – | 105 (97.22%) |
| Shi, Resurreccion | 1521 | 1265 (83.17%) | – | – | 681 (53.83%) | – | 584 (46.17%) | 256 (16.83%) | – | – | 154 (60.16%) | – | 102 (39.84%) |
| Riley | 120 620 | 120 461 (99.87%) | 2594 (2.15%) | – | – | 19 914 (16.53%) | 97 953 (81.32%) | 159 (0.13%) | 3 (1.89%) | – | – | 17 (10.69%) | 139 (87.42%) |
| Alizadehsani | 319 | 196 (61.44%) | – | – | – | – | 196 (100.00%) | 123 (38.56%) | – | – | 1 (0.81%) | – | 122 (99.19%) |
| Merkely | 10 474 | 10 336 (98.68%) | 2904 (28.10%) | 2107 (20.39%) | – | 5310 (51.37%) | 15 (0.15%) | 70 (0.67%) | 16 (22.86%) | 15 (21.43%) | – | 38 (54.29%) | 1 (1.43%) |
| McGrail | 209 | 118 (56.46%) | – | – | 31 (26.27%) | – | 87 (73.73%) | 91 (43.54%) | – | – | 8 (8.79%) | – | 83 (91.21%) |
| Izquierdo | 71 192 | NA | – | – | – | – | – | 1006 (1.41%) | 111 (11.03%) | – | – | – | 895 (88.97%) |
| Ward | 99 908 | 94 416 (94.50%) | 10 202 (10.81%) | – | – | – | 84 214 (89.19%) | 5492 (5.50%) | 433 (7.88%) | – | – | – | 5059 (92.12%) |
| Ebinger | 6062 | 5850 (96.50%) | 99 (1.69%) | – | – | – | 5668 (96.89%) | 212 (3.50%) | 3 (1.42%) | – | – | – | 205 (96.70%) |
| Salerno | 15 920 | 14 753 (92.67%) | – | – | 5517 (37.40%) | 8278 (56.11%) | 958 (6.49%) | 1167 (7.33%) | – | – | 339 (29.05%) | 626 (53.64%) | 202 (17.31%) |
| Iversen | 28 792 | 27 629 (95.96%) | 4430 (16.03%) | 1799 (6.51%) | – | 21 217 (76.79%) | 246 (0.89%) | 1163 (4.04%) | 177 (15.22%) | 78 (6.71%) | – | 898 (77.21%) | 10 (0.86%) |
| Hippisley‐Cox | 8 275 949 | NA | – | – | – | – | – | 19 486 (0.24%) | 1354 (6.95%) | 5715 (29.33%) | – | 12 036 (61.77%) | 381 (1.96%) |
| Fillmore | 22 914 | 21 120 (92.17%) | 8137 (38.53%) | 8416 (39.85%) | – | 3227 (15.28%) | 1340 (6.34%) | 1794 (7.83%) | 452 (25.20%) | 899 (50.11%) | – | 322 (17.95%) | 121 (6.74%) |
| Alkurt | 119 | NA | – | – | – | – | – | 119 (100.00%) | 14 (11.76%) | – | – | – | 105 (88.24%) |
Niedzwiedz et al. reported on SARS‐CoV‐2 infection by smoking status in multivariable analyses but did not present raw data. NA = not available.
FIGURE 3.
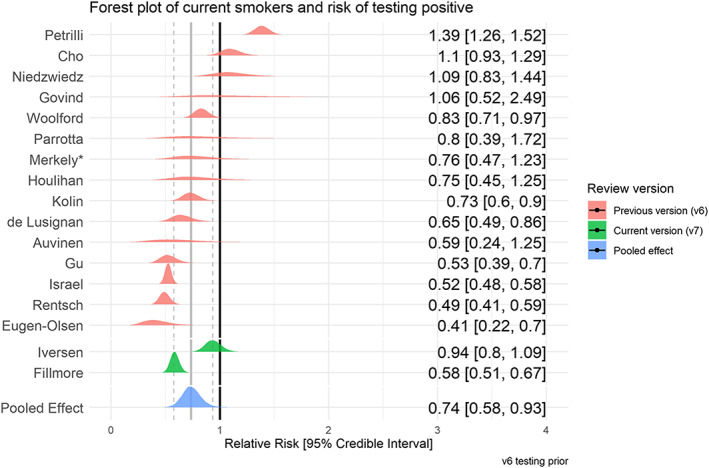
Forest plot for risk of testing positive for SARS‐CoV‐2 in current versus never smokers. *This was a ‘good’ quality study. [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
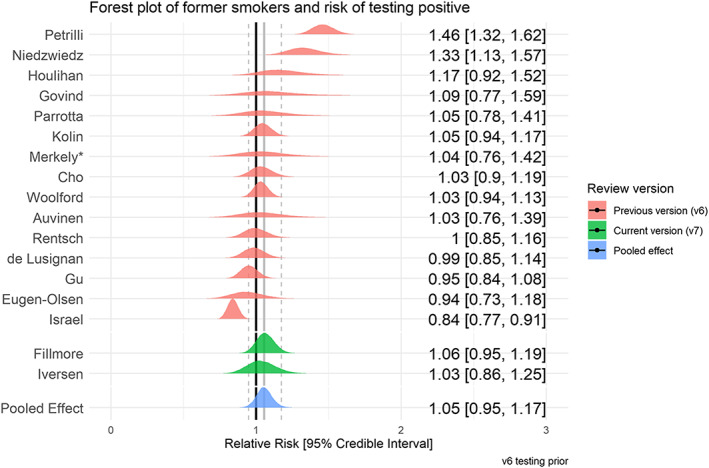
Forest plot for risk of testing positive for SARS‐CoV‐2 in former versus never smokers. *This was a ‘good’ quality study. [Colour figure can be viewed at wileyonlinelibrary.com]
Hospitalization for COVID‐19 by smoking status
Twenty‐nine studies examined hospitalization for COVID‐19 disease stratified by smoking status (see Table 3). Meta‐analyses were performed for eight ‘fair’ quality studies (see Figs 5 and 6). Current (RR = 1.06, CrI = 0.82–1.35, τ = 0.27, 95% CI = 0.08–0.55) and former (RR = 1.20, CrI = 1.03–1.44, τ = 0.17, 95% CI = 0.06–0.37) compared with never smokers were at increased risk of hospitalization with COVID‐19, but data for current smokers were inconclusive, and favoured there being no important association. The probability of current and former smokers being at increased risk of hospitalization compared with never smokers was 35 and 89%, respectively. Results were materially unchanged in two sensitivity analyses (see Supporting information, Fig. S3).
TABLE 3.
Hospitalization with COVID‐19 by smoking status.
| Community | Hospitalized | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Population with outcome | n (%) | Current smoker (%) | Former smoker (%) | Current/former smoker (%) | Never smoker (%) | Never/unknown smoker (%) | Not stated (%) | n (%) | Current smoker (%) | Former smoker (%) | Current/former smoker (%) | Never smoker (%) | Never/unknown smoker (%) | Not stated (%) |
| Rentsch | 554 | 269 (48%) | 69 (25.65%) | 90 (33.46%) | – | 110 (40.89%) | – | – | 285 (51%) | 90 (31.58%) | 89 (31.23%) | – | 106 (37.19%) | – | – |
| Chow (US CDC) | 6637 | 5143 (77%) | 61 (1.19%) | 80 (1.56%) | – | – | – | 5002 (97.26%) | 1494 (22%) | 27 (1.81%) | 78 (5.22%) | – | – | – | 1389 (92.97%) |
| Argenziano | 1000 | 151 (15%) | 14 (9.27%) | 18 (11.92%) | – | 119 (78.81%) | – | – | 849 (84%) | 35 (4.12%) | 161 (18.96%) | – | 653 (76.91%) | – | – |
| Lubetzky | 54 | 15 (27%) | – | – | 4 (26.67%) | – | – | 11 (73.33%) | 39 (72%) | – | – | 8 (20.51%) | – | – | 31 (79.49%) |
| Carillo‐Vega | 9946 | 3922 (39%) | 408 (10.40%) | – | – | – | – | 3514 (89.60%) | 6024 (60%) | 486 (8.07%) | – | – | – | – | 5538 (91.93%) |
| Yanover | 4353 | 4180 (96%) | 484 (11.58%) | 118 (2.82%) | – | 3578 (85.60%) | – | – | 173 (3%) | 30 (17.34%) | 11 (6.36%) | – | 132 (76.30%) | – | – |
| Hamer | 387 109 | 386 349 (99%) | 37 333 (9.66%) | 134 542 (34.82%) | – | 214 474 (55.51%) | – | – | 760 (0%) | 93 (12.24%) | 313 (41.18%) | – | 354 (46.58%) | – | – |
| Heili‐Frades | 4712 | 1973 (41%) | 121 (6.13%) | 222 (11.25%) | – | – | 1630 (82.62%) | 1630 (82.62%) | 2739 (58%) | 112 (4.09%) | 598 (21.83%) | – | – | 2029 (74.08%) | – |
| Freites | 123 | 69 (56%) | 1 (1.45%) | – | – | – | – | 68 (98.55%) | 54 (43%) | 3 (5.56%) | – | – | – | – | 51 (94.44%) |
| Berumen | 102 875 | 18 832 (18%) | – | – | 1546 (8.21%) | – | 17 286 (91.79%) | – | 12 690 (12%) | – | – | 1202 (9.47%) | – | 11 488 (90.53%) | – |
| Gianfrancesco | 600 | 323 (53%) | – | – | 61 (18.89%) | – | – | 262 (81.11%) | 277 (46%) | – | – | 68 (24.55%) | – | – | 209 (75.45%) |
| Chaudhry | 40 | 19 (47%) | – | – | 0 (0.00%) | – | – | 19 (100.00%) | 21 (52%) | – | – | 6 (28.57%) | – | – | 15 (71.43%) |
| Giannouchos | 89 756 | 58 485 (65%) | 4679 (8.00%) | – | – | – | 53 806 (92.00%) | – | 31 271 (34%) | 2721 (8.70%) | – | – | – | 28 550 (91.30%) | – |
| Wang, Oekelen | 57 | 22 (38%) | – | – | 6 (27.27%) | – | – | 16 (72.73%) | 36 (63%) | – | – | 15 (41.67%) | – | – | 20 (55.56%) |
| Miyara | 470 | 132 (28%) | 14 (10.61%) | 41 (31.06%) | – | 77 (58.33%) | – | – | 338 (71%) | 18 (5.33%) | 111 (32.84%) | – | 209 (61.83%) | – | – |
| Suleyman | 463 | 108 (23%) | – | – | 23 (21.30%) | – | – | 85 (78.70%) | 355 (76%) | – | – | 137 (38.59%) | – | – | 218 (61.41%) |
| Garassino | 196 | 48 (24%) | 10 (20.83%) | 27 (56.25%) | – | 11 (22.92%) | – | – | 152 (77%) | 38 (25.00%) | 84 (55.26%) | – | 26 (17.11%) | – | – |
| Siso‐Almirall | 260 | 119 (45%) | – | – | 31 (26.05%) | – | – | 88 (73.95%) | 141 (54%) | – | – | 50 (35.46%) | – | – | 91 (64.54%) |
| Gu | 884 | 511 (57%) | 30 (5.87%) | 126 (24.66%) | – | 355 (69.47%) | – | – | 373 (42%) | 10 (2.68%) | 138 (37.00%) | – | 225 (60.32%) | – | – |
| Killerby | 531 | 311 (58%) | – | – | 37 (11.90%) | 222 (71.38%) | – | 52 (16.72%) | 220 (41%) | – | – | 54 (24.55%) | 157 (71.36%) | – | 9 (4.09%) |
| Petrilli | 5279 | 2538 (48%) | 147 (5.79%) | 337 (13.28%) | – | 1678 (66.12%) | – | 376 (14.81%) | 2741 (51%) | 141 (5.14%) | 565 (20.61%) | – | 1590 (58.01%) | – | 445 (16.23%) |
| Nguyen | 689 | 333 (48%) | – | – | 57 (17.12%) | – | – | 276 (82.88%) | 356 (51%) | – | – | 114 (32.02%) | – | – | 242 (67.98%) |
| Mendy | 689 | 473 (68%) | – | – | 84 (17.76%) | – | – | 389 (82.24%) | 216 (31%) | – | – | 86 (39.81%) | – | – | 130 (60.19%) |
| Soares | 10 713 | 9561 (89%) | 132 (1.38%) | – | – | – | 9429 (98.62%) | – | 1152 (10%) | 77 (6.68%) | – | – | – | 1075 (93.32%) | – |
| Zobairy | 203 | 65 (32%) | 1 (1.54%) | – | – | – | 64 (98.46%) | – | 138 (67%) | 11 (7.97%) | – | – | – | 127 (92.03%) | – |
| Izquierdo | 1006 | 743 (73%) | 52 (7.00%) | – | – | – | 691 (93.00%) | – | 263 (26%) | 16 (6.08%) | – | – | – | 247 (93.92%) | – |
| Rizzo | 76 819 | 60 039 (78%) | 3931 (6.55%) | 11 379 (18.95%) | – | 30 042 (50.04%) | – | 14 687 (24.46%) | 16 780 (21%) | 1254 (7.47%) | 4585 (27.32%) | – | 8693 (51.81%) | – | 2248 (13.40%) |
| Dashti | 4140 | 2759 (66%) | – | – | 600 (21.75%) | 1541 (55.85%) | – | 618 (22.40%) | 1381 (33%) | – | – | 577 (41.78%) | – | 596 (43.16%) | 208 (15.06%) |
| Pan | 12 084 | 8548 (70%) | – | – | 1263 (14.78%) | – | – | 7285 (85.22%) | 3536 (29%) | – | – | 874 (24.72%) | – | – | 2662 (75.28%) |
NA = not available; CDC= Centers for Disease Control
FIGURE 5.

Forest plot for risk of hospitalization in current versus never smokers. [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 6.

Forest plot for risk of hospitalization in former versus never smokers. [Colour figure can be viewed at wileyonlinelibrary.com]
Disease severity by smoking status
Sixty studies reported disease severity in hospitalized patients stratified by smoking status (see Table 4). Severe (as opposed to non‐severe) disease was broadly defined as requiring intensive treatment unit (ITU) admission, requiring oxygen as a hospital inpatient or in‐hospital death. Meta‐analyses were performed for eight ‘fair’ quality studies (see Figs 7 and 8). Current (RR = 1.25, CrI = 0.85–1.93, τ = 0.34, 95% CI = 0.01–0.86) and former (RR = 1.52, CrI = 1.13–2.07, τ = 0.29, 95% CI = 0.47–0.66) compared with never smokers were at increased risk of greater disease severity; data for current smokers were inconclusive, but favoured there being a small but important association. The probability of current and former smokers having increased risk of greater disease severity compared with never smokers was 79 and 98%, respectively. Results were materially unchanged in two sensitivity analyses (see Supporting information, Fig. S4).
TABLE 4.
Disease severity by smoking status.
| Non‐severe disease | Severe disease | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Population with severity | n (%) | Current smoker (%) | Former smoker (%) | Current/former smoker (%) | Never smoker (%) | Never/unknown smoker (%) | Not stated (%) | n (%) | Current smoker (%) | Former smoker (%) | Current/former smoker (%) | Never smoker (%) | Never/unknown smoker (%) | Not stated (%) |
| Guan, Ni | 1085 | 913 (84%) | 108 (11.83%) | 12 (1.31%) | – | 793 (86.86%) | – | – | 172 (15%) | 29 (16.86%) | 9 (5.23%) | – | 134 (77.91%) | – | – |
| Zhang, Dong | 9 | 3 (33%) | 0 (0.00%) | 3 (100.00%) | – | 0 (0.00%) | – | – | 6 (66%) | 2 (33.33%) | 4 (66.67%) | – | 0 (0.00%) | – | – |
| Wan | 9 | 8 (88%) | 8 (100.00%) | 0 (0.00%) | – | 0 (0.00%) | – | – | 1 (11%) | 1 (100.00%) | 0 (0.00%) | – | 0 (0.00%) | – | – |
| Huang, Wang | 3 | 3 (100%) | 3 (100.00%) | 0 (0.00%) | – | 0 (0.00%) | – | – | 0 (0%) | 0 (−%) | 0 (−%) | – | 0 (−%) | – | – |
| Rentsch | 285 | 168 (58%) | 47 (27.98%) | 53 (31.55%) | – | 68 (40.48%) | – | – | 117 (41%) | 43 (36.75%) | 36 (30.77%) | – | 38 (32.48%) | – | – |
| Hu | 323 | 151 (46%) | – | – | 12 (7.95%) | – | 139 (92.05%) | – | 172 (53%) | – | – | 26 (15.12%) | – | 146 (84.88%) | – |
| Wang, Pan | 125 | 100 (80%) | – | – | 9 (9.00%) | – | 91 (91.00%) | – | 25 (20%) | – | – | 7 (28.00%) | – | 18 (72.00%) | – |
| Kim | 27 | 21 (77%) | 3 (14.29%) | – | – | – | 18 (85.71%) | – | 6 (22%) | 2 (33.33%) | 0 (0.00%) | – | – | 4 (66.67%) | – |
| Shi, Yu | 474 | 425 (89%) | – | – | 34 (8.00%) | – | 391 (92.00%) | – | 49 (10%) | – | – | 6 (12.24%) | – | 43 (87.76%) | – |
| Liao, Feng | 148 | 92 (62%) | – | – | 5 (5.43%) | – | – | 87 (94.57%) | 56 (37%) | 3 (5.36%) | – | – | – | – | 53 (94.64%) |
| Shi, Ren | 134 | 88 (65%) | – | – | 8 (9.09%) | – | – | 80 (90.91%) | 46 (34%) | – | – | 6 (13.04%) | – | – | 40 (86.96%) |
| Hadjadj | 50 | 15 (30%) | 1 (6.67%) | 2 (13.33%) | – | 12 (80.00%) | – | – | 35 (70%) | 0 (0.00%) | 7 (20.00%) | – | 28 (80.00%) | – | – |
| Zheng, Xiong | 73 | 43 (58%) | – | – | 6 (13.95%) | 37 (86.05%) | – | – | 30 (41%) | – | – | 2 (6.67%) | 28 (93.33%) | – | – |
| de la Rica | 48 | 26 (54%) | – | – | 6 (23.08%) | – | – | 20 (76.92%) | 20 (41%) | – | – | 4 (20.00%) | – | – | 16 (80.00%) |
| Yin, Yang | 106 | 47 (44%) | – | – | 6 (12.77%) | – | – | 41 (87.23%) | 59 (55%) | – | – | 12 (20.34%) | – | – | 47 (79.66%) |
| Allenbach | 147 | 100 (68%) | – | – | 9 (9.00%) | – | – | 91 (91.00%) | 47 (31%) | – | – | 0 (0.00%) | – | – | 47 (100.00%) |
| Goyal | 393 | 263 (66%) | 14 (5.32%) | – | – | – | – | 249 (94.68%) | 130 (33%) | 6 (4.62%) | – | – | – | – | 124 (95.38%) |
| Feng | 454 | 333 (73%) | 27 (8.11%) | – | – | – | – | 306 (91.89%) | 121 (26%) | 17 (14.05%) | – | – | – | – | 104 (85.95%) |
| Yao | 108 | 83 (76%) | 1 (1.20%) | – | – | – | – | 82 (98.80%) | 25 (23%) | 3 (12.00%) | – | – | – | – | 22 (88.00%) |
| Sami | 490 | 400 (81%) | 53 (13.25%) | – | – | – | – | 347 (86.75%) | 90 (18%) | 16 (17.78%) | – | – | – | – | 74 (82.22%) |
| Regina | 200 | 163 (81%) | 9 (5.52%) | – | – | – | – | 154 (94.48%) | 37 (18%) | 0 (0.00%) | – | – | – | – | 37 (100.00%) |
| Feuth | 28 | 21 (75%) | 1 (4.76%) | 7 (33.33%) | – | 13 (61.90%) | – | – | 7 (25%) | 2 (28.57%) | 1 (14.29%) | – | 4 (57.14%) | – | – |
| Mejia‐Vilet | 329 | 214 (65%) | – | – | 13 (6.07%) | – | – | 201 (93.93%) | 115 (34%) | – | – | 10 (8.70%) | – | – | 105 (91.30%) |
| Chen, Jiang | 135 | 54 (40%) | – | – | 4 (7.41%) | – | – | 50 (92.59%) | 81 (60%) | – | – | 9 (11.11%) | – | – | 72 (88.89%) |
| Vaquero‐Roncero | 146 | 75 (51%) | – | – | 4 (5.33%) | – | – | 71 (94.67%) | 71 (48%) | – | – | 6 (8.45%) | – | – | 65 (91.55%) |
| Kim, Garg | 2490 | 1692 (67%) | 112 (6.62%) | 395 (23.35%) | – | – | 1185 (70.04%) | – | 798 (32%) | 38 (4.76%) | 247 (30.95%) | – | – | 512 (64.16%) | – |
| Wu | 174 | 92 (52%) | – | – | 47 (51.09%) | – | 45 (48.91%) | – | 82 (47%) | 11 (13.41%) | – | – | – | 71 (86.59%) | – |
| Chaudhry | 40 | 34 (85%) | – | – | 5 (14.71%) | – | – | 29 (85.29%) | 6 (15%) | – | – | 1 (16.67%) | – | – | 5 (83.33%) |
| Garibaldi | 832 | 532 (63%) | 25 (4.70%) | 107 (20.11%) | – | – | – | 400 (75.19%) | 300 (36%) | 21 (7.00%) | 81 (27.00%) | – | – | – | 198 (66.00%) |
| Kuderer | 928 | 686 (73%) | 35 (5.10%) | 210 (30.61%) | – | 370 (53.94%) | – | 29 (4.23%) | 242 (26%) | 8 (3.31%) | 116 (47.93%) | – | 99 (40.91%) | 15 (6.20%) | 4 (1.65%) |
| Romao | 14 | 14 (100%) | – | – | 4 (28.57%) | – | – | 10 (71.43%) | 0 (0%) | – | – | – | – | – | – |
| Giannouchos | 89 756 | 78 050 (86%) | 6322 (8.10%) | – | – | – | 71 728 (91.90%) | – | 11 706 (13%) | 1089 (9.30%) | – | – | – | 10 617 (90.70%) | – |
| Cen | 1007 | 720 (71%) | – | – | 70 (9.72%) | – | – | 650 (90.28%) | 287 (28%) | – | – | 18 (6.27%) | – | – | 269 (93.73%) |
| Maraschini | 132 | 89 (67%) | – | 11 (12.36%) | – | 78 (87.64%) | – | – | 43 (32%) | – | 3 (6.98%) | – | 40 (93.02%) | – | – |
| Siso‐Almirall | 260 | 212 (81%) | – | – | 60 (28.30%) | – | – | 152 (71.70%) | 48 (18%) | – | – | 21 (43.75%) | – | – | 27 (56.25%) |
| Gu | 884 | 511 (57%) | 30 (5.87%) | 126 (24.66%) | – | 355 (69.47%) | – | – | 134 (15%) | 3 (2.24%) | 61 (45.52%) | – | 70 (52.24%) | – | – |
| Petrilli | 2729 | 1739 (63%) | 97 (5.58%) | 325 (18.69%) | – | 1067 (61.36%) | – | 250 (14.38%) | 990 (36%) | 44 (4.44%) | 236 (23.84%) | – | 517 (52.22%) | – | 193 (19.49%) |
| Mendy | 689 | 598 (86%) | – | – | 133 (22.24%) | – | – | 465 (77.76%) | 91 (13%) | – | – | 37 (40.66%) | – | – | 54 (59.34%) |
| Pongpirul | 193 | 161 (83%) | – | – | 25 (15.53%) | 106 (65.84%) | – | 30 (18.63%) | 32 (16%) | – | – | 4 (12.50%) | 21 (65.62%) | – | 7 (21.88%) |
| Jin, Gu | 6 | 2 (33%) | – | – | 0 (0.00%) | – | – | 4 (200.00%) | 4 (66%) | – | – | 2 (50.00%) | – | – | 2 (50.00%) |
| Senkal | 611 | 446 (73%) | 48 (10.76%) | – | – | – | – | 398 (89.24%) | 165 (27%) | 21 (12.73%) | – | – | – | – | 144 (87.27%) |
| Patel | 129 | 89 (68%) | 26 (29.21%) | – | – | – | 58 (65.17%) | 5 (5.62%) | 40 (31%) | 22 (55.00%) | – | – | – | 14 (35.00%) | 4 (10.00%) |
| Maucourant | 27 | 10 (37%) | 1 (10.00%) | 2 (20.00%) | – | 2 (20.00%) | – | 5 (50.00%) | 17 (62%) | 2 (11.76%) | 5 (29.41%) | – | 9 (52.94%) | – | 1 (5.88%) |
| Xie | 619 | 469 (75%) | – | – | 32 (6.82%) | – | – | 437 (93.18%) | 150 (24%) | – | – | 19 (12.67%) | – | – | 131 (87.33%) |
| Fox | 55 | 30 (54%) | 1 (3.33%) | 4 (13.33%) | – | 17 (56.67%) | – | 8 (26.67%) | 25 (45%) | 0 (0.00%) | 2 (8.00%) | – | 14 (56.00%) | – | 9 (36.00%) |
| Zhang, Cao | 240 | 162 (67%) | 2 (1.23%) | 6 (3.70%) | – | – | – | 154 (95.06%) | 78 (32%) | 4 (5.13%) | 4 (5.13%) | – | – | – | 70 (89.74%) |
| Kurashima | 53 | 10 (18%) | – | – | 3 (30.00%) | – | – | 7 (70.00%) | 43 (81%) | – | – | 24 (55.81%) | – | – | 19 (44.19%) |
| Zhan | 75 | NA | – | – | – | – | – | – | 75 (100%) | – | – | 9 (12.00%) | – | – | 66 (88.00%) |
| Omrani | 858 | 806 (93%) | – | – | 121 (15.01%) | – | – | 685 (84.99%) | 52 (6%) | – | – | 9 (17.31%) | – | – | 43 (82.69%) |
| Marcos | 918 | 555 (60%) | 38 (6.85%) | – | 69 (12.43%) | – | – | 448 (80.72%) | 363 (39%) | 18 (4.96%) | – | 71 (19.56%) | – | – | 292 (80.44%) |
| Hoertel, Sanchez‐Rico | 7345 | 6014 (81%) | 433 (7.20%) | – | – | – | – | 5581 (92.80%) | 1331 (18%) | 190 (14.27%) | – | – | – | – | 1141 (85.73%) |
| Qi | 267 | 217 (81%) | 22 (10.14%) | – | – | – | 195 (89.86%) | – | 50 (18%) | 31 (62.00%) | – | – | – | 19 (38.00%) | – |
| Monteiro | 112 | 84 (75%) | 3 (3.57%) | 14 (16.67%) | – | 63 (75.00%) | – | 4 (4.76%) | 28 (25%) | 4 (14.29%) | 6 (21.43%) | – | 14 (50.00%) | – | 4 (14.29%) |
| Dashti | 1381 | 619 (44%) | – | – | 239 (38.61%) | 292 (47.17%) | – | 88 (14.22%) | 762 (55%) | – | – | 338 (44.36%) | 304 (39.90%) | – | 120 (15.75%) |
| Morshed | 103 | 87 (84%) | 28 (32.18%) | – | – | – | 59 (67.82%) | – | 16 (15%) | 4 (25.00%) | – | – | – | 12 (75.00%) | – |
| Zhou, Sun | 144 | 108 (75%) | 11 (10.19%) | – | – | – | – | 97 (89.81%) | 36 (25%) | 2 (5.56%) | – | – | – | – | 34 (94.44%) |
| Hippisley‐Cox | – | NA | – | – | – | – | – | – | 1286 | 56 (4.35%) | 427 (33.20%) | – | 791 (61.51%) | – | 12 (0.93%) |
| Zhao, Chen | 641 | 398 (62%) | 87 (21.86%) | – | – | – | – | 311 (78.14%) | 195 (30%) | 52 (26.67%) | – | – | – | – | 143 (73.33%) |
| Qu | 246 | 226 (91%) | 90 (39.82%) | – | – | – | – | 136 (60.18%) | 20 (8%) | 14 (70.00%) | – | – | – | – | 6 (30.00%) |
FIGURE 7.

Forest plot for the risk of severe disease in current versus never smokers. [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 8.

Forest plot for the risk of severe disease in former versus never smokers. [Colour figure can be viewed at wileyonlinelibrary.com]
Mortality by smoking status
Fifty studies reported mortality from COVID‐19 by smoking status (see Table 5), with nine ‘fair’ quality studies included in meta‐analyses (see Figs 9 and 10). Current (RR = 1.22, 95% CrI = 0.78–1.94, τ = 0.49, 95% CI = 0.16–0.99) and former (RR = 1.39, 95% CrI = 1.09–1.87, τ = 0.27, 95% CI = 0.05–0.58) compared with never smokers were at increased risk of in‐hospital mortality from COVID‐19. Data for current smokers were inconclusive, but favoured there being no important association. The probability of current and former smokers being at greater risk of in‐hospital mortality compared with never smokers was 70 and 97%, respectively. Results were materially unchanged in two sensitivity analyses (see Supporting information, Fig. S5).
TABLE 5.
Mortality by smoking status.
| Recovered | Died | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Population with mortality | n (%) | Current smoker (%) | Former smoker (%) | Current/former smoker (%) | Never smoker (%) | Never/unknown smoker (%) | Not stated (%) | n (%) | Current smoker (%) | Former smoker (%) | Current/former smoker (%) | Never smoker (%) | Never/unknown smoker (%) | Not stated (%) |
| Chen | 274 | 161 (58%) | 5 (3.11%) | 5 (3.11%) | – | – | – | 151 (93.79%) | 113 (41%) | 7 (6.19%) | 2 (1.77%) | – | – | – | 104 (92.04%) |
| Zhou, Yu | 191 | 137 (71%) | 6 (4.38%) | – | – | – | – | 131 (95.62%) | 54 (28%) | 5 (9.26%) | – | – | – | – | 49 (90.74%) |
| Yang, Yu | 52 | 20 (38%) | 2 (10.00%) | – | – | – | 18 (90.00%) | – | 32 (61%) | – | – | – | – | 32 (100.00%) | – |
| Borobia | 2226 | 1766 (79%) | 113 (6.40%) | – | – | – | – | 1653 (93.60%) | 460 (20%) | 44 (9.57%) | – | – | – | – | 416 (90.43%) |
| Giacomelli | 233 | 185 (79%) | – | – | 53 (28.65%) | 132 (71.35%) | – | – | 48 (20%) | – | – | 17 (35.42%) | 31 (64.58%) | – | 0 (0.00%) |
| Yao | 108 | 96 (88%) | 1 (1.04%) | – | – | – | – | 95 (98.96%) | 12 (11%) | 3 (25.00%) | – | – | – | – | 9 (75.00%) |
| Carillo‐Vega | 9946 | 8983 (90%) | 795 (8.85%) | – | – | – | – | 8188 (91.15%) | 963 (9%) | 99 (10.28%) | – | – | – | – | 864 (89.72%) |
| Heng | 51 | 39 (76%) | 6 (15.38%) | – | – | – | – | 33 (84.62%) | 12 (23%) | 1 (8.33%) | – | – | – | – | 11 (91.67%) |
| Chen, Jiang | 135 | NA | – | – | – | – | – | – | 31 (22%) | – | – | 4 (12.90%) | – | – | 27 (87.10%) |
| Heili‐Frades | 4712 | 4086 (86%) | 210 (5.14%) | 659 (16.13%) | – | – | 3217 (78.73%) | – | 626 (13%) | 23 (3.67%) | 161 (25.72%) | – | – | 442 (70.61%) | – |
| Kim, Garg | 2490 | 2070 (83%) | 128 (6.18%) | 481 (23.24%) | – | – | 1461 (70.58%) | – | 420 (16%) | 22 (5.24%) | 161 (38.33%) | – | – | 236 (56.19%) | – |
| Al‐Hindawi | 31 | 15 (48%) | 0 (0.00%) | 10 (66.67%) | – | 5 (33.33%) | – | – | 16 (51%) | 1 (6.25%) | 12 (75.00%) | – | 3 (18.75%) | – | – |
| Louis | 22 | 16 (72%) | – | – | 7 (43.75%) | – | – | 9 (56.25%) | 6 (27%) | – | – | 3 (50.00%) | – | – | 3 (50.00%) |
| Soto‐Mota | 400 | 200 (50%) | – | – | 23 (11.50%) | – | – | 177 (88.50%) | 200 (50%) | – | – | 25 (12.50%) | – | – | 175 (87.50%) |
| Garibaldi | 747 | 634 (84%) | 36 (5.68%) | 129 (20.35%) | – | – | – | 469 (73.97%) | 113 (15%) | 6 (5.31%) | 36 (31.86%) | – | – | – | 71 (62.83%) |
| Docherty | 13 364 | 8199 (61%) | 370 (4.51%) | 1832 (22.34%) | – | 4179 (50.97%) | – | 1818 (22.17%) | 5165 (38%) | 214 (4.14%) | 1350 (26.14%) | – | 2105 (40.76%) | – | 1496 (28.96%) |
| Kuderer | 928 | 807 (86%) | 38 (4.71%) | 262 (32.47%) | – | 425 (52.66%) | – | 31 (3.84%) | 121 (13%) | 5 (4.13%) | 64 (52.89%) | – | 44 (36.36%) | – | 2 (1.65%) |
| Ramlall | 11 116 | 10 498 (94%) | – | – | 2771 (26.40%) | 7727 (73.60%) | – | – | 618 (5%) | – | – | 208 (33.66%) | 410 (66.34%) | – | – |
| Wang, Oekelen | 57 | 43 (75%) | – | – | 14 (32.56%) | – | – | 29 (67.44%) | 14 (24%) | – | – | 7 (50.00%) | – | – | 7 (50.00%) |
| Martinez‐Portilla | 224 | 217 (96%) | – | – | 7 (3.23%) | – | – | 210 (96.77%) | 7 (3%) | – | – | 0 (0.00%) | – | – | 7 (100.00%) |
| Cen | 1007 | 964 (95%) | – | – | 87 (9.02%) | – | – | 877 (90.98%) | 43 (4%) | – | – | 1 (2.33%) | – | – | 42 (97.67%) |
| Klang | 3406 | 2270 (66%) | – | – | 492 (21.67%) | – | – | 1778 (78.33%) | 1136 (33%) | – | – | 301 (26.50%) | – | – | 835 (73.50%) |
| Wang, Zhong | 5510 | 4874 (88%) | 247 (5.07%) | 1083 (22.22%) | – | 3544 (72.71%) | – | – | 636 (11%) | 28 (4.40%) | 214 (33.65%) | – | 394 (61.95%) | – | – |
| Miyara | 338 | 211 (62%) | 13 (6.16%) | 58 (27.49%) | – | 141 (66.82%) | – | – | 46 (13%) | 1 (2.17%) | 23 (50.00%) | – | 21 (45.65%) | – | – |
| Rajter | 255 | 209 (81%) | – | – | 28 (13.40%) | 181 (86.60%) | – | – | 53 (20%) | – | – | 18 (33.96%) | 28 (52.83%) | – | – |
| Zeng | 1031 | 866 (84%) | – | – | 69 (7.97%) | – | – | 797 (92.03%) | 165 (16%) | – | – | 36 (21.82%) | – | – | 129 (78.18%) |
| Chen, Yu | 1859 | 1651 (88%) | 32 (1.94%) | 54 (3.27%) | – | 1565 (94.79%) | – | – | 208 (11%) | 13 (6.25%) | 12 (5.77%) | – | 183 (87.98%) | – | – |
| Garassino | 190 | 124 (65%) | – | – | 92 (74.19%) | 32 (25.81%) | – | – | 66 (34%) | – | 61 (92.42%) | – | 5 (7.58%) | – | – |
| Gu | 884 | 864 (97%) | 40 (4.63%) | 250 (28.94%) | – | 219 (25.35%) | – | – | 20 (2%) | 0 (0.00%) | 14 (70.00%) | – | 6 (30.00%) | – | – |
| Sigel | 88 | 70 (79%) | – | – | 37 (52.86%) | – | – | 33 (47.14%) | 18 (20%) | – | – | 11 (61.11%) | – | – | 7 (38.89%) |
| Nguyen | 356 | 308 (86%) | – | – | 91 (29.55%) | – | – | 217 (70.45%) | 45 (12%) | – | – | 23 (51.11%) | – | – | 22 (48.89%) |
| de Souza | 8443 | 7826 (92%) | – | – | 95 (1.21%) | – | 7571 (96.74%) | 160 (2.04%) | 617 (7%) | – | – | 47 (7.62%) | – | 560 (90.76%) | 10 (1.62%) |
| Mendy | 532 | 663 (124%) | – | – | 160 (24.13%) | – | – | 502 (75.72%) | 26 (4%) | – | – | 10 (38.46%) | – | – | 16 (61.54%) |
| Shi, Resurreccion | 256 | 210 (82%) | – | – | 128 (60.95%) | – | – | 82 (39.05%) | 46 (17%) | – | – | 26 (56.52%) | – | – | 20 (43.48%) |
| Xie | 619 | 591 (95%) | – | – | 43 (7.28%) | – | – | 548 (92.72%) | 28 (4%) | – | – | 8 (28.57%) | – | – | 20 (71.43%) |
| Fox | 54 | 35 (64%) | 1 (2.86%) | 4 (11.43%) | – | 18 (51.43%) | – | 12 (34.29%) | 19 (35%) | 0 (0.00%) | 2 (10.53%) | – | 12 (63.16%) | – | 5 (26.32%) |
| Zhang, Cao | 289 | 240 (83%) | 10 (4.17%) | 6 (2.50%) | – | – | – | 224 (93.33%) | 49 (16%) | 4 (8.16%) | 8 (16.33%) | – | – | – | 37 (75.51%) |
| Gupta | 496 | 255 (51%) | – | – | 15 (5.88%) | – | 80 (31.37%) | 160 (62.75%) | 241 (48%) | – | – | 21 (8.71%) | 77 (31.95%) | – | 143 (59.34%) |
| Soares | 1075 | 696 (64%) | 38 (5.46%) | – | – | – | 658 (94.54%) | – | 456 (42%) | 39 (8.55%) | – | – | – | 417 (91.45%) | – |
| Thompson | 470 | 301 (64%) | 39 (12.96%) | 79 (26.25%) | – | 183 (60.80%) | – | – | 169 (35%) | 27 (15.98%) | 49 (28.99%) | – | 93 (55.03%) | – | – |
| Bernaola | 1645 | 1382 (84%) | 35 (2.53%) | 146 (10.56%) | – | 1201 (86.90%) | – | – | 263 (15%) | 6 (2.28%) | 33 (12.55%) | – | 218 (82.89%) | – | – |
| Islam | 654 | 631 (96%) | 103 (16.32%) | – | – | – | – | 507 (80.35%) | 23 (3%) | 3 (13.04%) | – | – | – | – | – |
| Philipose | 466 | 267 (57%) | 19 (7.12%) | 204 (76.40%) | – | 44 (16.48%) | – | – | 199 (42%) | 9 (4.52%) | 137 (68.84%) | – | 33 (16.58%) | – | 20 (10.05%) |
| Dashti | 4140 | 3953 (95%) | – | – | 1068 (27.02%) | 2078 (52.57%) | – | 804 (20.34%) | 187 (4%) | – | – | 109 (58.29%) | 56 (29.95%) | – | 22 (11.76%) |
| Fillmore | 1794 | 1566 (87%) | 408 (26.05%) | 758 (48.40%) | – | 279 (17.82%) | – | 98 (6.26%) | 228 (12%) | 44 (19.30%) | 141 (61.84%) | – | 43 (18.86%) | – | 23 (10.09%) |
| Pan | 3536 | 3302 (93%) | – | – | 862 (26.11%) | – | – | 2440 (73.89%) | 234 (6%) | – | – | 82 (35.04%) | – | – | 152 (64.96%) |
| Zhao, Chen | 474 | 398 (83%) | 87 (21.86%) | – | – | – | – | 311 (78.14%) | 82 (17%) | 36 (43.90%) | – | – | – | – | 46 (56.10%) |
| Holman | 10 989 | NA | – | – | – | – | – | – | 10 989 (100%) | 609 (5.54%) | 4684 (42.62%) | – | 5386 (49.01%) | – | 310 (2.82%) |
| Chand | 300 | 143 (47%) | 23 (16.08%) | – | – | – | – | 120 (83.92%) | 157 (52%) | 44 (28.03%) | – | – | – | – | 113 (71.97%) |
Solis et al. and the OpenSAFELY Collaborative reported on mortality by smoking status in a multivariable analysis but did not present raw data for both the exposure and outcome variables.
FIGURE 9.

Forest plot for the risk of mortality in current versus never smokers. [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 10.

Forest plot for the risk of mortality in former versus never smokers. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
This living rapid review found uncertainty in the majority of 233 studies arising from the recording of smoking status. Notwithstanding these uncertainties, compared with overall adult national prevalence estimates, recorded current smoking rates in most countries were lower than expected. In a subset of better of quality studies (n = 17), current smokers had a reduced risk of testing positive for SARS‐CoV‐2 but appeared more likely to present for testing and/or receive a test. Data for current smokers on the risk of hospitalization, disease severity and mortality were inconclusive, but favoured there being no important associations with hospitalization and mortality and a small but important increase in the risk of severe disease. Former smokers were at increased risk of hospitalization, disease severity and mortality compared with never smokers.
Issues complicating interpretation
Interpretation of results from studies conducted during the first phase of the SARS‐CoV‐2 pandemic is complicated by several factors (see Fig. 11):
FIGURE 11.

A schematic of some of the interpretation issues for the association of smoking and SARS‐CoV‐2/COVID‐19. *Indicates potential confounding with smoking status.
Exposure to SARS‐CoV‐2 is heterogeneous, with different subgroups at heightened risk of infection at different stages of the pandemic. This will likely introduce bias in studies assessing the rate of infection by smoking status conducted early on.
Current and former smokers may be more likely to meet local criteria for community testing due to increased prevalence of symptoms consistent with SARS‐CoV‐2 infection, such as cough, increased sputum production or altered sense of smell or taste [35]. Evidence from a small number of studies indicates that current smokers may be more likely to present for testing, hence increasing the denominator in comparisons with never smokers and potentially inflating the rate of negative tests in current smokers. Infection positivity rates estimated among random samples will be more informative than currently available data. We identified one population study conducted in Hungary reporting on seroprevalence and smoking status [36]; however, the response rate was fairly low, at 58.8%, and the current smoking rate was 10 percentage points below national prevalence estimates, thus questioning the representativeness of the final sample. Smoking status is being collected in at least two large representative infection and antibody surveys in the United Kingdom [37, 38].
Testing for acute infection requires swabbing of the mucosal epithelium, which may be disrupted in current smokers, potentially altering the sensitivity of assays [39].
Diagnostic criteria for SARS‐CoV‐2 infection and COVID‐19 have changed during the course of the pandemic [40]. It was not possible to extract details on the specific RT–PCR technique or platforms used across the included studies due to reporting gaps. Different platforms have varying sensitivity and specificity to detect SARS‐CoV‐2 infection.
Most included studies relied on EHRs as the source of information on smoking status. Research shows large discrepancies between EHRs and actual behaviour [41]. Known failings of EHRs include implausible longitudinal changes, such as former smokers being recorded as never smokers at subsequent hospital visits [41]. Misreporting on the part of the patient (perhaps due to perceived stigmatization) has also been observed, with biochemical measures showing higher rates of smoking compared with self‐report in hospitalized patients in the United States [42]. It is hence possible that under‐reporting of current and former smoking status in hospitals occurred across the included studies.
Individuals with severe COVID‐19 symptoms may have stopped smoking immediately before admission to hospital and may therefore not have been recorded as current smokers (i.e. reverse causality).
Smokers with COVID‐19 may be less likely to receive a SARS‐CoV‐2 test or present to hospital due to lack of access to healthcare, and may be more likely to die in the community from sudden complications (i.e. self‐selection bias) and thus not be recorded.
If there is a protective effect of nicotine on COVID‐19 disease outcomes, abrupt nicotine withdrawal upon hospitalization may lead to worse outcomes [12].
During periods of heightened demand of limited healthcare resources, current and former smokers with extensive comorbidities may have reduced priority for intensive care admission, thus leading to higher in‐hospital mortality.
Given the lack of knowledge of the disease progression and long‐term outcomes of COVID‐19, it is unclear whether studies conducted thus far in the pandemic have monitored patients for a sufficient time‐period to report complete survival outcomes or whether they are subject to early censoring.
Reasons for hospitalization vary by country and time in the pandemic. For example, early cases may have been hospitalized for isolation and quarantine reasons and not due to medical necessity. It is plausible that this may have skewed early data towards less severe cases. In addition, the observed association between former smoking and greater disease severity may be explained by collider bias [43], where conditioning on a collider (e.g. testing or hospitalization) by design or analysis may introduce a spurious association between current or former smoking (a potential cause of testing or hospitalization) and SARS‐CoV‐2 infection/adverse outcomes from COVID‐19 (potentially exacerbated by smoking) [44].
Limitations
This living rapid evidence review was limited by having a single reviewer extracting data with a second independently verifying the data extracted to minimize errors, restricting the search to one electronic database and one pre‐print server and by not including at least three large population surveys due to their reliance upon self‐reported suspected or confirmed SARS‐CoV‐2 infection (which means they do not meet our eligibility criteria) [35, 45, 46]. We also did not include a large, UK‐based, representative seroprevalence study [47] in our meta‐analyses, as the odds of testing positive in former smokers was not reported. However, the odds of infection for current smokers (odds ratio = 0.64, 95% CI = 0.58–0.71) was in concordance with the pooled estimate in our meta‐analysis. Population surveys—particularly with linked data on confirmed infection or antibodies—will be included in future review versions to help mitigate some of the limitations of healthcare based observational studies. The comparisons of current and former smoking prevalence in the included studies with national prevalence estimates did not adjust observed prevalence for the demographic profile of those tested/admitted to hospital. Other reviews focused on this comparison have applied adjustments for sex and age, and continue to find lower than expected prevalence—notwithstanding the issues complicating interpretation described above [17].
Implications for research, policy and practice
Further scientific research is needed to resolve the mixed findings summarized in our review. First, clinical trials of the posited therapeutic effect of nicotine could have important implications both for smokers and for improved understanding of how the SARS‐CoV‐2 virus causes disease in humans. Such trials should focus upon medicinal nicotine (as smoked tobacco is a dirty delivery mechanism that could mask beneficial effects) and potentially differentiate between different modes of delivery (i.e. inhaled versus ingested), as this can affect pharmacokinetics [48] and potential therapeutic effects. A second research priority would be a large, representative (randomly sampled) population survey with a validated assessment of smoking status which distinguishes between recent and long‐term ex‐smokers—ideally biochemically verified—and assesses seroprevalence and links to health records.
In the meantime, public‐facing messages about the possible protective effect of smoking or nicotine are premature. In our view, until there is further research, the quality of the evidence does not justify the huge risk associated with a message likely to reach millions of people that a lethal activity, such as smoking, may protect against COVID‐19. It continues to be appropriate to recommend smoking cessation and emphasize the role of alternative nicotine products to support smokers to stop as part of public health efforts during COVID‐19. At the very least, smoking cessation reduces acute risks from cardiovascular disease and could reduce demands on the health‐care system [49]. GPs and other health‐care providers can play a crucial role—brief, high‐quality and free on‐line training is available at National Centre for Smoking Cessation and Training.
Conclusion
Across 233 studies, recorded smoking prevalence was generally lower than national prevalence estimates. Current smokers were at reduced risk of testing positive for SARS‐CoV‐2 and former smokers were at increased risk of hospitalization, disease severity and mortality compared with never smokers.
Declaration of interests
D.S. and O.P. have no conflicts of interest to declare. L.S. has received a research grant and honoraria for a talk and travel expenses from manufacturers of smoking cessation medications (Pfizer and Johnson & Johnson). J.B. has received unrestricted research funding to study smoking cessation from companies who manufacture smoking cessation medications. All authors declare no financial links with tobacco companies or e‐cigarette manufacturers or their representatives.
Author contributions
David Simons: Conceptualization; data curation; formal analysis; methodology; writing‐original draft; writing‐review & editing. Lion Shahab: Conceptualization; data curation; formal analysis; methodology; writing‐original draft; writing‐review & editing. Jamie Brown: Conceptualization; data curation; formal analysis; methodology; writing‐original draft; writing‐review & editing. Olga Perski: Conceptualization; data curation; formal analysis; methodology; writing‐original draft; writing‐review & editing.
Future review versions
Previous review versions
Version 1: https://doi.org/10.32388/UJR2AW
Version 2: https://doi.org/10.32388/UJR2AW.3
Version 3: https://doi.org/10.32388/UJR2AW.4
Version 4: https://doi.org/10.32388/UJR2AW.5
Version 5: https://doi.org/10.32388/UJR2AW.6
Version 6: https://doi.org/10.32388/UJR2AW.7
Supporting information
Figure S1 Map of countries where included studies were conducted. Six studies were performed in multiple countries and are not included here.
Table S1 Study design, use of clinical diagnosis and stratification of smoking status by sex, age or socio‐economic position.
Table S2a Studies reporting complete smoking status
Table S2b Studies reporting partially complete smoking status
Table S2c Studies reporting incomplete smoking status
Table S3 Smoking prevalence in countries with included studies
Figure S2 Supporting Information
Figure S3 Supporting Information
Figure S4 Supporting Information
Figure S5 Supporting Information
Acknowledgements
An original short review for the Royal College of Physicians was converted to an extended living review after a request by Martin Dockrell, Tobacco Control Lead, Public Health England. All scientific decisions were made by the authors independently of funders and external organizations. The authors would like to thank Rosemary Koper for her assistance in running the electronic searches and data extraction. D.S. is supported by a PhD studentship from the UK Biotechnology and Biological Sciences Research Council (BB/M009513/1). O.P. receives salary support from Cancer Research UK (C1417/A22962). J.B., L.S. and O.P. are members of SPECTRUM, a UK Prevention Research Partnership Consortium (MR/S037519/1). UKPRP is an initiative funded by the UK Research and Innovation Councils, the Department of Health and Social Care (England) and the UK devolved administrations, and leading health research charities.
Simons, D. , Shahab, L. , Brown, J. , and Perski, O. (2021) The association of smoking status with SARS‐CoV‐2 infection, hospitalization and mortality from COVID‐19: a living rapid evidence review with Bayesian meta‐analyses (version 7). Addiction, 116: 1319–1368. 10.1111/add.15276.
References
- 1. Guan W., Ni Z., Hu Y. Y., Liang W. H., Ou C. Q., He J. X. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoffmann M., Kleine‐Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brake S. J., Barnsley K., Lu W., McAlinden K. D., Eapen M. S., Sohal S. S. Smoking upregulates angiotensin‐converting enzyme‐2 receptor: a potential adhesion site for novel coronavirus SARS‐CoV‐2 (Covid‐19). J Clin Med 2020; 9: 841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai G. Bulk and single‐cell transcriptomics identify tobacco‐use disparity in lung gene expression of ACE2, the receptor of 2019‐nCov. medRxiv 2020; 10.20944/preprints202002.0051.v3 [DOI] [Google Scholar]
- 5. Oakes J. M., Fuchs R. M., Gardner J. D., Lazartigues E., Yue X. Nicotine and the renin‐angiotensin system. Am J Physiol Regul Integr Comp Physiol 2018; 315: R895–R906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denholm J. T., Gordon C. L., Johnson P. D., Hewagama S. S., Stuart R. L., Aboltins C. et al. Hospitalised adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. Med J Aust 2010; 192: 84–86. [DOI] [PubMed] [Google Scholar]
- 7. Abadom T. R., Smith A. D., Tempia S., Madhi S. A., Cohen C., Cohen A. L. Risk factors associated with hospitalization for influenza‐associated severe acute respiratory illness in South Africa: a case‐population study. Vaccine 2016; 34: 5649–5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Almirall J., González C. A., Balanzó X., Bolíbar I. Proportion of community‐acquired pneumonia cases attributable to tobacco smoking. Chest 1999; 116: 375–379. [DOI] [PubMed] [Google Scholar]
- 9. Feldman C., Anderson R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J Infect 2013; 67: 169–184. [DOI] [PubMed] [Google Scholar]
- 10. Dye J. A., Adler K. B. Occasional review effects of cigarette smoke on epithelial cells of the respiratory tract. Thorax 1994; 49: 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vardavas C. I., Nikitara K. COVID‐19 and smoking: a systematic review of the evidence. Tob Induc Dis 2020; 18: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farsalinos K., Niaura R., Le Houezec J. Editorial: nicotine and SARS‐CoV‐2: COVID‐19 may be a disease of the nicotinic cholinergic system. Toxicol Rep 2020; 7: 658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID‐19: a systematic review and meta‐analysis. Arch Acad Emerg Med 2020; 8: e35. [PMC free article] [PubMed] [Google Scholar]
- 14. Alqahtani J. S., Oyelade T., Aldhahir A. M., Alghamdi S. M., Almehmadi M., Alqahtani A. S. et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID‐19: a rapid systematic review and meta‐analysis. medRxiv 2020; 15: e0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patanavanich R, Glantz SA. Smoking is associated with COVID‐19 progression: a meta‐analysis. medRxiv 2020. 10.14171/2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berlin I., Thomas D., Le Faou A.‐L., Cornuz J. COVID‐19 and smoking. Nicotine Tob Res 2020; 22: 1650–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Farsalinos K., Barbouni A., Niaura R. Systematic review of the prevalence of current smoking among hospitalized COVID‐19 patients in China: could nicotine be a therapeutic option? Intern Emerg Med 2020; 15: 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grundy E. J., Suddek T., Filippidis F. T., Majeed A., Coronini‐Cronberg S. Smoking, SARS‐CoV‐2 and COVID‐19: a review of reviews considering implications for public health policy and practice. Tob Induc Dis 2020; 18. 10.18332/tid/124788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Elliott J. H., Turner T., Clavisi O., Thomas J., Higgins J. P. T., Mavergames C. et al. Living systematic reviews: an emerging opportunity to narrow the evidence‐practice gap. PLOS Med 2014; 11: e1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tricco A. C., Antony J., Zarin W., Strifler L., Ghassemi M., Ivory J. et al. A scoping review of rapid review methods. BMC Med 2015; 13: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simons D., Brown J., Shahab L., Perski O. Smoking and COVID‐19: Rapid evidence review for the Royal College of Physicians, London (UK). Qeios 2020; 10.32388/VGJCUN. [Google Scholar]
- 22. R Core Team The R Project for Statistical Computing. Vienna, Austria: R core Team; 2013, pp. 1–12. [Google Scholar]
- 23. Bürkner P‐C. Advanced Bayesian Multilevel Modeling with the R Package brms. ArXiv170511123 Stat 2017. http://arxiv.org/abs/1705.11123 (accessed 26 July 2020).
- 24. Simons D., Shahab L., Brown J., Perski O. The association of smoking status with SARS‐CoV‐2 infection, hospitalization and mortality from COVID‐19: a living rapid evidence review (version 5). Qeios 2020; 10.32388/UJR2AW.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Efron B. Better bootstrap confidence intervals. J Am Stat Assoc 1987; 82: 171–185. [Google Scholar]
- 26. Miyara M., Tubach F., Martinez V., Morelot‐Panzini C., Pernet J., Haroche J. et al. Low rate of daily smokers in patients with symptomatic COVID‐19. medRxiv 2020; 10.1101/2020.06.10.20127514 [DOI] [Google Scholar]
- 27. Rimland C. A., Morgan C. E., Bell G. J., Kim M. K., Hedrick T., Marx A. et al. Clinical characteristics and early outcomes in patients with COVID‐19 treated with tocilizumab at a United States academic center. medRxiv 2020; 10.1101/2020.05.13.20100404 [DOI] [Google Scholar]
- 28. Girardeau Y., Gallois Y., de Bonnecaze G., Escudé B., Lafont C., Chatellier G. et al. Confirmed central olfactory system lesions on brain MRI in COVID‐19 patients with anosmia: a case‐series. medRxiv. 10.1101/2020.07.08.20148692 (accessed 25 August 2020). [DOI] [Google Scholar]
- 29. Ebinger J. Botwin G. J., Albert C. M., Alotaibi M., Arditi M., Berg A. H. et al. SARS‐CoV‐2 Seroprevalence across a diverse cohort of healthcare workers. medRxiv 2020; 10.1101/2020.07.31.20163055 [DOI] [Google Scholar]
- 30. Islam M. Z., Riaz B. K., Islam A. S., Khanam F., Akhter J., Choudhury R. et al. Risk factors associated with morbidity and mortality outcomes of COVID‐19 patients on the 14th and 28th day of the disease course: a retrospective cohort study in Bangladesh. medRxiv 2020; 10.1101/10.1101/2020.08.17.20176586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rentsch C. T., Kidwai‐Khan F., Tate J. P., Park L. S., King J. T., Skanderson M. et al. Covid‐19 testing, hospital admission, and intensive care among 2,026,227 United States Veterans aged 54–75 years. medRxiv 2020; 10.1101/2020.04.09.20059964 [DOI] [Google Scholar]
- 32. Odani S. Tobacco product use among military Veterans—United States, 2010–2015. Morb Mortal Wkly Rep 2018; 67: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niedzwiedz C. L., O'Donnell C. A., Jani B. D., Demou E., Ho F. K., Celis‐Morales C. et al. Ethnic and socioeconomic differences in SARS‐CoV‐2 infection: prospective cohort study using UK biobank. BMC Med 2020; 18: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trubiano J. A., Vogrin S., Smibert O. C., Marhoon N., Alexander A. A., Chua K. Y. et al. COVID‐MATCH65—a prospectively derived clinical decision rule for severe acute respiratory syndrome coronavirus 2. medRxiv 2020; 10.1101/2020.06.30.20143818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hopkinson N. S., Rossi N. N., Moustafa J. E.‐S. S. E., Laverty A. A., Quint J. K., Freydin M. B. et al. Current tobacco smoking and risk from COVID‐19 results from a population symptom app in over 2.4 million people. medrxiv 2020; 44; 10.1101/2020.05.18.20105288 [DOI] [PubMed] [Google Scholar]
- 36. Merkely B., Szabó A. J., Kosztin A., Berényi E., Sebestyén A., Lengyel C. et al. Novel coronavirus epidemic in the Hungarian population, a cross‐sectional nationwide survey to support the exit policy in Hungary. GeroScience 2020; 42: 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Department of Health and Social Care . Major home testing programme for coronavirus will track levels of infection in the community—GOV.UK. https://www.gov.uk/government/news/major-home-testing-programme-for-coronavirus‐will‐track‐levels‐of‐infection‐in‐the‐community (accessed 22 May 2020).
- 38. Office for National Statistics . COVID‐19 Infection Survey (CIS). https://www.ons.gov.uk/surveys/informationforhouseholdsandindividuals/householdandindividualsurveys/covid19infectionsurveycis (accessed 30 June 2020).
- 39. de Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G. et al. Risk factors for SARS‐CoV‐2 among patients in the Oxford Royal College of general practitioners research and surveillance centre primary care network: a cross‐sectional study. Lancet Infect Dis 2020; 20: 1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. World Health Organization . Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases. https://www.who.int/publications‐detail‐redirect/10665‐331501 (accessed 29 July 2020).
- 41. Polubriaginof F., Salmasian H., Albert D. A., Vawdrey D. K. Challenges with collecting smoking status in electronic health records. Annu Symp Proc AMIA Symp 2017; 2017: 1392–1400. [PMC free article] [PubMed] [Google Scholar]
- 42. Benowitz N. L., Schultz K. E., Haller C. A., Wu A. H. B., Dains K. M., Jacob P. Prevalence of smoking assessed biochemically in an urban public hospital: a rationale for routine cotinine screening. Am J Epidemiol 2009; 170: 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Griffith G., Morris T. T., Tudball M., Herbert A., Mancano G., Pike L. et al. Collider bias undermines our understanding of COVID‐19 disease risk and severity. medRxiv 2020; 10.1101/2020.05.04.20090506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murray E. Causation in smoking and COVID‐19. Twitter 2020. https://twitter.com/EpiEllie/status/1258607277357006849?s=20 (accessed 8 May 2020).
- 45. Bowyer R. C. E., Varsavsky T., Carole H. Geo‐social gradients in predicted COVID‐19 prevalence and severity in Great Britain: results from affiliations: corresponding authors: understanding the geographical distribution of COVID‐19 through the general population is key to the provision of ade. 2020. 10.1101/2020.04.23.20076521 [DOI]
- 46. Jackson S. E., Brown J., Shahab L., Steptoe A., Fancourt D. COVID‐19, smoking, and inequalities: a cross‐sectional survey of adults in the UK. medRxiv 2020. 10.1101/2020.04.30.20086074 [DOI] [Google Scholar]
- 47. Ward H., Atchison C. J., Whitaker M., Ainslie K. E., Elliot J., Okell L. C. et al. Antibody prevalence for SARS‐CoV‐2 in England following first peak of the pandemic: REACT2 study in 100,000 adults. medRxiv 2020; 10.1101/2020.08.12.20173690 [DOI] [Google Scholar]
- 48. Shahab L., Brose L. S., West R. Novel delivery systems for nicotine replacement therapy as an aid to smoking cessation and for harm reduction: rationale, and evidence for advantages over existing systems. CNS Drugs 2013; 27: 1007–1019. [DOI] [PubMed] [Google Scholar]
- 49. Stead L. F., Buitrago D., Preciado N., Sanchez G., Hartmann‐Boyce J., Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev 2013; 5: CD000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guan W., Liang W., Zhao Y., Liang H. R., Chen Z. S., Li Y. M. et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J 2020; 55: 2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lian J., Jin X., Hao S., Cai H., Zhang S., Zheng L. et al. Analysis of epidemiological and clinical features in older patients with coronavirus disease 2019 (COVID‐19) outside Wuhan. Clin Infect Dis 2020; 71: 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jin X., Lian J.‐S., Hu J.‐H., Gao J., Zheng L., Zhang Y. M. et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus‐infected disease 2019 (COVID‐19) with gastrointestinal symptoms. Gut 2020; 69: 1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020; 368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H. et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis 2020; 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang J., Dong X., Cao Y., Yuan Y. D., Yang Y. B., Yan Y. Q. et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy 2020; 75: 1730–1741. [DOI] [PubMed] [Google Scholar]
- 57. Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y. et al. Clinical features and treatment of COVID‐19 patients in Northeast Chongqing. J Med Virol 2020; 92: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu W., Tao Z.‐W., Wang L., Yuan M. L., Liu K., Zhou L. et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J 2020; 133: 1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang X., Cai H., Hu J., Lian J., Gu J., Zhang S. et al. Epidemiological, clinical characteristics of cases of SARS‐CoV‐2 infection with abnormal imaging findings. Int J Infect Dis 2020; 94: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020; 5: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu R., Ming X., Xu O., Song L., Gao Z., Gao L. et al. Association of cardiovascular manifestations with in‐hospital outcomes in patients with COVID‐19: a hospital staff data. medRxiv 2020; 10.1101/2020.02.29.20029348 [DOI] [Google Scholar]
- 63. Huang Y., Yang R., Xu Y., Gong P. Clinical characteristics of 36 non‐survivors with COVID‐19 in Wuhan, China. medRxiv 2020. 10.1101/2020.02.27.20029009 [DOI] [Google Scholar]
- 64. Xu H., Hou K., Xu H., Li Z., Chen H., Zhang N. et al. Acute myocardial injury of patients with coronavirus disease 2019. medRxiv 2020; 10.1101/2020.03.05.20031591 [DOI] [Google Scholar]
- 65. Li J., Li S., Cai Y., Liu Q., Li X., Zeng Z. et al. Epidemiological and clinical characteristics of 17 hospitalized patients with 2019 novel coronavirus infections outside Wuhan, China. medRxiv 2020; 10.1101/2020.02.11.20022053 [DOI] [Google Scholar]
- 66. Hu L., Chen S., Fu Y., Gao Z., Long H., Ren H. W. et al. Risk factors associated with clinical outcomes in 323 COVID‐19 patients in Wuhan, China. medRxiv 2020; 10.1101/2020.03.25.20037721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang R., Pan M., Zhang X., Han M., Fan X., Zhao F. et al. Epidemiological and clinical features of 125 hospitalized patients with COVID‐19 in Fuyang, Anhui, China. Int J Infect Dis 2020; 95: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. CDCMMWR Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12–March 28, 2020. Morb Mortal Wkly Rep 2020; 69: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dong X., Cao Y., Lu X., Zhang J. J., du H., Yan Y. Q. et al. Eleven faces of coronavirus disease 2019. Allergy 2020; 75: 1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kim E. S., Chin B. S., Kang C. K., Kim N. J., Kang Y. M., Choi J. P. et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID‐19. J Korean Med Sci 2020; 35: e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID‐19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care 2020; 24: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Argenziano M. G., Bruce S. L., Slater C. L., Tiao J. R., Baldwin M. R., Barr R. G. et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ 2020; 369: m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Solís P., Carreňo H. COVID‐19 fatality and comorbidity risk factors among confirmed patients in Mexico. medRxiv 2020. 10.1101/2020.04.21.20074591 [DOI] [Google Scholar]
- 75. Richardson S., Hirsch J. S., Narasimhan M., Crawford J. M., McGinn T., Davidson K. W. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the new York City area. JAMA 2020; 323: 2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fontanet A., Tondeur L., Madec Y., Grant R., Besombes C., Jolly N. et al. Cluster of COVID‐19 in northern France: a retrospective closed cohort study. medRxiv 2020; 10.1101/2020.04.18.20071134 [DOI] [Google Scholar]
- 77. Zheng K. I., Gao F., Wang X.‐B., Sun Q. F., Pan K. H., Wang T. Y. et al. Letter to the editor: obesity as a risk factor for greater severity of COVID‐19 in patients with metabolic associated fatty liver disease. Metabolism 2020; 108: 154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liao Y., Feng Y., Wang B., Wang H., Huang J., Wu Y. et al. Clinical characteristics and risk factors for developed COVID‐19 patients transferring to designated hospital from Jianghan Fangcang Shelter Hospital: a retrospective, observational study. medRxiv 2020. 10.1101/2020.04.21.20074724 [DOI] [Google Scholar]
- 79. Gil‐Agudo A., Rodriguez‐Cola M., Jimenez‐Velasco I., Gutierrez‐Henares F., Lopez‐Dolado E., Gambarrutta‐Malfatti C. et al. Clinical features of coronavirus disease 2019 (COVID‐19) in a cohort of patients with disability due to spinal cord injury. medRxiv 2020. 10.1101/2020.04.20.20072918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shi P., Ren G., Yang J., Li Z., Deng S., Li M. et al. Clinical characteristics of imported and second‐generation COVID‐19 cases outside Wuhan, China: a multicenter retrospective study. medRxiv 2020. 10.1101/2020.04.19.20071472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Pere H. et al. Impaired type I interferon activity and exacerbated inflammatory responses in severe Covid‐19 patients. medRxiv 2020. 10.1101/2020.04.19.20068015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gold J. A. W., Wong K. K., Szablewski C. M., Patel P. R., Rossow J., da Silva J. et al. Characteristics and clinical outcomes of adult patients hospitalized with COVID‐19—Georgia, March 2020. Morb Mortal Wkly Rep 2020; 69: 545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yu T., Cai S., Zheng Z., Cai X., Liu Y., Yin S. et al. Association between clinical manifestations and prognosis in patients with COVID‐19. Clin Ther 2020; 42: 964–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zheng Y., Xiong C., Liu Y., Qian X., Tang Y., Liu L. et al. Epidemiological and clinical characteristics analysis of COVID‐19 in the surrounding areas of Wuhan, Hubei Province in 2020. Pharmacol Res 2020; 157: 104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. de la Rica R., Borges M., Aranda M., del Castillo A., Socias A., Payeras A. et al. Low albumin levels are associated with poorer outcomes in a case series of COVID‐19 patients in Spain: a retrospective cohort study. medRxiv 2020. 10.1101/2020.05.07.20094987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yin R., Yang Z., Wei Y., Li Y., Chen H., Ma D. et al. Clinical characteristics of 106 patients with neurological diseases and co‐morbid coronavirus disease 2019: a retrospective study. medRxiv 2020. 10.1101/2020.04.29.20085415 [DOI] [Google Scholar]
- 87. Shi H., Zuo Y., Yalavarthi S., Gockman K., Zuo M., Madison J. A. et al. Neutrophil calprotectin identifies severe pulmonary disease in COVID‐19. medRxiv 2020. 10.1101/2020.05.06.20093070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cho E. R., Slutsky A. S., Jha P. Smoking and the risk of COVID‐19 infection in the UK Biobank Prospective Study. medRxiv 2020. 10.1101/2020.05.05.20092445 [DOI] [Google Scholar]
- 89. Allenbach Y., Saadoun D., Maalouf G., Vieira M., Hellio A., Boddaert J. et al. Multivariable prediction model of intensive care unit transfer and death: a French prospective cohort study of COVID‐19 patients. medRxiv 2020. 10.1101/2020.05.04.20090118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Robilotti E. V., Babady N. E., Mead P. A., Rolling T., Perez‐Johnston R., Bernardes M. et al. Determinants of severity in cancer patients with COVID‐19 illness. medRxiv 2020. 10.1101/2020.05.04.20086322 [DOI] [Google Scholar]
- 91. Williamson E. J., Walker A. J., Bhaskaran K., Bacon S., Bates C., Morton C. E. et al. OpenSAFELY: factors associated with COVID‐19 death in 17 million patients. Nature 2020; 584: 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Borobia A. M., Carcas A. J., Arnalich F., Álvarez‐Sala R., Monserrat‐Villatoro J., Quintana M. et al. A cohort of patients with COVID‐19 in a major teaching hospital in Europe. J Clin Med 2020; 9: 1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Giacomelli A., Ridolfo A. L., Milazzo L., Oreni L., Bernacchia D., Siano M. et al. 30‐day mortality in patients hospitalized with COVID‐19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res 2020; 158: 104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Shah S. J., Barish P. N., Prasad P. A., Kistler A., Neff N., Kamm J. et al. Clinical features, diagnostics, and outcomes of patients presenting with acute respiratory illness: a comparison of patients with and without COVID‐19. medRxiv 2020. 10.1101/2020.05.02.20082461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kolin D. A., Kulm S., Elemento O. Clinical and genetic characteristics of Covid‐19 patients from UK Biobank. medRxiv 2020. 10.1101/2020.05.05.20075507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lubetzky M., Aull M., Craig‐Shapiro R., Lee J., Lee J., Sultan S. et al. Kidney allograft recipients diagnosed with coronavirus disease‐2019: a single center report. medRxiv 2020. 10.1101/2020.04.30.20086462 [DOI] [Google Scholar]
- 97. Goyal P., Choi J. J., Pinheiro L. C., Schenck E. J., Chen R., Jabri A. et al. Clinical characteristics of Covid‐19 in new York City. N Engl J Med 2020; 382: 2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J. et al. COVID‐19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med 2020; 201: 1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yao Q., Wang P., Wang X., Qie G., Chu Y. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Polish Arch Intern Med 2020; 130: 390–399. [DOI] [PubMed] [Google Scholar]
- 100. Sami R., Soltaninejad F., Amra B., Naderi Z., Javanmard S. H., Iraj B. et al. A one‐year hospital‐based prospective COVID‐19 open‐cohort in the Eastern Mediterranean region: the Khorshid COVID cohort (KCC) study. medRxiv 2020. 10.1101/2020.05.11.20096727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Almazeedi S., Youha S. A., Jamal M. H., Al‐Haddad M., Al‐Muhaini A., Al‐Ghimlas F. et al. Clinical characteristics, risk factors and outcomes among the first consecutive 1,096 patients diagnosed with COVID‐19: the Kuwait experience. medRxiv 2020. 10.1101/2020.05.09.20096495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Carrillo‐Vega M. F., Salinas‐Escudero G., Garcia‐Peña C., Gutierrez‐Robledo L. M., Parra‐Rodriguez L. Early estimation of the risk factors for hospitalization and mortality by COVID‐19 in Mexico. medRxiv 2020. 10.1101/2020.05.11.20098145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yanover C., Mizrahi B., Kalkstein N., Marcus K., Akiva P., Barer Y. et al. What factors increase the risk of complications in SARS‐CoV‐2 positive patients? A cohort study in a nationwide Israeli health organization. medRxiv 2020; 6: e20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hamer M., Kivimäki M., Gale C. R., Batty G. D. Lifestyle risk factors, inflammatory mechanisms, and COVID‐19 hospitalization: a community‐based cohort study of 387,109 adults in UK. Brain Behav Immun 2020; 87: 184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Regina J., Papadimitriou‐Olivgeris M., Burger R., Filippidis P., Tschopp J., Desgranges F. et al. Epidemiology, risk factors and clinical course of SARS‐CoV‐2 infected patients in a Swiss university hospital: an observational retrospective study. medRxiv 2020. 10.1101/2020.05.11.20097741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Targher G., Mantovani A., Wang X.‐B., Yan H. D., Sun Q. F., Pan K. H. et al. Patients with diabetes are at higher risk for severe illness from COVID‐19. Diabetes Metab 2020; 46: 335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Valenti L., Bergna A., Pelusi S., Facciotti F., Lai A., Tarkowski M. et al. SARS‐CoV‐2 seroprevalence trends in healthy blood donors during the COVID‐19 Milan outbreak. medRxiv 2020. 10.1101/2020.05.11.20098442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Feuth T., Saaresranta T., Karlsson A., Valtonen M., Peltola V., Rintala E. et al. Is sleep apnoea a risk factor for Covid‐19? Findings from a retrospective cohort study. medRxiv 2020. 10.1101/2020.05.14.20098319 [DOI] [Google Scholar]
- 109. Ge H., Zhu M., Du J., Zhou Y., Wang W., Zhang W. et al. Cardiac structural and functional characteristics in patients with coronavirus disease 2019: a serial echocardiographic study. medRxiv 2020. 10.1101/2020.05.12.20095885 [DOI] [Google Scholar]
- 110. Parrotta E., Kister I., Charvet L., Sammarco C., Saha V., Charlson R. E. et al. COVID‐19 outcomes in MS: observational study of early experience from NYU multiple sclerosis comprehensive care center. Neuroimmunol Neuroinflammation 2020; 7: e835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Shekhar R., Sheikh A. B., Upadhyay S., Atencio J., Kapuria D. Early experience with COVID‐19 patients at academic hospital in southwestern United States. Infect Dis 2020; 52: 596–599. [DOI] [PubMed] [Google Scholar]
- 112. Mejia‐Vilet J. M., Cordova‐Sanchez B. M., Fernandez‐Camargo D., Mendez‐Perez R. A., Morales‐Buenrostro L. E., Hernandez‐Gilsoul T. A risk score to predict admission to intensive care unit in patients With COVID‐19: the ABC‐GOALS score. medRxiv 2020. 10.1101/2020.05.12.20099416 [DOI] [PubMed] [Google Scholar]
- 113. Chen C., Jiang J., Xu X., Hu Y., Hu Y., Zhao Y. Dynamic liver function indexes monitoring and clinical characteristics in three types of COVID‐19 patients. medRxiv 2020. 10.1101/2020.05.13.20099614 [DOI] [Google Scholar]
- 114. Li J., Chen Y., Chen S., Wang S., Zhang D., Wang J. et al. Derivation and validation of a prognostic model for predicting in‐hospital mortality in patients admitted with COVID‐19 in Wuhan, China: the PLANS (Platelet Lymphocyte Age Neutrophil Sex) model. medRxiv 2020. 10.1101/2020.05.13.20100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Palaiodimos L., Kokkinidis D. G., Li W., Karamanis D., Ognibene J., Arora S. et al. Severe obesity, increasing age and male sex are independently associated with worse in‐hospital outcomes, and higher in‐hospital mortality, in a cohort of patients with COVID‐19 in the Bronx, New York. Metabolism 2020; 108: 154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ip A., Berry D. A., Hansen E., Goy A. H., Pecora A. L., Sinclaire B. A. et al. Hydroxychloroquine and tocilizumab therapy in COVID‐19 patients—an observational study. medRxiv 2020. 10.1101/2020.05.21.20109207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Heili‐Frades S. COVID‐19 outcomes in 4712 consecutively confirmed SARS‐CoV2 cases in the city of Madrid. medRxiv 2020. 10.1101/2020.05.22.20109850 (accessed 27 July 2020). [DOI] [Google Scholar]
- 118. Vaquero L. M., Barrado M. E. S., Escobar D., Arribas P., Gonzalez J. R., Bermejo J. F. et al. C‐Reactive protein and SOFA score as early predictors of critical care requirement in patients with COVID‐19 pneumonia in Spain. medRxiv 2020. 10.1101/2020.05.22.20110429 [DOI] [Google Scholar]
- 119. Kim L., Garg S., O'Halloran A., Whitaker M., Pham H., Anderson E. J. et al. Interim analysis of risk factors for severe outcomes among a cohort of hospitalized adults identified through the U.S. coronavirus disease 2019 (COVID‐19)‐associated hospitalization surveillance network (COVID‐NET). medRxiv 2020. 10.1101/2020.05.18.20103390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wu M. A., Fossali T., Pandolfi L., Carsana L., Ottolina D., Frangipane V. et al. COVID‐19: the key role of pulmonary capillary leakage. An observational cohort study. medRxiv 2020. https://doi.org/10.1101/2020.05.17.20104877. [Google Scholar]
- 121. Shi Q., Zhao K., Yu J., Jiang F., Feng J., Zhao K. et al. Clinical characteristics of 101 COVID‐19 nonsurvivors in Wuhan, China: a retrospective study. medRxiv 2020. 10.1101/2020.03.04.20031039 [DOI] [Google Scholar]
- 122. Al‐Hindawi A., Sokhi J., Cuddihy J., Lockie C., Christie L., Davies R. et al. COVID‐19 in London, a case series demonstrating late improvement in survivors. medRxiv 2020. 10.1101/2020.05.16.20103853 [DOI] [Google Scholar]
- 123. Basse C., Diakite S., Servois V., Frelaut M., Noret A., Bellesoeur A. et al. Characteristics and outcome of SARS‐CoV‐2 infection in cancer patients. medRxiv 2020. 10.1101/2020.05.14.20101576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Freites D., Leon L., Mucientes A., Rodriguez‐Rodriguez L., Font J., Madrid A. et al. Risk factors for hospital admission related to COVID‐19 in inflammatory rheumatic diseases. medRxiv 2020. 10.1101/2020.05.14.20101584 [DOI] [PubMed] [Google Scholar]
- 125. Alshami A. A., Alattas R. A., Anan H. F., Al Qahtani H. S., Al Mulhim M. A., Alahilmi A. A. et al. Silent disease and loss of taste and smell are common manifestations of SARS‐COV‐2 Infection in a quarantine facility: first report from Saudi Arabia. medRxiv 2020. 10.1101/2020.05.13.20100222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Berumen J., Schmulson M., Alegre J., Guerrero G., Olaiz G., Wong‐Chew R. M. et al. Risk of infection and hospitalization by Covid‐19 in Mexico: a case–control study. medRxiv 2020. 10.1101/2020.05.24.20104414 [DOI] [Google Scholar]
- 127. Gianfrancesco M., Hyrich K. L., Al‐Adely S., Carmona L., Danila M. I., Gossec L. et al. Characteristics associated with hospitalization for COVID‐19 in people with rheumatic disease: data from the COVID‐19 global rheumatology Alliance physician‐reported registry. Ann Rheum Dis 2020; 79: 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Li J., Long X., Zhu C., Wang H., Wang T., Lin Z. et al. Olfactory dysfunction in recovered coronavirus disease 2019 (COVID‐19) patients. Mov Disord 202; 35: 1100–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Batty G. D., Deary I., Luciano M., Altschul D., Kivimaki M., Gale C. Psychosocial factors and hospitalizations for COVID‐19: Prospective cohort study of the general population. medRxiv 2020. 10.1101/2020.05.29.20100735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Israel A., Feldhamer I., Lahad A., Levin‐Zamir D., Lavie G. Smoking and the risk of COVID‐19 in a large observational population study. medRxiv 2020. 10.1101/2020.06.01.20118877 [DOI] [Google Scholar]
- 131. Valle D. M. D., Kim‐schulze S., Hsin‐hui H., Beckmann N. D., Nirenberg S., Wang B. et al. An inflammatory cytokine signature helps predict COVID‐19 severity and death. medRxiv 2020. 10.1101/2020.05.28.20115758 [DOI] [Google Scholar]
- 132. Chaudhry F., Bulka H., Rathnam A. S., Said O. M., Lin J., Lorigan H. et al. COVID‐19 in multiple sclerosis patients and risk factors for severe infection. medRxiv 2020. 10.1101/2020.05.27.20114827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Louis S., Dhawan A., Newey C., Nair D., Jehi L., Hantus S. et al. Continuous electroencephalography (cEEG) characteristics and acute symptomatic seizures in COVID‐19 patients. medRxiv 2020. 10.1101/2020.05.26.20114033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Soto‐Mota A., Garza B. A. M., Rodriguez E. M., Rodriguez J. O., Romo A. E., Minutti P. A. et al. The low‐harm score for predicting mortality in patients diagnosed with covid‐19: a multicentric validation study. medRxiv 2020; 10.1101/2020.05.26.20111120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Garibaldi B. T., Fiksel J., Muschelli J., Robinson M. L., Rouhizadeh M., Nagy P. et al. Patient trajectories and risk factors for severe outcomes among persons hospitalized for COVID‐19 in the Maryland/DC region. medRxiv 2020. 10.1101/2020.05.24.20111864 [DOI] [Google Scholar]
- 136. Docherty A. B., Harrison E. M., Green C. A., Hardwick H. E., Pius R., Norman L. et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO clinical characterization protocol: prospective observational cohort study. BMJ 2020; 369: m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Boulware D. R., Pullen M. F., Bangdiwala A. S., Pastick K. A., Lofgren S. M., Okafor E. C. et al. A randomized trial of Hydroxychloroquine as Postexposure prophylaxis for Covid‐19. N Engl J Med 2020; 383: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kuderer N. M., Choueiri T. K., Shah D. P., Shyr Y., Rubinstein S. M., Rivera D. R. et al. Clinical impact of COVID‐19 on patients with cancer (CCC19): a cohort study. Lancet 2020; 395: 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Romão V. C., Oliveira‐Ramos F., Cruz‐Machado A. R., Martins P., Barreira S., Silva‐Dinis J. et al. A COVID‐19 outbreak in a rheumatology department upon the early days of the pandemic. medRxiv 2020. 10.1101/2020.06.05.20107011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Giannouchos T., Sussman R., Mier J. M., Poulas K., Farsalinos K. Characteristics and risk factors for COVID‐19 diagnosis and adverse outcomes in Mexico: an analysis of 89,756 laboratory‐confirmed COVID‐19 cases. medRxiv 2020. 10.1101/2020.06.04.20122481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ramlall V., Thangaraj P., Meydan C., Foox J., Butler D., May B. et al. Identification of Immune complement function as a determinant of adverse SARS‐CoV‐2 infection outcome. medRxiv 2020. 10.1101/2020.05.05.20092452 [DOI] [Google Scholar]
- 142. Wang B., Oekelen O. V., Mouhieddine T., Del Valle D. M., Richter J., Cho H. J. et al. A tertiary center experience of multiple myeloma patients with COVID‐19: lessons learned and the path forward. medRxiv 2020. 10.1101/2020.06.04.20122846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Perrone F., Piccirillo M. C., Ascierto P. A., Salvarani C., Parrella R., Marata A. M. et al. Tocilizumab for patients with COVID‐19 pneumonia. The TOCIVID‐19 prospective phase 2 trial. medRxiv 2020. 10.1101/2020.06.01.20119149 [DOI] [Google Scholar]
- 144. Sharma A. K., Ahmed A., Baig V. N., Dhakad P., Dalela G., Kacker S. et al. Characteristics and outcomes of hospitalized young adults with mild to moderate Covid‐19 at a University Hospital in India. medRxiv 2020. 10.1101/2020.06.02.20106310 [DOI] [PubMed] [Google Scholar]
- 145. Eugen‐Olsen J., Altintas I., Tingleff J., Gamst‐Jensen H., Lindstroem M. B., Rasmussen L. J. et al. Low levels of the prognostic biomarker suPAR are predictive of mild outcome in patients with symptoms of COVID‐19—a prospective cohort study. medRxiv 2020. 10.1101/2020.05.27.20114678 [DOI] [Google Scholar]
- 146. Martinez‐Portilla R. J., Sotiriadis A., Torres‐Torres J., Christos C., Hawkins‐Villarreal A., Villafan‐Bernal J. R. et al. Risk factors for mortality in pregnant women with SARS‐CoV‐2 infection. medRxiv 2020. 10.1101/2020.05.31.20107276 [DOI] [Google Scholar]
- 147. Raisi‐Estabragh Z., McCracken C., Bethell M. S., Cooper J., Cooper C., Caulfield M. J. et al. Greater risk of severe COVID‐19 in black, Asian and minority ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)‐vitamin D status: study of 1326 cases from the UK biobank. J Public Health 2002; 42: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Luo H., Liu S., Wang Y., Phillips‐Howard P. A., Yang Y., Ju S. et al. Age differences in clinical features and outcomes in patients with COVID‐19, Jiangsu, China: a retrospective, multi‐center cohort study. medRxiv 2020. 10.1101/2020.06.01.20086025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Houlihan C. F., Vora N., Byrne T., Lewer D., Kelly G., Heaney J. et al. Pandemic peak SARS‐CoV‐2 infection and seroconversion rates in London frontline health‐care workers. Lancet 2020; 396: e6–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Cen Y., Chen X., Shen Y., Zhang X. H., Lei Y., Xu C. et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019—a multi‐Centre observational study. Clin Microbiol Infect 2020; 26: 1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Klang E., Kassim G., Soffer S., Freeman R., Levin M. A., Reich D. L. Morbid obesity as an independent risk Factor for COVID‐19 mortality in hospitalized patients younger than 50. Obesity 2002; 28: 1595–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Maraschini A., Corsi E., Salvatore M. A., Donati S. Coronavirus and birth in Italy: results of a national population‐based cohort study. medRxiv 2020. 10.1101/2020.06.11.20128652 [DOI] [PubMed] [Google Scholar]
- 153. Wang A.‐L., Zhong X., Hurd Y. Comorbidity and Sociodemographic determinants in COVID‐19 Mortality in an US Urban Healthcare System. medRxiv 2020. 10.1101/2020.06.11.20128926 [DOI] [Google Scholar]
- 154. McQueenie R., Foster H., Jani B. D., Katikireddi S. V., Sattar N., Pell J. P. et al. Multimorbidity, polypharmacy, and COVID‐19 infection within the UK Biobank cohort. medRxiv 2020. 10.1101/2020.06.10.20127563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Apea V. J., Wan Y. I., Dhairyawan R., Puthucheary Z. A., Pearse R. M., Orkin C. M. et al. Ethnicity and outcomes in patients hospitalised with COVID‐19 infection in East London: an observational cohort study. medRxiv 2020. 10.1101/2020.06.10.20127621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Woolford S. J., D'Angelo S., Curtis E. M., Parsons C. M., Ward K. A., Dennison E. M. et al. COVID‐19 and associations with frailty and multimorbidity: a prospective analysis of UK Biobank participants. medRxiv 2020. 10.1101/2020.06.09.20126292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Hultcrantz M., Richter J., Rosenbaum C., Patel D., Smith E., Korde N. et al. COVID‐19 infections and outcomes in patients with multiple myeloma in New York City: a cohort study from five academic centers. medRxiv 2020; 10.1101/2020.06.09.20126516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Rajter J. C., Sherman M., Fatteh N., Vogel F., Sacks J., Rajter J.‐J. ICON (Ivermectin in Covid nineteen) study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID19. medRxiv 2020. 10.1101/20.06.06.20124461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lan F.‐Y., Suharlim C., Kales S. N., Yang J. Association between SARS‐CoV‐2 infection, exposure risk and mental health among a cohort of essential retail workers in the United States. medRxiv 2020. 10.1101/2020.06.08.20125120 [DOI] [PubMed] [Google Scholar]
- 160. Zeng H., Zhang T., He X., Du Y., Tong Y., Wang X. et al. Impact of chronic comorbidities on progression and prognosis in patients with COVID‐19: a retrospective cohort study in 1031 hospitalized cases in Wuhan, China. medRxiv 2020. 10.1101/2020.06.14.20125997 [DOI] [Google Scholar]
- 161. Suleyman G., Fadel R. A., Malette K. M., Hammond C., Abdulla H., Entz A. et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open 2020; 3: e2012270–e2012270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chen L., Yu J., He W., Chen L., Yuan G., Dong F. et al. Risk factors for death in 1859 subjects with COVID‐19. Leukemia 2020; 34: 2173–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Garassino M. C., Whisenant J. G., Huang L.‐C., Trama A., Torri V., Agustoni F. et al. COVID‐19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry‐based, cohort study. Lancet Oncol 2020; 21: 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Hernández‐Garduño E. Obesity is the comorbidity more strongly associated for Covid‐19 in Mexico. A case–control study. Obes Res Clin Pract 2020; 14: 375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Govind R., de Freitas D. F., Pritchard M. R., Hayes R. D., MacCabe J. H. Clozapine treatment and risk of COVID‐19. medRxiv 2020. 10.1101/2020.06.17.20133595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Sisó‐Almirall A., Kostov B., Mas‐Heredia M., Vilanova‐Rotllan S., Sequeira‐Aymar E., Sans‐Corrales M. et al. Prognostic factors in Spanish Covid‐19 patients: a case series from barcelona. PLOS ONE 2020; 15: e0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Gu T., Mack J. A., Salvatore M., Sankar S. P., Valley T. S., Singh K. et al. COVID‐19 outcomes, risk factors and associations by race: a comprehensive analysis using electronic health records data in Michigan Medicine. medRxiv 2020. 10.1101/2020.06.16.20133140 [DOI] [Google Scholar]
- 168. Kibler M., Carmona A., Marchandot B., Matsushita K., Trimaille A., Kanso M. et al. Risk and severity of COVID‐19 and ABO blood group in transcatheter aortic valve patients. medRxiv 2020. 10.1101/2020.06.13.20130211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Ikitimur H., Borku Uysal B., Cengiz M., Ikitimur B., Uysal H., Ozcan E. et al. Determining host factors contributing to disease severity in a family cluster of 29 hospitalized SARS‐CoV‐2 patients: could genetic factors be relevant in the clinical course of COVID‐19? J Med Virol 2020. 10.1002/jmv.26106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Sierpiński R., Pinkas J., Jankowski M., Zgliczyński W. S., Wierzba W., Gujski M. et al. Sex differences in the frequency of gastrointestinal symptoms and olfactory or taste disorders among 1,942 non‐hospitalized patients with COVID‐19. Pol Arch Intern Med 2020; 13: 15414. [DOI] [PubMed] [Google Scholar]
- 171. Zhou Y., He X., Zhang J., e Xue Y., Liang M., Yang B. et al. Prolonged SARS‐CoV‐2 viral shedding in patients with COVID‐19 was associated with delayed initiation of arbidol treatment: a retrospective cohort study. medRxiv 2020. 10.1101/2020.06.09.20076646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Crovetto F., Crispi F., Llurba E., Figueras F., Gomez‐Roig M. D., Gratacos E. Seroprevalence and clinical spectrum of SARS‐COV‐2 infection in the first versus third trimester of pregnancy. medRxiv 2020. 10.1101/2020.06.17.20134098 [DOI] [Google Scholar]
- 173. Veras F. P., Pontelli M., Silva C., Toller‐Kawahisa J., de Lima M., Nascimento D. et al. SARS‐CoV‐2 triggered neutrophil extracellular traps (NETs) mediate COVID‐19 pathology. medRxiv 2020. 10.1101/2020.06.08.20125823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claer L. et al. IgA dominates the early neutralizing antibody response to SARS‐CoV‐2. medRxiv 2020. 10.1101/2020.06.10.20126532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Rossi B., Nguyen L. S., Zimmermann P., Boucenna F., Baucher L., Dubret L. et al. Effect of tocilizumab in hospitalized patients with severe pneumonia COVID‐19: a cohort study. medRxiv 2020. 10.1101/2020.06.06.20122341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Duan L., Zhang S., Guo M., Zhou E., Fan J., Wang X. et al. Epidemiological and clinical characteristics in patients with SARS‐CoV‐2 antibody negative probable COVID‐19 in Wuhan. medRxiv 2020. 10.1101/2020.06.18.20134619 [DOI] [Google Scholar]
- 177. Martin‐Jimenez P., Munoz‐Garcia M. I., Seoane D., Roca‐Rodriguez L., Garcia‐Reyne A., Lalueza A. et al. Cognitive impairment is a common comorbidity in COVID‐19 deceased patients. A hospital‐based retrospective cohort study. medRxiv 2020. 10.1101/2020.06.08.20125872 [DOI] [PubMed] [Google Scholar]
- 178. Elezkurtaj S., Greuel S., Ihlow J., Michaelis E., Bischoff P., Kunze C. A. et al. Causes of death and comorbidities in patients with COVID‐19. medRxiv 2020. 10.1101/2020.06.15.20131540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Lenka J., Chhabria M. S., Sharma N., Bryan E., Tan X., Boppana L. K. et al. Clinical characteristics and outcomes of critically ill patients with COVID‐19 in a tertiary community hospital in upstate New York. medRxiv 2020. 10.1101/2020.06.18.20135046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Olivares F., Munoz D., Fica A., Delama I., Alvarez I., Navarrete M. et al. Covid‐19 in Chile. The experience of a regional reference center. Preliminary report. medRxiv 2020. 10.1101/2020.06.14.20130898 [DOI] [PubMed] [Google Scholar]
- 181. Salton F., Confalonieri P., Santus P., Harari S., Scala R., Lanini S. et al. Prolonged low‐dose methylprednisolone in patients with severe COVID‐19 pneumonia. medRxiv 2020. 10.1101/2020.06.17.20134031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wei W., Ortwine J. K., Mang N. S., Joseph C., Hall B. C., Prokesch B. C. Limited role for antibiotics in COVID‐19: scarce evidence of bacterial coinfection. medRxiv 2020. 10.1101/2020.06.16.20133181 [DOI] [Google Scholar]
- 183. Zuo Y., Estes S. K., Gandhi A. A., Yalavarthi S., Ali R. A., Shi H. et al. Prothrombotic antiphospholipid antibodies in COVID‐19. medRxiv 2020. 10.1101/2020.06.15.20131607 [DOI] [Google Scholar]
- 184. Killerby M. E. Characteristics associated with hospitalization among patients with COVID‐19—metropolitan Atlanta, Georgia, March–April 2020. Morb Mortal Wkly Rep 2020; 69: 790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Petrilli C. M., Jones S. A., Yang J., Rajagopalan H., O'Donnell L., Chernyak Y. et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020; 369: m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Magagnoli J., Narendran S., Pereira F., Cummings T. H., Hardin J. W., Sutton S. S. et al. Outcomes of hydroxychloroquine usage in United States Veterans Hospitalized with COVID‐19. Med (NY) 2020; 10.1016/j.medj.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Bello‐Chavolla O. Y., Bahena‐López J. P., Antonio‐Villa N. E., Vargas‐Vázquez A., González‐Díaz A., Márquez‐Salinas A. et al. Predicting mortality due to SARS‐CoV‐2: a mechanistic score relating obesity and diabetes to COVID‐19 outcomes in Mexico. J Clin Endocrinol Metab 2020; 105: 2752–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Zuo Y., Zuo M., Yalavarthi S. Neutrophil extracellular traps and thrombosis in COVID‐19. medRxiv 2020. 10.1101/2020.04.30.20086736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Sigel K., Swartz T., Golden E., Paranjpe I., Somani S., Richter F. et al. Covid‐19 and people with HIV infection: outcomes for hospitalized patients in New York City. Clin Infect Dis 2020; 10.1093/cid/ciaa880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Nguyen A. B., Upadhyay G. A., Chung B., Smith B., Besser S. A., Johnson J. A. et al. Outcomes and cardiovascular comorbidities in a predominantly African‐American population with COVID‐19. medRxiv 2020. 10.1101/2020.06.28.20141929 [DOI] [Google Scholar]
- 191. de Melo A. C., Thuler L., da Silva J. L., de Albuquerque L. Z., Pecego A. C., de OR Rodrigues L. et al. Cancer inpatient with COVID‐19: a report from the Brazilian National Cancer Institute. medRxiv 2020. 10.1101/2020.06.27.20141499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Auvinen R., Nohynek H., Syrjänen R., Ollgren J., Kerttula T., Mäntylä J. et al. Comparison of the clinical characteristics and outcomes of hospitalized adult COVID‐19 and influenza patients: a prospective observational study. medRxiv 2020. 10.1101/2020.06.29.20140632 [DOI] [PubMed] [Google Scholar]
- 193. Souza F. S. H., Hojo‐Souza N. S., Santos E. B., Silva C. M., Guidoni D. L. Predicting the disease outcome in COVID‐19 positive patients through machine learning: a retrospective cohort study with Brazilian data. medRxiv 2020. 10.1101/2020.06.26.20140764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Mendy A., Apewokin S., Wells A. A., Morrow A. L. Factors associated with hospitalization and disease severity in a racially and ethnically diverse population of COVID‐19 Patients. medRxiv 2020. 10.1101/2020.06.25.20137323 [DOI] [Google Scholar]
- 195. Pongpirul W. A., Wiboonchutikul S., Charoenpong L., Panitantum N., Vachiraphan A., Uttayamakul S. et al. Clinical course and potential predicting factors of pneumonia of adult patients with coronavirus disease 2019 (COVID‐19): a retrospective observational analysis of 193 confirmed cases in Thailand. medRxiv 2020. 10.1101/2020.06.24.20139642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Jin C., Gu J., Yuan Y., Long Q., Zhang Q., Zhou H. et al. Treatment of six COVID‐19 patients with convalescent plasma. medRxiv 2020. 10.1101/2020.05.21.20109512 [DOI] [Google Scholar]
- 197. Favara D. M., Cooke A., Doffinger R., Houghton S., Budriunaite I., Bossingham S. et al. First results from the UK COVID‐19 Serology in Oncology Staff Study (CSOS). medRxiv 2020. 10.1101/2020.06.22.20136838 [DOI] [Google Scholar]
- 198. Fisman D., Greer A. L., Tuite A. Derivation and validation of clinical prediction rule for COVID‐19 mortality in Ontario, Canada. medRxiv 2020. 10.1101/2020.06.21.20136929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Madariaga M. L. L., Guthmiller J., Schrantz S., Jansen M. O., Christensen C., Kumar M. et al. Clinical predictors of donor antibody titer and correlation with recipient antibody response in a COVID‐19 convalescent plasma clinical trial. medRxiv 2020. 10.1101/2020.06.21.20132944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Senkal N., Chronic A. C. E. inhibitor use is associated with decreased odds of severe disease in patients with COVID‐19. Anatol J Cardiol 2020; 24: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Mohamud A. Y., Griffith B., Rehman M., Miller D., Chebl A., Patel S. C. et al. Intraluminal carotid artery thrombus in COVID‐19: another danger of cytokine storm? Am J Neuroradiol 2020; 41: 1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Magleby R., Westblade L. F., Trzebucki A., Simon M. S., Rajan M., Park J. et al. Impact of SARS‐CoV‐2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin Infect Dis 2020; 10.1093/cid/ciaa851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Kimmig L. M., Wu D., Gold M., Pettit N. N., Pitrak D., Mueller J. et al. IL6 inhibition in critically ill COVID‐19 patients is associated with increased secondary infections. medRxiv 2020. 10.1101/2020.05.15.20103531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Bello‐Chavolla O. Y., Antonio‐Villa N. E., Vargas‐Vázquez A., Fermín‐Martínez C. A., Márquez‐Salinas A., Bahena‐López J. P. Profiling pre‐symptomatic and asymptomatic cases with confirmed SARS‐CoV‐2 infection in Mexico City. medRxiv 2020. 10.1101/2020.07.02.20145516 [DOI] [Google Scholar]
- 205. Zacharioudakis I. M., Prasad P. J., Zervou F. N., Basu A., Inglima K., Weisenberg S. A. et al. Association of SARS‐CoV‐2 genomic load with COVID‐19 patient outcomes. medRxiv 2020. 10.1101/2020.07.02.20145151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Antonio‐Villa N. E., Bello‐Chavolla O. Y., Vargas‐Vazquez A., Fermin‐Martinez C. A., Marquez‐Salinas A., Bahena‐Lopez J. P. Health‐care workers with COVID‐19 living in Mexico City: clinical characterization and related outcomes. medRxiv 2020. 10.1101/2020.07.02.20145169 [DOI] [Google Scholar]
- 207. Patel M., Chowdhury J., Mills N., Marron R., Gangemi A., Dorey‐Stein Z. et al. ROX index predicts intubation in patients with COVID‐19 pneumonia and moderate to severe hypoxemic respiratory failure receiving high flow nasal therapy. medRxiv 2020. 10.1101/2020.06.30.20143867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Merzon E., Tworowski D., Gorohovski A., Vinker S., Golan Cohen A., Green I. et al. Low plasma 25(OH) vitamin D3 level is associated with increased risk of COVID‐19 infection: an Israeli population‐based study. medRxiv 2020. 10.1101/2020.07.01.20144329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Fan X., Yin C., Wang J., Yang M., Ma H., Jin G. et al. Pre‐diagnostic circulating concentrations of insulin‐like growth factor‐1 and risk of COVID‐19 mortality: results from UK Biobank. medRxiv 2020. 10.1101/2020.07.09.20149369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Shi Z., Resurreccion W. K., Wang C.‐H., Wei J., Na R., Zheng S. L. et al. Association of cancer with risk and mortality of COVID‐19: results from the UK Biobank. medRxiv 2020. 10.1101/2020.07.10.20151076 [DOI] [Google Scholar]
- 211. Maucourant C., Filipovic I., Ponzetta A., Aleman S., Cornillet M., Hertwig L. et al. Natural killer cell activation related to clinical outcome of COVID‐19. medRxiv 2020. 10.1101/2020.07.07.20148478 [DOI] [Google Scholar]
- 212. Elmunzer B. J., Spitzer R. L., Foster L. D., Merchant A. A., Howard E. F., Patel V. A. et al. Digestive manifestations in patients hospitalized with COVID‐19. medRxiv 2020. 10.1101/2020.07.07.20143024 [DOI] [Google Scholar]
- 213. Alizadehsani R., Sani Z. A., Behjati M., Hussain S., Abedini N., Hasanzadeh F. et al. Risk factors prediction, clinical outcomes, and mortality of COVID‐19 patients. medRxiv 2020. 10.1101/2020.07.07.20148569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Xie Y., Chen S., Wang X., Li B., Zhang T., He X. et al. Early Diagnosis and Clinical Significance of acute cardiac injury—under the iceberg: a retrospective cohort study of 619 non‐critically ill hospitalized COVID‐19 pneumonia patients. medRxiv 2020. 10.1101/2020.07.06.20147256 [DOI] [Google Scholar]
- 215. Fox T. A., Troy‐Barnes E., Kirkwood A. A., Chan W. Y., Day J. W., Chavda S. J. et al. Clinical outcomes and risk factors for severe COVID‐19 infection in patients with haematological disorders receiving chemo‐ or immunotherapy. Br J Haematol 2020. doi: 10.1111/bjh.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Martinez‐Resendez M. F., Castilleja‐Leal F., Torres‐Quintanilla A., Rojas‐Martinez A., Garcia‐Rivas G., Ortiz‐Lopez R. et al. Initial experience in Mexico with convalescent plasma in COVID‐19 patients with severe respiratory failure, a retrospective case series. medRxiv 2020. 10.1101/2020.07.14.20144469 [DOI] [Google Scholar]
- 217. Hoertel N., Rico M. S., Vernet R., Jannot A. S., Neuraz A., Blanco C. et al. observational study of haloperidol in hospitalized patients with Covid‐19. medRxiv 2020. 10.1101/2020.07.15.20150490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. McGrail D. E., Edwards D. COVID‐19 case series at UnityPoint Health St. Luke's Hospital in Cedar Rapids, IA. medRxiv 2020; 10.1101/2020.07.17.20156521 [DOI] [Google Scholar]
- 219. Pandolfi L., Fossali T., Frangipane V., Bozzini S., Morosini M., D'Amato M. et al. Broncho‐alveolar inflammation in COVID‐19 patients: a correlation with clinical outcome. medRxiv 2020. 10.1101/2020.07.17.20155978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Kurashima K., Kagiyama N., Ishiguro T., Takaku Y., Nakajima H., Shibata S. et al. IgG antibody seroconversion and the clinical progression of COVID‐19 pneumonia: A retrospective, cohort study. medRxiv 2020. 10.1101/2020.07.16.20154088 (accessed 25 August 2020). [DOI] [Google Scholar]
- 221. Zhan Z., Yang X., Du H., Zhang C., Song Y., Ran X. et al. Early improvement of acute respiratory distress syndrome in patients with COVID‐19: insights from the data of ICU patients in Chongqing, China. medRxiv 2020. 10.1101/2020.07.15.20154047 [DOI] [Google Scholar]
- 222. Omrani A. S., Almaslamani M. A., Daghfal J., Alattar R. A., Elgara M., Shaar S. H. et al. The first consecutive 5000 patients with Coronavirus disease 2019 from Qatar; a nation‐wide cohort study. medRxiv 2020. 10.1101/2020.07.15.20154690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Gupta R., Agrawal R., Bukhari Z., Jabbar A., Wang D., Diks J. et al. Higher comorbidities and early death is characteristic of hospitalized African‐American patients with COVID‐19. medRxiv 2020. 10.1101/2020.07.15.20154906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Hussein M. H., Toraih E. A., Attia A. S., Youssef M., Omar M., Burley N. et al. Asthma in COVID‐19: an extra chain fitting around the neck? medRxiv 2020. 10.1101/2020.07.13.20153130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Bian H., Zheng Z.‐H., Wei D., Zhang Z., Kang W. Z., Hao C. Q. et al. Meplazumab treats COVID‐19 pneumonia: an open‐labelled, concurrent controlled add‐on clinical trial. medRxiv 2020. 10.1101/2020.03.21.20040691 [DOI] [Google Scholar]
- 226. Eiros R., Barreiro‐Perez M., Martin‐Garcia A., Almeida J., Villacorta E., Perez‐Pons A. et al. Pericarditis and myocarditis long after SARS‐CoV‐2 infection: a cross‐sectional descriptive study in health‐care workers. medRxiv 2020. 10.1101/2020.07.12.20151316 [DOI] [Google Scholar]
- 227. Marcos M., Belhassen‐Garcia M., Puente A. S., Sampedro‐Gomez J., Azibeiro R., Dorado‐Diaz P. I. et al. Development of a severity of disease score and classification model by machine learning for hospitalized COVID‐19 patients. medRxiv 2020. 10.1101/2020.07.13.20150177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Hoertel N., Rico M. S., Vernet R., Beeker N., Jannot A. S., Neuraz A. et al. Association between SSRI antidepressant use and reduced risk of intubation or death in hospitalized patients with Coronavirus disease 2019: a multicenter retrospective observational study. medRxiv 2020. 10.1101/2020.07.09.20143339 [DOI] [Google Scholar]
- 229. Soares R. D., Mattos L. R., Raposo L. M. Risk factors for hospitalization and mortality due to COVID‐19 in Espírito Santo State, Brazil. Am J Trop Med 2020; 103: 1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Zobairy H., Shamsoddin E., Rasouli M. A., Khodlan N. V., Moradi G., Zareie B. et al. Association of olfactory dysfunction with hospitalization for COVID‐19: a multicenter study in Kurdistan. medRxiv 2020. 10.1101/2020.07.26.20158550 [DOI] [Google Scholar]
- 231. Altamimi H., Alahmad Y., Khazal F., El Hassan M., Al Binali H., Arabi A. et al. The outcome of COVID‐19 patients with acute myocardial infarction. medRxiv 2020. 10.1101/2020.07.21.20156349 [DOI] [Google Scholar]
- 232. Thompson J. V., Meghani N., Powell B. M., Newell I., Craven R., Skilton G. et al. Patient characteristics and predictors of mortality in 470 adults admitted to a district general hospital in England with Covid‐19. medRxiv 2020. 10.1101/2020.07.21.20153650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Reiter T., Pajenda S., Wagner L., Gaggl M., Atamaniuk J., Holzer B. et al. Covid‐19 serology in nephrology health care workers. medRxiv 2020. 10.1101/2020.07.21.20136218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Motta J. K., Ogunnaike R. O., Shah R., Stroever S., Cedeno H. V., Thapa S. K. et al. Clinical outcomes with the use of prophylactic versus therapeutic anticoagulation in COVID‐19. medRxiv 2020. 10.1101/2020.07.20.20147769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Santos C., Rhee Y., Hollinger E., Olaitan O., Schadde E., Peev V. et al. Comparative incidence and outcomes of COVID‐19 in kidney or kidney‐pancreas transplant recipients versus kidney or kidney‐pancreas waitlisted patients: a pilot study. medRxiv 2020. 10.1101/2020.07.20.20157990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Schneeweiss M. C., Leonard S., Weckstein A., Schneeweiss S., Rassen J. Renin–angiotensin–aldosterone‐system inhibitor use in patients with COVID‐19 infection and prevention of serious events: a cohort study in commercially insured patients in the US. medRxiv 2020. 10.1101/2020.07.22.20159855 [DOI] [Google Scholar]
- 237. Concha‐Mejia A., Rincon‐Sanchez R. A. CCOFEE‐GI study: Colombian COVID19 first experience in gastroentrology. Characterization of digestive manifestations in patients diagnosed with COVID‐19 at a highly complex institution in Bogota D.C., Colombia. medRxiv 2020. 10.1101/2020.07.24.20161604 [DOI] [Google Scholar]
- 238. Izquierdo J. L., Almonacid C., Gonzalez Y., Del Rio‐Bermudez C., Ancochea J., Cardenas R. et al. The impact of COVID‐19 on patients with asthma. medRxiv 2020. 10.1101/2020.07.24.20161596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Bernaola N., Mena R., Bernaola A., Lara A., Carballo C., Larranaga P. et al. Observational study of the efficiency of treatments in patients hospitalized with Covid‐19 in Madrid. medRxiv 2020. 10.1101/2020.07.17.20155960 [DOI] [Google Scholar]
- 240. Qi D., Yan X., Tang X., Peng J., Yu Q., Feng L. et al. Epidemiological and clinical features of 2019‐nCoV acute respiratory disease cases in Chongqing municipality, China: a retrospective, descriptive, multiple‐center study. medRxiv 2020. 10.1101/2020.03.01.20029397 [DOI] [Google Scholar]
- 241. Peters E. J., Collard D., van Assen S., Beudel M., Bomers M. K., Buijs J. et al. Outcomes of persons with COVID‐19 in hospitals with and without standard treatment with (hydroxy)chloroquine. medRxiv 2020. 10.1101/2020.08.14.20173369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Ouyang J., Shan X., Wang X., Zhang X., Chen Y., Qi M. et al. Clinical characteristics of COVID‐19 and the model for predicting the occurrence of critically ill patients: a retrospective cohort study. medRxiv 2020. 10.1101/2020.08.13.20173799 [DOI] [Google Scholar]
- 243. Valenzuela O., Ibanez S. E., Poli M., Roessler P., Aylwin M., Roizen G. et al. First report of tocilizumab use in a cohort of Latin American patients hospitalized for severe COVID‐19 pneumonia. medRxiv 2020. 10.1101/2020.08.12.20173104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Monteiro A. C. C., Suri R., Emeruwa I. O., Stretch R. J., Lopez R. Y., Sherman A. et al. Obesity and smoking as risk factors for invasive mechanical ventilation in COVID‐19: a retrospective, observational cohort study. medRxiv 2020; 10.1101/2020.08.12.20173849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Philipose Z., Smati N., Wong C. S. J., Aspey K., Mendall M. A. Obesity, old age and frailty are the true risk factors for COVID‐19 mortality and not chronic disease or ethnicity in Croydon. medRxiv 2020. 10.1101/2020.08.12.20156257 [DOI] [Google Scholar]
- 246. Weerahandi H., Hochman K. A., Simon E., Blaum C., Chodosh J., Duan E. et al. Post‐discharge health status and symptoms in patients with severe COVID‐19. medRxiv 2020; 10.1101/2020.08.11.20172742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Altibi A. M., Bhargava P., Liaqat H., Slota A. A., Sheth R., Al Jebbawi L. et al. Comparative clinical outcomes and mortality in prisoner and non‐prisoner populations hospitalized with COVID‐19: a cohort from Michigan. medRxiv 2020. 10.1101/2020.08.08.20170787 [DOI] [Google Scholar]
- 248. Izzi‐Engbeaya C., Distaso W., Amin A., Yang W., Idowu O., Kenkre J. S. et al. Severe COVID‐19 and diabetes: a retrospective cohort study from three London teaching hospitals. medRxiv 2020; 10.1101/2020.08.07.20160275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Rizzo S., Chawla D., Zalocusky K., Keebler D., Chia J., Lindsay L. et al. Descriptive epidemiology of 16,780 hospitalized COVID‐19 patients in the United States. medRxiv 2020; 10.1101/2020.07.17.20156265 [DOI] [Google Scholar]
- 250. Dashti H. T., Bates D., Fiskio J. M., Roche E. C., Mora S., Demler O. Clinical Characteristics and severity of COVID‐19 disease in patients from Boston area hospitals. medRxiv 2020. 10.1101/2020.07.27.20163071 [DOI] [Google Scholar]
- 251. Morshed M. S., Mosabbir A. A., Chowdhury P., Ashadullah S. M., Hossain M. S. Clinical manifestations of patients with Coronavirus disease 2019 (COVID‐19) attending at hospitals in Bangladesh. medRxiv 2020. 10.1101/2020.09.09.20191114 [DOI] [Google Scholar]
- 252. Jun T., Nirenberg S., Kovatch P., Huang K. Sex‐specificity of mortality risk factors among hospitalized COVID‐19 patients in New York City: prospective cohort study. medRxiv 2020; 10.1101/2020.07.29.20164640 [DOI] [Google Scholar]
- 253. Higuchi T., Nishida T., Iwahashi H., Morimura O., Otani Y., Okauchi Y. et al. Early clinical factors predicting the development of critical disease in Japanese patients with COVID‐19: a single‐center retrospective, observational study. medRxiv 2020; 10.1101/2020.07.29.20159442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Zhou K., Sun Y., Li L., Zang Z., Wang J., Li J. et al. Eleven routine clinical features predict COVID‐19 severity. medRxiv 2020. 10.1101/2020.07.28.20163022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Salerno S., Zhao Z., Sankar S. P., Salvatore M., Gu T., Fritsche L. G. et al. Understanding the patterns of repeated testing for COVID‐19: Association with patient characteristics and outcomes. medRxiv 2020. 10.1101/2020.07.26.20162453 [DOI] [Google Scholar]
- 256. Kumar A., Prasad G., Srivastav S., Gautam V. K., Sharma N. A retrospective study on efficacy and safety of Guduchi Ghan Vati for Covid‐19 asymptomatic patients. medRxiv 2020. 10.1101/2020.07.23.20160424 [DOI] [Google Scholar]
- 257. Hao S.‐R., Zhang S.‐Y., Lian J.‐S., Jin X., Ye C. Y., Cai H. et al. Liver enzyme elevation in coronavirus disease 2019: a multicenter, retrospective, cross‐sectional study. Am J Gastroenterol 2020; 10.14309/ajg.0000000000000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Iversen K., Bundgaard H., Hasselbalch R. B., Kristensen J. H., Nielsen P. B., Pries‐Heje M. et al. Risk of COVID‐19 in health‐care workers in Denmark: an observational cohort study. Lancet Infect Dis 2020. 10.1016/S1473-3099(20)30589-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Hippisley‐Cox J., Young D., Coupland C., Channon K. M., San Tan P., Harrison D. A. Risk of severe COVID‐19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart 2020; 106: 1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Fillmore N. R., La J., Szalat R. E., Tuck D. P., Nguyen V., Yildirim C. et al. Prevalence and outcome of Covid‐19 infection in cancer patients: a national VA study. medRxiv 2020. 10.1101/2020.08.21.20177923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Rashid M., Wu J., Timmis A., Curzen N., Zaman A., Clarke S. et al. Clinical characteristics and outcomes of COVID‐19 positive acute coronary syndrome patients; a multisource electronic healthcare records study from England. medRxiv 2020. 10.1101/2020.08.20.20175091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Pan A., Khan O., Meeks J., Boom M., Masud F., Andrieni J. et al. Disparities in covid‐19 hospitalizations and mortality among black and hispanic patients: cross‐sectional analysis from the Greater Houston Metropolitan Area. medRxiv 2020. 10.1101/2020.08.19.20177956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Alkurt G., Murt A., Aydin Z., Tatli O., Agaoglu N. B., Irvem A. et al. seroprevalence of coronavirus disease 2019 (covid‐19) among health care workers from three pandemic hospitals of Turkey. medRxiv 2020. 10.1101/2020.08.19.20178095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Zhao Z., Chen A., Hou W., Graham J. M., Li H., Richman P. S. et al. Prediction model and risk scores of ICU admission and mortality in COVID‐19. PLOS ONE 2020; 15: e0236618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Holman N., Knighton P., Kar P., O'Keefe J., Curley M., Weaver A. et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endocrinol 2020; 8: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Qu J., Chang L. K., Tang X., Du Y., Yang X., Liu X. et al. Clinical characteristics of COVID‐19 and its comparison with influenza pneumonia. Acta Clin Belg 2020; 75: 348–356. [DOI] [PubMed] [Google Scholar]
- 267. Chand S., Kapoor S., Orsi D., Fazzari M. J., Tanner T. G., Umeh G. C. et al. COVID‐19‐associated critical illness—report of the first 300 patients admitted to intensive care units at a new York City medical center. J Intens Care Med 2020; 35: 963–970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Map of countries where included studies were conducted. Six studies were performed in multiple countries and are not included here.
Table S1 Study design, use of clinical diagnosis and stratification of smoking status by sex, age or socio‐economic position.
Table S2a Studies reporting complete smoking status
Table S2b Studies reporting partially complete smoking status
Table S2c Studies reporting incomplete smoking status
Table S3 Smoking prevalence in countries with included studies
Figure S2 Supporting Information
Figure S3 Supporting Information
Figure S4 Supporting Information
Figure S5 Supporting Information
Data Availability Statement
All data contributing to the current and future review versions are https://doi.org/10.6084/m9.figshare.12756020. All code required to reproduce the current and future analyses are https://doi.org/10.5281/zenodo.4002046.


