Abstract
Objective
Fibrosis is the most characteristic pathological hallmark of SSc, a connective tissue disease characterized by vascular and immunological abnormalities, inflammation and enhanced extracellular matrix production, leading to progressive fibrosis of skin and internal organs. We previously demonstrated that parvovirus B19 (B19V) can infect normal human dermal fibroblasts (NHDFs) and that B19V persists in SSc fibroblasts. In this study, we investigated whether parvovirus B19V is able to activate in vitro NHDFs and to induce in these cells some phenotypic features similar to that observed in the SSc fibroblasts.
Methods
We preliminarily analysed the time course of B19V infection in cultured NHDFs, then we investigated the ability of B19V to induce cell migration, invasive phenotype and mRNA expression of some profibrotic and/or proinflammatory genes.
Results
We confirmed our previous findings that B19V infects NHDFs, but the infection is not productive. After incubation with B19V, NHDFs showed a significant increase of both migration and invasiveness, along with mRNA expression of different profibrotic genes (α-SMA, EDN-1, IL-6, TGF-β1 receptors 1 and 2, Col1α2), some genes associated with inflammasome platform (AIM2, IFI16, IL-1β, CASP-1) and genes for metalloprotease (MMP 2, 9 and 12).
Conclusion
These data suggest that B19V can activate dermal fibroblasts and may have a role in the pathogenesis of fibrosis. B19V-induced fibroblast migration and invasiveness could be due to the B19V-associated MMP9 overexpression and activation. Moreover, the up-regulation of MMP12, typical of SSc, could link the B19V infection of fibroblasts to the anti-angiogenic process.
Keywords: parvovirus B19 infection, systemic sclerosis, normal human dermal fibroblasts, fibrosis
Rheumatology key messages
Parvovirus B19 (B19V) activates normal human dermal fibroblasts in vitro.
B19V-activated fibroblasts showed a significant increase of both migration and invasiveness.
B19V-activated fibroblasts showed a significant increase of mRNA expression of profibrotic, inflammasome-associated and metalloproteases-coding genes.
Introduction
Pathologic fibrosis is the hallmark of SSc, a connective tissue disease characterized by vascular and immunological abnormalities, inflammation and enhanced extracellular matrix production, leading to fibrotic damage of skin and internal organs produced by chronically activated fibroblasts [1]. The trigger(s) inducing fibroblasts’ activation in SSc remains unknown. Besides the host’s genetic predisposition, several environmental agents have been considered, including toxic and infectious agents such as EBV, CMV and parvovirus B19 (B19V) [2]. B19V is a small non-enveloped ssDNA virus with a capsid composed of VP1 and VP2 proteins, which differ for 227 amino acids at the amino-terminal end of the VP1-protein, the so-called VP1-unique region (VP1u). The left part of viral genome encodes non-structural (NS1) protein involved in viral replication and in the pathogenesis of several B19V-associated diseases [3].
After primary infection, B19V DNA persists lifelong in several tissues, mainly bone marrow, heart, liver, synovia and skin [4], with still unknown functional consequences. Chronic B19V infection implies autoimmune clinical features that are suggested to depend on ‘molecular mimicry’ [3].
The hypothesis that B19V can contribute to the onset and/or evolution of SSc is supported by studies demonstrating B19V persistence in bone marrow and skin of SSc patients and more frequent detection of anti-B19V NS1 circulating antibodies [5]. Endothelia, fibroblasts and perivascular inflammatory cells of SSc skin patients showed the presence of both B19V DNA and TNF-α mRNAs, and degeneration of endothelial cells related with the degree of B19V RNA expression, suggesting a causal role of B19V in the propagation of the endothelial cell dysfunction [6]. So far, no mechanism has been proposed to explain such features.
Recently, we demonstrated that B19V abortive infection increases TNF-α production and induces NLR family pyrin domain containing 3 (NLRP3)-mediated caspase-1 activation and IL-1β secretion in phorbol 12-myristate 13-acetate-differentiated THP-1 monocytic cell line and that B19V-infected monocytes from SSc patients express NLRP3 and activate caspase-1 [7]. These data indicate that B19V-mediated activation of inflammatory pathways in monocytes might contribute to the disease progression and/or development of specific clinical phenotypes.
In addition, we previously demonstrated that B19V infects cultured normal human dermal fibroblasts (NHDFs) [8] and persists in SSc fibroblasts propagated in vitro [9].
In the present study, we first analysed the time course of B19V infection in NHDFs, then we investigated whether B19V infection can activate NHDFs and induce phenotypic features similar to those observed in SSc fibroblasts, evaluating cell migration, invasion and expression of some profibrotic and/or proinflammatory genes.
Methods
Cells and virus
NHDFs (Sigma, Milan, Italy) were cultured in Medium-199 (M199) with 10% foetal bovine serum (FBS). Erythropoietin (Epo)-dependent bone marrow megakaryoblastic leukaemia UT7EpoS1 cell line was cultured in Iscove’s Modified Dulbecco’s Medium (IMDM) containing 10% FBS and 2 U/ml of Epo at 37°C.
For infection experiments, both viremic plasma I700 and recombinant virus B19V-EC [10] were used at a multiplicity of infection (moi) 10 000 geq/cell. As controls (mock infected cells) B19V negative plasma or M199 alone were used.
For inhibition studies I700 or B19V-EC were pre-incubated with commercial immunoglobulin (Venital; Kedrion, Italy), containing a high titre of anti-B19V antibodies, for 1 h at 37°C.
B19V infection of NHDFs
NHDFs (2.5×105) were infected with I700 or with B19V-EC for 2 h at 37°C. At different time points post-infection, the cells were analysed to quantify viral DNA, mRNAs and for the presence of viral proteins. Control infection of UT7EpoS1 cells was performed as described [8].
Nucleic acid extraction and amplification
Real-time PCR was carried out to quantify the B19V DNA [11] extracted from cell cultures using E.Z.N.A. Tissue DNA Kit (Omega Biotek, Norcross, GA, USA). All samples were also analysed by real-time PCR for housekeeping sequence (AP3B1) (Bio-Rad, Hercules, CA, USA).
The RNA was extracted using the E.Z.N.A. Total RNA Kit 1 (Omega Bio-tek, Norcross, GA, USA), retro-transcribed using the PrimeScript RT reagent kit (Takara Clontech, St-Germainen-Laye, France) and analysed for the presence of B19V spliced transcripts by qualitative PCR modified from Bostic [12]. To quantify B19V NS1 mRNA, cDNA was amplified as described [11]. Real-time quantitative PCR for 18 s mRNA was done using validated PrimePCR SYBR Green Assays (Bio-Rad, Hercules, CA, USA).
Immunofluorescence
NHDFs (1×105) seeded onto glass slides were infected with I700 and incubated for 24, 48 and 72 h. UT7EpoS1-infected cells were spotted onto glass slides (1×105/slide) at 48 hpi. Immunofluorescence was performed as described [13] using -B19V VP1/VP2 monoclonal antibody (antiMillipore, Merck Millipore, Burlington, MA, MAB8293).
Wound-healing assay
Fibroblasts (4 × 105) were incubated with I700 or B19V-EC and with B19V-negative plasma or M199 alone. After 2 h, a wound was generated on the cell monolayer using a micropipette tip and microphotographs were taken at 2 and 24 hpi. Images were analysed with the ImageJ MRI Wound healing software (ImageJ National Institutes of Health, Bethesda, MD) and reported as the percentage of the wound closure compared with initial wound area.
Invasion assays
The I700 or B19-EC-infected or mock infected NHDFs were harvested after 48 h, re-suspended in M199 (1.8×104 cells/200 µl) and placed in the upper compartment of the Boyden chamber (Bioamp, Italy) with wells separated by 8 µm-pore size polycarbonate Matrigel-coated filters (BD Europe, San Jose, CA). After 24 h incubation at 37°C with I700 or B19V-EC and respective controls, filters were fixed and invasive cells on the lower filter surface were stained with Diff-Quik reagent (Bioamp, Italy) and counted using a light microscope.
Expression studies
NHDFs (2.5×105) were incubated for 48 or 72 h at 37°C with I700 or with B19V negative plasma. The mRNA expression of selected genes such as α-smooth muscle actin (α-SMA), endothelin-1 (EDN-1), TGF-ß1, TGF-ß1 receptors 1 (TGF-ßR1) and 2 (TGF-ßR2), connective tissue growth factor, collagen chain 2 (COL1α2), metalloproteases (MMPs) 2, 9 and 12, IL-1β and IL-6, caspase-1 (casp-1), NOD-like receptors (NLRP3, AIM2, IFI16) and Toll-like receptors (TLRs) 3, 4, 7, 8, 9 was analysed by comparative RT-PCRs using validated PrimePCR SYBR Green Assays (Bio-Rad, Hercules, CA, USA). MMP12 expression was analysed using (IDT, Thema Ricerca, Italy) primers.
Expression of selected genes in three to nine independent B19V-infected cultures was displayed as fold-change relative to mock-infected cultures using ΔΔCt method. The expression of target genes was normalized to the expression of 18 s gene. The means between two groups (B19V-infected and mock-infected) were analysed by Student’s two-tailed t test.
Gelatine zymography analyses in conditioned media
At 48 hpi, the conditioned media from B19V-infected and mock-infected NHDFs were collected, centrifuged, diluted with Tris–Glycine SDS Native Sample Buffer (Invitrogen, Italy) and loaded on 10% Novex Zymogram Gelatine Gels (Invitrogen, Italy). The gels were developed following manufacturer’s instructions and quantified using ImageJ software.
Results
B19V infects NHDFs
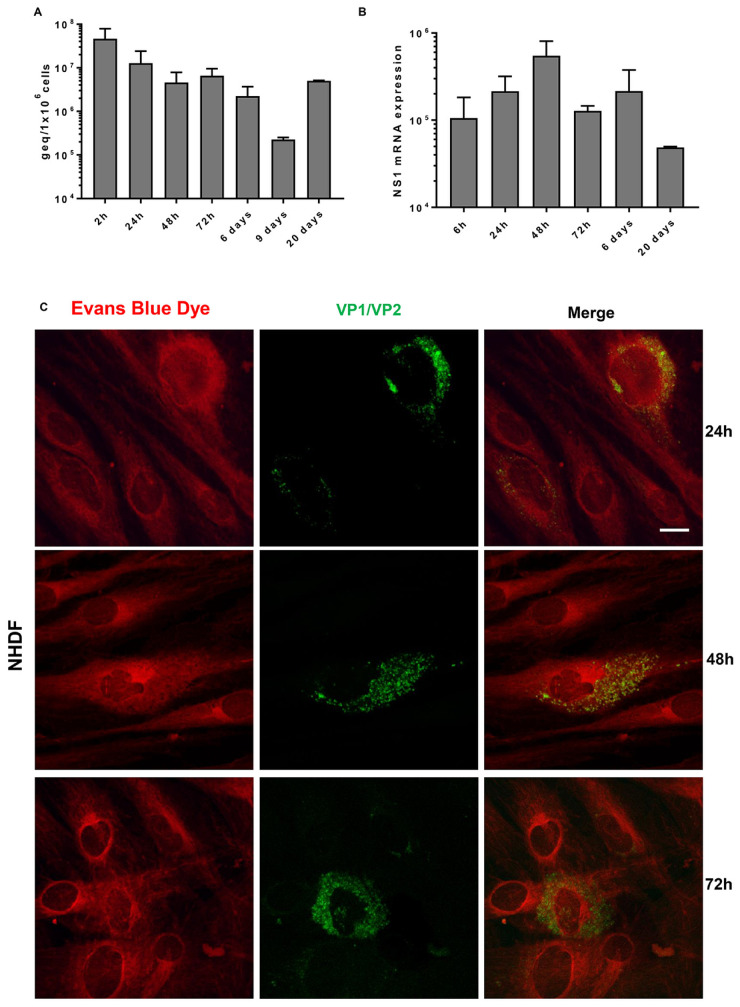
No increase of viral DNA nor mRNA for NS1 protein was observed during the time course of infection of NHDFs (Fig. 1A and B). The spliced mRNAs were detected but not in all cultures and not at all time points. B19V VP1/VP2 proteins were detected in 10%, 8% and 6% of infected fibroblasts at 24, 48 and 72 hpi, respectively (Fig. 1C).
Fig. 1.
Time course of B19V infection of primary NHDF cultures
(A) The levels of viral DNA were evaluated at the end of absorption/penetration period (2 hpi), at 24, 48 and 72 h and at 6, 9 and 20 days post infection. (B) The mRNAs for NS1 protein were carried out at 6, 24, 48 and 72 h and at 6 and 20 days post infection. Bars represent mean (S.e.m.) values from four independent cultures. (C) Immunofluorescence assay for the detection of B19V VP1/VP2 proteins was carried out at 24, 48 and 72 hpi. Positive cells are stained in green. A counterstain with Evans Blue was performed in order to highlight the cell structure. Scale bar=5 µm.
In the UT7EpoS1 cells, permissive for B19V multiplication, continuous increase of both DNA and NS1 mRNA was observed and the spliced mRNAs were detected in all tested cultures and at each time point. At 48 hpi, the VP1/VP2 proteins were present in 10% of infected cells.
B19V induces migration and invasive phenotype of NHDFs
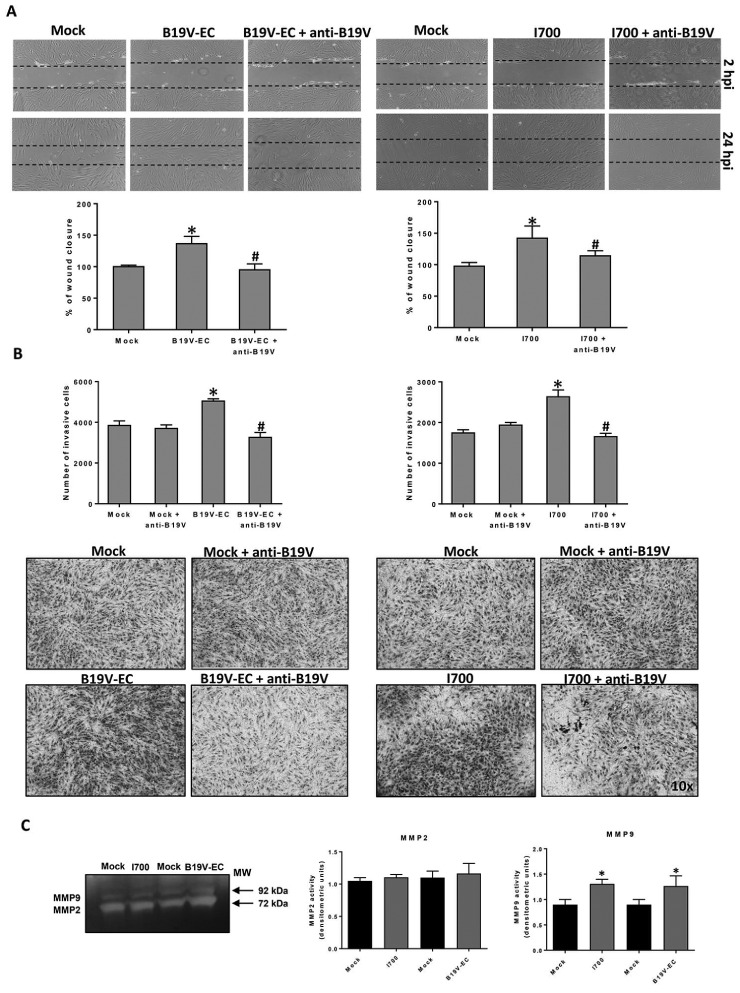
Compared with mock-infected fibroblasts, both I700 and B19V-EC-infected NHDFs showed a significant increase of migration ability (143%; P =0.0063 and 138%; P =0.0486 respectively). In the presence of antibodies, we observed significant decrease of cells migration compared with antibody untreated samples: 44% (P =0.0220) for antibody-treated I700 and 28% (P =0.0435) for antibody-treated B19V-EC (Fig. 2A).
Fig. 2.
B19V-induced wound healing, invasion and MMPs in NHDFs cultures
NHDFs were incubated with B19V-viremic plasma I700, with anti-B19V antibody-treated I700 or with B19V negative serum (mock) and with recombinant virus (B19V-EC), withanti-B19V-treated B19V-EC or with M199 (mock). (A) Wound healing: values are given as percentage of wound closure from four experiments. (B) Invasion: values are given as number of invasive cells from two independent experiments, in triplicate. *P<0.05 vs NHDFs mock control, #P<0.05 vs B19V-infected NHDFs. (C) MMP2 and MMP9 activities by gelatine zymography. Results are reported as densitometric units [mean of three different experiments (S.d.)]. *P<0.05 vs mock control NHDFs.
Both I700 and B19V-EC infection induced invasion in all fibroblast’s cultures tested, with an increase of 150% (P =0.0324) and 130% (P =0.0042) compared with mock-infected cells. The pre-incubation of the virus with the specific antibodies significantly reduced the invasion response to B19V: 55% (P =0.004) and 45% (P =0.0039) decrease of the invasiveness in the case of I700 or of B19V-EC, respectively (Fig. 2B).
B19V induces MMPs expression and MMP9 activity in NHDFs
We observed significant increase of MMP2 (fold change 1.7; P =0.016) and MMP9 (fold change: 2.9; P =0.007) transcripts at 72 hpi. Moreover, using gelatine zymography, we found significant increase in the activity of MMP9 in the supernatants from B19V infected cultures compared with mock infected cells (P ˂ 0.05) (Fig. 2C).
We also observed increased levels of MMP12 transcripts (fold change: 1.6; P =0.002) at 72 hpi.
B19V induces activation markers in NHDFs
Compared with mock-infected cells, the B19V-infected fibroblasts showed a significant increase in expression of profibrotic genes such as α-SMA (fold change: 3.5; P =0.0000), EDN-1 (fold change: 2.4; P =0.0007), IL-6 (fold change: 1.3; P =0.0008), TGFßR1 (fold change: 3.6; P =0.014) and TGFßR2 (fold change: 10.9; P =0.0004) and COL1α2 (fold-change 1.5; P =0.01) at 48 hpi.
Moreover, upregulation of some inflammasome-associated genes such as AIM2 (fold change: 4.5; P =0.0004), IFI16 (fold change: 3.6; P =0.0000), caspase-1 (fold change: 7.2; P =0.004) and IL-1ß (fold change: 7.6; P =0.0004) was observed in B19V-infected fibroblasts compared with mock-infected cells.
B19V induces expression of PRRs transcripts in NHDFs
Compared with mock-infected cells, the B19V infected fibroblasts showed significant increased levels of TLR3 (fold change: 2.4; P =0.04) and TLR4 (fold change: 3.3; P =0.016) genes.
Discussion
Although several reports have suggested a role of B19V in the aetiology of autoimmune connective tissue diseases [3] and an association with SSc [5–9], the mechanisms of virus-driven pathogenesis is unknown.
The results of the present study confirm that cultured NHDFs can be infected by B19V, but they are not able to sustain viral replication.
We demonstrated that B19V stimulates migration and invasion of cultured NHDFs, as previously shown by others for synovial fibroblasts stimulated with B19V viremic serum [14] or with B19V VP1u protein [15], with comparable results using viremic plasma or recombinant virus. Migration and invasiveness decreased significantly in the presence of B19V-neutralizing antibodies, confirming that cell activation was due to the virus. Moreover, B19V-infected cultures showed a significant increase of mRNA transcripts of MMP2 and MMP9 and significantly higher activity of MMP9 by gelatine zymography, suggesting that B19V-induced MMPs could contribute to the invasiveness of infected fibroblasts observed in our study.
We showed that B19V infection also induced a significant increase of mRNA transcripts of different profibrotic genes such as α-SMA, EDN-1 and COL1α, which were found to be overexpressed also in SSc fibroblasts [1]. The EDN-1 acts not only as a potent vasoconstrictor but also as a mediator of fibrosis, resulting increased in the serum of SSc patients, promoting myofibroblast differentiation, expression of collagen, and inhibiting MMP1 expression and activity [16].
We also found increased mRNA levels of TGF-β1 receptors but not TGF-β1 cytokine, in line with other reports on SSc fibroblasts [17].
Moreover, in our study, B19V-infected fibroblasts produced significantly higher levels of IL-6 mRNA, as previously reported in a patient with acute B19V infection and polyarthritis [18]. It has been demonstrated that parvovirus B19 NS1 protein trans-activates IL-6 promoter [19].
We also found significant increase of TLR4 expression in B19V-infected fibroblasts, whose transcription up-regulation has been previously observed in SSc skin [20].
In the present study, NHDFs stimulated with B19V produced significantly higher transcript levels of some genes involved in inflammasome signalling, suggesting that B19V could contribute to fibrosis by activating the inflammasome pathway, that is considered a central driver of SSc-associated fibrosis [21]. The NLRP3, caspase-1 and inflammasome-dependent proinflammatory cytokines are overexpressed in SSc skin [22]. Many viruses activate inflammasomes, including B19V [7]. Further, B19V/NS1 protein induces expression of IL-1β and IL-18 through activation of caspase-1-associated NLRP3 inflammasome in PBMCs from Still’s disease patients [23] and monocytes from SSc patients highly express the NLRP3 gene and activate caspase-1 following B19V infection [7].
Our study revealed significant upregulation of MMP12 mRNA in B19V-infected cells, according to our previous observation showing that SSc fibroblasts overexpress MMP12, which cleaves urokinase-type plasminogen activator receptor (uPAR) in human microvascular endothelial cells [24], thereby preventing its recognized pro-angiogenic role and contributing to SSc vascular desertification.
Altogether, the data suggest that B19V can activate dermal fibroblasts and may have a role in the pathogenesis of fibrosis, the hallmark of SSc. B19V-induced fibroblasts’ migration and invasiveness could be due to the B19V-associated MMP9 activation and the upregulation of MMP12 could link the B19V infection of fibroblasts to the anti-angiogenic process.
Because B19V-dependent modification of normal fibroblasts phenotype suggests a role for the virus as a cofactor to trigger fibrosis, it would, therefore, be interesting to extend our investigation on protein expression and functional role using fibroblasts from SSc patients.
Acknowledgements
The authors thank Kedrion S.p.A. (Italy) for the generous gift of B19V viremic plasma.
Funding: This work was supported by a grant from Ministero dell’Istruzione, dell’Università e della Ricerca ‘Progetti di Rilevante Interesse Nazionale (PRIN) 2015’ (grant number: 2015YZB22C-002 LS6; CUP: B16J15001970001). The funders had no role in the data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest 2007;117:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farina A, Farina AG. Fresh insights into disease etiology and the role of microbial pathogens. Curr Rheumatol Rep 2016;18:doi: 10.1007/s11926-015-0552-x. [DOI] [PubMed] [Google Scholar]
- 3. Kerr JR. The role of parvovirus B19 in the pathogenesis of autoimmunity and autoimmune disease. J Clin Pathol 2016;69:279–91. [DOI] [PubMed] [Google Scholar]
- 4. Norja P, Hokynar K, Aaltonen LM et al. Bioportfolio: lifelong persistence of variant and prototypic erythrovirus DNA genomes in human tissue. Proc Natl Acad Sci USA 2006;103:7450–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferri C, Zakrzewska K, Longombardo G et al. Parvovirus B19 infection of bone marrow in systemic sclerosis patients. Clin Exp Rheumatol 1999;17:718–20. [PubMed] [Google Scholar]
- 6. Magro CM, Nuovo G, Ferri C et al. Parvoviral infection of endothelial cells and stromal fibroblasts: a possible pathogenetic role in scleroderma. J Cutan Pathol 2004;31:43–50. [DOI] [PubMed] [Google Scholar]
- 7. Zakrzewska K, Arvia R, Torcia MG et al. Effects of parvovirus B19 in vitro infection on monocytes from patients with systemic sclerosis: enhanced inflammatory pathways by caspase-1 activation and cytokine production. J Invest Dermatol 2019;139:2125–33. [DOI] [PubMed] [Google Scholar]
- 8. Zakrzewska K, Cortivo R, Tonello C et al. Human parvovirus B19 experimental infection in human fibroblasts and endothelial cells cultures. Virus Res 2005;114:1–5. [DOI] [PubMed] [Google Scholar]
- 9. Ferri C, Giuggioli D, Sebastiani M et al. Parvovirus B19 infection of cultured skin fibroblasts from systemic sclerosis patients: comment on the article by Ray et al. Arthritis Rheum 2002;46:2262–3; author reply 2263–4. [DOI] [PubMed] [Google Scholar]
- 10. Manaresi E, Conti I, Bua G, Bonvicini F, Gallinella G. A parvovirus B19 synthetic genome: sequence features and functional competence. Virology 2017;508:54–62. [DOI] [PubMed] [Google Scholar]
- 11. Toppinen M, Norja P, Aaltonen LM et al. A new quantitative PCR for human parvovirus B19 genotypes. J Virol Methods 2015;218:40–5. [DOI] [PubMed] [Google Scholar]
- 12. Bostic JR, Brown KE, Young NS, Koenig S. Quantitative analysis of neutralising immune response to human parvovirus B1 9using a novel reverse transcriptase-polymerase chain reaction-based assay. J Infect Dis 1999;179:619–26. [DOI] [PubMed] [Google Scholar]
- 13. Pasquinelli G, Bonvicini F, Foroni L, Salfi N, Gallinella G. Placental endothelial cells can be productively infected by parvovirus B19. J Clin Virol 2009;44:33–8. [DOI] [PubMed] [Google Scholar]
- 14. Ray NB, Nieva DR, Seftor EA, Khalkhali-Ellis Z, Naides SJ. Induction of an invasive phenotype by human parvovirus B19 in normal human synovial fibroblasts. Arthritis Rheum 2001;44:1582–6. [DOI] [PubMed] [Google Scholar]
- 15. Lu J, Zhi N, Wong S, Brown KF. Activation of synoviocytes by the secreted phospholipase A2 motif in the VP1-unique region of parvovirus B19 minor capsid protein. J Infect Dis 2006;193:582–90. [DOI] [PubMed] [Google Scholar]
- 16. Shi-Wen X, Denton CP, Dashwood MR et al. Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. J Invest Dermatol 2001;116:417–25. [DOI] [PubMed] [Google Scholar]
- 17. Yamane K, Ihn H, Kubo M, Tamaki K. Increased transcriptional activities of transforming growth factor beta receptors in scleroderma fibroblasts. Arthritis Rheum 2002;46:2421–8. [DOI] [PubMed] [Google Scholar]
- 18. Wagner AD, Goronzy JJ, Matteson EL, Weyand CM. Systemic monocyte and T-cell activation in a patient with human parvovirus B19 infection. Mayo Clin Proc 1995;70:261–5. [DOI] [PubMed] [Google Scholar]
- 19. Moffatt S, Tanaka N, Tada K et al. A cytotoxic nonstructural protein, NS1, of human parvovirus B19 induces activation of interleukin-6 gene expression. J Virol 1996;70:8485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Reilly S. Toll-like receptors in systemic sclerosis: an emerging target. Immunol Lett 2018;195:2–8. [DOI] [PubMed] [Google Scholar]
- 21. Artlett CM, Sassi-Gaha S, Rieger JL et al. The inflammasome activating caspase- 1 mediates fibrosis and myofibroblast differentiation in systemic sclerosis. Arthritis Rheum 2011;63:3563–74. [DOI] [PubMed] [Google Scholar]
- 22. Martínez-Godínez MA, Cruz-Domínguez MP, Jara LJ et al. Expression of NLRP3 inflammasome, cytokines and vascular mediators in the skin of systemic sclerosis patients. IMAJ 2015;17:5–10. [PubMed] [Google Scholar]
- 23. Chen DY, Chen YM, Chen HH, Hsieh CW et al. Human parvovirus B19 nonstructural protein NS1 activates NLRP3 inflammasome signaling in adult-onset Still’s disease. Mol Med Rep 2018;17:3364–71. [DOI] [PubMed] [Google Scholar]
- 24. Serrati S, Cinelli M, Margheri F et al. Systemic sclerosis fibroblasts inhibit in vitro angiogenesis by MMP-12-dependent cleavage of the endothelial cell urokinase receptor. J Pathol 2006;210:240–8. [DOI] [PubMed] [Google Scholar]