Abstract
Objectives
To compare membranous lupus nephritis (MLN) and proliferative lupus nephritis (PLN) with respect to survival, demographic, clinical and laboratory characteristics; and to investigate predictors of renal and patient survival.
Methods
Single-centre retrospective observational study. Patients with biopsy-proven PLN, MLN and mixed lupus nephritis were included. Groups were compared using appropriate statistical tests and survival was analysed through the Kaplan-Meier method. Cox regression analysis was performed to investigate predictors of renal and patient survival.
Results
A total of 187 patients with biopsy-proven lupus nephritis (135 with PLN, 38 with MLN and 14 with mixed LN) were followed for up to 42 years (median 12 years). There was a higher proportion of MLN amongst Afro-Caribbeans than amongst Caucasians (31% vs 15%, P = 0.010). Patients with MLN had significantly lower anti-dsDNA antibodies (P = 0.001) and higher C3 levels (P = 0.018) at diagnosis. Cumulative renal survival rates at 5, 10, 15 and 20 years were 91, 81, 75 and 66% for PLN and 100, 97, 92 and 84% for MLN, respectively (P = 0.028). Cumulative patient survival at 5, 10, 15 and 20 years was 94, 86, 80 and 76%, with no difference between PLN and MLN. Urinary protein-creatinine ratio above 42 mg/mmol and eGFR below 76 ml/min/1.73 m2, one year after the diagnosis of LN, were the strongest predictors of progression to end-stage renal disease. eGFR below 77 ml/min/1.73 m2, at one year, development of end-stage renal disease and Afro-Caribbean ethnicity were associated with higher mortality.
Conclusion
Patients with MLN and PLN differ significantly regarding serological profiles and renal survival, suggesting different pathogenesis. Renal function at year one predicts renal and patient survival.
Keywords: systemic lupus erythematosus, lupus nephritis, outcomes, survival
Rheumatology key messages
We compared MLN and PLN in a large multi-ethnic cohort, with a very long follow-up.
Patients with MLN and PLN differ significantly regarding anti-dsDNA and complement levels, and renal survival.
Renal function at year one predicts renal and patient survival in LN patients.
Introduction
SLE is a complex and heterogeneous autoimmune systemic disease, which can affect multiple organs and systems. Lupus nephritis (LN) is one of its most severe manifestations. A recently published international inception cohort study demonstrated renal involvement in 38.3% of patients with SLE [1] but, depending on the populations studied, this proportion can be higher than 50% [2].
Despite the improvement in survival of patients with SLE and LN over the last 30 years, LN is associated with end-stage renal disease (ESRD) and death; with chronic kidney disease associated with a poorer quality of life [1].
LN is currently classified histopathologically according to the 2003 International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification system [3]. More than 50% of patients have proliferative LN (PLN). Membranous LN (MLN) is less frequent, accounting for 10–20% of cases [4]. In some patients, there is a combination of membranous and proliferative changes (mixed LN) [3].
There are several studies analysing the long-term outcomes of patients with PLN [5–7], MLN [8–10], and even comparing pure PLN or pure MLN with mixed LN [11–13]. However, to our knowledge there are no recent studies directly comparing the very long-term outcomes of PLN vs MLN. Our objectives were to compare patients with MLN and PLN with respect to survival, demographic, clinical and laboratory characteristics; and to investigate predictors of renal and patient survival.
Methods
Study population
All patients with LN followed at the Rheumatology Department of University College London Hospitals (UCLH) between January 1978 and December 2017 were studied. We included not only patients diagnosed at UCLH, but also those transferred from other centres. Study subjects had to fulfil ACR 1997 [14] or SLICC 2012 [15] classification criteria for SLE, with a renal biopsy showing LN class III, IV, V or a combination of these, according to the ISN/RPS 2003 classification system [3]. We did not have any exclusion criteria. We divided the patients into three groups, according to the classification of their first renal biopsy: PLN (class III and IV), MLN (class V) and mixed LN (III+V or IV+V).
This is an observational retrospective study of medical records collected over a period of over 30 years. All data were derived from normal clinical management and no patients underwent extra questionnaires or research procedures. No individualized or identifiable data are presented in this study. Therefore, ethical approval and informed consent were not required.
Data collection
Individual clinical files were reviewed in order to collect the following data:
Demographic data: year of birth, sex, ethnicity.
Clinical data: year of diagnosis of SLE, date of diagnosis of nephritis, number of renal biopsies, date of each renal biopsy, class of LN in each biopsy, development of ESRD, date of last visit, year and cause of death (if occurred), comorbidities, blood pressure (recorded as the proportion of visits when blood pressure was higher that the target of 130/80 mmHg), treatment with antimalarials, immunosuppressants and corticosteroids.
Laboratory data: ever-positive ANA, anti-dsDNA, anti-Sm, anti-RNP, anti-Ro, anti-La; antiphospholipid antibodies (anti-cardiolipin, anti-beta2glycoprotein1, lupus anticoagulant), ever-low C3; urinary protein/creatinine ratio (uPCR), serum creatinine, albumin, anti-dsDNA (ELISA) and C3 levels – all at the time of biopsy, 12 months afterwards, and at the time of last visit. The same assays were used, over time, for determination of ANA, ENA and anti-dsDNA at UCLH clinical laboratory.
We determined the estimated glomerular filtration rate (eGFR) for all patients using the CKD-EPI creatinine 2009 equation [16].
Renal survival refers to the proportion of patients without ESRD after the diagnosis of LN. ESRD corresponds to the final stage of chronic kidney disease, where renal function is very poor (eGFR < 15) and renal replacement therapy (dialysis or transplant) is necessary.
Statistical analysis
Statistical analysis was performed with IBM® SPSS® statistics version 22. For categorical variables, we compared groups using Pearson’s chi-squared test. For continuous numerical variables, we used one-way ANOVA, with Tukey’s post-hoc test (Welch’s ANOVA and Games-Howell post-hoc test were used when homogeneity of variances was not met), or Kruskal–Wallis test. A paired-t test or a Wilcoxon signed-rank test were performed to compare laboratory results for paired samples, at different time points. Patient and renal cumulative survival were analysed through the Kaplan–Meier method. Patients were censored if they were lost to follow-up or reached the end of the study. Cox regression analysis was performed to investigate predictors of shorter survival: first, each of the possible predictive variables (chosen according to the clinical question) was tested in a univariable Cox regression. For continuous numerical variables showing a significant result, we constructed receiver operating characteristic curves (Supplementary Figs S1 and S2, available at Rheumatology online) to investigate whether there was a cut-off for each variable, which could be a good predictor of the event (ESRD or death). Then we tested that cut-off in the Cox regression, to find its hazard ratio. Afterwards, the variables showing significant results were tested together, using a maximum of one covariate for each 10 events in the multivariable Cox regression model. Significance level was defined at 0.05.
Results
Patients with MLN have lower anti-dsDNA and higher C3 levels than those with PLN, at the time of biopsy
A total of 187 patients with biopsy-proven PLN (n = 135), MLN (n = 38) or mixed LN (n = 14) were included in this study. However, because of some missing laboratory data, the number of patients for certain analyses was lower (please see detailed number of patients in Table 1). Median time of follow-up was 12 years (interquartile range (IQR) = 13 years, maximum 42 years). Nineteen patients were diagnosed with LN before the age of 16. Age at diagnosis and time between the diagnosis of SLE and LN did not differ between the three groups. However, the groups differ significantly regarding ethnic distribution (Table 1). In fact, there was a significantly higher proportion of MLN amongst Afro-Caribbean patients than amongst Caucasian patients (31% vs 15%, P = 0.010).
Table 1.
Comparative description of the UCLH cohort of patients with proliferative, membranous and mixed LN
| Class III and IV | Class V | III+V or IV+V | P | ||
|---|---|---|---|---|---|
| Total, n | 135 | 38 | 14 | ||
| Females, n (%) | 123 (91) | 33 (87) | 11 (79) | 0.303 | |
| Ethnicity | Caucasian, n (%) | 67 (50) | 12 (32) | 3 (21) | 0.037 a |
| Afro-Caribbean, n (%) | 34 (25) | 18 (47) | 6 (43) | ||
| Asian, n (%) | 34 (25) | 8 (21) | 5 (36) | ||
| Age LN diagnosis (y), mean (S.d.) | 29 (12) | 29 (14) | 26 (8) | 0.581 | |
| Time SLE-LN (y), median (IQR) | 1 (4) | 1 (6) | 0 (7) | 0.842 | |
| uPCR at LN diagnosis, median (IQR) | 212 (411) | 332 (346) | 126 (209) | 0.370 | |
| n = 70 | n = 26 | n = 8 | |||
| Nephrotic-range proteinuria, n (%) | 27 (39) | 14 (54) | 1 (13) | 0.098 | |
| n = 70 | n = 26 | n = 8 | |||
| Creatinine at LN diagnosis, median (IQR) | 81 (37) | 63 (28) | 70 (55) | 0.009 b | |
| n = 69 | n = 25 | n = 10 | |||
| eGFR at LN diagnosis, mean (S.d.) | 95 (35) | 120 (34) | 113 (40) | 0.007 c | |
| n = 69 | n = 25 | n = 10 | |||
| Albumin at LN diagnosis, mean (S.d.) | 31 (7) | 30 (6) | 32 (7) | 0.647 | |
| n = 63 | n = 21 | n = 10 | |||
| C3 at LN diagnosis, mean (S.d.) | 0.62 (0.24) | 0.89 (0.37) | 0.66 (0.24) | 0.018 d | |
| n = 72 | n = 23 | n = 9 | |||
| Anti-dsDNA at LN diagnosis, median (IQR) | 488 (1541) | 81 (129) | 292 (206) | 0.001 e | |
| n = 72 | n = 21 | n = 8 | |||
| > 1 renal biopsy, n (%) | 29 (22) | 10 (26) | 6 (43) | 0.192 | |
| Different class in subs. biopsy, n (%) | 6 (21) | 5 (50) | 0 (0) | 0.058 | |
| Ever low C3, n (%) | 107 (80) | 35 (92) | 11 (79) | 0.203 | |
| Ever anti-dsDNA positive, n (%) | 111 (83) | 32 (84) | 12 (86) | 0.950 | |
| Ever anti-Sm positive, n (%) | 25 (19) | 16 (42) | 6 (43) | 0.004 f | |
| Ever anti-Ro positive, n (%) | 54 (40) | 16 (42) | 10 (71) | 0.081 | |
| Ever anti-La positive, n (%) | 21 (16) | 3 (8) | 4 (29) | 0.168 | |
| Ever anti-RNP positive, n (%) | 42 (31) | 19 (50) | 8 (57) | 0.030 g | |
| Use of antimalarials, n (%) | 82 (66) | 27 (73) | 9 (69) | 0.732 | |
| Use of immunosupressants, n (%) | 121 (95) | 35 (95) | 14 (100) | 0.669 | |
| Use of steroids, n (%) | 125 (97) | 36 (95) | 13 (93) | 0.668 | |
| ESRD – total, n (%) | 34 (25) | 3 (8) | 2 (14) | 0.056 | |
| ESRD – subgroup transplant, n (%) | 18 (53) | 1 (33) | 1 (50) | 0.126 | |
| Deaths, n (%) | 30 (22) | 4 (11) | 1 (7) | 0.135 | |
Note: There were missing data for some variables, therefore, we present the number of patients analysed for each group below the statistics for those variables.
For variables with a statistically significant difference between groups, values are presented in bold.
Ethnicity: chi-squared test: P =0.028 when comparing class III and IV vs V; P =0.525 for class V vs mixed; P =0.125 for class III and IV vs mixed.
Creatinine at LN diagnosis: Kruskal–Wallis test was used to compare the three groups; Mann–Whitney’s test was used to compare the groups in pairs: P =0.002 when comparing class III and IV vs V; P =0.270 for class V vs mixed; P =0.484 for class III and IV vs mixed.
eGFR at LN diagnosis: one-way ANOVA with Tukey HSD post hoc test: P =0.008 when comparing class III and IV vs V; P =0.850 for class V vs mixed; P =0.289 for class III and IV vs mixed.
C3 at LN diagnosis: Welch ANOVA with Games-Howell post hoc test: P =0.009 when comparing class III and IV vs V; P =0.124 for class V vs mixed; P =0.903 for class III and IV vs mixed.
Anti-dsDNA at LN diagnosis: Kruskal–Wallis test was used to compare the three groups; Mann–Whitney’s test was used to compare the groups in pairs: P <0.001 when comparing class III and IV vs V; P =0.002 for class V vs mixed; P =0.558 for class III and IV vs mixed.
Ever anti-Sm positive: chi-squared test: P =0.003 when comparing class III and IV vs V; P =0.961 for class V vs mixed; P =0.034 for class III and IV vs mixed.
Ever anti-RNP positive: chi-squared test: P =0.034 when comparing class III and IV vs V; P =0.647 for class V vs mixed; P =0.052 for class III and IV vs mixed.
eGFR: estimated glomerular filtration rate, ml/min/1.73 m2; ESRD: end-stage renal disease; IQR: interquartile range; LN: lupus nephritis; UCLH: University College London Hospitals; uPCR: urinary protein-creatinine ratio, mg/mmol; y: years. Creatinine is presented in μmol/l, albumin in g/l and C3 in g/l.
At the time of diagnosis of LN, mean eGFR was significantly lower in patients with PLN than MLN or mixed LN (95 vs 120 vs 113 ml/min/1.73 m2 respectively, P = 0.007). There was no significant difference between groups regarding levels of uPCR or serum albumin, as all three groups had high levels of proteinuria and serum hypoalbuminemia (Table 1).
At the time of biopsy, mean C3 levels were significantly lower in the PLN (0.62 (S.D. 0.24) g/l) and mixed LN (0.66 (S.D.0.24) g/l) groups than the MLN group (0.89 (S.D. 0.37) g/l) (P = 0.018 for difference between groups). In fact, patients with MLN had near normal C3 levels (lower limit of normal at the UCLH laboratory =0.90 g/l). They also had near normal anti-dsDNA levels (median 81 IU/l, IQR 129; upper limit of normal at UCLH =50 IU/l) which were significantly lower than in those with PLN (median 488, IQR 1541) and mixed histology (median 292, IQR 206) (P = 0.001 for difference between groups).
Conversely, the groups do not differ in the proportion of patients with ever-positivity for anti-dsDNA, anti-Ro and anti-La antibodies, as well as in the proportion of patients with ever-low C3 levels. However, the proportion of patients ever-positive for anti-Sm and anti-RNP antibodies was lower in patients with PLN. This was not surprising given that this group had a higher proportion of Caucasians, who, in turn, had a significantly lower proportion of anti-Sm (14% vs 38% in Afro-Caribbeans and 30% in Asians; P = 0.004) and anti-RNP antibodies (21% vs 53% in Afro-Caribbeans and 45% in Asians; P < 0.001).
The proportion of patients ever treated with antimalarials, steroids or immunosuppressants did not differ between groups. There was also no difference regarding the proportion of patients treated with each immunosuppressant drug: cyclophosphamide (49.5%, 45.9% and 35.7% for PLN, MLN and mixed LN, respectively; P = 0.607), mycophenolate mofetil (57%, 59.5% and 78.6%; P = 0.297), calcineurin inhibitors (7.8%, 10.8% and 14.3%; P = 0.654), azathioprine (66.1%, 70.3% and 78.6%; P = 0.605) and B cell-depleting therapy using rituximab (42.2%, 39.5% and 57.1%; P = 0.505).
One year after the diagnosis of LN, uPCR decreased significantly and serum albumin increased significantly in patients with both PLN and MLN, but not mixed LN (Table 2). At this time point, levels of C3 increased and levels of anti-dsDNA decreased significantly in patients with PLN and mixed LN, but these changes were not significant in patients with MLN (who had near-normal levels to start with).
Table 2.
Evolution of laboratory parameters for each group of LN patients
| LN diagnosis | 12 months after diagnosis | P (diagnosis vs 12 M) | 7 years after diagnosisa | Last visita | P (diagnosis vs LV) | |
|---|---|---|---|---|---|---|
| Class III and IV | n = 70 | n = 69 | n = 70 | n = 74 | ||
| uPCR, median (IQR) | 212 (411) | 42 (116) | <0.001 | 21 (39) | 0.242 | |
| Creatinine, median (IQR) | 81 (37) | 75 (30) | 0.016 | 71 (27) | 72 (34) | 0.312 |
| eGFR, mean (S.d.) | 95 (35) | 98 (33) | 0.124 | 96 (30) | 87 (33) | 0.081 |
| Albumin, mean (S.d.) | 31 (7) | 39 (6) | <0.001 | 42 (5) | <0.001 | |
| C3, mean (S.d.) | 0.62 (0.24) | 0.85 (0.29) | <0.001 | 0.99 (0.22) | <0.001 | |
| Anti-dsDNA, median (IQR) | 488 (1541) | 91 (299) | <0.001 | 17 (80) | <0.001 | |
| Class V | n = 26 | n = 20 | n = 24 | n = 31 | ||
| uPCR, median (IQR) | 332 (346) | 39 (76) | <0.001 | 13 (82) | 0.266 | |
| Creatinine, median (IQR) | 63 (28) | 67 (29) | 0.559 | 70 (24) | 65 (30) | 0.523 |
| eGFR, mean (S.d.) | 120 (34) | 115 (28) | 0.559 | 108 (30) | 97 (26) | 0.001 |
| Albumin, mean (S.d.) | 30 (6) | 38 (5) | 0.001 | 40 (5) | <0.001 | |
| C3, mean (S.d.) | 0.89 (0.37) | 0.92 (0.35) | 0.708 | 1.02 (0.26) | 0.189 | |
| Anti-dsDNA, median (IQR) | 81 (129) | 34 (79) | 0.103 | 27 (109) | 0.616 | |
| Class III+V or IV+V | n = 8 | n = 7 | n = 6 | n = 9 | ||
| uPCR, median (IQR) | 126 (209) | 148 (262) | 0.893 | 79 (154) | 0.686 | |
| Creatinine, median (IQR) | 70 (55) | 69 (21) | 0.889 | 65 (13) | 70 (11) | 0.767 |
| eGFR, mean (S.d.) | 131 (38) | 117 (29) | 0.683 | 112 (21) | 104 (23) | 0.620 |
| Albumin, mean (S.d.) | 32 (7) | 35 (9) | 0.362 | 35 (14) | 0.574 | |
| C3, mean (S.d.) | 0.66 (0.24) | 0.78 (0.27) | 0.020 | 0.86 (0.32) | 0.089 | |
| Anti-dsDNA, median (IQR) | 292 (206) | 136 (155) | 0.046 | 204 (368) | 0.161 |
Note: There were missing data for these variables, therefore, the number of patients analysed for each group does not correspond to the total number of patients.
For variables with a statistically significant difference between time points, p values are presented in bold.
For creatinine levels and eGFR at 7 years and at the last visit, patients with ESRD were excluded from this analysis, as they constituted significant outliers.
12 M: 12 months after diagnosis of LN; eGFR: estimated glomerular filtration rate; IQR: interquartile range; LN: lupus nephritis; LV: last visit; uPCR: urinary protein-creatinine ratio.
Progression to ESRD is faster in patients with PLN and is associated with Afro-Caribbean ethnicity, not taking antimalarials, and eGFR and uPCR at diagnosis and at one year
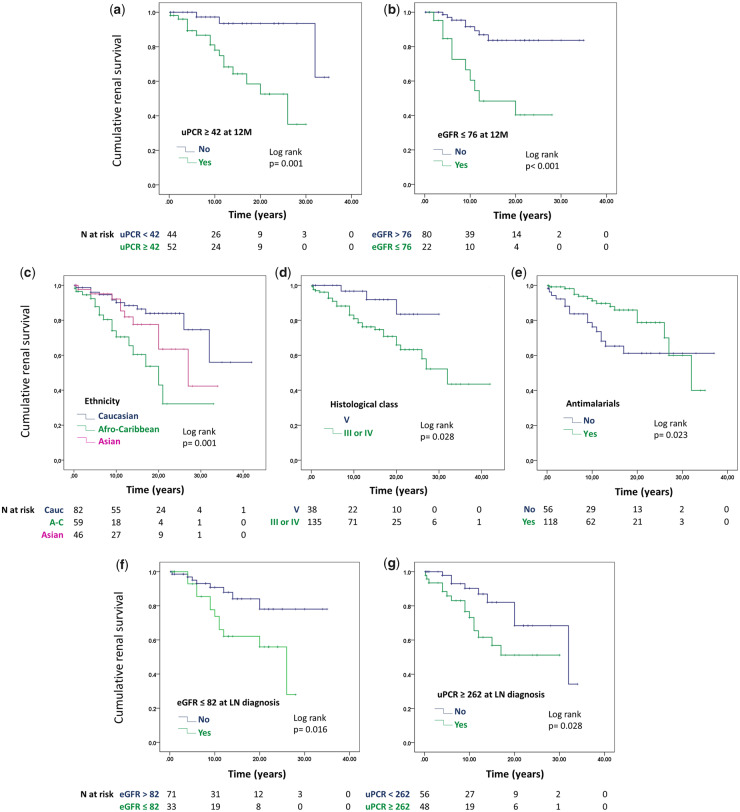
During the time of follow-up, 39 patients progressed to ESRD and 20 of these had a renal transplant (Table 1). Cumulative renal survival rates at five, 10, 15 and 20 years were 91, 81, 75 and 66%, respectively, for proliferative LN, and 100, 97, 92 and 84% for membranous LN, with a significant difference between these two groups (P = 0.028).
Having uPCR above 42 mg/mmol or eGFR below 76 ml/min/1.73 m2, one year after the diagnosis of LN, were the strongest predictors of progression to ESRD, in univariable analysis, with hazard ratios (HR) of eight-fold and five-fold, respectively (Table 3). HR for uPCR and eGFR at the time of diagnosis were considerably smaller (2.5 and 2.8 respectively). Other factors associated with increased risk of ESRD were Afro-Caribbean ethnicity (HR = 3.9), PLN (HR = 3.4), not having taken antimalarials (HR = 2.1) and poorly controlled diastolic blood pressure. The effect of uPCR and eGFR at one year remained significant after adjusting for ethnicity, histological class, uPCR and eGFR at the time of diagnosis, the use of antimalarials and diastolic blood pressure (Table 4). eGFR at the time of diagnosis and one year afterwards are strongly correlated (r = 0.778, P < 0.001).
Table 3.
Hazard ratios (HR) for possible predictors of ESRD and mortality, identified by univariable regression Cox analysis
| Univariable Cox Regression | HR [95% CI] | P |
|---|---|---|
| Predictors of ESRD | ||
| uPCR ≥ 42 at 12 M | 8.081 [1.856, 35.179] | 0.005 |
| eGFR ≤ 76 at 12 M | 4.985 [1.964, 12.651] | 0.001 |
| Ethnicity (AC) | 3.861 [1.817, 8.206] | <0.001 |
| Histological class (III or IV) | 3.423 [1.049, 11.173] | 0.041 |
| eGFR ≤ 82 at LN diagnosis | 2.833 [1.156, 6.945] | 0.023 |
| uPCR ≥ 262 at LN diagnosis | 2.508 [1.062, 5.922] | 0.036 |
| No antimalarials | 2.180 [1.089, 4.363] | 0.028 |
| % Diastolic BP > 80 mmHg | 1.016 [1.001, 1.030] | 0.032 |
| Predictors of mortality | ||
| eGFR ≤ 77 at 12 M | 6.591 [2.252, 19.294] | 0.001 |
| No antimalarials | 3.799 [1.820, 7.929] | <0.001 |
| No steroids | 3.719 [1.119, 12.359] | 0.032 |
| ESRD | 3.299 [1.694, 6.424] | <0.001 |
| UPCR ≥ 67 at 12 M | 3.102 [1.039, 9.266] | 0.043 |
| Age LN diagnosis > 33 | 2.278 [1.162, 4.465] | 0.016 |
| Ethnicity (AC) | 2.241 [1.053, 4.770] | 0.036 |
| Year LN diagnosis | 0.959 [0.924, 0.996] | 0.032 |
| % Diastolic BP > 80 mmHg | 1.022 [1.005, 1.039] | 0.009 |
See Supplementary Tables S1 and S2, available at Rheumatology online, for full list of variables tested and respective HR.
BP: blood pressure; eGFR: estimated glomerular filtration rate, ml/min/1.73 m2; uPCR: urinary protein-creatinine ratio, mg/mmol.
Table 4.
HR for predictors of ESRD and mortality (variables analysed in pairs with Cox regression)
| Cox regression | HR [95% CI] | P |
|---|---|---|
| Predictors of ESRD | ||
| uPCR ≥ 42 at 12 M | 6.365 [1.431, 28.321] | 0.015 |
| eGFR ≤ 76 at 12 M | 8.041 [2.831, 22.834] | <0.001 |
| uPCR ≥ 42 at 12 M | 8.027 [1.820, 35.401] | 0.006 |
| Ethnicity (AC) | 1.200 [0.361, 3.989] | 0.766 |
| eGFR ≤ 76 at 12 M | 6.800 [2.490, 18.572] | <0.001 |
| Ethnicity (AC) | 4.138 [1.235, 13.870] | 0.021 |
| uPCR ≥ 42 at 12 M | 9.229 [2.104, 40.481] | 0.003 |
| Histological class (III or IV) | 286141.61 [<0.001-7.149E+187] | 0.953 |
| eGFR ≤ 76 at 12 M | 4.300 [1.633, 11.321] | 0.003 |
| Histological class (III or IV) | 233853.34 [<0.001-1.219E+192] | 0.955 |
| uPCR ≥ 42 at 12 M | 5.204 [1.169, 23.163] | 0.030 |
| eGFR ≤ 82 at LN diagnosis | 4.660 [1.476, 14.713] | 0.009 |
| uPCR ≥ 42 at 12 M | 5.489 [1.226, 24.574] | 0.026 |
| uPCR ≥ 262 at LN diagnosis | 2.739 [0.939, 7.992] | 0.065 |
| eGFR ≤ 76 at 12 M | 4.030 [1.548, 10.495] | 0.004 |
| uPCR ≥ 262 at LN diagnosis | 2.195 [0.826, 5.836] | 0.115 |
| uPCR ≥ 42 at 12 M | 7.119 [1.615, 31.385] | 0.010 |
| No antimalarials | 3.828 [1.360, 10.778] | 0.011 |
| eGFR ≤ 76 at 12 M | 3.659 [1.337, 10.011] | 0.012 |
| No antimalarials | 2.382 [0.857, 6.621] | 0.096 |
| uPCR ≥ 42 at 12 M | 5.051 [1.097, 23.349] | 0.038 |
| % Diastolic BP > 80 mmHg | 1.010 [0.0990, 1.030] | 0.347 |
| eGFR ≤ 76 at 12 M | 3.554 [1.214, 10.407] | 0.021 |
| % Diastolic BP > 80 mmHg | 1.012 [0.992, 1.032] | 0.239 |
| Predictors of mortality | ||
| eGFR ≤ 77 at 12 M | 5.288 [1.603, 17.447] | 0.006 |
| No antimalarials | 1.514 [0476, 4.813] | 0.482 |
| eGFR ≤ 77 at 12 M | 6.040 [1.995, 18.283] | 0.001 |
| No steroids | 2.420 [0.525, 11.159] | 0.257 |
| eGFR ≤ 77 at 12 M | 3.543 [1.120, 11.210] | 0.031 |
| ESRD | 5.807 [1.817, 18.555] | 0.003 |
| eGFR ≤ 77 at 12 M | 9.242 [2.418, 35.319] | 0.001 |
| UPCR ≥ 67 at 12 M | 2.119 [0.611, 7.354] | 0.237 |
| eGFR ≤ 77 at 12 M | 14.927 [4.053, 54.982] | <0.001 |
| Ethnicity | 11.506 [2.669, 49.598] | 0.001 |
| eGFR ≤ 77 at 12 M | 5.440 [1.770, 16.719] | 0.003 |
| Age LN diagnosis > 33 | 1.887 [0.638, 5.584] | 0.251 |
| eGFR ≤ 77 at 12 M | 6.866 [2.290, 20.581] | 0.001 |
| Year LN diagnosis | 1.014 [0.938, 1.096] | 0.723 |
| eGFR ≤ 77 at 12 M | 4.665 [1.308, 16.641] | 0.018 |
| % Diastolic BP > 80 mmHg | 1.013 [0.989, 1.037] | 0.288 |
Note: Due to small number of events (ESRD and death), multivariable Cox regression analysis was limited to two covariates. Therefore, instead of including all the eight or nine explicative variables at the same time in a regression model predicting ESRD or death, respectively, we included only a pair of variables at a time. Each possible explicative variable was tested against the variables with the highest HR on univariable analysis (uPCR and eGFR at one year in the case of ESRD, and eGFR at one year in the case of mortality).
Variables that keep statistical significance in the multivariable model are presented in bold.
eGFR: estimated glomerular filtration rate, ml/min/1.73 m2; uPCR: urinary protein-creatinine ratio, mg/mmol.
Fig. 1 shows Kaplan–Meier curves demonstrating the effect of each of the factors shown in Table 3 on development of ESRD over time. All the graphs show significant separation of the curves. With respect to the main aim of this paper, Fig. 1d shows that patients with MLN had significantly better renal survival than those with PLN, although this significance was lost in the multivariable analysis due to the much stronger predictive effect of uPCR and eGFR (Table 4).
Fig. 1.
Kaplan–Meier curves showing cumulative renal survival for different groups of patients with membranous and proliferative LN
At the last follow-up visit, excluding the patients who developed ESRD, there were three patients (8%) with MLN and 18 patients (13%) with PLN with an eGFR below 60 ml/min/1.73 m2.
Mortality does not differ between patients with MLN and PLN but is associated with Afro-Caribbean ethnicity, ESRD and eGFR at one year
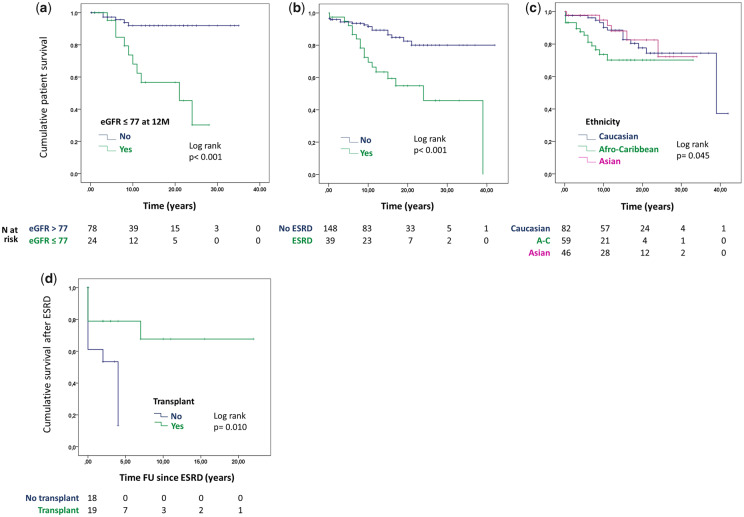
Thirty-five of the 187 patients died. The main causes of death were infection (n = 11) and cardiovascular (n = 11), followed by cancer (n = 7) and other causes (n = 5). For one patient, the cause of death was uncertain. Cumulative survival figures at five, 10, 15 and 20 years are 94, 86, 80 and 76%, respectively.
Table 3 shows that a number of variables were associated with increased mortality on univariable analysis, with eGFR below 77 ml/min/1.73 m2, one year after the diagnosis of LN, being associated with the highest HR (6.6) for death. After adjusting for eGFR at one year, only Afro-Caribbean ethnicity and ESRD were independently associated with mortality (Table 4). Fig. 2a–c represents the Kaplan–Meier survival curves showing the significant effects of these three factors. Of note, there was no significant difference in survival between patients with MLN and PLN on Kaplan–Meier analysis (log rank P = 0.122). Amongst patients with ESRD, there was a significant difference in survival when comparing those who received a transplant with those who stayed on dialysis (Fig. 2d).
Fig. 2.
Kaplan–Meier curves showing cumulative patient survival for different groups of patients with MLN and PLN
Discussion
Our results suggest that patients with MLN differ significantly from patients with PLN regarding ethnic background, serologic profiles (anti-dsDNA and C3 levels at the time of diagnosis) and renal survival. We identified uPCR above 42 mg/mmol and eGFR below 76 ml/min/1.73 m2, one year after the diagnosis of LN, as the strongest predictors of progression to ESRD. An eGFR below 77 ml/min/1.73 m2 one year after the diagnosis of LN, development of ESRD and Afro-Caribbean ethnicity are associated with higher mortality.
Active SLE is often characterized by low complement and raised anti-dsDNA levels, and there were striking differences in these parameters between the membranous and proliferative groups. Other authors have described the differences we found with respect to anti-dsDNA and complement levels at the time of diagnosis. In fact, it is generally accepted that patients with pure MLN can present with normal complement levels and negative anti-dsDNA binding [17, 18]. However, this is the first study directly comparing the actual levels of these serological markers in a large multi-ethnic cohort of patients with MLN or PLN.
The higher proportion of positive anti-Sm and anti-RNP antibodies in patients with MLN and mixed LN probably reflects the higher proportion of Afro-Caribbeans in these two groups. Other studies have shown these antibodies to be more prevalent in Afro-Caribbean patients [19]. It is accepted that the overall prevalence of LN is higher in people of African ancestry, Hispanics and Asians, compared with Caucasians [20]. An epidemiologic study published in 2006, aiming to investigate the prevalence and incidence of biopsy-proven LN in the north-west of England, estimated that the proportion of SLE patients with LN was 10% in Caucasian patients, 27% in Indo-Asian, and 58% in Afro-Caribbean patients [21]. However, no previous studies have shown a difference in ethnic background between patients with PLN and MLN.
Renal survival and mortality rates in our patients are similar to most other cohorts [22], as are the main causes of death [23–26]. In contrast, a Japanese study with 186 LN patients showed lower ESRD and mortality rates; however, more than one-third of the patients included had mesangial LN (class I and II), which is associated with a significantly better prognosis and does not require the same treatment as class III, IV or V [27].
It is important to note that five of our 38 patients classified as MLN on the first renal biopsy developed proliferative changes on a subsequent biopsy (three patients had class IV and two patients had mixed LN), carried out due to relapse of proteinuria. This included two of the only three MLN patients who developed ESRD. Therefore, whereas we are confident that the prognostic predictors for ESRD shown in Fig. 1 are true for PLN, we do not have enough data to confirm their validity for pure MLN. However, a recent retrospective multicentre study by Silva-Fernandez et al. analysed the outcome of 150 patients with pure MLN from Spain and the US (65% Caucasians), with a mean follow up of 7.6 years [8]. By the end of follow-up, eight patients (5.3%) had developed ESRD and nine patients (6%) had died. Predictors of ESRD and death were only investigated with univariable logistic regression analysis due to the small number of events. As in our study, the authors found high proteinuria, high serum creatinine and low creatinine clearance at time of diagnosis to be associated with ESRD. Other parameters associated with ESRD were male sex, hypertension and dyslipidaemia. Male sex was also identified in some other studies as a predictor of worse prognosis [28–30]. In our study, which included 20 males, the Cox regression analysis did not show a significant effect of sex on renal or patient survival (Supplementary Tables S1 and S2, available at Rheumatology online).
Predictors of death in the study by Silva-Fernandez et al. [8] included age, haemodialysis and not having received mycophenolate mofetil or antimalarials. We found both age at diagnosis and the absence of treatment with antimalarials to be associated with death, on univariable analysis. However, the effect was not significant after adjusting for eGFR. Several other studies mention the protective role of antimalarials in patients with LN [8, 31]. We did not analyse the effect of each individual immunosuppressant separately, but did not find a significant effect from the use of immunosuppressants as a whole. Our patients on dialysis also had poorer survival than those who received a transplant, and this has been already shown by other studies [32, 33]. ESRD in general has been found to predict higher mortality in patients with LN [34].
Although most studies suggest baseline proteinuria and serum creatinine as predictors of prognosis [8, 28–31], several other studies, like ours, support the importance of renal parameters at 12 months [6, 35, 36]. Dall’Era et al. looking at long-term outcomes in 76 patients of the Euro-Lupus Nephritis Cohort, found proteinuria below 0.8 g/day, 12 months after beginning treatment, to be the single best predictor of good long-term renal function (sensitivity 81% and specificity 78%) [6]. Similarly, in 90 patients from the MAINTAIN Nephritis Trial, a cut-off of 0.7 g/day for proteinuria was the best predictor of renal outcome (sensitivity 71%, specificity 75%) [36]. Furthermore, a Japanese study with 81 patients followed for a median period of 4.25 years showed that achieving a complete renal remission (uPCR < 50 mg/mmol and a normal or near normal eGFR) at 12 months after induction therapy was associated with a higher flare-free rate [35].
Different studies showed that sustained renal remission is associated with a better prognosis in LN [37], notably with reduced progression to chronic kidney disease, ESRD and mortality [38]. A Korean study suggested that, in diffuse PLN, remission of proteinuria is an independent prognostic marker associated with improved renal and patient survival, regardless of the time between the biopsy and the normalization of proteinuria or the recurrence of proteinuria after remission [7].
Alarcón and collaborators, in the USA, showed that while outcomes were poorer in ethnic minority groups, poverty, rather than ethnicity, was independently associated with mortality of SLE patients [20, 39]. In the UK, where access to the health system is free, socioeconomic factors should play a less preponderant role, as suggested by a 25-year follow up of our cohort [40]. A further formal study, assessing socioeconomic factors such as education, employment status and others, would help to answer this question definitively.
One of the main limitations of our study is the high number (44%) of patients with some missing laboratory data at the time of the renal biopsy. These patients could not be included in the Cox regression analyses investigating the effect of those specific laboratory parameters on ESRD and death. However, patients included in the analyses do not differ significantly from those with missing data regarding sex, age of diagnosis, class of LN, treatment received, ESRD and deaths (Supplementary Table S3, available at Rheumatology online).
We believe that the main strengths of our study relate to our large multi-ethnic cohort with a very long period of follow-up, often exceeding 30 years. We used survival analysis to investigate predictors of ESRD and death, which, as opposed to linear or logistic regression, takes into account the time when the event occurred rather than only whether it occurred or not. Furthermore, this kind of analysis considers the subjects in whom the event did not occur during the time of follow-up (censored); therefore, the risk of bias due to underestimating the occurrence of the event is lower.
In conclusion, patients with MLN and PLN differ significantly regarding serological profiles and renal survival, suggesting different pathogenesis. Notably, anti-dsDNA antibodies, which have been associated with the pathogenesis of PLN, may not have a preponderant role in MLN. Antigen discovery studies using proteomic techniques, for instance, would be important to investigate the antigens and antibodies involved in LN. Studies with other multi-ethnic cohorts would be important to confirm our finding of a higher proportion of MLN amongst patients with African ancestry. This might represent a different genetic susceptibility associated with MLN. Proteinuria and renal function at year one appear to be the best predictors of progression to ESRD. Renal function at year one, ESRD and ethnicity are associated with mortality.
Supplementary Material
Acknowledgements
This work was carried out at a centre supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Funding: F.F. and T.M. were funded by LUPUS UK (Grants 1543411 and 1579346). T.M. was supported by the Medical Research Council (Grant MR/P017371/1).
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Hanly JG, O’Keeffe AG, Su L et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology 2016;55:252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ortega LM, Schultz DR, Lenz O, Pardo V, Contreras GN. Review: lupus nephritis: pathologic features, epidemiology and a guide to therapeutic decisions. Lupus 2010;19:557–74. [DOI] [PubMed] [Google Scholar]
- 3. Weening JJ, D’Agati VD, Schwartz MM et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 2004;15:241–50. [DOI] [PubMed] [Google Scholar]
- 4. Austin HA III, Illei GG, Balow JE. Lupus membranous nephropathy In: Lewis EJ, Schwartz MM, Korbet SM, Chan DTM, eds. Lupus nephritis. 2nd edn. Oxford: Oxford University Press, 2011: 169–98. [Google Scholar]
- 5. Hanaoka H, Iida H, Kiyokawa T, Takakuwa Y, Kawahata K. Early achievement of deep remission predicts low incidence of renal flare in lupus nephritis class III or IV. Arthritis Res Ther 2018;20:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dall’Era M, Cisternas MG, Smilek DE et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus Nephritis cohort. Arthritis Rheumatol 2015;67:1305–13. [DOI] [PubMed] [Google Scholar]
- 7. Koo HS, Kim S, Chin HJ. Remission of proteinuria indicates good prognosis in patients with diffuse proliferative lupus nephritis. Lupus 2016;25:3–11. [DOI] [PubMed] [Google Scholar]
- 8. Silva-Fernandez L, Oton T, Askanase A et al. Pure membranous lupus nephritis: description of a cohort of 150 patients and review of the literature. Reumatol Clin 2019;15:34–42. [DOI] [PubMed] [Google Scholar]
- 9. Mejia-Vilet JM, Cordova-Sanchez BM, Uribe-Uribe NO, Correa-Rotter R. Immunosuppressive treatment for pure membranous lupus nephropathy in a Hispanic population. Clin Rheumatol 2016;35:2219–27. [DOI] [PubMed] [Google Scholar]
- 10. Pastén V R, Massardo V L, Rosenberg G H et al. Curso clínico de la nefropatía membranosa lúpica pura. Rev Méd Chile 2005;133:23–32. [DOI] [PubMed] [Google Scholar]
- 11. Ikeuchi H, Hiromura K, Kayakabe K et al. Renal outcomes in mixed proliferative and membranous lupus nephritis (Class III/IV + V): a long-term observational study. Mod Rheumatol 2016;26:908–13. [DOI] [PubMed] [Google Scholar]
- 12. Moroni G, Quaglini S, Gravellone L et al. Membranous nephropathy in systemic lupus erythematosus: long-term outcome and prognostic factors of 103 patients. Semin Arthritis Rheum 2012;41:642–51. [DOI] [PubMed] [Google Scholar]
- 13. Ilori TO, Enofe N, Oommen A et al. Comparison of outcomes between individuals with pure and mixed lupus nephritis: a retrospective study. PLoS One 2016;11:e0157485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 15. Petri M, Orbai AM, Alarcon GS et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mok CC. Membranous nephropathy in systemic lupus erythematosus: a therapeutic enigma. Nat Rev Nephrol 2009;5:212–20. [DOI] [PubMed] [Google Scholar]
- 18. Austin HA, Illei GG. Membranous lupus nephritis. Lupus 2005;14:65–71. [DOI] [PubMed] [Google Scholar]
- 19. Arroyo-Avila M, Santiago-Casas Y, McGwin G Jr et al. Clinical associations of anti-Smith antibodies in PROFILE: a multi-ethnic lupus cohort. Clin Rheumatol 2015;34:1217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alarcón GS. Multiethnic lupus cohorts: what have they taught us? Reumatol Clin 2011;7:3–6. [DOI] [PubMed] [Google Scholar]
- 21. Patel M, Clarke AM, Bruce IN, Symmons DP. The prevalence and incidence of biopsy-proven lupus nephritis in the UK: evidence of an ethnic gradient. Arthritis Rheum 2006;54:2963–9. [DOI] [PubMed] [Google Scholar]
- 22. Tektonidou MG, Dasgupta A, Ward MM. Risk of end-stage renal disease in patients with lupus nephritis, 1971-2015: a systematic review and Bayesian meta-analysis. Arthritis Rheumatol 2016;68:1432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tselios K, Gladman DD, Sheane BJ, Su J, Urowitz M. All-cause, cause-specific and age-specific standardised mortality ratios of patients with systemic lupus erythematosus in Ontario, Canada over 43 years (1971-2013. Ann Rheum Dis 2019;78:802–806. [DOI] [PubMed] [Google Scholar]
- 24. Lerang K, Gilboe IM, Steinar Thelle D, Gran JT. Mortality and years of potential life loss in systemic lupus erythematosus: a population-based cohort study. Lupus 2014;23:1546–52. [DOI] [PubMed] [Google Scholar]
- 25. Yurkovich M, Vostretsova K, Chen W, Avina-Zubieta JA. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res 2014;66:608–16. [DOI] [PubMed] [Google Scholar]
- 26. Bernatsky S, Boivin JF, Joseph L et al. Mortality in systemic lupus erythematosus. Arthritis Rheum 2006;54:2550–7. [DOI] [PubMed] [Google Scholar]
- 27. Kono M, Yasuda S, Kato M et al. Long-term outcome in Japanese patients with lupus nephritis. Lupus 2014;23:1124–32. [DOI] [PubMed] [Google Scholar]
- 28. Yang J, Liang D, Zhang H et al. Long-term renal outcomes in a cohort of 1814 Chinese patients with biopsy-proven lupus nephritis. Lupus 2015;24:1468–78. [DOI] [PubMed] [Google Scholar]
- 29. Moroni G, Vercelloni PG, Quaglini S et al. Changing patterns in clinical-histological presentation and renal outcome over the last five decades in a cohort of 499 patients with lupus nephritis. Ann Rheum Dis 2018;77:1318–25. [DOI] [PubMed] [Google Scholar]
- 30. Siso A, Ramos-Casals M, Bove A et al. Outcomes in biopsy-proven lupus nephritis: evaluation of 190 white patients from a single center. Medicine 2010;89:300–7. [DOI] [PubMed] [Google Scholar]
- 31. Zheng ZH, Zhang LJ, Liu WX et al. Predictors of survival in Chinese patients with lupus nephritis. Lupus 2012;21:1049–56. [DOI] [PubMed] [Google Scholar]
- 32. Sabucedo AJ, Contreras G. ESKD, transplantation, and dialysis in lupus nephritis. Semin Nephrol 2015;35:500–8. [DOI] [PubMed] [Google Scholar]
- 33. Tsai WT, Chang HC, Wang CT, Chiang BL, Lin YT. Long-term outcomes in lupus patients receiving different renal replacement therapy. J Microbiol Immunol Infect 2019;52:648–53. [DOI] [PubMed] [Google Scholar]
- 34. Galindo-Izquierdo M, Rodriguez-Almaraz E, Pego-Reigosa JM et al. Characterization of patients with lupus nephritis included in a large cohort from the Spanish Society of Rheumatology Registry of Patients With Systemic Lupus Erythematosus (RELESSER). Medicine 2016;95:e2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ichinose K, Kitamura M, Sato S et al. Complete renal response at 12 months after induction therapy is associated with renal relapse-free rate in lupus nephritis: a single-center, retrospective cohort study. Lupus 2019;28:501–9. [DOI] [PubMed] [Google Scholar]
- 36. Tamirou F, Lauwerys BR, Dall'Era M et al. A proteinuria cut-off level of 0.7 g/day after 12 months of treatment best predicts long-term renal outcome in lupus nephritis: data from the MAINTAIN Nephritis Trial. Lupus Sci Med 2015;2:e000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernandes das Neves M, Irlapati RV, Isenberg D. Assessment of long-term remission in lupus nephritis patients: a retrospective analysis over 30 years. Rheumatology 2015;54:1403–7. [DOI] [PubMed] [Google Scholar]
- 38. Pakchotanon R, Gladman DD, Su J, Urowitz MB. Sustained complete renal remission is a predictor of reduced mortality, chronic kidney disease and end-stage renal disease in lupus nephritis. Lupus 2018;27:468–74. [DOI] [PubMed] [Google Scholar]
- 39. Duran S, Apte M, Alarcon GS. Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc 2007;99:1196–8. [PMC free article] [PubMed] [Google Scholar]
- 40. Adler M, Chambers S, Edwards C, Neild G, Isenberg D. An assessment of renal failure in an SLE cohort with special reference to ethnicity, over a 25-year period. Rheumatology 2006;45:1144–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.