Abstract
Research on the immunosuppression of cancer cells has attracted much attention in recent years. The present study sought to provide a new strategy for tumor immunotherapy targeting mast cells by studying the mechanisms underlying mast cell function in cancer immunosuppression. Between January 2015 and December 2017, the tumor tissues of 40 patients with gastric cancer (GC) were collected and grouped in Lihuili Hospital of Ningbo City, China. Pathological sections were prepared and an immunofluorescence assay was performed to analyze the expression of forkhead Box Protein P3 (FOXP3), tryptase, TGFβ1, TGF-βR, IL-9, IL-9R and Oxford 40 ligand (OX40L). Then, the correlations between FOXP3 and tryptase, TGFβ1 and tryptase expression, and the expression of OX40L in patients with GC with different stages were analyzed. The results revealed that high levels of mast cells were present in patients GC, and tryptase and FOXP3 expressions were positively correlated. Mast cells regulate T regulatory (reg) cells in the gastric tumor microenvironment by secreting TGFβ1. Tregs, in turn, promote the survival of mast cells in the tumor microenvironment by producing IL-9. Furthermore, OX40L expression in mast cells was significantly associated with Tumor-Node-Metastasis staging of GC. Overall, the present study reported a positive feedback system that functions through TGFβ1 and IL-9 to allow cross-talk between Tregs and mast cells. Moreover, OX40L may be a potential target for the diagnosis and treatment of GC. These results may provide a new strategy for tumor immunotherapy targeting mast cells.
Keywords: mast cell, T regulatory cell, TGFβ1, IL-9, Oxford 40 ligand
Introduction
Gastric cancer (GC) is one of the most common digestive malignancies throughout the world (1). It remains an important cancer worldwide and was responsible for over 1,000,000 new cases in 2018 and an estimated 783,000 mortalities, making it the fifth most frequently diagnosed cancer and the third leading cause of cancer-associated mortality (1). Despite the declining morbidity and mortality rates among patients with GC in the majority of developed countries, the disease remains largely incurable and is still the second leading cause of cancer-associated death worldwide (2,3). Meanwhile, the treatment options available to patients with GC remain limited. Due to the lack of obvious early symptoms of GC and insufficient popularization of routine gastroscopy test, about 80% of GC cases have been diagnosed at an advanced stage. For GC treatment, besides surgical treatment, radiotherapy and chemotherapy are still frequently used adjuvant treatment methods (4). Immunotherapy has received great interest in recent years; however, it has not become a major treatment option for GC (5). Tumor immunotherapy is gaining significant popularity in the field of cancer treatment, benefiting from the in-depth study of cancer immunosuppression in recent years (5). However, few tumor immunosuppression studies have focused on the role of mast cells (MCs) in this process. Therefore, the present study aimed to investigate the mechanism by which MCs regulate cancer immunosuppression, in the hope that improving our understanding of these functions efforts may provide novel insights and possibly a new strategy for tumor immunotherapy by targeting MCs.
MCs are a group of long-lived heterogeneous cells originating from bone marrow (6). Their effects on tumor development are numerous and complex as MCs were originally identified by their roles in angiogenesis (7) and inflammation. For example, accumulation of MCs has been found to accelerate inflammation and aggravate immunosuppression in the tumor microenvironment via the stem cell factor (SCF)/c-kit signaling pathway (8). However, previous work has also revealed a role for MCs in regulating the adaptive immune response (6).
Tumor-induced immune suppression hinders the cytotoxic responses of T lymphocytes, as well as natural killer cells, and promotes tumor progression (9). There are various immunosuppressive mechanisms in which tumors and regulatory T cells (Tregs) play a vital role (9,10). Treg cells, which are characterized by expression of the transcription factor forkhead box protein (Foxp)3 in the nucleus, are a functionally distinct subset of T lymphocytes with immunosuppressive capacities necessary for maintaining immune tolerance (11,12). Through the interaction between MCs and Tregs influence the intensity of tumor-induced inflammation, and can result in either the promotion or inhibition of tumor growth (6). An increase in MCs has been observed in numerous experimental animal tumor models, as well as human tumor specimens (13,14). Our previous study provided evidence of a clear correlation between the number of MCs and expression of Foxp3 in human GC (15), but the mechanism underlying this correlation remains unclear. Therefore, the present study aimed to resolve these mechanisms in GC.
OX40, the receptor of OX40L, could be expressed by effector and memory CD4+ T cells (16). Studies have shown that inhibition of OX40/OX40L signaling pathway can regulate inflammation and immune response and thereby promote GC patient recovery (17), suggesting OX40L may be related to the progression of GC, and can act as a marker to determine the malignancy and prognosis of GC. However, no research on this has been reported so far. Therefore, the results presented in this study may help to elucidate a new strategy for GC immunotherapy targeting MCs.
Materials and methods
Patients and specimens
Samples were collected from 40 patients with GC at the Ningbo Medical Center of Lihuili Hospital (Ningbo, China) following approval from the Ethics Committee of Lihuili Hospital (approval no. KY2020PJ020). Written consent was obtained from the patients prior to the collection of samples. The inclusion and exclusion criteria were as follows: i) Diagnosis of GC confirmed by pathology; ii) without anticancer treatment before surgery; iii) underwent curative resection for GC between January 2015 and December 2017 (patients in stage IV underwent resection only); iv) with complete clinicopathological and follow-up data. Postoperatively, all the specimens were stored using paraffin. The specimens were fixed in 4% paraformaldehyde at 25°C for 4 h, and then transferred to 70% ethanol. The individual lobes of tumor biopsy material were placed in processing cassettes, dehydrated through a serial alcohol gradient, embedded in paraffin wax blocks, and then subjected to paraffin section with a thickness of 5 µm. Clinical stages were classified according to the 7th Tumor-Node-Metastasis (TNM) staging system (18). The clinical characteristics of all patients are summarized in Table I.
Table I.
Clinical characteristics and the stages of the patients with gastric cancer.
| Characteristics | Value |
|---|---|
| Sex, male/femalea | 23/17 (57.5/42.5) |
| Age, yearsb | 30-75 |
| TNM stagea | |
| I | 10 (20) |
| II | 10 (20) |
| III | 10 (20) |
| IV | 10 (20) |
Presented as n (%).
Presented as range. TNM, tumor-node-metastasis.
Immunofluorescence
Primary rabbit anti-Mast Cell Tryptase (MCT) (1:500; cat. no. bs-2725R; BIOSS) and mouse anti-FoxP3 (1:500; cat. no. sc-166212; Santa Cruz Biotechnology, Inc.) antibodies were used to detect MCT+ cells and FoxP3 expression, respectively. Other primary antibodies including rat anti-OX40 (1:500; cat. no. sc-71768; Santa Cruz Biotechnology, Inc.), mouse anti-IL-9R (1:500; cat. no. sc-515622; Santa Cruz Biotechnology, Inc.), rabbit anti-IL-9 (1:500; cat. no. bs-10435R; BIOSS) and rabbit anti-TGF β1 (1:500; cat. no. bs-0086R; BIOSS) antibodies were also used. Before immunofluorescence analysis, the sections were blocked with donkey serum albumin (1:50; cat. no. BMS0140; Abbkine Scientific Co., Ltd.) in PBS for 1 h at room temperature and then incubated at 4°C with the aforementioned primary antibodies. Then the sections were incubated with secondary goat anti-rat IgG antibody (1:500; cat. no. bs-0293G), goat anti-rabbit IgG antibody (1:500; cat. no. bs-0295G) or goat anti-Mouse IgG antibody (1:500; bs-0296G) (all BIOSS) at 37°C for 1 h. Slides were then mounted with Vectashield containing DAPI (Vector Laboratories, Inc.; Maravai LifeSciences) and visualized and images captured using a fluorescence microscope (Olympus Corporation; magnification, ×200) coupled to a CCD camera (Nikon Corporation). Negative controls, in which PBS was used in place of primary antibodies, were included for each marker. The mean intensity of fluorescence was analyzed using ImageJ (version 1.45r; National Institutes of Health).
Statistical analysis
All statistical analyses were performed using SPSS version 14.0 software (SPSS Inc.). The results are expressed as mean ± standard error of the mean of three independent experiments. The means of the tumor and non-tumor groups were compared using a paired Student's t-test. Multiple comparisons were performed using a one-way analysis of variance followed by Tukey's post hoc test, which was used to analyze clinical stages in relation to mean levels of OX40L. P<0.05 was considered to indicate a statistically significant difference. Relationships between tryptase and Foxp3, and tryptase and TGFβ1 in GC tissue or from The Cancer Genome Atlas database (https://tcga-data.nci.nih.gov/tcga) were analyzed with the Spearman's rank correlation coefficient.
Results
The role of MCs in regulating microenvironment immunity of GC
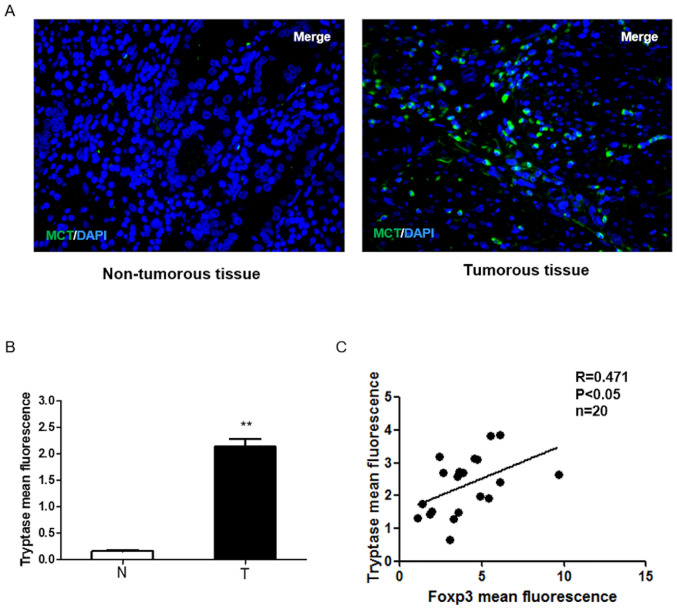
MCs play an important role in the tumor microenvironment of GC (15), therefore, to clarify the role of MCs in GC, an immunofluorescence assay to detect the expression of MCs in 40 human paired GC and non-tumor tissue samples. As shown in Fig. 1A and B, tryptase was upregulated in GC specimens compared with the surrounding non-tumorous tissues. In total, 20 of the 40 samples were used to detect Foxp3 expression in GC tissues. The results showed that MCs displayed high levels of Foxp3 in GC samples and tryptase and Foxp3 expressions were positively correlated in human GC (P<0.05; Fig. 1C), which suggests that the infiltration of MCs in GC may increase the number of Tregs and MCs may play an important role in regulating tumor immunity.
Figure 1.
Role of mast cells in regulating microenvironment immunity of gastric cancer. (A) Immunofluorescence analysis of tryptase (green) expression in a set of 40 human gastric cancer specimens and surrounding non-tumorous tissues (magnification, ×200). (B) Tryptase expression was upregulated in gastric cancer specimens. Mean intensity of Tryptase analyzed by ImageJ software fluorescence was separated into non-tumorous tissue and tumor tissue (**P<0.01 vs. non-tumorous tissue group). (C) Positive correlation between tryptase and Foxp3. Mean intensities of TGFβ1 and tryptase fluorescence were analyzed using ImageJ software. Spearman's rank correlation analysis indicated a significant positive correlation (r=0.471; P<0.05). N, non-tumorous tissue; T, tumor tissue; Foxp3, forkhead box protein 3; MCT, Mast Cell Tryptase.
MCs regulate Treg cells in the GC tumor microenvironment by secreting TGFβ1
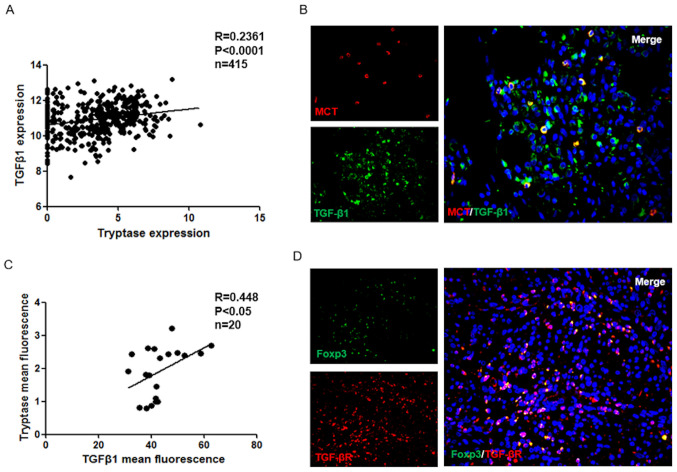
It has been previously reported that GC cells induce the increased human CD4+ Foxp3+ Tregs via the production of TGFβ1 (19), but whether mast cells can secrete TGFβ1 in GC is unclear. Using TCGA database, it was demonstrated that TGFβ1 expression has a positive correlation with MCs in the GC group (P<0.001; Fig. 2A). In addition, immunofluorescence was used to analyze the localization of MCs and TGFβ1 in GC tissues (Fig. 2B), and the results were examined using correlation analysis. As presented in Fig. 2C, the TGFβ1 expression exhibited a positive correlation with MCs in GC tissues (P<0.05). Furthermore, the expression of TGFβR in GC tissues was also examined. The results indicated that TGFβR is expressed in Treg cells (Fig. 2D). In summary, these data suggested that the cytokine TGFβ1 may be a molecule involved in crosstalk between MC and Treg cells.
Figure 2.
Mast cells regulate Treg cells in gastric cancer tumor microenvironment by producing TGFβ1. (A) Expression of TGFβ1 was positively correlated with tryptase in human gastric cancer specimens from The Cancer Genome Atlas database (r=0.2361; P<0.0001). (B and C) Mast cells express TGF-β1 in gastric cancer (merged in yellow, right panel; magnification, ×200), and the correlation between MCT cell and TGF-β1 protein levels in human gastric cancer. Mean intensities of TGFβ1 and tryptase fluorescence were analyzed using ImageJ software. Spearman's correlation analysis indicated a significant positive correlation (r=0.448; P<0.05). (D) Colocalization of TGF-βR and Foxp3+ Treg cells (merged in yellow, right panel; magnification, ×200). Treg, T regulatory cell; MCT, Mast Cell Tryptase.
Tregs regulate MCs in the tumor microenvironment by producing IL-9
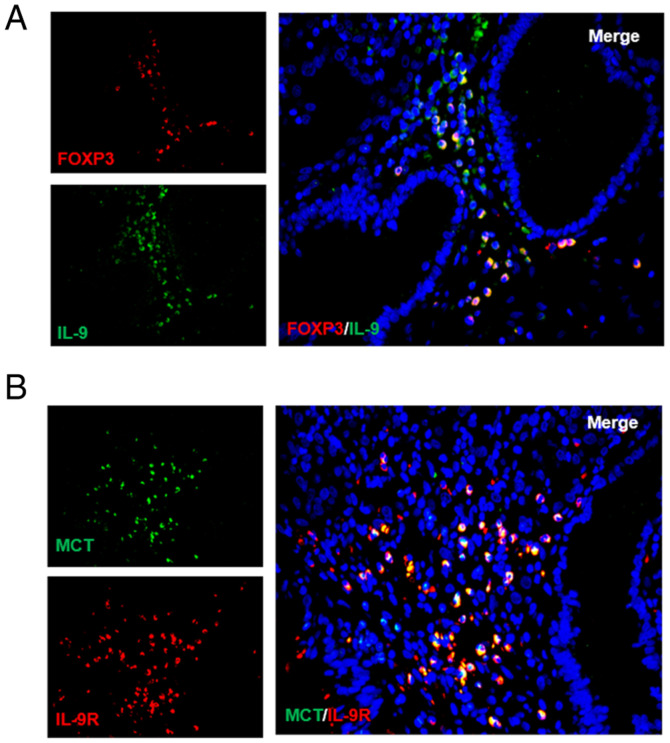
Considering that IL-3, IL-4 and IL-9 affect MC proliferation but only IL-9 is able to promote the growth of MCs from bone marrow and MC progenitors (20,21), it was hypothesized that Tregs may regulate MCs through IL-9. To test this hypothesis, IL-9 and Treg cells were probed for using immunofluorescence. As expected, it was reported that Tregs secreted IL-9 (Fig. 3A). High IL-9R expression was also detected on the surface of MCs for the first time (Fig. 3B). Together, these results showed that a positive feedback regulation system may exist between MCs and Treg cells that operates through TGFβ1 and IL-9.
Figure 3.
Tregs regulate mast cells in the tumor microenvironment by producing IL-9. (A) Colocalization of IL-9 (green) and Foxp3+ Treg cells (red) in human gastric cancer. (B) IL-9R expression was detected on the surface of mast cells by immunofluorescence. Treg, T regulatory cell; Foxp3, forkhead box protein 3; MCT, Mast Cell Tryptase.
Expression of OX40L in MCs is an important marker for determining GC malignancy and prognosis
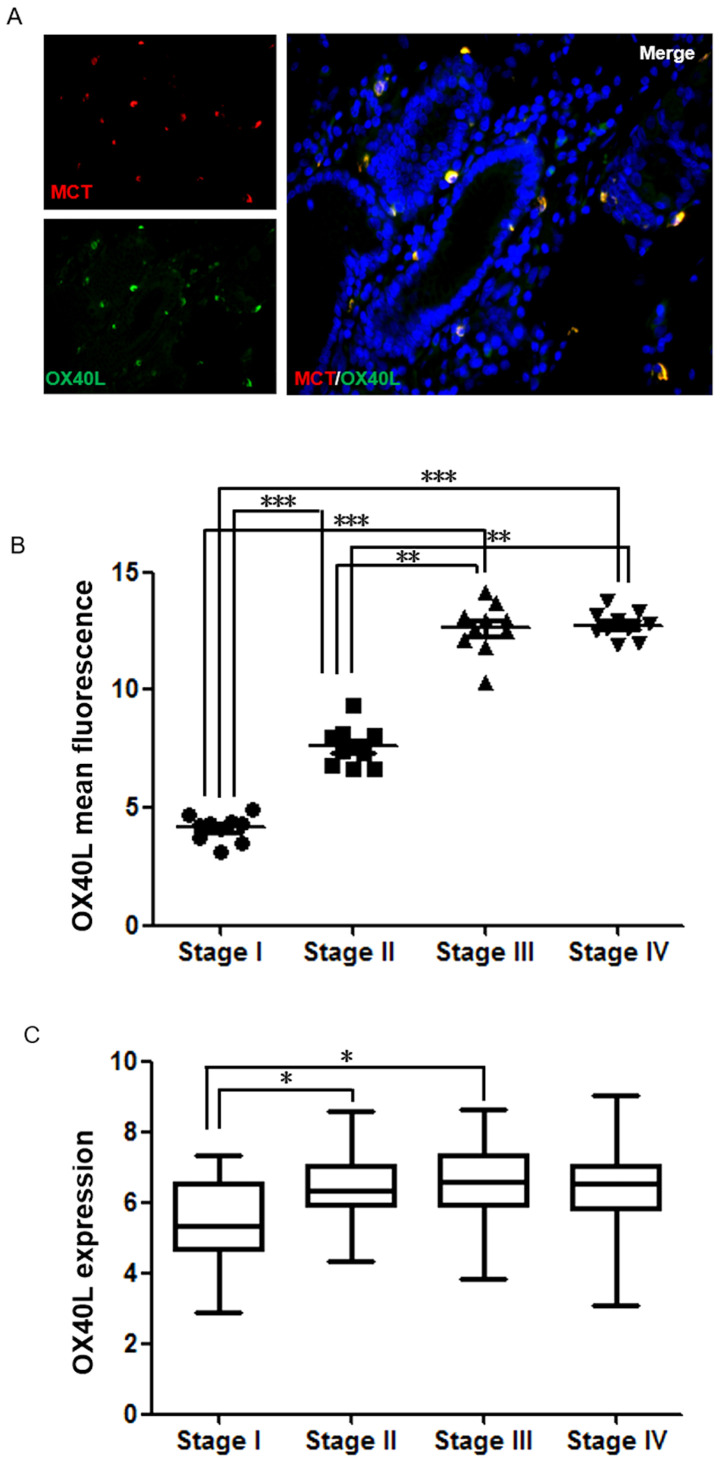
OX40L was also a protein of interest. Notably, in GC tissues, it was demonstrated that the majority of OX40L was expressed on MCs, which has not been reported previously to the best of our knowledge (Fig. 4A). By analyzing clinical samples, it was found that OX40L expression in GC was significantly associated with TNM stage. As presented in Fig. 4B, there was a significant difference in OX40L levels between Stage I and Stages II–IV (P<0.001). To further confirm whether the expression of OX40L is an important marker for determining GC malignancy and prognosis, TCGA data were analyzed and there was a significant difference in OX40L levels between Stages I and II (P<0.05; Fig. 4C). This suggests that OX40L expressed by MCs may be an important marker for determining GC malignancy and prognosis.
Figure 4.
The expression of OX40L in mast cells has positive effects on malignancy determination and prognosis of gastric cancer. (A) Colocalization of OX40L (green; magnification, ×200) and mast cells (red; magnification, ×200) in human gastric cancer. (B) Mean intensity of OX40L analyzed using ImageJ software fluorescence was separated into four groups by the TNM stage. (C) Expression of OX40L in different TNM stage of gastric cancer tissues, and the data shown are expression profiles of 406 human gastric cancer specimens from The Cancer Genome Atlas database. *P<0.05, **P<0.01, ***P<0.001. OX40L, Oxford 40 ligand; TNM, Tumor-Node-Metastasis.
Discussion
GC is the most common gastrointestinal tumor (1). Although the medical understanding of this disease is improving, the rates of incidence and mortality of GC are still high, especially in China. In 2018, 42.6% of new GC cases and 45% of GC-induced deaths occurred in China (22). The research on tumor immunity observed GC has led to several important discoveries (23), yet further exploration is needed, despite the progress made.
Our previous study focused on the roles of MCs in GC, reporting that the frequency of MCs is higher in tumors compared with in healthy tissues, and MC levels are correlated with TNM stage (13). Ribatti et al (14) also demonstrated that MC density is correlated with the progression gastric carcinoma and the density of MCs is positively correlated with the development of the disease from stage I to stage IV. Some studies have suggested that MCs play a protective role in human cancer (24–27); however, in GC, the overall role of MCs in promoting cancer is unclear. The tumor promoting mechanism of MCs is exceedingly complicated, and involves tissue remodeling, angiogenesis and immune regulation (14). Therefore, conducting research on MCs can be very difficult, leading the present study to focus on the immunoregulatory function of MCs. Several studies have demonstrated increased presence of intratumoral and circulating Treg cells in gastric adenocarcinomas (28–30). In gastric tumors, Treg abundance is also correlated with decreased overall survival (28,31), and, in particular, a high Foxp3 level is an independent factor associated with worse overall survival time and rate (30). In our previous study a positive correlation was identified between MCs and Tregs using flow cytometry (15). In the present study, immunofluorescence was used to further confirm this correlation and to further explore the possible mechanisms of interaction.
It is widely recognized that TGFβ plays several central roles in carcinogenesis (31–33). The TGFβ family of proteins regulates numerous cellular functions, including cell growth, differentiation, adhesion, migration and apoptosis. TGFβ is also able to manipulate the tumor microenvironment to promote carcinogenesis. Malignant tumor cells secrete a large amount of TGFβ protein, which not only accelerates the proliferation and migration of cancer cells, but also enables cancer cells to evade the immune system. TGFβ also induces Tregs to inhibit effector T cells, a set of cells which have the capability of recognizing and killing cancer cells like Cytotoxic T lymphocyte (34). In addition, TGFβ can act directly on effector T cells, natural killer cells and B cells, to inhibit their immunological activities (34). Yuan et al (19) found that GC cells could induce human CD4+ Foxp3+ Tregs through the production of TGFβ1. TGFβ1 is also an important product of MCs (35), but whether MCs can regulate Tregs in human GC by secreting TGFβ1 has not been previously reported. The present study demonstrated that there is a correlation between TGFβ1 and MCs in GC (Fig. 2), suggesting that MCs are also involved in TGFβ1 secretion, which can have a positive effect on Tregs.
IL-9 was also investigated, another important factor linking Tregs and MCs, which has been shown to promote MC proliferation and function (36). Previous work has shown that the number of basal MCs was normal without IL-9 (37); however the presence of IL-9, in combination with Stem cell factor, was able to promote the proliferation of MCs from bone marrow and MC progenitors (36). The primary source of IL-9 is T lymphocytes, including natural Tregs and inducible Tregs, both of which are Foxp3+ populations that are able to secrete IL-9 (38,39). However, there is conflicting evidence regarding the production of IL-9 from human Treg cells (40,41). Additionally, in human donors the co-expression of Foxp3 and IL-9 has not been reported either. There are several pieces of evidence connecting IL-9 and MCs (42). IL-9 is a key proliferation or differentiation factor and chemoattractant for MCs (43,44) and has been previously implicated as a key cytokine important for regulating the interactions between Tregs and MCs in other systems, such as inflammation (39). Furthermore, IL-9 production by Tregs recruits MCs that are essential for Treg-induced immune-suppression (45). Thus, the present study sought to analyze the functional role of IL-9 in Treg-MC interactions in GC. The current study analyzed Foxp3 and IL-9 expression in GC, and reported that these were co-expressed, indicating that Treg cells can secrete IL-9 in GC tissues. In addition, we discovered for the first time that IL-9R is expressed on the surface of MC. Based on these results, it is reasonable to speculate that Tregs can regulate MCs by secreting IL-9 in GC. These results suggested that a positive feedback regulation system exists between Treg cells and MCs that operates through TGFβ1 and IL-9. This feedback may have an inhibitory effect and result in cancer-mediated immunosuppression.
The present study also investigated the important molecule OX40L. It was found that patients with high expression of OX40L had a worse prognosis (Fig. 4). However, the expression of OX40L did not significantly affect the survival curve (data not shown). Further analysis showed that OX40L expression was also correlated with TNM stage (Fig. 4), and OX40L expression was higher in GC patients at late-stage (stages II–IV) compared with that in stage I patients. Previous studies have found an association between MC and TNM staging of GC (13,15), leading to the speculation that changes in OX40L expression could be caused by MCs. Previous work has shown that OX40L expression is upregulated in response to antigen presentation on multiple antigen-presenting cells (46). The type of cells that can be induced to express OX40L is broader compared with that for OX40, and studies have reported expression of OX40L on MCs (47,48), as well as vascular endothelial cells (49). In addition, MCs can also promote angiogenesis (24), suggesting that the increased expression of OX40L is likely caused by neovascularization. The results of the present study demonstrated a significant difference in OX40L levels between stage I and stages II–IV GC patients, suggesting that OX40L can be used as a potential novel GC marker for clinical evaluation of occurrence, development and metastasis of GC. Although OX40L has not been clinically used as a prognostic factor for GC and the immune-promoting effect of OX40L may not be dominant in GC, the present study does provide a new perspective on the unique role of MCs in GC.
In summary, the present results showed increased expression of both MCs and Foxp3 in GC samples compared with normal tissues. The significant correlation between MCs and Foxp3 supports the hypothesis that MCs play a role in the immune suppression seen in GC and may, at least partially, affect the prognosis. The mechanism of action between these two cell types was further investigated, revealing that there be a regulatory feedback mechanism involving TGFβ1 and IL-9. TGFβ1 has been shown to play an important role in GC (50). The current study showed that MCs are involved in the secretion of TGFβ1, and can promote Tregs through TGFβ1. Tregs can also positively regulate MCs by producing IL-9 to promote MC function. OX40L may serve as a potential prognostic indicator of GC and could provide a new perspective to study the angiogenesis in this disease. However, the present study has some limitations. First, the study only focused on tumor immunity and did not thoroughly analyze detailed clinicopathological data, hence the present data lack some important clinicopathological characteristics, such as lymph node metastasis and liver metastasis. In addition, the present study only provided preliminarily evidence to support the hypothesis that there may be a positive feedback regulation system between Treg cells and MCs operating through TGFβ1 and IL-9. Therefore, further research is needed to validate the current results. Ultimately, further experiments could further improve our understanding of the mechanistic interactions between Tregs and MCs.
Acknowledgements
The authors of the present study would like to thank Professor Wu Ke (Wuhan Union Hospital) for critically reading this manuscript.
Funding
The present study was funded by the Natural Science Foundation of Ningbo (grant no. 2015A610223).
Availability of data and materials
All data generated or analyzed during this study are included within the article.
Authors' contributions
HY conceived the study. YZ and HY designed the study. YZ searched the literature and collected the data. YZ, SY and KD performed the experiments and interpreted the data. SY, JS, KD, QL, YW and WC performed the statistical analysis. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Human samples were obtained with informed written or oral consent from the donors. The study was approved by the Ethical committee of Ningbo Medical Center Lihuili Hospital (Ningbo, China; approval no. approval no. KY2020PJ020).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wang LH, Su L, Wang JT. Correlation between elevated FOXP3 expression and increased lymph node metastasis of gastric cancer. Chin Med J (Engl) 2010;123:3545–3549. [PubMed] [Google Scholar]
- 3.Yoshii M, Tanaka H, Ohira M, Muguruma K, Iwauchi T, Lee T, Sakurai K, Kubo N, Yashiro M, Sawada T, Hirakawa K. Expression of forkhead box P3 in tumour cells causes immunoregulatory function of signet ring cell carcinoma of the stomach. Br J Cancer. 2012;106:1668–1674. doi: 10.1038/bjc.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan Z. Recent advances in the surgical treatment of advanced gastric cancer: A review. Med Sci Monit. 2019;25:3537–3541. doi: 10.12659/MSM.916475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magalhães H, Fontes-Sousa M, Machado M. Immunotherapy in advanced gastric cancer: An overview of the emerging strategies. Can J Gastroenterol Hepatol. 2018;2018:2732408. doi: 10.1155/2018/2732408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khazaie K, Blatner NR, Khan MW, Gounari F, Gounaris E, Dennis K, Bonertz A, Tsai FN, Strouch MJ, Cheon E, et al. The significant role of mast cells in cancer. Cancer Metastasis Rev. 2011;30:45–60. doi: 10.1007/s10555-011-9286-z. [DOI] [PubMed] [Google Scholar]
- 7.Ribatti D, Crivellato E. Mast cells, angiogenesis, and tumour growth. Biochim Biophys Acta. 2012;1822:2–8. doi: 10.1016/j.bbadis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Huang B, Lei Z, Zhang GM, Li D, Song C, Li B, Liu Y, Yuan Y, Unkeless J, Xiong H, Feng ZH. SCF-mediated mast cell infiltration and activation exacerbate the inflammation and immunosuppression in tumor microenvironment. Blood. 2008;112:1269–1279. doi: 10.1182/blood-2008-03-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piconese S, Colombo MP. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 11.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 12.Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, Vatan L, Finlayson E, Huang E, Simeone D, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69:3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]
- 13.Ribatti D, Crivellato E. Chapter 4 the controversial role of mast cells in tumor growth. Int Rev Cell Mol Biol. 2009;275:89–131. doi: 10.1016/S1937-6448(09)75004-X. [DOI] [PubMed] [Google Scholar]
- 14.Ribatti D, Guidolin D, Marzullo A, Nico B, Annese T, Benagiano V, Crivellato E. Mast cells and angiogenesis in gastric carcinoma. Int J Exp Pathol. 2010;91:350–356. doi: 10.1111/j.1365-2613.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, WU K, Cai K, Zhai R, Tao K, Wang G, Wang J. Increased numbers of gastric-infiltrating mast cells and regulatory T cells are associated with tumor stage in gastric adenocarcinoma patients. Oncol Lett. 2012;4:755–758. doi: 10.3892/ol.2012.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Smedt T, Smith J, Baum P, Fanslow W, Butz E, Maliszewski C. Ox40 costimulation enhances the development of T cell responses induced by dendritic cells in vivo. J Immunol. 2002;168:661–670. doi: 10.4049/jimmunol.168.2.661. [DOI] [PubMed] [Google Scholar]
- 17.Huang L, Wang M, Yan Y, Gu W, Zhang X, Tan J, Sun H, Ji W, Chen Z. OX40L induces helper T cell differentiation during cell immunity of asthma through PI3K/AKT and P38 MAPK signaling pathway. J Transl Med. 2018;16:74. doi: 10.1186/s12967-018-1436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edge SB, Compton CC. The American joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 19.Yuan XL, Chen L, Zhang TT, Ma YH, Zhou YL, Zhao Y, Wang WW, Dong P, Yu L, Zhang YY, Shen LS. Gastric cancer cells induce human CD4+Foxp3+ regulatory T cells through the production of TGF-β1. World J Gastroenterol. 2011;17:2019–2027. doi: 10.3748/wjg.v17.i15.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Zhang Y, Zeng Y, Ge S, Sun X, Jia M, Wu Y, Wang N. Isoimperatorin reduces the effective dose of dexamethasone in a murine model of asthma by inhibiting mast cell activation. Phytother Res. 2020 doi: 10.1002/ptr.6726. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 21.Feng LL, Gao JM, Li PP, Wang X. IL-9 contributes to immunosuppression mediated by regulatory T cells and mast cells in B-cell non-hodgkin's lymphoma. J Clin Immunol. 2011;31:1084–1094. doi: 10.1007/s10875-011-9584-9. [DOI] [PubMed] [Google Scholar]
- 22.Yu F, Tian T, Deng B, Wang T, Qi Q, Zhu M, Yan C, Ding H, Wang J, Dai J, et al. Multi-marker analysis of genomic annotation on gastric cancer GWAS data from Chinese populations. Gastric Cancer. 2019;22:60–68. doi: 10.1007/s10120-018-0841-y. [DOI] [PubMed] [Google Scholar]
- 23.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 24.Lis R, Touboul C, Mirshahi P, Ali F, Mathew S, Nolan DJ, Maleki M, Abdalla SA, Raynaud CM, Querleu D, et al. Tumor associated mesenchymal stem cells protects ovarian cancer cells from hyperthermia through CXCL12. Int J Cancer. 2011;128:715–725. doi: 10.1002/ijc.25619. [DOI] [PubMed] [Google Scholar]
- 25.Ogino S, Shima K, Baba Y. Colorectal cancer expression of peroxisome proliferator-activated receptor-gamma (PPARG, PPARgamma)is associated with good prognosis. Gastroenterology. 2009;136:1242–1250. doi: 10.1053/j.gastro.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinnamon MJ, Carter KJ, Sims LP, Lafleur B, Fingleton B, Matrisian LM. A protective role of mast cells in intestinal tumorigenesis. Carcinogenesis. 2008;29:880–886. doi: 10.1093/carcin/bgn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao YB, Wang JL, Wang GB. The function of mast cells in gastric cancer. Gastroenterology. 2011;19:2246–2250. [Google Scholar]
- 28.Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: Possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089–1099. doi: 10.1002/cncr.11618. [DOI] [PubMed] [Google Scholar]
- 29.Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, Sun Y. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136:1585–1595. doi: 10.1007/s00432-010-0816-9. [DOI] [PubMed] [Google Scholar]
- 30.Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. Localisation pattern of Foxp3+ regulatory T cells is associated with clinical behaviour in gastric cancer. Br J Cancer. 2008;98:148–153. doi: 10.1038/sj.bjc.6604149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bataller A, Montalban-Bravo G, Soltysiak KA, Garcia-Manero G. The role of TGFβ in hematopoiesis and myeloid disorders. Leukemia. 2019;33:1076–1089. doi: 10.1038/s41375-019-0420-1. [DOI] [PubMed] [Google Scholar]
- 32.Zhu H, Luo H, Shen Z, Hu X, Sun L, Zhu X. Transforming growth factor-β1 in carcinogenesis, progression, and therapy in cervical cancer. Tumour Biol. 2016;37:7075–7083. doi: 10.1007/s13277-016-5028-8. [DOI] [PubMed] [Google Scholar]
- 33.Syed V. TGF-β signaling in cancer. J Cell Biochem. 2016;117:1279–1287. doi: 10.1002/jcb.25496. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz M, Zhang Y, Rosenblatt JD. B cell regulation of the anti-tumor response and role in carcinogenesis. J Immunother Cancer. 2016;4:40. doi: 10.1186/s40425-016-0145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elieh Ali Komi D, Grauwet K. Role of mast cells in regulation of T cell responses in experimental and clinical settings. Clin Rev Allergy Immunol. 2018;54:432–445. doi: 10.1007/s12016-017-8646-z. [DOI] [PubMed] [Google Scholar]
- 36.Matsuzawa S, Sakashita K, Kinoshita T, Ito S, Yamashita T, Koike K. IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. J Immunol. 2003;170:3461–3467. doi: 10.4049/jimmunol.170.7.3461. [DOI] [PubMed] [Google Scholar]
- 37.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13:573–583. doi: 10.1016/S1074-7613(00)00056-X. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt E, Van Brandwijk R, Van Snick J, Siebold B, Rüde E. Tcgfiii/p40 is produced by naive murine cd4+ t cells but is not a general t cell growth factor. Eur J Immunol. 1989;19:2167–2170. doi: 10.1002/eji.1830191130. [DOI] [PubMed] [Google Scholar]
- 39.Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 40.Beriou G, Bradshaw EM, Lozano E, Costantino CM, Hastings WD, Orban T, Elyaman W, Khoury SJ, Kuchroo VK, Baecher-Allan C, Hafler DA. TGF-beta induces IL-9 production from human Th17 cells. J Immunol. 2010;185:46–54. doi: 10.4049/jimmunol.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Putheti P, Awasthi A, Popoola J, Gao W, Strom TB. Human CD4+ memory T cells can become CD4+IL-9+ T cells. PLoS One. 2010;5:e8706. doi: 10.1371/journal.pone.0008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sehra S, Yao W, Nguyen ET, Glosson-Byers NL, Akhtar N, Zhou B, Kaplan MH. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol. 2015;136:433–40.e1. doi: 10.1016/j.jaci.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hauber HP, Bergeron C, Hamid Q. IL-9 in allergic inflammation. Int Arch Allergy Immunol. 2004;134:79–87. doi: 10.1159/000078384. [DOI] [PubMed] [Google Scholar]
- 44.Renga G, Moretti S, Oikonomou V, Borghi M, Zelante T, Paolicelli G, Costantini C, De Zuani M, Villella VR, Raia V, et al. IL-9 and mast cells are key players of candida albicans commensalism and pathogenesis in the gut. Cell Rep. 2018;23:1767–1778. doi: 10.1016/j.celrep.2018.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eller K, Wolf D, Huber JM, Metz M, Mayer G, McKenzie ANJ, Maurer M, Rosenkranz AR, Wolf AM. IL-9 production by regulatory T cells recruits mast cells that are essential for regulatory T cell-induced immune-suppression. J Immunol. 2011;186:83–91. doi: 10.4049/jimmunol.1001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb GJ, Hirschfield GM, Lane PJL. OX40, OX40L and autoimmunity: A comprehensive review. Clin Rev Allergy Immunol. 2016;50:312–332. doi: 10.1007/s12016-015-8498-3. [DOI] [PubMed] [Google Scholar]
- 47.Kashiwakura J, Yokoi H, Saito H, Okayama Y. T cell proliferation by direct cross-talk between OX40 ligand on human mast cells and OX40 on human T cells: Comparison of gene expression profiles between human tonsillar and lung-cultured mast cells. J Immunol. 2004;173:5247–5257. doi: 10.4049/jimmunol.173.8.5247. [DOI] [PubMed] [Google Scholar]
- 48.Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci USA. 2005;102:6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imura A, Hori T, Imada K, Ishikawa T, Tanaka Y, Maeda M, Imamura S, Uchiyama T. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183:2185–2195. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebert MP, Yu J, Miehlke S, Fei G, Lendeckel U, Ridwelski K, Stolte M, Bayerdörffer E, Malfertheiner P. Expression of transforming growth factor beta-1 in gastric cancer and in the gastric mucosa of first-degree relatives of patients with gastric cancer. Br J Cancer. 2000;82:1795–1800. doi: 10.1054/bjoc.1999.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included within the article.