Abstract
Purpose:
Contrast-enhanced digital mammography (CEDM) is an advanced breast imaging technique using iodinated intravenous contrast to detect breast cancer. This article describes imaging features of skin contamination artifact on CEDM that mimic in situ carcinoma in a case series.
Materials and Methods:
Five patients were identified whose CEDM images demonstrated apparent calcifications and non-mass enhancement suspicious for in-situ carcinoma, with no subsequent evidence of disease. Retrospective image analysis was performed on the unprocessed image data, processed images, and imaging parameters. Dual-energy mammographic technique was performed on two breast phantoms with varying degrees of topical contrast contamination.
Results:
Temporal analysis confirmed the suspicious finding was neither an abnormality of the compression paddle nor the receptor. Comparison of LE and HE images demonstrated the suspicious finding attenuated near the K-edge of iodine, suggesting contrast contamination. Iodinated contrast applied to the surface of breast phantoms replicated the artifact, with a pattern of apparent enhancement similar to the appearance of in-situ carcinoma.
Conclusion:
Skin contamination with iodinated contrast can result in an artifact on post-contrast digital mammography that mimics the appearance of in-situ carcinoma.
Keywords: Contrast Enhanced Digital Mammography, Contamination Artifact, Iodinated Contrast
INTRODUCTION
Contrast Enhanced Digital Mammography (CEDM) is a relatively new imaging technique for breast cancer detection. As with Magnetic Resonance Imaging (MRI), it uses intravenous contrast material to identify tumor neoangiogenesis, at times before a discrete mass can be detected.
All initial studies have demonstrated that CEDM is more sensitive than conventional digital mammography, and in one study tomosynthesis. This is of particular importance in women with dense breasts. [1–4]. Preliminary results also demonstrate that in the diagnostic setting, cancer detection rates of CEDM are comparable to MRI [1, 3, 5]. Therefore CEDM has great potential for routine clinical practice and is being increasingly used in mammography centers internationally. Different centers have different indications for its use. Many centers use it to further evaluate clinical symptoms or abnormal screening examinations, to stage known cancers or for problem solving. Others are also using it for screening patients at increased risk for breast cancer. Its use for evaluating patients undergoing neoadjuvant chemotherapy is under investigation.
During CEDM, the patient receives an intravenous injection of iodinated contrast after which mammography is performed with nearly simultaneous image acquisition using Low Energy (LE) (below the K-edge of iodine) and a High Energy (HE) (above the K-edge of iodine) X-ray spectra. The low energy image is essentially identical to standard mammography despite prior administration of intravenous contrast [6–8]. The high energy image uses stronger filtration to maximize iodine attenuation. Post-processing of the LE/HE image pair suppresses background tissue and highlights iodinated contrast, producing a recombined image [9].
As with all new modalities, however, there are pitfalls to be better understood by the interpreting physician [10]. The goal of this article is to describe imaging features and etiology of an artifact seen on CEDM which mimics in situ carcinoma using a case series of five patients.
METHODS
Imaging Centers
The breast imaging departments at two cancer centers participated in this study. The center in the United States began using CEDM in 2010 for research and since 2011 uses CEDM for research and clinically for both diagnostic and screening mammography. The center in France began using CEDM in 2000 for research and currently uses CEDM in the diagnostic and research settings. Over 2000 CEDM exams were performed between 2013 and 2016 across both institutions.
Radiologists
Four academic radiologists subspecialized in breast imaging were involved in the care of these 5 patients. Three radiologists were from the United States institution with 1–7 years of experience with CEDM. One radiologist from the French counterpart had 14 years of experience reading CEDM.
Study Database
During routine clinical practice at the United States breast center, three patients undergoing CEDM for screening were identified as having a similar finding of apparent non-mass enhancement and what appeared to be calcifications, but may retrospectively have been a shine-through artifact, on CEDM in a pattern suggestive of in situ carcinoma. The finding did not persist on further diagnostic evaluation including MRI and, in one case, a negative stereotactic biopsy in a region of apparent non-mass enhancement.
Discussion with another breast radiologist in France who was experienced with performing and interpreting CEDM revealed an additional two patients with similar imaging findings. These 2 patients underwent CEDM for diagnostic evaluation.
The median age of the patients was 59 (range, 57–74). Three patients underwent CEDM as mammographic screening for increased risk of breast cancer, while two patients underwent CEDM for diagnostic workup following abnormal screening mammography. All patients were followed for at least 1 year. Patient summary is presented in Table 1.
Table 1.
Patient Summary
| Patient | Age (years) | Risk factors | Indication for CEDM | CEDM findings | Evaluation of questioned lesion | Length of available imaging followup (months) |
|---|---|---|---|---|---|---|
| A | 65 | Mastectomy for IDC and DCIS 16y prior. FHx. | Research | Possible calcifications and NME | Magnification views, MRI, stereotactic biopsy, short interval followup CEDM | 29 |
| B | 59 | ADH excised 1 year prior. | High risk screening mammogram | Possible calcifications and NME | Magnification views, ultrasound, MRI | 12 |
| C | 74 | Breast Conservation for DCIS 8y prior. BRCA2 variant. FHx. | Research | Possible calcifications and NME | Magnification views, ultrasound, MRI, short interval followup MRI | 15 |
| D | 57 | None. | Abnormal screening mammogram | Contrast appearance suggested artifact. Ipsilateral mass biopsied (fibroadenoma) | Ultrasound | 27 |
| E | 59 | None. | Abnormal screening mammogram | Possible NME. Contralateral mass biopsied (IDC and DCIS) | MRI | 36 |
Note: FHx (Family History), IDC (Invasive Ductal Carcinoma), DCIS (Ductal Carcinoma In Situ), ADH (Atypical Ductal Hyperplasia), NME (Non-mass enhancement)
Institutional Review Board (IRB) approval was not required for a case study of three or fewer patient cases at each institution.
CEDM Image Acquisition
Prior to imaging, an IV line was placed by a departmental nurse. Nonionic iodinated contrast, either Omnipaque 350 (iohexol, GE, Shanghai, China) or Xenetix 350 (Guerbet, France), was injected via a power injector at a rate of 3ml/seconds and a dose of 1.5cc/kg while the patient was seated. After injection the patient was disconnected from the injector by the nurse. The intravenous access was maintained until after the mammogram. Gloves were worn by the nurse manipulating the IV line.
Mammographic imaging was initiated approximately 2.5–3 minutes after intravenous contrast administration and continued for up to 5 minutes. Images were obtained by a certified mammography technologist using standard mammographic technique and positioning. On average, the technologist required 60 to 70 seconds to reposition the patient between left and right cranial-caudal (CC) imaging and 120 to 180 seconds between CC and mediolateral-oblique (MLO) imaging; the time taken varied depending on patient morphology and fitness. Gloves were not usually worn by technologists while positioning the breast.
At the end of imaging, the radiology technologist or nurse wore gloves while removing the IV line and discarding all contrast contaminated equipment. Mammography equipment was cleansed by the technologist between patients.
CEDM images from both institutions were acquired using a Senographe Essential mammography unit with a SenoBright upgrade (GE Medical Systems, Milwaukee, WI, USA). Standard LE images were obtained using a 0.3 mm molybdenum or rhodium target and either a 0.03 mm molybdenum or 0.025 mm rhodium filter, with 28–31kVp. HE images were obtained using a filter constructed of 0.3 mm aluminum and 0.3 mm copper, with 47–49 kVp. A dual energy recombination algorithm processed the LE and HE images into iodine images.
Image Analysis
For this study, two radiologists and the medical physicist reviewed the unprocessed image data, processed images, imaging sequences, imaging parameters, and acquisition times.
Images were reviewed on institutional-approved display monitors for mammography (Barco, Kortrijk, Belgium) using the Centricity Picture Archiving and Communications System (GE Medical Systems, Milwaukee, WI, USA). Both centers use Premium View software (GE Medical Systems) to view mammography images.
Breast Phantoms
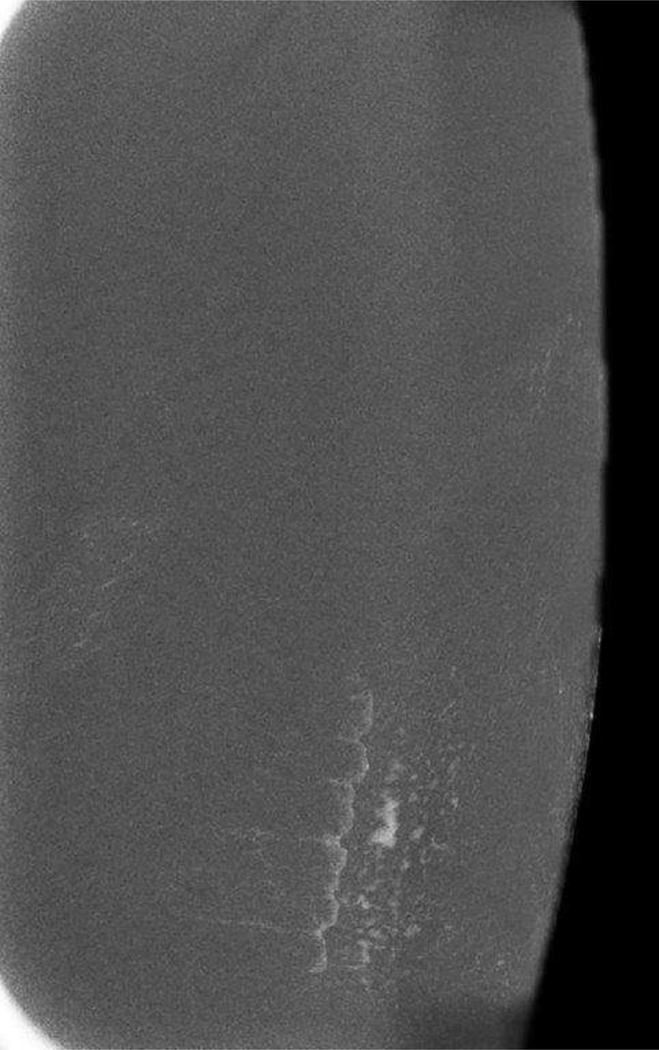
Two phantoms were used to test the hypothesis of skin contamination. Mammographic images utilizing the dual-energy technique were acquired with a StereoTactic Needle Biopsy Phantom, Model 013 (CIRS Inc, Norfolk, Virginia). The compressed phantom is 4.1 cm thick and composed of a gelatinous material designed to approximate the radiological properties of breast tissue. A small quantity of Omnipaque 350 was spread thinly across the surface of the phantom, which was then imaged with a LE exposure of 28 kVp using the molybdenum target and rhodium filter, and a HE exposure of 45 kVp using the molybdenum target and copper filter.
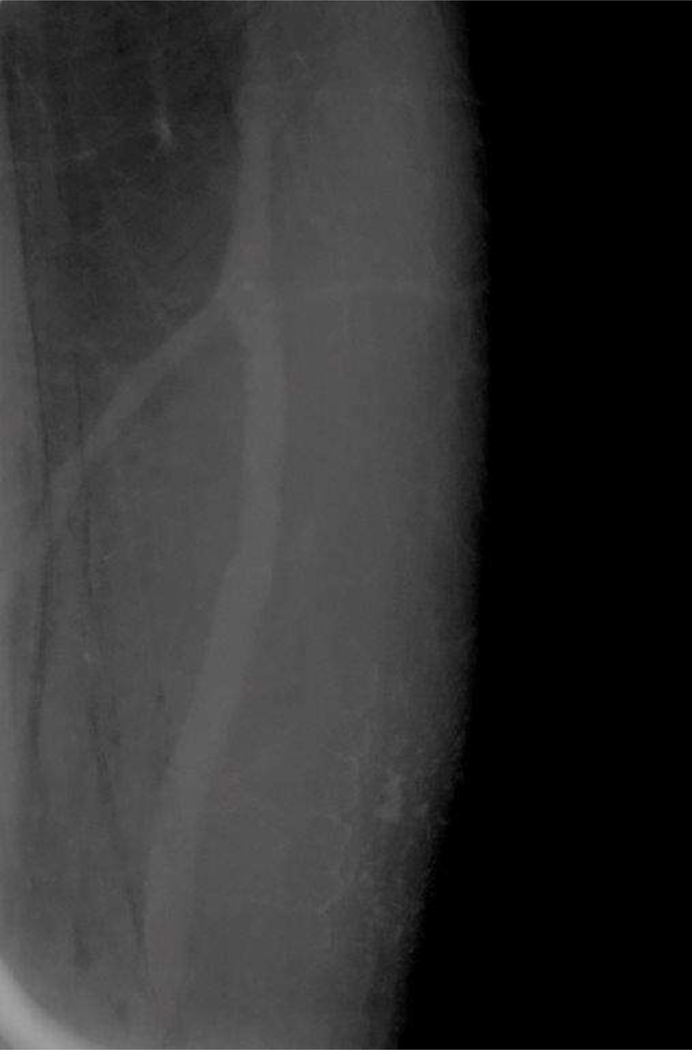
A medical physicist voluntarily imaged the superficial soft tissues of his left forearm with a dual energy technique after spreading a small amount of Omnipaque 350 on his skin, which more closely mimicked breast tissue with porous skin, subcutaneous fat, and blood vessels. The left forearm soft tissues were 4.4 cm thick when compressed. This in vivo specimen was imaged with a LE exposure of 28 kVP using the molybdenum target and rhodium filter, and a HE exposure of 45 kVp using the molybdenum target and copper filter.
RESULTS
Unprocessed Image Analysis
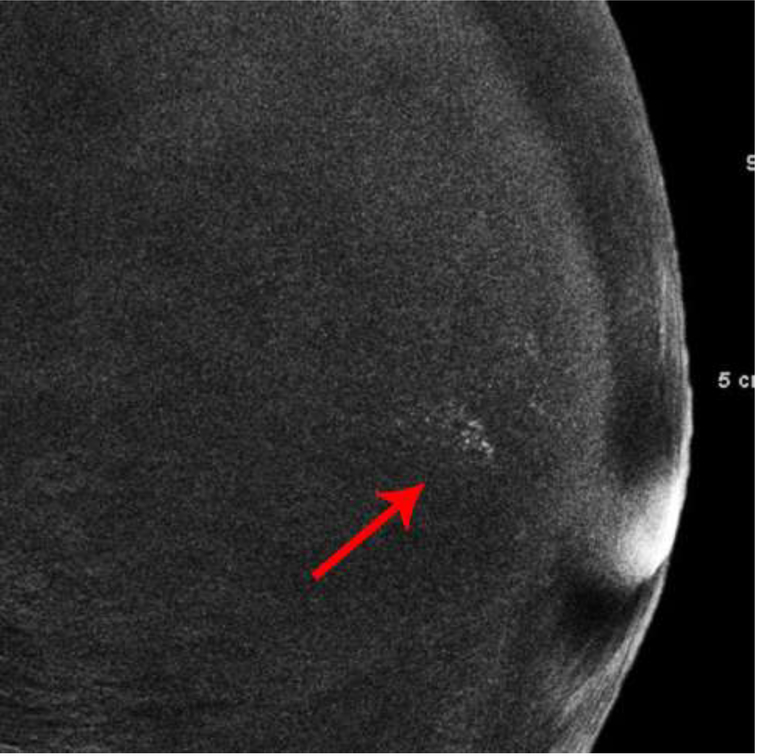
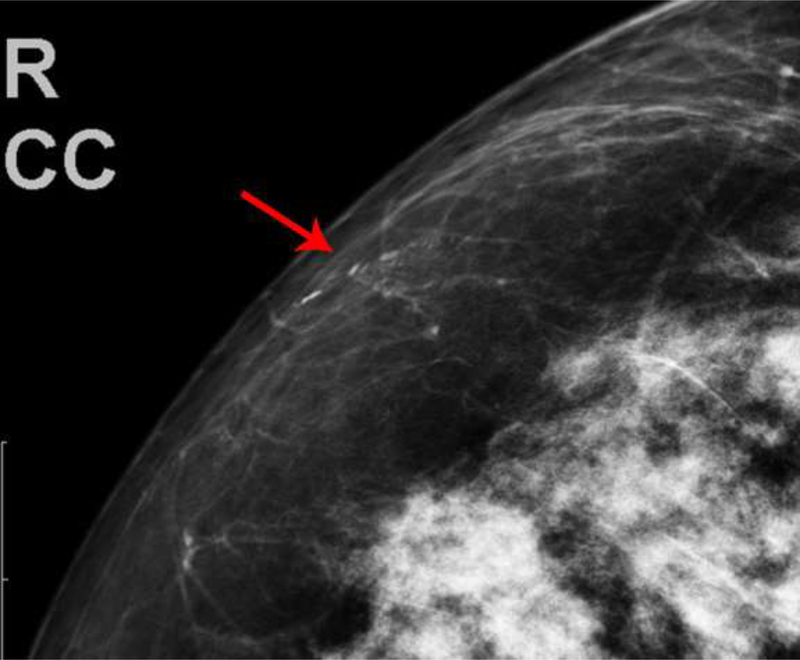
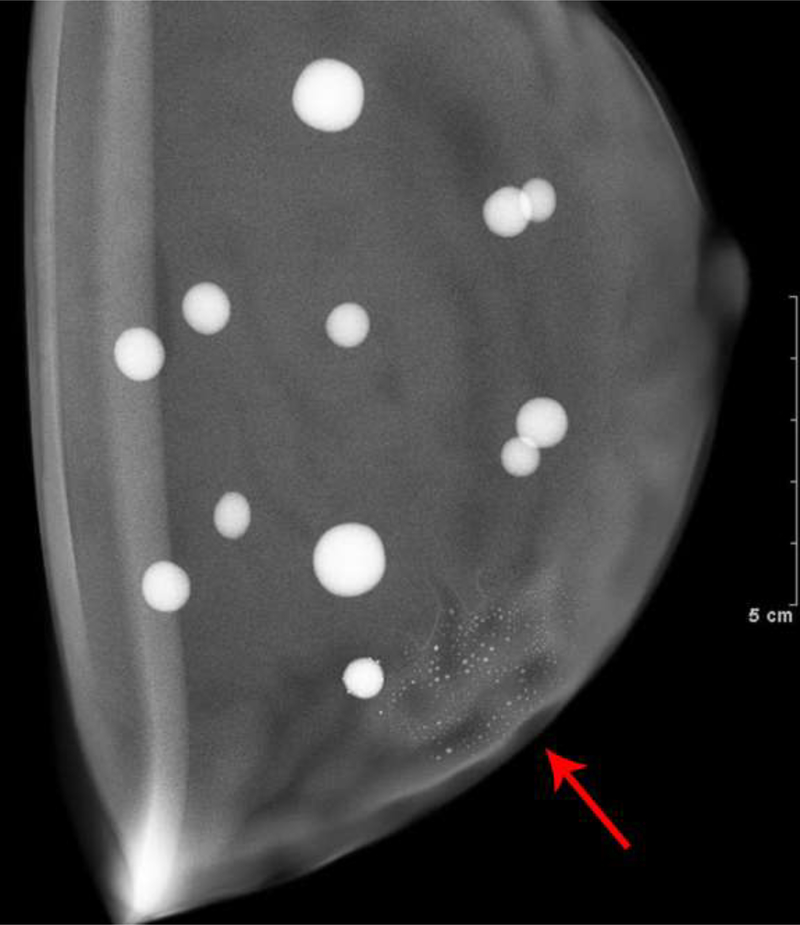
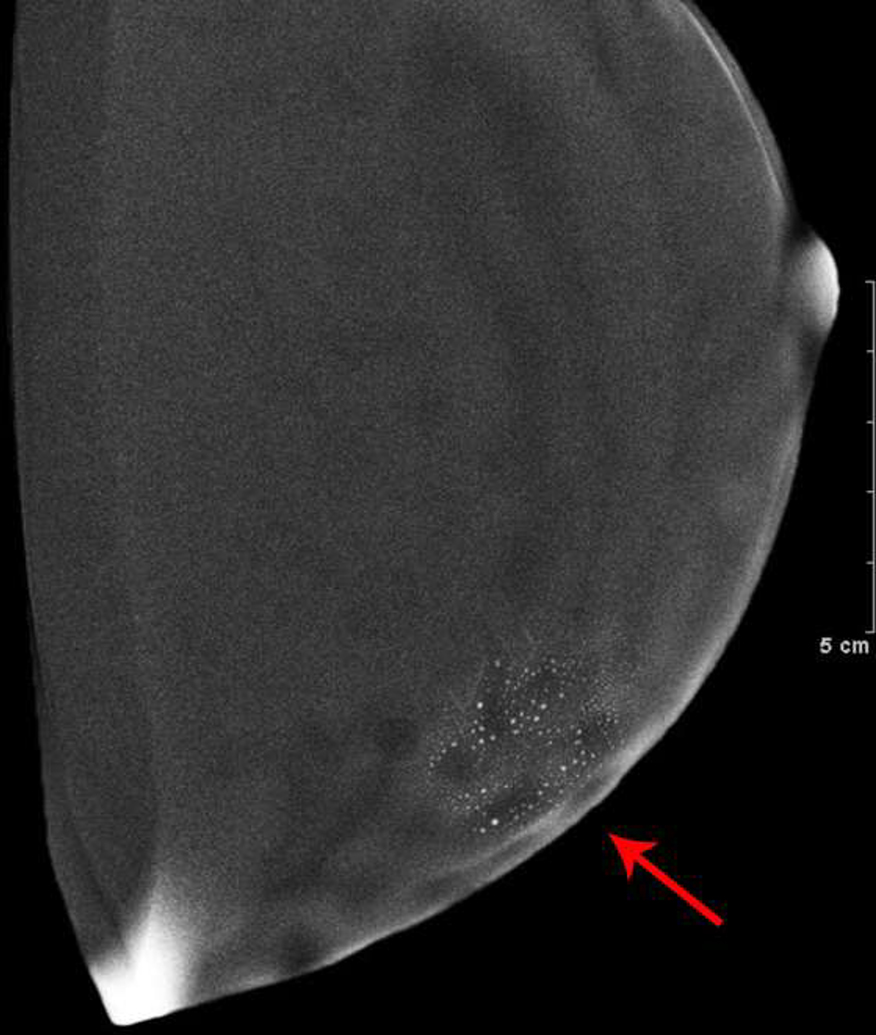
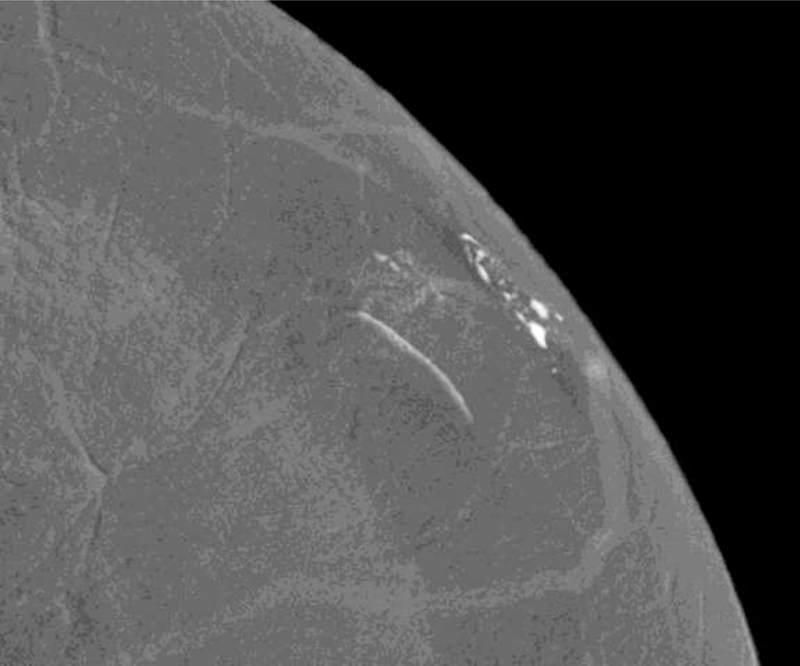
The first patient presented with questioned calcifications and non-mass enhancement in the left breast [Fig 1]. Scheduled same-day breast MRI did not show a corresponding abnormality. Patient returned for spot magnification views which did not demonstrate calcifications. A stereotactic biopsy was performed due to the suspicious non-mass enhancement, with targeting using architectural landmarks, which resulted in benign pathology. No calcifications were identified on specimen radiography or pathologic analysis.
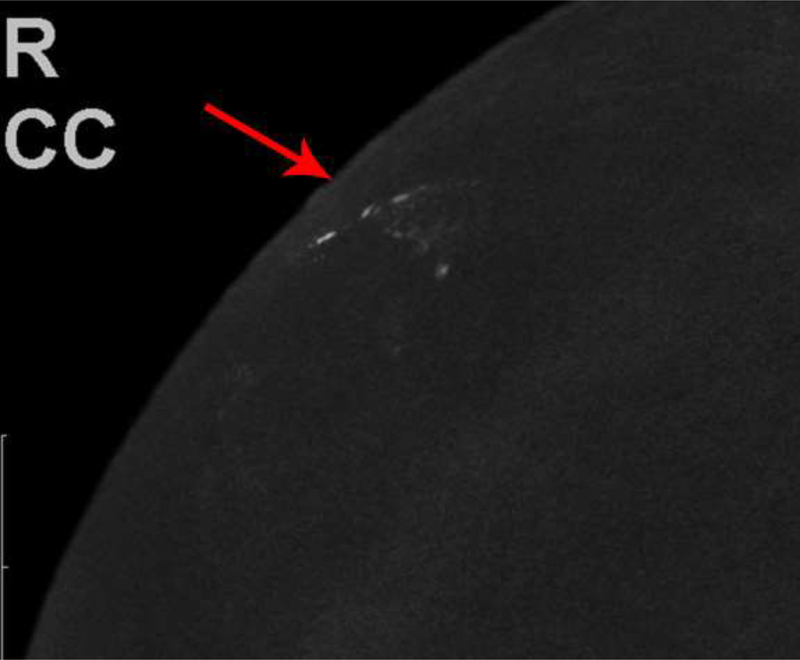
Fig. 1—
Left digital mammogram of Patient A, a 65-year-old female with remote history of right mastectomy for invasive ductal carcinoma and DCIS. Left mediolateral-oblique image (A) demonstrates densities which appear to be fine linear calcifications (arrows). Recombined left mediolateral-oblique (B) and cranial caudal (C) images demonstrate apparent linear non-mass enhancement in the same distribution as apparent calcifications. Spot magnification views did not demonstrate calcifications. No suspicious enhancement on same day MRI. Stereotactic biopsy was performed in area of suspicious non-mass enhancement on CEDM, resulting in benign pathology. No calcifications on specimen radiography or pathologic analysis.
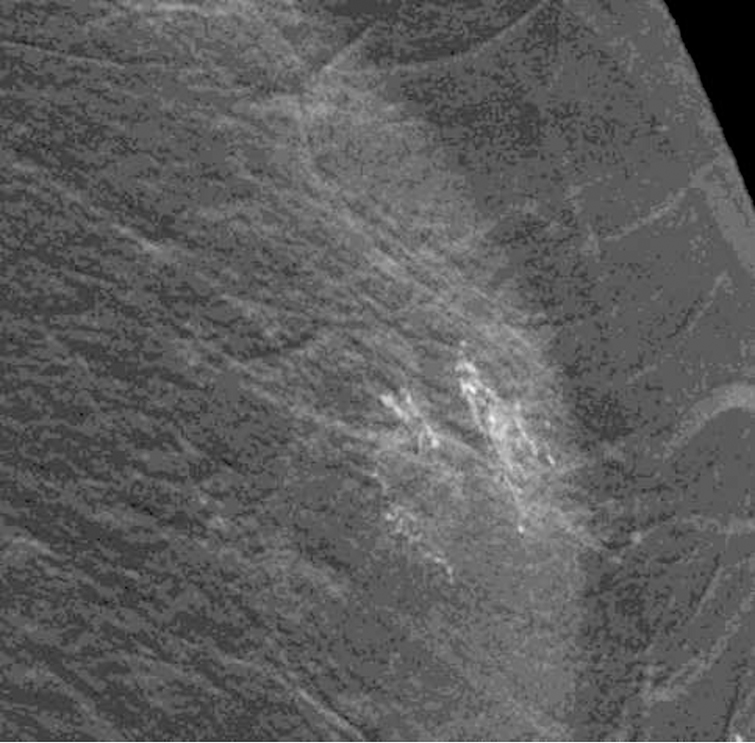
Subsequently, two patients had a similar appearing artifact [Figs 2 and 3]. In both, the apparent contrast artifact on initial imaging was not seen on subsequent imaging of the contralateral breast, suggesting the abnormality was not on the mammography equipment nor caused by malfunction of the imaging system. For these two patients, the affected breast was cleansed prior to magnification views for questioned calcifications. No calcifications were seen on magnification views. Because of the preceding experience, both patients were referred for an MRI which did not demonstrate correlative non-mass enhancement. Based on our previous experience, we determined that this was an artifact and opted to follow the patients with imaging.
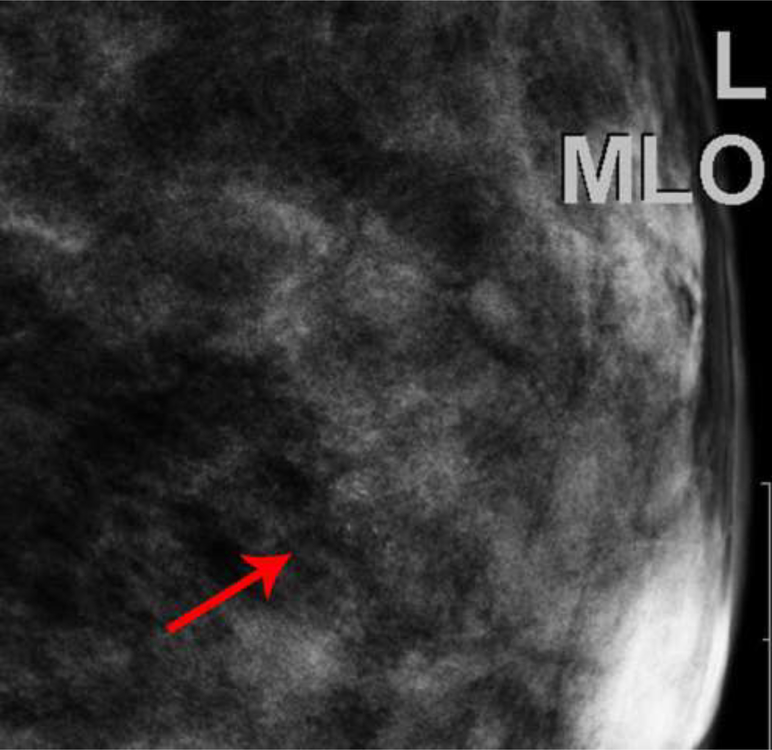
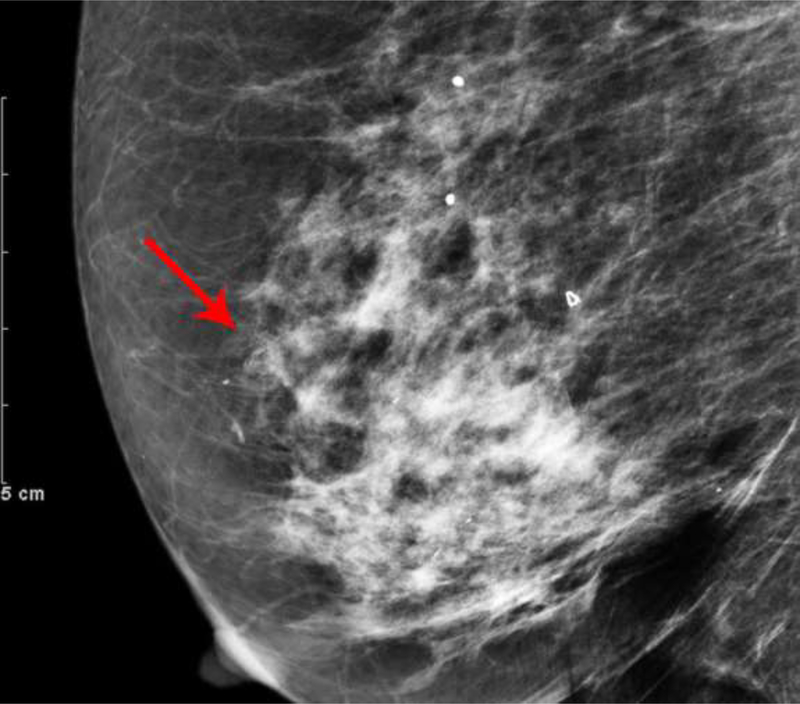
Fig. 2—
Left digital mammogram of Patient B, a 59-year-old female with history of surgical excision of left breast Atypical Ductal Hyperplasia one year prior. Mediolateral-oblique (A) and craniocaudal (C) projections demonstrate apparent fine linear calcifications in the lower inner left breast. Recombined images in the MLO (B) and CC (D) projections demonstrate apparent linear non-mass enhancement in a similar distribution. These findings were not present on contralateral breast images. Spot magnification views performed after breast cleansing showed no calcifications (not shown). A follow-up breast MRI performed nine days later demonstrated no suspicious findings.
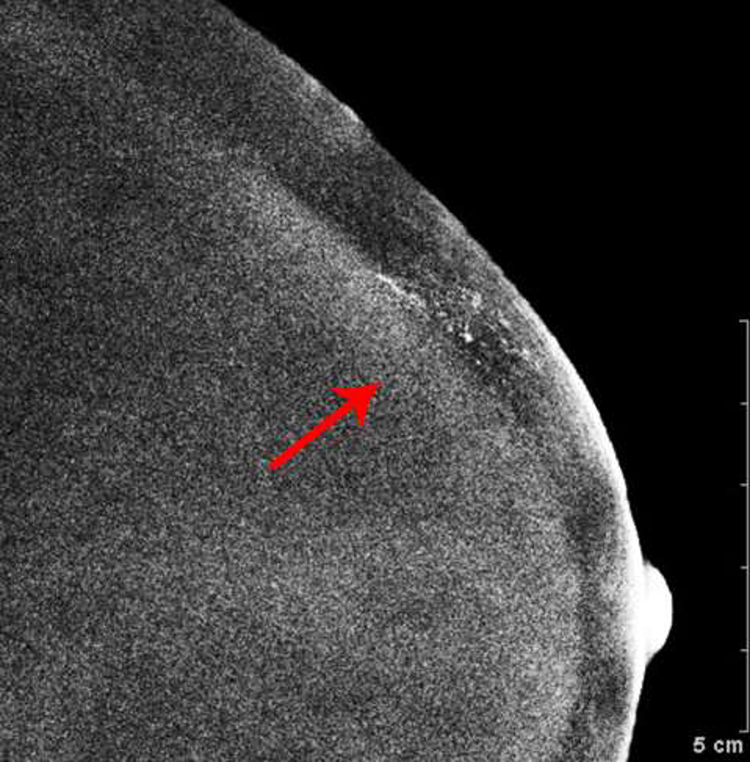
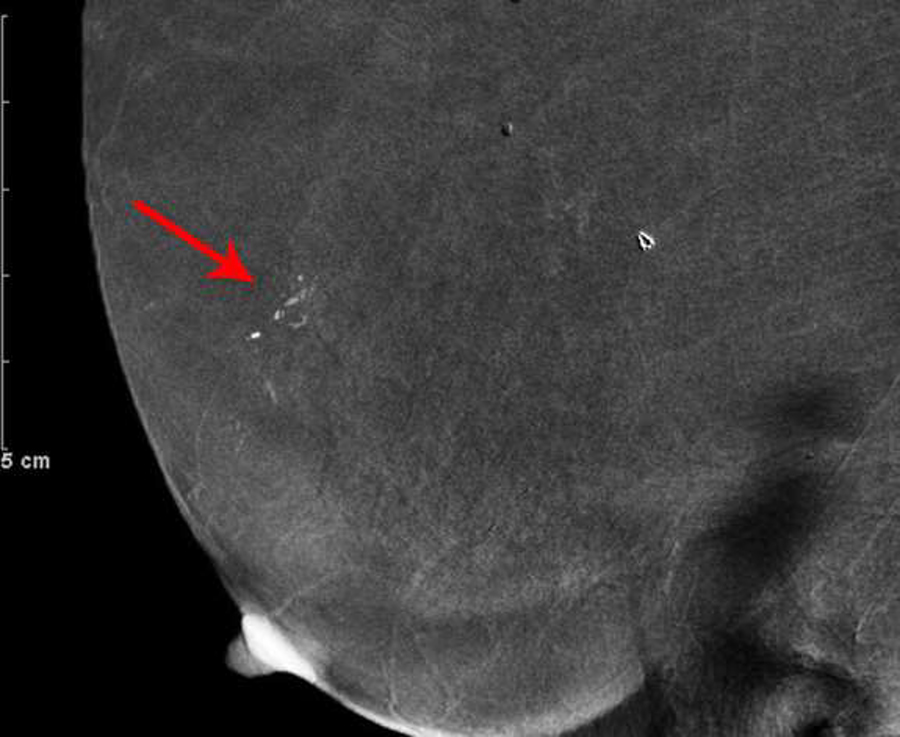
Fig. 3—
Right digital mammogram of Patient C, a 74-year-old female with remote history of treatment for right breast DCIS. Right cranial caudal (A) and mediolateral-oblique (C) views demonstrate what appear to be grouped fine-linear branching calcifications in the upper outer breast. Recombined images in the right cranial caudal (B) and mediolateral-oblique (D) projections demonstrate apparent linear non-mass enhancement in a similar distribution. These findings were not present on contralateral breast images. Spot magnification views performed after breast cleansing showed no calcifications (not shown). Breast MRI performed 21 days later was negative.
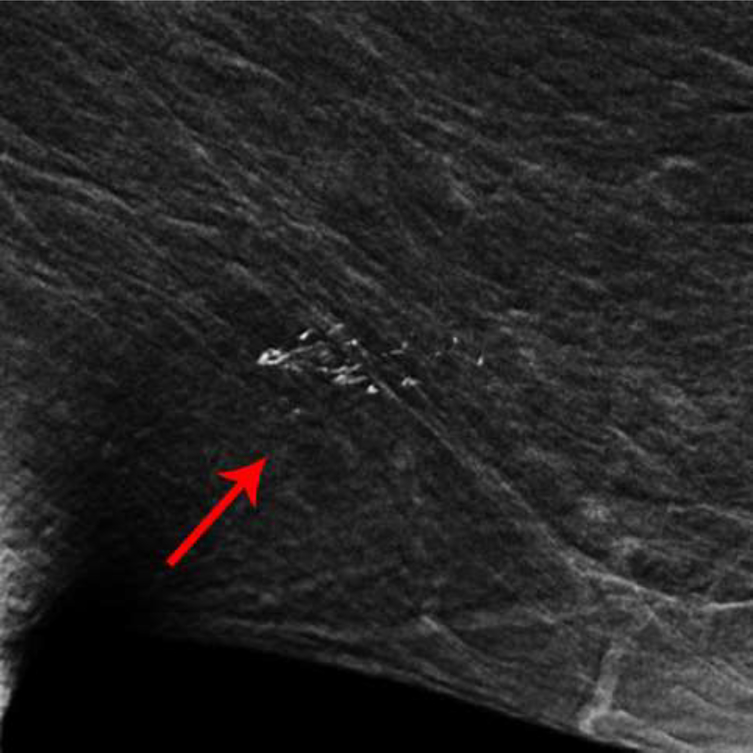
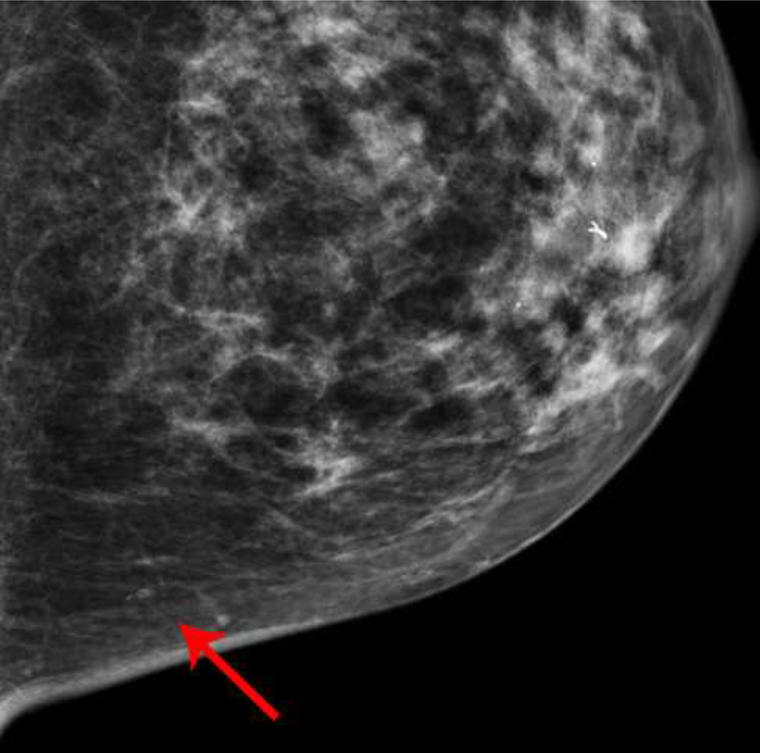
Two additional patients had CEDM performed for diagnostic evaluation following abnormal screening mammograms. The apparent non-mass enhancement was superficially located in both patients, and in one patient appeared to be located within skin folds behind the nipple (Fig 4), suggestive of artifact. Both patients had directed ultrasound to the area of non-mass enhancement, yielding no correlative findings, and one patient (Patient E) had followup MRI, which showed no correlative enhancement.
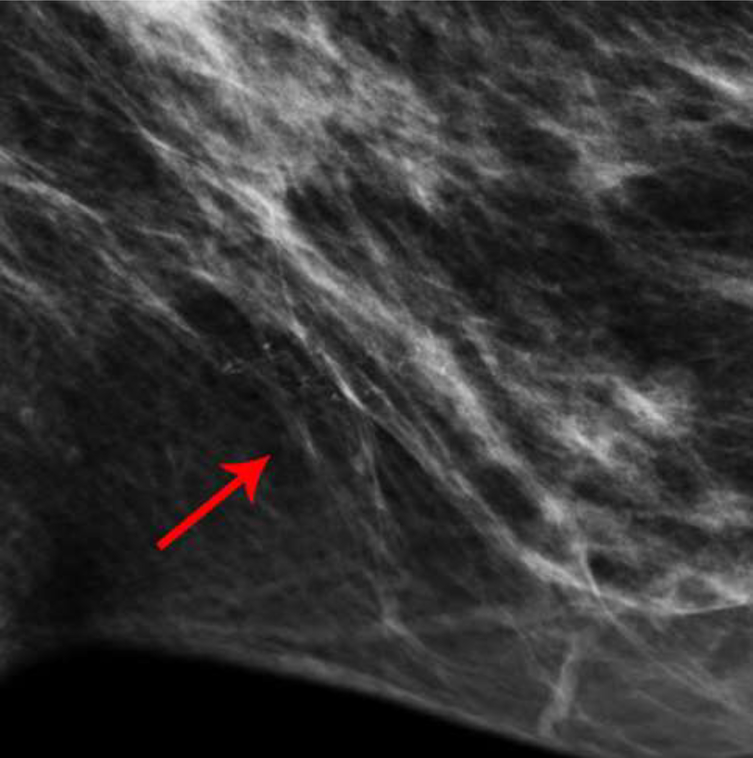
Fig. 4—
Left mediolateral-oblique mammogram of Patient D, a 57-year-old female with abnormal screening mammogram, demonstrating a suspicious mass in the far posterior left breast, which was subsequently biopsied with pathology yielding fibroadenoma (not included in cropped image). Recombined image of the left breast in mediolateral-oblique projection demonstrates contrast which appears to be within the skin folds just posterior to the nipple.
Review of the unprocessed images for all 5 patients showed the high attenuation areas were more prominent on the HE exposure than the LE exposure. This indicates the hyperdense material attenuated more strongly above the K-edge of iodine, suggestive of iodinated contrast material.
Together, these findings raised the hypothesis that skin contamination with iodinated intravenous contrast could lead to artifacts which mimic calcifications and non-mass enhancement. This hypothesis was then tested and replicated using breast phantoms.
Phantom Imaging
Small quantities of iodinated contrast spread thinly on the 2 phantoms (breast phantom and forearm) reproduced the appearance of calcifications and non-mass enhancement seen on the patient mammograms (Figs. 6, 7). The commonality of pattern appearance on the phantom mammograms with the artifact on the breast mammograms confirmed the origin of this artifact.
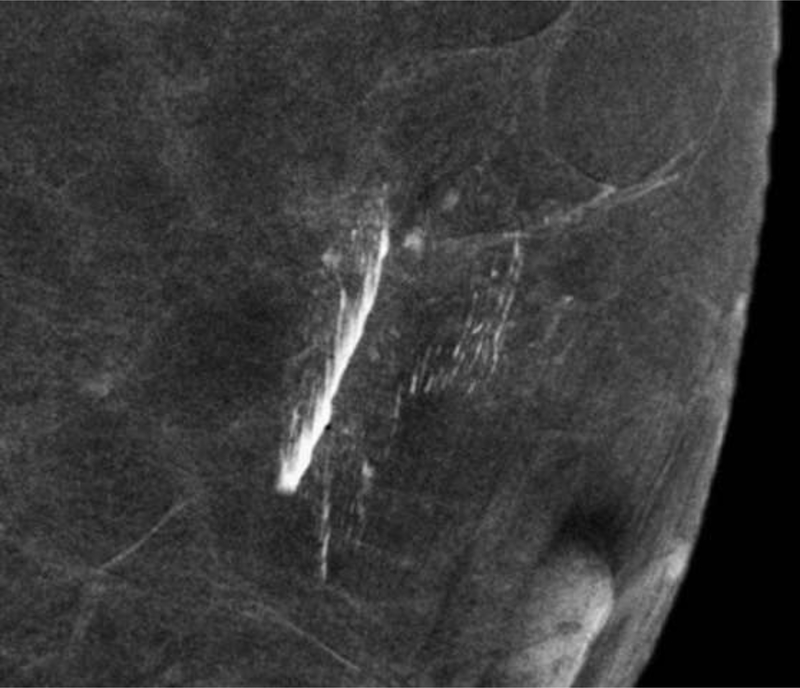
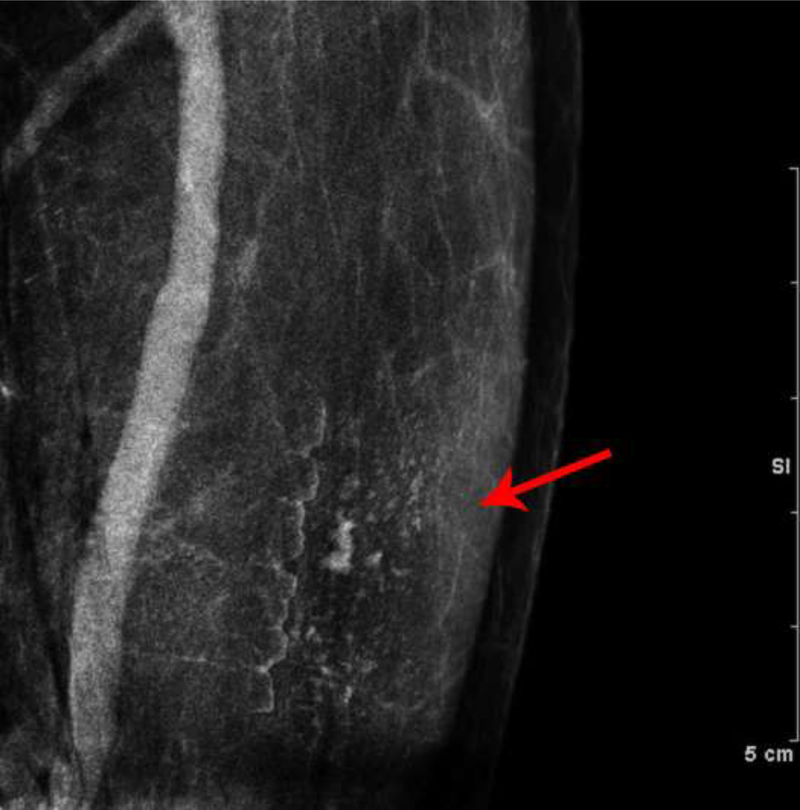
Fig. 6—
Phantom Images: Dual-energy images of a breast phantom after application of trace Omnipaque 350 contrast to the surface of the phantom. The low energy (A) and recombined (B) images demonstrate contamination artifact with iodinated contrast. This confirms that surface contamination with iodinated contrast is visible on LE images.
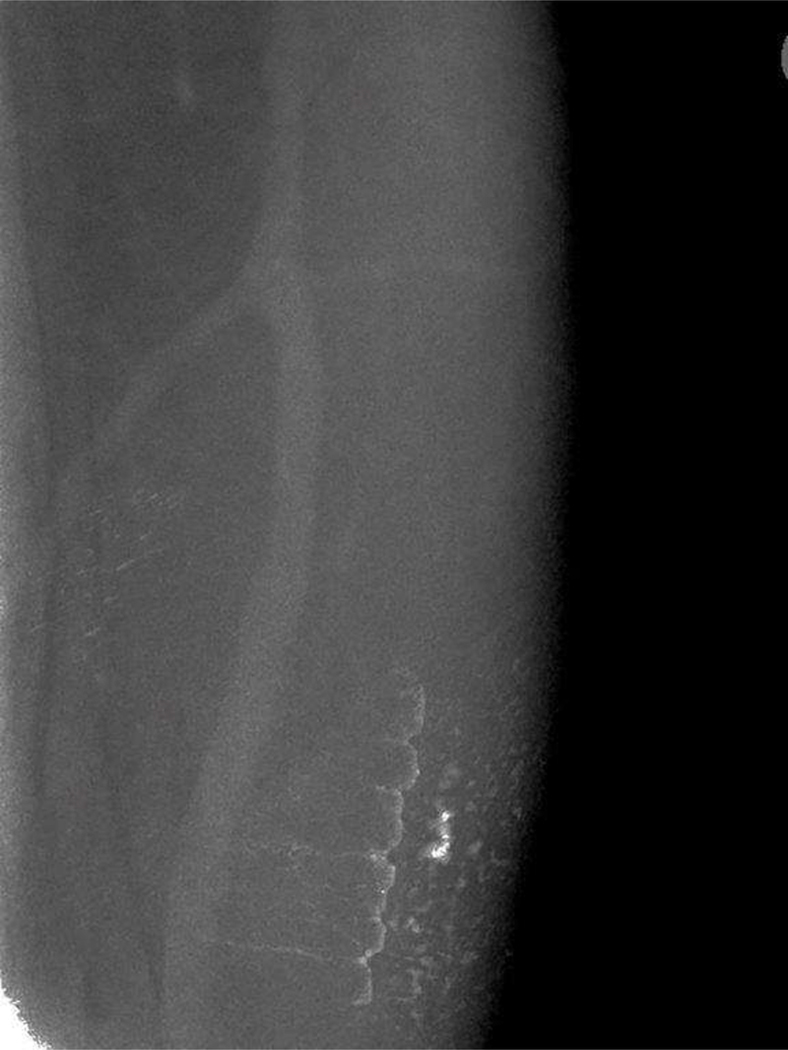
Fig. 7—
Skin Texture Model Images: Images of the superficial soft tissues of a volunteer’s left forearm were obtained with dual-energy mammographic technique, after a small amount of Omnipaque 350 contrast was spread thinly onto the skin. A) Low energy processed image, B) High energy unprocessed image, C) Low energy unprocessed image, and D) Recombined image. All images show the appearance of skin contamination with iodinated intravenous contrast material. This confirms that skin contamination with iodinated contrast is visible on LE images.
DISCUSSION
In this article we describe a previously unreported artifact of CEDM. We identified five patients with a suspicious pattern of non-mass enhancement and questioned calcifications on CEDM. These findings were similar in appearance to linear, non-mass enhancement on MRI and analogous to the branched linear distribution of calcifications on mammography, both of which are suggestive of ductal carcinoma in situ (DCIS). Each of these patients was further evaluated with additional imaging and one also had a core-needle biopsy. All additional imaging and the biopsy produced negative results. Thorough review of the image acquisition process suggested that the densities were not true calcifications nor enhancement, but potential artifact. The origin of this potential artifact was further investigated by imaging two breast phantoms, which replicated and confirmed the finding of skin contamination with iodinated contrast used for the CEDM study. A final diagnosis of iodinated contrast contamination artifact was made in each of these patients. All patients have been followed for at least one year with no evidence of cancer at the sites of artifact. Had this artifact been described previously, additional diagnostic workup and followup imaging could have been prevented.
Although the LE image is obtained after contrast injection, the iodinated contrast within the body is not captured on the low energy mammographic images. [6] This is because LE images are obtained at 26–30 kVp, below the K-edge of iodine at 33keV, thus minimizing iodine attenuation. [9] This study demonstrates that iodinated contrast on the skin is visible on the LE image, while the intravenous contrast is not visible. We hypothesize this is due to the higher concentration of iodinated contrast on the skin, which produces greater attenuation of photons, relative to the intravenous contrast distributed throughout the body. Increased visibility may also be due to slight magnification of iodine on the skin further from the detector.
If this finding is encountered in clinical practice, there are several scenarios which suggest that the CEDM finding may be artifactual: 1) if non-mass enhancement is only seen on one view and does not persist on additional or repeat imaging, and resolves after washing the breast; 2) if mammographic images suggest calcifications that do not persist on high quality magnification views, then the suspicious calcifications are likely an artifact, possibly shine-through from iodine contamination of the skin; and, 3) if suspicious non-mass enhancement does not persist on repeat contrast enhanced studies, such as CEDM or MRI, then an artifact is suggested. Nevertheless, a short interval follow-up CEDM should be performed to ensure that there is no underlying pathology.
Recognition of this potential artifact suggests safeguards to be implemented during acquisition of CEDM. To reduce skin contamination, the technologist or nurse handling the iodinated contrast equipment should wear gloves which are then removed prior to positioning the patient. Hand washing is recommended between handling contrast materials, such as IV tubing, and positioning the patient. The technologist should be mindful of possible contamination of the breast by contrast, either by sight or by recognizing that the breast is wet. If contrast-enhanced tomosynthesis is available, then skin contamination could be readily demonstrated.
CONCLUSION
We present five patients with artifactual apparent calcification and non mass enhancement on CEDM, not previously described in the literature. Our investigation identified this artifact as skin contaminated by the iodinated contrast used for the CEDM examination. This artifact, which may cause unnecessary diagnostic work up and followup exams, can be eliminated by using appropriate protocols when performing the exam.
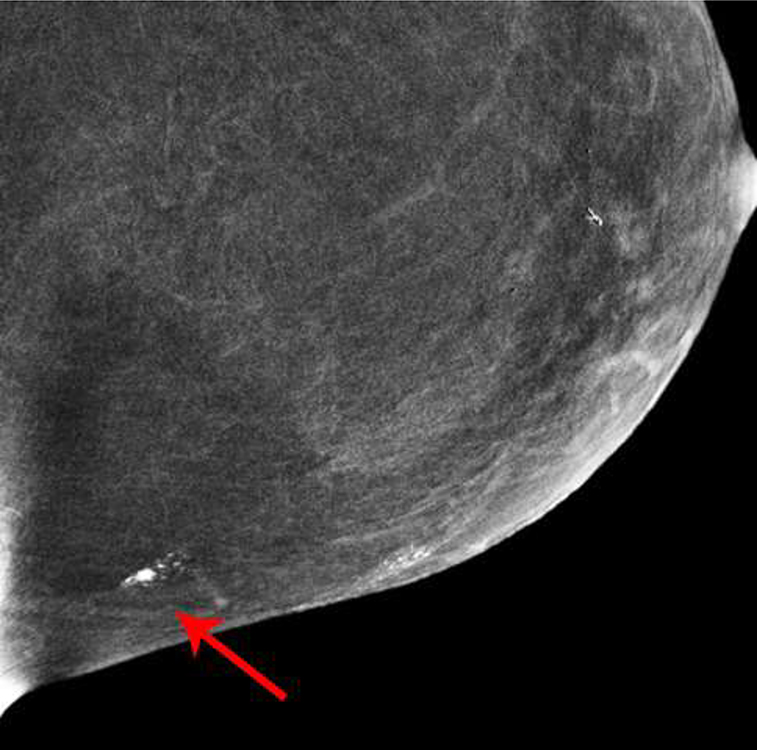
Fig. 5—
Left mammogram of Patient E, a 59-year-old female with right breast abnormality on screening mammogram, which was subsequently biopsied yielding Invasive Ductal Carcinoma and DCIS. Recombined images of the left breast CEDM demonstrate apparent linear non-mass enhancement in a superficial location in the outer (A) upper (B) quadrant.
Highlights.
IV contrast contamination of breast skin can mimic DCIS on CEDM.
Dot-dash pattern of enhancement may represent contamination artifact.
Suggest artifact if enhancement is only on one view.
Suggest artifact if enhancement doesn’t persist on repeat imaging.
If artifact is suggested, cleanse the breast prior to additional imaging.
Acknowledgments:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Funding:
This work was supported by the NIH/NCI (grant number Cancer Center Support Grant P30 CA008748). This funding source had no role in the design of this study and did not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Footnotes
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dromain C, Thibault F, Muller S, Rimareix F, Delaloge S, Tardivon A, Balleyguier C, Dual-energy contrast-enhanced digital mammography: initial clinical results, European radiology 21(3) (2011) 565–74. [DOI] [PubMed] [Google Scholar]
- [2].Chou CP, Lewin JM, Chiang CL, Hung BH, Yang TL, Huang JS, Liao JB, Pan HB, Clinical evaluation of contrast-enhanced digital mammography and contrast enhanced tomosynthesis--Comparison to contrast-enhanced breast MRI, Eur J Radiol 84(12) (2015) 2501–8. [DOI] [PubMed] [Google Scholar]
- [3].Fallenberg EM, Dromain C, Diekmann F, Engelken F, Krohn M, Singh JM, Ingold-Heppner B, Winzer KJ, Bick U, Renz DM, Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size, European radiology 24(1) (2014) 256–64. [DOI] [PubMed] [Google Scholar]
- [4].Cheung YC, Lin YC, Wan YL, Yeow KM, Huang PC, Lo YF, Tsai HP, Ueng SH, Chang CJ, Diagnostic performance of dual-energy contrast-enhanced subtracted mammography in dense breasts compared to mammography alone: interobserver blind-reading analysis, European radiology 24(10) (2014) 2394–403. [DOI] [PubMed] [Google Scholar]
- [5].Jochelson MS, Dershaw DD, Sung JS, Heerdt AS, Thornton C, Moskowitz CS, Ferrara J, Morris EA, Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma, Radiology 266(3) (2013) 743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Francescone MA, Jochelson MS, Dershaw DD, Sung JS, Hughes MC, Zheng J, Moskowitz C, Morris EA, Low energy mammogram obtained in contrast-enhanced digital mammography (CEDM) is comparable to routine full-field digital mammography (FFDM), European journal of radiology 83(8) (2014) 1350–5. [DOI] [PubMed] [Google Scholar]
- [7].Fallenberg EM, Dromain C, Diekmann F, Renz DM, Amer H, Ingold-Heppner B, Neumann AU, Winzer KJ, Bick U, Hamm B, Engelken F, Contrast-enhanced spectral mammography: Does mammography provide additional clinical benefits or can some radiation exposure be avoided?, Breast cancer research and treatment 146(2) (2014) 371–81. [DOI] [PubMed] [Google Scholar]
- [8].Lalji UC, Jeukens CR, Houben I, Nelemans PJ, van Engen RE, van Wylick E, Beets-Tan RG, Wildberger JE, Paulis LE, Lobbes MB, Evaluation of low-energy contrast-enhanced spectral mammography images by comparing them to full-field digital mammography using EUREF image quality criteria, European radiology 25(10) (2015) 2813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carton AK, Saab-Puong S, Suminski M, SenoBright Contrast Enhanced Spectral Mammography Technology, General Electric Company, Wauwatosa, WI, 2012, p. 7. [Google Scholar]
- [10].Yagil Y, Shalmon A, Rundstein A, Servadio Y, Halshtok O, Gotlieb M, Sklair-Levy M, Challenges in contrast-enhanced spectral mammography interpretation: artefacts lexicon, Clinical radiology 71(5) (2016) 450–7. [DOI] [PubMed] [Google Scholar]